Abstract
Taste buds are the sensory end organs for gustation, mediating sensations of salty, sour, bitter, sweet and umami as well as other possible modalities, e.g. fat and kokumi. Understanding of the structure and function of these sensory organs has increased greatly in the last decades with advances in ultrastructural methods, molecular genetics, and in vitro models. This review will focus on the cellular constituents of taste buds, and molecular regulation of taste bud cell renewal and differentiation.
Keywords: Taste Receptors, neurotransmitter, ATP, SOX2, LGR5, Sonic Hedgehog, WNT, adult stem cells
Taste buds are distributed widely within the oropharynx with their relative sensitivity to different taste qualities being related to their location. The lingual taste buds – on fungiform, foliate and circumvallate papillae – and those on the soft palate of mammals, are the best studied, but recent reports have described taste buds of the larynx (Jette, Clary et al. 2019, Prescott, Umans et al. 2020) and even retromolar taste buds associated with salivary glands (Nguyen, Beck-Coburn et al. 2020). Although morphological details of these populations differ, the general organization of taste buds is similar; each bud comprises 40-100 specialized columnar epithelial cells organized into a garlic bulb-like assembly where the majority of cells extend an apical process through the taste pore to access the contents of the oral cavity. Like other epithelial cells, the cells of a taste bud (TBCs) have a limited lifespan (7-30 days or longer) (Barlow 2015) and are replaced by proliferative progenitor cells resident along the basement membrane of the epithelium. In this short review, we (1) highlight new findings on the cellular organization of taste buds, and (2) present an overview of our current understanding of cellular and molecular mechanisms of TBC renewal.
Historically identified taste bud cell types: modern molecular and functional correlates
Cellular Composition of Taste Buds
The different cell types of taste buds were described morphologically at the advent of biological electron microscopy in the 1960s. At the ultrastructural level, 3 elongate TBC types are recognizable in rodent taste buds according to features such as: nuclear shape, overall shape of the cell and elaboration of processes (Yang, Dzowo et al. 2020). Type I cells have highly involuted nuclei and are generally fusiform but with many fine, lamellate processes wrapping around nerve fibers and TBCs, tapering apically to end in multiple microvilli. Type II cells have relatively round and smooth nuclei and a smooth fusiform shape terminating apically, as a single slender microvillus. Type III cells are slender and tall, tapering to a single stout apical microvillus, with nuclei that tend to be elongate with a moderate number of shallow involutions. In addition to these 3 elongate, fusiform cell types (I, II and III), a population of ovoid cells situated in the basal compartment of taste buds have collectively been termed Type IV cells, and have been postulated to represent immature taste cells – a topic discussed further below. With the development of correlative molecular, genetic and anatomical methods in the last decades, functional correlates have been revealed corresponding to and further subdividing the original morphological TBC types (Roper and Chaudhari 2017).
Type II and Type III cells directly participate in transduction of different taste qualities and synaptic activation of afferent gustatory nerve fibers. Type III and II TBCs also have been termed Presynaptic Cells and Receptor Cells, respectively (DeFazio, Dvoryanchikov et al. 2006), but we prefer the original nomenclature since both cell types transduce particular taste qualities and both form synaptic connections with afferent nerves although, as described below, the nature of the synapses is quite different for the two types of taste cells. Molecular expression data strongly suggest that each taste cell expresses receptors for a single taste modality, e.g. a subset of Type II cells express the sweet receptor Tas1R2+Tas1R3, while a different subset of Type II cells express subsets of bitter taste receptor genes, the Tas2Rs. Similarly, Type III taste cells express Otop1, the ion channel required for sour transduction along with several other molecular features (See Table 1) (Lossow, Hermans-Borgmeyer et al. 2017, Wilson, Finger et al. 2017, Teng, Wilson et al. 2019).
TABLE 1:
Functional and Molecular Features of Conventional TBC Types
| Cell Type | Type I | Type II | Type III |
|---|---|---|---|
| Function | Glial-like: | Transduction | Transduction |
| Ionic Homeostasis Neurotransmitter reuptake and degradation |
Sweet, Bitter or Umami T1Rs and T2Rs |
Sour Otop1 |
|
| Molecular Features | NTPDase2 ROMK GAD65 GLAST |
PLCβ2 TRPM5 CALHM1/3 IP3R3 Skn1a |
PGP9.5 PKD2L1 Car4 GAD67 Mash1 Serotonin |
| Synaptic Mechanism | none | Channel Synapses (CALHM1/3) | Vesicular Synapses (SNAP25) |
Type III cells, which transduce ionic taste qualities of sour (Teng, Wilson et al. 2019) and aspects of salty taste (Kinnamon and Finger 2019, Teng, Wilson et al. 2019, Dutta Banik, Benfey et al. 2020), are smooth, elongate cells (Fig. 1) with a single stout microvillus extending into the taste pore (Yang, Dzowo et al. 2020) and form classical synapses onto the afferent nerve fibers, with both clear and dense-core vesicles. Type III cells express SNAP25 and other elements of the SNARE complex in keeping with the conventional vesicular synapses described in Type III cells (Yang, Crowley et al. 2000, Kinnamon and Finger 2019). Consistent with vesicular release of transmitter, the membrane capacitance of Type III cells increases with activation, due to fusion of vesicular membrane with the plasma membrane of the cell (Kinnamon and Finger 2019). Transduction of sour involves direct depolarization of the cell by entry of cations (H+ or Na+) into the apical compartment of the cell, (Teng, Wilson et al. 2019), ultimately leading to opening of voltage-gated Ca++ channels to allow influx of Ca++, which in turn triggers vesicular release of neurotransmitter. The best-established neurotransmitter of Type III cells is serotonin (Roper and Chaudhari 2017), which acts on 5-HT3a receptors on the afferent nerves (Kinnamon and Finger 2019) but which, in the absence of activation of purinergic receptors, is insufficient to drive a neural response (Kinnamon and Finger 2019). Type III cells also synthesize GABA, via GAD67, and release GABA although no clear roll for GABA in neural transmission is known (Roper and Chaudhari 2017).
