Abstract
Background:
Transcranial focused ultrasound (tFUS) is a noninvasive brain stimulation method that may modulate deep brain structures. This study investigates whether sonication of the right anterior thalamus would modulate thermal pain thresholds in healthy individuals.
Methods:
We enrolled 19 healthy individuals in this three-visit, double-blind, sham-controlled, crossover trial. Participants first underwent a structural MRI scan used solely for tFUS targeting. They then attended two identical experimental tFUS visits (counterbalanced by condition) at least one week apart. Within the MRI scanner, participants received two, 10-min sessions of either active or sham tFUS spread 10 min apart targeting the right anterior thalamus [fundamental frequency: 650 kHz, Pulse repetition frequency: 10 Hz, Pulse Width: 5 ms, Duty Cycle: 5%, Sonication Duration: 30s, Inter-Sonication Interval: 30 s, Number of Sonications: 10, ISPTA.0 995 mW/cm2, ISPTA.3 719 mW/cm2, Peak rarefactional pressure 0.72 MPa]. The primary outcome measure was quantitative sensory thresholding (QST), measuring sensory, pain, and tolerance thresholds to a thermal stimulus applied to the left forearm before and after right anterior thalamic tFUS.
Results:
The right anterior thalamus was accurately sonicated in 17 of the 19 subjects. Thermal pain sensitivity was significantly attenuated after active tFUS. The pre-post x active-sham interaction was significant (F(1,245.95) = 4.03, p = .046). This interaction indicates that in the sham stimulation condition, thermal pain thresholds decreased 1.08 °C (SE = 0.28) pre-post session, but only decreased .51 °C (SE = 0.30) pre-post session in the active stimulation group.
Conclusions:
Two 10-min sessions of anterior thalamic tFUS induces antinociceptive effects in healthy individuals. Future studies should optimize the parameter space, dose and duration of this effect which may lead to multi-session tFUS interventions for pain disorders.
Keywords: Focused ultrasound, Fus, tfus, lifup, Low intensity focued ultrasound, Pain, Thalamus, MRI, Sonication
Introduction
Transcranial focused ultrasound (tFUS) is a promising new technology that is both noninvasive and may be focally applied to deep brain targets([1-4]). tFUS utilizes transducers which contain piezoelectric elements to produce pulses of ultrasonic waves that summate deep in the brain [5-7]. tFUS noninvasively stimulates deep brain targets with a high level of spatial resolution. No other current technologies exist for noninvasive deep brain stimulation which makes tFUS a highly promising technology that may have substantial research and clinical potential [5,8,9].
Ultrasound neuromodulation may be administered at two intensities, each with their own biologic effects. When delivered at a high intensity, also known as high intensity focused ultrasound (HIFU), multiple ultrasound beams may be spatially and temporally summated at deep brain structures to induce permanent, irreversible lesions([10-12]). These lesions can be used to thermoablate hyperactive tissues in debilitating neurologic disorders such as essential tremor([13-15]). Due to its irreversible nature, HIFU is often approached as a last-line treatment. Reducing the intensity (wattage) of the ultrasound beam allows for neuromodulation of neural tissues without thermal damage, often referred to as transcranial focused ultrasound (tFUS) [16]. It is hypothesized that tFUS acts on neurons via a mechanical force mechanism of action, leading to increased conductance of neurons and opening of ion channels([17]).
Prior preclinical research has helped to establish tFUS parametric safety limits and suggests that tFUS is safe and can induce biologic effects in the central and peripheral nervous system [18-21]. The primary safety concern of tFUS is causing cavitation or thermal damage to tissue receiving sonication([22-25]). Indeed high doses of ultrasound are used for surgical ablation[14, 26], however previous studies using low intensity tFUS administered below the FDA-regulated power limits for diagnostic ultrasound have not induced negative physiological changes within the sonicated tissue [27,28].
Since establishing the safety of tFUS at certain intensities, researchers are beginning to explore the parametric and neurophysiologic effects of tFUS in humans. Preliminary work has suggested different sonication parameters can induce reversible physiological effects on the nervous system, ranging from increased excitation in regions of interest([29]) to suppression of visual evoked potentials [16]. There have been no prior studies of tFUS with neuropsychiatric changes or pain, with limited consensus on the optimal parameters for modulation of the networks involved in brain disorders.
If neuromodulation by focused ultrasound could affect deep-brain activity to alter sensory and pain thresholds, then perhaps it could be developed therapeutically. In this study, we investigated whether tFUS could modulate nociception in healthy individuals as there is a current need for new, non-opioid methods of modulating pain. While safe and successful in most clinical settings, opioid medications to manage pain are limited by dose-dependent side effects and potential abuse [30]. [31] This problem is well demonstrated by the major increase within the last decade of US emergency room visits and fatal poisonings caused by nonmedical use of opioid analgesics [32,33].
Building on the existing body of research, we administered tFUS to the anterior nuclei of the thalamus – a central relay structure involved in pain perception in healthy adults [34,35]. Based on earlier work by Martin and colleagues [36], we hypothesized that active tFUS would modulate pain perception by interacting with the thalamus and affecting afferent sensory-discriminative component of pain(37). As a first step, we investigated whether MRI-guided sonication of the anterior thalamus could alter pain perception in healthy adults and whether tFUS could produce quantifiable behavioral effects from a deep brain target. Using a validated thermal pain paradigm [38-41] applied to the left forearm before, during, and after tFUS sonication within the MRI scanner, we investigated whether tFUS would modulate pain perception via sonication of the right anterior thalamus as measured by Quantitative Sensory Threshold (QST). During these tFUS sessions we also acquired BOLD fMRI data, which will be reported in a separate manuscript, due to the complexity of those methods and analysis.
