Abstract
Background
Rapid coronavirus disease 2019 (COVID-19) diagnosis and isolation of infectious persons are critical to stopping forward transmission, and the care cascade framework can identify gaps in the COVID-19 response.
Methods
We described a COVID-19 symptom to isolation cascade and barriers among symptomatic persons who tested polymerase chain reaction positive for severe acute respiratory disease coronavirus 2 (SARS-CoV-2) at a low-barrier testing site serving a low-income Latinx community in San Francisco. Steps in the cascade are defined as days from symptom onset to test, test to result, and result to counseling on self-isolation. We examined SARS-CoV-2 cycle threshold (Ct) values to assess the likelihood of infectiousness on the day of testing and during missed isolation days.
Results
Among 145 persons, 97% were Latinx and 81% had an income of <$50 000. The median time from symptom onset to isolation (interquartile range [IQR]) was 7 (5–10) days, leaving a median (IQR) of 3 (0–6) days of isolation. Eighty-three percent had moderate to high levels of virus (Ct <33), but by disclosure 23% were out of their isolation period. The longest intervals were symptom onset to test (median [IQR], 4 [2–9] days) and test to results notification (median [IQR], 3 [2–4] days). Access to a test site was the most common barrier to testing, and food and income loss was the most common barrier to isolation.
Conclusions
Over half of the 10-day isolation period passed by the time of disclosure, and over a fifth of people were likely outside the window of infectiousness by the time they received results. Improvements in test access and turnaround time, plus support for isolation, are needed for epidemic control of SARS-CoV-2 in highly impacted communities.
Keywords: COVID-19, care cascade, Latino, Latinx, Hispanic, vulnerable populations
Severe acute respitatory syndrome coronavirus 2 (SARS-Co-V-2) is disproportionately impacting communities of color in the United States [1–5]; in California the highest number of cases and deaths are in the Latinx population [6]. A nationwide survey revealed longer testing turnaround times for Latinx and Black persons compared with White persons, and this delay can decrease the efficacy of containment strategies in these populations [7]. For the Latinx population and other communities of color, there are long-standing structural barriers to care access and systemic inequities and racism that can increase the time from symptom onset to effective isolation [3].
Reducing SARS-CoV-2 transmission relies on prompt isolation of people during a period of infectiousness—typically a short 10-day window from symptom onset for symptomatic immunocompetent individuals [8–10]. To effectively reduce transmission, individuals with coronavirus disease 2019 (COVID-19) must have rapid access to testing, get results quickly, and have the capacity to isolate effectively. Modeling studies emphasize the importance of effective isolation for those persons identified as at risk of or diagnosed with COVID-19 [11].
The care cascade framework is a powerful tool for understanding key barriers to effective epidemic control and is used widely for other infectious diseases such as HIV and tuberculosis [12, 13]. For COVID-19, effective isolation starts with accessible testing, but few studies to date have quantified the gaps and delays to obtain COVID-19 testing from symptoms to effective isolation in socio-economically vulnerable populations. Our overall aims were to expose the challenges of a symptom to isolation cascade and to identify actionable steps to improve the overall effectiveness of testing in a predominantly Latinx population participating in community testing. A central goal of testing is to isolate people during their infectious period in order to reduce forward transmission. As such, a secondary objective of this study was to use SARS-CoV-2 polymerase chain reaction (PCR) cycle threshold (Ct) values, a proxy for infectiousness, to assess whether testing and isolation recommendations reach this population during the time they are infectious.
METHODS
Study Setting and Testing Campaign
From July 29 to August 7, 2020, we offered twice-weekly outdoor walk-up testing at the 24th and Mission Bay Area Rapid Transit (BART) station in the Mission District of San Francisco, California. Testing dates coincided with a second wave of COVID-19 cases in San Francisco [14]. To date, the Mission District has the highest number of COVID-19 cases within San Francisco neighborhoods [14], and in 2018 Latinx residents comprised 58% of the population of the census tract surrounding the transport hub [15]. Before the transit hub pop-up testing, low-barrier community-based testing was only available 1 day a week in the Mission District.
