Abstract
Previous studies found significant modification in spatiotemporal parameters of backward walking in healthy older adults, but the age-related changes in the neuromuscular control have been considered to a lesser extent. The present study compared the intersegmental coordination, muscle activity and corresponding modifications of spinal montoneuronal output during both forward and backward walking in young and older adults. Ten older and ten young adults walked forward and backward on a treadmill at different speeds. Gait kinematics and EMG activity of 14 unilateral lower-limb muscles were recorded. As compared to young adults, the older ones used shorter steps, a more in-phase shank and foot motion, and the activity profiles of muscles innervated from the sacral segments were significantly wider in each walking condition. These findings highlight age-related changes in the neuromuscular control of both forward and backward walking. A striking feature of backward walking was the differential organization of the spinal output as compared to forward gait. In addition, the resulting spatiotemporal map patterns also characterized age-related changes of gait. Finally, modifications of the intersegmental coordination with aging were greater during backward walking. On the whole, the assessment of backward walk in addition to routine forward walk may help identifying or unmasking neuromuscular adjustments of gait to aging.
Introduction
Age-related changes in the gait features of forward locomotion have been studied extensively [1–4], and have been related to risk of falling [5] and/or to health status in older adults [6]. The modifications of walking between young and older adults occur in parallel with, amongst other factors, changes in the musculoskeletal system [7,8] as well as the central and peripheral nervous systems [9,10]. A number of studies have provided new insights about the plasticity of the neuromuscular control of gait to adapt to those age-related physiological changes (e.g., [11–13]).
In particular, a distal-to-proximal redistribution of joint efforts has been established as a gait feature of older adults [14]. Part of this so-called biomechanical plasticity has been related to a reduction of muscle strength [12,15–17]. However, other studies have revealed that older adults may retain the muscular potential to develop higher ankle power, and in turn reduce the distal-to-proximal redistribution [18] under specific situations (e.g., during walking uphill [19] or using biofeedback [20]). The decline of propulsive power generation during push-off is thus not only due to a reduced muscular capacity but might also emerge from a different neuromuscular control strategy [19]. Therefore, recent efforts have been made to understand the age-related plasticity of the neuromuscular system during forward walking [1,13,21–23]. For example, using the planar covariation law [24,25], significant adjustments of the intersegmental coordination related to aging, and especially of the shank-foot coordination, have been documented. In addition, aging also involves modifications of muscle activations [26–29], suggesting a loss of fine neural control [29,30]. These changes are reflected at the level of the alpha-motoneuronal (MN) activity [28], however the modifications of spinal locomotor output have never been quantified.
Although continuous backward walking occurs rarely, it is still critical for independence in daily life, e.g., when stepping back in front of a forthcoming vehicle, when opening a door, when backing up to sit down [31]. Backward walking has been extensively studied in the context of theories on the organization of central pattern generators (CPGs). As first hypothesized by Grillner [32], it has been suggested that backward walking is basically forward in reverse [33–36]. While the kinematics seems to support this idea [36], suggesting sharing circuitry [35,37], such reversal is not present for muscle activity, especially for ankle muscles [33,36]. Ivanenko et al. [38] have also noted important differences in the spinal cord MN activity between backward and forward walking, suggesting a partial reconfiguration of lower level networks [39]. In addition, backward walking requires the involvement of specialized control circuits [40] mainly at supraspinal levels [41], suggesting that it is more challenging to the nervous system than standard forward walking.
For this reason, there has been a growing interest in the use of backward walking for rehabilitation purposes. Recent studies suggest that backward walking can be used for rehabilitation or for diagnostics in patients with neurological injuries [42–47]. Since older adults rely more on visual feedback during both standing and walking than young adults [48–51], it has been hypothesized that backward gait may be used to unmask mobility impairments and assess risk of falling. Compared to young adults, backward walking in older adults is characterized by higher stride frequency, slower speed, and increased gait variability [2,31,52]. However, perhaps due to subtle neuromuscular adjustments associated with normal aging [22,28,53], it is still unclear how the neuromuscular control adapts to backward walking with aging.
To the best of our knowledge, the present study is the first to provide quantitative comparisons of the pattern generator output during forward and backward walking between young and older adults. We intended to better pinpoint underlying mechanisms of age-related neuromuscular adaptations in both backward and forward walking. In particular, because backward walking is more challenging than forward walking and because patterns of neuromuscular control are direction specific in humans [54], we wondered whether backward walking can reveal age-related modifications of gait that are not otherwise apparent during forward walking.
Altered spatiotemporal stride parameters [2,31], altered coordination patterns among the elevation angles of the lower limb segments [1,13], and wider bursts of muscle activity [28,29] have been previously documented for the forward locomotion of older adults. Here, we expected that some of these alterations might apply also to backward walking. In particular, we expected age-related adjustments of the intersegmental coordination, namely a more in-phase shank and foot motion, as well as a widening of muscle activities. Importantly, we also expected that some of these age-related modifications might be reflected in the pattern of rostrocaudal activation of the motoneuron pools. Finally, we hypothesized that these age-related differences of neuromuscular control would be more pronounced during backward walking compared with forward walking.
Methods
Subject and experimental procedure
Ten young (4 ♀; age: 28.7±5.1 yrs, mass: 74.5±10.7 kg, height: 1.75±0.07 m, means±SD) and 10 older adults (1 ♀; age: 73.5±4.5 yrs, mass: 81.5±5.9 kg, height: 1.76±0.05 m, mean±SD) participated to the study. Mass and height were not significantly different between young and older adults (mass: t = 0.5; p = 0.605; height: t = 1.8; p = 0.086). The number of subjects was determined by a priori power analysis using the G*Power program. Based on the age-related difference of stride length during forward walking on a treadmill [1], a total sample of 18 participants (9 per group) would be sufficient to detect a large effect size (ƞp2 = 0.20) with 90% power, using one-way ANOVA with p = 0.05. No subject had a recent history of falling. All participants were able to walk without assistance and did not complain about musculoskeletal disorders. All participants gave written informed consent. Experiments were performed according to the Declaration of Helsinki and were approved by the ethics committee of IRCCS Santa Lucia Foundation (CE/PROG749).
Participants were asked to walk forward and backward while they wore their own walking shoes on a treadmill at two different fixed speeds [2 (0.56) and 3 (0.83) km h-1(m s-1) backward, 2 (0.56) and 4 (1.11) km h-1 (m s-1) forward]. Two young adults did not perform the walking tasks at 2 km h-1 and one older adult was unable to walk backward at 3 km h-1. During backward walking, subjects were allowed to hold a hand rail with their left hand for balance. For each trial, at least 8 consecutive strides were analysed (Table 1). Bilateral, full-body three-dimensional (3D) kinematics was recorded at 200 Hz by means of a Vicon-612 system (Oxford, UK) with nine cameras placed around the treadmill. Twelve reflective markers were attached to the skin of the subjects overlying the following bilateral landmarks: gleno-humeral joint, lateral epicondyle of the elbow, ulnar process of the wrist, greater trochanter, lateral femur epicondyle and lateral malleolus. In addition, four markers were placed on each shoe in approximate correspondence with the heel, and fifth metatarso-phalangeal joint. The EMG data were recorded at 2000 Hz by means of a Delsys Trigno Wireless System (Boston, MA). The following 14 muscles were recorded on the right side of the body: erector spinae (ES) at L2 level, gluteus maximus (Gmax), gluteus medius (Gmed), tensor fasciae latae (TFL), vastus medialis (VM), vastus lateralis (VL), rectus femoris (RF), long head of the biceps femoris, (BF), semitendinosus (ST), tibialis anterior (TA), medial gastrocnemius (MG), lateral gastrocnemius (LG), soleus (SOL) and peroneus longus (PERL). EMG electrodes were placed based on suggestions from SENIAM (seniam.org), the European project on surface EMG. To ensure correct placement of EMG electrodes, muscle bellies were located by means of palpation and the electrodes were oriented along the main direction of the fibers [55]. In certain conditions, some electrodes became partially detached and the data series produced by these electrodes were removed from the analysis (replaced by a not-a-number vector) on a subject-specific basis (S1 Table). Table 1 presents the number of muscles and strides analysed for each subject in each walking condition. Kinematic and EMG recordings were synchronized on-line. All analyses were performed using custom Matlab sofware (MathWorks Inc., MA, USA).
