Abstract
Thermotoga maritima is a hyperthermophilic bacterium but its genome encodes a number of archaeal proteins including S-adenosyl-L-homocysteine hydrolase (SAHase), which regulates cellular methylation reactions. The question of proper folding and activity of proteins of extremophilic origin is an intriguing problem. When expressed in E.coli and purified (as a homotetramer) at room temperature, the hyperthermophilic SAHase from T.maritima was inactive. ITC study indicated that the protein undergoes heat-induced conformational changes, and enzymatic activity assays demonstrated that these changes are required to attain enzymatic activity. To explain the mechanism of thermal activation, two crystal structures of the inactive form of T. maritima SAHase (iTmSAHase) were determined for an incomplete binary complex with the reduced cofactor (NADH), and in a mixture of binary complexes with NADH and with adenosine. In contrast to active SAHases, in iTmSAHase only two of the four subunits contain a bound cofactor, predominantly in its non-reactive, reduced state. Moreover, the closed-like conformation of the cofactor-containing subunits precludes substrate delivery to the active site. The two other subunits cannot be involved in the enzymatic reaction either; although they have an open-like conformation, they do not contain the cofactor, whose binding site may be occupied by an adenosine molecule. The results suggest that this enzyme, when expressed in mesophilic cells, is arrested in the activity-incompatible conformation revealed by its crystal structures.
Keywords: Cellular methylation, Heat-induced activation, X-ray crystallography
1. Introduction
Most biological transmethylation reactions are catalyzed by methyltransferases that utilize S-adenosyl-L-methionine (SAM) as the methyl group donor [1]. A byproduct of these reactions is S-adenosyl-L-homocysteine (SAH), which is a potent negative-feedback inhibitor of SAM-dependent methyltransferases. Therefore, SAH concentration has to be strictly controlled to ensure proper methylation activity in a living cell [2–4], In all organisms, this control function is fulfilled by two distinct enzymes, namely methylthioadenosine/S-adenosyl-L-homocysteine nucleosidase (MTAN, EC 3.2.2.9) or/and S-adenosyl-L-homocysteine hydrolase (SAHase, EC 3.3.1.1). MTAN hydrolyzes SAH to adenine and S-ribosyl-L-homocysteine, whereas SAHase catalyzes the reversible hydrolysis of SAH to adenosine (Ado) and L-homocysteine (Hcy). Depending on the organism, the genomes encode both enzymes or only one of them [5].
Thermotoga maritima is a hyperthermophilic bacterium with optimal growth temperature of 353 K [6], adapted to geothermally heated environment, such as hot springs or hydrothermal vents. Its genome contains two distinct genes involved in SAH metabolism, namely mtaN encoding methylthioadenosine/S-adenosyl-L-homocysteine nucleosidase and ahcY encoding S-adenosyl-L-homocysteine hydrolase (TmSAHase) [7]. The former gene encodes a bacterial enzyme with numerous orthologs in Eubacteria. On the other hand, ahcY is one of many genes of archaeal origin found in the T. maritima genome. The acquisition of the archeal-type ahcY gene was most likely a consequence of massive lateral gene transfer related to the adaptation to hyperthermophilic environment [5,7].
Insight into SAHase structure and function is based mainly on crystallographic studies reported for several enzymes. The first crystal structures were determined for mammalian SAHases [8,9]. Currently, the Protein Data Bank (PDB) stores models of SAHases from diversified organisms representing eubacteria Mycobacterium tuberculosis [10], Bradyrhizobium elkanii [11], Burkholderia pseudomallei (PDB entry 3GLQ; unpublished), Brucella melitensis (3N58; unpublished)], eukaryotic microorganisms [Plasmodium falciparum [12], Trypanosoma brucei (3H9U, unpublished), Leishmania major (3G1U; unpublished), Cryptosporidium parvum (5HM8; unpublished)], plants (Lupinus luteus [13]), and mammals (Mus musculus [14]). Recently, the first crystal structure of SAHase from the hyperthermophilic Thermotoga maritima in its active form has been determined and deposited in the PDB in the open (3X2E) and closed (3X2F) conformations, between which the enzyme oscillates during the catalytic cycle [15]. SAHases are oligomeric enzymes, usually active as homotetramers. The only exception are plant SAHases, which form active dimers in solution [13,16,17]. All SAHases require for their activity a tightly but non-covalently bound NAD+ cofactor, which is present in each subunit of the oligomeric protein.
A typical SAHase subunit is comprised of the substrate binding domain (residues 1–175 and 330–369 in TmSAHase numbering), cofactor binding domain (176–369) and a small C-terminal tail (370–404). The substrate and cofactor domains oscillate between two conformational states, known as open and closed, during the catalytic cycle. A channel leading to the active site can be open or shut depending on the conformation of a molecular gate formed by a tandem of His and Phe residues (284–285). In most SAHases, the C-terminal tail is a dimerization domain, mutually swapped between two tightly associated subunits, and participates in cofactor binding of the adjacent subunit. The tetramer is formed by side-by-side association of two intimate dimers. However, this is not the case with TmSAHase, where the C-terminal domain is shorter by eleven residues and therefore cannot be involved in cofactor binding across the intimate dimer [15].
Enzyme samples that are directly isolated form hyperthermophiles are usually properly folded and therefore fully active. A similar situation has been encountered in numerous cases of recombinant hyperthermophilic proteins expressed in mesophilic organisms and purified at room or even lower temperature. However, some exceptions have been reported, where the recombinant enzymes were expressed and purified in an inactive form as a result of improper folding. These include, for instance, D-glyceraldehyde-3-phosphate dehydrogenase from T. maritima [18], glutamate dehydrogenase from Pyrococcus sp. [19–22], indolepyruvate ferredoxin oxidoreductase from Pyrococcus kodakaraensis KOD1 [23], or sulfur oxygenase reductase from Aquifex aeolicus [24]. It is of note that most of these examples are enzymes utilizing a redox cofactor during the enzymatic reaction. Usually, the enzyme obtained from a mesophilic expression system can be converted to the active form using heat- or urea-induced activation, which involves changes in the oligomeric state or structural rearrangements within the same oligomeric form [21].
Here, we present a thermal activation study of an inactive form of E. coli-expressed SAHase from T. maritima (iTmSAHase). The results of enzymatic assays and isothermal titration calorimetry experiments suggest that the protein might be arrested in an activity-incompatible conformation, and that it can be converted to the catalytically competent enzyme by heat-induced activation. These observations were corroborated by our crystallographic structural characterization of iTmSAHase. This inactive form of TmSAHase was crystallized as an incomplete binary complex with the reduced form of the cofactor, NADH, and as a mixture of binary complexes with NADH and with adenosine, which is a substrate/product of the catalytic reaction. Both crystal structures show that the enzyme cannot be active because of the conformation adopted by the subunits. Moreover, the cofactor is present only in two subunits. Our results also indicate that the cofactor is incorporated into the protein structure during the expression step mainly in its reduced form, which does not support the catalytic reaction.
2. Material and methods
2.1. Cloning, expression, purification and preliminary characterization of the protein
The coding sequence of the ahcY gene was amplified by PCR from T. maritima MSB8 genomic DNA. The amplicon was cloned into pMCSG9 expression vector using the ligation independent cloning reaction and sequenced. The correct construct was transformed into BL21-CodonPlus(DE3)®-RIPL E. coli cells and expressed. 15mL of LB medium containing 34 μgmL−1 chloramphenicol and 100 μg mL−1 ampicillin were inoculated and grown overnight at 310 K and the overnight culture was used for inoculation of 1 L of LB medium, which was subsequently cultivated with appropriate antibiotics to an OD600 of ~1.0. The temperature was decreased to 291 K and protein expression was induced with IPTG at a final concentration of 0.3 mM. The cells were harvested 18 h after induction and flash-frozen in liquid nitrogen.
