Abstract
In female mammals, reproduction, and in particular lactation, is the energetically most exigent life-history phase. Reproduction is strongly controlled by body reserves and food availability, so females with better body condition or food supply are believed to have higher reproductive output. Additionally, the growth and mortality of young mammals depends on their postnatal development. Therefore, the degree of precociality affects energetic demands for both mothers and young. To study the reproductive performance of the precocial European hare (Lepus europaeus), we analysed relationships between six predictor variables describing maternal and environmental effects and nine response variables relating to reproduction from 217 captive females. We compared the data with those of precocial and altricial mammal species from an extensive literature search. For hares, we found: (1) Heavier females had heavier litters at birth. (2) In summer and spring, total litter mass was larger than in winter. (3) At the end of lactation, the litters of multiparous females were heavier than those of primiparous females. (4) Both older females and females giving birth for the first time had relatively high leveret mortality during lactation. Comparing our results with the literature for other mammals revealed that the body condition (i.e., body mass) of females before birth is predictive of reproductive parameters in both precocial and altricial species. In the precocial hare, female body condition is no longer predictive of reproductive parameters at the end of lactation, whereas in altricial species, female body condition remains predictive of reproduction (litter mass at the end of lactation, offspring mortality) until the end of lactation. We conclude that these effects are caused by precocial offspring feeding on solid food soon after birth and, thus, being less dependent on the mother’s body condition during lactation than altricial offspring. In line with this, precociality might have evolved as a way of buffering offspring against maternal effects.
Introduction
Reproduction is a costly process [1–4] and is directly linked to the evolution of reproductive strategies [5] and population dynamics [6]. In female mammals, reproduction is the most energetically demanding life-history phase [7], so it is firmly regulated by body condition and food availability [8]. Of the reproductive processes, lactation is the most energetically demanding for female mammals; it results in maximum metabolic rates and energetic costs [1, 2, 9, 10]. Females in optimal body condition are able to make high reproductive investments without impairing their future survival and reproduction [11, 12]. In several mammalian species, offspring mass is indeed positively correlated with the mother’s body mass [13–16] although fathers have an influence on offspring mass too due to their contribution to the offspring genotype. However, reproduction can reduce the mother’s body condition [17–20] and, hence, future reproductive performance [21–23]. Two strategies in maternal investment have evolved: capital breeders use their own body fat reserves to cover the high energetic costs of reproduction, whereas income breeders increase their energy or food intake [24]. Questions relating energy to reproductive performance are difficult to address in wild mammals. In captive females, it is possible to vary diet composition in order to quantify the influence of energy availability, female body mass and length of lactation on reproductive output (i.e. the number of young born) and reproductive success (i.e. the number of young that survive until the end of lactation).
Reproductive performance in wild female mammals may vary seasonally. Due to seasonal fluctuations in climate and day length, ecosystems at higher latitudes and in continental habitats are characterized by large seasonal changes in ecologically important parameters such as temperature and the availability of food [25]. Correlated with these variations, most mammals dwelling in such habitats show seasonal changes in breeding activity [25, 26], including seasonal variations in litter size [27–29] and offspring body mass at birth [30, 31].
Further, fertility rate is typically low at the beginning of the reproductive life of a female mammal [32], increases thereafter and starts to decrease again from about the middle of the reproductive span onwards [32, 33]. However, age-related variation in fertility is almost impossible to study in wild populations because the number of senescent individuals is low, due to predation and disease [34–36].
The growth and mortality of young mammals depend not only on characteristics of their mother, but also on their postnatal development. Two strategies have evolved in mammals–altricial and precocial postnatal development–such that a continuum exists between them. Precocial young are born, after a prolonged gestation period, with fur and open eyes, and are well-developed and mobile soon after birth. In contrast, altricial young are born without fur and with closed eyes, are rather immobile, and have a long postnatal period of parental dependency. In mammals, altricial young are more common, but precocial young occur in numerous mammalian taxa [37]. Small mammals generally have large litters of altricial young and high reproductive rates, whereas large mammals usually produce precocial young in small litters [37]. Female precocial rodents have a lower peak energy demand during lactation than female altricial rodents, as precocial offspring start to take solid food earlier [38].
In both precocial and altricial reproduction, output (number of offspring) is positively correlated with female body mass (e.g., [9, 39, 40]), decreases in older females (e.g., [33]) and varies during the year in seasonal environments (e.g., [41]). Both female characteristics (e.g., body mass at mating, age, parturition sequence, lactation duration) and environmental characteristics (e.g., season of birth, food availability) can affect the growth and mortality of young in both precocial and altricial species during the lactation period. However, little is known about the influence of female and environmental characteristics on reproductive parameters (e.g., litter size, litter mass, mean body mass of offspring) at the end of lactation in precocial and altricial mammals, and the trade-offs associated with the different strategies of postnatal development during the lactation period are not well understood. Especially in precocial mammals, reproductive parameters at the end of lactation are scarce (but see [30, 42]).
This study focuses on the European hare (Lepus europaeus), a precocial polygynandrous species without social hierarchy [43, 44]. Several previous studies described reproductive biology and strategy in this lagomorph, but important questions remain unanswered. Hackländer et al. [45] showed that female diet during lactation affects the energy budget of mothers, but does not influence total offspring body mass gain during lactation. Litter size within each reproductive season increases from the first litter and then declines towards the last litter in wild European hares [46–50], but whether season of birth influences leveret body mass and total litter mass is unknown. Offspring body mass at birth is decisive for offspring survival [51–54], especially in precocial mammalian species that reach maternal independence rather early [51]. Moreover, the fertility of female European hares depends on age. Most leverets are born during a mother’s third year of life. After this peak, fertility decreases and senescence takes place even if body condition is high [48, 55]. It is important to understand the influence of maternal age on reproductive parameters such as litter size, leveret body mass, and litter mass, in order to quantify maternal effects in the precocial hare.
The goal of this study was to investigate the reproductive performance of female European hares and to compare the results with data from other precocial and altricial mammals. Our hypotheses were: (1) female hares’ diet and body mass affect females’ reproductive output and success; (2) litter mass is highest and leveret body mass is lowest in the middle of the breeding season; (3) reproductive parameters that are decisive for the reproductive performance (i.e. litter size, litter mass, leveret body mass and leveret survival during lactation) are lower in primiparous females and in females at the end of their reproductive life; and (4) in European hares and other precocial mammals, but not in altricial mammals, female and environmental characteristics affect reproductive parameters at birth and at the end of lactation in different ways (e.g. female body mass is only relevant until birth, whereas the season is relevant until the end of lactation). To test these hypotheses, we analysed data from captive female European hares and conducted an extensive literature search on reproductive parameters of precocial and altricial mammals.
