Abstract
Background
Nephrotoxicity is a known adverse effect of polymyxin antibiotics, including colistin. Although previous meta-analyses have aimed to characterize colistin-associated nephrotoxicity risk relative to other antibiotics, included studies were observational in nature with high risk of confounding and heterogeneity. We conducted this systematic review and meta-analysis of exclusively randomized controlled trials (RCTs) to evaluate the incidence of nephrotoxicity associated with colistin versus minimally nephrotoxic antibiotics.
Methods
We searched PubMed, EMBASE, Cochrane Library, and 3 trial registries for RCTs comparing the nephrotoxicity of colistin to nonpolymyxin antibiotics. Randomized controlled trials that used aminoglycosides were excluded. Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using random-effects models. The study outcome was the rate of nephrotoxicity.
Results
Five RCTs with a total of 377 patients were included. Most patients received colistin for pneumonia in the intensive care unit, and the comparators were β-lactam-based regimens. Colistimethate sodium was dosed at 9 million units/day (300 mg/day of colistin base activity), with administration of a loading dose in 4 studies. The nephrotoxicity incidence in patients who received colistin was 36.2% (95% CI, 23.3% to 51.3%). The nephrotoxicity rate was significantly higher in the colistin arm than comparators (RR, 2.40; 95% CI, 1.47 to 3.91; P ≤ .001; I2 = 0%), and the number needed to harm was 5. Findings persisted upon one-study-removed-analysis.
Conclusions
This meta-analysis of RCTs found a colistin-associated nephrotoxicity rate of 36.2% and an increase in this risk compared with β-lactam-based regimens by 140%. Colistin should be regarded as a last-line agent and safer alternatives should be considered when possible.
Keywords: colistimethate, colistin, kidney, nephrotoxicity, polymyxin
In this meta-analysis of randomized controlled trials, colistin was associated with a nephrotoxicity rate of 36%. Nephrotoxicity risk was 140% higher with colistin compared to a beta-lactam-based regimen.
Although used sparingly compared with an array of other antibiotics, polymyxins remain relevant in the current era of antibiotic-resistant infections [1]. The polymyxins (polymyxin B, and polymyxin E, or colistin) have a unique mechanism of disrupting outer cell membrane integrity, leading to their retained activity against multiresistant or extensively drug-resistant Gram-negative pathogens. Despite potentially favorable in vitro activity, widespread use of polymyxins is largely limited by dosing controversies pertaining to pharmacokinetic/pharmacodynamic (PK/PD) optimization as well as adverse effects, particularly nephrotoxicity. Nephrotoxicity rates for both polymyxins are variable and are reported to be 20%–50%. Consensus guidelines for the optimal use of polymyxins were recently published in 2019 to provide clinical guidance on the use of these agents [1]. Colistin is recommended as the preferred agent for urinary tract infections, whereas the guidelines generally favor use of polymyxin B over colistin for serious systemic infections due to its more predictable PK properties and potentially less nephrotoxicity. Despite guideline recommendations, some institutions may still favor colistin over polymyxin B for systemic infections, possibly due to availability, familiarity of use, and/or its more favorable compounding properties. Prescribing information for polymyxin B, unlike colistin, recommends preparation in several hundred milliliters of dextrose, which may not be optimal for certain populations (eg, critically ill or volume-restricted patients) [2].
Given that there are niche roles for both polymyxins, it is of clinical importance to have an appreciable sense of the true nephrotoxicity risk, particularly in the setting of guideline-recommended dosing. For colistin, there is variability in reported nephrotoxicity rates that may be due to several factors, including lack of treatment randomization in most studies, the presence of confounders (eg, concomitant receipt of other nephrotoxic medications), and dosing inconsistencies. This heterogeneity was seen in a recent meta-analysis of observational cohorts [3]. Recent publication of several randomized controlled trials (RCTs) permits pooling of robust data and a less biased evaluation of nephrotoxicity outcomes with colistin use. This meta-analysis was conducted to identify the incidence of colistin-associated nephrotoxicity from RCTs, particularly in relation to minimally nephrotoxic comparators.