Table 1. Number of muscles (and number of strides) analysed per subject in each walking condition (e.g. FW 2 is forward walk at 2 km h-1).
| Young | Older | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | FW 2 | FW 4 | BW 2 | BW 3 | Subject | FW 2 | FW 4 | BW 2 | BW 3 |
| Y1 | 14 (12) | 14 (16) | 12 (17) | 13 (16) | E1 | 12 (11) | 11 (10) | 11 (10) | 11 (8) |
| Y2 | 13 (12) | 14 (12) | 14 (13) | 14 (17) | E2 | 14 (11) | 14 (15) | 14 (10) | 14 (8) |
| Y3 | 14 (13) | 14 (12) | 13 (12) | 13 (13) | E3 | 13 (12) | 13 (11) | 11 (12) | 13 (9) |
| Y4 | 8 (10) | 10 (11) | 8 (11) | 8 (11) | E4 | 13 (12) | 13 (11) | 13 (11) | 13 (8) |
| Y5 | 13 (11) | 13 (12) | 13 (11) | 13 (11) | E5 | 14 (13) | 13 (17) | 14 (15) | 14 (15) |
| Y6 | 12 (10) | 12 (11) | 11 (11) | 13 (10) | E6 | 7 (11) | 8 (10) | 12 (11) | 10 (10) |
| Y7 | 14 (11) | 11 (12) | 12 (10) | 13 (12) | E7 | 9 (14) | 9 (12) | 12 (11) | 11 (10) |
| Y8 | 13 (13) | 13 (16) | 13 (13) | 13 (11) | E8 | 14 (13) | 13 (11) | 13 (11) | 10 (10) |
| Y9 | – | 13 (10) | – | 14 (8) | E9 | 13 (12) | 12 (13) | 13 (15) | 13 (19) |
| Y10 | – | 14 (9) | – | 14 (8) | E10 | 13 (10) | 10 (10) | 12 (11) | – |
Kinematic data analysis
The stride was defined as the period between two ground contacts of the right foot. Foot-contact was estimated according to the local minima of the vertical displacement of the heel marker [56], while the timing of the lift-off was estimated from the maximum excursion of the lower limb elevation angle, defined as the angle between the vertical axis and the whole limb segment (from the greater trochanter to the lateral malleolus), projected on the sagittal plane [24].
From the marker locations, the orientation of the thigh, shank, foot and trunk relative to the vertical axis (elevation angle) were computed as described in Borghese et al. [24]. For each participant, the duration of different strides of each trial was normalized by interpolating individual gait cycles over 200 points. To analyse the relative phase of the time-course of the elevation angles during a stride, the phase lags between two adjacent limb-segments were computed by means of cross-correlation function.
As reported in prior studies, a principal component analysis was applied to determine the covariance matrix of the segment elevation angles [57], after subtraction of the mean value. Notice that, for this analysis, the amplitude of these angles was not normalized. Eigenvalues and eigenvectors ui were computed by factoring the covariance matrix from the set of original signals by means of a singular value decomposition algorithm. The first two eigenvectors (u1 and u2) lied on the best-fitting plane of angular covariation, and the data projected onto the corresponding axes corresponded to the first (PC1) and second (PC2) principal components. The planarity was evaluated for each condition by calculating the percentage of variance that was explained by u1 (PV1), u2 (PV2) and u3 (PV3). If the data lay perfectly on a plane, PV1 + PV2 would be 100% (and PV3 would be 0%). By definition, the third eigenvector u3 is orthogonal to the plane defined by u1 and u2. The parameter u3t corresponds to the direction cosine with the positive semi-axis of the thigh and provides one measure of the orientation of the plane.
EMG data analysis
The collected raw EMG signals were high-pass filtered (30 Hz), then rectified and low-pass filtered with a zero-lag third-order Butterworth filter (10 Hz). As for the kinematic data, the time scale was normalized by interpolating individual gait cycles over 200 points. For each condition and for each EMG (rectified, filtered) waveform, the full width at half maximum (FWHM) was calculated as the period during which the EMG activity exceeded the half of its maximum [29,58,59].
The EMG activities were normalized to unit variance across all trials [60] and then mapped onto the estimated rostro-caudal location of the MN pools in the human spinal cord from the L2 to S2 segments based on Kendall’s myotomal charts [55], as in Ivanenko et al [61,62]. To account for size differences in MN pools at each spinal level, this fractional activity value was then multiplied by the estimated segment-specific number of MNs (MNj), based on Tomlinson and Irving [63]. Note that, consistent with previous work [64], the spinal maps were relatively insensitive to the subset of muscles analysed (Table 1). Indeed, spinal maps reconstructed from a subset of seven muscles (minimum number of muscles recorded) were strongly correlated with the maps computed from the full set of muscles, with average correlation coefficients between 0.9–0.99 for each task and at each individual spinal segment (S2 Table).
To compute the relative activation of the lumbar and sacral segments in each condition, we averaged the motor output patterns over the gait cycle in the upper part of the lumbar segments (sum of the activity from L2 to L4) and the sacral segments (sum of activity from S1 to S2). To reduce overlaps due to maps smoothing, the spinal segment L5 was not taken into account [65,66]. The FWHM, the maximal activation, and its timing were calculated for both lumbar and sacral segments.
Statistics
The statistical analysis was designed to assess the effect of progression speed, direction (backward vs. forward), age group (young vs. older), and the interaction between these factors. A general linear mixed model was applied, with the direction and speed defined as repeated measures. The normality of the residuals was checked by means of the Kolmogorov-Smirnov test. Normality was not assumed for 5 variables (range of motion of the trunk elevation angle, PV3, range of motion of the thigh elevation angle and FWHM of Gmax and TFL). In these cases, an inverse (for the parameter trunk and thigh ROM) or log (for the other parameters) transform was applied, and the normality of the residuals was then assumed. In each Figure, the asterisks indicate significant student t-tests with Benjamini-Hochberg p-level adjustment [67] comparing the age groups. The effect size, measure by the eta square (ƞp2), is reported for age group comparisons.
Results
Gait and kinematic parameters
The stride period (and stride length) decreased with speed (F2,67 = 32.3; p< 0.001; Fig 1C) and was significantly affected by both the age and the direction of progression: the stride period decreased when walking backward as compared to walking forward (F1,67 = 18.7; p< 0.001), and in older as compared to young adults (F1,67 = 71.1; p< 0.001; ƞp2 = 0.49). The relative duration of the stance phase was shorter with increasing speeds (F2,67 = 17.6; p< 0.001) and in older adults (F1,67 = 32.3; p< 0.001; ƞp2 = 0.34), but was not significantly affected by the direction of progression (F1,67 = 2.1; p = 0.155).