The cell pellet was resuspended in buffer A (20 mM imidazole, 500 mM NaCl, 20 mM Tris·HCl pH 8.0, 10% glycerol) with the addition of 1 mM TCE·HCl, 1 mM PMSF, l00 μg mL−1 lysozyme and 500 units of benzonase. Cells were disrupted by sonication on ice and centrifuged for removal of cell debris. Thermal precipitation, the standard step of purification of proteins from thermophilic organisms, was deliberately omitted in this study. The supernatant was loaded onto a HisTrap column equilibrated with 0.1 M NiSO4. The protein was eluted with a buffer containing 500 mM imidazole, 500 mM NaCl, 20 mM Tris·HCl pH 8.0,10% glycerol and 1 mM TCEP·HCl. TEV protease was added to the final concentration of 0.1 mg mL−1 to remove the His tag and the protein solution was extensively dialyzed against buffer A. After overnight incubation at 292 K, the solution was loaded onto a HisTrap column equilibrated with 0.1 M NiSO4 and the protein was eluted with buffer A, subsequently exchanged for buffer B (100 mM NaCl, 25 mM Tris·HCl pH 8.0) via dialysis. The protein solution was concentrated using Amicon Ultra 30 ultracentrifugation filters and loaded onto a Superdex 200 (Pharmacia) gel filtration column pre-equilibrated with buffer B supplemented with 1 mM TCEP·HCl. The protein was eluted with buffer B as a tetramer. Fractions with iTmSAHase were concentrated to 10 mg mL−1 by ultracentrifugation and the fresh protein solution was used for crystallization experiments. Protein concentration was estimated by UV absorption measured at 280 nm. The purified protein has the TmSAHase sequence, extended at the N-terminus by a short tripeptide (SNA-) cloning artifact. SDS-PAGE analysis confirmed the size of the expressed protein (~45 kDa). Finally, mass spectrometry was used to confirm the size and sequence of the recombinant enzyme.
2.2. SAHase activity assays
Assays for the enzymatic activity in the direction of SAH synthesis were carried out using ACQUITY UPLC® H-Class high performance liquid chromatograph (HPLC) with a photodiode array (PDA) eλ detector (Waters Corp., USA). Chromatographic separations were carried out using an XBridge BEH Shield RP18 column (5 μm, 4.6 mm × 150 mm) with Sentry Guard Cartridge (5 μm, 4.6 mm × 20 mm) (Waters, USA). For elution, a mobile phase containing methanol and water (12:88, v:v) was used. The flow rate was 0.8 mL min−1 and the injection volume was 20 μL. All chromatographic separations were performed at 298 K. Under the experimental conditions, the retention times of NAD+, SAH and Ado were 3.9, 4.4 and 6.5 min, respectively. The rate of SAH formation was monitored at 260 nm. The assays were performed in 1 mL volume in a buffer containing 150 mM KCl and 25 mM Tris·HCl pH 8.0, supplemented with 2 mM Ado and 4 mM L-Hcy. As a variable, tests were performed in the absence or presence of 5mM NAD+. Three different temperature-dependent conditions of the reaction were tested: (i) assays were performed at 298 K and monitored after 2, 6 and 24 h; (ii) iTmSAHase samples were incubated at 358 K for 30 min and subsequently chilled to 298 K prior to the assays conducted at 298 K and monitored after 2, 6 and 24 h; (iii) reactions were performed at 358 K and monitored after 1,2 and 4 h. As a negative control, reaction mixtures without the addition of the enzyme were used.
2.3. Determination of the oligomeric state in solution
The oligomeric state of the protein in solution was determined as a function of concentration and temperature. Size exclusion chromatography (SEC) was applied to confirm the homotetrameric state in the concentration range from 1 to 10 mg mL−1 at a constant, room temperature. The experiments were carried out using a Superdex 200 gel filtration column pre-equilibrated with buffer B and calibrated with a gel filtration standard (BioRad).
Dynamic light scattering (DLS) experiments were performed to establish the oligomeric state of the enzyme at various temperatures but at a constant concentration of 5 mg mL−1. Three scenarios were utilized: (i) unheated protein sample measured at 298 K; (ii) protein sample heated to 358 K for 30 min and then measured at 298 K; and (iii) protein sample heated to 358 K for 30 min and then measured at 358 K. All samples were filtered (0.1 μm) prior to the experiment.
2.4. Isothermal titration calorimetry
ITC measurements were performed at 293 K using a MicroCal iTC200 calorimeter (GE Healthcare). Prior to the titrations, the protein was dialyzed against a buffer containing 100 mM NaCl and 25 mM HEPES·NaOH pH 7.5. Two differently treated protein samples were used for the measurements: (i) protein incubated at 358 K for 30 min and cooled down to 293 K and (ii) unheated protein. Prior to the titrations, the protein concentration was estimated by the Bradford method. The final protein concentration was adjusted to 86 μM or 106 μM. Adenosine, NAD+ and adenosine 5′-monophosphate (AMP) were dissolved in the dialysis buffer to the concentrations of 1.5 mM, 0.5 mM and 1.0 or 1.5 mM, respectively. The ligands were injected to the protein solution (300 μL) in 2 μL aliquots until saturation was observed. The raw ITC data were analyzed with the ORIGIN 7.0 software (OriginLab) to obtain the thermodynamic parameters of the complexation reactions: stoichiometry (N), dissociation constant (Kd), as well as changes in enthalpy (ΔH) and entropy (ΔS). For sigmoidal titration curves, the one set of sites model was fitted and N was obtained from the experiment. For hyperbolic curves, for which the determination of N is impossible, N was fixed while fitting the same model. All measurements were performed in triplicates.
2.5. Determination of the redox state of the cofactor
The oxidized:reduced ratio of the cofactor in a sample of iTmSAHase was determined spectrofluorimetrically by excitation at 340 nm and measurement of emission at 460 nm, as described previously [25].
2.6. Crystallization
Initial screening for crystallization conditions was performed with Robotic Sitting Drop Vapor Diffusion setup (Mosquito) using Crystal Screen 1 from Hampton Research. The protein was subjected to crystallization in the absence or presence of adenosine. In the latter case, prior to crystallization trials, a portion of the protein solution in buffer B was incubated overnight at room temperature with 2 mM adenosine. For both samples, initial crystals were obtained from 20 mM CaCl2, 0.1 M sodium acetate pH 4.6 and 30% (v/v) MPD. The conditions were manually refined by adjusting the protein concentration, pH and type of divalent cation. Crystallization drops were mixed from 2 μL of the protein solution (6 mg mL−1) incubated without (crystal 1) or with (crystal 2) adenosine, and 2 μL of the reservoir solution containing 20 mM MgCl2 or CaCl2, 0.1 M sodium acetate, pH 4.6 or 5.3, and 30% (v/v) MPD for crystal 1 or 2, respectively. Diffraction-quality crystals were grown within a few days at 292 K using the hanging-drop vapor-diffusion method.
2.7. X-Ray data collection and processing
X-ray diffraction data were measured at the Advanced Photon Source (ANL, Argonne, IL, USA) SER-CAT 22-BM beamline at 100K using a Mar 225 mm CCD detector. Crystals were fished out directly from the mother liquor and vitrified in liquid nitrogen. Diffraction data extending to 1.90/1.75 Å resolution were obtained from 180/360 images recorded with 1.0°/0.5° oscillation at 200/180 mm crystal-to-detector distance for crystal 1/2, respectively. Indexing and integration of all images was performed in DENZO and scaling in SCALEPACK from the HKL2000 package [26]. The crystals are isomorphous, with the monoclinic space group C2. The final data sets are characterized in Table 1.
Table 1.
Crystallographic data and data collection and structure refinement statistics.
| Data collection and processing statistics | |||
| Data set | iTmSAHase-NADH | iTmSAHase-NADH-Ado | |
| Beamline | APS SER-CAT 22-BM | ||
| Wavelength (Å) | 0.97242 | 0.97856 | |
| Temperature (K) | 100 | ||
| Space group | C2 | ||
| Cell dimensions (Å,°) | |||
| a | 120.4 | 120.4 | |
| b | 106.7 | 105.5 | |
| c | 85.9 | 85.5 | |
| β | 109.2 | 108.8 | |
| Mosaicity (°) | 0.27 | 0.52 | |
| Resolution range (Å) | 3(1.0 1.9(1(1.93 1.90)a | 30.0–1.75(1.81–1.75)a | |
| Total/unique reflections | 306144/80548 | 387679/101542 | |
| Completeness (%) | 99.6 (92.7) | 99.9(100) | |
| Multiplicity | 3.8 (3.2) | 3.8 (3.8) | |
| <I/σ(I)> | 23.4(2.1) | 21.9 (2.3) | |
| Rmerge b | 0.056 (0.505) | 0.051 (0.589) | |
| Refinement statistics | |||
| Resolution (Å) | 30.0–1.90 | 30.0–1.75 | |
| No. of reflections in working/test set | 79463/1069 | 100299/1169 | |
| R/Rfreec (%) | 16.1/19.7 | 16.6/18.8 | |
| No. of atoms (protein/ligand/water/Cl−) | 6198/44/491/2 | 6215/71/581/2 | |
| R.m.s. deviation from ideal | |||
| bond lengths (Å) | 0.014 | 0.015 | |
| bond angles (°) | 1.595 | 1.674 | |
| Average B factor (Å2) | 40.3 | 39.5 | |
| Rama chandran statistics (%) most favored/allowed regions | 98/2 | 99/1 | |
| PDB code | 5TOV | 5TOW | |
Values in parentheses correspond to the last resolution shell.