Material and methods
Captive hares and data collection
Our study animals were born, individually marked and maintained in the outbred breeding colony of European hares at the Research Institute of Wildlife Ecology in Vienna (Austria). The study was discussed and approved by the institutional ethics and animal welfare committee of the University of Veterinary Medicine, Vienna, in accordance with GSP guidelines and national legislation. Data were collected over a period of 16 years (1989–2004). The hares were kept in outdoor cages with wire-netting floors (0.5 m2). They were sheltered from the wind and direct sunlight, but exposed to the natural photoperiod and ambient temperatures (mean annual air temperature: 10.4°C, annual minimum air temperature: -19.6°C, annual maximum air temperature: 37.6°C, data provided by the Austrian Central Institute for Meteorology and Geodynamics, weather station Wien Hohe Warte, 48°14’N, 16°21’E). All individuals had access to food and water ad libitum, and were given regular anti-parasitic treatment. Generally, males, females, and leverets were kept separately. Only for mating, a male was placed into a female’s cage for two nights. Males were selected randomly in order to avoid bias due to the influence of male body condition on leveret body condition. Females that did not mate successfully were removed from the breeding program; this mostly happened when they were between four and six years old. Animals were housed for use in further research following this research. Because leverets are nursed only once a day in the wild [56], we separated them from their mothers except for between 08:00 and 09:00 h each day, when they were placed into their mother’s cage and were allowed to suckle. Females always accepted their young for suckling irrespective of any potential disturbing factor, e.g. human odour. We stopped putting leverets with their mothers for suckling after four to five weeks, depending on each female’s level of aggression towards her offspring and the body mass of the leverets. This period of weaning corresponds to that found in the wild [56].
At birth, and at the end of lactation, litter mass was measured by weighing the entire litter, and mean leveret body mass was determined by dividing litter mass by litter size. All weights were taken to the nearest gram. Leverets were sexed according to secondary sexual characteristics. Sex ratio at birth was calculated by the number of male leverets at birth divided by the litter size at birth. Leveret mortality during lactation was determined by litter size at the end of lactation subtracted from litter size at birth divided by litter size at birth. The age of each hare was known as all birth dates were recorded.
We determined female mass and age at mating. Lactation duration was defined as the number of days leverets were put with their mothers for suckling. Female hares were in two diet groups: one was fed with standard hare pellets (‘low-fat diet’, Raiffeisen, Salzburg, n = 157; number of litters = 947) and the other was fed with high-fat pellets (‘high-fat diet’, n = 77; number of litters = 473). Note that 17 females changed diet group once but never during reproductive season. The nutrient composition of the low-fat diet corresponded to chemical analysis of stomach contents from free-ranging hares [57, 58]. The high-fat diet was produced by adding 1 kg of sunflower oil that was rich in fatty acids to 10 kg standard pellets. This procedure resulted in a profound increase in dietary fat content (for further information see [45]). We defined ‘season of birth’ as winter (December to February), spring (March to May), summer (June to August), or autumn (September to November).
Data analysis
Data were collected partly repeatedly from the same females in different years. Therefore, we used generalized linear models (GLMMs), with separate intercepts for females, nested into each year, as random effects. This allowed us to adjust for repeated measurements from a female and under different environmental conditions in each study year. GLMMs were computed using R [59] packages ‘brms’ [60] and ‘rstan‘ [61]. The Bayesian GLMM approach implemented in these libraries has the advantage that it can readily estimate random effects even when data are obtained partly as repeated measures, and partly from a single reproductive bout in one female. This data structure often causes singularities and prevents random effect estimates with other methods. Multicollinearity between the predictor variables was checked calculating the Variance Inflation Factor (VIF) with R package ‘car’ [62] for all independent variables in each model. The variables litter size at birth and litter mass at birth had a VIF>8. Therefore, we removed litter size at birth from the one full model including these two predictors.
All GLMM samples were drawn with the NUTS algorithm using four chains and at least 4000 iterations (6000 for more complex models). We only report models that converged with all Rhat<1.05.
In addition to the above random effects, all full models included the following five predictor variables describing maternal and environmental effects: female mass at mating, female age (both variables scaled), reproductive state (primiparous/multiparous), female diet (high/low fat) and season of birth (factor with levels winter, spring, summer, autumn) as well as the two-way interactions between these variables. Some models included additional fixed predictors (for an overview of all models see S1 Table). We eliminated model terms to determine the model that minimized leave-one-out cross-validation Information Criterion (LOOIC; [63]) using the R package ‘loo’ [64]. As a measure of support for the inclusion of a variable, we used the increment of the increase caused by its removal, Δ LOO.
The response variables litter size at birth and litter size at the end of lactation were Poisson distributed without overdispersion (inspected with library ’fitdistrplus’, [65]), and were analysed accordingly. For the response variables litter mass at birth and mean leveret body mass at birth, we used the family function ‘gaussian’. GLMMs for normally distributed data were also used to model the mass gain of litters and mean body mass gain of leverets during lactation. The response variables in these models were litter mass and mean leveret body mass at the end of lactation, which were always adjusted for litter mass or mean leveret body mass at birth, entered as fixed predictors. Litter mass and body mass gain were only analysed for litters in which there was no mortality to avoid the effect of a sudden change in sibling rivalry on the response variables.
Sex ratio and leveret mortality (during lactation) were analysed using binomial models. For each reproductive bout, we considered litter size as the ‘trial’ number, and the number of male leverets (for the sex ratio) or the number of leverets dying (for mortality) as the ‘incidence’ number. We used only flat (uniform) prior distributions, as we had no prior information on expected slopes, and because this avoids bias on the resulting posterior distributions [66, 67].
To test the effect of female age on milk quantity, we reanalysed data on 21 females published by Hackländer et al. [45] with Bayesian GLMM similar to the other analyses of this study. The daily milk quantity was examined using the family function ‘gaussian’. The full model included female age, litter size, leveret age (in weeks), season of birth, and female diet (low- or high-fat), with intercepts for females, nested into each year, as random effects.
Literature search
The Web of Science (Clarivate Analytics, Boston, MA, USA) was used as a research tool for finding scientific articles on mammalian reproduction (date limit: 30.06.2020). Search terms were the parameters used to describe reproduction (i.e. litter size, mean young mass, litter mass, young mortality, lactation duration) in combination with mammals. Furthermore, terms describing postnatal development (e.g., altricial, precocial), maternal effects (i.e. female body mass, female age, parturition sequence, lactation duration) and effects of environmental characteristics (i.e. season of birth, diet availability) were included in the literature search. We included synonyms of the search terms (e.g., offspring, young).
Results
We analysed data on reproductive performance of 217 females producing 909 leverets in 442 litters. Female mass at mating ranged from 2210 g to 4905 g with a mean of 3562 g (± 13.42 SE). The youngest female to give birth produced her litter at the age of 109 days, so she had mated successfully at the very young age of about two months (67 days). This female was born in April and had her first litter of only one leveret in July (leveret’s body mass at birth: 124 g). The oldest female to give birth produced her litter at the age of 2317 days (~ 6.3 years). On average, females gave birth for the first time at the age of 423 days (± 27.34 SE, ~ 1 year and 2 months old), and for the last time at the age of 740 days (± 43.85 SE; about two years old). The sex ratio of offspring was balanced (at birth: 51% males; at the end of lactation: 48% males).