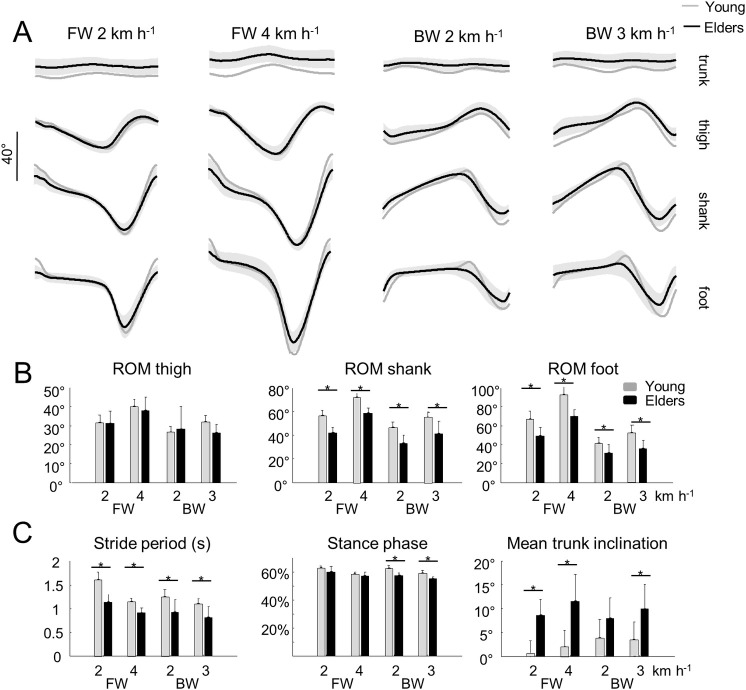
Fig 1. Elevation angles of lower-limb segments and general gait parameters during forward (FW) and backward (BW) walking in young and older adults.
A—Elevation angles of the trunk, thigh, shank and foot over a stride. All the curves of each subject walking at a given walking condition were first averaged (mean-curve). The curves presented here are the average of the mean-curves of all the young (grey lines) and older (black lines) adults. The grey zone represents±1 SD for the older adults. B—Average range of motion of the thigh, shank and foot over one stride. C—Average, stride period, relative stance phase and mean trunk inclination over one stride. In panel B and C, the bars represent the grand mean of all the young (grey) and the older (black) adults. Thin lines represent one standard deviation. The * indicates a significant effect of age.
When speed increased in both backward and forward walking, the range of motion (ROM) of the thigh, shank and foot elevation angles increased (thigh: F2,67 = 8.2; p = 0.001; shank: F2,67 = 63.9; p< 0.001; foot: F2,67 = 37.7; p< 0.001) in both groups (Fig 1A and 1B), whereas the ROM of the trunk elevation angle and its mean inclination were not significantly affected (ROM: F2,67 = 0.8; p = 0.476; mean: F2,67 = 1.6; p = 0.203). During backward walking, the thigh, shank and foot ROM significantly decreased (thigh: F2,67 = 4.8; p = 0.032; shank: F1,67 = 26.6; p< 0.001; foot: F1,67 = 57.9; p< 0.001), the trunk ROM increased (F1,67 = 8.9; p = 0.006), whereas the trunk mean inclination was not significantly different relative to forward walking (trunk: F1,67 = 1.2; p = 0.289).
In both age groups, the time-varying waveform of the elevation angles remained fairly similar across walking conditions (Fig 1A). However, the ROM of the shank and the foot segments were significantly smaller (shank: F1,67 = 87.0; p< 0.001; ƞp2 = 0.58; foot: F1,67 = 75.1; p< 0.001; ƞp2 = 0.53), and the mean trunk inclination was significantly greater (F1,67 = 51.7; p< 0.001; ƞp2 = 0.45) in older than in young adults. The trunk and thigh ROM were not significantly different between young and older adults (trunk: F1,67 = 1.2; p = 0.288; thigh: F1,67 = 0.617; p< 0.543).
Intersegmental coordination
The coordination between thigh, shank and foot elevation angles was evaluated using principal component analysis (Fig 2). Fig 2A illustrates the averaged gait loops plotted in 3D during backward and forward walking in both age groups. Notice the appreciably smaller loops in backward walking and in older adults (along with smaller ROM).
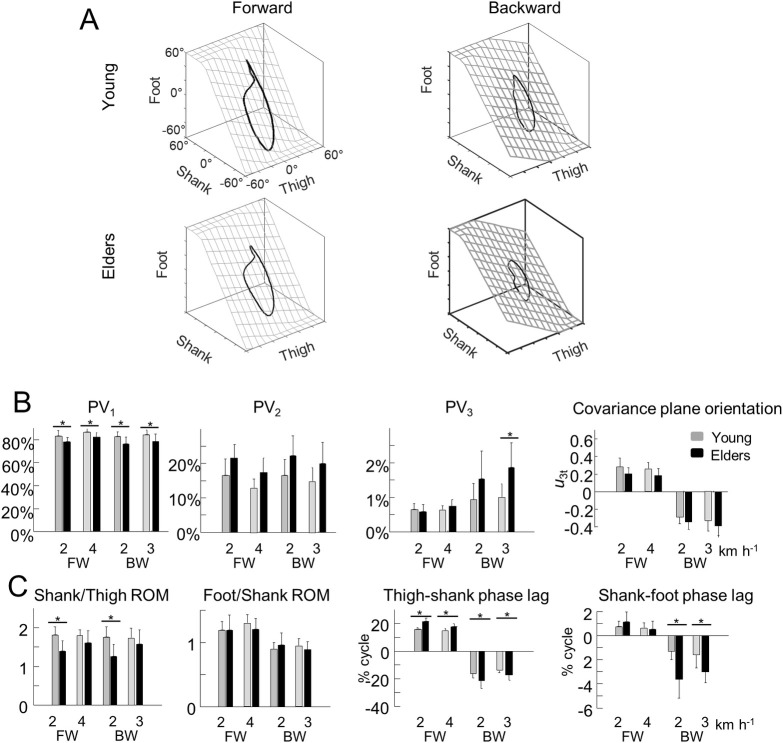
Fig 2. Planar covariation of elevation angles.
A–Covariation of the ensemble-average limb-segment elevation angles during forward (right—1.11 m s−1) and backward (left– 3 km h-1) walking in young (up) and older adults (bottom). Note that when the elevation angles of thigh, shank and foot are plotted one versus the other in a x-y-z space, they co-vary along a loop constrained on a plane (x–y). Grids show the best-fitting plane. B–Average percentage of variance accounted for by the first (left—PV1), second (middle—PV2) and third (right–PV3) eigenvector of the principal component analysis and the direction cosines of the normal to the covariation plane with the positive semi-axis of the thigh angular coordinates (u3t). C–Average amplitude ratios between the range of motion of adjacent segments and phase lags between time curves of elevation angles of adjacent segments. In panel A and B, grey bars correspond to young adults, whereas black bars correspond to older adults. The * indicates a significant effect of age.
In each condition, PV1 + PV2 > 97% (Fig 2B). However, PV1 was significantly smaller (and PV2 greater) in older than in young adults (PV1: F1,67 = 23.7; p< 0.001; ƞp2 = 0.26; PV2: F1,67 = 20.9; p< 0.001; ƞp2 = 0.24). Furthermore, PV1 slightly but significantly increased (and PV2 decreased) with speed (PV1: F2,67 = 4.7; p = 0.012; PV2: F2,67 = 4.9; p = 0.010), but was not significantly affected by the walking direction (PV1: F1,67 = 0.4; p = 0.534; PV2: F1,67 = 0.6; p = 0.802). The percentage of variance accounted for by the third PC (PV3), which represents the deviation from planarity, did not change significantly with speed (F2,67 = 1.4; p = 0.259), but increased significantly during backward walking (F1,67 = 13.2; p = 0.002) and in older adults (F1,67 = 13.2; p = 0.001; ƞp2 = 0.16). In addition, the effect of walking direction was significantly greater in older adults (interaction: F1,67 = 4.4; p = 0.039).