Rmerge = Σhkl Σi|Ii(hkl) − <I(hkl)>|/ΣhklΣiIi(hkl), where <I(hkl)> is the average intensity of reflection hkl.
R= 100·Σhkl||Fo(hkl)| − |Fc(hkl)||/Σhkl|Fo(hkl)|, where Fo and Fc are the observed and calculated structure factors, respectively. Rfree is calculated analogously for the test reflections, randomly selected and excluded from the refinement.
2.8. Structure solution and refinement
2.8.1. iTmSAHase-NADH-adenosine complex
The crystal structure of iTmSAHase was solved by molecular replacement, as implemented in the program PHASER [27] from the CCP4 suite [28]. The substrate- (residues 18–181 and 355–421) and cofactor-binding (182–354) domains of a model of H. sapiens SAHase (PDB entry 1LI4) [29] were used as search probes. The correct solution was found in space group C2 for two different-conformation subunits in the asymmetric unit. Automatic model building was carried out with the online version of ARP/wARP [30]. Isotropic stereochemically-restrained structure-factor refinement was carried out in REFMAC5 [31] with maximum-likelihood targets and with the inclusion of three TLS groups [32] per protein chain, defined as suggested by the TLSMD server [33]. In the final model, one subunit (B) has the cofactor-binding domain occupied by a molecule of the reduced form of the cofactor, NADH, while the other subunit (A) has a molecule of adenosine in the cofactor binding site. The ligands were identified without ambiguity in mFo-DFc omit electron density maps phased with the contribution of the protein atoms only. Additionally, 581 water molecules, one molecule of MPD and two chloride anions were identified in the asymmetric unit. Geometrical restraints for the NADH and adenosine molecules were generated using the Grade Web server [34]. Electron density was not defined for some parts of the structure and residues 169–174, 330–334 and 401–404 of chain A, as well as four N-terminal residues (including the artifactual tripeptide) of both chains were not modeled.
2.8.2. iTmSAHase-NADH complex
The iTmSAHase-NADH-adenosine complex model stripped of the adenosine molecule was transferred to the nearly identical unit cell of the iTmSAHase-NADH crystal. The refinement was carried out as for the above crystal structure. The final rounds of refinement included 491 water molecules, in addition to one NADH molecule and two chloride anions. As in the iTmSAHase-NADH-adenosine structure, electron density was not defined for some parts of the structure and residues 172–177, 283–284, 290, 330–334 and 498404 from chain A, as well as four N-terminal residues from both chains were not modeled.
2.8.3. Final refinement and validation
The COOT program [35] was used for manual modeling in electron density maps. Stereochemical quality of the models was assessed using the wwPDB validation pipeline [36]. Final refinement statistics for both crystal structures are reported in Table 1. The atomic coordinates and structure factors have been deposited in the Protein Data Bank with the accession codes 5TOV (iTmSAHase-NADH, incomplete binary complex), and 5TOW (iTmSAHase-NADH-adenosine, a mixture of two binary complexes). Raw diffraction images for both structures were deposited in the Integrated Resource for Reproducibility in Macromolecular Crystallography (ProteinDiffraction.org) with DOI numbers 10.18430/M35TOV and 10.18430/M35TOW for the 5TOV and 5TOW models, respectively.
2.9. Other software used
The SSM algorithm [37] was used for Cα superposition of protein models. Protein-ligand interactions were analyzed with PDBsum [38]. Molecular figures were generated in PyMOL [39], with mFo-DFc and 2mFo-DFc electron density maps calculated in PHENIX [40]. Protein domain movements were analyzed with the DynDom server [41] using chain A of the native TmSAHase models (PDB IDs 3X2E and 3X2F) and chains A and B of iTmSAHase in the mixture of binary complexes with NADH and adenosine from this work. Interaction-interface areas were calculated with the PDBePISA server [42].
3. Results and discussion
3.1. Evaluation of enzymatic activity
Since the reaction catalyzed by SAHase is reversible and strongly shifted in the direction of SAH synthesis, we used this synthetic direction (spectrophotometric detection of SAH) in our assays of catalytic activity. In all assays conducted at room temperature and at 358 K without the addition of NAD+, no traces of SAH were detected even after 24 h of incubation. Conversely, all tests performed in the presence of the oxidized form of the cofactor, led to SAH synthesis, although the efficiency was highly variable. These activity assays clearly indicate that the addition of NAD+ to the reaction mixture is essential for enzymatic activity. Very high enzymatic activity was observed when the reaction was carried out at 358 K. However, only traces of SAH could be detected even after 24 h for the reactions performed at 298 K using either heated or unheated enzyme in the presence of NAD+. The efficiency of these reactions was very low and the enzymatic activity was at 0.9 and 0.4%, respectively, when compared with the reaction at 358 K.
3.2. Determination of the oligomeric state
iTmSAHase is inactive in solution at room temperature, although, similarly to the native enzyme, it also exists exclusively as a homotetramer, even at concentrations as low as 1 mg mL−1 and at a broad range of temperatures, as confirmed by SEC FPLC and DLS experiments (Supplementary materials, Figs. S1A, B). The DLS experiments indicated a small but measurable change of the molecular size of differently heat-treated samples. Similar averaged sizes of 94.5 ± 9.9 Å and 97.0 ± 12.4 Å were established at 298 K for the unheated and heated iTmSAHase samples, respectively. The homotetramer average size increased to 105.3 ± 6.4 Å at 358 K.
3.3. Calorimetric evaluation of heat-induced conformational changes
The ITC isotherms obtained upon NAD+ titration of unheated protein were hyperbolic and the stoichiometry (N) was fixed at 0.25 (calculated for the monomer) while fitting the one set of sites model. This indicates that only one subunit out of four could bind NAD+; fixing N at 0.5 was questionable as it resulted in poor fit and the error in Kd determination was close to 100%. For the isotherms obtained for adenosine titrations, N also had to be fixed, while fitting the one set of sites model, again because of the hyperbolic shape of the curves. In this case N was fixed at 0.5 (corresponding to ligand binding by two out of four subunits) and not at 1 (which would correspond to ligand binding by all four subunits, as observed in active SAHases). The reason for this choice of N was the same as in the case of the NAD+ isotherms, namely fixing N at 0.75 or 1 resulted in large errors, while assuming N equal to 0.5 resulted in the best fit.
After the heat treatment, an increase of affinity for NAD+ (~15 times higher) as well as for adenosine (~2 times higher) is observed. Moreover, binding of NAD+ by heat-treated protein is more specific, as the shape of the isotherm changes from hyperbolic to sigmoidal. Additionally, a decrease of the enthalpy contribution with a simultaneous increase of the entropic contribution to the complexation reactions is observed for both ligands after the heat treatment. These observations suggest that upon heat treatment at 358 K, iTmSAHase undergoes conformational changes that consequently result in stronger and more specific ligand binding, as compared to the treatment at 293 K. For the sigmoidal isotherms obtained upon titrations of the heat-treated protein with NAD+, a one set of sites model was fitted and the Hill constant N of 0.26 was estimated experimentally. This stoichiometry could be explained by a situation where only one cofactor molecule is bound to one of the two cofactor-free subunits of the tetramer with a change of enthalpy. Therefore, we suggest that under the experimental conditions used, NAD+ binding by the two cofactor-free subunits of the tetramer is not identical, i.e. that the first ligand must bind with an enthalpy change, while the second one with unmeasurable heat effect.
