Abstract
Objective
Elevated levels of pro-inflammatory cytokines are observed in severe COVID-19 infections, and cytokine storm is associated with disease severity. Tocilizumab, an interleukin-6 receptor antagonist, is used to treat chimeric antigen receptor T cell-induced cytokine release syndrome and may attenuate the dysregulated immune response in COVID-19. We compared outcomes among tocilizumab-treated and non-tocilizumab-treated critically ill COVID-19 patients.
Design, setting, and participants
This was a retrospective observational study conducted at a tertiary referral center investigating all patients admitted to the intensive care unit for COVID-19 who had a disposition from the hospital because of death or hospital discharge between March 1 and May 18, 2020 (n = 96). The percentages of death and secondary infections were compared between patients treated with tocilizumab (n = 55) and those who were not (n = 41).
Measurements and main results
More tocilizumab-treated patients required mechanical ventilation (44/55, 80%) compared to non-treated patients (15/41, 37%; P < 0.001). Of 55 patients treated with tocilizumab, 32 (58%) were on mechanical ventilation at the time of administration, and 12 (22%) progressed to mechanical ventilation after treatment. Of patients treated with tocilizumab requiring mechanical ventilation, 30/44 (68%) were intubated within 1 day of administration. Fewer deaths were observed among tocilizumab-treated patients, both in the overall population (15% vs 37%; P = 0.02) and among the subgroup of patients requiring mechanical ventilation (14% vs 60%; P = 0.001). Secondary infections were not different between the 2 groups (tocilizumab: 31%, non-tocilizumab: 17%; P = 0.16) and were predominantly related to invasive devices, such as urinary and central venous catheters.
Conclusions
Tocilizumab treatment was associated with fewer deaths compared to non-treatment despite predominantly being used in patients with more advanced respiratory disease.
Keywords: COVID-19, Tocilizumab, SARS-CoV2, Acute respiratory distress syndrome, Cytokine release syndrome, Pneumonia
Introduction
Since the advent of Coronavirus Disease 2019 (COVID-19) in Wuhan, China in December 2019 (Zhou et al., 2020), the virus has spread to virtually every country in the world and now accounts for more than 29 million cases worldwide with 937 519 deaths https://www.worldometers.info/coronavirus/https://www.worldometers.info/coronavirus/ (accessed September 15). Despite efforts to develop vaccines and generate data from controlled clinical trials, the morbidity and mortality imposed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia require the implementation of therapies that, based on logical deduction from known mechanisms of action and case reports, could be useful.
Early reports from patients with SARS-CoV-2 pneumonia identified interleukin 6 (IL-6) as a potential pathogenic factor in the initiation of acute respiratory distress syndrome (ARDS) (Parsons et al., 2005). IL-6 is a pleiotropic cytokine that functions as a mediator of both innate and adaptive immune functions. IL-6 has diverse immune and biologic actions, including direction of immune cell differentiation, sentinel responses to invading pathogens, and ischemic injury. IL-6 is also critical for plasma cell growth and immunoglobulin production. Excessive and unregulated IL-6 transcription is commonly seen in patients with autoimmune or inflammatory disorders. Emerging data from patients with SARS-CoV-2 infection suggests that IL-6 transcription is initiated and sustained after respiratory epithelium is infected. The virus has a proclivity for activation of alveolar and circulating macrophages resulting in copious and sustained IL-6 production manifesting as the cytokine storm, endothelial cell damage, capillary leak and the clinical and pathological features of ARDS (Roschewski et al., 2020). This data suggests that inhibiting IL-6 production and/or blocking receptor binding could be an important therapeutic option for limiting morbidity and mortality.
Tocilizumab (anti-interleukin-6 receptor [anti-IL-6R] monoclonal antibody) is of interest due to its ability to reduce ARDS after chimeric antigen receptor (CAR) T cell therapy. Tocilizumab is a recombinant immunoglobulin(Ig)G1 humanized monoclonal antibody that inhibits the binding of IL-6 to the soluble and membrane-bound forms of the IL-6R. Tocilizumab is approved by the USA Food and Drug Administration for the treatment of severe rheumatoid arthritis, systemic juvenile idiopathic arthritis, giant cell arteritis, and more recently, for cytokine release syndrome occurring after chimeric antigen receptor (CAR) T-cell therapy (Le et al., 2018).