Figure 1:

A. Reconstructions from a serial blockface scanning electron microscopic image series through a taste bud from a circumvallate papilla showing the characteristic morphology of the 4 canonical types of taste cells (Adapted from (Kinnamon and Finger 2019). Two Type IV cells aare shown (magenta). The smaller one at lower right is typical of a recently post-mitotic cell likely expressing SHH; the more elongate Type IV cell at left is relatively older and has begun differentiating into one of the other cell types. The surface of the epithelium is indicated by the gray horizontal line; the basal lamina by the gray bar at the bottom. B. Electron micrograph showing a dividing progenitor cell (PC) apposed to the basal lamina of the epithelium below a taste bud outlined in yellow. One of the daughter cells of this multipotent dividing cell may enter the taste bud to become a Type IV taste precursor cell. Roman numerals I-IV denote nuclei of identifiable taste cell types. Inset: Higher magnification of the PC showing the bilobed nucleus – a hallmark of telophase of cell division.
Successful transmission of sour and salt information from Type III cells to afferent nerves requires activation of P2X3 receptors (Kinnamon and Finger 2019, Larson, Vandenbeuch et al. 2020), but the source of the requisite ATP is enigmatic. No one has succeeded in measuring ATP release from Type III cells (Kinnamon and Finger 2019) and Type III cells exhibit little or no VNUT (vesicular nucleotide transporter) required for loading ATP into synaptic vesicles (Kinnamon and Finger 2019). Other mechanisms for non-vesicular release of ATP, e.g. hemichannels or transporters (Taruno 2018), are not obvious in these cells. Whether the inability to measure ATP release from Type III cells reflects reflects a difficult-to-measure, focal release of a small amount of ATP, or whether the requisite ATP for neurotransmission originates from other sources, is unclear.
Type II cells, in contrast, show distinct markers of non-vesicular synaptic release of their neurotransmitter, ATP, (Ma, Taruno et al. 2018, Romanov, Lasher et al. 2018, Kashio, Wei-Qi et al. 2019, Nomura, Nakanishi et al. 2020) but none of the features of conventional vesicle-mediated synaptic transmission – no voltage-gated calcium channels, no increase in capacitance with transmitter release and no obvious sites of accumulation of synaptic vesicles along presynaptic membranes (Romanov, Lasher et al. 2018, Kinnamon and Finger 2019). Instead, synaptic transmission from Type II cells relies on a voltage-gated channel formed by CALHM1/CALHM3 to regulate activity-dependent release of ATP (Ma, Taruno et al. 2018, Kinnamon and Finger 2019). The release sites are further characterized, in rodents, by the presence of a large mitochondrion with tubular cristae, previously termed an “atypical mitochondrion”. These “atypical” mitochondria appear to serve as a local reservoir for ATP to permit repeated release via opening of CAHLM1/CALHM3 channels in the plasma membrane. Transduction of bitter, sweet, or umami tastants occurs by activation of the cognate GPCR (Tas2Rs for bitter; Tas1Rs for sweet and umami) triggering a PLCß2/IP3R3-mediated cascade to affect release of Ca++ from intracellular stores, which activates TRPM5 channels causing strong depolarization to activate Na+ channels to generate action potentials. These action potentials then are sufficient to directly gate the CAHLM1/CAHLM3 channels to allow release of ATP (Kinnamon and Finger 2019, Taruno, Nomura et al. 2020). The amount of ATP released is directly proportional to the number of spikes produced by the Type II cell (Romanov, Lasher et al. 2018, Kinnamon and Finger 2019). Accordingly, neurotransmitter release in Type II cells is independent of Ca++ influx through voltage-gated Ca++ channels, unlike the situation in Type III cells. Indeed, the presence of voltage-gated Ca++ channels is one of the key features used to distinguish Type III from Type II taste cells (Kinnamon and Finger 2019).