Since adenosine can be considered to be a fragment of NAD+, the question arises where exactly this nucleoside binds to the protein upon ITC titration of unheated and heated protein. Due to structural mimicry, adenosine could bind in the substrate or cofactor binding sites, or simultaneously in both places. To clarify this point, a titration with adenosine 5′-monophosphate (AMP), a larger fragment of the cofactor, was also performed. Titrations of the unheated and heated protein with AMP produced similar results as in the case of adenosine. For both isotherms, N was successfully fixed at 0.5 while fitting the one set of binding sites model to the hyperbolic titration curves. These observations suggest that adenosine and AMP occupy the same position. Additionally, in both experiments, only two ligand molecules are bound to the protein tetramer. Moreover, our crystallographic results obtained for iTmSAHase incubated with high concentration of adenosine (2 mM) indicate that the nucleoside is bound only in the cofactor binding domain (vide infra). Therefore we suggest that adenosine (and AMP) is bound in the cofactor binding domain during the ITC experiments as well.
Ihe main conclusion from the calorimetric experiments is that there is an increase in the strength and specificity of NAD+ binding after heat treatment, which is an indication of a conformational change. The results of the ITC measurements are summarized in Table 2 and Fig. 1.
Table 2.
Thermodynamic parameters for interactions of the inactive (iTmSAHase) and heat-treated (htTmSAHase) protein with adenosine (Ado), NAD+ and adenosine 5′-monophosphate (AMP), obtained from ITC titration (protein/titrant).
| iTmSAHase/Ado | htTmSAHase/Ado | iTmSAHase/NAD+ | htTmSAHase/NAD+ | iTmSAHase/AMP | htTmSAHase/AMP | |
|---|---|---|---|---|---|---|
| Kd [μM] | 19.0± 1.5 | 8.8 ±1.2 | 9.4 ±1.2 | 0.63 ±0.04 | 30.0 ±3.0 | 25.0 ±2.0 |
| N | 0.5/fixed | 0.5/fixed | 0.25/fixed | 0.262 ±0.002 | 0.5/fixed | 0.5/fixed |
| ΔH [cal/mol] | −5709± 157 | −4612 ±160 | −8839 ±362 | −5026 ± 54 | −1923 ±88 | −1832 ±44 |
| ΔS [cal/mol/deg] | 2.1 | 7.4 | −7.2 | 11.2 | 14.1 | 14.8 |
Fig. 1.
Calorimetric titration of the protein with adenosine (A, B), NAD+ (C, D) and AMP (E, F). Experiments (A), (C) and (E) were conducted using unheated protein (iTmSAHase) whereas in (B), (D) and (F) the protein was preincubated at 358 K (htTmSAHase). The top plot of each panel represents the raw heat data obtained from 20 consecutive 2 μL injections of 1.5 mM (E) or 1.0 mM (F) of AMP, and 1.5 mM adenosine or 0.5 mM NAD+ into the sample cell (200 μM) containing 86 μM (A,B,D,F) or 106 μM (C,E) protein. The heat peak areas have been plotted against the molar ratio of adenosine/NAD+/AMP added to the protein, creating the binding isotherm at the bottom of each panel. The best fit of the model of one set of binding sites is represented by the solid line. All titrations were performed at 293 K.
3.4. Experimental conditions of ITC analysis
A note is required to discuss the level of compatibility of the crystallographic experiments, based on crystals grown at acidic pH, and the ITC experiments carried out at neutral conditions. Firstly, we note that our numerous attempts to obtain iTmSAHase crystals at neutral or basic pH have been unsuccessful. In fact, under those conditions hardly any precipitate was observed. The only crystals were obtained at pH 4.6 (iTmSAHase-NADH) and 5.3 (iTmSAHase-NADH-Ado). These observations suggested that acidic pH promotes iTmSAHase crystallization by reducing protein solubility. It is of note that although the two crystals of iTmSAHase complexes were obtained at different pH values of sodium acetate (4.6 and 5.3), their structures are nevertheless very similar. This might suggest that pH does not affect the structure significantly, at least in this pH range. We also note that the real pH values in crystallization drops were somehow higher than nominal, mainly as the result of the presence of Tris buffer of pH 8.0 in the protein solution. To test this, we prepared mock solutions imitating the crystallization drop conditions, and their pH values were higher, 5.2 and 5.7, respectively.
We also tried to conduct the ITC analysis at acidic conditions. Unfortunately, such a study was not possible in the pH range between 5 and 6, for two reasons. (i) The solubility of iTmSAHase is much lower at acidic conditions. We tried to dialyze the protein at 4 °C or room temperature against buffers containing MES, sodium citrate or sodium acetate. After dialysis and centrifugation the protein concentration could be increased to 50 μM, but it was too low to detect any ITC heat effect. Additionally, we observed slow but gradual precipitation of the protein in the supernatant with time (in the crystallization drops the protein was additionally stabilized by high MPD content; however, due to its high viscosity MPD cannot be used in ITC titrations). Our further study indicated that iTmSAHase is not denatured at acidic pH, as the protein was dissolved completely when the pH was increased to 7.5. DLS analysis confirmed that the protein is a tetramer in solution, (ii) When acidic solutions (including MES, sodium acetate or citrate) of iTmSAHase for ITC analysis were heated to 85 °C, protein denaturation was observed. It is of note that no denaturation occurred at neutral pH.
Although it was not possible to directly compare the conditions of the crystallographic and biophysical studies, the experiments performed at acidic and neutral pH lead to similar conclusions. The results indicate that only two of four subunits can bind adenosine molecules in the cofactor binding domains. Moreover, the crystal structure of the iTmSAHase-NADH-Ado complex explains why only two of the four subunits can bind adenosine molecules.
3.5. Overall structure of the inactive form of TmSAHase (iTmSAHase)
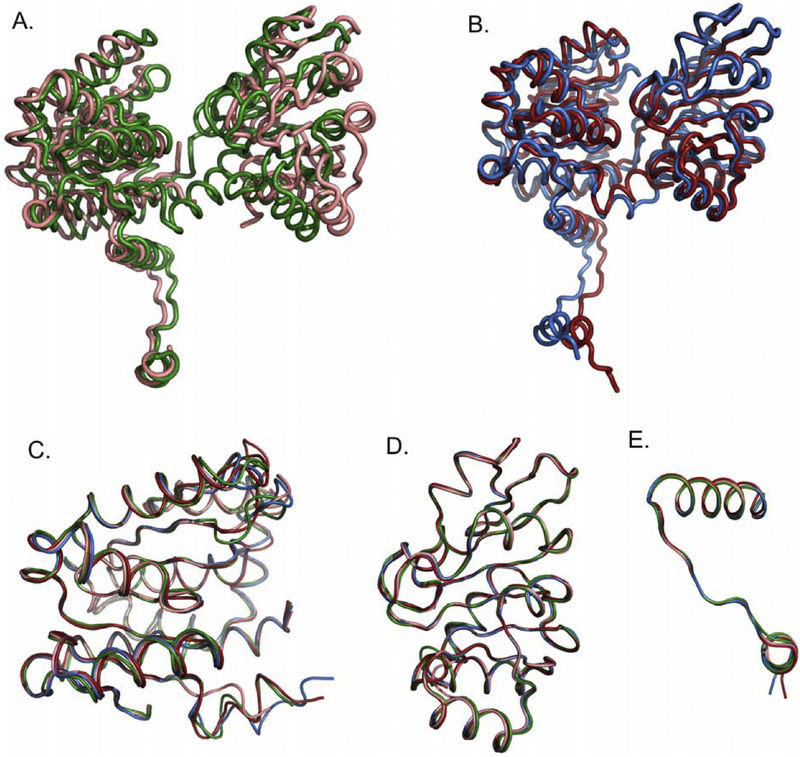
iTmSAHase crystalizes in space group C2 with two subunits (A and B) in the asymmetric unit. Interestingly, these two subunits have different conformation and do not interact with each other to form the intimate SAHase dimer. Crystallographic symmetry generates two intimate dimers from the two subunits, i.e. AA’ and BB’, and the complete tetramer, composed of two intimate dimers of different conformation, has the form AA’BB’ (Fig. 2). The structures of the incomplete binary and mixed binary complexes of iTmSAHase are very similar. The r.m.s. deviation between the two complete tetramers is 0.35 Å over 1560 Cα atoms. Also, the mode of NADH binding in the two structures is nearly identical. Since the crystal structure of the mixture of binary complexes with NADH and Ado was determined at higher resolution (1.75Å), it will be used for further descriptions unless stated otherwise.
Fig. 2.