Study planning and mobilization was done in partnership with the Latino Task Force–COVID-19, a collaborative of local Latinx community-based organizations and leaders. The community-led team advertised testing to local businesses and gave fast track tickets to local frontline essential workers, which allowed ticket holders to bypass the testing line. Walk-up testing was provided at no cost, regardless of symptoms or insurance. After providing informed consent, we conducted a brief survey on testing history, symptoms, employment, and transportation use before and during the COVID-19 pandemic. After the survey, medical staff performed anterior nares nasal swab for quantitative reverse transcription PCR (RT-PCR). All participants received post-test guidance on the day of testing on how to quarantine while awaiting test results if they were experiencing symptoms or had a recent exposure to someone with COVID-19.
Results Disclosure and Care of Participants With COVID-19
A bilingual team disclosed positive results over the telephone. During the disclosure calls, they performed a clinical assessment, confirmed the date of symptom onset, and provided guidance on self-isolation and quarantine per guidelines [16, 17]. Symptomatic patients with a positive PCR were advised to end their period of isolation 10 days after symptom onset if their fever had resolved for at least 24 hours and if their symptoms had improved. If the date of symptom onset was >14 days before the time of assessment, then participants were advised to isolate from the date of the test. Using our previously described “Test to Community Care Model,” a community health worker–led team provided regular check-in calls, delivered food and cleaning supplies, and assisted participants in linking to primary care [18].
Laboratory Assays
Swabs were collected in DNA/RNA Shield (Zymo Research) to inactivate the virus and preserve RNA stability. RT-PCR of viral N and E genes and human RNAse P gene was performed on extracted RNA at a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory operated by UCSF and the Chan Zuckerberg Biohub using a laboratory-developed test with a limit of detection of 100 viral genome copies/mL [19]. Assay results returned were qualitative, but Ct values were recorded for both genes for research purposes.
Study Definitions, Outcomes, and Statistical Analyses
COVID-19 Symptom to Isolation Cascade Among a Predominantly Low-Income Latinx Community
This analysis included people with a PCR positive for SARS-CoV-2 who were symptomatic. Symptomatic persons were defined as (a) reporting COVID-19 symptoms [20] at the time of testing or (b) reporting symptoms within 30 days before testing or (c) reporting symptoms between testing and result disclosure (presymptomatic at the time of testing). We excluded asymptomatic persons and all persons with a known prior positive PCR, as the onset of infection course could not be accurately defined by testing alone. We calculated descriptive statistics for each step across the symptom to isolation cascade.
We defined steps in the cascade as number of days from symptom onset to obtaining a test for COVID-19 (Gap: Testing Access), test to receiving the result (Gap: Test turnaround time), and positive results to verbal disclosure of results and counseling on self-isolation (Gap: Notification Time). We calculated the number of days of isolation missed, which we defined as the days of the infectious period minus the number of isolation days recommended at the time of the disclosure. At the time of disclosure, we asked a random sample of persons with a positive PCR and an interval of >3 days between symptom onset and testing about barriers they experienced to obtaining testing earlier.
Assessment of Adherence to Isolation and Quarantine
There are no validated surveys to assess adherence to isolation and quarantine, so we explored 2 different methods. The first was 3-day self-reported adherence to isolation: “Over the last 3 days, how many times did you leave the house to go to buy food, to run errands, to go to work, to go to school, to go to a doctor’s visit, or other?” Nonadherence was defined as leaving the house for any reason aside from seeing a health care provider. We administered this questionnaire to all people who were still in their period of self-isolation during 3 predefined interview days. Our second method was through a direct assessment by random home visit and calls by the community health worker (CHW) team. A person met our definition of nonadherence to self-isolation if they failed to answer the phone or door while the CHW was outside (after 3 attempts) for a home delivery or if work, transport, or street noise was noted while talking on the phone. Random home visits and calls were made to a randomly selected subsample of 13 households.