Methods
Study Overview
This MUSC Institutional Review Board-approved study utilized a three-visit, double-blind, randomized, sham-controlled crossover trial in this concurrent tFUS/MRI study. We recruited 29 healthy adults between 18 and 45 years old from the local community through email broadcast. Exclusionary criteria included the following: seizure history (individual or family), history of depression, hospitalizations or surgeries in the previous 6 months, current pain episode or a history of chronic pain, pregnancy, or alcohol dependence or any illicit drug use in the previous 6 months. This study was registered on Clinicaltrials.gov (NCT04339972), and a preprint of this manuscript was hosted on the medRxiv website.
During their initial visit, participants were screened and signed written informed consent. Of the 29 consented, 19 completed the study and yielded useable data (11 women, mean age = 24.5 SD 4.6, range = 18–38). The 10 who were not used in the final dataset were withdrawn for: scheduling complications (n = 6), dropout (n = 2), scanner technical issues (n = 1), and non-tFUS related scanner claustrophobia (n = 1). All of these participants were removed from the dataset before any analysis was conducted and before unblinding.
After screening and signing MUSC-approved written consent, a T1-weighted MPRAGE (Siemens Prisma 3T Scanner, TR: 2300 ms TE: 2.32 ms, TI: 900 ms, acquisition time: 5.21s, 0.9 mm isotropic) anatomical image was acquired for each participant. This MPRAGE scan was used in conjunction with Brainsight neuro-navigation (Rogue Industries, Quebec, Montreal, Canada) to determine the optimal location on the participant’s scalp where the tFUS transducer would attach in order to direct a sonication beam to the right anterior thalamus, considering both beam angle and depth.
Participants subsequently returned on two separate experimental tFUS/MRI visits at least one week apart during which they received two, 10-min sessions of either active or sham tFUS within the bore of the MRI scanner (Fig. 1). Participants were randomly assigned to receive either active or sham tFUS during the first visit and the opposite the second visit in a randomized, counterbalanced design.
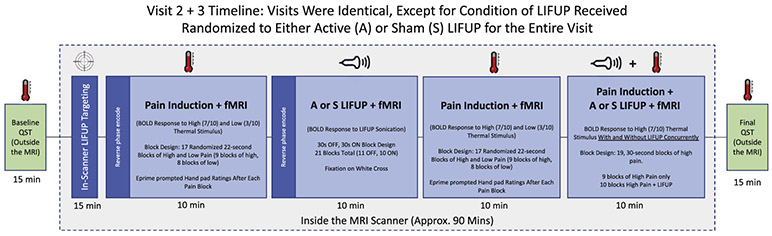
Fig. 1.
Study Overview. Experimental visits 2 and 3 were identical except for sham or active tFUS, with outside the scanner QST conducted at baseline and after in-scanner tFUS administration.
Quantitative Sensory Thresholding (QST) – Outside of MRI Scanner
Quantitative sensory thresholding (QST) systematically determines information about Ab fibers (sensory), Ad fibers (pain), and C fibers (pain tolerance). We acquired threshold levels by attaching a 30 × 30 mm thermode on the left forearm of participants (ATS thermode Medoc, Durham, NC, USA). Using the Medoc Pathway System, we determined sensory, pain, and tolerance thresholds by administering incremental periods of thermal stimulus beginning at 32 °C and increasing by 0.5 °C per second. Participants were asked to verbally indicate when the stimulus was first detected (sensory), when it was painful (pain), and then pressed a button when the stimulus was intolerable (pain tolerance). When ‘intolerable’ was indicated, the thermode ceased heating and quickly returned to 32 °C. This was repeated for 5 trials to obtain average sensory, pain, and tolerance thresholds. We acquired QST data at baseline and after tFUS (approximately 90 min post-baseline QST, 45 min after initiation of the first tFUS session and 20 min after initiation of the second tFUS session).
As a secondary outcome measure, we recorded pain ratings using an MRI-compatible handpad within the bore of the scanner in an attempt to capture acute pain effects, as well as concurrent tFUS/Pain thresholds. See supplemental methods 1 for description of these secondary methods and findings.
Transcranial focused ultrasound (tFUS)
tFUS targeting outside of the MRI
An initial MRI visit was conducted on all participants in order to acquire an anatomical T1-weighted MRI scan to be used for outside of scanner targeting approximation. This involved using Brainsight (Rogue Industries, Quebec, Montreal, Canada) to 3D reconstruct the anatomical scan and use an ultrasound transducer holder to mark the scalp target on the right temporal window that gives the nearest approximation to target the thalamus using an extrapolated straight line connecting the surface marker to the anterior 1/3 of the right thalamus. This scalp area was then marked with a permanent marker and used as the initial ultrasound transducer target during scanning. Participants’ hair was not shaved as part of the procedures for this study.
After marking the skin, we connected the scalp surface and right anterior thalamus using Brainsight and measured the distance from scalp to target, which determined whether a 1 or 2 cm Aquaflex Ultrasound Gel Pad (Parker Laboratories Inc, USA) would be needed. As the ultrasound transducer focal length was fixed, we used this method to accommodate for different cranial sizes and shapes, as well as scalp-to-target distances.