Overall structure of iTmSAHase, described as a dimer of intimate dimers (AA’-BB’). Schematic diagram of the inactive enzyme crystallized as an incomplete binary complex (A) and as a mixture of binary complexes (B) with the ligands, and after 90° rotation presenting a view of subunits BB’ in the closed conformation with NADH bound (C), and a view of subunits AA’ in the open conformation with adenosine bound (D). The NADH cofactor (red) and adenosine (blue) are shown in space-filling representation. Panels (B), (D) illustrate the iTmSAHase-NADH-Ado complex. In the iTmSAHase-NADH complex (A), (C) the sites filled in iTmSAHase-NADH-Ado with adenosine (blue) are empty.
The organization of the iTmSAHase tetramer is different when compared with that observed in all crystal structures of active SAHases. Moreover, the arrangement of the domains within the subunits differs from the typical scheme characteristic of active SAHases. In most cases reported to date, all four subunits of the tetramer uniformly adopt the open conformation in ligand-free from, or the closed conformation when complexed with an active-site ligand. An exception is the crystal structure of SAHase from Bradyrhizobium elkanii, where only three subunits bind the serendipitous adenosine ligand and adopt the closed conformation, while the fourth subunit is open [11], or where a mixture of open, closed and intermediate states is possible in different ligand complexes [43].
In the mixed binary complex of iTmSAHase, two subunits (A and A’) exist in an open-like conformation and each of them binds one Ado molecule in the cofactor-binding domain if this ligand is added to the protein. Intriguingly, the cofactor-binding site of the subunits in the open-like conformation is not occupied by the cofactor even when the protein samples have been incubated overnight with 5 mM NAD+ (~50 fold molar excess) prior to the last step of FPLC purification (data not shown). The two other subunits (B and B’) are in a closed-like conformation and each contains one cofactor molecule in the cofactor binding site, but no ligand in the substrate binding site. The presence of the two cofactor molecules in these two subunits is independent of the purification procedure, which implies that they are incorporated into the complex during protein expression. The tetramer structures, together with the associated ligands are shown in Fig. 2A-D. The Cα traces of the open-like and closed-like subunits deviate by ~4.40 Å, attesting to their significantly different conformation, illustrated in Fig. 3A.
Fig. 3.
Overall structure of iTmSAHase subunits. (A) Superposition of the subunits in the closed (blue) and open (salmon) conformation. The cofactor (red) and adenosine (blue) are shown in space-filling representation. (B) Superposition of the closed and open subunits as in (A) but with an indication of main-chain rigidity, as expressed by the ADP values of main-chain atoms, ranging from 20 (blue, thin line) to 80 Å2 (red, thick line). Encircled numbers indicate the molecular hinges connecting the substrate- and cofactor-binding domains, and involved in domain rearrangement: 1 (residues 162–181) and 2 (residues 327–336).
An important consequence of the asymmetric structure of the iTmSAHase tetramer is that there are significant differences between the interface areas and numbers of polar contacts within the interacting subunits. Specifically, the interaction interface area between the two subunits in the open-like conformation (AA’) is 1822 Å2, with 18 polar contacts, whereas for the two subunits in the closed-like conformation (BB’) the area is much larger, 2414 Å2, with 28 polar contacts. The interface area between subunits with different conformation (AB or A’B’) is 1376 Å2 with 29 polar contacts.
Such a quaternary structure of the tetramer explains why the iTmSAHase protein is inactive. The two subunits in the open-like conformation do not contain the cofactor molecule that is essential for the enzymatic reaction. The two other subunits adopt a closed-like conformation, and the entrance (access channel) to the substrate-binding pocked is shut by a molecular gate formed by a pair of highly conserved His284 and Phe285 residues [10,11,13]. In consequence, this conformation precludes any possibility of substrate binding. Moreover, even though the closed-like subunits do contain a cofactor molecule, it is predominantly in the reduced state, as determined spectrofluorimetrically for freshly purified iTmSAHase samples (72 ± 12% of total cofactor content). Therefore, the majority of the bound cofactor molecules are incapable of supporting the key step of the catalytic reaction, i.e. hydride anion abstraction from the C3 atom of the substrate [8,9,44–46].
A detailed comparison indicates additional differences between the subunits in the two states, including their rigidity and completeness of the models. The subunit in the open-like conformation is more flexible, with the mean ADP (atomic displacement parameter) value for main-chain atoms of 40.4 Å2. The corresponding value for the subunit in the closed-like conformation is lower, 36.4 Å2. However, rigid and flexible regions map to the same parts of the subunits, as illustrated in Fig. 3B. Additionally, the model of the subunit in the open-like conformation is less complete and some amino acid residues (169–174 and 330–334) of the two hinge regions linking the substrate and cofactor domains are not defined in the electron density.
A closer inspection of the subunit conformations, as well as detailed comparisons with the structure of the catalytically active protein, clearly indicate that the present subunits cannot be described as having the classical open or closed form. To find out if the structural differences arise from different subunit/domain structure or/and from different domain/subunit orientation, a series of Secondary-Structure Matching (SSM) comparisons of the full subunits as well as of the substrate-, cofactor- and dimerization-domains were performed. The results are illustrated in Fig. 4 and summarized in Table 3. A superposition of the iTmSAHase subunit referred to as “open-like" with the open and closed subunits of the native TmSAHase structure (PDB IDs 3X2E and 3X2F) reveals a poor match, with Cα r.m.s.d. values of 3.42 and 3.82 Å, respectively. A closer match is observed when the iTmSAHase subunit referred to as “closed-like" is superposed on the open and closed subunits of native TmSAHase, the r.m.s.d. values being 2.78 and 1.63 Å, respectively. Thus, the closest similarity is observed when the closed subunits are compared. In this comparison, the substrate-and cofactor-binding domains superpose nearly perfectly. However, the relatively high overall r.m.s.d. of 1.63 Å results from a markedly different orientation of the C-terminal dimerization domains. Superpositions of all corresponding individual domains extracted from the open and closed conformations of the native and inactive TmSAHase models are characterized by low r.m.s.d. values between 0.27 and 0.89 Å. These comparisons indicate that the individual domains of the inactive protein are properly folded, but oriented in an incorrect way. Evidently, this aberrant tertiary structure of domain orientations is linked to the low temperature of the protein expression and purification protocols.
Fig. 4.
Superpositions of subunits and individual domains extracted from models of the inactive (present work, mixture of binary complexes) and active (PDB entries 3X2E/3X2F for open/closed conformation, respectively) forms of S-adenosyl-L-homocysteine hydrolase from T. maritima. (A) Subunits in the open conformation; (B) subunits in the closed conformation; (C) substrate-binding domains; (D) cofactor-binding domains; (E) C-terminal dimerization domains. The colour code indicates the subunits/domains as follows: salmon, iTmSAHase, open-like conformation; green, TmSAHase, open conformation; blue, iTmSAHase, closed-like conformation; red, TmSAHase, closed conformation.
Table 3.
Secondary-Structure Matching (SSM) comparison of subunits and individual domains of native (TmSAHase) and inactive (iTmSAHase) forms of S-adenosyl-L-homocysteine hydrolase from T. maritima in various conformations. The results are presented as r.m.s.d. (Nalign), where r.m.s.d. is the Cα root-mean-square deviation (Å) between probe and target, and Nalign is the number of matched residues. SBD is substrate-binding domain (residues 2–175 and 330–369), CBD is cofactor-binding domain (176–329), and CTD is C-terminal domain (370–402). The subunits and domains have been extracted from chain A of the native TmSAHase models in the open (PDB ID 3X2E) and closed (3X2F) conformations, and from chains A and B of iTmSAHase in the binary complexes (this work).