Reproductive performance
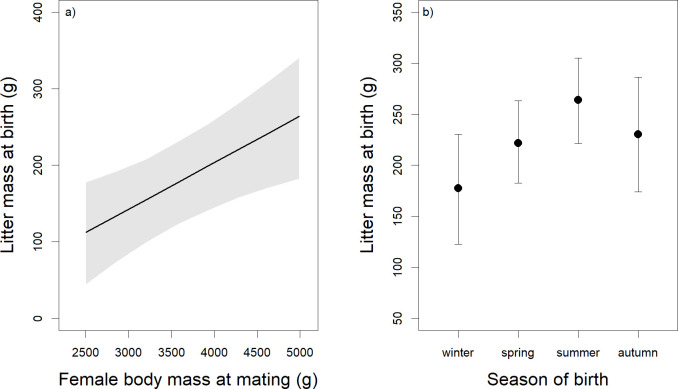
The coefficients of the best model for each of the nine response variables are shown in Table 1. Litter size at birth was not closely associated with by any predictor variable. However, the best model suggested that heavier females had larger litters (-0.03 to 0.19; β = 0.08; Δ LOO 0.8; for), but litter size at birth showed no strong seasonal effect (litter size at birth in winter: mean = 1.65 ± 0.15 SE; spring: mean = 1.77 ± 0.09 SE, summer: mean = 2.13 ± 0.11 SE; autumn: mean = 1.94 ± 0.21 SE). Heavier females had heavier litters at birth (β = 21.51; Δ LOO 4.7; Fig 1A), and litter mass at birth was related to the season of birth (Δ LOO 10.0): it was lower in winter and higher in spring and summer (Fig 1B). Mean leveret body mass at birth was lower in larger litters (β = -8.71; Δ LOO 29.9; Table 2), and leverets of primiparous females were lighter at birth than leverets of multiparous females (β = -9.94; Δ LOO 7.2).
Table 1. Coefficients of the best models for all nine response variables describing reproduction.
| Litter size at birth | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 0.61 | 0.10 | [0.40, 0.80] |
| Female body mass at mating | 0.08 | 0.05 | [-0.03, 0.19] |
| Litter mass at birth | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 176.58 | 26.91 | [123.72, 228.40] |
| Female body mass at mating | 21.43 | 6.82 | [8.04, 34.60] |
| Season of birth: Spring | 45.65 | 23.19 | [0.16, 90.68] |
| Season of birth: Summer | 87.07 | 22.80 | [42.17, 131.38] |
| Season of birth: Autumn | 53.91 | 28.21 | [-1.25, 108.96] |
| Mean leveret body mass at birth | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 130.36 | 2.49 | [125.54, 135.12] |
| Litter size at birth | -8.71 | 1.61 | [-11.81, -5.54] |
| Reproductive state | -9.94 | 3.58 | [-16.99, -3.06] |
| Litter size at the end of lactation | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 0.50 | 0.10 | [0.31, 0.69] |
| Litter size at birth | 0.33 | 0.07 | [0.19, 0.46] |
| Litter mass at the end of lactation | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 0.11 | 0.07 | [-0.03, 0.25] |
| Female diet | -0.14 | 0.09 | [-0.31, 0.03] |
| Lactation duration | 0.26 | 0.05 | [0.16, 0.35] |
| Litter mass at birth | 0.64 | 0.11 | [0.42, 0.85] |
| Litter size at birth | 0.31 | 0.11 | [0.10, 0.53] |
| Reproductive state | -0.26 | 0.09 | [-0.44, -0.086] |
| Mean leveret body mass at the end of lactation | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 0.09 | 0.08 | [-0.06, 0.23] |
| Lactation duration | 0.24 | 0.05 | [0.14, 0.34] |
| Mean leveret body mass at birth | 0.92 | 0.04 | [0.84, 1.00] |
| Reproductive state | -0.21 | 0.10 | [-0.40, -0.02] |
| Sex ratio at birth | Estimate | Est. Error | 95% Credible Interval |
| Intercept | -0.06 | 0.20 | [-0.44, 0.35] |
| Female body mass at mating | -0.02 | 0.13 | [-0.29, 0.23] |
| Leveret mortality during lactation | Estimate | Est. Error | 95% Credible Interval |
| Intercept | -1.60 | 0.53 | [-2.74, -0.66] |
| Female age | 0.54 | 0.30 | [-0.03, 1.18] |
| Reproductive state | 1.41 | 0.50 | [0.45, 2.41] |
| Daily milk quantity | Estimate | Est. Error | 95% Credible Interval |
| Intercept | 44.46 | 23.64 | [-7.01, 89.06] |
| Leveret age | 7.68 | 0.62 | [6.49, 8.90] |
| Litter size at birth | 13.69 | 1.52 | [10.63, 16.61] |
| Season of birth: Spring | -7.41 | 3.69 | [-14.63, -0.23] |
| Season of birth: Summer | 1.31 | 3.34 | [-5.36, 7.92] |
| Season of birth: Autumn | 13.90 | 12.27 | [-9.87, 37.71] |
Coefficients of the best models for the response variables litter size at birth (n = 54; number of litters = 178), litter mass at birth (n = 52; number of litters = 174), mean leveret body mass at birth (n = 52; number of litters = 174), litter size at the end of lactation (n = 40; number of litters = 99), litter mass at the end of lactation (n = 40; number of litters = 98), mean leveret body mass at the end of lactation (n = 40; number of litters = 98), sex ratio at birth (n = 50; number of litters = 162), leveret mortality during lactation (n = 54; number of litters = 178), and daily milk quantity (n = 21, litters = 23).
Fig 1.
Relationships between a) female body mass at mating and litter mass at birth and b) season of birth and litter mass at birth. Relationships between a) female body mass at mating and litter mass at birth, with the line representing the predicted median values and the grey area displaying the Bayesian credible intervals; and b) season of birth and litter mass at birth, with the points representing the predicted median values and the whiskers displaying the Bayesian credible intervals (n = 52; number of litters = 174). See text for details on statistics.
Table 2. Mean leveret body mass at birth of leverets from different litter sizes.
| Litter size at birth | Mean leveret body mass at birth (g) | SE | n |
|---|---|---|---|
| 1 | 125.5 | 2.54 | 118 |
| 2 | 118.6 | 1.73 | 152 |
| 3 | 108.8 | 2.00 | 96 |
| 4 | 102.8 | 4.34 | 18 |
| 5 | 89.5 | 6.50 | 2 |
Mean leveret body mass, standard error and sample size of different litter sizes at birth (number of females = 114; number of litters = 386). Mean leveret body mass was determined by dividing litter mass by litter size.
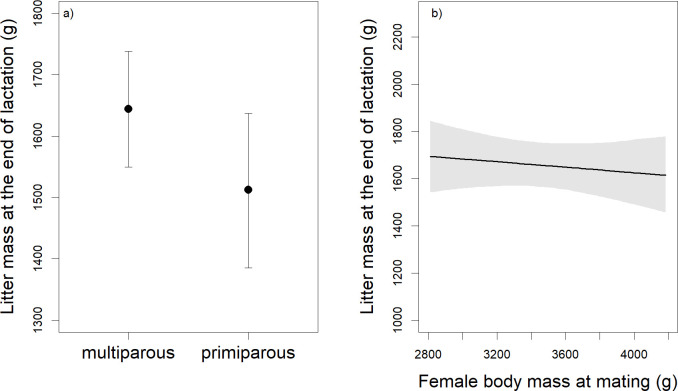
Litter mass at the end of lactation was positively associated with lactation duration (β = 0.24; Δ LOO 17.5), and with litter mass at birth (β = 0.92; Δ LOO 184.9). Litters of primiparous females were lighter at the end of lactation than those of multiparous females (β = -0.21; Δ LOO 4.4; Fig 2A), but, by the end of lactation, litter mass was no longer related to female body mass at mating as it had been at birth (Fig 2B) and the effect of the season of birth was no longer significant. By the end of lactation, leverets of primiparous females remained lighter than those of multiparous females (β = -0.36; Δ LOO 2.4). Mean leveret body mass at the end of lactation was also positively associated with lactation duration (β = 0.52; Δ LOO 22.9) and with mean leveret body mass at birth (β = 0.42; Δ LOO 22.0).