Realtime tFUS targeting inside the MRI
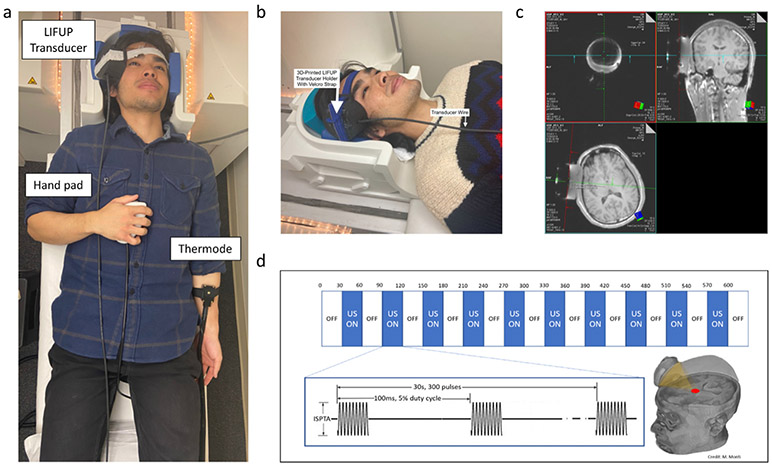
Inside the scanner, the transducer was coupled to the participant’s scalp using the Aquaflex pad in a custom, 3D-printed head-worn wearable mount that helps tune the distance of the ultrasound beam as the focal length was fixed (80 mm) (Fig. 2a and b).
Fig. 2.
Administration of tFUS in the MRI Scanner. a) compound picture of setup in scanner with thermode, hand pad, tFUS device, b) Photograph of how tFUS is attached to participant in the scanner head coil (32ch), c) Overview of tFUS sonication block design paradigm, d) screenshot of the MRI console screen demonstrating how active-time tFUS targeting is conducted.
After the participant was in the bore of the scanner, a quick (1 min) structural scout sequence (TR: 3.15 ms; TE: 1.37 ms; Voxel size: 1.6 mm3; 128 slices, FA: 8 deg) was acquired and study personnel used the MRI console computer to determine whether the transducer was correctly engaged with the pre-planned right anterior thalamic target.
Targeting was confirmed using a two-man observational approach on the Siemens Prisma Scanner Console computer. We acquired a rapid structural scout set with an acquisition window wide enough to captured both brain anatomy and the tFUS transducer. Digitized lines were created that intersect the fixed fiducials that are incorporated into the tFUS transducer and projected 80 mm from the center of the face of transducer and extended orthogonal into the underlying neuroanatomy. One researcher for all participants (BB) then visually determined whether the 80 mm projected line intersects the pre-planned target (thalamus). A secondary individual confirmed this targeting visually. If the line was not intersected with the target, both targeters would concur and subsequently reposition the transducer by removing the subject from the scanner bore and adjusting the head mount to better engage the target. The same scout image was repeated to assess the new ultrasonic beam target (Fig. 2c). No participant required more than one repositioning.
tFUS parameters and administration
We administered ultrasound using the BrainSonix BXPulsar 1002 tFUS System (BrainSonix Corp., Sherman Oaks, CA, USA) using a single-element, air-backed, spherical ultrasound transducer with a 61 mm diameter and 80 mm focal length. The transducer was coupled to the scalp of the participant using a 3D-printed transducer holder that allows for sonication to occur in conjunction with ultrasonic standoff pads and ultrasound gel (Fig. 2b).
Sonication parameters were as follow: [Fundamental frequency: 650 kHz, Pulse repetition frequency: 10 Hz, Pulse Width: 5 ms, Duty Cycle: 5%, Sonication Duration: 30s, Inter-Sonication Interval: 30 s, Number of Sonications: 10, ISPTA.0 995 mW/cm2, ISPTA.3 719 mW/cm2, Peak rarefactional pressure 0.72 MPa]. The intensity ISPTA.3 is based on the US FDA derating approach for diagnostic ultrasound systems. This assumes a uniform tissue attenuation of 0.3dB/cm-MHz (and so it is denoted ISPTA.3) [www.fda.gov/media/71100/download. 2019][42]. While it is not completely predictive of the therapeutic transcranial situation, it does provide an upper bound on in-situ exposure. The two tFUS sessions were spread 10 min apart. E-Prime 3.0 software (Psychology Software Tools, Inc., Pittsburgh, PA, USA) was used to trigger tFUS in a conventional 30s ON, 30s OFF block design (Fig. 2d). This Brainsonix system has an onboard integrated interface that allows for the custom manipulation of tFUS parameters. We have included a screenshot of the output screen as a supplemental figure (Sup. 1) to demonstrate the settings used in this study and exemplify the various parameter settings that are provided to the user.
Blinding
Randomization of the active or sham tFUS order was performed in MATLAB 2015a (Mathworks Inc., Natick, MA, USA) and entered into a spreadsheet and subjects were assigned numbers. One researcher (PMS) knew the status of the subjects. However, this researcher was in the control room behind the MRI scanner during the study and he never interacted with the subject or the rest of the staff during the scans, and he did not conduct any behavioral data analysis. During the scanning, PMS either armed the BrainSonix, or not, depending on the subject’s status on that visit. He also checked before each study that the puck was working by placing the ultrasound transducer in a water bath and checking for waves.
Statistical analysis of pain data
The primary outcome measure was thermal sensory thresholds measured using the QST paradigm. These data QST data were hand recorded then digitized for analysis. Five consecutive QST trials were conducted at the beginning and end of each experimental visit. These trials quantified the thermal pain, sensory and tolerance thresholds for each participant (Fig. 1). The first of each of the 5 trials was discarded to avoid novelty and orienting effects, as well as to ensure consistent A-delta fiber activation suppression during each testing period. Therefore, 4 threshold values were used at baseline and post-tFUS for each experimental visit.
We used linear mixed modeling with unstructured covariance matrices to examine pre vs. post session effects, active vs. sham stimulation effects and the pre-post x active-sham interaction while controlling for trial number. Participant intercepts, trial number and pre-post slopes were entered into the model as random effects at level-1 to account for uncontrollable, nonconstant effects [43].