| TmSAHase closed conformation | TmSAHase open conformation | |
|---|---|---|
| iTmSAHase subunit open-like conformation | 3.82 (335J/3.79 (333) | 3.42 (359)/3.48 (356) |
| iTmSAHase subunit closed-like conformation | 1.63 (390)/1.56 (391) | 2.78 (371)/2.76 (380) |
| iTmSAHase SBD open-like conformation | 0.76 (196)/0.72 (200) | 0.62(1935/0.69 (200) |
| iTmSAHase SBD closed-like conformation | 0.89 (208)/0.91 (208) | 0.79 (203)/0.83 (205) |
| iTmSAHase CBD open-like conformation | 0.36 154),«1.4 3 149 | 0.44 (150)/0.53 (149) |
| iTmSAHase CBD closed-like conformation | 0.27 (154)/0.26 (154) | 0.44 (152)/0.51 (154) |
| iTmSAHase CTD open-like conformation | 0.38 (31 )/0.40 (28) | 0.40 (31)/0.38 (28) |
| iTmSAHase CTD closed-like conformation | 0.60 (35)/0.63 (35) | 0.36 (32)/0.37 (32) |
3.6. Analysis of domain movement
As concluded above, the domains of iTmSAHase are properly folded but their spatial arrangement is different from that observed in the native enzyme. To elucidate this behavior, domain movement analysis was performed to compare the inactive and active forms of the protein. In general, the rearrangement is seen as a movement of the cofactor-binding domain towards the substrate-binding domain with a concomitant closure of the gap between these domains. As a consequence, the substrate and the cofactor are brought together to allow the enzymatic reaction to proceed. However, there are significant differences between TmSAHase and iTmSAHase in the mode of “conformational breathing" during the closed-open transition. For the native enzyme, the domain movement can be described as a pure rotation by about 14° around the molecular hinge section. A different situation is observed for the inactive protein, where the rotation angle around the molecular hinge is larger (~20°) and is coupled with a translation along the rotation axis by ~10 Å. The existence of two modes of reorientation of the cofactor-binding domain is also reflected in differences in the molecular hinge sections. Although the two distinct hinge elements are formed by similar regions of the active and inactive enzyme, they differ slightly in their length and precise location. According to DynDom analysis, in iTmSAHase the molecular hinge elements are longer and cover residues 162–181 and 327–336, while in the active enzyme both elements are shorter, 169–182 and 335–336.
3.7. The mode of adenosine binding in the cofactor-binding domain
In the mixed binary complex of iTmSAHase, two subunits in the open-like conformation bind adenosine molecule in the cofactor binding domain (Fig. 5A). The nucleoside occupies a small cavity at the protein surface (Fig. 5C) that is different from the original substrate binding site. In this complex of iTmSAHase, the adenine moiety is docked within the cavity, while the ribose ring is partially exposed to the solvent region. Amino acid residues of the subunit (chain A) in which the ligand is docked participate only indirectly, via water molecules, in adenosine binding. Direct hydrogen bonds to the adenosine molecule are provided only by amino acid residues from the cofactor binding domain of the symmetry-related subunit (chain A’) within the intimate dimer. In the active form of the enzyme, these residues would be involved in cofactor binding. Specifically, these interactions are formed by side-chain atoms of residues including Glu226 (Oε2…O2′), Lys231 (Nζ…O3′), Asn261 (Nδ2…N7) and by the main-chain carbonyl O atom of Ser259 (O…O5′). The adenine ring is sandwiched between the side chains of Val227 and Ser259, accepting C—H…π hydrogen bonds. Additionally, three water molecules form hydrogen bonds with the N1 and N6 atoms of the adenine base and the O2′/O3′ hydroxyl groups of the ribose moiety. This binding mode of the adenosine molecule is very similar to that observed for the adenosine moiety of the NADH cofactor bound in subunits B and B’ of the tetramer (Fig. 5A,B).
Fig. 5.
The mode of adenosine (A) and NADH (B) binding in the cofactor binding site of iTmSAHase. Only side chains involved in polar or hydrophobic interactions and water molecules (red spheres) are shown. Potential hydrogen bonds are indicated by broken lines. The mF0-DFc difference electron density map is contoured at 3.0σ. (C) Illustration of adenosine binding in the cofactor binding pocket. The ligand and protein are shown in space-filling and surface representation, respectively. (D) Illustration of the non-planar conformation of the six-membered ring of the nicotinamide moiety of NADH in close proximity of two alternative conformations of Cys207. The 2mF0-DFc electron density map is contoured at 1.0σ.
The absence of adenosine in the substrate/product binding pocket suggests that the inactive protein has low affinity for adenosine binding in this site. This seems to be the result of different domain organization in iTmSAHase, where the angle between the two principal domains of the subunit in the open-like conformation is larger (Fig. 4A). In consequence, the different domain organization may affect substrate binding and ligand-induced domain rearrangement.
3.8. The mode of NADH binding
Each subunit in the closed-like conformation binds one molecule of the reduced form of the cofactor in the cofactor-binding domain. The closed-like conformation is adopted despite the fact that no substrate/product is bound in the substrate-binding domain. The cofactor binding mode, shown in Fig. 5B, is very similar to that observed in other SAHases, including the active form of T. maritima SAHase, where all subunits adopt the canonical closed conformation (PDB entry 3X2F). It is of note that the active form of SAHase from T. maritima adopts the closed conformation only in the presence of SAH although the substrate (or products of its hydrolysis) is not defined in the electron density maps [15]. Eight water molecules and amino acid residues from both the cofactor- and substrate-binding domains of iTmSAHase are involved in hydrogen-bonding interactions with the cofactor. These interactions include the side chains of Thrl41 (Oγ1…O2N), Thrl42 (Oγ1…O3D), Glu226 (Oε1…O2B and Oε2…O3B), Lys231 (Nζ…O3′), Asn261 (Nδ2…N7A) and Asn329 (Nδ2…O7N and Oδ1…N7N), as well as main-chain atoms of residues Cys207, Ala282 and His284. Additionally, the side chains of Val227 and Ser259, which restrict the size of the cofactor-binding cavity, are involved in C—H…π interactions with the adenine ring. The nicotinamide moiety of the co factor is partially disordered and has been modeled with 0.7 partial occupancy. The disorder is coupled to repulsion by one of the alternative side-chain conformations of Cys207. Also, the nicotinamide ring is non-planar, at least in the visible portion, and this buckled structure is a hallmark of its reduced form [47] (Fig. 5D).
To find out it the presence ot NADH could be the result ot NAD reduction in the X-ray beam during data collection, or if there was an earlier reduction event during the protein expression procedure, we determined the NADH:NAD+ ratio in freshly purified protein samples. The results indicate that the NADH fraction significantly predominates over NAD+ (72 ± 12% of total cofactor content), thus confirming that the reduced form of the cofactor was present in abundance in the protein preparations prior to the crystallization experiments. Numerous attempts at cofactor exchange in iTmSAHase all failed to yield a homogeneous protein-NAD+ complex [25]. Obviously, this is a consequence of the iTmSAHase structure, where the wrong (i.e. reduced) cofactor is trapped in the cofactor binding domain of a closed-like subunit at an early stage of protein production. In this conformational state, the bound NADH cofactor cannot be removed from the protein and substituted with its oxidized NAD+ form.
3.9. Biochemical and structural implications of thermal activation of iTmSAHase
The active form of TmSAHase was characterized biochemically in two studies before [48,49]. In both those studies, the recombinant enzyme was purified according to a protocol that included thermal precipitation of the cell lysate at 353 K. Additionally, the crystal structure of the active form of TmSAHase has been recently described [15]. In that study, the protein sample was purified with the application of thermal precipitation at the same temperature (353 K). One of the conclusions from those studies is that the activity of the purified enzyme is low or even undetectable, unless the reaction mixture has been supplemented with the cofactor in the correct, oxidized, NAD+ form. Also, crystallization of active TmSAHase required the addition of the NAD+ cofactor to the protein solution. The present results provide a rational explanation of those observations.
In the present study, as well as in the previous work, the recombinant enzyme was expressed in the mesophilic E. coli at 291 –315 K, i.e. well below the temperature required for heat-induced activation of the enzyme. This implies that after the expression step, in all experiments that were not preceded by heat-treatment, the protein existed in its inactive conformation.
Our crystallographic results indicate that the iTmSAHase tetramer binds only two molecules of the cofactor, and that it is predominantly in its reduced form. As demonstrated by our enzymatic assays, no catalytic activity could be detected, not only at room temperature but also at 358 K, without the addition of NAD+. Although our spectrofluorimetric measurements indicated that iTmSAHase might contain a small fraction of the oxidized cofactor, which in principle could be involved in the enzymatic reaction, in reality, as demonstrated by the enzymatic assays, this is not the case and evidently other factors are also involved. The most obvious reason is the wrong conformation of the iTmSAHase molecule. In conclusion, the presence of the NAD+ cofactor in the reaction environment is essential for the restoration of the activity of the enzyme at 358 K. A corollary to this observation might be that all four subunits of the tetrameric enzyme have to bind the correctly oxidized cofactor molecule to attain enzymatic activity.