METHODS
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search Strategy and Selection Criteria
Articles were identified via MEDLINE (via PubMed), EMBASE, and Cochrane Library bibliographic databases using the search terms “colistin” or “colistimethate” and were restricted to RCTs (Supplementary Material). No restrictions were performed on language or publication date. In addition, the references of included studies and unpublished studies on the EU Clinical Trials registry, ClinicalTrails.gov, and WHO International Clinical Trials Registry Platform were searched. The searches and data extractions were completed independently by 2 authors until June 1, 2020. Any disagreement in the literature screening or data extraction was resolved through discussion. We only included comparative RCTs evaluating nephrotoxicity in adults treated with colistin-based therapy and excluded any RCT that used aminoglycosides in any arm to minimize confounding given their relatively high potential to cause or contribute to nephrotoxicity.
Outcomes, Data Analysis, and Risk of Bias
The primary outcome was nephrotoxicity. The incidence of nephrotoxicity in the colistin arm was estimated and evaluated relative to a comparator antibiotic arm. Mantel-Haenszel risk ratios (RRs) with 95% confidence intervals (CIs) were assessed using random-effects models, and heterogeneity (I2) was evaluated using Cochran’s Q test. The robustness of the meta-analysis finding was evaluated in a one-study-removed analysis. The number needed to treat for an additional harmful outcome (number needed to harm [NNH]) was estimated. We assessed study quality using the Cochrane risk of bias tool for RCTs (low, unclear, or high). To enhance clarity, we expressed doses in both Million International Units (MU)/day of colistimethate sodium (CMS) and mg/day of colistin base activity (CBA) [4]. We performed all analyses using the Comprehensive Meta-Analysis Version 3 software (Biostat, Englewood, NJ).
RESULTS
Search Results and Study Characteristics
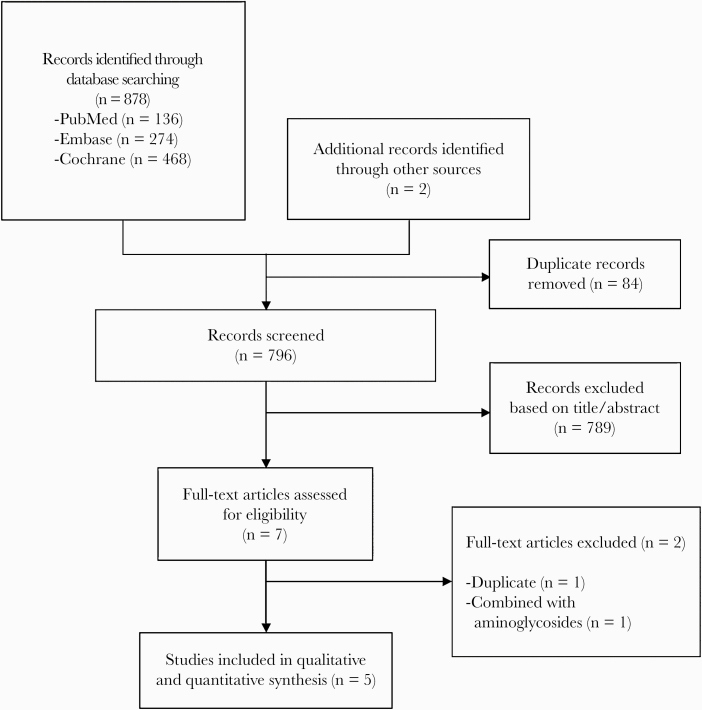
The search process identified 878 articles, and a total of 5 RCTs [5–9] were included after screening by title and/or abstract followed by full text (Figure 1). A total of 377 patients were included in the meta-analysis. The characteristics of included studies are summarized in Table 1. Three RCTs were open-label [5–7], 1 was single-blind [8], and 1 was double-blind [9]. Studies were conducted in 4 continents. Mean or median patient age ranged from 54 to 72 years. One study did not mention the percentage of patients with chronic kidney disease [5], whereas 4 studies either excluded those patients or the cohort percentage did not exceed 4% [5–9]. Four studies included patients who were in the intensive care unit and had ventilator-associated pneumonia [5–8], and 1 study included any hospitalized patient with ventilator-associated or hospital-acquired pneumonia, complicated urinary tract, or intra-abdominal infections [9]. The duration of antibiotic therapy ranged from 8 to 14 days. The colistin dose used was 9 MU/day of CMS (300 mg/day of CBA), and 4 studies mentioned use of a loading dose [6–9]. The comparator was (1) ampicillin/sulbactam in 3 RCTs [5, 7, 8] and (2) meropenem [6] and imipenem-relebactam [9] in the remaining RCTs. Colistin or comparator was combined with another agent (meropenem, imipenem, and levofloxacin) in 3 studies [6–8] and was used as monotherapy in the remaining studies [5, 6]. Vancomycin was used in 2 studies in 16%–36% of patients but was balanced between the study groups (15.8% vs 17% [P = .816] in the Cisneros et al [6] study and 41.7% vs 30.4% [P = .55] in the Khalili et al [7] study in the colistin arm vs the comparator arm, respectively). The definitions of nephrotoxicity in each study are summarized in Table 2 and the quality assessment of these studies is provided in Table 3.