Fig 2.
Relationships between a) female reproductive state and litter mass at the end of lactation and b) female body mass at mating and litter mass at the end of lactation. Relationships between a) female reproductive state and litter mass at the end of lactation, with the points representing the predicted median values and the whiskers displaying the Bayesian credible intervals (n = 40; number of litters = 98); and b) female body mass at mating and litter mass at the end of lactation, with the line representing the predicted median values and the grey area displaying the Bayesian credible intervals. See text for details on statistics.
Sex ratio at birth was not related to female body mass at mating, season of birth, female age, female diet or female reproductive state (all credible intervals including 0). Mean leveret mortality between birth and the end of lactation was 0.34. Leverets of older females (β = 0.55; Δ LOO 3.2; Fig 3A) and primiparous females (β = 1.42; Δ LOO 9.3; Fig 3B) had higher mortality during lactation than those of other females.
Fig 3.
Relationships between a) female age and leveret mortality and b) female reproductive state and leveret mortality. Relationships between a) female age and leveret mortality, with the line representing the predicted median values and the grey area displaying the Bayesian credible intervals; and b) female reproductive state and leveret mortality, with the points representing the predicted median values and the whiskers displaying the Bayesian credible intervals (n = 54; number of litters = 178). See text for details on statistics.
Milk production
The daily milk quantity provided to offspring during lactation increased with litter size (β = 13.69; Δ LOO 48.4) and leveret age (β = 7.68; Δ LOO 142.1), and was lower for litters born in spring than for those born in winter (Δ LOO 7.0). Daily milk quantity was not significantly related to female diet (low- or high-fat) or female age.
Literature search
Out of 71 articles on mammalian reproduction, we found 36 published articles in which authors provided information on reproductive parameters (i.e. litter size at birth, mean offspring mass at birth, litter mass at birth, litter size at the end of lactation, mean offspring mass at the end of lactation, litter mass at the end of lactation, offspring mortality between birth and the end of lactation; S2 Table) of mammals within the altricial and precocial postnatal development continuum (excluding parent-clinging mammals such as primates). Among them, 31 articles also included information on maternal effects (i.e., female body mass, female age, parturition sequence, lactation duration) and/or effects of environmental characteristics (i.e., season of birth, diet availability) on reproductive parameters (S3 Table). Among them, 22 articles analysed relationships between female body mass and reproduction (Table 3). No other maternal or environmental parameter was analysed regarding its effect on reproduction in the 36 published articles. Note that none of the studies on species near the middle of the continuum provided information on relationships between female body mass and reproduction. Hence, Table 3 only includes studies on precocial and altricial mammal species.
Table 3. Comparison of studies investigating relationships between female body mass and reproductive parameters in precocial and altricial mammal species.
| Influence | Precocial | Altricial | |||
|---|---|---|---|---|---|
| Study | Species | Study | Species | ||
| Litter mass at birth | + | This study | European hare Lepus europaeus | [39] | Hispid cotton rat Sigmodon hispidus |
| + | [40] | Deer mouse Peromyscus maniculatus | |||
| + | [68] | European rabbit Oryctolagus cuniculus | |||
| Litter mass at the end of lactation | no | This study | European hare Lepus europaeus | ||
| + | [40] | Deer mouse Peromyscus maniculatus | |||
| Mean offspring mass at birth | no | This study | European hare Lepus europaeus | ||
| + | [42] | Reindeer Rangifer tarandus | [40] | Hispid cotton rat Sigmodon hispidus | |
| + | [69] | Reindeer Rangifer tarandus | [40] | Deer mouse Peromyscus maniculatus | |
| + | [13] | Reindeer Rangifer tarandus | [70] | White-footed mouse Peromyscus leucopus | |
| + | [71] | Reindeer Rangifer tarandus | [72] | Richardson’s ground squirrel Spermophilus richardsonii | |
| + | [73] | Red deer Cervus elaphus | |||
| + | [14] | Red deer Cervus elaphus | |||
| + | [74] | Red deer Cervus elaphus | |||
| + | [15] | White-tailed deer Odocoileus virginianus | |||
| + | [16] | Fallow deer Dama dama | |||
| Mean offspring mass at the end of lactation | no | This study | European hare Lepus europaeus | ||
| + | [40] | Deer mouse Peromyscus maniculatus | |||
| + | [75] | Columbian ground squirrel Spermophilus columbianus | |||
| Litter size at birth | no | This study | European hare Lepus europaeus | [68] | Norway rat Rattus norvegicus |
| no | [76] | Guinea pig Cavia porcellus | |||
| + | [77] | Guinea pig Cavia apere | [78] | Meadow vole Microtus pennsylvanicus | |
| + | [79] | Flying squirrel Glaucomys volans | [39] | Hispid cotton rat Sigmodon hispidus | |
| + | [40] | Deer mouse Peromyscus maniculatus | |||
| + | [68] | European rabbit Oryctolagus cuniculus | |||
| + | [70] | White-footed mouse Peromyscus leucopus | |||
| + | [80] | Richardson’s ground squirrel Spermophilus richardsonii | |||
| Litter size at the end of lactation | no | This study | European hare Lepus europaeus | ||
| Postnatal mortality until the end of lactation | no | This study | European hare Lepus europaeus | ||
| + | [52] | Leaf-eared mouse Phyllotis darwini | |||
| + | [81] | European rabbit Oryctolagus cuniculus |
Comparison of studies investigating relationships between female body mass and reproductive parameters in precocial and altricial mammal species. Numbers refer to the studies in the reference list.
Discussion
Female diet and body mass effects
Our hypothesis (1), that female hares’ diet and body mass affect females’ reproductive output and success, was only partly supported by our results. Female diet (low- or high-fat) had no effect on reproductive parameters. This is in line with Hackländer et al. [45], who found no difference in litter size or mean leveret body mass at birth or at the end of lactation between the low- and high-fat diet group. However, a high-fat diet positively affects the energy budget of a female, enabling her to shorten the lactation period [45]. Hackländer et al.’s study was conducted on a subset of 18 of our 217 female hares.
Litter mass at birth was positively associated with female body mass at mating. Litter mass is a good measure of reproductive output, as it integrates litter size and intrauterine growth. Thus, our results support the assumption that females in optimal body condition show higher reproductive investment [11, 12]. In addition, our findings indicate that female European hares are, to a certain extent, capital breeders [24]. In contrast to numerous other studies of mammals, we did not find any effect of female body mass on mean offspring body mass at birth [13–16, 39, 40, 42, 69–74], or on litter size at birth [39, 40, 68, 70, 77–80].