Cycle Thresholds at Testing and Relationship With Days Since Symptom Onset and Recommended Isolation Times
We used RT-PCR as a proxy for infectiousness and conservatively defined Ct <24 as a high viral load (infectious), Ct between 24–33 as a moderate viral load, and Ct >33 as a low viral load (low probability of being infectious). These strata are based on data from the CDC’s finding of 33 as a cutoff for replication-competent virus [21], as well as studies correlating Ct and probability of culturing the SARS-CoV-2 virus [10, 22]. In our RT-PCR assay, a Ct of 33 corresponds to 2.6 ×103 viral RNA copies. We calculated the mean and median days of isolation by Ct strata.
RESULTS
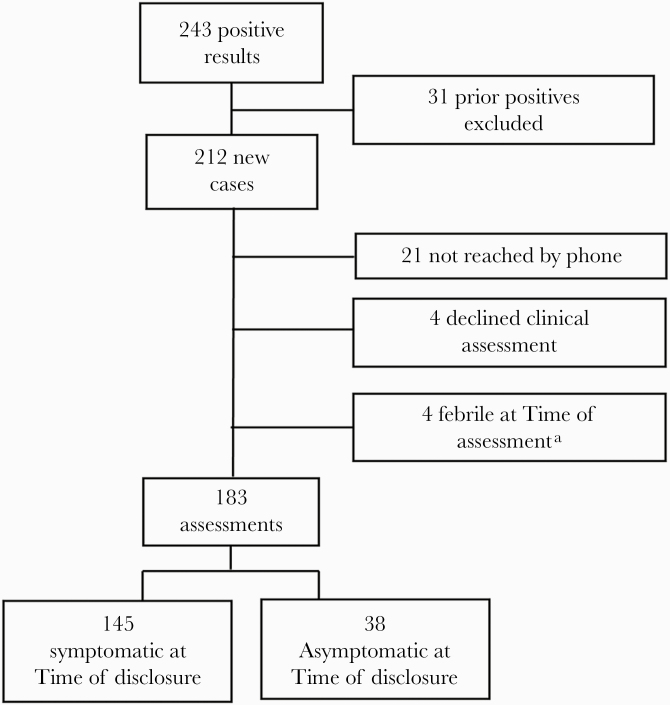
Overall, 2622 persons were tested by SARS-CoV-2 RT-PCR. Of those who were tested, 1937 (74%) were Latinx, 1677 (64%) were between the ages of 18 and 50, and 1491 (69%) were testing for the first time. Among testers, 243 (9%) tested PCR positive and 212 people (8%) were newly positive. Of the 243 people with a positive PCR, 240 (99%) retrieved their results via text message or email and 191 (79%) were reached by phone. Demographics for people with a positive PCR are in Table 1. A total of 145 participants met our definitions of symptomatic and were included in the cascade analysis (Figure 1).
Table 1.
Demographics of 145 Participants With Symptoms Compatible With COVID-19 and PCR Positive for SARS-CoV-2
| No. (%) | |
|---|---|
| Gender/sex at birth | |
| Male | 84 (58) |
| Female | 59 (41) |
| Nonbinary | 2 (1) |
| Race/ethnicity | |
| Latinx | 141 (97) |
| Black/African American | 2 (1.3) |
| Asian | 3 (2.1) |
| White | 2 (1.3) |
| Pacific Islander | 0 (0) |
| American Indian | 1 (0.7) |
| Underlying medical condition | |
| Diabetes mellitus | 16 (11) |
| Hypertension | 15 (10) |
| Lung disease | 3(2) |
| Malignancy | 2 (1) |
| Symptoms of COVID-19 | |
| Fever | 87 (60) |
| Cough | 74 (51) |
| Myalgias | 73 (50) |
| Severe fatigue | 56 (38) |
| Trouble breathing | 21(14) |
| Diarrhea | 27 (18) |
| Loss of smell | 61(42) |
| Loss of taste | 58 (40) |
| Headaches | 35 (24) |
| Sore throat | 15 (10) |
| Congestion | 10 (7) |
| Prior exposure to someone with COVID-19 before testing | 73 (50) |
Abbreviations: COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
Study participant diagram and inclusion to COVID-19 symptom to isolation cascade. aUnable to provide an end date for isolation given ongoing fever. Abbreviation: COVID-19, coronavirus disease 2019.
