Coronavirus disease 2019 (COVID-19), a major healthcare problem of this century, has already claimed hundreds of thousands of lives. Rigorous research is examining potential therapeutic options. An early study reported that oral famotidine 80 mg 3 times a day was associated with improved patient-reported outcomes in nonhospitalized COVID-19 patients.1 Since then, studies evaluating outcomes in hospitalized COVID-19 patients treated with famotidine have reported conflicting results.2, 3, 4 It has also been suggested that proton pump inhibitors (PPIs) may be associated with increased risks of severe disease5 and death6 in infected patients. We conducted this meta-analysis to evaluate any association between famotidine or PPI use and outcomes in patients with COVID-19.
Methods
Methods are described in more detail in the Supplementary Methods. Briefly, we included studies that evaluated any association between famotidine or PPI use and outcomes in patients with COVID-19 and reported summary results as adjusted odds ratio (OR) or hazard ratio (HR). We assessed severity of disease and mortality among infected patients. The indicators of disease severity included endotracheal intubation, intensive care unit admission, and mortality; Supplementary Table 1 provides details of study characteristics. We analyzed data using a random-effects model. Heterogeneity was assessed using the I2 statistic.
Results
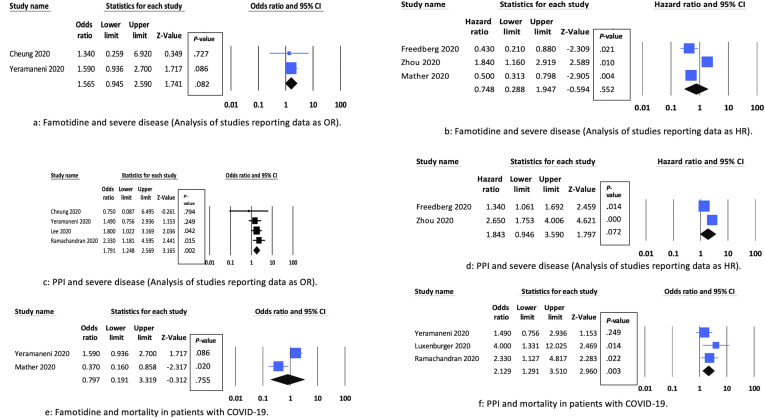
We included 9 observational studies with 21,285 patients.2, 3, 4, 5, 6, 7, 8, 9, 10 All studies were retrospective in design. We found no association between famotidine use and disease severity on unadjusted analysis (risk ratio, 1.02; 95% confidence interval [CI], 0.58–1.81; I2 = 86%). Adjusted analysis including studies3 , 4 reporting data as OR or HR showed similar results: 2 studies2 , 7 , 9, OR, 1.57 (95% CI, 0.95–2.59; I2 = 0%) (Figure 1 A); 3 studies, HR, 0.75 (95% CI, 0.29–1.95; I2 = 89%) (Figure 1 B). Sensitivity analysis excluding each study at a time showed substantial reduction in heterogeneity (I2 = 0%) on exclusion of Zhou et al9 but did not change on exclusion of either of the other 2 studies2 , 7 (Supplementary Table 2). We found no association between famotidine use and mortality (OR, 0.79; 95% CI, 0.19–3.32; I2 = 88%) (Figure 1 E) (2 studies4 , 7).
Figure 1.
Forest plots showing associations between PPI or famotidine use and severe disease and mortality. (A) Famotidine and severe disease: analysis of studies reporting data as OR. (B) Famotidine and severe disease: analysis of studies reporting data as HR. (C) PPI and severe disease: analysis of studies reporting data as OR. (D) PPI and severe disease: analysis of studies reporting data as HR. (E) Famotidine and mortality in patients with COVID-19. (F) PPI and mortality in patients with COVID-19.