Obviously, during the stance phase of backward walking the foot relative to the hip moves from back to front, whereas in forward walking the foot moves from front to back. Accordingly, the orientation of the loop formed by the thigh, shank and foot elevation angles is reversed during backward walking as compared to forward walking [36], resulting in an opposite sign of the direction cosine u3t (Fig 2B; F1,67 = 397.3; p< 0.001). In both conditions, u3t was significantly smaller in older than in young adults (F1,67 = 8.2; p = 0.006; ƞp2 = 0.12) but was not significantly affected by the speed of progression (F2,67 = 1.2; p = 0.295).
Both the shape of the loop and the orientation of the plane depend on the amplitude ratio and the time relationship characteristics of adjacent elevation angles (Fig 2C). The amplitude ratio between thigh and shank segments was significantly smaller in older adults (F1,67 = 21.1; p< 0.001; ƞp2 = 0.25), but was not significantly affected by speed (F2,67 = 2.1; p = 0.126) or walking direction (F1,67 = 1.0; p = 0.316). The amplitude ratio between shank and foot segments was significantly smaller in backward walking (F1,67 = 17.7; p< 0.001), but was not significantly affected by speed (F2,67 = 0.7; p = 0.507) or age groups (F1,67 = 0.9; p = 0.330).
At each speed, the phase lags between adjacent segments were greatly affected by walking direction (thigh-shank: F1,67 = 914.8; p< 0.001; shank-foot: F1,67 = 101.6; p< 0.001): in forward walking, the phase lags were positive, showing that the oscillation of the proximal segment lead the distal ones, whereas in backward walking the phases lags were negative. In addition, the phase lags were significantly greater in older adults (thigh-shank: F1,67 = 33.7; p< 0.001; ƞp2 = 0.31; shank-foot: F1,67 = 22.7; p< 0.001; ƞp2 = 0.24), and the effect of age was significantly greater during backward walking (thigh-shank: F1,67 = 19.5; p< 0.001; shank-foot: F1,67 = 16.8; p< 0.001).
EMG activities and spinal motor output
Fig 3A illustrates the ensemble averages of rectified EMG envelopes at all walking conditions in young and older adults. EMGs for forward walking were qualitatively consistent with those reported in the literature [28,68]. The activity patterns of some muscles for backward walking were strikingly different from those of forward walking. For example, BF and ST were mostly active during early stance and the end of swing in forward walk, whereas they were mostly active during early stance in backward walk. The ankle extensors GM, GL, SOL and PERL were mostly active during mid-stance in forward walking, whereas they were active during late swing and early stance in backward walking.
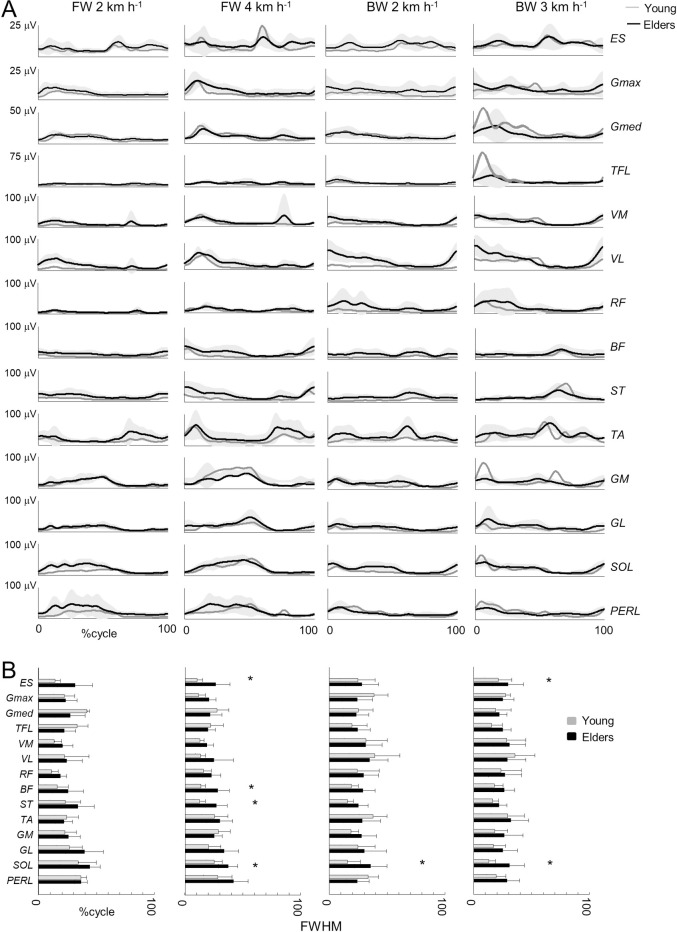
Fig 3. Ensemble-averaged electromyogram (EMG) patterns during forward and backward walking in young and older adults.
A–ensemble-averaged EMG patterns over one stride. ES, erector spinae; GM, gluteus maximus; Gmed, gluteus medius; SART, sartorius; TFL, tensor fascia latae; ADD, adductor longus; VM, vastus medialis; VL, vastus lateralis; RF, rectus femoris; BF, biceps femoris; ST, semitendinous; TA, tibialis anterior; MG, gastrocnemius medialis; LG, lateral gastrocnemius; SOL, soleus; PERL, peroneus longus. The curves presented here are the average of the mean-curves of all the young (grey lines) and older (black lines) adults. The grey zone represents±1 SD for the older adults. B–Full Width Half Maximum (FWHM) of the 14 lower-limb muscles at each walking condition. The bars represent the grand mean of all the young (grey) and the older (black) adults. Thin lines represent one standard deviation. The * indicates a significant effect of age.
In older adults, the EMG data remained roughly similar to young adults. However, some muscles were characterized by a different duration of activation, which we estimated as the FWHM (Fig 3B). In particular, the trunk extensor muscles (ES: F1,59 = 12.4; p = 0.001; ƞp2 = 0.17), the hamstrings (BF: F1,61 = 10.0; p = 0.002; ƞp2 = 0.14; ST: F1,57 = 10.8; p = 0.002; ƞp2 = 0.16), and the ankle extensors (GM: F1,66 = 7.0; p = 0.010; ƞp2 = 0.09; GL: F1,59 = 9.4; p = 0.003; ƞp2 = 0.14; SOL: F1,61 = 39.9; p< 0.001; ƞp2 = 0.39; PERL: F1,56 = 9.7; p = 0.003; ƞp2 = 0.15) presented significantly longer burst durations in older than in young adults.
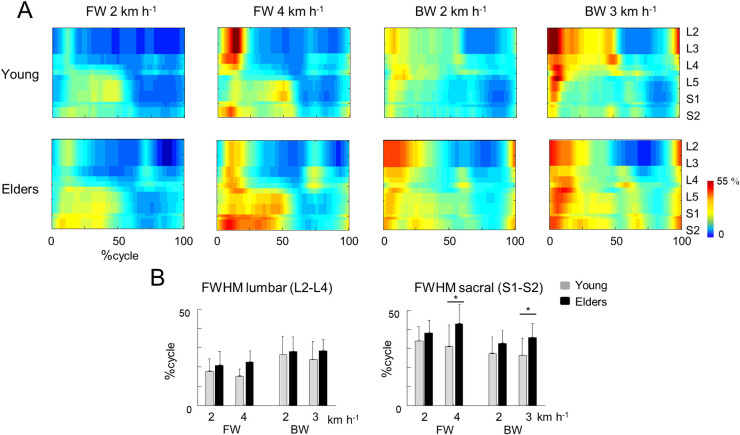
Fig 4A presents the EMG of Fig 3A normalized to unit variance across all trials [60], mapped onto the estimated rostro-caudal location of the MN pools in the spinal cord (see Methods). The lumbar segments showed one major spot of activity around touchdown, involving primarily hip and knee extensors, whereas the sacral segments showed one major spot of activity around lift-off, mainly corresponding to the ankle extension at the end of stance [65,69,70]. The burst timing and duration of the spinal segments differed with age and direction of progression. As compared to young adults, the FWHM of the sacral MN activation was significantly greater in older adults (F1,65 = 19.9; p< 0.001; ƞp2 = 0.23), whereas FWHM of the lumbar MN activation was not significantly different (F1,65 = 2.9; p = 0.091; Fig 4). In addition, the occurrence of the maximal activation of the lumbar segment occurred significantly earlier in older than in young adults (F1,65 = 11.1; p = 0.001; ƞp2 = 0.14). Instead, no significant difference with age was observed at the sacral level (F1,65 = 1.1; p = 0.307), except for an earlier activation in backward than forward walking (F1,65 = 40.3; p< 0.001). In addition, the effect of walking direction was significantly greater in older adults (interaction: F1,65 = 7.4; p = 0.008).