COVID-19 Symptom to Isolation Care Cascade
Symptom to Test
Days from symptom onset to test date ranged from –4 days (negative value used for symptoms that developed after testing) to 33 days, with a median time (IQR) of 4 (2–9) days (Table 2). Among the 36 people who completed the questionnaire on testing delays, reasons for not testing earlier included not feeling sick enough to get a test (31%), being unable to get an appointment (25%) or not knowing where or how to make an appointment (14%), not having insurance or a doctor (14%), results not returning fast enough to be useful (8%), and testing sites being too far (3%).
Table 2.
Duration in Days for Each Step of the Symptom to Isolation Cascade
| Metric | Average (SD) | Median (IQR) | Range |
|---|---|---|---|
| Days from symptom onset to test | 6.3 (6.4) | 4 (2–9) | –4 to 33 |
| Days from test performed to result | 2.5 (1.4) | 2 (2–3) | 1 to 10 |
| Days from test result finalized to verbal disclosure | 0.8 (1.1) | 1 (0–1) | 0 to 9 |
| Days of isolation days recommended | 3.7 (2.8) | 4 (0–6) | 0 to 10 |
| Days of missed isolation | 7.4 (2.6) | 7 (5–10) | 0 to 10 |
Abbreviation: IQR, interquartile range.
Test to Results Notification
The median time from sample collection to verbal results notification (IQR) was 3 (2–4) days, with times ranging from 1 to 9 days. The median time from the time the results were finalized by the lab to reaching the participant on the phone was 0.8 days, with times ranging from 0 to 9 days.
Overall Time From Symptom Onset to Isolation
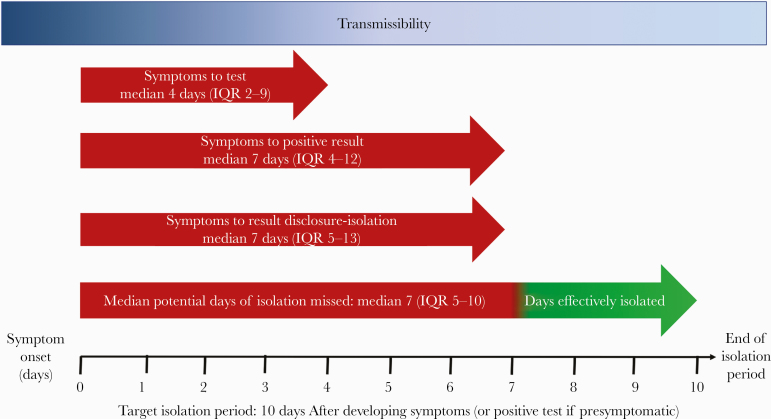
The median time from symptom onset to verbal results notification and isolation (IQR) was 7 (5–13) days, and 38 of 145 (26%) people had 0 of 10 days left of isolation by the time they were notified (Figure 2).
Figure 2.
This schematic of the COVID-19 symptom to isolation cascade describes the steps and time between symptom onset and effective isolation among 145 persons testing positive for SARS-CoV-2 at a low-barrier testing campaign at a transport hub. The longest delays were noted in participants presenting for testing (median [IQR], 4 [2–9] days) and test turnaround time (median [IQR], 3 [2–4] days), resulting in a total duration of time from symptom onset to effective isolation (IQR) of 7 (5–13) days. The target recommended time for self-isolation is 10 days from symptom onset. Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Adherence to Isolation and Quarantine
One hundred seven people were eligible for the survey on barriers to isolation, and of these, 63 (59%) participants reported at least 1 barrier to successfully self-isolating. Barriers included loss of income (92%), insufficient food access (88%), insufficient space to isolate (35%), caregiving needs (27%), and mental health (8%). Among the 32 persons called for the 3-day self-reported adherence to isolation survey, 29 (91%) met the definition of self-reported adherence to self-isolation. One person left his or her house to move their car, another left to attend an appointment with their lawyer, and the last left isolation to get food. We also conducted “random checks” for 7 participants, and 4 (57%) met the criteria for adhering to self-isolation. Three did not meet the criteria due to environmental cues—transport, street, or work noises noted during the time of the call, or the individual was noted by the community health worker to not be at home.