We found no association between PPI use and disease severity on unadjusted analysis (risk ratio, 1.95; 95% CI, 0.94–4.07; I2 = 96%). Analysis of 4 studies3, 4, 5, 6 reporting data as adjusted OR showed that PPI use was associated with an increased risk of severe disease (OR, 1.79; 95% CI, 1.25–2.57; I2 = 0%) (Figure 1 C). Sensitivity analysis excluding each study at a time did not change the results or the heterogeneity (Supplementary Table 2). Subgroup analysis including 2 studies2 , 9 from Western countries showed similar results (OR, 1.86; 95% CI, 1.15–3.0; I2 = 0%). However, subgroup analysis including 2 studies from Eastern countries showed no significant effect (OR, 1.70; 95% CI, 0.98–2.94; I2 = 0%). Subgroup analyses based on method of adjustment of confounders also showed increased risk of severe disease with PPI use. An analysis of 2 studies reporting data as HR showed no significant difference (pooled HR, 1.84; 95% CI, 0.95–3.59; I2 = 87%) (Figure 1 D). PPI use was associated with increased risk of mortality (pooled OR, 2.12; 95% CI, 1.29–3.51; I2 = 16%) (Figure 1 F) (3 studies4 , 6 , 8).
Discussion
Some studies have suggested that famotidine improves outcomes in COVID-19 patients.2 , 7 The exact mechanism for this alleged benefit is unclear. However, famotidine might bind to and inhibit the SARS-CoV-2 protease that is necessary for breakdown of immature SARS-CoV-2 protein particles that contribute to the inflammatory response in COVID-19 patients.7
PPIs are prescribed for a variety of indications, including gastroesophageal reflux disease, risk reduction for gastric ulcer associated with nonsteroidal anti-inflammatory drug use, Helicobacter pylori infection, healing of erosive esophagitis, maintenance of healed erosive esophagitis, and Zollinger-Ellison syndrome. We found slight increases in risks of severe disease and mortality among PPI users. Of note, there was discrepancy in the disease severity analyses between studies reporting data as OR or HR, perhaps because studies reporting data as HR did not adjust data for some important variables that can affect outcomes in COVID-19 patients such as steroid or remdesivir use. In contrast, most studies reporting data as OR adjusted for these variables. There is no clear mechanism to explain why PPIs might be associated with worse outcomes in COVID-19 patients. However, suppression of gastric acid by PPIs increases intragastric pH and may result in impaired ability to destroy ingested pathogens.5
This meta-analysis has several limitations. All included studies were observational and retrospective in nature, with attendant risks of measured and unmeasured confounding. Although there was low heterogeneity in the analyses of PPI studies, the analysis of famotidine studies was limited by substantial to considerable heterogeneity. On sensitivity analysis, the heterogeneity was decreased by excluding 1 study (Zhou et al9) that considered both inpatient and outpatient exposure to famotidine compared with other studies that mainly considered inpatient exposure. Also, the median total dose of famotidine in the study by Zhou et al9 was much higher compared with other studies (Supplementary Table 1). H2-receptor antagonists other than famotidine have not been evaluated in COVID-19. Only 9 studies were included in this meta-analysis. However, only between 2 and 5 studies could be included in individual analyses. Therefore, analyses may not be adequately powered to make firm conclusions. The small number of studies with conflicting results included in the analysis of famotidine and severe disease raises the possibility of a Type II error responsible for the lack of significance. Data on dosing of PPIs or famotidine were not consistently reported. Some studies reported median cumulative dose; others did not report it at all. Some patients in these studies received additional medicines that could have affected outcomes such as steroids and remdesivir. However, such data were reported inconsistently. Some studies also had risks of confounding bias, immortal time bias (Supplementary Table 1), and reverse causality bias.11 These biases may be responsible for discrepancies in findings across the studies and heterogeneity in data.
Because of these limitations, definite conclusions cannot be made based on the results of this meta-analysis. Because of obvious practical concerns, randomized controlled trials may not be feasible to evaluate this issue. Future prospective, multicenter, observational studies, adjusting for all potential confounders and taking appropriate measures to avoid biases such as immortal time bias, are required to further evaluate this issue and make any firm conclusions. In accordance with recommendations from the Infectious Diseases Society of America,12 there is insufficient evidence to administer famotidine to a patient with COVID-19 outside of a randomized controlled trial. Similarly, there is no compelling evidence to withhold or withdraw PPI treatment from a patient with COVID-19 who has a valid indication for it.
