Dear Editor:
Transcranial magnetic stimulation (TMS) administration currently necessitates trained operators and equipment to hold the coil in place over specific brain targets [1], limiting its application in ambulatory, hospital or home settings.
We have developed custom, individually-tailored TMS helmets that allow one to administer TMS in diverse settings where trained TMS operators are unavailable or the experiment or treatment involves movement such as ambulatory, extreme environments, or potentially at home. Here we overview how we constructed helmets that fix a TMS coil into position over a participant’s motor hotspot (Fig. 1a and b) and describe its stepwise fabrication with video accompaniment (Supplemental Video). We also present within-and between-visit reliability resting motor thresholds (rMT) while wearing the helmets.
Fig. 1.
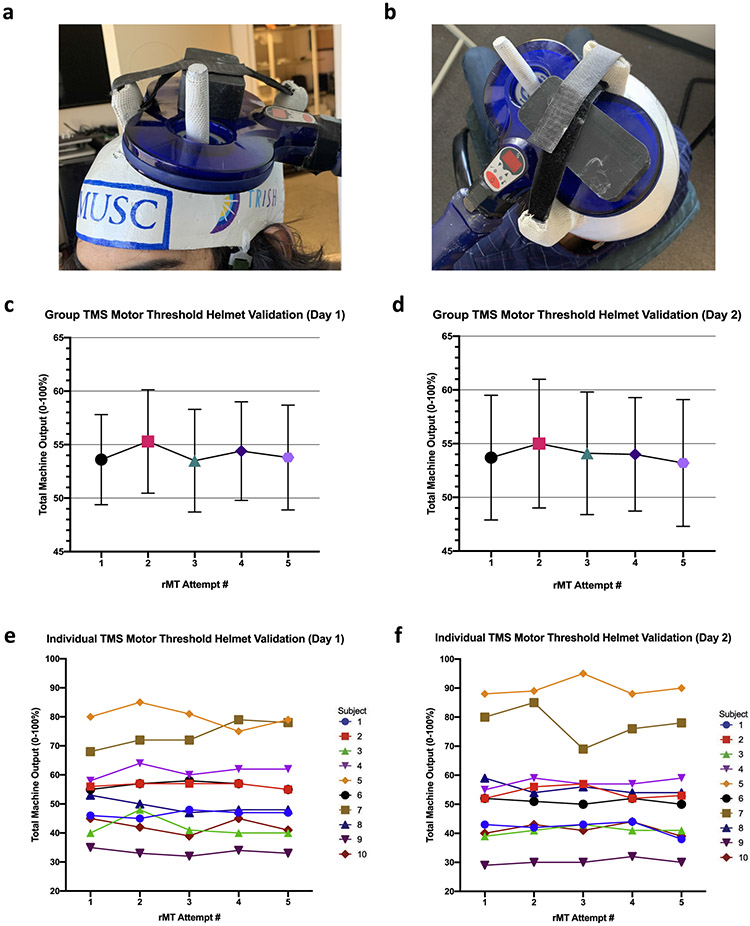
a,b: photos of TMS coil fixed into custom helmets fabricated for the purpose of collecting resting motor thresholds in non-laboratory settings. c,d: Group-level stability of resting motor thresholds over five rMT attempts each day. e,f: Individual-level stability of resting motor thresholds over five rMT attempts each day. Each line represents an individual subject.
How to make a TMS helmet
Each helmet required: casting fiberglass (BSN Medical Delta-LITE Cast Tape), 3D printed components (custom fabricated), Epoxy adhesive (J-B Weld MinuteWeld), Velcro strap (18in), and chin strap (Pyramex elastic chin strap with chin cup). The estimated cost of these materials is $50. We describe our construction below.
Fiberglass casting (0:08)
Wearing a cloth liner to protect hair, cover the top of the head with fiberglass and use the remaining roll to wrap the head of the participant, creating a base cast of the head. Hold downward pressure until the helmet cures.
Mark ears and remove cast (1:07)
Mark the ears and midline of the helmet on the participant to ensure proper future positioning. Remove the cast and liner from the head and cut arches for the ears.
Determine motor hotspot (1:38)
Wear helmet and deliver supra-threshold TMS pulses to the motor cortex of the participant to determine the optimal “motor hotspot” (scalp target that elicits largest motor activation in contralateral thumb). Mark the front edge of the TMS coil and the coil “hole” position on the cap.
Cut stimulation window (2:04)
Cut a window out of the helmet positioned under the center of the TMS coil to minimize the thickness of the helmet at the point of stimulation.
Attach 3D-printed anchors (3:04)
3D-print anchors to attach to the helmet that allow the attachment of a TMS coil. Our system is for use with a Magstim Fig. 8 coil. These anchors ensure the TMS coil sits precisely over the motor hotspot.
Reinforce helmet with fiberglass (4:13)
Attach Velcro strap to anchor and strengthen helmet with additional fiberglass.
Attach chin strap (5:27)
Attach elastic chin strap to the helmet (1 cm anterior of ear).
Attach TMS coil accessory (6:15)
The TMS coil accessory is a plastic brick and provides a pressure point for the strap. This allows for increased downward force on the TMS coil while it is in the helmet and is critical to a “tight fit.”
Test helmet (6:29)
Final fitting determines whether modification is needed (sanding, expanding the TMS stimulation window, readjusting straps, etc). Note that we do visually observed thumb twitch as a demonstration, however the described experiment uses EMG as detailed below.