SARS-CoV-2 PCR Cycle Thresholds and Recommended Isolation
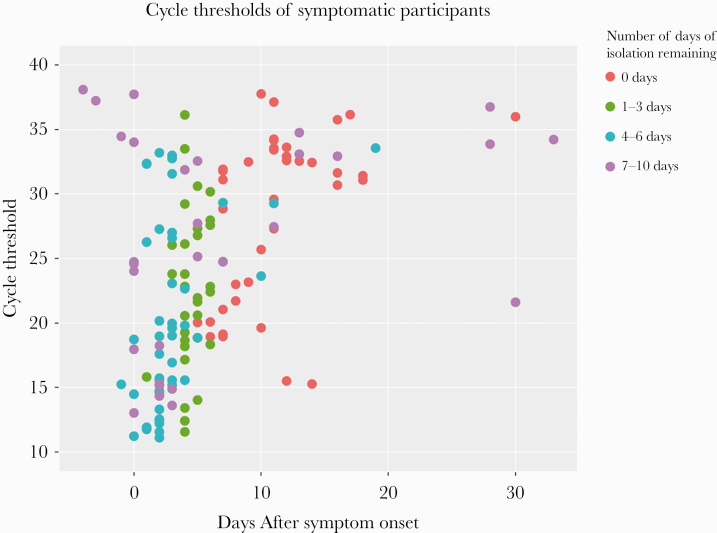
After disclosures were completed, we compared Ct values with days of symptom onset and the number of days of isolation recommended at disclosure (Figure 3). The geometric mean ± SD of cycle threshold was 24 ± 10 among presymptomatic patients, 23 ± 7 for patients who were tested within 14 days of symptoms, and 33 ± 4 for people tested >14 days from symptoms. Overall, 74 (52%) people had a Ct <24, 47 (32%) had a Ct of 24–33, and 24 (17%) had a Ct >33, and the median number of days of isolation did not differ across strata (P = .48): 4 (2–5), 3 (0–6), and 4 (0–8) days, respectively. Among those with high to moderate viral loads (Ct <33), 81% received <3 days of isolation and 23% received 0 days of isolation. Forty-five percent (14/31) of people presenting >10 days after symptom onset had a cycle threshold of >33, but 6 of these participants received 7–10 days of isolation. Among people reporting symptom onset >14 days before their test, 6 of 7 had a Ct >33, but 1 had a Ct of 21.6.
Figure 3.
Relationship between SARS-CoV-2 cycle thresholds, days from symptom onset (negative days indicate that symptoms began after testing), and number of days of isolation recommended among 145 persons diagnosed with COVID-19 at a low-barrier testing campaign. Days since symptom onset were assessed at the time of disclosure and the days of isolation were based on day of symptom onset and the day of disclosure. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
The effectiveness of a COVID-19 testing program in decreasing forward transmission depends on the speed with which it can both identify infectious individuals and provide counseling and support for adherence to self-isolation. Yet, in this cross-sectional analysis of low-income Latinx persons testing at a low-barrier COVID-19 pop-up testing site, we found long delays across the symptom to isolation cascade. By the time participants received counseling on isolation and quarantine, a median of only 3 days of isolation remained and a quarter of people were already outside the 10-day window of isolation. Likewise, of the people with Ct values <33 (moderate to high levels of virus), over one-fifth had already completed their 10-day period of isolation. Interventions to improve testing access, reduce test turnaround time, and provide language-concordant education about when to seek testing are urgently needed to improve this cascade, especially among Latinx communities and communities of color in the United States.