Results
tFUS safety
There were no adverse events (AEs) observed throughout this experiment. One subject who was not included in the analysis experienced an in-scanner panic attack, and had been randomized to active tFUS that visit. This panic attack occurred during non-tFUS scan sequences and resulted in the termination of the visit. Panic symptoms resolved 15 min after the incident and the participant was withdrawn from the study.
Location of the sonication
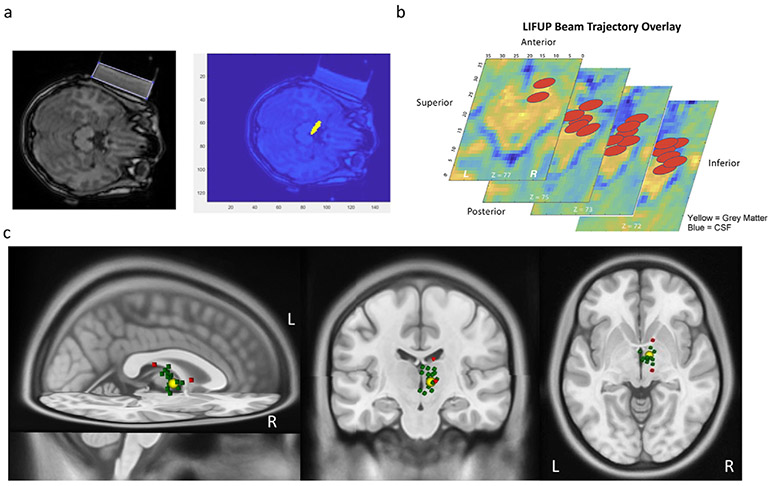
Using a custom-written MATLAB-based graphical user interface, we analyzed the location of sonication for each individual to confirm the thalamic targeting position. This method determines the scalp location and angle of approach of the transducer to estimate the location of the peak sonication by calculating a beam 80 mm from the center of the surface of the tFUS transducer, along the path determined to be orthogonal to the transducer. The software then determines the central focal point of the area of sonication, which extends 1.5 cm (0.075 cm in each direction from the center of the sonication beam) at a width of 0.5 cm (Fig. 3a). The program then gives, as output, the location of stimulation (in pixels and mm), distance from stimulation site to right anterior thalamic target (cm), and overlap between the area of stimulation and the anterior thalamus (in raw area, as a percentage of the total area of the anterior thalamus, and as a percentage of the stimulation area that falls within the target). The target engagement data is then overlaid on the MNI-152 template brain (Fig. 3b).
Fig. 3.
Software-based confirmation of deep brain sonication. a) individual MRI images were used to create trajectory models of the sonication beam and b) the center of the sonication beam coordinates were then mapped onto an MNI-152 template brain. c) The MNI coordinates of the sonication beam were then overlaid on a standard MNI template brain to visualize the center of sonication beam. The large yellow sphere represents the actual size of the stimulation in the target (right anterior thalamus) and the small spheres represent the center of each subject’s individual sonication beam (green = on target, N = 17/19; red = off target, N = 2/19).
This post-hoc analysis of target engagement revealed that our tFUS targeting approach successfully engaged the anterior thalamus in 17 of 19 (89.5%) of participants (Table 1). The coordinates of the peak intensity of the tFUS beam was then overlaid on a 3D MNI brain to serve as a visualization of where we sonicated in each participant (Fig. 3c).
Table 1 –
Confirmation of sonication beam target engagement.
| MNI Vectors from Target (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Subject # |
Sonication On Target? |
Pixels overlapping Target |
mm3 overlapping Target |
Distance (mm) of Focal Center from Target Center |
X (Post. to Ant.) |
Y (Left to Right) |
Z (Inf. To Sup.) |
| 1 | Y | 17 | 71.825 | 11.38 | −2.85 | 4.89 | −9.87 |
| 2 | Y | 19 | 80.275 | 10.72 | −4.72 | 7.08 | 6.52 |
| 3 | Y | 6 | 25.35 | 13.61 | −3.78 | 6.49 | 11.35 |
| 4 | Y | 83 | 350.675 | 3.71 | −2.37 | 1.81 | 2.21 |
| 5 | Y | 55 | 232.375 | 6.33 | −1.77 | 1.96 | 5.75 |
| 6 | Y | 77 | 325.325 | 4.32 | −3.39 | 2.03 | −1.75 |
| 7 | Y | 72 | 304.2 | 6.29 | 3.3 | −4.57 | 2.79 |
| 8 | N | 0 | 0 | 14.72 | 13.83 | −3.25 | 3.83 |
| 9 | Y | 26 | 109.85 | 8.08 | −2.44 | −0.79 | −7.66 |
| 10 | Y | 14 | 59.15 | 11.26 | 3.11 | 8.79 | −6.32 |
| 11 | Y | 31 | 130.975 | 7.03 | 6.84 | −1.22 | 1.08 |
| 12 | Y | 17 | 71.825 | 12.25 | −8.11 | −3.04 | 8.66 |
| 13 | Y | 22 | 92.95 | 8.06 | −3.84 | −3.07 | 6.39 |
| 14 | Y | 91 | 384.475 | 1.34 | 0.87 | 0.39 | 0.94 |
| 15 | Y | 36 | 152.1 | 6.86 | −4.49 | 1.57 | −4.94 |
| 16 | Y | 28 | 118.3 | 6.01 | −2.41 | −1.22 | 5.37 |
| 17 | N | 0 | 0 | 21.06 | −13.67 | −2.5 | 15.83 |
| 18 | Y | 8 | 33.8 | 11.71 | −6.05 | 2.1 | 9.8 |
| 19 | Y | 88 | 371.8 | 0.76 | −0.11 | 0.24 | 0.71 |
Pre and post tFUS quantitative sensory thresholds (QST)
For thermal “sensory” thresholds (“indicate when can you feel the initiation of thermal stimulus”), while controlling for trial number (F(1,14.16) = 32.97, p < .0001), no main effects for pre-post stimulation (F(1,18.08) = 1.79, ns) nor active vs. sham were found (F(1,250.55 = 1.98, ns). The pre-post x active-sham interaction trended toward significance (F(1,250.54 = 3.55, p = .06). In the active stimulation condition, sensory thresholds increased, on average 0.83 °C (SE = 0.40) after the session, whereas, in the sham group, sensory thresholds increased 0.12 °C (SE = 0.38). These sensory changes were in the hypothesized direction (tFUS made subjects more insensitive to the stimulus) but did not meet significance.