Non-canonical Taste Transducing Cells
Intriguingly, a population of salt-responsive TBCs in the fungiform papillae of rodents do not fit neatly into either Type II or III categories (Nomura, Nakanishi et al. 2020) having some Type II cell features, e.g. CALHM1 channels (Bigiani 2017), but lacking other classical molecular features of Type II cells such as TrpM5. For these salt responsive cells, entry of Na+ into the apical region depolarizes the cell sufficiently to activate voltage-gated Na+ channels to generate an action potential thereby gating the ATP-release channels directly without the necessity for initial increases intracellular Ca++. Accordingly, TRPM5 channels, which are gated by high intracellular Ca++, are not necessary for the sodium salt transduction cascade, as they are in conventional Type II TBCs. Better delineation of the morphology and gene expression profiles of these salt-responsive cells will determine if they do indeed represent a distinct morphological type of TBC, or a specialized subset of Type II cells.
Another novel population of TBCs is a recently described class of broadly responsive Type III cells described by Medler and co-workers (Dutta Banik, Benfey et al. 2020). Using KCl-induced depolarization and responsiveness to citric acid as well known defining features for Type III cells, these investigators found that roughly half of Type III cells responded narrowly to ionic stimuli, i.e., acids or NaCl. Unexpectedly, the remaining half of the Type III cells, although responding to conventional ionic stimuli, also responded to bitter, sweet or umami substances via a PLCβ3-dependent signaling system. The extent to which these broadly responsive Type III cells differ morphologically and/or genetically from the narrowly responsive Type III cells is unexplored.
Type I cells, classically described as “glial-like” are irregular in shape, and extend flattened, wing-like processes that envelop adjacent cells and nerves (Yang, Dzowo et al. 2020). In addition to wrapping other cell types, Type I cells express several molecular features of astrocytes including features for neurotransmitter degradation or re-uptake, e.g. GLAST, and the norepinephrine transporter, NET (Roper and Chaudhari 2017, Kinnamon and Finger 2019). Also like astrocytes, Type I cells are important for buffering K+ (Roper and Chaudhari 2017). Type I cells respond with increases in intracellular Ca++ to a variety of neurotransmitters and modulators including oxytocin (Sinclair, Perea-Martinez et al. 2010) and substance P (Huang and Wu 2018). Although Type I cells lack obvious synaptic specializations, they can release GABA (Roper and Chaudhari 2017, Huang and Wu 2018) and probably other neurotransmitters and modulators. What the overall impact of these Type I cell functions on taste processing may be remains to be determined.
A recent report (Baumer-Harrison, Raymond et al. 2020) suggests that optogenetic activation of Type I cells can activate gustatory afferents mimicking stimulation of amiloride-sensitive (AS) salt cells (discussed above – (Nomura, Nakanishi et al. 2020)). But two other findings call into question this conclusion. First, AS-salt responses depend on the Pou2f3 (Skn-1a) transcription factor, which is required for Type II but not Type I cell differentiation (Ohmoto, Jyotaki et al. 2020). Second, Nomura et al (Nomura, Nakanishi et al. 2020) find that AS salt responses rely on the CALHM1/3 channel as well as voltage-gated Na+ channels, neither of which are present in Type I cells. Taken together, these latter findings call into question the specificity of the genetic driver, GAD65, used to drive expression of the light-sensitive channel rhodopsin in the Baumer-Harrison et al study.
Type IV cells are round cells situated in the basal compartment of taste buds (See Figs. 1, 2). These cells are post-mitotic, and represent an immature cell type within buds. The Type IV cells arise from a population of multipotent, proliferative basal keratinocytes situated along the basement membrane of the epithelium (See Fig. 1B and Fig 2 below). Following terminal cell division, the post-mitotic cells enter the taste bud and begin to express the morphogen, Sonic Hedgehog (SHH) in a nerve-dependent fashion (Miura et al. 2006)(Barlow 2015). As Type IV cells mature, they downregulate SHH and transition from a flattened round profile at the margins of the taste bud to a more centrally located and apically elongated form closely associated with nerve fibers and other TBCs as illustrated in Fig. 1A (Yang, Dzowo et al. 2020). During this transition, the Type IV cells begin to assume characteristics of the elongate TBC types. When a cell stops being a Type IV cell and more properly should be called, an immature Type I or Type II or Type III cell, is unclear. The molecular signaling events leading up to formation of the Type IV cells will be described below.
