Key Findings.
-
▪
Without evidence-based guidance, the pacemaker lower rate limit is typically left at 60 beats per minute, which is much lower than the average adult resting heart rate of 71–79 beats per minute based on large cohorts.
-
▪
While low heart rates are beneficial for patients with systolic dysfunction, pacing at a more physiologic heart rate may be a therapeutic target for patients with diastolic dysfunction or heart failure with a preserved ejection fraction (HFpEF).
-
▪
Using data from the Centers for Disease Control and Prevention growth charts, the National Health and Nutrition Examination Survey, and the Health-eHeart Study, we demonstrate a negative linear relationship between height and resting heart rate both during human growth and among healthy adult individuals.
-
▪
We derived a simple linear regression equation that defines the relationship between height and resting heart rate, which could be used in future studies to investigate a personalized pacemaker lower rate in patients with diastolic dysfunction or HFpEF.
Introduction
When Walton Lillehei and Earl Bakken pioneered the use of pacemakers for heart block following cardiac surgery, they reasoned that the programmed lower rate limit (LRL) should be set to a heart rate (HR) that the patient would be expected to have if conduction disease was not present.1 While the adult resting HR is known to average between 71 and 79 beats per minute (bpm),2,3 the expected resting HR for a given individual is not known. Owing to the desire to limit dyssynchronous pacing from conventional pacing sites4 and because a method to predict an individual’s resting HR is unknown, the pacemaker LRL is typically left at or near the factory setting of 60 bpm.5 This may not be the ideal backup pacing rate for all pacemaker-reliant patients.
The 60-bpm-fits-all approach dates back to an era before conduction system pacing—His bundle, left bundle, fascicular, and Bachmann bundle pacing—existed. With the potential to implant a fully physiologic pacing system, the pacemaker LRL could be customized without pacemaker-mediated dyssynchrony. Recent evidence suggests that backup rates better approximating physiologic resting HRs benefit pacemaker-reliant patients with heart failure and a preserved ejection fraction (HFpEF) (Supplemental Tables 1 and 2).6 In patients with HFpEF, atrial pacing to achieve a higher HR reduces cardiac filling pressures, whereas pharmacologic HR lowering increases filling pressures and worsens heart failure symptoms.6 Increasing the pacemaker LRL from 60 bpm to 80 bpm in patients with diastolic dysfunction and/or HFpEF improves quality of life, functional capacity, and NTproBNP levels, particularly in patients with a paced QRS <150 ms or pacing from the Bachmann and His bundles.7
Rather than suggest an arbitrary lower rate target, we sought to identify a readily available metric to predict resting HR individualized to each person. Although resting HR is influenced by many variables,2,3 we hypothesized that height could serve as a useful predictor and sought to better define the height–HR relationship. Height is a predictor of resting HR during the growing process2,8 and may also predict resting HR in adults. As humans grow, the average resting HR falls from about 120 bpm in infants to 70–74 bpm in adult men and 73–79 bpm in adult women.2,3,8
Methods and results
Heart rates and height during human growth
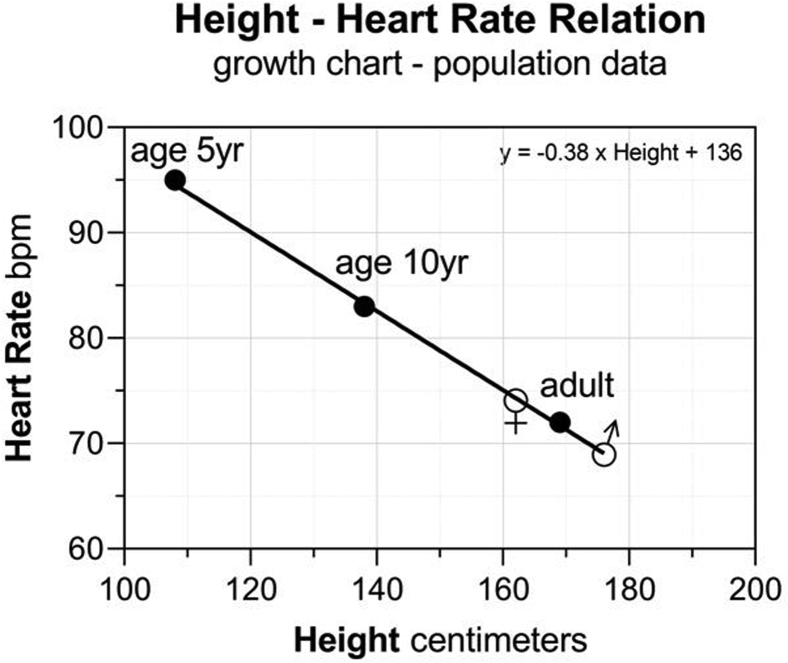
Median height data from published national survey data2 and CDC growth charts8 were collected to establish the relationship of growth-associated height and HR differences, producing evidence of a linear relationship. For each 1-centimeter increase in height, there was a 0.38 bpm reduction in HR (Figure 1).