Figure 1.
Flow diagram of the study selection process.
Table 1.
Characteristrics of Included Studies
| Study | Design | Location | Funding Source | Number of Patients | Patient Characteristics | Colistin vs Comparator Therapy | Duration of Therapy (Days) |
|---|---|---|---|---|---|---|---|
| Betrosian et al [5] | Superiority, open-label RCT | 2 sites in Greece | Nonindustry | 28; 15 vs 13 | ICU patients with ventilator-associated pneumonia; Age: 67 vs 72 years; APACHE II score: 14 | CMS 3 MU (100 mg CBA) IV q8h vs ampicillin/sulbactam 6/3 g IV q8h | 9 vs 10 |
| Cisneros et al [6] | Superiority, open-label RCT | 32 sites in Europe | Nonindustry | 232; 120 vs 112 | ICU patients with ventilator-associated pneumonia; Age: 63 vs 60.5 years; APACHE II score: 18; diabetes mellitus in 18%; CKD in 2%; vancomycin in 16% | CMS 4.5 MU (150 mg CBA) IV LD, then 3 MU (100 mg CBA) q8 vs meropenem 2 g IV q8h | 9 vs 8 |
| Khalili et al [7] | Superiority, open-label RCT | 1 site in Iran | Nonindustry | 47; 24 vs 23 | ICU patients with ventilator-associated pneumonia; Age: 61 vs 56 years; cardiovascular disease in 43%; diabetes mellitus in 28%; CKD in 4%; vancomycin in 36% | CMS 9 MU (300 mg CBA) IV LD, then 4.5 MU (150 mg CBA) q12 (+meropenem) vs ampicillin/sulbactam 2/1 g IV 4h | 14 vs 14 |
| Mosaed et al [8] | Superiority, single-blind RCT | 1 site in Iran | Nonindustry | 23; 11 vs 12 | ICU patients with ventilator-associated pneumonia; Age: 67 vs 64 years; APACHE II score: 19; cardiovascular disease in 13%; diabetes mellitus in 4%; CKD excluded | CMS 9 MU (300 mg CBA) IV LD, then 4.5 MU (150 mg CBA) q12 (+levofloxacin) vs ampicillin/sulbactam 4/2 g IV 6h (+levofloxacin) | 8 vs 9 |
| Motsch et al [9] | Superiority, double-blind RCT | 16 sites in North and South America, Europe | Industry | 47; 31 vs 16 | Hospitalized patients with nosocomial pneumonia, complicated urinary tract infection, or complicated intraabdominal infection; Age: 61 vs 59; Patients with CrCl <15 mL/min excluded | CMS 9 MU (300 mg CBA) IV LD, then 4.5 MU (150 mg CBA) q12h (+imipenem) vs imipenem/relebactam 500/250 mg IV q6h | 11 vs 11 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CBA, colistin base activity; CKD, chronic kideny disease; CMS, colistimethate sodium; CrCl, creatinine clearance; ICU, intensive care unit; IV, intravenous; LD, loading dose; MU, million unit; RCT, randomized controlled trial.
Table 2.
Nephrotoxicity Definition in the Included Studies
| Study | Nephrotoxicity Definition |
|---|---|
| Betrosian et al [5] | In patients with “normal” renal function: A SCr value >2 mg/dL or reduction in CrCl of 50% or initiation of renal replacement therapy. |
| In patients with pre-existing renal dysfunction: An increase of >50% of the baseline SCr or reduction in calculated CrCl of 50% relative to value at start of therapy. | |
| Cisneros et al [6] | Based on the RIFLE score. |
| Khalili et al [7] | An increase of ≥0.3 mg/dL in SCr within 48 hours of therapy or 1.5-times increase in the baseline within 7 days, or urine volume of <0.5 mL/kg per hour for 6 hours. |
| Mosaed et al [8] | A 30% increase in SCr |
| Motsch et al [9] | In patients with “normal” renal function: SCr doubling (to >1.2 mg/dL) or ≥50% CrCl reduction. |
| In patients with pre-existing renal impairment: SCr increases ≥1 mg/dL, ≥20% CrCl reduction, or need for renal replacement therapy. |
Abbreviations: SCr, serum creatinne; CrCl, creatinine clearance.