Seasonal effects
Our hypothesis (2), that litter mass is highest and leveret body mass is lowest in the middle of the breeding season, could only be confirmed with respect to litter mass. In contrast to the results of several mammal studies, including some on the European hare (litter size: [27–29, 46–48, 50, 82], offspring body mass: [30, 31]), we did not find any effect of season of birth on litter size or on mean offspring body mass, either at birth or at the end of lactation. In fact, our findings were very close to those of researchers working on natural populations of hares under similar climatic conditions (e.g., in the Netherlands [83]). In captive animals, a lack of effect of season on litter size and mean offspring body mass at birth can probably be attributed to the unlimited food availability over the whole year [25]. Moreover, our captive animals were partly sheltered from harsh weather conditions and were free from endoparasites. A lack of seasonal influence on litter size has also been recorded in captive Savi’s pine voles (Microtus savii) [84]. We suggest that the modified environmental conditions in our captive population act to reduce the strong seasonal effects that occur under continental climate conditions and mimic the conditions found in regions with an oceanic climate [85]. In line with this notion, Broekhuizen and Maaskamp [83] reported lower seasonal variation in litter size in hares in the Netherlands (mean litter size was between 1.5 and 2 in January/February, around 3 in summer and around 2.5 in autumn).
We did find a seasonal influence on litter mass at birth. This suggests that females giving birth in spring and summer invest their surplus energy in having not only more, but also heavier leverets. Litter mass at birth, which is a direct indicator of the female’s reproductive investment, seems to be unchanged by artificial conditions (including constant food availability) in captivity, and therefore may be regulated in a different way than litter size and mean offspring body mass. An explanation might be that litter mass at birth is under photoperiod control.
Female reproductive stage and age effects
The results of the present study support our hypothesis (3), that reproductive parameters that are decisive for the reproductive performance are lower in primiparous females and in females at the end of their reproductive life, only for the parameter leveret mortality during lactation. We detected only one age-related effect on reproductive performance of European hare females in our study: leveret mortality during lactation increased with maternal age. This is in contrast to evidence of reproductive senescence causing reduced reproductive output in various mammalian species (e.g., [86–89]), including the European hare [48, 55]. Our finding that leveret mortality during lactation increases with the age of the mother suggests that maternal-effect senescence occurs in the European hare, such that senescence affects postnatal maternal care. Maternal-effect senescence has been described in another precocial mammal, the bottlenose dolphin (Tursiops aduncus), a species which, in contrast to the European hare, provides extensive maternal care [90]. However, our reanalysis of data published by Hackländer et al. [45] to investigate whether older females produced less daily milk than younger females showed that daily milk quantity was affected by litter size and age of leverets, but not by female age. Higher offspring mortality in primiparous females has been described repeatedly in mammals (e.g., [91–93]), and is perhaps explained by primiparous females being relatively less physically mature and less experienced in reproduction [94].
Differences related to precociality and altriciality at birth and at the end of lactation
We did find support for a part of our hypothesis (4), that in European hares and other precocial mammals, but not in altricial mammals, female and environmental characteristics affect reproductive parameters at birth and at the end of lactation in different ways. Litter mass at birth is positively associated with female body mass in mammals, irrespective of their altricial or precocial postnatal developmental strategy (for our study see Fig 1A; for an overview of published studies see Table 3). However, we found no relationship between female body mass and litter mass at the end of lactation in our precocial hares (Fig 2B), whereas in altricial deer mice (Peromyscus maniculatus) female body mass is positively correlated with litter mass at the end of lactation [40]. This difference might be caused by precocial offspring feeding on solid food soon after birth and, thus, being less dependent on the mother’s body condition during lactation than altricial offspring. In hares, leverets eat solid food soon after birth, and also consume proportionally more solid food if the mother’s dietary energy content during gestation and lactation is low [95]. Thus, leverets can compensate for poor milk quality or quantity by additional foraging. However, in the natural environment this increased foraging certainly comes at the cost of increased predation risk.
Mean offspring mass at birth was not related to female body mass in our hares, but these parameters are positively correlated in several other larger precocial mammals [13–16, 42, 69, 71, 73, 74] and in all altricial mammals that are represented in our dataset ([39, 40, 70, 72]). Offspring mass at birth is negatively correlated with litter size, irrespective of postnatal development strategy (e.g., precocial: [15, 77, 96, 97], this study; altricial: [40, 52, 70, 75]). Therefore, the difference in the relationship between offspring mass at birth and female body mass is most certainly due to the difference in litter size: smaller litter sizes occur in large species (such as large ungulates) and larger litter sizes occur in small species (such as our European hare). Mean offspring mass at the end of lactation was not related to female body mass in our precocial hares, but these parameters are positively related in altricial species [40, 75]. Consistently, this might be because precocial offspring consume solid food in addition to their mother’s milk.
In line with other differences in precociality and altriciality at the end of lactation, postnatal mortality until the end of lactation was not related to female body mass in our precocial hares, whereas mortality is lower in the offspring of heavier females in altricial mammal species [52, 81].
We found no published data on maternal and environmental effects on litter size at the end of lactation in our literature search. Hence, we could not compare differences in litter size at birth and at the end of lactation in relation to precociality and altriciality. The scarcity of published data on female characteristics other than body mass and on environmental effects on other reproductive parameters at the end of lactation did not allow further comparisons between precocial and altricial mammals. We recommend that future researchers should record reproductive parameters not only at birth, but also at the end of lactation, to allow further investigation of differences between precocial and altricial mammals.
Maternal effects on reproduction
At birth, litter size and mass in the precocial hare are strongly positively correlated with the maternal body mass, but, by the end of lactation, correlations with maternal body mass no longer exist. Litters born in spring and summer, and those born to females in the middle of their reproductive lives, are the most likely to have high litter mass and to survive lactation. In altricial mammals, reproductive parameters can be predicted from female mass, not just at birth, but also at the end of lactation. The differences of maternal effects on reproduction between altricial and precocial mammals may have a meaning for evolution. Hence, precociality might have evolved as a way of buffering offspring against maternal effects.