Fig 4.
Spatiotemporal spinal motor outputs computed from normalized EMGs (A) and average full width half maximum and mean activation of the lumbar (top) and sacral (bottom) segments (B) during forward and backward walking. For each individual, EMG signals from each muscle were normalized to unit variance across all trials [60]. The bars represent the grand mean of all the young (grey) and the older (black) adults. Thin lines represent one standard deviation. The * indicates a significant effect of age.
Another age-related difference was represented by the intensities of the sacral and lumbar segments, when evaluated from non-normalized EMGs (Fig 3A and S1 Fig). The sacral mean intensity increased significantly with speed (F2,67 = 5.1; p = 0.009), whereas the lumbar mean intensity did not change significantly with speed (F2,67 = 2.15; p = 0.124). Reversing the walking direction augmented the engagement of lumbar segments (F1,65 = 5.3; p = 0.025), without affecting the sacral ones (F1,65 = 0.5; p = 0.470). Older adults presented significantly greater intensities at both lumbar and sacral levels (lumbar: F1,65 = 18.3; p< 0.001; ƞp2 = 0.21; sacral: F1,65 = 8.3; p = 0.005; ƞp2 = 0.11). Notice that the effect of age on the burst duration did not depend on normalization (S1 Fig): the FWHM of the sacral MN activation was greater in older adults (F1,65 = 19.4; p< 0.001; ƞp2 = 0.23), whereas FWHM of the lumbar MN activation was not different (F1,65 = 1.8; p = 0.180).
Discussion
In this study, we investigated the effect of aging on the neuromuscular control of both forward and backward walking. Changing direction of locomotion is performed rather readily by both young and older adults. The effect of age on forward gait pattern has been often associated with a reduction of ankle propulsion force, for instance [19]. Despite the inverted plantigrade–digitigrade sequence during backward walking, we found similar age-related modifications of kinematic coordination and muscle activities in both backward and forward walking, suggesting specific adjustments of the motor control. In addition, we found that the age-related modifications on the intersegmental coordination were greater during backward walking.
With aging, motor weakness is due in part to neuromuscular degeneration, but also to degenerative changes in the central nervous system. Thus, reduction in grey matter volume [71], number of motor cortical [72] and spinal motor neurons [73], synaptic density [74], white matter integrity [75], and descending commands for motor activation [76] are some of the factors that may contribute to age-related motor impairment.
To date, several studies have dealt with the effects of aging on forward walking. Our results are aligned with prior results showing that older subjects take shorter steps [77] (Fig 1) and adapt their intersegmental coordination mainly by changing the amplitude and phase of shank and foot motion [1,13,21,22] (Fig 2A). Most authors of previous work discuss a reduction in mechanical power generated by the plantarflexor muscles as the hallmark biomechanical features of older gait. More recently, it has also been shown that older adults display longer bursts of muscle activation [29] (Fig 3) that could be related to a more robust neuromuscular control (i.e. more able to cope with errors) to deal with poorer balance control [78,79]. Again, the reduced dynamic stability in older adults has been associated with a diminished ankle push-off [18].
Here, we found that the modifications of the intersegmental coordination during backward walking are similar to those during forward walking. In particular, the changes in the orientation of the covariation plane with age (Fig 2B) are mainly related to a change of the phase shift between shank and foot elevation angles (Fig 2C; [1]). The more in-phase oscillation of the shank and the foot in older adults may explain the reduction of ankle ROM with aging (Fig 1B), which is not only due to shorter steps (Fig 1C) since the ratio between proximal and distal segments also changed (Fig 2C). This reduced angular excursion at the ankle in older adults has already been ascribed to co-contractions of distal antagonist muscles, in part due to EMG widening [58]. Accordingly, the activity profiles of the muscles innervated by the sacral segments were significantly wider in older adults (Fig 4A). The reconstructed spinal maps of MN activity further illustrate this finding. Similar results during forward walking at matched cadence were previously documented by Monaco et al. [28], suggesting that the widening of EMG is not dependent on spatiotemporal gait parameters. In addition, this result does not simply reflect the documented distal-to-proximal modification of kinematics or kinetics, since the human spinal topography does not reflect the muscle topography on the lower limbs. Indeed, both distal (GM, GM, SOL, PERL) and proximal (BF, ST) muscles mainly innervated by distal segments of the spinal cord [55,80] displayed wider activations (Fig 3B).
The present results of a caudal-cranial gradient of involvement of the spinal locomotor segments in older adults remain to be explained. It is well established that there exists a cranio-caudal gradient of corticospinal development in infancy [81], but less is known about differential degeneration of different portions of the corticospinal tract with aging. In general, it appears that projection tracts, such as the corticospinal tract, which develop earlier than association tracts in infancy, degenerate later than association tracts in older subjects [82].
Normal aging and the development of neurodegeneration are two processes that are closely linked [83,84]. Indeed with aging, neurodegeneration might occur when cells fail to adapt to the increases in oxidative, metabolic and ionic stress [85]. In addition, the disks between the vertebrae become hard and brittle when aging. As a result, more pressure is put on the spinal cord and on the spinal nerve roots, especially in distal segments. These observations may partly underlie the distal-to-proximal age-related changes in the neuromuscular system with aging. Accordingly, when the spinal excitability is estimated using the Hoffmann reflex technique, no difference is found between young and older adults on VM muscle [86], whereas age-related modulations of the reflex response have been reported in SOL muscle [87]. Further, during standing, when a perturbation is delivered, older adults exhibit intermittent reversals of the classical distal-proximal postural synergy used in young adults [88]. Similarly, proximal muscles tend to be activated first in the paretic limb of hemiplegic subject [89]. Interestingly, Martino et al. [90] found that the spinal maps of patients affected by hereditary spastic paraplegia were characterized by a spread of the loci of activation at the sacral segments and, at more severe stages, the lumbar segments, somewhat reminiscent of what happens in older adults. It is theoretically possible that the age-related changes in the neuromuscular control of gait are, at least in part, related to the progression of the aging degenerative process within the corticospinal tract, involving initially the sacral segments and later the lumbar segments. However, this possibility must be corroborated by studies specifically investigating changes in corticospinal innervation of different spinal segments.
The fact that the age-related modifications of neuromuscular control of gait observed during forward walking are also observed during backward walking indirectly supports the idea that walking impairment is not solely dependent on the reduction of force generated by the plantar-flexor muscles. Indeed, during backward walking, plantar flexion plays only a small role in propulsion [91]. The similarity of age-related modifications between the two walking directions indirectly supports the idea that somewhat similar spinal automatisms are used for forward and backward walking, as proposed by Grasso et al [36], Earhart et al. [92] and Ivanenko et al. [38], with a partial reconfiguration of lower-level networks [39] plus the probable intervention of supraspinal elements specifically for backward walking [40,41].