Acknowledgments
CRediT Authorship Contributions
Faisal Kamal, MD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Methodology: Lead; Writing – original draft: Lead). Muhammad Ali Khan, MD (Data curation: Supporting; Formal analysis: Lead; Methodology: Lead; Supervision: Supporting; Writing – review & editing: Supporting). Sachit Sharma, MD (Methodology: Supporting; Writing – original draft: Lead; Writing – review & editing: Supporting). Zaid Imam, MD (Writing – review & editing: Equal). Colin W. Howden, MD (Conceptualization: Lead; Methodology: Supporting; Supervision: Lead; Writing – review & editing: Lead).
Footnotes
Conflicts of interest This author discloses the following: Colin W. Howden is a consultant for Phathom Pharmaceuticals, Ironwood, RedHill Biopharma, Alfasigma, and Clexio; speaker for RedHill Biopharma, Alnylam, and Alfasigma; and stockholder in Antibe Therapeutics. The remaining authors disclose no conflicts.
Funding None.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2021.02.028.
Supplementary Methods
Inclusion and Exclusion Criteria
We reviewed PubMed and MEDLINE, Embase, and Web of Science Core Collection from January 1, 2020 to December 28, 2020 to identify studies evaluating any association between famotidine or PPI use and outcomes in patients with COVID-19. We also reviewed the bibliographies of retrieved articles to identify any additional pertinent studies. We only included studies with hospitalized COVID-19 patients in our analysis and only assessed outcomes in patients with a diagnosis of COVID-19 who received a PPI or famotidine. We did not assess the association between PPI use and the odds of acquiring COVID-19 in the community. Our search yielded 2 studies1 , 2 that evaluated an association between PPI use and the odds of acquiring COVID-19; these were excluded from our analysis. We only included published studies and did not include any unpublished data. Our search yielded 4 studies3, 4, 5, 6 that were unpublished data from medRxiv; these were excluded from our analyses.
Statistical Analysis
We used the DerSimonian and Laird random-effects model to analyze the data. Heterogeneity was assessed using the I2 statistic. We initially performed an unadjusted analysis by pooling all studies together and reported data as pooled risk ratios with 95% CI. We used raw data from studies to calculate risk ratios. To analyze and report adjusted data and to explore heterogeneity, we separately pooled studies reporting data as adjusted HR or OR. To assess the robustness of our results and further investigate heterogeneity, we performed sensitivity analyses by excluding each study at a time (influential analysis) (Supplementary Table 2). When possible, we also performed subgroup analyses based on methods of adjustment for confounders (propensity score matching vs multivariable logistic regression) and region (Eastern vs Western countries).
Supplementary Table 1.
Study Characteristics
| Study, Year, Country | Study Design | No. of Patients | Dose of Famotidine or PPI | Exposure to PPI or Famotidine | Patients on Supplemental Oxygen | No. of Intubated Patients | No. of Deaths | No. of Patients Receiving Steroids or Remdesivir | No. of Patients With Severe Disease | Methods for Controlling Confounders | Confounders Adjusted | Indicators of Disease Severity | Immortal Time Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheung et al, 2020, Hong Kong | Retrospective, cohort | 952 | NA | Exposure to PPI or famotidine on day of admission | NA | NA | NA | NA | 51 | Multivariable logistic regression model | Age, sex, comorbidities (DM, HTN, ischemic heart disease, stroke, and atrial fibrillation), other medications | Presence of (1) critical complication (respiratory failure, septic shock, and/or multiple-organ | Unclear risk of immortal time bias. Exposure to PPI or famotidine was defined as on day of admission. It is not reported when the outcome assessment was started. |