Supporting information
(XLS)
(XLS)
(XLS)
(CSV)
Acknowledgments
We would like to thank Elisabeth Hammerschmidt, Birgit Hauhart, Nicole Hilgers and Michaela Salaba for their help with data collection. English language editing and review services were supplied by Nancy Jennings, www.dotmoth.co.uk.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by a PhD grant to KH from the German Academic Exchange Service (DAAD), by a grant to KH from the Deutsche Wildtier Stiftung, by the Austrian Federal Ministry of Education, Science and Culture, the Austrian hunting associations, the City of Vienna, and the Department of Science and Culture of the Government of Lower Austria. Open access funding was provided by BOKU Vienna Open Access Publishing Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Clutton-Brock T. Reproductive effort and terminal investment in iteroparous animals. Am Midl Nat. 1984;123:212–29. [Google Scholar]
- 2.Gittleman J, Thompson S. Energy allocation in mammalian reproduction. Amer Zool. 1988;28:863–75. [Google Scholar]
- 3.Speakman JR. The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond B, Biol Sci. 2008;363:375–98. 10.1098/rstb.2007.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debeffe L, Poissant J, McLoughlin PD. Individual quality and age but not environmental or social conditions modulate costs of reproduction in a capital breeder. Ecol Evol. 2017;7:5580–91. 10.1002/ece3.3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roff DA. Life history evolution. Sunderland, Mass: Sinauer Associates; 2002. [Google Scholar]
- 6.Proaktor G, Coulson T, Milner-Gulland E. The demographic consequences of the cost of reproduction in ungulates. Ecol. 2008;89:2604–11. 10.1890/07-0833.1 [DOI] [PubMed] [Google Scholar]
- 7.Nemeth M, Millesi E, Siutz C, Wagner K-H, Quint R, Wallner B. Reproductive performance and gestational effort in relation to dietary fatty acids in guinea pigs. J Anim Sci Biotechnol. 2017;8:28 10.1186/s40104-017-0158-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauffman AS, Bojkowska K, Rissman EF. Critical periods of susceptibility to short-term energy challenge during pregnancy: Impact on fertility and offspring development. Physiol Behav. 2010;99:100–8. 10.1016/j.physbeh.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rödel HG, Valencak TG, Handrek A, Monclús R. Paying the energetic costs of reproduction: reliance on postpartum foraging and stored reserves. Behav Ecol. 2016;27:748–56. 10.1093/beheco/arv217 [DOI] [Google Scholar]
- 10.Richard Q, Toïgo C, Appolinaire J, Loison A, Garel M. From gestation to weaning: Combining robust design and multi-event models unveils cost of lactation in a large herbivore. J Anim Ecol. 2017;86:1497–509. 10.1111/1365-2656.12736 [DOI] [PubMed] [Google Scholar]
- 11.Cichon M, Olejniczak P, Gustafsson L. The effect of body condition on the cost of reproduction in female collared flycatchers Ficedula albicollis. Ibis. 1998;140:128–30. [Google Scholar]
- 12.Stearns SC. The evolution of life histories. Oxford: Oxford Univ. Press; 2004. [Google Scholar]
- 13.Mysterud A, Røed KH, Holand Ø, Yoccoz NG, Nieminen M. Age-related gestation length adjustment in a large iteroparous mammal at northern latitude. J Anim Ecol. 2009;78:1002–6. 10.1111/j.1365-2656.2009.01553.x [DOI] [PubMed] [Google Scholar]
- 14.Landete-Castillejos T, García A, Gómez JA, Molina A, Gallego L. Subspecies and body size allometry affect milk production and composition, and calf growth in red deer: comparison of Cervus elaphus hispanicus and Cervus elaphus scoticus. Physiol Biochem Zool. 2003;76:594–602. 10.1086/375660 [DOI] [PubMed] [Google Scholar]
- 15.Michel ES, Demarais S, Strickland BK, Belant JL. Contrasting the effects of maternal and behavioral characteristics on fawn birth mass in white-tailed deer. PLoS One. 2015;10:e0136034 10.1371/journal.pone.0136034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birgersson B, Ekvall K. Early growth in male and female fallow deer fawns. Behav Ecol. 1997;8:493–9. 10.1093/beheco/8.5.493 [DOI] [Google Scholar]
- 17.Testa J, Adams G. Body condition and adjustments to reproductive effort in female moose (Alces alces). J Mammal. 1998;79:1345–54. [Google Scholar]
- 18.Monteith KL, Stephenson TR, Bleich VC, Conner MM, Pierce BM, Bowyer RT. Risk-sensitive allocation in seasonal dynamics of fat and protein reserves in a long-lived mammal. J Anim Ecol. 2013;82:377–88. 10.1111/1365-2656.12016 [DOI] [PubMed] [Google Scholar]
- 19.Simard MA, Huot J, Bellefeuille S de, Côté SD. Linking conception and weaning success with environmental variation and female body condition in a northern ungulate. J Mammal. 2014;95:311–27. 10.1644/13-MAMM-A-036 [DOI] [Google Scholar]
- 20.Gélin U, Wilson ME, Coulson G, Festa-Bianchet M. Experimental manipulation of female reproduction demonstrates its fitness costs in kangaroos. J Anim Ecol. 2015;84:239–48. 10.1111/1365-2656.12266 [DOI] [PubMed] [Google Scholar]
- 21.Festa-Bianchet M, Gaillard J-M, Jorgenson J. Mass-and density-dependent reproductive success and reproductive costs in a capital breeder. Am Nat. 1998;152:367–79. 10.1086/286175 [DOI] [PubMed] [Google Scholar]
- 22.Hamel S, Gaillard J-M, Yoccoz NG, Loison A, Bonenfant C, Descamps S. Fitness costs of reproduction depend on life speed: empirical evidence from mammalian populations. Ecol Lett. 2010;13:915–35. 10.1111/j.1461-0248.2010.01478.x [DOI] [PubMed] [Google Scholar]
- 23.Moyes K, Morgan B, Morris A, Morris S, Clutton-Brock T, Coulson T. Individual differences in reproductive costs examined using multi-state methods. J Anim Ecol. 2011;80:456–65. 10.1111/j.1365-2656.2010.01789.x [DOI] [PubMed] [Google Scholar]
- 24.Drent RH, Daan S. The prudent parent: energetic adjustments in avian breeding 1). Ardea. 2002;38–90:225–52. 10.5253/arde.v68.p225 [DOI] [Google Scholar]
- 25.Bronson FH. Mammalian reproductive biology. Chicago, USA: University of Chicago Press; 1989. [Google Scholar]
- 26.Prendergast BJ. Internalization of seasonal time. Horm Behav. 2005;48:503–11. 10.1016/j.yhbeh.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 27.Jánová E, Heroldová M, Bryja J. Conspicuous demographic and individual changes in a population of the common vole in a set-aside alfalfa field. Ann Zool Fennici. 2008;45:39–54. 10.5735/086.045.0104 [DOI] [Google Scholar]
- 28.Sale MG, Wilson BA, Arnould JPY. Comparison of life-history characteristics of island and mainland populations of the swamp antechinus. J Zool. 2009;277:119–25. 10.1111/j.1469-7998.2008.00514.x [DOI] [Google Scholar]