We recently reported on our experience using tocilizumab to treat SARS-CoV-2 pneumonia, where we observed favorable responses in temperature and oxygen usage and vasopressor requirements after treatment (Jordan et al., 2020). Most of the tocilizumab-treated patients in this report were critically ill, requiring mechanical ventilation. The association of tocilizumab with reduced mortality in critically ill patients has also been reported in other observational studies (Gupta et al., 2020, Somers et al., 2020). Further, data from the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial reported that the most significant benefit of dexamethasone on mortality was seen among patients receiving mechanical ventilation, suggesting that immunomodulation may be more effective when given to patients with more advanced disease (RECOVERY Collaborative Group et al., 2020). Given these observations, we have expanded on our initial report with a larger cohort of patients treated in the intensive care unit (ICU) with tocilizumab and compare outcomes to ICU patients with SARS-CoV-2 pneumonia patients not treated with tocilizumab.
Materials and methods
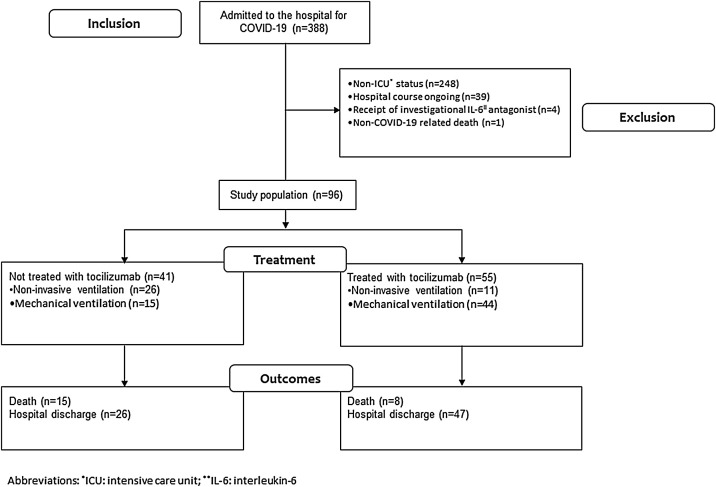
This observational study was supported by Cedars-Sinai Medical Center and approved by the Institutional Review Board of Cedars-Sinai Medical Center (STUDY00000678; Tocilizumab Effectiveness for Treatment of COVID-19). The flowchart of study inclusion and exclusion is provided in Figure 1 . We retrospectively analyzed the medical records of all patients admitted to Cedars-Sinai Medical Center between March 1 and May 18, 2020, for a COVID-19-related-admission with diagnosis confirmed by a positive nasopharyngeal real-time polymerase chain reaction test for SARS-CoV2 (n = 388). We were specifically interested in investigating mortality outcomes associated with tocilizumab treatment in the highest-risk population and therefore restricted the study population to all patients admitted to the ICU (n = 140). To allow for comparison of outcomes between study groups, we only included patients who had a disposition from the hospital by the end of the study period on May 18, 2020, either because of death or hospital discharge (n = 101). Of these, the following were excluded: 4 patients because of treatment with an investigational IL-6 antagonist, clazakizumab, and 1 patient for a non-COVID related death. The final study population consisted of 96 patients; 55 were treated with tocilizumab, 41 were not.
Figure 1.
Study flowchart.
Patient demographics and clinical observations were extracted from the electronic health record (Epic, version May 2019). Co-morbidities were defined using diagnoses present on admission or historical diagnoses identified from hospital billing records. Obesity was defined as a body mass index (BMI) ≥30 kg/m2 based on height and weight measurements recorded at the time of hospital admission.
Treatment protocol
Per institutional protocol, patients diagnosed with COVID-19 were assessed with a COVID-19 laboratory panel which includes a serum IL-6 level, C-reactive protein (CRP), ferritin, d-dimer, lactate dehydrogenase, and troponin I. Patients were approved for treatment with tocilizumab according to the following criteria: signs of respiratory compromise consisting of tachypnea, dyspnea OR peripheral capillary oxygen saturation (SpO2) < 90% on at least 4 L of oxygen OR increasing oxygen requirements over 24 h, PLUS 2 or more of the following predictors for severe disease:
-
•
IL-6 > 10 pg/mL
-
•
CRP > 35 mg/L
-
•
Ferritin > 500 ng/mL
-
•
D-dimer > 1 mcg/L
-
•
Neutrophil-Lymphocyte Ratio > 4
-
•
Lactate dehydrogenase > 200 U/L
-
•
Increased troponin in a patient without known cardiac disease
The treatment protocol consists of a single 400 mg dose of tocilizumab given by intravenous infusion. Antimicrobial prophylaxis is not routinely given after tocilizumab administration. Follow-up measurements of inflammatory markers, including interleukin-6 levels, C-reactive protein, ferritin, d-dimer, and lactate dehydrogenase are periodically done post-administration at the discretion of the treating physician.