Figure 1.
The relationship of growth-associated height and resting heart rate. Linear regression of height and resting heart rate obtained from group medians of national survey and growth chart data.
Heart rates and height in an adult population
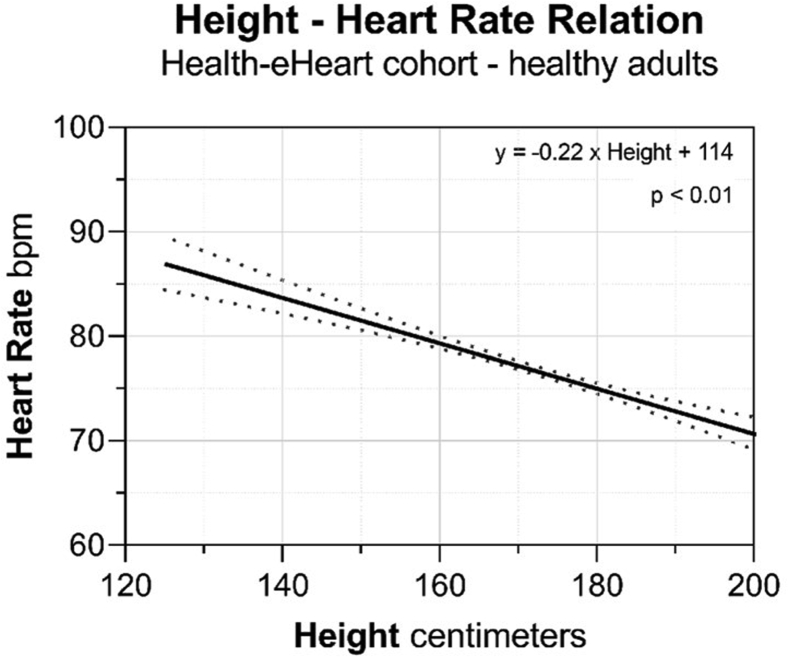
We validated the height–HR relationship in healthy adults enrolled in the Health-eHeart Study with available sex and self-reported height data (n = 4795; n = 2111 female). Resting HR was obtained using photoplethysmography through a smartphone camera; resting HR was assured by excluding measurements preceded by accelerometer-recorded activity (Supplemental Methods).3 Using linear regression, for every 1-centimeter increase in height, there was a 0.22 ± 0.02 bpm reduction in resting HR (P < .001) (Table 1, Figure 2). The height–HR relationship by sex is shown in Supplemental Figure 1 and Supplemental Tables 3 and 4). Additional anthropomorphic variables were assessed as predictors of resting HR (Supplemental Tables 3–10). In univariate analysis, female sex and body mass index were positive predictors of resting HR (P < .001), whereas weight was not a significant predictor of resting HR (Supplemental Tables 5–7). In multivariate analysis adjusting for height, sex, and weight, all 3 variables were independent predictors of resting HR (Table 2).
Table 1.