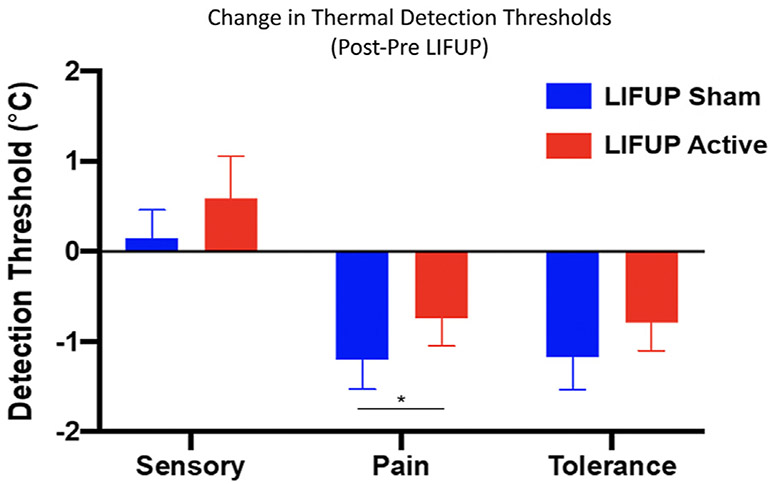
With respect to thermal pain thresholds (“indicate when you find the stimulus painful”), a main effect for pre vs. post was observed (F(1,17.76) = 8.97, p = .008) but no main effect for active vs. sham was seen (F(1,245.95) = 0.50, ns) while controlling for trial number (F(1,12.60) = 24.21, p < .0001). However, the pre-post x active-sham interaction was significant (F(1,245.95) = 4.03, p = .046). (Fig. 4). This interaction indicates that in the sham stimulation condition, thermal pain thresholds decreased 1.08 °C (SE = 0.28) pre-post session, but only decreased .51 °C (SE = 0.30) pre-post session in the active stimulation group. That is, tFUS significantly attenuated the increase in sensitivity that occurred as a result of the 2-h study procedures and repeated painful stimuli, suggesting an antinociceptive effect of active but not sham tFUS.
Fig. 4.
Overall change in QST pain threshold by condition. Temperature sensitivity increases were significantly attenuated (timeXcondition p = 0.046) after active tFUS (0.51° change) relative to sham stimulation (1.08°). That is, across the 2 h study, subjects became more sensitized to the painful stimulus (forearm heat). On the day they received active tFUS stimulation, subjects were not as sensitive over time.
For thermal pain tolerance (“indicate when you can no longer stand this pain, please stop now”), there was a main effect for pre-post session on thermal pain tolerance (F(1,17.93) = 6.51, p = .02) but not in active vs. sham (F(1,247.51) = 2.05, ns) while controlling for trial number (F(1,443.23) = 33.22, p < .0001). The active-sham x pre-post interaction was not significant (F(1,247.46) = 1.15, ns). Mathematically, on the day they received tFUS, subjects tolerated a higher temperature after the stimulation than on the day they received sham. The sham stimulation condition was associated with a decreased pain tolerance of 0.85 °C (SE = 0.24) pre-post session, while active stimulation was associated with a decrease of 0.60 °C (SE = 0.31) pre-post session (NS).
In summary, there was a significant antinociceptive effect of tFUS on pain thresholds. However, tFUS did not significantly change sensory or tolerance thresholds, although the group differences for tolerance were in the direction of an antinociceptive effect.
Immediate hand pad pain ratings in the scanner
There was no significant effect of tFUS on these ratings. See supplement 1 for details.
Integrity of the blind
Subjects were asked after each MRI tFUS visit whether they thought they received active or sham, and how confident they were on a scale of 1–10. Six participants declined to guess which intervention they received after the first visit, and 2 declined after the second visit. Overall, participants were not confident in their guesses (average confidence score 4.13/10). Despite the low confidence, overall and as a group, subjects were able to guess better than chance which intervention they received. Combining guesses from both visits, more participants assigned sham thought they received sham (32%), and more participants assigned active thought they received active (42%) than should have occurred by chance (χ2(3,n = 19) = 7.8, p = 0.04).
In examining the comments about what informed participants’ guesses, two themes emerged. Some participants thought they heard a clicking or humming sound, which they presumed was active. Notably one subject guessed wrongly that they had received active based on this noise when in fact they received sham. The second theme is that interestingly, of the 5 sessions where subjects rated a 9 or 10 confidence, they based their decision on the perception that the pain felt less during that run, and they guessed active correctly. This suggests that the statistically significant tFUS changes in pain tolerance were also observed and noticed by the subjects.