| (ACEIs, ARBs, aspirin, statins, and prednisolone), and laboratory parameters | dysfunction), (2) ventilatory support (invasive or noninvasive), (3) ICU admission, and/or (4) death | ||||||||||||
| (leukocyte, platelet, C-reactive protein, urea, creatinine, sodium, potassium, bilirubin, alkaline phosphatase, alanine aminotransferase, albumin, globulin, and lactate dehydrogenase | |||||||||||||
| Freedberg et al, 2020, USA | Retrospective, cohort | 1620 | Total median dose of famotidine: 136 mg (range, 63–233), over median 5.8 days. 28% of all famotidine doses were intravenous; 47% were 20 mg, 35% were 40 mg, and 17% | Exposure to famotidine within 24 h of admission | 1217 | 142 | 238 | NA | 340 | Cox proportional hazard model and PS matching | DM, HTN, coronary artery disease, heart failure, end-stage renal disease, CKD, chronic pulmonary disorders, obesity (classified based on BMI), and age (classified as <50 y, 50–65 y, and | Composite of death or endotracheal intubation | Immortal time bias not present. Exposure to famotidine was defined as within 24 h of admission and outcome assessment started from day 2. |
| were 10 mg. | >65 y). To assess severity of COVID-19, the first recorded form of supplemental oxygen after triage was captured and classified as room air, nasal cannula oxygen, or non-rebreather/similar. | ||||||||||||
| Yeramaneni et al, 2020, USA | Retrospective, cohort | 7158 | Median cumulative dose of 160 mg (range, 80– | Exposure to famotidine within 24 h of admission | 2922 | 220 | 687 | Remdisivir: 32 | 687 | Coarsened exact matching and multivariable logistic regression model | Age, sex, race, ethnicity, BMI, coronary artery | Mortality | Immortal time bias not present. Exposure to famotidine within 24 h of admission and deaths or intubations within 48 h of admission were excluded. |
| 300) over median of 6 days | Steroids: 1177 | disease, DM, renal disease, COPD, congestive heart failure, HTN, World Health Organization severity index, smoking status, in hospital medications such as azithromycin, ACEIs, ARBs, antivirals, remdisivir, tocilizumab, steroids, and PPI use. | |||||||||||
| Mather et al, 2020, USA | Retrospective, cohort | 878 | 83% received famotidine orally and 17% received it intravenously. Dose of oral famotidine was 20 mg/d in 95.2% of cases and 40 mg/d in 4.8% of cases. Dose of intravenous famotidine was | Exposure to famotidine within ±7 days of COVID-19 screening and/or hospital admission | NA | NA | 191 | Remdisivir: 27 | 430 | Multivariable logistic regression model and PS matching | Age, sex, race, smoking status, BMI, HTN, DM, obesity (BMI 30 kg/m2), coronary artery disease, heart failure, atrial fibrillation, chronic obstructive pulmonary disease, CKD, prior history of malignancy, use of hydroxychloroquine, azithromycin, | Mortality, requirement for mechanical ventilation, composite of death, or requirement for mechanical ventilation | High risk of immortal time bias. Exposure to famotidine was defined as within ±7 days of COVID-19 screening and/or hospital admission and assessment of outcome was started after hospitalization. |
| 20 mg. Median total famotidine dose was 80 mg | Steroids: 377 | remdesivir, and corticosteroids | |||||||||||
| (range, 40–160) over a median of 4 days (range, 2–8). | |||||||||||||
| Luxenburger et al, 2020, Germany | Retrospective, cohort | 152 | PPI: 20 mg daily (4.8%), 40 mg daily (87%), 80 mg daily (8%) | Patients already on PPI as outpatient | NA | NA | 17 | NA | 45 | NA | NA | ARDS, mortality | Could not be assessed because adequate information was not available regarding when the outcome assessment was started. |
| Lee et al, 2019, Korea | Retrospective, cohort | 4785 | NA | Current or past use of PPI as outpatient | NA | NA | NA | Steroids: 224 | 81 | PS matching | Age; sex; region of residence (urban or rural); history of DM, cardiovascular disease, cerebrovascular disease, COPD, HTN, or CKD; Charlson comorbidity index (0, 1, or ≥2); and current use of systemic steroid, metformin, or aspirin. | Composite endpoint 1 (requirement for oxygen therapy, ICU admission, invasive ventilation, or death). | Low risk of immortal time bias |