Table 3.
Quality Assessment of Included Studiesa
| Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | ||
|---|---|---|---|---|---|---|---|
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
| Betrosian et al [5] | + | ? | - | - | + | + | + |
| Cisneros et al [6] | + | + | - | - | + | + | + |
| Khalili et al [7] | + | ? | - | - | + | + | + |
| Mosaed et al [8] | + | ? | - | - | ? | + | + |
| Motsch et al [9] | + | + | + | + | + | + | ? |
a+, low risk of bias; “?” Unclear risk of bias; “-” high risk of bias.
Study Outcomes
The pooled incidence of nephrotoxicity in patients who received colistin was 36.2% (95% CI, 23.3% to 51.3%). The rate of nephrotoxicity was significantly higher with colistin than with comparators (RR, 2.40; 95% CI, 1.47 to 3.91; P ≤ .001; I2 = 0%) (Figure 2). The absolute risk difference was 19.1% (33.9% in the colistin arm vs 14.8% in the comparator arm) and the NNH was 5. The differences remained statistically significant in the one-study-removed analysis.
Figure 2.
Forest plot showing the risk ratios of nephrotoxicity using random-effects models in patients receiving colistin versus comparators. Vertical line, “no difference” point between the 2 groups; horizontal line, 95% confidence interval; squares, risk ratios; diamonds, pooled risk ratios. CI, confidence interval; MH, Mantel-Haenszel.
DISCUSSION
Our meta-analysis of RCTs confirms a high rate of nephrotoxicity, occurring in approximately one third of treated patients, with colistin-based regimens relative to β-lactam-based comparators. The 2019 polymyxin use guidelines estimate nephrotoxicity rates of 20%–50% [1]. However, individual studies have described incidences ranging from 0% to 60%. This variability is due to heterogeneous patient populations, variable definitions of nephrotoxicity, ranges of doses used, severity of illness differences, and presence of potential confounders such as concomitant use of other nephrotoxic agents. A recent meta-analysis by Oliota et al [10] found that the colistin-associated nephrotoxicity rate was 26.7% (95% CI, 22.8%–30.9%). Although this was a large observational analysis of 7911 patients, the included studies demonstrated high heterogeneity, possibly due to a lack of comparator groups and the clinical variability among the cohorts. In contrast, our analysis mainly identified studies of critically ill populations, and it has low heterogeneity. In addition, we analyzed studies that were prospective and randomized.
There are several risk factors for polymyxin-associated nephrotoxicity that are described in the literature. Those that are consistently reported include advanced age, extremes of weight, presence of chronic comorbid conditions, hypoalbuminemia, renal impairment, and receipt of other nephrotoxic agents [1]. In our analysis, the mean/median age of patients in most included studies was above 60 years of age and the overall percentage of baseline renal insufficiency was low. Other patient-specific data was not uniformly reported across the included studies. Risk may also be associated with duration of polymyxin therapy. One study indicated the rate of colistin-associated acute kidney injury to be 12% at 48 hours of treatment and 29% at day 7 [11]. In our analysis, patients received treatment for 8–14 days. Our finding of a 36% nephrotoxicity risk appears consistent with those studies that have characterized colistin nephrotoxicity on more of a continuum rather than at a single point in time [11].
There are several strengths to our analysis. We only included studies that were prospective and randomized, and we specifically excluded those that permitted receipt of other potentially nephrotoxic Gram-negative agents such as the aminoglycosides. In addition, colistin dosing in the 5 included studies (9 MU/day; 300 mg of CBA) is consistent with that recommended in the 2019 polymyxin guidelines (9–10.9 MU/day, or 300–360 mg of CBA). Guidelines also recommend an initial loading dose, which was administered in 4 of the 5 studies. This dosing is expected to produce concentrations associated with the maximal tolerability threshold, which suggests that our finding of a 36% nephrotoxicity incidence is representative of the risk associated with optimal dosing. The majority of RCTs were not funded by industry, and the population was representative of patients from 4 continents, increasing the external validity of this meta-analysis. Finally, given that colistin recipients frequently are complicated patients with severe illness, the critically ill population in this analysis could be expected to be representative of other likely candidates for colistin therapy in a real-world setting.