Discussion
This controlled, double-blind study investigated whether sonication of the right anterior thalamus using tFUS produces quantifiable antinociceptive effects in healthy adults. Our findings reveal that two, 10-min sessions of tFUS delivered to the right anterior thalamus administered within the FDA-guidelines of power intensity is feasible and safe, with no serious adverse events related to sonication. Using a novel device targeting system, we were able to accurately sonicate the right anterior thalamus in 17/19 subjects. tFUS produced significant antinociceptive effects in thermal pain threshold ratings that were measured 25–45 min after sonication. Active tFUS blunted sensitization to thermal stimuli compared to sham. This is a promising early finding demonstrating the utility of MRI-guided tFUS to modulate deep brain structures.
The integration of afferent pain signals are often described as sensory discriminative (pain intensity and quality), cognitive (attention and anticipation), and affective motivational (unpleasantness) [37]. All three pathways receive signals from pain fibers in the periphery which relay up to the thalamus in the central nervous system. The thalamus has been a therapeutic target for the treatment of pain for over 60 years, with early reports of the removal of thalamic neural tissue via thalamotomy as a treatment for pain reported in 1965[44] as well as implanted electrical stimulation via deep brain stimulation (DBS) for chronic, refractory neuropathic pain with variable stimulation parameters and outcomes[45,46].For this reason, we chose the anterior thalamus as the primary target for sonication, as this central node is the hub for pain processing signals which project up to various cortical regions with the objective of delivering a believed inhibitory sonication parameter to suppress thalamocortical relay of sensory pain signals.
Our findings suggest that 20 min of tFUS delivered to the right anterior thalamus (contralateral to the site of peripheral pain administration) reduces the sensory component of pain perception. Thermal pain thresholds were increased as a result of active tFUS, suggesting that more afferent pain signal is required to indicate sensory pain, compared to sham tFUS. These findings are preliminary and serve as a proof of principle that tFUS to a deep nucleus can cause behavioral changes. Moreover, this study supports further research, building toward tFUS as a noninvasive deep brain stimulation therapy for pain.
Our study was designed to capture the behavioral effects of a full session of tFUS, however we also measured the immediate effects of tFUS as a theoretical “pain block” by delivering tFUS with and without pain administration – asking participants to rate whether pain was more intense or unpleasant (using a hand pad rating from 1 to 5) with or without tFUS. Although we failed to find an immediate “blocked” effect of tFUS when delivered concurrent with pain (see supplemental data), the five-point discrimination scale limited the observational bandwidth of our collected data, skewing towards large effects rather than more subtle effects which may have occurred. Further interpretation of this failed blockade could be that the antinociceptive effects of tFUS are purely a cumulative effect as a function of energy delivered over time. This is consistent with data from frog sciatic nerve where tFUS causes a reduction in excitability that persists for up to 45 min, and is not necessarily quickly turned on or off [47]. The mechanism or mechanisms of action of tFUS are still unclear, however without the confirmation of sonication-induced action potentials in the thalamus, it is difficult to extrapolate what likely causes these pain effects.
Limitations and future study considerations
This study was an early, proof-of-concept, double-blind pilot trial to establish the groundwork for future tFUS studies. There are several limitations that should considered and incorporated into future tFUS studies. Although we performed this study inside the MRI scanner, the targeting could be improved in the future. In our MATLAB-based program, we post-hoc determined that 17 of 19 participants had the tFUS beam focused within the anterior thalamus. However, deviations in subject skull surface may perturb the sonication beam trajectory in configurations. Some groups have proposed using 3D printed lenses to account for skull curvature and these likely can correct for inconsistencies[48, 49].
Secondly, there currently is no established paradigm for real-time imaging of ultrasonic perturbations of a magnetic field in the MRI space, making target engagement less conclusive. Unlike High Intensity Focused Ultrasound[50] which is used for ablation by creating thermal lesions in tissue, we did not perform MR thermometry[51] as tFUS operates at power levels that do not induce thermal changes in tissue. This results in “invisible” tFUS trajectories that are based on offline computer modelling and needs refinement and technological advancement. Some technologies are in development and are being tested pre-clinicaly to address these limitations, including imaging the micrometric displacements induced by the ultrasound radiation force pressure by using a new technique called Magnetic Resonance Acoustic Radiation Force Imaging (MR ARFI) [52,53]. These imaging techniques can measure the displacements produced by focused ultrasound pulses when systematically applied to different locations of phantoms or tissue.
Additionally, we did not sonicate another brain region as a control. Thus, theoretically our results are not necessarily the result of thalamic stimulation. The effects could be a global tFUS effect and not due to thalamic stimulation. tFUS studies with active brain regions as control conditions are needed for better regional brain behavior causal statements – and some groups are have begun to employ this method in their study design[54, 55]. It is known that transkull transmission will make the focus both shallower and broader than it is when measured in degassed water [2]. This effect is dependent on the skull density ratio, which was not measured [56]. Therefore, it is possible that some subjects did not receive stimulation of the anterior thalamus at all. Our control condition simply had the tFUS device turned off. Some MRI studies have reported that certain parameters of tFUS can produce noise carried via bone conduction and may be responsible for brain effects via the cochlear pathway[57]. Future studies will need to potentially control for this confound, perhaps employing active noise masking via headphones.
In addition, this was a relatively small sample size study and we were only powered to detect moderate to large effects. Based on the effect size, future pain studies like this should likely enroll between 25 and 30 participants per condition arm to effectively determine biologic effects. Furthermore, the dosing of tFUS is regulated primarily at the transducer emission power levels set for diagnostic ultrasonic imaging (non-neuromodulatory) in a publication issued by the FDA in 2019[42]. As more studies are done and the safety profile of tFUS is further assessed, perhaps higher doses can be delivered in the future, which may have larger effects. Increasing dosing may not only include increasing wattage of ultrasound, but also increasing the number of sonication sessions within a day or over multiple days. Multiple daily sessions within an MRI scanner are expensive and the field should work to create robust and reliable ways to administer tFUS outside of the MRI scanner after the initial within scan study.