Fig. 2:
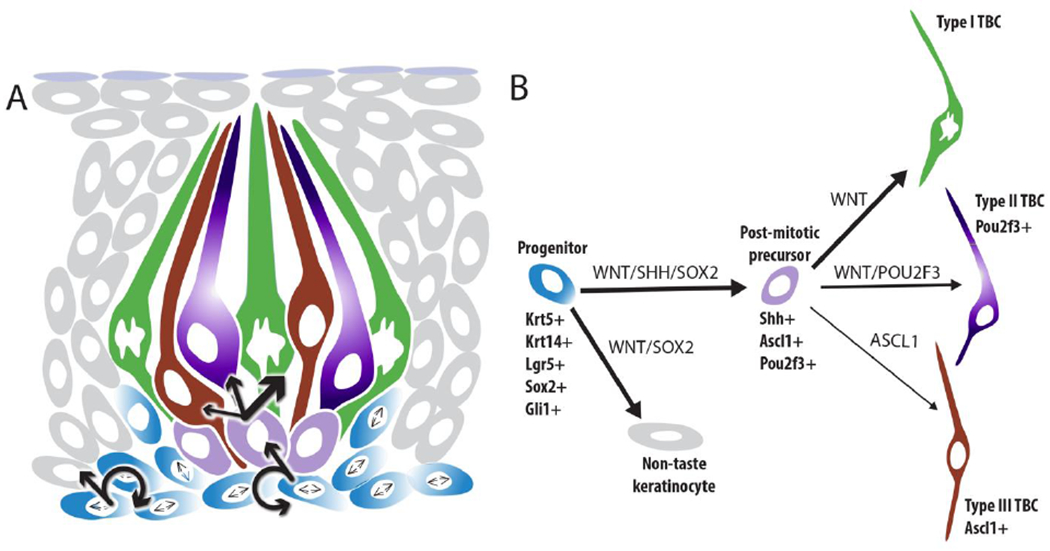
Schematic figures of lineage relationships and molecular regulators of taste bud cell renewal. A. Lingual progenitors (blue) sit at the basement membrane and are highly proliferative. Progenitors divide to replace themselves (circular arrows), as well as give rise to non-taste keratinocytes (grey - straight arrow, left), and SHH+ immediate taste precursor cells (light purple – straight arrow center). Each SHH+ cell can differentiate into a Type I (green), Type II (dark purple) or Type III (brick red) TBC. B. Production of non-taste keratinocytes (grey) from taste progenitors (blue) requires WNT and SOX2 function (noted along arrow connecting the 2 populations), while taste lineage production via SHH+ postmitotic precursors (light purple) requires WNT, SHH and SOX2 (noted along along arrow connecting the 2 populations). High levels of WNT (thick arrow) are hypothesized to be necessary for Type I TBC differentiation (green), while moderate WNT signaling may promote Type II TBC differentiation (dark purple)(see Barlow 2015). POU2F3 is required for Type II TBCs, while ASCL1 function is required for Type III TBCs (brick red). Gene products in all capitals aligned with lineage arrows indicate functional role in TBC renewal, genes listed in beneath or adjacent to cell lineage steps in sentence case are expressed in each cell population.
Taste bud cells are continuously and reliably renewed.
Regardless of cell type, all TBCs constantly turnover and each taste cell has been ascribed an average lifespan of ~10 days (Barlow 2015). This metric is often cited in textbooks, based on results of birthdating experiments of rodent taste buds where taste cells have a median longevity of 10 days (Barlow 2015). In fact, TBC lifespan is highly variable with both shorter (2 days) and longer (24 days) lived cells (Hamamichi, Asano-Miyoshi et al. 2006, Perea-Martinez, Nagai et al. 2013, Barlow 2015), which may reflect differences in the lifespans of different taste cell types, e.g. Type III appear longer lived than Type II cells (Perea-Martinez, Nagai et al. 2013), but may also be attributable to replacement of injured taste cells regardless of taste cell type ((Beidler and Smallman 1965)).
TBCs are produced by lingual stem cells situated outside of taste buds. These proliferative basal keratinocytes reside at the basement membrane of the tongue epithelium, and the majority of these progenitors is actively engaged in the cell cycle_(Barlow 2015) (Fig. 2A). In well studied adult stem cell models, such as skin/hair follicles and intestine, basal cells represent a complex mix of functional cell types, including stem cells that can give rise to all lineages within the epithelium, as well as continually renew themselves. In producing the spectrum of differentiated epithelial cell types, stem cells generate transit amplifying (TA) cells that have significant proliferative potential with limited ability to self-renew. Rather repeated TA cell divisions expand epithelial cell number before daughter cells undergo terminal differentiation. While stem and TA progenitor subpopulations are well recognized in skin and intestine, distinct populations within lingual epithelium have not been firmly established, hence our use of the term “lingual progenitor” which encompasses stem and TA cells of the taste epithelial lineage.
“Will the real taste stem cells please step forward?”
To date, a number of multipotent lingual progenitor populations have been identified, including those expressing cytokeratins (KRT) 5 and KRT14, SOX2, GLI1 and LGR5.
Using genetic lineage tracing in mice, KRT5+ progenitors within circumvallate and fungiform gustatory papillae replace themselves, generate both taste and non-taste epithelial lineages, and do so for up to a year (Ohmoto, Lei et al. 2020). By contrast, KRT14+ lineagetraced clones extinguish over time, suggesting this population may comprise mostly TA cells that have limited capacity to replenish themselves (Okubo, Clark et al. 2009). One caveat is that the genetically engineered tools used to follow the fate of KRT14 daughter cells may not fully reflect the potential of these progenitors and thus the stem potential of KRT14+ cells remains an open question.