The relationship between height and resting heart rate from the Health-eHeart Study cohort
| Variable | Coefficient | Standard error | 95% confidence interval | P value |
|---|---|---|---|---|
| Intercept | 114.153 | 3.686 | 106.926, 121.380 | <.001 |
| Height | -0.218 | 0.021 | -0.259, -0.176 | <.001 |
Figure 2.
The relationship of height and resting heart rate in healthy adults. The linear regression of height and resting heart rate from real-world heart rate–photoplethysmography data from the Health-eHeart Study.
Table 2.
Multivariate analysis of height, weight, and sex on resting heart rate from the Health-eHeart Study cohort
| Variable | Coefficient | Standard error | 95% confidence interval | P value |
|---|---|---|---|---|
| Intercept | 103.003 | 5.368 | 92.479, 113.526 | <.001 |
| Height | -0.206 | 0.032 | -0.268, -0.143 | <.001 |
| Weight | 0.094 | 0.012 | 0.070, 0.118 | <.001 |
| Female sex | 3.024 | 0.597 | 1.853, 4.195 | <.001 |
This project adhered to the guidelines set forth by the Office of Human Research Protection that is supported by the U.S. Department of Health & Human Services. The University of Vermont and the UCSF Institutional Review Board deemed this ancillary analysis using data from the Health-eHeart Study to be exempt from review, as it was a retrospective analysis performed on de-identified data. Enrolled subjects in the Health-eHeart Study provided written informed consent.
Discussion
In this analysis, we demonstrate a negative linear relationship between height and resting HR both during human growth2,8 and among healthy adult individuals in an out-of-clinic dataset from the Health-eHeart Study.3 Among animal species of different body sizes, body length is consistently and negatively correlated with HR.9 In humans, height correlates with cardiac stroke volume10 and a prior investigation evaluating arterial hemodynamics by stature found height to be a strong predictor of resting HR,11 consistent with our findings. We derived a simple linear regression equation, which defines the height–resting HR relationship that could be used to predict an individual’s resting HR. For example, the predicted average resting HR for a patient who is 150 cm (4.9 feet) tall is 82 bpm, while the predicted HR for a patient who is 195 cm (6.4 feet) tall is 72 bpm (Figure 2).
Our analyses reproduce the positive relationships between female sex, body mass index, weight, and resting HR, which have been previously described.2,3 While many variables are associated with resting HR (Supplemental Appendix), height is an easily measured and relatively constant variable that is unique to individuals. Given the relatively linear correlation between height and resting HR, we propose height as a simple and pragmatic variable to serve as a starting point toward individualizing the pacemaker LRL for patients with diastolic dysfunction or HFpEF and conduction system pacing.
Despite rapid innovation in cardiac devices, pacing algorithms, and programmable pacemaker features tailored to the individual patient, the pacemaker LRL is rarely changed from the factory setting of 60 bpm.5 In the systolic heart failure population, pharmacologic HR lowering has well-established benefits.12 In contrast, among patients with HFpEF low HRs may be detrimental (Supplemental Table 1) by increasing central arterial pressures and left ventricular end-diastolic pressure, both of which contribute to increased wall stress and chronic adverse remodeling.6 Atrial pacing above 60 bpm improves cardiac filling pressures,6 symptoms, and functional capacity in HFpEF patients,7 and reverses concentric left ventricular hypertrophy in animal models13 (Supplemental Table 2).
We acknowledge several limitations with respect to our analysis of the height–HR relationship. The CDC and national survey data from which the growth-associated height–HR curves were derived use a single resting HR measurement.2,8 HR data from the Health-eHeart Study were derived using the geometric mean of all HR values of a participant; however, participants were free to measure their HR at any frequency and time of day.3 These measures underestimate diurnal variations in resting HR and atrioventricular conduction. In addition, most patients with rate-responsive pacemakers do not pace at the programmed LRL during waking hours or activity. However, physical activity levels are reported to be low among HFpEF patients14 and increased physiologic pacing with a customized LRL at rest or during sleep would be expected to reduce cardiac filling pressures and induce long-term beneficial remodeling. Finally, the proposed height–HR algorithm is a starting point to improve beyond the arbitrary 1-size-fits-all nominal LRL setting of 60 bpm. Further refinements incorporating more variables might be an area for further study. Additional limitations and future directions are detailed in the Supplemental Appendix.