Additionally, our temporal understanding of the tFUS induced changes is limited. Because tFUS was delivered inside the scanner, no QST was delivered between the two tFUS doses. Thus, we do not know if these two doses were additive or not, or how long it takes for the antinociceptive effects to occur. Similarly, we did not perform pain threshold tests later on the tFUS day or the following days to test the durability of effects. A study in macaques showed that a single 40s sonication can modulate brain activity for over an hour [58]. As such, follow-up studies in the pain lab outside of the scanner are needed, so that one could administer the QST more frequently and obtain more nuanced temporal understanding of the antinociceptive effects we have found here.
Conclusions
Although preliminary, our findings suggest sonication of the right thalamus likely modulates thalamic activity, antinociceptive effects on pain processing networks and reduces pain thresholds in healthy individuals. tFUS appears to be a promising new form of brain stimulation that is able to focally target deep brain structures. Further refinement and research is needed to explore targeting, dosing, and parameter optimization in order to move tFUS forward as a potential therapy for pain or other neuropsychiatric disorders.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Dr. Mark Schafer of Brainsonix for his help with technical and practical considerations for administration of tFUS.
Funding
Funding for this study was provided by the Tiny Blue Dot Foundation (TBD).
Footnotes
Declaration of competing interest
AB is employed by BrainSonix, which manufactures the ultrasound device. He holds patents in this area. BWB owns minority stake in Bodhi NeuroTech Inc, which manufactures meditation enhancing devices and holds patents in this area. No other authors have any other conflicts.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2020.10.007.
References
- [1].Aubry J-F, Tanter M. Therapeutic ultrasound In: MR-guided transcranial focused ultrasound. Springer; 2016. p. 97–111. [DOI] [PubMed] [Google Scholar]
- [2].Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998;24(2):275–83. [DOI] [PubMed] [Google Scholar]
- [3].Hynynen K MRI-guided focused ultrasound treatments. Ultrasonics 2010;50(2):221–9. [DOI] [PubMed] [Google Scholar]
- [4].Sun J, Hynynen K. Focusing of therapeutic ultrasound through a human skull: a numerical study. J Acoust Soc Am 1998;104(3):1705–15. [DOI] [PubMed] [Google Scholar]
- [5].Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul 2011;4(3):125–36. [DOI] [PubMed] [Google Scholar]
- [6].Younan Y, Deffieux T, Larrat B, Fink M, Tanter M, Aubry JF. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med Phys 2013;40(8). 082902. [DOI] [PubMed] [Google Scholar]
- [7].Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry J-F, et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron 2019;101(6):1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caulfield KA, George MS. The future of brain stimulation treatments. Psychiatr Clin 2018;41(3):515–33. [DOI] [PubMed] [Google Scholar]
- [9].Bowary P, Greenberg BD. Noninvasive focused ultrasound for neuromodulation: a review. Psychiatr Clin 2018;41(3):505–14. [DOI] [PubMed] [Google Scholar]
- [10].Köhler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys 2009;36(8):3521–35. [DOI] [PubMed] [Google Scholar]
- [11].Kennedy JE, Ter Haar G, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol 2003;76(909):590–9. [DOI] [PubMed] [Google Scholar]
- [12].ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperther 2007;23(2):89–104. [DOI] [PubMed] [Google Scholar]
- [13].Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369(7):640–8. [DOI] [PubMed] [Google Scholar]
- [14].Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2016;375(8):730–9. [DOI] [PubMed] [Google Scholar]
- [15].Dobrakowski PP, Machowska-Majchrzak AK, Łabuz-Roszak B, Majchrzak KG, Kluczewska E, Pierzchała KB. MR-guided focused ultrasound: a new generation treatment of Parkinson’s disease, essential tremor and neuropathic pain. Intervent Neuroradiol 2014;20(3):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci 2014;17(2):322–9. [DOI] [PubMed] [Google Scholar]
- [17].Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci 2012;13(12):867–78. [DOI] [PubMed] [Google Scholar]
- [18].Yoon K, Lee W, Lee JE, Xu L, Croce P, Foley L, et al. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PloS One 2019;14(10). e0224311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee W, Chung YA, Jung Y, Song IU, Yoo SS. Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound. BMC Neurosci 2016;17(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim H, Lee SD, Chiu A, Yoo SS, Park S. Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation. Neuroreport 2014;25(7):475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoo SS, Kim H, Min BK, Franck E, Park S. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport 2011;22(15):783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maxwell AD, Cain CA, Hall TL, Fowlkes JB, Xu Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med Biol 2013;39(3):449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gateau J, Aubry J, Chauvet D, Boch A, Fink M, Tanter M. In vivo bubble nucleation probability in sheep brain tissue. Phys Med Biol 2011;56(22):7001. [DOI] [PubMed] [Google Scholar]
- [24].Gateau J, Taccoen N, Tanter M, Aubry J-F. Statistics of acoustically induced bubble-nucleation events in in vitro blood: a feasibility study. Ultrasound Med Biol 2013;39(10):1812–25. [DOI] [PubMed] [Google Scholar]
- [25].Constans C, Mateo P, Tanter M, Aubry J-F. Potential impact of thermal effects during ultrasonic neurostimulation: retrospective numerical estimation of temperature elevation in seven rodent setups. Phys Med Biol 2018;63(2). 025003. [DOI] [PubMed] [Google Scholar]
