ABSTRACT
We compared the expression of mitochondrial alternative oxidase (AOX) and other non-phosphorylating respiratory components (NPhPs) in wild type and AOX1a transgenic Arabidopsis thaliana following short-term transfer of plants to higher irradiance conditions to gain more insight into the mechanisms of AOX functioning under light. The AOX1a overexpressing line (XX-2) showed the highest amount of AOX1a transcripts and AOX1A synthesis during the entire experiment, and many NPhPs genes were down-regulated after 6–8 h under the higher light conditions. Antisense AS-12 plants displayed a compensatory effect, typically after 8 h of exposure to higher irradiance, by up-regulating their expression of the majority of genes encoding AOX and other respiratory components. In addition, AS-12 plants displayed ‘overcompensation effects’ prior to their transfer to high light conditions, i.e., they showed a higher expression level of certain genes. As a result, the ROS content in AS-12, as in XX-2, was consistently lower than in the wild type. All NPhPs genes share, in common with AOX1a, light- and stress-related cis-acting regulatory elements (CAREs) in their promoters. However, the expression of respiratory genes does not always depend on the level of AOX1a expression. This suggests the presence of multiple combinations of signaling pathways in gene induction. Based on our results, we outline possible directions for future research.
KEYWORDS: Alternative oxidase, AtAOX1a-transformed plants, cis-acting regulatory elements, gene expression, high light stress, non-phosphorylating respiratory components, signaling pathways
It is known that mitochondrial respiration under light optimizes photosynthesis and maintains the cellular redox balance.1–4 In many respects, it is connected with the functioning of the mitochondrial electron transport chain (mETC), which is in contrast to mammalian mETC as it consists not only of a phosphorylating cytochrome pathway (CP) but also of several non-phosphorylating pathways (NPhPs). These pathways are not coupled with a transmembrane proton producing an electrochemical gradient. As a result, ATP synthesis is bypassed. Additional enzymes involved include the rotenone-insensitive internal and external type II NAD(P)H dehydrogenases (NDs) ,5 uncoupling proteins (UCP) which uncouple electron transport to ADP phosphorylation ,6 and another protein which has a strong effect on cell energy, known as the terminal cyanide-insensitive alternative oxidase (AOX).7,8 AOX transfers electrons directly from the ubiquinone pool to oxygen and produces water. Consequently, the AOX pathway bypasses proton-pumping Complexes III and IV and reduces the energy yield of respiration by at least two thirds. These energy-dissipating systems (AOX, NDs, and UCP) allow for the fine-tuning of mitochondrial membrane potential and the alleviation of ROS production. Among them, the alternative oxidase pathway (AP) plays a crucial role in the photosynthesizing cell.1,9–13 AOX is involved in the dissipation of excess reductants exported from chloroplasts, thereby ‘unloading’ photosynthetic ETC and maintaining cell redox homeostasis.
AOX and other enzymes of NPhPs are encoded by nuclear genes. AOX gene expression is thought to be directly (anterograde) light induced and mediated through photoreceptor control.12,14,15 Photosensitive cis-acting regulatory elements (CAREs) that regulate gene expression are found to be evenly distributed over the 1.0 kb AtAOX1a promoter.14,16 The G-box sequence, the known CARE in the promoters of many light-regulated genes, has been also identified in the promoter region of AOX1a in Arabidopsis.17 The expression of AOX is also regulated by retrograde signals induced by perturbed mitochondrial function. The mitochondrial retrograde regulation (MRR) region (a 93 bp portion) in the promoter of AOX1a in Arabidopsis was found to be essential for gene induction by the mETC