Our data highlight a long lag between symptom onset and testing—a median time of 4 days and up to 33 days. Participants cited lack of access to testing (testing site too far, unable to get an appointment, or perception that insurance was needed) as a common reason for not testing earlier. Regular access to low-barrier testing sites that are open 7 days a week, have a walk-up option, and are conveniently located within the communities most affected may shrink the time between symptom onset and testing among socio-economically vulnerable populations, and we have previously shown the effectiveness of low-barrier testing in reaching low-income Latinx communities [1]. Additionally, language and culturally concordant messaging on how and when to access COVID-19 testing, spearheaded by trusted community health workers, can help vulnerable populations navigate complex care systems and address testing barriers related to medical mistrust, immigration, and language that Latinx and other communities of color face [23–25].
Reducing testing turnaround around time to effective isolation can have a large impact on reducing forward transmission [11, 26]. Even with perfect contact tracing, a modeling study by Kretzchmar et al. [27] suggested that a test turnaround time of more than 3 days will be insufficient to reduce the reproductive number to <1 without other control measures. In the present study, the average time from test to result was 2 days. While this is faster than the 4-day turnaround time reported in a national survey in July [7], there is room to improve, and ideally results would return immediately with the patient still on site, or at a maximum of 24 hours. Improvements in test turnaround can be achieved with rapid antigen testing or increasing laboratory capacity to ensure PCR test turnaround. We previously demonstrated accuracy of rapid antigen tests in a small low-barrier testing campaign [28], but larger studies are needed to measure the accuracy and impact on symptom to isolation cascade for symptomatic and asymptomatic people being tested in low-barrier community-based testing sites.
The financial and psychological ramifications of adhering to self-isolation are high [30, 31], and among socio-economically vulnerable populations these costs are further amplified by insufficient social protections. In this study, >85% of persons with a positive SARS-CoV-2 PCR test with SARS-CoV-2 had a household income <$50 000 per year, and only one-fifth of people working reported having sick leave. Three-quarters of respondents reported not being able to isolate safely without additional support, and access to food and income were among the most common challenges to maintaining isolation. To our knowledge, there are no studies that measure adherence to self-isolation for COVID-19, but existing data from the SARS and H1N1 outbreaks highlight the importance of having a clear rationale for isolation, such as a positive test, backstopped by sufficient food support and access to financial assistance if needed [31]. In a study of household members of persons with H1N1, the most common reason for not adhering to quarantine was needing to go to work [32]. Consistent with these findings, our data support the importance of timely access to social and financial support as a key determinant of isolation and quarantine adherence. Examples of such support structures include our community-led Test to Community Care model [18] or wage replacement programs such as San Francisco’s Right to Recover program [33]. Inability to safely social distance at home was common, and having access to hotel rooms provided by the City of San Francisco [34] was fundamental to supporting adherence to isolation among people living in crowded or multigenerational households.
Measuring adherence to isolation and quarantine was challenging given the potential for social desirability bias during self-reported surveys and privacy concerns with tracking via mobile applications. Here, we piloted 2 metrics for adherence to self-isolation. As expected, adherence by self-report was higher than random home visits. We acknowledge limitations in our random home visits, as people may not have answered their doors or phones if they were asleep or did not recognize the phone number, and it may be challenging to differentiate whether one is hearing street noise because the person is outside or if they are inside but live on a busy street. Validation of these metrics and qualitative studies on isolation are needed, as mobility tracking devices that objectively measure isolation may be met with mistrust, especially in communities of color.