On the other hand, it has been shown that the plasticity associated with locomotor adaptation in human is direction specific, suggesting separate functional networks controlling forward and backward walking [54]. Moreover, backward walking is more challenging for the nervous system [40,41], and this style of walking is much less practiced than forward walking. Accordingly, we expected that the age-related differences of the neuromuscular control of gait would be greatly evidenced during backward walking. Indeed, several findings support the idea that backward walking may unmask mobility impairments in adult stroke patients [43], Parkinson disease [93], and in children with cerebral palsy [42]. By comparing older to young adults, greater adjustments of spatiotemporal gait parameters have been observed during backward than forward walking [2,31,52]. However, in these studies, subjects walked at self-selected speed and their velocity was significantly lower for older adults. In addition, the reduction of velocity was greater in backward than forward walking, and it was therefore difficult to differentiate the effect of age from the effect of speed.
By comparing young and older adults at matched speeds, we showed an interaction between age group and the direction of progression on the relative duration of the stance phase (Fig 1C), on PV3 (Fig 2B), on the phase lags between adjacent segment (Fig 2C), and on the timing of maximal sacral MN activity. By separating the effects of concomitant issues, such as age and speed, these changes of gait parameters, kinematics and muscle activity suggest that older persons have greater deficits in backward performance than during forward walking.
Our sample size was relatively small and the older subjects tended to be active and had no recent history of falls. Future studies involving a more heterogeneous population of individuals should be designed to focus on specific gait abnormalities in challenging conditions as a function of physical functioning. It is also important to note that the familiarization time for the backward walking task was limited. Whether similar differences between age groups would be still apparent after longer familiarization to backward walking is also an open question. Nevertheless, the findings of this study extend the available information on age-related differences in the neuromuscular control of gait occurring both during backward and forward walking. In addition, the results suggest that assessing backward walking in clinical practice may shed light on or even unmask neuromuscular adjustments of gait in older adults.
Supporting information
A—Ensemble-averaged normalized electromyogram (EMG) patterns. For each individual, EMG signals from each muscle were normalized to unit variance across all trials. B–Motor output (reported in μV) is plotted as a function of gait cycle in young (top) and older (bottom) adults. C–Average full width half maximum and mean activation of the lumbar (top) and sacral (bottom) segments. The bars represent the grand mean of all the young (grey) and the older (black) adults. Thin lines represent one standard deviation. The * indicates a significant effect of age.
(TIF)
List of muscles analysed (1) or removed (0) for each condition (from left to right: Forward 2 km h-1; Forward 4 km h-1; Backward 2 km h-1; Backward 3 km h-1) in young (Y) and older (O) adults.
(DOCX)
(DOCX)
(XLS)
Abbreviations
- EMG
electromyography
- FWHM
full width at half maximum
- MN
motoneurons
- ƞp2
eta square
- ui
eigenvectors
- PV
percentage of variance explained by eigenvectors
- ROM
range of motion
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Italian Ministry of Health (Ricerca corrente, IRCCS Fondazione Santa Lucia), Italian Space Agency (grants I/006/06/0 and ASI-MARS-PRE DC-VUM - 2017-006), the H2020-779963 EUROBENCH FSTP-1 grant (sub-project PEPATO), and Italian University Ministry (PRIN grant 2017CBF8NJ_005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dewolf AH, Meurisse GM, Schepens B, Willems PA. Effect of walking speed on the intersegmental coordination of lower-limb segments in elderly adults. Gait Posture. 2019;70: 156–161. 10.1016/j.gaitpost.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Laufer Y. Effect of age on characteristics of forward and backward gait at preferred and accelerated walking speed. J Gerontol A Biol Sci Med Sci. 2005;60: 627–632. 10.1093/gerona/60.5.627 [DOI] [PubMed] [Google Scholar]
- 3.Samson MM, Crowe A, de Vreede PL, Dessens JAG, Duursma SA, Verhaar HJJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging Clin Exp Res. 2001;13: 16–21. 10.1007/BF03351489 [DOI] [PubMed] [Google Scholar]
- 4.Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70: 340–347. 10.1093/ptj/70.6.340 [DOI] [PubMed] [Google Scholar]
- 5.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45: 313–320. 10.1111/j.1532-5415.1997.tb00946.x [DOI] [PubMed] [Google Scholar]
- 6.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305: 50–58. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGibbon CA. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exerc Sport Sci Rev. 2003;31: 102–108. 10.1097/00003677-200304000-00009 [DOI] [PubMed] [Google Scholar]
- 8.Perry MC, Carville SF, Smith ICH, Rutherford OM, Newham DJ. Strength, power output and symmetry of leg muscles: effect of age and history of falling. Eur J Appl Physiol. 2007;100: 553–561. 10.1007/s00421-006-0247-0 [DOI] [PubMed] [Google Scholar]
- 9.Skinner HB, Barrack RL, Cook SD. Age-related Decline in Proprioception. Clinical Orthopaedics and Related Research®. 1984;184: 208–211. [PubMed] [Google Scholar]
- 10.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, et al. Motor Control and Aging: Links to Age-Related Brain Structural, Functional, and Biochemical Effects. Neurosci Biobehav Rev. 2010;34: 721–733. 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monaco V, Rinaldi LA, Macrì G, Micera S. During walking elders increase efforts at proximal joints and keep low kinetics at the ankle. Clinical Biomechanics. 2009;24: 493–498. 10.1016/j.clinbiomech.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Hortobágyi T, Rider P, Gruber AH, DeVita P. Age and muscle strength mediate the age-related biomechanical plasticity of gait. Eur J Appl Physiol. 2016;116: 805–814. 10.1007/s00421-015-3312-8 [DOI] [PubMed] [Google Scholar]
- 13.Gueugnon M, Stapley PJ, Gouteron A, Lecland C, Morisset C, Casillas J-M, et al. Age-Related Adaptations of Lower Limb Intersegmental Coordination During Walking. Front Bioeng Biotechnol. 2019;7: 173 10.3389/fbioe.2019.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. Journal of Applied Physiology. 2000;88: 1804–1811. 10.1152/jappl.2000.88.5.1804 [DOI] [PubMed] [Google Scholar]
- 15.Sepic SB, Murray MP, Mollinger LA, Spurr GB, Gardner GM. Strength and range of motion in the ankle in two age groups of men and women. Am J Phys Med. 1986;65: 75–84. [PubMed] [Google Scholar]