and TCA cycle inhibitors.18 Afterward, 10 distinct CAREs, associated with stress- and light responses, in the AtAOX1a promoter were discovered.17 ROS production and changes in the TCA cycle and TCA acids under high light conditions may trigger the activation of CAREs in the MRR region via interactions with appropriate transcription factors.18,19 Changes in the redox state in the photosynthetic electron transport chain (for example, at the level of the plastoquinone pool) may also act as a signal transduced to the nucleus and trigger gene expression, including components of mitochondrial respiration.20 Close correlations between the plastoquinone redox state and the expression of AOX and chloroplast light-harvesting complex II (LHCB2) components under light- and drought-induced stress were recently shown.21 The signaling pathways involved in light induction of AOX gene expressions are still not fully understood.15,20
The important role of AOX in supporting efficient photosynthetic performance has been confirmed in studies, which involved the manipulation of AOX gene expression. In Arabidopsis thaliana (L.) Heynh., AOX1a is the most stress-responsive gene, among all AOX genes, in terms of the variety of stress inducers it responds to and the magnitude of its response.17,18,22 In AOX1a-knockout A. thaliana mutants (aox1a) or SHAM-treated wild type plants during greening under higher light (500 μmol m−2 s−1) conditions a reduced NADP+/NADPH ratio decreases plastidial protein import efficiency.23 In addition, overexpression of AOX1a rescues the aox1a mutant, and induces chlorophyll accumulation and plastidial protein import. The decline in the maximum quantum efficiency of photosystem II is significantly faster in the aox1a mutants than in the wild type.24 A reduction in ZAT10 mRNA (a transcriptional repressor involved in abiotic stress responses) in aox1a A. thaliana plants has been shown to impair tolerance to ROS and HL conditions and to decrease the expression of antioxidant genes.25 AOX1a-antisense plants suffer from more severe PSII photoinhibition after exposure to high light (800 μmol m−2 s−1) conditions than wild type (WT) or AOX1a-overexpressed plants.26 This decreased ability of aox1a mutants to withstand oxidative stress can be explained by the fact that other AOX isoforms cannot fully compensate for the lack of AOX1A.27,28 There are also studies where mutants that suppress or lack AOX display a compensatory effect by activating other NPhPs and ROS defense systems in response to stress.16,25,29–32 These contradictory findings may be explained by the nature, duration, and intensity of the stress treatment.
Strong alterations in the phenotype of AOX-transformed plants under stress, including HL conditions as well as early light induction of AOX expression ,12,14,20,33 suggests that changes in the AOX amount will lead to compensatory changes in the expression of other respiratory components. It cannot be ruled out that AOX expression, as a component integrated into a wider transcriptional stress response, changes the redox status and affects the involvement of other defense metabolic pathways. In experiments on the de-etiolation of a wheat leaf, the expression of several NPhP genes lagged behind the expression of AOX1a.12,34 This is consistent with the idea that AOX is not only a target but a regulator of stress responses.35 It is also been proposed that AOX is a marker of genetic variation in cell reprogramming and yield stability.36 However, the role of AOX in triggering and/or mediating the induction of other stress-response genes is not conclusive and is currently the subject of research.
Here, a wild type and two AOX1a-transformed A. thaliana lines (an overexpressing line and an antisense line with strongly decreased protein expression) were transferred to higher irradiance conditions to gain more insight into the mechanisms involved in the regulation of AOX under light and the role of AtAOX1a in the expression of other AOX and NPhPs genes. A search for common CAREs in the promoters of AOX1a and other studied genes was performed to detect possible interactions between genes at the transcriptional level.