Statistical analysis
Baseline characteristics between tocilizumab-treated and non-tocilizumab treated patients were compared using a t-test or Mann–Whitney U test for numerical variables, as appropriate, and the Fisher’s exact test for categorical variables. Data are presented as frequencies (percentage, %) for categorical variables and mean (±standard deviation, SD) or median (range) for numerical variables. The primary outcome of interest was the proportion of patients who died, with statistical comparison performed using the Fisher’s exact test. Secondary outcomes included the number of culture-confirmed bacterial or fungal infections, which were extracted by manual chart review. Ventilator-free days at 28 days were calculated as previously described (Yehya et al., 2019). Briefly, this metric was defined as the number of ventilator-free hospital days within 28 days of initiation of mechanical ventilation. Ventilator-free days were considered 0 if the patient died or was ventilated for longer than 28 days. Complications were identified from diagnoses codes extracted from hospital billing records reflecting acute diagnoses or diagnoses occurring after ICU admission.
Analyses were performed on the overall population in addition to a subgroup of patients considered to have more severe disease, defined as a requirement for mechanical ventilation at any time during the hospital stay (tocilizumab, n = 44; non-tocilizumab, n = 15). All calculations were performed using IBM SPSS Statistics for Windows v. 24.0 (IBM Corp.; Armonk, NY).
Results
Table 1 compares the baseline characteristics of tocilizumab-treated and non-tocilizumab treated patients. The median number of hospital days prior to tocilizumab treatment was 2 days (range 0−16 days). A higher proportion of tocilizumab-treated patients were male and obese (body mass index ≥30 kg/m2); there were no differences in the distribution of age, race/ethnicity, or other co-morbidities between the 2 groups. The distribution of sequential organ failure assessment scores (a mortality prediction score) at ICU admission was not different between patients in the non-tocilizumab treated and tocilizumab-treated groups. However, the vast majority of patients in the tocilizumab group required mechanical ventilation (80%) as opposed to only 37% of patients in the non-tocilizumab group (P < 0.001). Initial laboratory values were similar in the 2 groups aside from a trend toward higher alanine aminotransferase and lactate dehydrogenase levels in the tocilizumab-treated group. Notably, serum IL-6 and CRP levels were markedly elevated but did not differ between the 2 groups. A higher proportion of tocilizumab-treated patients received additional medications investigated as therapies for SARS-CoV-2, including hydroxychloroquine, azithromycin, and remdesivir. Only 1 patient in each group was treated with dexamethasone.
Table 1.
Comparison of baseline characteristics between non-tocilizumab treated and tocilizumab-treated patients.