The transcription factor SOX2 regulates adult stem cells in a spectrum of tissues (Arnold, Sarkar et al. 2011), including adult stem cells in the tongue, where it is required for differentiation of taste cells (Okubo, Pevny et al. 2006, Castillo-Azofeifa, Seidel et al. 2018, Ohmoto, Lei et al. 2020). In adult mice, SOX2 is expressed by lingual progenitors (Fig 2A) (Okubo, Pevny et al. 2006, Ohmoto, Ren et al. 2017, Castillo-Azofeifa, Seidel et al. 2018), and genetic lineage tracing of SOX2+ cells results in labeling of both taste and non-taste epithelium, which persists for months after induction (Ohmoto, Ren et al. 2017). These data support an adult stem cell function for SOX2+ progenitors.
The Hedgehog (Hh) signaling pathway is key regulator of epithelial homeostasis, including that of the tongue (Mistretta and Kumari 2019). GLI1 is a Hh target gene and is expressed by lingual progenitors (Fig 2A) in response to secreted Hh protein. Specifically, Sonic hedgehog (SHH) is synthesized by recently generated Type IV cells within buds as well as by the gustatory innervation, and SHH supplied by both tissues combine to support TBC renewal (Castillo-Azofeifa, Losacco et al. 2017). Genetic lineage tracing of GLI1+ cells produces clones of cells within taste buds and non-taste epithelia (Liu, Lu et al. 2013) indicating that GLI1 also marks a population of multipotent lingual progenitors. The extent to which GLI1 marks a persistent stem and/or TA populations is unknown; GLI1+ lineage label appears to diminish over 3 months (Liu, Lu et al. 2013), suggesting GLI1+ cells may represent a TA rather than a long lived stem cell population.
The best experimentally supported multipotent lingual stem cell population to date are lingual progenitors in the circumvallate papilla that express LGR5 (Fig 2A). Genetic lineage tracing of LGR5+ cells leads to long term labeling of taste and non-taste lineages within the CVP epithelium, as well as persistent labeling of LGR5+ progenitor cells (Yee, Li et al. 2013). In addition to lineage tracing in vivo, a key test of stem cell identity is the ability to generate the appropriate lineages from single progenitors in vitro. This has been demonstrated for LGR5+ intestinal crypt stem cells, which when cultured under appropriate conditions generate “miniguts” that contain the multiple cell types resident in the intestinal villi (de Sousa and de Sauvage 2019). Building on this technology, Jiang and colleagues cultured isolated LGR5+ progenitors which gave rise to lingual organoids housing differentiated Type I, II and III TBCs (Ren, Lewandowski et al. 2014). All in vivo lineage tracing studies discussed above have demonstrated that populations of basal cells expressing specific genes, e.g., KRT5, GLI1 etc., are multipotent, giving rise to all TBC types and non-taste epithelial cells (Fig 2A). However, lineage tracking data have left open the question of the capacity or potency of individual lingual progenitors. Using organoid technology, single LGR5+ progenitors now have been shown to be multipotent. The extent to which other progenitor populations can propogate TBC-replete organoids remains to be determined.
Importantly, all of these lineage-traced progenitor populations partially overlap in vivo, and the extent to which they subdivide functionally as stem cells and/or TA cells is an important question, as we learn more of the plasticity of progenitor populations in other epithelia. Several cellular strategies have been reported to maintain intestinal epithelium in the face of injury. For example, ablation of one stem cell population can be rescued by activation of another; further in response to injury differentiated cells can dedifferentiate and acquire stem function to repair the epithelium (see (de Sousa and de Sauvage 2019) for review). Given how many drugs and viruses negatively impact taste function, exploring how these taste progenitor populations behave and compensate for one another in injury models will likely be a fruitful area of future investigation.
How do different TBC types emerge from a common progenitor population?
Taste bud cell renewal is regulated by the WNT/ß-catenin pathway (Fig 2B) as well as by Sonic Hedgehog (SHH). Specifically, WNT signaling is required for progenitor proliferation and survival, and promotes differentiation of Type I and Type II TBCs (Gaillard, Xu et al. 2015, Gaillard, Bowles et al. 2017, Xu, Horrell et al. 2017). SHH, by contrast, is required for differentiation of TBCs (Barlow 2015), and further, SOX2 is required downstream of SHH to permit TBC formation (Castillo-Azofeifa, Seidel et al. 2018)(Fig 2B), although SHH may also impact progenitor proliferation (see (Mistretta and Kumari 2019)).
Once daughter cells have been generated by progenitors outside of buds, these cells exit the cell cycle, enter the bud within 12-24 hrs and transiently turn on SHH expression (Miura, Kusakabe et al. 2006). SHH+ cells lie in the basal compartment of taste buds and appear to be a subset of the historically defined Type IV cells (see above and Fig. 1). Lineage tracing of SHH+ cells in adult mice revealed that individual SHH+ cells are postmitotic (Barlow 2015), and differentiate into Type I, II or III cells, in proportions consistent with the standing ratios of these different cell types in adult buds (Miura, Scott et al. 2014). Thus, SHH+ Type IV cells are competent to differentiate into each of the 3 TBC types. Given these cells are no longer proliferative, we have termed them “postmitotic taste precursor cells” to reflect their functional position in the taste cell lineage (Miura, Scott et al. 2014) (Fig 2).