Our enzymatic activity study revealed that the heat treatment of iTmSAHase at 358 K changes the enzymatic properties significantly. These changes are also reflected in the thermodynamic parameters of NAD+ and adenosine binding, which are dramatically altered after the heat treatment. Surprisingly, the ITC experiments indicate that the heated enzyme can bind only one instead of two cofactor molecules (in the two formally “empty” subunits). This result is in contrast with the structural studies of enzymatically active SAHases, which bind one cofactor molecule in each subunit. However, it should be stressed that all ITC experiments were performed at 293 K. Similarly, the enzymatic assays in the presence of NAD+ indicate very low (<1%) activity of both heated and unheated samples at 298 K when compared with the heated enzyme tested at 358 K. These results may suggest that the protein heated to 358 K and subsequently cooled down, could exist in other non-native, low-activity conformation(s), but with a higher affinity for the cofactor and the ligand when compared with the unheated protein. This hypothesis is additionally supported by DLS analyses, which indicate some changes in the average size of the homotetramer depending on the heating history and the temperature of the measurements (Fig. S1B).
4. Conclusions
S-adenosyl-L-homocysteine hydrolase from Thermotoga maritima was recombinantly expressed in the mesophilic Escherichia coli cells and purified as a homotetramer at room temperature. Enzymatic activity study indicated that the enzyme expressed and purified at the room temperature conditions is inactive. Isothermal titration calorimetry experiments revealed that the inactive form of TmSAHase undergoes heat-induced conformational changes and enzymatic activity assays demonstrated that these changes are required to attain enzymatic activity. To elucidate the mechanism of thermal activation, two crystal structures of the inactive form of T. maritima SAHase (iTmSAHase) were determined, for a binary complex with the reduced form of the cofactor (NADH), and for a mixture of binary complexes with NADH and a reaction byproduct/substrate (adenosine). The homotetramers in the two structures are very similar and are composed in each case of two subunits in the open conformation and two subunits in the closed conformation. However, the results clearly indicate that the protein has not adopted a structure that is compatible with the catalytically-competent, native enzyme. In contrast to the catalytically active form of SAHase from several sources, in iTmSAHase only two of the four subunits contain a tightly bound cofactor. Additionally, the cofactor exists mainly in its reduced NADH state and, therefore, cannot participate in substrate oxidation, which is a key step of the catalytic cycle. Moreover, the closed-like conformation formation of the cofactor-containing subunits precludes substrate delivery to the active site. The remaining two subunits cannot be involved in the enzymatic reaction either. Although they have an open-like conformation, they do not contain the cofactor, whose binding site is vulnerable to clogging by an adenosine molecule. The results indicate that the inactive form of the enzyme, iTmSAHase, can be compared to a clockwork that is assembled from more-or-less correct parts, but is functioning incorrectly: its “open"-“closed" transitions are only a poor imitation of the genuine structural transformations required for the catalytic cycle of the active enzyme.
Supplementary Material
Acknowledgments
This project was supported by the Polish National Science Centre, grant #2013/09/B/NZ1/01880 to KB. The BioNanoTechno Center at the Institute of Chemistry, University of Bialystok, was created with the support of European Fund for Regional Development and national funds from the Ministry of Science and Higher Education, as part of the Operational Programme Development of Eastern Poland 2007–2013, project POPW.Ol.03.00–20-034/09–00. X-ray diffraction data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-BM beamline of the APS/ANL. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31–109-Eng-38.
Abbreviations:
- Ado
adenosine
- ADP
atomic displacement parameter
- AMP
adenosine 5′-monophosphate
- Hcy
L-homocysteine
- DLS
dynamic light scattering
- FPLC
fast protein liquid chromatography
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- iTmSAHase
inactive form of S-adenosyl-L-homocysteine hydrolase from T. maritima
- ITC
isothermal titration calorimetry
- MES
2-(N-morpholino)ethanesulfonic acid
- MPD
2-methyl-2,4-pentanediol
- NAD+
oxidized form of nicotinamide adenine dinucleotide
- NADH
reduced form of nicotinamide adenine dinucleotide
- PMSF
phenylmethylsulfonyl fluoride
- SAH
S-adenosyl-L-homocysteine
- SAHase
S-adenosyl-L-homocysteine hydrolase
- SAM
S-adenosyl-L-methionine
- SEC
size exclusion chromatography
- TCEP
tris(2carboxyethyl)phosphine
- TEV
Tobacco Etch Virus
- TmSAHase
native (active) S-adenosvl-L-homocvsteine hydrolase from T. maritima
Footnotes
Conflict of interest
None declared.
Appendix A.Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijbiomac.2017.06.065.
References
- [1].Richards HH, Chiang PK, Cantoni GL, Adenosylhomocysteine hydrolase. Crystallization of the purified enzyme and its properties, J. Biol. Chem 253 (1978)4476–4480. [PubMed] [Google Scholar]
- [2].Poulton JE, Butt VS, Purification and properties of S-adenosyl-L-methionine: caffeic acid O-methyltransferase from leaves of spinach beet (Beta vulgaris L), Biochim. Biophys. Acta 403 (1975) 301–314. [DOI] [PubMed] [Google Scholar]
- [3].Chiang PK, L Cantoni G, Perturbation of biochemical transmethylations by 3-deazaadenosine in vivo, Biochem. Pharmacol 28 (1979) 1897–1902. [DOI] [PubMed] [Google Scholar]
- [4].Chiang PK, Biological effects of inhibitors of S-adenosylhomocysteine hydrolase, Pharmacol. Ther 77 (1998) 115–134. [DOI] [PubMed] [Google Scholar]
- [5].Stepkowski T, Brzeziński K, Legocki AB, Jaskólski M, Béna G, Bayesian phylogenetic analysis reveals two-domain topology of S-adenosylhomocysteine hydrolase protein sequences, Mol. Phylogenet. Evol 34 (2005) 15–28. [DOI] [PubMed] [Google Scholar]
- [6].Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO, Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C, Arch. Microbiol 144 (1986)324–333. [Google Scholar]
- [7].Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EIC, Peterson JD, Nelson WC, Ketchum ICA, McDonald L, Utterback TR, Malek JA, Linher ICD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, White O, Salzberg SL, Smith HO, Venter JC, Fraser CM, Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima, Nature 399 (1999) 323–329. [DOI] [PubMed] [Google Scholar]
- [8].Turner MA, Yuan CS, Borchardt RT, Hershfield MS, Smith GD, L Howell P, Structure determination of selenomethionyl S-adenosylhomocysteine hydrolase using data at a single wavelength, Nat. Struct. Biol 5 (1998) 369–376. [DOI] [PubMed] [Google Scholar]
- [9].Hu Y, Komoto J, Huang Y, Gomi T, Ogawa H, Takata Y, Fujioka M, Takusagawa F, Crystal structure of S-adenosylhomocysteine hydrolase from rat liver, Biochemistry 38 (1999) 8323–8333. [DOI] [PubMed] [Google Scholar]
- [10].Reddy MCM, Kuppan G, Shetty ND, L Owen J, Ioerger TR, Sacchettini JC, Crystal structures of Mycobacterium tuberculosis S-adenosyl-L-homocysteine hydrolase in ternary complex with substrate and inhibitors, Protein Sci 17 (2008)2134–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manszewski T, Singh K, Imiolczyk B, Jaskolski M, An enzyme captured in two conformational states: crystal structure of S-adenosyl-L-homocysteine hydrolase from Bradyrhizobium elkanii, Acta Crystallogr. D Biol. Crystallogr 71 (2015)2422–2432. [DOI] [PubMed] [Google Scholar]
- [12].Tanaka N, Nakanishi M, Kusakabe Y, Shiraiwa K, Yabe S, Ito Y, Kitade Y, Nakamura KT, Crystal structure of S-adenosyl-L-homocysteine hydrolase from the human malaria parasite Plasmodium falciparum, J. Mol. Biol 343 (2004) 1007–1017. [DOI] [PubMed] [Google Scholar]