| Composite endpoint 2 (severe clinical outcomes of COVID-19, ICU admission, invasive ventilation, or death) | |||||||||||||
| Ramachandran et al, 2020, USA | Retrospective, cohort | 295 | NA | PPI exposure before admission | NA | NA | 56 | NA | 129 | Multivariable logistic regression | NA | Mortality, ARDS | Low risk of immortal time bias. PPI exposure was defined as PPI use before admission and outcome assessment started after hospitalization |
| Zhou et al, 2020, Hong Kong | Retrospective, cohort | 4445 | Median cumulative PPI dose: 1080 mg (range, 600–2430) | Exposure to famotidine and PPI as inpatient and outpatient | NA | NA | NA | Steroids: 632 | 212 | PS matching | Age, cardiovascular disease, renal disease, stroke, Kaletra, diuretics for heart failure, other antihypertensives, | Need for ICU admission or intubation, or death | High risk of immortal time bias. Both inpatient and outpatient exposure to famotidine was considered and outcome assessment started after admission. Only exposure to famotidine after ICU admission was not considered. |
| Median cumulative famotidine dose: 1040 mg (range, 480–2440) | Remdesivir: NA | PPI/famotidine, neutrophils, lymphocytes, platelets, urea, | |||||||||||
| creatinine, albumin and glucose | |||||||||||||
| Argenziano et al, 2020 | Retrospective, case series | 1000 | NA | NA | NA | 233 | 211 | Steroids: 178 | 233 | NA | NA | ICU admission | Could not be assessed |
| Remdesivir: 18 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; ARDS, acute respiratory distress syndrome; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; ICU, intensive care unit; NA, not available; PS, propensity score.
Supplementary Table 2.
Sensitivity Analysis by Excluding Each Study One at a Time
| Study Excluded | Pooled HR or OR (95% CI) | Heterogeneity (I2) (%) |
|---|---|---|
| Famotidine and severe disease: analysis of studies reporting data as HR | ||
| Freedberg 2020 | 0.96 (0.27–3.44) | 93 |
| Mather 2020 | 0.91 (0.22–3.79) | 91 |
| Zhou 2020 | 0.48 (0.32–0.71) | 0 |
| PPI and severe disease: analysis of studies reporting data as OR | ||
| Cheung 2020 | 1.84 (1.27–2.64) | 0 |
| Lee 2020 | 1.79 (1.12–2.85) | 0 |
| Ramachandran 2020 | 1.62 (1.05–2.47) | 0 |
| Yeramaneni 2020 | 1.93 (1.26–2.95) | 0 |
References
- 1.Janowitz T., et al. Gut. 2020;69:1592–1597. doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedberg, et al. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung K.S., et al. Gastroenterology. 2020;160:1898–1899. doi: 10.1053/j.gastro.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeramaneni S., et al. Gastroenterology. 2020;160:919–921.e3. doi: 10.1053/j.gastro.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S.W., et al. Gut. 2020;70:76–84. [Google Scholar]
- 6.Ramachandran P., et al. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000002013. [DOI] [Google Scholar]
- 7.Mather J.F., et al. Am J Gastroenterol. 2020;115:1617–1623. doi: 10.14309/ajg.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luxenburger H., et al. J Intern Med. 2021;289:121–124. doi: 10.1111/joim.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J., et al. Gut. 2020 [Google Scholar]
- 10.Argenziano M.G., et al. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etminan M., et al. Gastroenterology. 2020;160:1443–1446. doi: 10.1053/j.gastro.2020.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.idsociety.org/practice-guideline/covid–19-guideline-treatment-and-management/
References
- 1.Almario C.V., et al. Am J Gastroenterol. 2020;115:1707–1715. doi: 10.14309/ajg.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarlow B., et al. Am J Gastroenterol. 2020;115:1920–1921. doi: 10.14309/ajg.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKeigue P.M., et al. medRxiv. 2020 [Google Scholar]
- 4.Ullah A., et al. medRxiv. 2020 [Google Scholar]
- 5.Yan S., et al. medRxiv. 2020 [Google Scholar]
- 6.Jimenez L., et al. medRxiv. 2020 [Google Scholar]