- 29.Qu J, Liu M, Yang M, Zhang Y, Ji W. Reproduction of plateau pika (Ochotona curzoniae) on the Qinghai–Tibetan plateau. Eur. J. Wildl. Res. 2012;58:269–77. 10.1002/ps.2302 [DOI] [PubMed] [Google Scholar]
- 30.Holand Ø, Mysterud A, Røed KH, Coulson T, Gjøstein H, Weladji RB, et al. Adaptive adjustment of offspring sex ratio and maternal reproductive effort in an iteroparous mammal. Proc Biol Sci. 2006;273:293–9. 10.1098/rspb.2005.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKay AE, Forsyth DM, Coulson G, Festa-Bianchet M. Maternal resource allocation adjusts to timing of parturition in an asynchronous breeder. Behav Ecol Sociobiol. 2018;72:295 10.1007/s00265-017-2419-9 [DOI] [Google Scholar]
- 32.vom Saal F, Finch C. Reproductive senescence: Phenomena and mechanisms in mammals and selected vertebrates. In: Knobil E, Neill J, editors. The physiology of reproduction. 2nd ed New York: Raven Press; 1988. [Google Scholar]
- 33.Finch C. Longevity, senescence and the genome. Chicago: University of Chicago Press; 1990. [Google Scholar]
- 34.Arking R. Biology of aging: observations and principles. 2nd ed Sunderland: Sinauer Associates Inc.; 1998. [Google Scholar]
- 35.Holliday R. Aging is no longer an unsolved problem in biology. Ann NY Acad Sci. 2006;1067:1–9. 10.1196/annals.1354.002 [DOI] [PubMed] [Google Scholar]
- 36.Alberts SC, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, et al. Reproductive aging patterns in primates reveal that humans are distinct. Proc Natl Acad Sci. 2013;110:13440–5. 10.1073/pnas.1311857110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derrickson EM. Comparative reproductive strategies of altricial and precocial eutherian mammals. Funct Ecol. 1992;6:57 10.2307/2389771 [DOI] [Google Scholar]
- 38.Künkele J, Trillmich F. Are precocial young cheaper? Lactation energetics in the guinea pig. Physiol Zool. 1997;70:589–96. 10.1086/515863 [DOI] [PubMed] [Google Scholar]
- 39.Campbell MT, Slade NA. The effect of maternal mass on litter size and offspring survival in the hispid cotton rat (Sigmodon hispidus). Can J Zool. 1995;73:133–40. 10.1139/z95-015 [DOI] [Google Scholar]
- 40.Myers P, Master LL. Reproduction by Peromyscus maniculatus: Size and Compromise. J Mammal. 1983;64:1–18. 10.2307/1380746 [DOI] [Google Scholar]
- 41.Knobil E, Neill J, editors. The physiology of reproduction. 2nd ed New York: Raven Press; 1988. [Google Scholar]
- 42.Rognmo A, Markussen KA, Jacobsen E, Grav HJ, Blix AS. Effects of improved nutrition in pregnant reindeer on milk quality, calf birth weight, growth, and mortality. Ran. 1983;3:10 10.7557/2.3.2.476 [DOI] [Google Scholar]
- 43.Schai-Braun S, Hackländer K. Family Leporidae. Hares and rabbits. In: Wilson D, Lacher T, Mittermeier R, JR., editors. Handbook of the mammals of the world: Volume 6 Lagomorphs and rodents I. Barcelona: Lynx Editions; 2016. p. 62–148. [Google Scholar]
- 44.Hackländer K, Schai-Braun SC. Lepus europaeus. In: Smith AT, Johnston CH, Alves PC, Hackländer K, editors. Lagomorphs—Pikas, Rabbits, and Hares of the World. Baltimore, Maryland, USA: Johns Hopkins University Press; 2018. [Google Scholar]
- 45.Hackländer K, Tataruch F, Ruf T. The effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus). Physiol Biochem Zool. 2002;75:19–28. 10.1086/324770 [DOI] [PubMed] [Google Scholar]
- 46.Lincoln G. Reproduction and “March madness” in the Brown hare, Lepus europaeus. J Zool. 1974;174:1–14. 10.1111/j.1469-7998.1974.tb03140.x [DOI] [PubMed] [Google Scholar]
- 47.Flux J. Reproduction and body weights of the hare Lepus europaeus Pallas, in New Zealand. New Zeal J Sci. 1976;10:357–401. [Google Scholar]
- 48.Frylestam B. Reproduction in the European hare in southern Sweden. Holarctic Ecol. 1980;3:74–80. 10.1111/j.1600-0587.1980.tb00710.x [DOI] [Google Scholar]
- 49.Flux J. Reproductive strategies in the genus Lepus. In: Myers K, MacInnes C, editors. Proceedings of the world lagomorph conference. Guleph, Ontario: University of Guelph; 1981. p. 155–174. [Google Scholar]
- 50.Hansen K. Reproduction in European hare in a Danish farmland. Acta Theriol. 1992;37:27–40. 10.4098/AT.arch.92-3 [DOI] [Google Scholar]
- 51.Mukasa-Mugerwa E, Said AN, Lahlou-Kassi A, Sherington J, Mutiga ER. Birth weight as a risk factor for perinatal lamb mortality, and the effects of stage of pregnant ewe supplementation and gestation weight gain in Ethiopian Menz sheep. Preventive Veterinary Medicine. 1994;19:45–56. 10.1016/0167-5877(94)90014-0 [DOI] [Google Scholar]
- 52.Nespolo RF, Bacigalupe LD. Viability selection on early body mass and the effect of female body size on fecundity: a study on the leaf-eared mouse Phyllotis darwini. Ecol Res. 2009;24:997–1002. 10.1007/s11284-008-0570-5 [DOI] [Google Scholar]
- 53.Ronget V, Gaillard J-M, Coulson T, Garratt M, Gueyffier F, Lega J-C, et al. Causes and consequences of variation in offspring body mass: meta-analyses in birds and mammals. Biol Rev. 2018;93:1–27. 10.1111/brv.12329 [DOI] [PubMed] [Google Scholar]
- 54.Blomquist GE, Williams LE. Quantitative genetics of costly neonatal sexual size dimorphism in squirrel monkeys (Saimiri boliviensis). J Evol Biol. 2013;26:756–65. 10.1111/jeb.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bensinger S, Kugelschafter K, Eskens U, Sobiraj A. Untersuchungen zur jährlichen Reproduktionsleistung von weiblichen Feldhasen (Lepus europaeus PALLAS, 1778) in Deutschland. Z Jagdwiss. 2000;46:73–83. 10.1007/BF02241570 [DOI] [Google Scholar]
- 56.Broekhuizen S, Maaskamp F. Behaviour of does and leverets of the European hare (Lepus europaeus) whilst nursing. J Zool. 1980;191:487–501. 10.1111/j.1469-7998.1980.tb01480.x [DOI] [Google Scholar]
- 57.Brüll U. Nahrungsbiologische Studien am Feldhasen in Schleswig-Holstein: ein Beitrag zur Äsungsverbesserung. In: Pielowski Z, Pucek Z, editors. Ecology and management of the European hare populations. Warszawa; 1976. p. 93–99. [Google Scholar]
- 58.Onderscheka K., Tataruch F. Ein Versuch zur Erstellung von Normalwerten wildlebender Tiere und die Anwendung dieser Daten in der Wildbiologie. Wien Tierärztl Monschr. 1982;69:274–9. [Google Scholar]
- 59.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 60.Bürkner P-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J Stat Softw 2017. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- 61.Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, et al. Stan: A Probabilistic Programming Language. J Stat Softw 2017. 10.18637/jss.v076.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox J, Weisberg S. An R Companion to Applied Regression. Thousand Oaks CA: Sage; 2019. [Google Scholar]