Conclusion
In conclusion, conduction system pacing at an increased, individualized backup rate may be an important therapeutic target for patients with HFpEF. In this analysis, we derived a simple linear regression equation that defines the relationship between height and HR and can be used in future studies to investigate a personalized pacemaker lower rate in this population.
Funding Sources
This research was supported by research grants from the National Institutes of Health grant R01 HL-122744 (M. Meyer) and the Fonds de Recherche du Québec, Grant 274831 (R. Avram).
Disclosures
Dr Meyer and the University of Vermont have licensed patents for the use of pacemakers for the prevention and treatment of heart failure with preserved ejection fraction. Dr Marcus and Dr Meyer have received research funding from Medtronic. The other authors report no conflicts.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.09.004.
Appendix. Supplementary data
References
- 1.Lillehei C.W., Gott V.L., Hodges P.C., Jr. Transitor pacemaker for treatment of complete atrioventricular dissociation. J Am Med Assoc. 1960;172:2006–2010. doi: 10.1001/jama.1960.03020180016003. [DOI] [PubMed] [Google Scholar]
- 2.Ostchega Y., Porter K.S., Hughes J. Resting pulse rate reference data for children, adolescents, and adults: United States, 1999-2008. Natl Health Stat Report. 2011:1–16. [PubMed] [Google Scholar]
- 3.Avram R., Tison G.H., Aschbacher K. Real-world heart rate norms in the Health eHeart study. NPJ Digit Med. 2019;2:58. doi: 10.1038/s41746-019-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillis A.M., Russo A.M., Ellenbogen K.A. HRS/ACCF expert consensus statement on pacemaker device and mode selection. Heart Rhythm. 2012;9:1344–1365. doi: 10.1016/j.hrthm.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Biffi M., Melissano D., Rossi P. The OPTI-MIND study: a prospective, observational study of pacemaker patients according to pacing modality and primary indications. Europace. 2014;16:689–697. doi: 10.1093/europace/eut387. [DOI] [PubMed] [Google Scholar]
- 6.Meyer M., LeWinter M.M. Heart rate and heart failure with preserved ejection fraction: time to slow beta-blocker use? Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahlberg K., Arnold M.E., Lustgarten D. Effects of a higher heart rate on quality of life and functional capacity in patients with left ventricular diastolic dysfunction. Am J Cardiol. 2019;124:1069–1075. doi: 10.1016/j.amjcard.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC Growth Charts. National Center for Health Statistics and the National Center for Chronic Disease Prevention and Health Promotion. May 30, 2000. https://www.cdc.gov/growthcharts/data/set1clinical/Cj41cs021bw.pdfhttps://www.cdc.gov/growthcharts/data/set1clinical/Cj41cs022bw.pdf Accessed February 21, 2020.
- 9.O’Rourke M. Churchill-Livingstone; Edinburgh: 1982. Arterial Function in Health and Disease; pp. 170–182. [Google Scholar]
- 10.Jegier W., Sekelj P., Auld P.A. The relation between cardiac output and body size. Br Heart J. 1963;25:425–430. doi: 10.1136/hrt.25.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smulyan H., Marchais S.J., Pannier B., Guerin A.P., Safar M.E., London G.M. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol. 1998;31:1103–1109. doi: 10.1016/s0735-1097(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 12.Swedberg K., Komajda M., Bohm M. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 13.Klein F.J., Bell S., Runte K.E. Heart rate-induced modifications of concentric left ventricular hypertrophy: exploration of a novel therapeutic concept. Am J Physiol Heart Circ Physiol. 2016;311:H1031–H1039. doi: 10.1152/ajpheart.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S.J., Heitner J.F., Sweitzer N.K. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.