| Discharged ICU Patient Population (N = 96*) Data through 5/18/2020 |
|||
|---|---|---|---|
| Non-TOCI | TOCI | P-value | |
| (N = 41) | (N = 55) | ||
| Outcomes | |||
| Invasive ventilation | 15(36.6) | 44 (80.0) | <0.001 |
| Death | 15 (36.6) | 8 (14.5) | 0.02 |
| Overall Vent-free days | 4.33 (±3.735) | 9.68 (7.136) | 0.008 |
| Demographics | |||
| Gender | |||
| Female | 21 (51.2) | 11 (20.0) | 0.002 |
| Male | 20 (48.8) | 44 (80.0) | |
| Age | 66.59 (±19.10) | 63.22 (±16.29) | 0.36 |
| Race | |||
| White | 20 (48.8) | 21 (38.2) | 0.31 |
| Asian | 4 (9.8) | 2 (3.6) | |
| Black or African American | 8 (19.5) | 12 (21.8) | |
| Hispanic | 8 (19.5) | 14 (25.5) | |
| Other/Unknown | 1 (2.4) | 6(10.9) | |
| Comorbidities | |||
| Hypertension | 29 (70.7) | 33 (60.0) | 0.29 |
| Cardiac Arrhythmias | 16 (39.0) | 17 (30.9) | 0.52 |
| CHF | 12 (29.3) | 8 (14.5) | 0.13 |
| Renal Failure | 11 (26.8) | 15 (27.3) | 1.00 |
| Obesity (BMI > 30) | 11 (26.8) | 27 (49.1) | 0.04 |
| Diabetes | 20 (48.8) | 27 (49.1) | 1.00 |
| Initial Lab Values | |||
| ALT | 29 (10−160) | 37 (10−3460) | 0.07 |
| AST | 41 (16−291) | 55 (16−8000) | 0.27 |
| CRP | 122.3 (2.40−327.20) | 122.5 (11.10−343.10) | 0.94 |
| D-DIMER | 2.28 (.42−14.28) | 1.12 (.37−20.00) | 0.11 |
| IL-6 | 42.70 (3.20−1682.80) | 28.5 (3.60−6604.50) | 0.98 |
| LDH | 444 (160−1193) | 497.5 (210−3325) | 0.01 |
| Lymphocytes | 0.875 (.06−2.89) | .44 (.00−1.63) | 0.16 |
| Neutrophils | 7.12 (2.14−20.51) | 5.84 (2.56–22.72) | 0.50 |
| Troponin | 0.02 (.01−36.69) | 0.02 (.01−25.33) | 0.97 |
| WBC | 8.560 (3.27−25.96) | 7.45 (3.34−26.42) | 0.47 |
| SOFA Score | 5 (0−13) | 4 (0−13) | 0.66 |
| Hospital days prior to tocilizumab treatment, median (range) | NA | 2 (0−16) | NA |
| Concomitant Medications | |||
| Hydroxychloroquine | 20 (48.8) | 43 (78.2) | 0.004 |
| Azithromycin | 22 (53.7) | 47 (85.5) | 0.001 |
| Remdesivir | 1 (2.4) | 8 (14.5) | 0.07 |
| Dexamethasone | 1 (2.4) | 1 (1.8) | 1.00 |
Data are n (%), mean (±SD), and median (range). P-values were calculated using Mann–Whitney U test, t-test, chi–square test or Fisher’s exact test, as appropriate.
Given that tocilizumab-treated patients had more severe respiratory disease, as indicated by a significantly higher percentage of patients requiring mechanical ventilation, baseline characteristics and initial laboratory values among the subgroup of patients who required mechanical ventilation were also compared in Table 2 . The median number of hospital days prior to tocilizumab treatment was 2 days (range 0−16 days). Of 55 patients treated with tocilizumab, 32 (58%) were on mechanical ventilation at the time of administration, and 12 (22%) progressed to mechanical ventilation after treatment (44 ventilated patients total). The distribution of gender, race/ethnicity, and co-morbidities among patients treated with mechanical ventilation was similar to that observed in the overall population. The initial laboratory values in this subgroup were similar to the overall population.