- 16.Anderson DE, Madigan ML. Healthy older adults have insufficient hip range of motion and plantar flexor strength to walk like healthy young adults. Journal of Biomechanics. 2014;47: 1104–1109. 10.1016/j.jbiomech.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhman D, Willson J, Mizelle JC, DeVita P. The relationships between physical capacity and biomechanical plasticity in old adults during level and incline walking. J Biomech. 2018;69: 90–96. 10.1016/j.jbiomech.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Browne MG, Franz JR. Ankle power biofeedback attenuates the distal-to-proximal redistribution in older adults. Gait and Posture. 2019;71: 44–49. 10.1016/j.gaitpost.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franz JR. The Age-Associated Reduction in Propulsive Power Generation in Walking. Exerc Sport Sci Rev. 2016;44: 129–136. 10.1249/JES.0000000000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franz JR, Maletis M, Kram R. Real-time feedback enhances forward propulsion during walking in old adults. Clin Biomech (Bristol, Avon). 2014;29: 68–74. 10.1016/j.clinbiomech.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 21.Noble JW, Prentice SD. Intersegmental coordination while walking up inclined surfaces: age and ramp angle effects. Exp Brain Res. 2008;189: 249–255. 10.1007/s00221-008-1464-z [DOI] [PubMed] [Google Scholar]
- 22.Bleyenheuft C, Detrembleur C. Kinematic covariation in pediatric, adult and elderly subjects: Is gait control influenced by age? Clinical Biomechanics. 2012;27: 568–572. 10.1016/j.clinbiomech.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 23.Hafer JF, Boyer KA. Age related differences in segment coordination and its variability during gait. Gait Posture. 2018;62: 92–98. 10.1016/j.gaitpost.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 24.Borghese NA, Bianchi L, Lacquaniti F. Kinematic determinants of human locomotion. J Physiol. 1996;494: 863–879. 10.1113/jphysiol.1996.sp021539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchi L, Angelini D, Orani GP, Lacquaniti F. Kinematic coordination in human gait: relation to mechanical energy cost. J Neurophysiol. 1998;79: 2155–2170. 10.1152/jn.1998.79.4.2155 [DOI] [PubMed] [Google Scholar]
- 26.Winter DA. The biomechanics and motor control of human gait: normal, elderly and pathological. University of Waterloo Press; 1991. [Google Scholar]
- 27.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19: 1085–1091. 10.1016/j.jelekin.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaco V, Ghionzoli A, Micera S. Age-related modifications of muscle synergies and spinal cord activity during locomotion. J Neurophysiol. 2010;104: 2092–2102. 10.1152/jn.00525.2009 [DOI] [PubMed] [Google Scholar]
- 29.Santuz A, Brüll L, Ekizos A, Schroll A, Eckardt N, Kibele A, et al. Neuromotor Dynamics of Human Locomotion in Challenging Settings. iScience. 2020;23: 100796 10.1016/j.isci.2019.100796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43: 268–279. 10.1212/wnl.43.2.268 [DOI] [PubMed] [Google Scholar]
- 31.Fritz NE, Worstell AM, Kloos AD, Siles AB, White SE, Kegelmeyer DA. Backward walking measures are sensitive to age-related changes in mobility and balance. Gait Posture. 2013;37: 593–597. 10.1016/j.gaitpost.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 32.Grillner S. Control of locomotion in bipeds, tetrapods and fish. American Physiological Society Handbook of Physiology: Section 1: The Nervous System, volume II, Part1 Motor Control. American Physiological Society; Bethesda, MD: Vernon B. Brooks, John M. Brookhart, Vernon B. Mountcastle; 1981. pp. 1179–1236. [Google Scholar]
- 33.Thorstensson A. How is the normal locomotor program modified to produce backward walking? Exp Brain Res. 1986;61: 664–668. 10.1007/BF00237595 [DOI] [PubMed] [Google Scholar]
- 34.Winter DA, Pluck N, Yang JF. Backward walking: a simple reversal of forward walking? J Mot Behav. 1989;21: 291–305. 10.1080/00222895.1989.10735483 [DOI] [PubMed] [Google Scholar]
- 35.Duysens J, Tax AA, Murrer L, Dietz V. Backward and forward walking use different patterns of phase-dependent modulation of cutaneous reflexes in humans. J Neurophysiol. 1996;76: 301–310. 10.1152/jn.1996.76.1.301 [DOI] [PubMed] [Google Scholar]
- 36.Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80: 1868–1885. 10.1152/jn.1998.80.4.1868 [DOI] [PubMed] [Google Scholar]
- 37.Lamb T, Yang JF. Could different directions of infant stepping be controlled by the same locomotor central pattern generator? J Neurophysiol. 2000;83: 2814–2824. 10.1152/jn.2000.83.5.2814 [DOI] [PubMed] [Google Scholar]
- 38.Ivanenko YP, Cappellini G, Poppele RE, Lacquaniti F. Spatiotemporal organization of alpha-motoneuron activity in the human spinal cord during different gaits and gait transitions. Eur J Neurosci. 2008;27: 3351–3368. 10.1111/j.1460-9568.2008.06289.x [DOI] [PubMed] [Google Scholar]
- 39.Jansen K, De Groote F, Massaad F, Meyns P, Duysens J, Jonkers I. Similar muscles contribute to horizontal and vertical acceleration of center of mass in forward and backward walking: Implications for neural control. Journal of Neurophysiology. 2012;107: 3385–3396. 10.1152/jn.01156.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogkamer W, Meyns P, Duysens J. Steps forward in understanding backward gait: from basic circuits to rehabilitation. Exerc Sport Sci Rev. 2014;42: 23–29. 10.1249/JES.0000000000000000 [DOI] [PubMed] [Google Scholar]
- 41.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage. 2012;59: 1602–1607. 10.1016/j.neuroimage.2011.08.084 [DOI] [PubMed] [Google Scholar]
- 42.Cappellini G, Sylos-Labini F, MacLellan MJ, Sacco A, Morelli D, Lacquaniti F, et al. Backward walking highlights gait asymmetries in children with cerebral palsy. Journal of Neurophysiology. 2018;119: 1153–1165. 10.1152/jn.00679.2017 [DOI] [PubMed] [Google Scholar]
- 43.Hawkins KA, Balasubramanian CK, Vistamehr A, Conroy C, Rose DK, Clark DJ, et al. Assessment of backward walking unmasks mobility impairments in post-stroke community ambulators. Topics in Stroke Rehabilitation. 2019;26: 382–388. 10.1080/10749357.2019.1609182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C-Y, Lee J-S, Kim H-D. Comparison of the Effect of Lateral and Backward Walking Training on Walking Function in Patients with Poststroke Hemiplegia: A Pilot Randomized Controlled Trial. American Journal of Physical Medicine and Rehabilitation. 2017;96: 61–67. 10.1097/PHM.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 45.Makino M, Takami A, Oda A. Comparison of forward walking and backward walking in stroke hemiplegia patients focusing on the paretic side. Journal of Physical Therapy Science. 2017;29: 187–190. 10.1589/jpts.29.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20: 183–190. 10.3233/nre-2005-20305 [DOI] [PubMed] [Google Scholar]
- 47.Rose DK, Demark L, Fox EJ, Clark DJ, Wludyka P. A Backward Walking Training Program to Improve Balance and Mobility in Acute Stroke: A Pilot Randomized Controlled Trial. Journal of Neurologic Physical Therapy. 2018;42: 12–21. 10.1097/NPT.0000000000000210 [DOI] [PubMed] [Google Scholar]
- 48.Yeh TT, Cluff T, Balasubramaniam R. Visual reliance for balance control in older adults persists when visual information is disrupted by artificial feedback delays. PLoS ONE. 2014;9: e91554 10.1371/journal.pone.0091554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franz JR, Francis CA, Allen MS, O’Connor SM, Thelen DG. Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Hum Mov Sci. 2015;40: 381–392. 10.1016/j.humov.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connor KW, Loughlin PJ, Redfern MS, Sparto PJ. Postural adaptations to repeated optic flow stimulation in older adults. Gait Posture. 2008;28: 385–391. 10.1016/j.gaitpost.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. J Mot Behav. 2010;42: 197–208. 10.1080/00222895.2010.481693 [DOI] [PubMed] [Google Scholar]
- 52.Laufer Y. Age- and gender-related changes in the temporal-spatial characteristics of forwards and backwards gaits. Physiother Res Int. 2003;8: 131–142. 10.1002/pri.281 [DOI] [PubMed] [Google Scholar]
- 53.Monaco V, Ghionzoli A, Dario P, Micera S. Muscle synergies during walking: comparison between young and elderly people. Preliminary results. Conf Proc IEEE Eng Med Biol Soc. 2008;2008: 5370–5373. 10.1109/IEMBS.2008.4650428 [DOI] [PubMed] [Google Scholar]