Arabidopsis (A. thaliana) plants, the Col-0 ecotype (wild type, WT), and AtAOX1a antisense (AS-12) and overexpression (XX-2) lines (for further details, see37) were grown at 22°C for 10 h under 90 μmol m−2 s−1 light conditions (using luminescent lamps TL-D 30 W and TL-D 30 W (Aquarelle, Philips, Amsterdam, the Netherlands) and 14 h of darkness. Seeds from the WT and the AOX-modified plants were obtained from the Nottingham Arabidopsis Stock Center (NASC, Nottingham University, UK), and stock numbers were N6707 and N6591 for AS-12 and XX-2, respectively (kindly presented by Dr. V. I. Tarasenko and Dr. O. I. Grabelnych from the Siberian Institute of Plant Physiology, Irkutsk, Russia).38 Four-week-old plants were transferred to the higher irradiance (400 μmol m−2 s−1) treatment for 8 h. A value of 400 µmol m–2 s–1 was found to be the level of illumination in the upper part of the curvature region (the transition from the light-limited phase to the light-saturated plateau) on the light-response curve obtained for the net photosynthetic rate of the mature rosette leaf (Supplementary Fig. S1). Gene expression analysis was performed using Real-Time quantitative Reverse Transcription PCR (qRT-PCR) (for further details see:16). Primer pairs were designed using the Primer-BLAST online tool39 to simultaneously amplify fragments of all known splice variants of each of the studied genes16 The expressions of the studied genes were normalized using the housekeeping genes AT2G28390 and AT4G34270.40 For Western-blotting the combined leaf extracts from the wild type were separated on 12.5% gel and blotted on a nitrocellulose membrane using a wet transfer (25 mM Tris, 192 mM glycine, and 10% (v/v) methanol at pH 8.3).16 Filters were blocked (1.5 h) in 2% milk in TBST and incubated with polyclonal antibodies (Agrisera, Vännäs, Sweden) at a dilution factor of 1:1000. Anti-AOX1/2 (AS04 054), anti-COXII (AS04 052), and anti-VDAC1 (AS07 212) were incubated at a dilution factor of 1:5000. Polyclonal antibodies, anti-NDA1 (PHY 1037S), anti-NDA2 (PHY1038S), anti-NDB2 (PHY1555S), anti-UCP1 (PHY1574S) (PhytoAB Inc., San Francisco, California, USA), were incubated at a dilution factor of 1:1000. Secondary antibodies linked to horseradish peroxidase were then used and the signal was detected by chemiluminescence using Quantity One 1-D Analysis Software, Version 4.6.9. (Bio-Rad, California, USA). Superoxide (O2−) and peroxide were spectrophotometrically determined according to Chaitanya abd Naithani41 and to Bellincampi et al.,42 respectively, and described in detail by Garmash et al.12 Thiobarbituric acid reactive substance (TBARS) determinations were performed using the method described by Heath and Packer.43
After the plants were transferred to higher light conditions, elevated expressions of four AOX genes, including AOX1a in the antisense line and three genes (AOX1a, AOX1c, and AOX2) in the WT, were detected. XX-2 plant samples were characterized by stable high levels of AOX1a expression, and some genes were even down-regulated (Figure 1A). AOX1b expressions were very low in all lines, although AOX1b is in tandem with AOX1a on chromosome 3 in A. thaliana. However, AOX1b expression is limited to flowers and floral buds.22 The antisense line had low and quite leaky expression of AOX1a, also, following the transfer to higher irradiance conditions. Interestingly, the highest AOX1d expression in the AS-12 line was evident 8 h after transfer of plants to HL conditions. In A. thaliana, AOX1d (along with AOX1a) is known to be highly responsive to stress, in contrast to other AOX genes.22,27,46 In some cases stress-induced expressions of AOX1c and AOX2 have also been identified.47,48