Table 2.
Comparison of baseline characteristics between non-tocilizumab treated and tocilizumab-treated patients who required mechanical ventilation.
| Discharged Ventilated Patient Population (N = 59*) Data through 5/18/2020 |
|||
|---|---|---|---|
| Non-TOCI | TOCI | P-value | |
| (N = 15) | (N = 44) | ||
| Outcomes | |||
| Death | 9 (60.0) | 6 (13.6) | 0.001 |
| Overall Vent-free days | 4.33 (±3.735) | 9.68 (7.13) | 0.008 |
| Demographics | |||
| Gender | |||
| Female | 9 (60.0) | 8 (18.2) | 0.006 |
| Male | 6 (40.0) | 36 (81.8) | |
| Age | 73.47 (±14.0) | 63.61 (±14.83) | 0.03 |
| Race | |||
| White | 5 (33.3) | 16 (36.4) | 0.18 |
| Asian | 2 (13.3) | 0 (0.0) | |
| Black or African American | 3 (20.0) | 12 (27.3) | |
| Hispanic | 4 (26.7) | 12 (27.3) | |
| Other/Unknown | 1 (6.7) | 4 (9.1) | |
| Comorbidities | |||
| Hypertension | 11 (73.3) | 26 (59.1) | 0.37 |
| Cardiac Arrhythmias | 7 (46.7) | 16 (36.4) | 0.55 |
| CHF | 5 (33.3) | 8 (18.2) | 0.28 |
| Renal Failure | 5 (33.3) | 14 (31.8) | 1.00 |
| Obesity (BMI > 30) | 3 (20.0) | 23 (52.3) | 0.04 |
| Diabetes | 8 (53.3) | 21 (47.7) | 0.77 |
| Initial Lab Values | |||
| ALT | 34 (13−160) | 36.5 (10−3460) | 0.84 |
| AST | 64 (26−291) | 52.5 (16−8000) | 0.63 |
| CRP | 151.05 (21.3−283.2) | 125.15 (11.10−343.10) | 0.78 |
| D-DIMER | 2.335 (.42−14.28) | 1.48 (.38−20.00) | 0.34 |
| IL-6 | 67.7 (4.60−955) | 28.3 (3.60−6604.50) | 0.14 |
| LDH | 483 (199−1110) | 500.0 (210−3325) | 0.37 |
| Lymphocytes | 0.50 (.06−2.89) | .53 (.00−1.63) | 0.58 |
| Neutrophils | 8.3 (2.99–16.05) | 5.78 (2.91−22.72) | 0.12 |
| Troponin | 0.08 (.01−36.69) | .02 (.01−25.33) | 0.07 |
| WBC | 9.73 (4.27−17.98) | 7.44 (4.16−26.42) | 0.11 |
| SOFA Score | 6 (3−13) | 5 (0−13) | 0.10 |
| Hospital days prior to tocilizumab treatment, median (range) | NA | 2 (0−16) | NA |
| Concomitant Medications | |||
| Hydroxychloroquine | 5 (33.3) | 33 (75.0) | 0.006 |
| Azithromycin | 7 (46.7) | 37 (84.1) | 0.01 |
| Remdesivir | 1 (6.7) | 5 (11.4) | 1.00 |
| Dexamethasone | 0 (0.0) | 1 (2.3) | 1.00 |
Data are n (%), mean (±SD), and median (range). P-values were calculated using Mann–Whitney U test, t-test, chi–square test or Fisher’s exact test, as appropriate.
Outcomes
Fewer deaths were observed in the tocilizumab group. Mortality in the non-tocilizumab group was 15/41 (37%) compared to 8/55 (15%) in the tocilizumab-treated patients (P = 0.02). No patients treated with remdesivir died. After excluding remdesivir-treated patients from the analysis, the proportion of deaths in the non-tocilizumab group remained higher than the tocilizumab group (38% vs 17%; P = 0.05). Among patients requiring mechanical ventilation, the mortality difference between the two groups was more pronounced. Of non-tocilizumab treated patients maintained on mechanical ventilation 9/15 (60%) died compared to 6/44 (14%) tocilizumab-treated patients (P = 0.001). Among the 6 deaths in the subgroup of tocilizumab recipients who required mechanical ventilation, 2 patients were treated with tocilizumab before progressing to mechanical ventilation, and 4 patients were treated after mechanical ventilation was initiated.