Ascl1 (Mash1), a transcription factor that functions in the Notch pathway, is required for development of Type III TBCs in both zebrafish and mouse (Kapsimali, Kaushik et al. 2011, Seta, Oda et al. 2011). In mice, a subset of basal cells within buds express Ascl1, including some but not all SHH+ cells Fig 2B (Miura, Kusakabe et al. 2006), suggesting ASCL1 may function within postmitotic precursor cells destined to become Type III cells. Recent ASCL1 lineage tracing labeled primarily Type III cells, with some labeling of Type II TBCs (Hsu, Seta et al. 2020); one model to account for this pattern is that ASCL1 may be co-expressed initially in SHH+ cells, but is downregulated in immature cells destined for a Type II cell fate, and maintained in those that acquire a Type III cell fate (Fig 2B).
Another transcription factor, POU2F3 (Skn-1a) is required for Type II cell production (Matsumoto, Ohmoto et al. 2011). POU2F3 is expressed by Type II cells; genetic loss of POU2F3 leads to loss of Type II TBCs and expansion of Type III cell number with no obvious impact on Type I cell populations. POU2F3 is also expressed in a subset of Type IV cells (Fig 2B), suggesting POU2F3 may be required in postmitotic taste precursors for the acquisition of Type II TBC fate (Matsumoto, Ohmoto et al. 2011). In mouse olfactory epithelium, where Pou2f3 is required for development of microvillar sensory cells (Yamaguchi, Yamashita et al. 2014) and Ascl1 is required for ciliated olfactory sensory neurons (Cau, Gradwohl et al. 1997), Ascl1 and Pou2f3 are rarely co-expressed. However, in taste buds, the extent of Ascl1 and Pou2f3 co-expression has not been explored. Thus, the intriguing possibility exists that regulation of Ascl1 vs Pou2f3 in SHH+ precursors may underlie TBC fate decisions; identifying the genetic pathways upstream of their potential differential regulation may shed light on cell fate selection in taste buds.
CONCLUSION
Taste buds comprise a limited set of distinctive TBC cell types including both a glial-like type and several different taste transducing types distinguished morphologically, molecularly and functionally. In keeping with the general turnover of epithelium, the TBCs are all replaced over a period of weeks by proliferative multipotent keratinocytes in the surrounding epithelium. The post-mitotic cells enter the base of the taste bud, initiate expression of SHH and assume different molecular trajectories to generate the different TBC types. Ultimate cell fate depends on expression of several transcription factors including POU2F3 (Skn-1a) and ASCL1 (Mash1) necessary for formation of the transducing TBC types, which are likely regulated by both SHH and WNT/ß-catenin signaling as well as by other gene pathways still to be identified.
Acknowledgements:
The authors thank Sue Kinnamon for helpful discussion and comments on drafts of this contribution. We also thank Yannick Dzowo and Robert Lasher for their contributions to Fig. 1.
Funding Sources: This work has been supported by grants DC018489 and DC012383 to LAB, DC014728 to TEF and DC017679 to S. Kinnamon from the National Institute on Deafness and Other Communication Disorders of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI Statement
The authors (LB and TF) declare no conflicts of interest.
References
Highlighted references:
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N and Hochedlinger K (2011). “Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice.” Cell Stem Cell 9(4): 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow LA (2015). “Progress and renewal in gustation: new insights into taste bud development.” Development 142(21): 3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer-Harrison C, Raymond MA, Myers TA, Sussman KM, Rynberg ST, Ugartechea AP, Lauterbach D, Mast TG and Breza JM (2020). “Optogenetic Stimulation of Type I GAD65(+) Cells in Taste Buds Activates Gustatory Neurons and Drives Appetitive Licking Behavior in Sodium-Depleted Mice.” J Neurosci 40(41): 7795–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler LM and Smallman RL (1965). “Renewal of cells within taste buds.” J Cell Biol 27(2): 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigiani A (2017). “Calcium Homeostasis Modulator 1-Like Currents in Rat Fungiform Taste Cells Expressing Amiloride-Sensitive Sodium Currents.” Chem Senses 42(4): 343–359. [DOI] [PubMed] [Google Scholar]
- Castillo-Azofeifa D, Losacco JT, Salcedo E, Golden EJ, Finger TE and Barlow LA (2017). “Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance.” Development 144(17): 3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]; Taste buds are maintained by an intact innervation, but the molecular factor(s) that provide trophic support have proved difficult to identify. Sonic hedgehog promotes TBC renewal in adult mice, and this study reveals that in addition to SHH+ cells in taste buds, the gustatory innervation also supplies SHH that is sufficient to maintain taste buds.