- [13].Brzezinski K, Dauter Z, Jaskolski M, High-resolution structures of complexes of plant S-adenosyl-L-homocysteine hydrolase (Lupinus luteus), Acta Crystallogr. D Biol. Crystallogr 68 (2012) 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kusakabe Y, Ishihara M, Umeda T, Kuroda D, Nakanishi M, Kitade Y, Gouda H, Nakamura KT, Tanaka N, Structural insights into the reaction mechanism of S-adenosyl-L-homocysteine hydrolase, Sci. Rep 5 (2015) 16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng Y, Chen C-C, Ko T-P, Xiao X, Yang Y, Huang C-H, Qian G, Shao W, Guo R-T, Crystal structures of S-adenosylhomocysteine hydrolase from the thermophilic bacterium Thermotoga maritima, J. Struct. Biol 190 (2015) 135–142. [DOI] [PubMed] [Google Scholar]
- [16].Guranowski A, Pawelkiewicz J, Adenosylhomocysteinase from yellow lupin seeds. Purification and properties, Eur. J. Biochem 80 (1977) 517–523. [DOI] [PubMed] [Google Scholar]
- [17].Brzezinski K, Bujacz G, Jaskolski M, Purification, crystallization and preliminary crystallographic studies of plant S-adenosyl-L-homocysteine hydrolase (Lupinus luteus), Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun 64 (2008) 671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schultes V, Jaenicke R, Folding intermediates of hyperthermophilic D-glyceraldehyde-3-phosphate dehydrogenase from Thermotoga maritima are trapped at low temperature, FEBS Lett 290 (1991) 235–238. [DOI] [PubMed] [Google Scholar]
- [19].Diruggiero J, Robb FT, Expression and in vitro assembly of recombinant glutamate dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus, Appl. Environ. Microbiol 61 (1995) 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goda S, Kojima M, Nishikawa Y, Kujo C, Kawakami R, Kuramitsu S, Sakuraba H, Hiragi Y, Ohshima T, Intersubunit interaction induced by subunit rearrangement is essential for the catalytic activity of the hyperthermophilic glutamate dehydrogenase from Pyrobacuium islandicum †, Biochemistry 44 (2005) 15304–15313. [DOI] [PubMed] [Google Scholar]
- [21].Ohshima T, Structural characteristics of active and inactive glutamate dehydrogenases from the hyperthermophile Pyrobacuium islandicum, Biosci. Biotechnol. Biochem 76 (2012) 1601–1610. [DOI] [PubMed] [Google Scholar]
- [22].Abd Rahman RN, Fujiwara S, Takagi M, Kanaya S, Imanaka T, Effect of heat treatment on proper oligomeric structure formation of thermostable glutamate dehydrogenase from a hyperthermophilic archaeon, Biochem. Biophys. Res. Commun 241 (1997) 646–652. [DOI] [PubMed] [Google Scholar]
- [23].Siddiqui MA, Fujiwara S, Takagi M, Imanaka T, In vitro heat effect on heterooligomeric subunit assembly of thermostable indolepyruvate ferredoxin oxidoreductase, FEBS Lett 434 (1998) 372–376. [DOI] [PubMed] [Google Scholar]
- [24].Pelletier N, Leroy G, Guiral M, Giudici-Orticoni M-T, Aubert C, First characterisation of the active oligomer form of sulfur oxygenase reductase from the bacterium Aquifex aeolicus, Extremophiles 12 (2008) 205–215. [DOI] [PubMed] [Google Scholar]
- [25].Yuan CS, Yeh J, Liu S, Borchardt RT, Mechanism of inactivation of S-adenosylhomocysteine hydrolase by (Z)-4’,5’-didehydro-5’-deoxy-5’-fluoroadenosine, J. Biol. Chem 268 (1993) 17030–17037. [PubMed] [Google Scholar]
- [26].Otwinowski Z, Minor W, Processing of X-ray diffraction data collected in oscillation mode, Methods Enzymol 276 (1997) 307–326. [DOI] [PubMed] [Google Scholar]
- [27].McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, Phaser crystallographic software, J. Appl. Crystallogr 40 (2007) 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS, Overview of the CCP4 suite and current developments, Acta Crystallogr. D Biol. Crystallogr 67 (2011) 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang X, Hu Y, Yin DH, Turner MA, Wang M, Borchardt RT, Howell PL, Kuczera K, Schowen RL, Catalytic strategy of S-adenosyl-L-homocysteine hydrolase: transition-state stabilization and the avoidance of abortive reactions, Biochemistry 42 (2003) 1900–1909. [DOI] [PubMed] [Google Scholar]
- [30].Langer G, Cohen SX, Lamzin VS, Perrakis A, Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7, Nat. Protoc 3 (2008) 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA, REFMAC 5 for the refinement of macromolecular crystal structures, Acta Crystallogr. D Biol. Crystallogr 67 (2011)355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Winn MD, Isupov MN, Murshudov GN, Use of TLS parameters to model anisotropic displacements in macromolecular refinement, Acta Crystallogr. D Biol. Crystallogr 57 (2001) 122–133. [DOI] [PubMed] [Google Scholar]
- [33].Painter J, Merritt EA, TLSMD web server for the generation of multi-group TLS models, J. Appl. Crystallogr 39 (2006) 109–111. [Google Scholar]
- [34].Smart OS, Womack TO, Sharff A, Flensburg C, Keller P, Paciorek W, Vonrhein C, Bricogne G, grade, version 1.1.02,2011. http://www.globalphasing.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Emsley P, Lohkamp B, Scott WG, Cowtan K, Features and development of Coot, Acta Crystallogr. D Biol. Crystallogr 66 (2010) 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Berman H, Henrick K, Nakamura H, Announcing the worldwide protein data bank, Nat. Struct. Biol 10 (2003), 980-980. [DOI] [PubMed] [Google Scholar]
- [37].Krissinel E, Henrick K, Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions, Acta Crystallogr. D Biol. Crystallogr 60 (2004) 2256–2268. [DOI] [PubMed] [Google Scholar]
- [38].de Beer TAP, Berka K, Thornton JM, Laskowski RA, PDBsum additions, Nucleic Acids Res 42 (2014) D292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schrodinger LLC, The PyMOL Molecular Graphics System, Version 1.8,2015.
- [40].Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH, PHENIX: a comprehensive Python-based system for macromolecular structure solution, Acta Crystallogr. D Biol. Crystallogr 66 (2010)213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hayward S, Berendsen HJC, Systematic analysis of domain motions in proteins from conformational change: new results on citrate synthase and T4 lysozyme, Proteins Struct. Funct. Genet 30 (1998) 144–154. [PubMed] [Google Scholar]
- [42].Krissinel E, Henrick K, Inference of macromolecular assemblies from crystalline state, J. Mol. Biol 372 (2007) 774–797. [DOI] [PubMed] [Google Scholar]
- [43].Manszewski T, Szpotkowski K, Jaskolski M, Crystallographic and SAXS studies on S-adenosyl-L-homocysteine hydrolase from Bradyrhizobium elkanii, IUCrJ 4 (2017) 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Palmer JL, Abeles RH, The mechanism of action of S-adenosylhomocysteinase, J. Biol. Chem 254 (1979) 1217–1226. [PubMed] [Google Scholar]
- [45].Komoto J, Huang Y, Gomi T, Ogawa H, Takata Y, Fujioka M, Takusagawa F, Effects of site-directed mutagenesis on structure and function of recombinant rat liver S-adenosylhomocysteine hydrolase. Crystal structure of D244E mutant enzyme, J. Biol. Chem 275 (2000) 32147–32156. [DOI] [PubMed] [Google Scholar]
- [46].Takata Y, Yamada T, Huang Y, Komoto J, Gomi T, Ogawa H, Fujioka M, Takusagawa F, Catalytic mechanism of S-adenosylhomocysteine hydrolase, site-directed mutagenesis of asp-130, Lys-185 Asp-189, and Asn-190, J. Biol. Chem 277 (2002) 22670–22676. [DOI] [PubMed] [Google Scholar]
- [47].Meijers R, Morris RJ, Adolph HW, Merli A, Lamzin VS, Cedergren-Zeppezauer ES, On the enzymatic activation of NADH, J. Biol. Chem 276(2001)9316–9321. [DOI] [PubMed] [Google Scholar]
- [48].Lozada-Ramírez JD, Sánchez-Ferrer A, Garcáa-Carmona F, Recombinant S-Adenosylhomocysteine hydrolase from Thermotoga maritima: cloning, overexpression, characterization, and thermal purification studies, Appl. Biochem. Biotechnol 170 (2013) 639–653. [DOI] [PubMed] [Google Scholar]
- [49].Qian G, Chen C, Zhou R, He Y, Shao W, A thermostable S-adenosylhomocysteine hydrolase from Thermotoga maritima: Properties and its application on S-adenosylhomocysteine production with enzymatic cofactor regeneration, Enzyme Microb. Technol 64-65 (2014) 33–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.