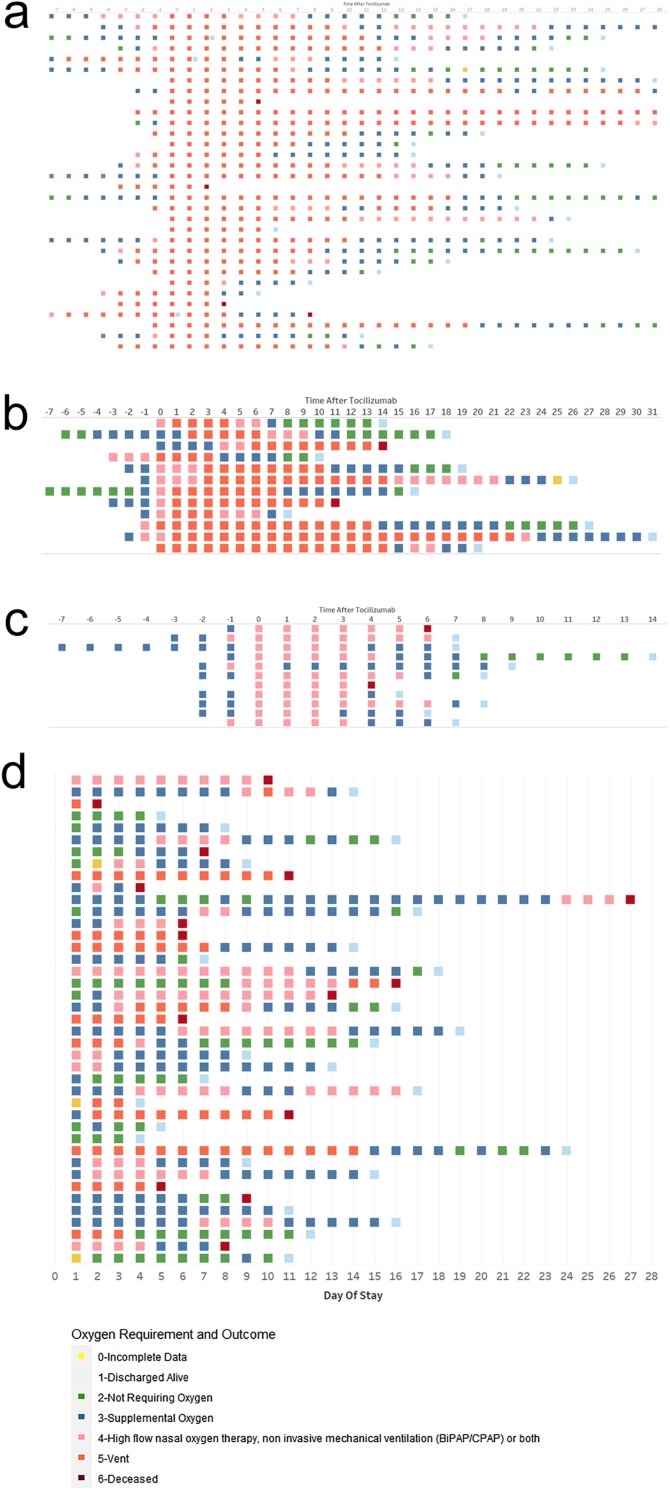
Figure 2 illustrates the hospital course and oxygen support requirements for each individual. Of the 32 patients on mechanical ventilation at the time of tocilizumab treatment, for 22, mechanical ventilation was initiated within one day of administration of tocilizumab ( Figure 2a). Of the 4 patients that died, 2 died shortly after treatment (2 and 3 days, respectively). A total of 22 patients were discharged alive at a median of 17.5 days after treatment (range 5–28 days). At 28 days after treatment, 6 patients remained hospitalized; of these, 2 patients were free of supplemental oxygen, and only 1 remained on mechanical ventilation.
Figure 2.
Individual patient hospital course among patients (a) treated with tocilizumab after mechanical ventilation (n = 32); (b) treated with tocilizumab before mechanical ventilation (n = 12); (c) treated with tocilizumab not requiring mechanical ventilation (n = 11); and (d) patients not treated with tocilizumab (n = 41).
A total of 12 patients received tocilizumab before progressing to mechanical ventilation (Figure 2b), with a median number of days in the ICU before tocilizumab administration of 1.5 days (range 0–7 days). Most patients (8/12; 66.7%) were intubated immediately following tocilizumab treatment on the same or next day after administration. Two patients died, one at 11 days after treatment, and one at 14 days; ten patients recovered and were discharged from the hospital.
Figure 2c shows the hospital course of 11 patients who received tocilizumab and did not require mechanical ventilation through the course of hospitalization. All 11 patients required oxygen by high-flow nasal cannula at the time of treatment. Of these, 2 patients died at 4 and 6 days after tocilizumab; the remaining 9 patients recovered and were discharged from the hospital.
Figure 2d shows the hospital course of the 41 patients not treated with tocilizumab. Fifteen patients died at a median of 8 days after ICU admission (range 2–27 days). Among the 26 survivors, the median number of hospital days after ICU admission was 12.5 days (range 4–24 days).
Infectious complications
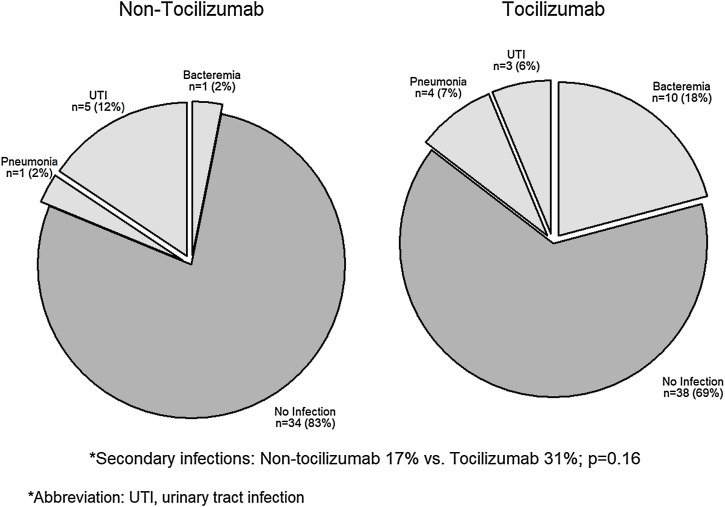
Figure 3 compares the frequency of secondary infectious complications between both groups. There were 7 cases of secondary infections in the non-tocilizumab treated group (17%) compared to 17 cases in the tocilizumab group (31%; P = 0.16). Infections in both groups were predominantly nosocomial and related to invasive devices, including catheter-associated urinary tract infections, ventilator-associated pneumonia, and bacteremia secondary to central venous catheter infections.