Figure 1.
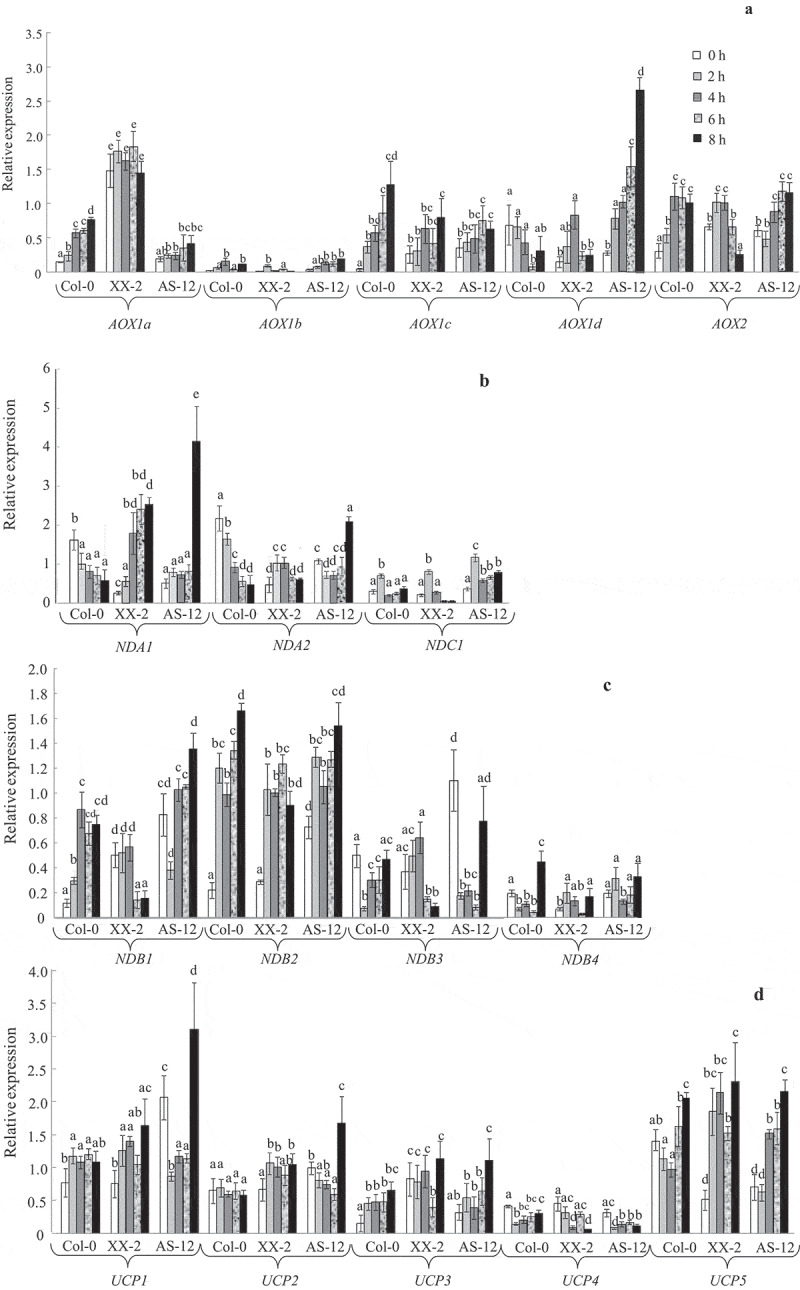
Expression profiles of alternative oxidase genes (AOX1a-d and AOX2) (A) and genes encoding other non-phosphorylating respiratory components (B-D) in leaves of wild type (WT), XX-2, and AS-12 Arabidopsis thaliana plants grown in control (at 90 μmol m−2 s−1, i.e. 0 h) and in higher light conditions (at 400 μmol m−2 s−1, i.e. 2–8 h). The genes encoding internal NAD(P)H dehydrogenases (NDA1,2 and NDC1) (B), external NAD(P)H dehydrogenases (NDB1-4) (C), and uncoupling proteins (UCP1-5) (D) were examined. (E) A hierarchical cluster analysis was performed with the R-program package pvclast using the Ward method of clustering and absolute correlation values as distance.44,45 Three plants were combined into one common sample to isolate total RNA and synthesize cDNA. Each data point represents three pooled samples (i.e. nine plants). Three RT-PCR reactions were performed on each pooled sample. The final number of expression values (nine) was used as n for statistical calculations. Significant differences between mean values in each gene analysis are indicated by different letters (ANOVA, Duncan’s test, P < 0.05)
Figure 1.

(continued)
In order to analyze a possible interaction between AOX genes at the transcriptional level, a study of a 1000 bp fragment belonging to the upstream regions of the transcription start sites (TSS) of all AtAOX genes was carried out by manually searching the NCBI (National Center of Biotechnology Information) Gene data bases and by using the Arabidopsis Gene Regulatory Information Server (Agris) (Figure 2). Results showed that all AtAOX genes have several light- and stress-regulated cis-acting regulatory elements (CAREs) in common (with the exception of AOX1b, which has only one CARE in common) with AOX1a. The presence of several G-box elements (CACGTG) in AOX1a, AOX2 promoters, and the co-expression of these genes indicate that their light induction is connected with the HY5-activation signal pathway. HY5 is a positive regulator of photomorphogenesis under broad-spectrum light conditions, suggesting that it acts downstream of phyto- and crypto-chromes (reviewed in49). It encodes the G-box binding factor (GBF1), which is a basic leucine zipper transcription factor (TF) (bZIP) that binds to G-box and promotes many light-regulated genes including those related to photosynthesis.50,51 The expressions of AOX1a, AOX1c, and AOX1d, which are enriched by CAREs such as the MYB4 binding site motif (BSM), SORLEP3, SORLIP2, and CCA1 BSM in their promoters, are possibly more dependent on perturbations in the photosynthetic electron transport chain under changing light conditions. CCA1 is known to bind to TFs in the MYB family, and is involved in the phytochrome-regulated expression of the LHCB gene, which encodes light-harvesting chlorophyll a/b proteins associated with photosystem II in Arabidopsis.52,53 LHCB plays an important role in the photoprotection of PSII when plants are exposed to HL conditions.52,54 MYB4, like other MYB TFs, is implicated in different plant stress responses.55 SORLEP3 and SORLIP2 may be involved in mediating responses downstream of phyA, perhaps in combination with other elements under stress conditions including high light.53 It can be concluded that the AOX1a promoter region contains light- and stress-responsive CAREs that occur in the promoters of other AOX genes. This suggests that other AOX genes may compensate for AOX1a suppression at the transcriptional level, at certain illumination levels. Contrarily, expression of AOX genes may be regulated by distinctive signaling pathways that converge to act on shared CAREs. This was indicated by the hierarchical clustering algorithm we used, which selected AOX genes from three different clusters (Figure 1E). However, much more research is needed to understand the regulation of AOX genes’ expression at the transcriptional level under light stress.