The prescribed period of isolation depends on self-reported symptom onset. Our examination of cycle thresholds of symptomatic participants highlights missed opportunities for isolation and identifies scenarios where consideration of the cycle threshold, as a proxy for viral load, may aid in guidance on isolation and quarantine. In this study, 53% of people presenting after 14 days of symptoms yielded high cycle thresholds >33 (<1e4 viral genomes), suggesting a lower probability of being infectious [10, 22]. However, despite measuring high cycle thresholds, 57% of these individuals were required to isolate 7–10 days, as their symptoms were outside of the 14-day window. Aside from in vitro cell culture viral recovery experiments, which themselves are imperfect proxies for human infection, it is difficult to measure the relationship between viral load and probability of infectiousness in a given individual. Nevertheless, isolating people with very low probabilities of being infectious can have negative financial and psychological ramifications—especially for vulnerable populations who cannot work from home and who have job insecurity. Conversely, 3 people in this study with a symptom onset of >14 days after the disclosure call had a cycle threshold of <22 but were not advised to isolate, based on symptoms and the fact that they were beyond of the 10-day isolation window. Though these cases were rare, these findings suggest potential underisolation. It may be the case that lower cycle threshold values could be used to advise longer isolation times as part of the disclosure. In this way, cycle thresholds may be helpful in determining the amount of isolation days for people who receive results >10 days after symptom onset, thus reducing the possibility of both over- and underisolation.
These findings must be interpreted in the context of the study design. The data are from a cross-sectional convenience sample of persons testing at 1 “pop-up” low-barrier testing site. Though this design limits generalizability, the characteristics of the sample are also a strength, as the sample was drawn from a testing site that tested a majority Latinx population of >2600 people, and to our knowledge, there are no other cascades focusing specifically on a socio-economically vulnerable Latinx population. Other limitations include the following: (1) the sample size of our exploratory assessment of adherence to self-isolation is small and was meant as a proof-of-concept study to pilot the use of 2 different metrics, and validation with larger studies is needed; and (2) we assessed symptoms at the time of testing and results disclosure, so we may have missed presymptomatic persons who developed symptoms after disclosure. Lastly, it is important to note that we make the assumption that people did not quarantine until they received a positive result. It is possible that some people were quarantining while awaiting their results, but we think this is unlikely as prior studies highlight the importance of a test result for adherence to isolation [30, 31]. There are significant financial costs of self-isolation among this population, and a large proportion of people diagnosed with COVID-19 in this study could not isolate safely at home due to household crowding.
Our symptom to isolation cascade analysis among low-income Latinx persons highlights testing access and test turnaround time as high-impact areas to reduce the time from symptoms to effective isolation. Improving testing access and turnaround time will be futile without adherence to isolation. The majority of people in this study needed additional support to isolate from provision of food to referrals to the city’s isolation and quarantine hotels, and addressing these needs is paramount. COVID-19 is disproportionately affecting the Latinx and Black persons in the United States, and a focus on shortening the symptom to effective isolation cascade is crucial to ensuring equity and effectiveness of public health interventions to reduce SARS-CoV-2 transmission.
Acknowledgments
We thank Bevan Dufty and the BART Team, Jeff Tumlin, and the San Francisco MUNI, Supervisor Hillary Ronen, Mayor London Breed, Dr. Grant Colfax and the Department of Public Health, Salu Ribiero and Bay Area Phlebotomy and Laboratory services, the PrimaryBio COVID testing platform, Program for Breakthrough Biomedical Research, which is partially funded by the Sandler Foundation, and our community ambassadors and volunteers.
Financial support. This study was supported by UCSF, the Chan Zuckerberg Biohub, and the Chan Zuckerberg Initiative. L.R. was funded by T32 AI060530.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Ethical approval. The UCSF Human Research Protection Program Institutional Review Board determined that the study met the criteria for public health surveillance activities.
Patient consent. We obtained verbal and written informed consent in the participant’s preferred language before study participation.
References
- 1. Chamie G, Marquez C, Crawford E, et al. SARS-CoV-2 community transmission disproportionately affects Latinx population during shelter-in-place in San Francisco. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis 2020; 222:890–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macias Gil R, Marcelin JR, Zuniga-Blanco B, et al. COVID-19 pandemic: disparate health impact on the Hispanic/Latinx population in the United States. J Infect Dis 2020; 222:1592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The COVID Tracking Project. The COVID Racial Data Tracker. Available at: https://covidtracking.com/race. Accessed 2 October 2020.