- [26].Schlesinger D, Lee M, Ter Haar G, Sela B, Eames M, Snell J, et al. Equivalence of cell survival data for radiation dose and thermal dose in ablative treatments: analysis applied to essential tremor thalamotomy by focused ultrasound and gamma knife. Int J Hyperther 2017;33(4):401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO. Ultrasound neuromodulation: a review of results, mechanisms and safety. Ultrasound Med Biol 2019;45(7):1509–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pasquinelli C, Hanson LG, Siebner HR, Lee HJ, Thielscher A. Safety of transcranial focused ultrasound stimulation: a systematic review of the state of knowledge from both human and animal studies. Brain Stimul 2019;12(6):1367–80. [DOI] [PubMed] [Google Scholar]
- [29].Gibson BC, Sanguinetti JL, Badran BW, Yu AB, Klein EP, Abbott CC, et al. Increased excitability induced in the primary motor cortex by transcranial ultrasound stimulation. Front Neurol 2018;9:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gallagher RM, Rosenthal LJ. Chronic pain and opiates: balancing pain control and risks in long-term opioid treatment. Arch Phys Med Rehabil 2008;89(3 Suppl 1):S77–82. [DOI] [PubMed] [Google Scholar]
- [31].Bohnert ASB, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. J Am Med Assoc: JAMA, J Am Med Assoc 2011;305(13):1315–21. [DOI] [PubMed] [Google Scholar]
- [32].Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief 2009;(22):1–8. [PubMed] [Google Scholar]
- [33].Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep 2010;59(23):705–9. [PubMed] [Google Scholar]
- [34].Clasca F, Rubio-Garrido P, Jabaudon D. Unveiling the diversity of thalamocortical neuron subtypes. Eur J Neurosci 2012;35(10):1524–32. [DOI] [PubMed] [Google Scholar]
- [35].Vogt BA, Sikes RW, Vogt LJ. Anterior cingulate cortex and the medial pain system In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. p. 313–45. [Google Scholar]
- [36].Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM. Non-invasive ultrasonic thalamic stimulation in disorders of consciousness after severe brain injury: a first-in-man report. Brain Stimul 2016;9(6):940–1. [DOI] [PubMed] [Google Scholar]
- [37].Xiao X, Zhang Y-Q. A new perspective on the anterior cingulate cortex and affective pain. Neurosci Biobehav Rev 2018;90:200–11. [DOI] [PubMed] [Google Scholar]
- [38].Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, et al. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology 2013;38(7):1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brown J, Younger J, Madan A, Borckardt J. Pain in chronic medical illness, vol. 2013 Pain research and treatment; 2013. p. 615967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain 2012;153(6):1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain 2012;153(7):1350–63. [DOI] [PubMed] [Google Scholar]
- [42].FDA) UFaDA. Marketing clearance of diagnostic ultrasound systems and transducers.
- [43].Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 1998;23(4):323–55. [Google Scholar]
- [44].Mark VH, Ervin FR. Role of thalamotomy in treatment of chronic severe pain. PGM (Postgrad Med) 1965;37(5):563–71. [DOI] [PubMed] [Google Scholar]
- [45].Wallace BA, Ashkan K, Benabid A-L. Deep brain stimulation for the treatment of chronic, intractable pain. Neurosurg Clin 2004;15(3):343. [DOI] [PubMed] [Google Scholar]
- [46].Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Fo6cus 2006;21(6):1–8. [DOI] [PubMed] [Google Scholar]
- [47].Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol 2009;35(10):1737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maimbourg G, Houdouin A, Deffieux T, Tanter M, Aubry J-F. 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys Med Biol 2018;63(2). 025026. [DOI] [PubMed] [Google Scholar]
- [49].Maimbourg G, Houdouin A, Deffieux T, Tanter M, Aubry J-F. Steering capabilities of an acoustic lens for transcranial therapy: numerical and experimental studies. IEEE (Inst Electr Electron Eng) Trans Biomed Eng 2019;67(1):27–37. [DOI] [PubMed] [Google Scholar]
- [50].Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol 2013;12(5):462–8. [DOI] [PubMed] [Google Scholar]
- [51].Rieke V MR thermometry Interventional magnetic resonance imaging. Springer; 2011. p. 271–88. [Google Scholar]
- [52].Paquin R, Vignaud A, Marsac L, Younan Y, Lehéricy S, Tanter M, et al. Keyhole acceleration for magnetic resonance acoustic radiation force imaging (MR ARFI). Magn Reson Imag 2013;31(10):1695–703. [DOI] [PubMed] [Google Scholar]
- [53].Ozenne V, Constans C, Bour P, Santin MD, Valabrègue R, Ahnine H, et al. MRI monitoring of temperature and displacement for transcranial focus ultrasound applications. Neuroimage 2020;204 116236. [DOI] [PubMed] [Google Scholar]
- [54].Wattiez N, Constans C, Deffieux T, Daye PM, Tanter M, Aubry J-F, et al. Transcranial ultrasonic stimulation modulates single-neuron discharge in macaques performing an antisaccade task. Brain Stimul 2017;10(6):1024–31. [DOI] [PubMed] [Google Scholar]
- [55].Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry J-F. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr Biol 2013;23(23):2430–3. [DOI] [PubMed] [Google Scholar]
- [56].Boutet A, Gwun D, Gramer R, Ranjan M, Elias GJB, Tilden D, et al. The relevance of skull density ratio in selecting candidates for transcranial MR-guided focused ultrasound. J Neurosurg 2019:1–7. [DOI] [PubMed] [Google Scholar]
- [57].Guo H, Hamilton II M, Offutt SJ, Gloeckner CD, Li T, Kim Y, et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron 2018;98(5):1020–30. [DOI] [PubMed] [Google Scholar]
- [58].Verhagen L, Gallea C, Folloni D, Constans C, Jensen DE, Ahnine H, et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife 2019;8 e40541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.