Figure 2.
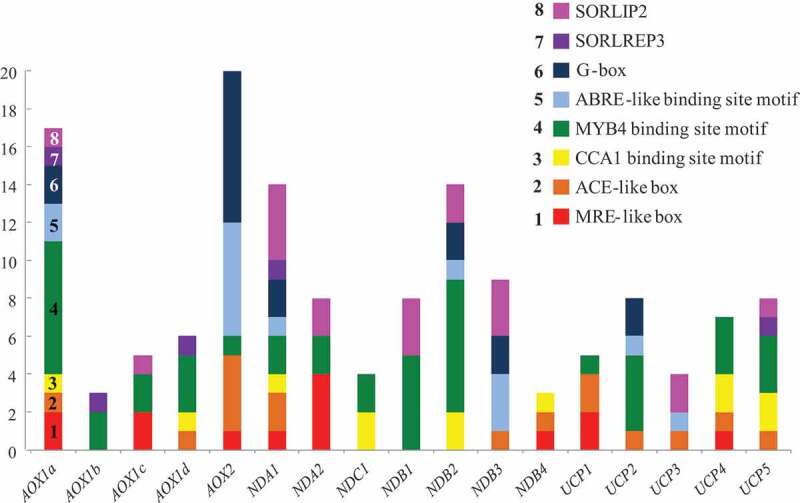
Amounts of cis-acting regulatory elements (CAREs) in promoters of Arabidopsis thaliana genes encoding components of alternative and cytochrome respiratory pathways. Analysis of a 1000 bp fragment belonging to the upstream regions of all genes, made by manual search using the NCBI databases and known sequences of CAREs (MRE- and ACE-like boxes) as well as the analysis of promoters using the Agris database. Light-responsive CAREs: G-box (CACGTG), MRE-like box (ACCTA), ACE-like box (CACGTG), CCA1 binding site motive (BSM) (AA(A/C)AATCT), SORLIP2 (GGGCC), and SORLREP3 (TGTATATAT). Stress responsive CAREs: MYB4 BSM (A(A/C)C(A/T)A(A/C)C), and ABRE BSM ((C/G/T)ACGTG(G/T)(A/C))
We found that light induction of AOX gene expression was followed by an increase in the amount of different AOX proteins in the WT and transformant lines (Figure 3, Supplementary Fig. S2). Two bands on the AOX immunoblots at approximately 34 and 30 kDa, probably corresponding to different isoforms of the AOX1 reduced form, were detected (Figure 3). The presence of a second lower molecular band (30 kDa) in AOX Western-blot analysis has also been described in previous studies on isolated mitochondria from Arabidopsis plants.56–58The AOX protein associated with the 34 kDa band was found in abundance in leaves from the XX-2 line. This is likely a product of AOX1a expression (AOX1A).37 WT and AS-12 plants, before exposure to higher irradiance conditions, contained a very low amount of AOX1 (at the 34 kDa band). After exposure to higher irradiance, levels of the AOX1 protein at the 34 kDa band markedly increased in leaves from the WT and AS-12 plants. Since the amount of sense AOX1a can be present at up to 50% in AS-12 plants relative to the wild type ,37 AOX (at the 34 kDa band) in samples of the antisense line under the higher light conditions was likely a product of AOX1a expression.
Figure 3.