- 54.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nature Neuroscience. 2007;10: 1055–1062. 10.1038/nn1930 [DOI] [PubMed] [Google Scholar]
- 55.Kendall F, McCreary E, Provance P, Rodgers M, Romani W. Muscles Testing and Function with Posture and Pain. Baltimore: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 56.Ivanenko YP, Dominici N, Lacquaniti F. Development of independent walking in toddlers. Exerc Sport Sci Rev. 2007;35: 67–73. 10.1249/JES.0b013e31803eafa8 [DOI] [PubMed] [Google Scholar]
- 57.Ivanenko YP, d’Avella A, Poppele RE, Lacquaniti F. On the origin of planar covariation of elevation angles during human locomotion. J Neurophysiol. 2008;99: 1890–1898. 10.1152/jn.01308.2007 [DOI] [PubMed] [Google Scholar]
- 58.Martino G, Ivanenko YP, Serrao M, Ranavolo A, d’Avella A, Draicchio F, et al. Locomotor patterns in cerebellar ataxia. J Neurophysiol. 2014;112: 2810–2821. 10.1152/jn.00275.2014 [DOI] [PubMed] [Google Scholar]
- 59.Dewolf AH, Mesquita RM, Willems PA. Intra-limb and muscular coordination during walking on slopes. Eur J Appl Physiol. 2020. 10.1007/s00421-020-04415-4 [DOI] [PubMed] [Google Scholar]
- 60.Cheung VCK, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci U S A. 2009;106: 19563–19568. 10.1073/pnas.0910114106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol. 2006;95: 602–618. 10.1152/jn.00767.2005 [DOI] [PubMed] [Google Scholar]
- 62.Ivanenko YP, Dominici N, Cappellini G, Di Paolo A, Giannini C, Poppele RE, et al. Changes in the spinal segmental motor output for stepping during development from infant to adult. J Neurosci. 2013;33: 3025–3036a. 10.1523/JNEUROSCI.2722-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34: 213–219. 10.1016/0022-510x(77)90069-7 [DOI] [PubMed] [Google Scholar]
- 64.La Scaleia V, Ivanenko YP, Zelik KE, Lacquaniti F. Spinal motor outputs during step-to-step transitions of diverse human gaits. Front Hum Neurosci. 2014;8: 305 10.3389/fnhum.2014.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dewolf AH, Ivanenko YP, Zelik KE, Lacquaniti F, Willems PA. Differential activation of lumbar and sacral motor pools during walking at different speeds and slopes. J Neurophysiol. 2019;122: 872–887. 10.1152/jn.00167.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dewolf AH, Ivanenko YP, Mesquita RM, Lacquaniti F, Willems PA. Neuromechanical adjustments when walking with an aiding or hindering horizontal force. Eur J Appl Physiol. 2020;120: 91–106. 10.1007/s00421-019-04251-1 [DOI] [PubMed] [Google Scholar]
- 67.Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71: 353–360. 10.4097/kja.d.18.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. Waterloo, ON: Waterloo Biomechanics; 1991. [Google Scholar]
- 69.Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. Journal of Neurophysiology. 2006;95: 602–618. 10.1152/jn.00767.2005 [DOI] [PubMed] [Google Scholar]
- 70.Cappellini G, Ivanenko YP, Dominici N, Poppele RE, Lacquaniti F. Migration of motor pool activity in the spinal cord reflects body mechanics in human locomotion. J Neurophysiol. 2010;104: 3064–3073. 10.1152/jn.00318.2010 [DOI] [PubMed] [Google Scholar]
- 71.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14: 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 72.Henderson G, Tomlinson BE, Gibson PH. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. J Neurol Sci. 1980;46: 113–136. 10.1016/0022-510x(80)90048-9 [DOI] [PubMed] [Google Scholar]
- 73.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985). 2003;95: 1717–1727. 10.1152/japplphysiol.00347.2003 [DOI] [PubMed] [Google Scholar]
- 74.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging. 1991;12: 336–338; discussion 352–355. 10.1016/0197-4580(91)90013-a [DOI] [PubMed] [Google Scholar]
- 75.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46: 530–541. 10.1016/j.neuroimage.2009.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol A Biol Sci Med Sci. 1999;54: M249–253. 10.1093/gerona/54.5.m249 [DOI] [PubMed] [Google Scholar]
- 77.Herssens N, Verbecque E, Hallemans A, Vereeck L, Van Rompaey V, Saeys W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture. 2018;64: 181–190. 10.1016/j.gaitpost.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 78.Martino G, Ivanenko YP, d’Avella A, Serrao M, Ranavolo A, Draicchio F, et al. Neuromuscular adjustments of gait associated with unstable conditions. J Neurophysiol. 2015;114: 2867–2882. 10.1152/jn.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santuz A, Ekizos A, Eckardt N, Kibele A, Arampatzis A. Challenging human locomotion: stability and modular organisation in unsteady conditions. Sci Rep. 2018;8: 2740 10.1038/s41598-018-21018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharrard WJW. The Segmental Innervation of the Lower Limb Muscles in Man. Ann R Coll Surg Engl. 1964;35: 106–122. [PMC free article] [PubMed] [Google Scholar]
- 81.Payne VG, Isaacs LD. Human Motor Development: A Lifespan Approach. Routledge; 2017. [Google Scholar]
- 82.de Groot M, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, et al. Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimers Dement. 2015;11: 321–330. 10.1016/j.jalz.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 83.Chung T, Park Js, Kim S, Montes N, Walston J, Höke A. Evidence for dying-back axonal degeneration in age-associated skeletal muscle decline. Muscle Nerve. 2017;55: 894–901. 10.1002/mus.25267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salvadores N, Sanhueza M, Manque P, Court FA. Axonal Degeneration during Aging and Its Functional Role in Neurodegenerative Disorders. Front Neurosci. 2017;11 10.3389/fnins.2017.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mattson MP, Magnus T. Aging and Neuronal Vulnerability. Nat Rev Neurosci. 2006;7: 278–294. 10.1038/nrn1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mau-Moeller A, Behrens M, Lindner T, Bader R, Bruhn S. Age-related changes in neuromuscular function of the quadriceps muscle in physically active adults. J Electromyogr Kinesiol. 2013;23: 640–648. 10.1016/j.jelekin.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 87.Baudry S, Collignon S, Duchateau J. Influence of age and posture on spinal and corticospinal excitability. Exp Gerontol. 2015;69: 62–69. 10.1016/j.exger.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 88.Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev. 1986;23: 97–114. 10.2190/VXN3-N3RT-54JB-X16X [DOI] [PubMed] [Google Scholar]
- 89.Badke MB, Duncan PW, Di Fabio RP. Influence of prior knowledge on automatic and voluntary postural adjustments in healthy and hemiplegic subjects. Phys Ther. 1987;67: 1495–1500. 10.1093/ptj/67.10.1495 [DOI] [PubMed] [Google Scholar]
- 90.Martino G, Ivanenko Y, Serrao M, Ranavolo A, Draicchio F, Rinaldi M, et al. Differential changes in the spinal segmental locomotor output in Hereditary Spastic Paraplegia. Clin Neurophysiol. 2018;129: 516–525. 10.1016/j.clinph.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 91.Soda N, Ueki T, Aoki T. Three-dimensional Motion Analysis of the Ankle during Backward Walking. J Phys Ther Sci. 2013;25: 747–749. 10.1589/jpts.25.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Earhart GM, Jones GM, Horak FB, Block EW, Weber KD, Fletcher WA. Forward versus backward walking: transfer of podokinetic adaptation. J Neurophysiol. 2001;86: 1666–1670. 10.1152/jn.2001.86.4.1666 [DOI] [PubMed] [Google Scholar]
- 93.Hackney ME, Earhart GM. Backward Walking in Parkinson Disease. Mov Disord. 2009;24: 218–223. 10.1002/mds.22330 [DOI] [PMC free article] [PubMed] [Google Scholar]