Several limitations related to this analysis are worthy of consideration. The studies in this analysis used variable definitions of nephrotoxicity, which can make clinical application challenging. However, all definitions incorporated an element of elevated serum creatinine, which is generally consistent with how nephrotoxicity is identified and characterized in clinical settings. In addition, this definition variability is typical in meta-analyses assessing nephrotoxicity. Moreover, removing one study from the analysis showed no difference in the meta-analysis conclusion. This was true even when data from the Cisneros et al [6] study was removed, which comprised approximately two thirds of patients in our analysis. Although we aimed to control for confounders related to nephrotoxicity by limiting included studies to RCTs, several factors may have impacted our findings. There is a possibility that colistin-associated nephrotoxicity incidence was inflated due to patients’ critical illness, because all were treated in the intensive care unit; however, few had a history of chronic kidney disease. In addition, although we did exclude studies that included patients who received other nephrotoxic Gram-negative agents, in 2 studies [6, 7], some patients received concurrent vancomycin. Concurrent use of vancomycin and polymyxins has shown to increase risk of kidney injury in other studies [1, 11]; therefore, the impact of this combination on our findings cannot be excluded, although the overall numbers in our analysis were relatively small, and no significant differences were found between the 2 groups in the percentage of patients who received vancomycin. No information was provided on the use of other nephrotoxic agents. We also did not correlate nephrotoxicity with a particular PK/PD or concentration threshold, which would be of interest given guideline recommendations to target an area under the curve (24-hour steady state) and average steady-state concentration of 50 mg.hour/L and 2 mg/L, respectively. Exposures above these thresholds have been associated with increased nephrotoxicity [1]. In addition, we could not evaluate the role of a colistin loading dose on nephrotoxicity rates, although PK/PD principles still support administration of a loading dose, as was given to most patients in our analysis. Finally, there was insufficient detail to examine the progression and resolution of nephrotoxicity along a continuum among the individual studies. It is also unclear whether different colistin brands and lots may have different rates of nephrotoxicity due to differences in conversion rate from CMS to colistin [1].
CONCLUSIONS
Colistin remains a clinically relevant polymyxin agent. In a meta-analysis of RCTs, the incidence of colistin-associated nephrotoxicity was approximately 36% and the relative risk compared with β-lactams increased by 140%. This analysis had low heterogeneity and characterized risk associated with contemporary colistin dosing recommendations. Clinicians should weigh the risks versus benefits of using colistin therapy given its toxicity potential in critically ill patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019; 39:10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polymyxin B Product Information. Bedford, OH: Bedford Laboratories; 2012. [Google Scholar]
- 3. Chien HT, Lin YC, Sheu CC, et al. Is colistin-associated acute kidney injury clinically important in adults? A systematic review and meta-analysis. Int J Antimicrob Agents 2020; 55:105889. [DOI] [PubMed] [Google Scholar]
- 4. Eljaaly K Dose and duration of intraventricular antibiotic therapy in meningitis. Clin Microbiol Infect 2016; 22:817. [DOI] [PubMed] [Google Scholar]
- 5. Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect 2008; 56:432–6. [DOI] [PubMed] [Google Scholar]
- 6. Cisneros JM, Rosso-Fernández CM, Roca-Oporto C, et al. ; Magic Bullet Working Group WP1 Colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia (Magic Bullet study): an investigator-driven, open-label, randomized, noninferiority controlled trial. Crit Care 2019; 23:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalili H, Shojaei L, Mohammadi M, et al. Meropenem/colistin versus meropenem/ampicillin-sulbactam in the treatment of carbapenem-resistant pneumonia. J Comp Eff Res 2018; 7:901–11. [DOI] [PubMed] [Google Scholar]
- 8. Mosaed R, Haghighi M, Kouchak M, et al. Interim study: comparison of safety and efficacy of levofloxacin plus colistin regimen with levofloxacin plus high dose ampicillin/ sulbactam infusion in treatment of ventilator-associated pneumonia due to multi drug resistant acinetobacter. Iran J Pharm Sci 2018; 16:206–13. [PMC free article] [PubMed] [Google Scholar]
- 9. Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: A multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis 2020; 70:1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliota AF, Penteado ST, Tonin FS, et al. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis 2019; 94:41–9. [DOI] [PubMed] [Google Scholar]
- 11. Shields RK, Anand R, Clarke LG, et al. Defining the incidence and risk factors of colistin-induced acute kidney injury by KDIGO criteria. PLoS One 2017; 12:e0173286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.