- 5. Rodriguez-Diaz CE, Guilamo-Ramos V, Mena L, et al. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann Epidemiol 2020; 52:46–53.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.California Department of Public Health. Data on racial demographics Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/Race-Ethnicity.aspx. Accessed 2 October 2020.
- 7. Chwe H, Quintana A, Lazer D, et al. The state of the nation: a 50-state COVID-19 survey report #17: COVID-19 result times. 2020. Available at: www.covidstates.org. Accessed 2 October 2020.
- 8. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med 2021; 174:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perera RAPM, Tso E, Tsang OTY, et al. SARS-CoV-2 virus culture and subgenomic RNA for respiratory specimens from patients with mild coronavirus disease. Emerg Infect Dis 2020; 26:2701–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilinski A, Mostashari F, Salomon JA. Modeling contact tracing strategies for COVID-19 in the context of relaxed physical distancing measures. JAMA Netw Open 2020; 3:e2019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Subbaraman R, Nathavitharana RR, Mayer KH, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med 2019; 16:e1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.City and County of San Francisco. Maps of COVID-19 cases Available at: https://data.sfgov.org/stories/s/Maps-of-COVID-19-Cases/adm5-wq8i/. Accessed 29 September 2020.
- 15.US Census Bureau. American Community Survey (ACS) Available at: https://www.census.gov/programs-surveys/acs. Accessed 6 October 2020.
- 16. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed 2 October 2020.
- 17. San Francisco Department of Public Health. Guidance isolation and quarantine 2020 Available at: https://www.sfdph.org/dph/alerts/covid-guidance/Guidance-Isolation-and-Quarantine.pdf. Accessed 29 September 2020.
- 18. Kerkhoff AD, Sachdev D, Mizany S, et al. Evaluation of a novel community-based COVID-19 ‘test-to-care’ model for low-income populations. PLoS One 2020; 15:e0239400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The CLIAHUB Consortium. Rapid deployment of SARS-CoV-2 testing: the CLIAHUB. PLOS Pathog. 2020; 16:e1008966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) – symptoms 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 14 October 2020.
- 21.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-duration of isolation 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed 15 October 2020.
- 22. Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis 2020; 71:2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landers SJ, Stover GN. Community health workers—practice and promise. Am J Public Health 2011; 101:2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peretz PJ, Islam N, Matiz LA. Community health workers and Covid-19 — addressing social determinants of health in times of crisis and beyond. N Engl J Med. 2020; 383:e108. [DOI] [PubMed] [Google Scholar]
- 25. Kim HN, Lan KF, Nkyekyer E, et al. Assessment of disparities in COVID-19 testing and infection across language groups in Seattle, Washington. JAMA Netw Open 2020; 3:e2021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hellewell J, Abbott S, Gimma A, et al. ; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health 2020; 8:e488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kretzschmar ME, Rozhnova G, Bootsma MCJ, et al. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health 2020; 5:e452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pilarowski G, Lebel P, Sunshine S et al. Performance characteristics of a rapid SARS-CoV-2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis 2020. doi: 10.1093/infdis/jiaa802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubin GJ, Smith LE, Melendez-Torres G, Yardley L. Improving adherence to “test, trace and isolate.” J R Soc Med 2020; 113:335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Webster RK, Brooks SK, Smith LE, et al. How to improve adherence with quarantine: rapid review of the evidence. Public Health 2020; 182:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teh B, Olsen K, Black J, et al. Impact of swine influenza and quarantine measures on patients and households during the H1N1/09 pandemic. Scand J Infect Dis 2012; 44:289–96. [DOI] [PubMed] [Google Scholar]
- 32.Fracassa D. SF to pay low-wage workers who get COVID-19 to stay home and isolate - SFChronicle.com San Francisco Chronicle. 28 May 2020.
- 33.City and County of San Francisco. COVID-19 alternative housing Available at: https://data.sfgov.org/stories/s/COVID-19-Alternative-Housing/4nah-suat/. Accessed 12 December 2020.