Validating TMS helmets – A motor threshold study
We fabricated 10 of these custom TMS helmets (individually tailored to the heads of each participant). We then conducted a 2-visit study exploring the use of these TMS helmets to collect five resting motor thresholds (rMT) per visit using a closed-loop TMS system. Importantly, the TMS coils were simply placed into the helmet and were not held or guided by any administrator.
TMS motor Threshold collection
Following written informed consent, TMS motor thresholds were collected using the closed-loop TMS system, utilizing the Magstim BiStim TMS system (70mm coil) connected to the Cambridge Electronics EMG system (CED 1401, 1902). This validated system uses electromyography (EMG) to measure the amplitude of the TMS motor evoked potentials in the contralateral abductor pollicis brevis (APB) to determine whether a thumb twitch occurred (>150μV) and is comparable to visually observed determination [2,3]. This information is real-time assessed in Spike 2 software and triggers the subsequent TMS stimulus intensity to administer based on the parametric estimation via sequential testing (PEST) paradigm [2,4].
Statistical analysis
A repeated measures ANOVA was conducted to determine the stability of the motor threshold within visit for the group using Prism 8 (GraphPad, USA). Within- and between-visit intraclass correlation coefficients (ICC) were calculated to determine individual test-retest reliability and consistency of the five collected motor thresholds ((IBM SPSS 25, SPSS Inc).
One participant had only four rMTs collected during their second visit due to coil overheating (subject 5 visit 2). This missing data point was interpolated from her 4 prior rMTs collected that visit.
Results
Helmet-facilitated rMTs were not significantly different over the five within-visit rMT attempts (Fig. 1c and d).
Individual reliability of the within-visit reliability of five consecutive rMT attempts as measured by ICC is high (alpha, 95%CI) [(day 1 = 0.968,0.925–0.991); (day 2 = 0. 979,0.949–0.994)], confirming consistent individual helmet rMT (p < 0.001) (Fig. 1e and f). Furthermore, helmets demonstrate high individual reliability between-visits (alpha, 95%CI) 0.956, 0.833–0.989.
Conclusions
Custom, individually-fitted helmets are feasible, reliable and can help administer TMS in variable gravity or ambulatory, at-home, and other non-laboratory settings.
As described, this TMS helmet is compatible with the Magstim 70mm TMS coil targeting the motor cortex, however simple modifications would allow its use with any TMS coil. The stimulation target site can be relocated to target non-motor brain regions (i.e.: DLPFC, mPFC, etc.). Another advantage of this helmet is that the TMS coil snaps in place and is firmly affixed to the participant – allowing for safe administration of TMS with minimal training. Individuals may also be able to self-administer stimulation using this method – which is appealing in environments such as the at-home setting, although other safety issues would need to be addressed in terms of correct dosing and limitations of the intensity and number of stimuli.
One limitation of this helmet is it introduces distance between the scalp and the TMS coil. On average, this distance was between 2 and 3mm for each helmet. Kozel et al. describe the impact of distance in an equation for motor threshold (y) as a function of distance in mm (x) as the following: y = 2.944x+34.957 [5]. Our helmet therefore likely increases the true motor threshold between 5.89% and 8.83% (total machine output units).
Personalized helmets like these allow TMS to be delivered outside of the clinic setting with similar precision of expert operators [2] in otherwise unconventional settings such as the home. This system can administer TMS to the correct location in diverse settings, with minimal to no training or experience regarding coil placement after the initial laboratory determinations by trained individuals.
Supplementary Material
Acknowledgements
Financial support from: This work is supported by the Translational Research Institute for Space Health (TRISH) through NASA NNX16AO69A, National Center of Neuromodulation for Rehabilitation (NC NM4R) (5P2CHD086844-03), and the COBRE Brain Stimulation Core (5P20GM109040-04).
Footnotes
Declaration of competing interest
BWB is listed as an inventor on a provisional patent related to the helmets described in this manuscript which is assigned to the Medical University of South Carolina. BWB holds minority ownership in Bodhi NeuroTech Inc, which develops meditation brain stimulation technology. All other authors have no conflicts to report.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2020.01.009.
Contributor Information
Bashar W. Badran, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA.
Kevin A. Caulfield, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA.
James W. Lopez, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA
Claire Cox, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA.
Sasha Stomberg-Firestein, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA.
William H. DeVries, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA
Lisa M. McTeague, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA
Mark S. George, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, 29425, USA; Ralph H. Johnson VA Medical Center, Charleston, SC, 29401, USA
Donna Roberts, Department of Radiology, Medical University of South Carolina, Charleston, SC, 29425, USA.
References
- [1].Nauczyciel C, Hellier P, Morandi X, Blestel S, Drapier D, Ferre JC, et al. Assessment of standard coil positioning in transcranial magnetic stimulation in depression. Psychiatry Res 2011;186(2–3):232–8. [DOI] [PubMed] [Google Scholar]
- [2].Badran BW, Ly M, DeVries WH, Glusman CE, Willis A, Pridmore S, et al. Are EMG and visual observation comparable in determining resting motor threshold? A reexamination after twenty years. Brain Stimul 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pridmore S, Fernandes Filho JA, Nahas Z, Liberatos C, George MS. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. JECT 1998;14(1):25–7. [PubMed] [Google Scholar]
- [4].Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT 2004;20(3):160–5. [DOI] [PubMed] [Google Scholar]
- [5].Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;12(3):376–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.