Figure 3.
Comparison of the frequency of secondary infections among non-tocilizumab treated and tocilizumab-treated patients.
Discussion
In this paper, we compared mortality outcomes following ICU admission between patients who received tocilizumab for COVID-19 and those who did not. Although tocilizumab-treated recipients had more severe respiratory disease compared to non-tocilizumab treated patients, fewer deaths were observed among patients treated with tocilizumab. There were numerically more secondary infections in the tocilizumab group, mostly related to nosocomial infections from invasive devices or catheters, but no statistical difference compared to untreated patients.
Early observations of patients with COVID-19 were that severe infections were associated with markedly elevated levels of pro-inflammatory cytokines and that cytokine storm was associated with disease severity (Huang et al., 2020). This phenomenon was similarly observed in prior studies involving patients infected with SARS and MERS-CoV (Mahallawi et al., 2018, Wong et al., 2004). Given that dysregulated inflammation is implicated as one of the primary mechanisms that drive the development of ARDS (Imai et al., 2003, Ranieri et al., 2000), several studies have now investigated the use of immunomodulatory agents as a means of mitigating the effects of cytokine storm associated with SARS-CoV-2 (Biran et al., 2020, Copaescu et al., 2020, Guaraldi et al., 2020, Price et al., 2020, Somers et al., 2020, Zhao et al., 2020). Inhibition of the IL-6 pathway is an attractive target, particularly because tocilizumab, a monoclonal antibody directed against the IL-6 receptor α, is known to effectively control cytokine storm associated with CAR T-cell therapy (Le et al., 2018).
This study is a follow-up to a recent report of our initial experience using tocilizumab in 27 patients with COVID-19, 21 of whom required mechanical ventilation at the time of administration (Jordan et al., 2020). Of the 21 mechanically ventilated patients, 18 met the Berlin criteria for ARDS with PaO2/FiO2 ≤ 300 mm Hg. Oxygen and vasopressor requirements diminished within 1 week after treatment, and 15/21 patients were extubated at a median of 8 days after tocilizumab (IQR: 4–10 days). One additional patient was treated with tocilizumab before mechanical ventilation and required intubation 4 days after tocilizumab administration. However, this patient recovered and was extubated 7 days later. Only 2 patients had died at the time of publication.
Based on our favorable early experience, tocilizumab became the drug of choice at our institution for patients who were rapidly deteriorating. This utilization is reflected in the current study, where 80% of tocilizumab-treated patients required mechanical ventilation and 30/44 (68%) of ventilated patients were intubated within 1 day of tocilizumab treatment. Despite having more severe respiratory disease compared to non-tocilizumab treated patients, we observed a significant mortality difference favoring tocilizumab-treated patients. This finding is consistent with other recent observational studies that have similarly reported a lower risk of death associated with tocilizumab treatment (Biran et al., 2020, Guaraldi et al., 2020, Price et al., 2020, Somers et al., 2020).
A series of randomized controlled trials comparing tocilizumab treatment to placebo were recently published (Hermine et al., 2020, Salvarani et al., 2020, Stone et al., 2020). All 3 trials evaluated the use of tocilizumab at earlier stages of illness and were designed to test whether treatment can prevent disease progression. Although the primary endpoints in these studies were not met, fewer tocilizumab-treated patients needed noninvasive or mechanical ventilation or died by day 14 in 1 of the trials (Hermine et al., 2020). Press releases from 2 other randomized controlled trials reported that IL-6 blockade did not improve clinical status or mortality in patients with COVID-19; however, a third randomized controlled trial reported that non-ventilated patients treated with tocilizumab were less likely to need mechanical ventilation compared to placebo [Anon, 2020, Anon, 2021a, Anon, 2021b. The benefit of IL-6 blockade may be limited to specific subgroups, perhaps those with more advanced disease, which will require further study. In the phase III trial of sarilumab, there was a positive signal among critical patients who were mechanically ventilated [https://www.prnewswire.com/news-releases/regeneron-and-sanofi-provide-update-on-kevzara-sarilumab-phase-3-us-trial-in-covid-19-patients-301087849.html (July 2)]. This observation is consistent with findings from our study and others (Biran et al., 2020, Somers et al., 2020). Similar observations were found in the RECOVERY trial evaluating the use of dexamethasone in patients hospitalized with COVID-19, where treatment with dexamethasone resulted in lower mortality among patients receiving oxygen therapy, but not among patients who required no respiratory support (RECOVERY Collaborative Group et al., 2020). It should be noted that results from a sub-study of RECOVERY, comparing the additive effects of treatment with tocilizumab vs control on top of the original treatment assignment, have not yet been released.