- 63.Vehtari A, Gelman A, Gabry J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. 2017;27:1413–32. 10.1007/s11222-016-9696-4 [DOI] [Google Scholar]
- 64.Vehtari A, Gabry J, Magnusson M, Yao Y, Gelman A. loo: Efficient leave-one-out cross-validation and WAIC for Bayesian models. R package version 2.2.0. 2019. https://mc-stan.org/loo. [Google Scholar]
- 65.Delignette-Muller ML, Dutang C. fitdistrplus: An R Package for Fitting Distributions. J Stat Softw 2015. 10.18637/jss.v064.i04 [DOI] [Google Scholar]
- 66.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. Boca Raton, London, New York: CRC Press Taylor and Francis Group; 2014. [Google Scholar]
- 67.Kruschke JK. Doing Bayesian data analysis: A tutorial with R, JAGS, and Stan. 2nd ed Amsterdam: AP Academic Press/Elsevier; 2015. [Google Scholar]
- 68.Rödel HG, Prager G, Stefanski V, Holst D von, Hudson R. Separating maternal and litter-size effects on early postnatal growth in two species of altricial small mammals. Physiol Behav. 2008;93:826–34. 10.1016/j.physbeh.2007.11.047 [DOI] [PubMed] [Google Scholar]
- 69.Eloranta E, Nieminen M. Calving of the experimental reindeer herd in Kaamanen during 1970–85. Ran. 1986;6:115 10.7557/2.6.2.635 [DOI] [Google Scholar]
- 70.Millar JS. Energetics of reproduction in Peromyscus leucopus: The cost of lactation. Ecol. 1978;59:1055–61. 10.2307/1938558 [DOI] [Google Scholar]
- 71.Kojola I. Early maternal investment and growth in reindeer. Can J Zool. 1993;71:753–8. 10.1139/z93-099 [DOI] [Google Scholar]
- 72.Dobson FS, Michener GR. Maternal traits and reproduction in Richardson’s ground squirrels. Ecol. 1995;76:851–62. 10.2307/1939350 [DOI] [Google Scholar]
- 73.Blaxter KL, Hamilton WJ. Reproduction in farmed red deer. 2. Calf growth and mortality. J. Agric. Sci. 1980;95:275–84. 10.1017/s0021859600039290 [DOI] [Google Scholar]
- 74.Moore GH, Littlejohn GM, Cowie GM. Factors affecting liveweight gain in red deer calves from birth to weaning. New Zeal J Agr Res. 1988;31:279–83. 10.1080/00288233.1988.10423416 [DOI] [Google Scholar]
- 75.Skibiel AL, Dobson FS, Murie JO. Maternal influences on reproduction in two populations of Columbian ground squirrels. Ecol Monogr. 2009;79:325–41. 10.1890/08-0718.1 [DOI] [Google Scholar]
- 76.Künkele J. Effects of litter size on the energetics of reproduction in a highly precocial rodent, the guinea pig. J Mammal. 2000;81:691–700. [DOI] [Google Scholar]
- 77.Kasparian K, Geissler E, Trillmich F. Optimal offspring size in a small mammal: an exception to the tradeoff invariant life-history rule. Oikos. 2005;111:271–8. 10.1111/j.0030-1299.2005.13973.x [DOI] [Google Scholar]
- 78.Dobson FS, Myers P. The seasonal decline in the litter size of meadow voles. J Mammal. 1989;70:142–52. 10.2307/1381677 [DOI] [Google Scholar]
- 79.Fokidis HB, Risch TS, Glenn TC. Reproductive and resource benefits to large female body size in a mammal with female-biased sexual size dimorphism. Anim Behav. 2007;73:479–88. 10.1016/j.anbehav.2006.08.010 [DOI] [Google Scholar]
- 80.Risch TS, Michener GR, Dobson FS. Variation in litter size: a test of hypotheses in Richardson’s ground squirrels. Ecol. 2007;88:306–14. 10.1890/06-0249 [DOI] [PubMed] [Google Scholar]
- 81.Rödel HG, Starkloff A, Seltmann MW, Prager G, Holst D von. Causes and predictors of nest mortality in a European rabbit population. Mamm Biol. 2009;74:198–209. 10.1016/j.mambio.2008.04.003 [DOI] [Google Scholar]
- 82.Raczynski J. Studies on the European hare: V. Reproduction. Acta Theriol. 1964;9:305–52. [Google Scholar]
- 83.Broekhuizen S, Maaskamp F. Annual production of young in European hares (Lepus europaeus) in the Netherlands. J Zool. 1981;193:499–516. 10.1111/j.1469-7998.1981.tb01500.x [DOI] [Google Scholar]
- 84.Caroli L, Luiselli L, Capizzi D. Reproductive strategies and life-history traits of the Savi’s pine vole, Microtus savii. Zool Sci. 2000;17:209–16. [Google Scholar]
- 85.Hackländer K, Zeitlhofer C, Ceulemans T, Suchentrunk F. Continentality affects body condition and size but not yearly reproductive output in female European hares (Lepus europaeus). Mamm Biol. 2011;76:662–4. 10.1016/j.mambio.2011.05.001 [DOI] [Google Scholar]
- 86.Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–11. 10.1038/33910 [DOI] [PubMed] [Google Scholar]
- 87.Bérubé C, Festa-Bianchet M, Jorgenson J. Individual differences, longevity, and reproductive senescence in bighorn ewes. Ecol. 1999;80:2555–65. [Google Scholar]
- 88.Ericsson G, Wallin K, Ball J, Broberg M. Age-related reproductive effort and senescence in free-ranging moose, Alces alces. Ecol. 2001;82:1613–20. [Google Scholar]
- 89.Sharp S, Clutton-Brock T. Reproductive senescence in a cooperatively breeding mammal. J Anim Ecol. 2010;79:176–83. 10.1111/j.1365-2656.2009.01616.x [DOI] [PubMed] [Google Scholar]
- 90.Karniski C, Krzyszczyk E, Mann J. Senescence impacts reproduction and maternal investment in bottlenose dolphins. Proc Biol Sci 2018. 10.1098/rspb.2018.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zedrosser A, Dahle B, Støen O-G, Swenson JE. The effects of primiparity on reproductive performance in the brown bear. Oecol. 2009;160:847–54. 10.1007/s00442-009-1343-8 [DOI] [PubMed] [Google Scholar]
- 92.Paul A, Kuester J. Infant handling by female Barbary macaques (Macaca sylvanus) at Affenberg Salem: testing functional and evolutionary hypotheses. Behav Ecol Sociobiol. 1996;39:133–45. 10.1007/s002650050275 [DOI] [Google Scholar]
- 93.Festa-Bianchet M, Jorgenson JT, Lucherini M, Wishart WD. Life history consequences of variation in age of primiparity in bighorn ewes. Ecol. 1995;76:871–81. 10.2307/1939352 [DOI] [Google Scholar]
- 94.Clutton-Brock TH. Reproductive success. In: Clutton-Brock TH, editor. Reproductive success: Studies of individual variation in contrasting breeding systems. Chicago, London: The University of Chicago Press; 1988. pp 472–486. [Google Scholar]
- 95.Valencak T, Ruf T. Energy turnover in European hares is centrally limited during early, but not during peak lactation. J Comp Physiol B. 2009;179:933–43. 10.1007/s00360-009-0376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Jaarsveld AS. A comparative investigation of hyaenid and aardwolf life-histories, with notes on spotted hyaena mortality patterns. Trans R Soc S Afr. 1993;48:219–32. 10.1080/00359199309520272 [DOI] [Google Scholar]
- 97.Singh MD, Singh S, Garcia GW. Understanding mammary activity in red-rumped agouti and implications for management and conservation of this Neotropical game species. Braz J Biol. 2018;78:540–7. 10.1590/1519-6984.172814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
(XLS)
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.