- Castillo-Azofeifa D, Seidel K, Gross L, Golden EJ, Jacquez B, Klein OD and Barlow LA (2018). “SOX2 regulation by hedgehog signaling controls adult lingual epithelium homeostasis.” Development 145(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C and Guillemot F (1997). “Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors.” Development 124(8): 1611–1621. [DOI] [PubMed] [Google Scholar]
- de Sousa EMF and de Sauvage FJ (2019). “Cellular Plasticity in Intestinal Homeostasis and Disease.” Cell Stem Cell 24(1): 54–64. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD and Chaudhari N (2006). “Separate populations of receptor cells and presynaptic cells in mouse taste buds.” J Neurosci 26(15): 3971–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta Banik D, Benfey ED, Martin LE, Kay KE, Loney GC, Nelson AR, Ahart ZC, Kemp BT, Kemp BR, Torregrossa AM and Medler KF (2020). “A subset of broadly responsive Type III taste cells contribute to the detection of bitter, sweet and umami stimuli.” PLoS Genet 16(8): e1008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Bowles SG, Salcedo E, Xu M, Millar SE and Barlow LA (2017). “beta-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice.” PLoS Genet 13(8): e1006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D, Xu M, Liu F, Millar SE and Barlow LA (2015). “beta-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates.” PLoS Genet 11(5): e1005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamichi R, Asano-Miyoshi M and Emori Y (2006). “Taste bud contains both short-lived and long-lived cell populations.” Neuroscience 141(4): 2129–2138. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Seta Y, Matsuyama K, Kataoka S, Nakatomi M, Toyono T, Gunjigake KK, Kuroishi KN and Kawamoto T (2020). “Mash1-expressing cells may be relevant to type III cells and a subset of PLCbeta2-positive cell differentiation in adult mouse taste buds.” Cell Tissue Res. [DOI] [PubMed] [Google Scholar]
- Huang AY and Wu SY (2018). “Substance P as a putative efferent transmitter mediates GABAergic inhibition in mouse taste buds.” Br J Pharmacol 175(7): 1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette ME, Clary MS, Prager JD and Finger TE (2019). “Chemical receptors of the arytenoid: A comparison of human and mouse.” Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimali M, Kaushik AL, Gibon G, Dirian L, Ernest S and Rosa FM (2011). “Fgf signaling controls pharyngeal taste bud formation through miR-200 and Delta-Notch activity.” Development 138(16): 3473–3484. [DOI] [PubMed] [Google Scholar]
- Kashio M, Wei-Qi G, Ohsaki Y, Kido MA and Taruno A (2019). “CALHM1/CALHM3 channel is intrinsically sorted to the basolateral membrane of epithelial cells including taste cells.” Sci Rep 9(1): 2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC and Finger TE (2019). “Recent advances in taste transduction and signaling.” F1000Res 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ED, Vandenbeuch A, Anderson CB and Kinnamon SC (2020). “Function, Innervation, and Neurotransmitter Signaling in Mice Lacking Type-II Taste Cells.” eNeuro 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Lu M and Guthrie KM (2013). “Anterograde trafficking of neurotrophin-3 in the adult olfactory system in vivo.” Exp Neurol 241: 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossow K, Hermans-Borgmeyer I, Behrens M and Meyerhof W (2017). “Genetic Labeling of Car4-expressing Cells Reveals Subpopulations of Type III Taste Cells.” Chem Senses 42(9): 747–758. [DOI] [PubMed] [Google Scholar]
- Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, Payne R, Demuro A, Parker I, Mitchell CH, Henao-Mejia J, Tanis JE, Matsumoto I, Tordoff MG and Foskett JK (2018). “CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes.” Neuron 98(3): 547–561 e510. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper fully resolves the nature of the ATP release channel in Type II taste cells. The previously-identified CAHLM1 subunit did not fully meet the characteristics of the taste cell release channel although it was clearly necessary for functionality. Only when forming a heterologous channel with CAAHLM3 does the complex meet the physiological properties of the channel from Type II taste cells.
- Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y and Abe K (2011). “Skn-1a (Pou2f3) specifies taste receptor cell lineage.” Nat Neurosci 14(6): 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM and Kumari A (2019). “Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation.” Int J Mol Sci 20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y and Harada S (2006). “Cell lineage and differentiation in taste buds.” Arch Histol Cytol 69(4): 209–225. [DOI] [PubMed] [Google Scholar]
- Miura H, Scott JK, Harada S and Barlow LA (2014). “Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells.” Dev Dyn 243(10): 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Beck-Coburn GE, Karabucak B and Tizzano M (2020). “Mucous Salivary Glands Associated with the Retromolar Taste Papillae.” Chem Senses in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Nakanishi M, Ishidate F, Iwata K and Taruno A (2020). “All-Electrical Ca(2+)-Independent Signal Transduction Mediates Attractive Sodium Taste in Taste Buds.” Neuron 106(5): 816–829 e816 [DOI] [PubMed] [Google Scholar]; (2 star) This work definitively identifies the population of taste cells in fungiform taste buds that underlie amiloride-sensitive salt responses. Previous investigators had characterized these cells in terms of what they weren’t: neither Type III cells nor TrpM5-expressing Type II cells. But this new works identifies them as a unique cell population showing many, but not all, characteristics of Type II taste cells.