Limitations of this study stem from its retrospective, observational design. Baseline characteristics in the tocilizumab and non-tocilizumab groups were not balanced, which was a consequence of pre-specified criteria established at our institution for patients to receive treatment with tocilizumab. These criteria were implemented to ration our supply of tocilizumab, which was in a nationwide shortage at the beginning of the COVID-19 pandemic, and were meant to select patients with severe respiratory disease and evidence of a pro-inflammatory state. Given this, tocilizumab-treated patients in this study generally had more advanced respiratory disease than untreated patients. We were unable to statistically adjust for covariate imbalance, given that there were only 23 deaths in the study population as a whole. Additionally, other investigational therapies for COVID-19 were not restricted. However, these therapies mostly consisted of hydroxychloroquine and azithromycin, both of which are now generally considered ineffective for COVID-19 (Cavalcanti et al., 2020). Relatively few patients in this study received concomitant treatment with remdesivir and/or dexamethasone.
In summary, treatment with tocilizumab in critically ill patients with COVID-19 was associated with lower mortality than the standard of care treatment, without a significant increase in serious secondary infection complications. While we await further data from randomized controlled trials, this study adds to a growing body of literature suggesting that IL-6 blockade can improve survival in patients with severe COVID-19.
Conclusions
In this study, there were fewer deaths among critically ill patients with COVID-19 treated with tocilizumab, suggesting that IL-6 blockade could be an important therapeutic option for limiting morbidity and mortality.
Conflicts of interest
Dr. Edmund Huang and Dr. Stanley Jordan have received research grant funding from Vitaeris. Dr. Stanley Jordan has received consulting fees from Vitaeris.
Funding source
All authors receive salary support from Cedars-Sinai Medical Center.
Ethical approval
This observational study was supported by Cedars-Sinai Medical Center and approved by the Institutional Review Board of Cedars-Sinai Medical Center (STUDY00000678; Tocilizumab Effectiveness for Treatment of COVID-19).
Acknowledgements
Edmund Huang and Sharon Isonaka contributed equally to this work.
We remember all those who have suffered from COVID-19. Our thoughts and prayers are with them and their families.
References
- https://www.clinicaltrialsarena.com/news/roche-actemra-covid-data/ (August 7 RoO, 2020).
- https://www.prnewswire.com/news-releases/regeneron-and-sanofi-provide-update-on-kevzara-sarilumab-phase-3-us-trial-in-covid-19-patients-301087849.html (July 2 Ro).
- https://www.roche.com/media/releases/med-cor-2020-09-18.htm [Accessed 23 September].
- https://www.worldometers.info/coronavirus/ [Accessed 15 September].
- Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Coalition Covid-19 Brazil II. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146(3) doi: 10.1016/j.jaci.2020.07.001. 518-534 e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Parodo J., Kajikawa O., de Perrot M., Fischer S., Edwards V. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289(16):2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- Jordan S.C., Zakowski P., Tran H.P., Smith E.A., Gaultier C., Marks G. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons P.E., Eisner M.D., Thompson B.T., Matthay M.A., Ancukiewicz M., Bernard G.R. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-232. [DOI] [PubMed] [Google Scholar]
- Price C.C., Altice F.L., Shyr Y., Koff A., Pischel L., Goshua G. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest. 2020 doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri V.M., Giunta F., Suter P.M., Slutsky A.S. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA. 2000;284(1):43–44. doi: 10.1001/jama.284.1.43. [DOI] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Cui W., Tian B.P. Efficacy of tocilizumab treatment in severely ill COVID-19 patients. Crit Care. 2020;24(1):524. doi: 10.1186/s13054-020-03224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]