- Ohmoto M, Jyotaki M, Foskett JK and Matsumoto I (2020). “Sodium-Taste Cells Require Skn-1a for Generation and Share Molecular Features with Sweet, Umami, and Bitter Taste Cells.” eNeuro 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmoto M, Lei W, Yamashita J, Hirota J, Jiang P and Matsumoto I (2020). “SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue.” PLoS One 15(10): e0240848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmoto M, Ren W, Nishiguchi Y, Hirota J, Jiang P and Matsumoto I (2017). “Genetic Lineage Tracing in Taste Tissues Using Sox2-CreERT2 Strain.” Chem Senses 42(7): 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Clark C and Hogan BL (2009). “Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate.” Stem Cells 27(2): 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Pevny LH and Hogan BL (2006). “Sox2 is required for development of taste bud sensory cells.” Genes Dev 20(19): 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Martinez I, Nagai T and Chaudhari N (2013). “Functional cell types in taste buds have distinct longevities.” PLoS One 8(1): e53399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Umans BD, Williams EK, Brust RD and Liberles SD (2020). “An Airway Protection Program Revealed by Sweeping Genetic Control of Vagal Afferents.” Cell 181(3): 574–589 e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF and Jiang P (2014). “Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo.” Proc Natl Acad Sci U S A 111(46): 16401–16406. https://pubmed.ncbi.nlm.nih.gov/25368147/. [DOI] [PMC free article] [PubMed] [Google Scholar]; In vitro models for taste buds and taste bud cell renewal have been limited. Development of organoids from cultured adult lingual stem cells of mice represents a significant advance; all 3 major taste cell types are present in these lingual organoids, including subsets of TBCs that are responsive to sweet or bitter taste stimuli.
- Romanov RA, Lasher RS, High B, Savidge LE, Lawson A, Rogachevskaja OA, Zhao H, Rogachevsky VV, Bystrova MF, Churbanov GD, Adameyko I, Harkany T, Yang R, Kidd GJ, Marambaud P, Kinnamon JC, Kolesnikov SS and Finger TE (2018). “Chemical synapses without synaptic vesicles: Purinergic neurotransmission through a CALHM1 channel-mitochondrial signaling complex.” Sci Signal 11(529). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD and Chaudhari N (2017). “Taste buds: cells, signals and synapses.” Nat Rev Neurosci 18(8): 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta Y, Oda M, Kataoka S, Toyono T and Toyoshima K (2011). “Mash1 is required for the differentiation of AADC-positive type III cells in mouse taste buds.” Dev Dyn 240(4): 775–784. [DOI] [PubMed] [Google Scholar]
- Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD and Chaudhari N (2010). “Oxytocin signaling in mouse taste buds.” PLoS One 5(8): e11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A (2018). “ATP Release Channels.” Int J Mol Sci 19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Nomura K, Kusakizako T, Ma Z, Nureki O and Foskett JK (2020). “Taste transduction and channel synapses in taste buds.” Pflugers Arch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng B, Wilson CE, Tu YH, Joshi NR, Kinnamon SC and Liman ER (2019). “Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1.” Curr Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR and Kinnamon SC (2008). “Amiloride-sensitive channels in type I fungiform taste cells in mouse.” BMC Neuroscience 9(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CE, Finger TE and Kinnamon SC (2017). “Type III Cells in Anterior Taste Fields Are More Immunohistochemically Diverse Than Those of Posterior Taste Fields in Mice.” Chem Senses 42(9): 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Horrell J, Snitow M, Cui J, Gochnauer H, Syrett CM, Kallish S, Seykora JT, Liu F, Gaillard D, Katz JP, Kaestner KH, Levin B, Mansfield C, Douglas JE, Cowart BJ, Tordoff M, Liu F, Zhu X, Barlow LA, Rubin AI, McGrath JA, Morrisey EE, Chu EY and Millar SE (2017). “WNT10A mutation causes ectodermal dysplasia by impairing progenitor cell proliferation and KLF4-mediated differentiation.” Nat Commun 8: 15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Yamashita J, Ohmoto M, Aoude I, Ogura T, Luo W, Bachmanov AA, Lin W, Matsumoto I and Hirota J (2014). “Skn-1a/Pou2f3 is required for the generation of Trpm5-expressing microvillous cells in the mouse main olfactory epithelium.” BMC Neurosci 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME and Kinnamon JC (2000). “Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity.” J Comp Neurol 424(2): 205–215. [DOI] [PubMed] [Google Scholar]
- Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, Lasher RS, Kinnamon JC and Finger TE (2020). “Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud.” J Comp Neurol 528(5): 756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed reconstructions from serial electron micrographs demonstrate the morphology and inter-relationships between the different cell types in taste buds of the circumvallate papilla of mice. Clear differences are made obvious in the synaptic relationships between nerve fibers and Type II comparred to Type III cells.
- Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF and Jiang P (2013). “Lgr5-EGFP Marks Taste Bud Stem/Progenitor Cells in Posterior Tongue.” Stem Cells 31(5): 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]