Immunoblots of AOX (two bands around 34 and 30 kDa), internal NAD(P)H dehydrogenases (NDA1, and NDA2), external NAD(P)H dehydrogenases (NDB2), an uncoupling protein (UCP1), and mitochondrial voltage-dependent anion channel (VDAC1) proteins in whole-leaf tissue extracts from the wild type (Col-0) and two AOX1a-transformed lines grown in control (at 90 μmol m−2 s−1, i.e. 0 h) and in higher light conditions (at 400 μmol m−2 s−1, i.e. 2–8 h) (A). Fifteen micrograms of protein were loaded per lane, separated by an SDS-PAGE, immunoblotted, and visualized by chemiluminescence. Relative values of proteins levels, using VDAC1 as a reference, were obtained by dividing the densitometry of the appropriate protein and the VDAC1 bands (B). Blots were quantified by densitometry using Quantity One 4.6.9 software (Bio-Rad, USA). Data are presented as mean values ± SE of three replicates. Significant differences between mean values in each protein analysis are indicated by different letters (ANOVA, Kruskal-Wallis test, P < 0.05)
The AOX1A, AOX1B, AOX1C, and AOX1D precursor proteins deduced from the nucleotide sequences of AOX1a, AOX1b, AOX1c, and AOX1d are 354, 325, 329, and 318 amino acid residues, respectively (,59 TAIR, AOX1D: AT1G32350.1). We cannot attribute the AOX isoform at the 30 kDa band to a specific gene and, thus, believe that the 30 kDa AOX isoform is a mixed product resulting from the expressions of AOX1b-d genes.
Stress-induced co-expression of AOX and genes related to other NPhPs in plants, including A. thaliana, has often been observed. This suggests that the presence of multiple members in the alternative respiratory components pathway provide flexibility and fine control in response to various stresses.12,16,20,60,61 There is evidence of AOX1a-dependent expression of genes of other NPhPs.20 Contrarily, it has been shown that AOX1a expression is unaffected by the silencing of, for example, NDs genes.62–64 With the incorporation of the AtNDB2 overexpression construct into the AtAOX1a-overexpressed background line XX1, the expression of AtNDB2 in the mitochondria has been found to strongly increase.65 However, the amount of AOX protein was unchanged.65 It has also been shown that other respiratory components may compensate for the absence of functional AOX1a ,16,30,47 suggesting a modulating role for AOX1a in the functioning of the mETC, especially during oxidative challenges in response to stress.
Following transfer to higher irradiance conditions, gene expression in the NPhPs components was mainly correlated with the synthesis of the studied encoding proteins and depended on the level of AOX1a expression (Figure 1). In the AS-12 line, increases in the expressions of most NPhPs genes (NDA1,2, NDC1, NDB1,2,3, and UCP1,2,3,5) were observed. The relative transcript amounts of NDA2, NDB3, and UCP1 increased exclusively in AS-12, and NDA1 and UCP2 increased in both the AOX1a-transformed lines. This indicates that the expression of NPhPs genes is dependent on the level of AOX1a expression.
We analyzed the available information on expression patterns of the above listed genes and their co-expression with AOX1a under changing light conditions, as well as the common light- and stress-regulated CAREs in promoter regions of these genes. In the WT line, the gene expression and protein synthesis of one of the internal NDs, NDA1, were relatively high even before exposure to the higher light conditions, but then these decreased by the end of the experiment (8 h) (Figures 1 and 3). This may be attributed to a diurnal NDA1 expression pattern, which is at its highest expression level 1–2 h after the onset of day, followed by a steep decrease.20,66,67 In the present study, plants were transferred to higher irradiance conditions 2 h after the onset of day. Thus, NDA1 expression and encoding protein synthesis may be fully induced before transfer due to diurnal changes. In AOX1a transformants, NDA1 expression and NDA1 synthesis were noticeably different compared to the WT line, as they were amplified under HL conditions. The highest numbers of the NDA1 promoter were observed in identical light-related CAREs related to the AOX1a promoter. In addition, AOX1a and NDA1 were combined in one close cluster (Figure 1E). Thus, the cooperation of NDA1 and AOX1a occurs at the mRNA level. It was demonstrated that NDA1 expression, as well as that of AOX1a, is maintained at a high level under methyl viologen treatment and high CO2 levels under higher light conditions 400 μmol m−2 s−1).20 This suggests that ROS generated from the photosynthetic system and the cellular carbon status respectively contribute to the regulation of both gene expression and their co-expression.20 Our results indicate that the higher expression of NDA1 and the synthesis of NDA1 in the XX-2 line are related to the co-expression of genes (AOX1a and NDA1) and, in the AS-12 line, these genes interact in a compensatory manner.
NDA2 encodes an internal NADH dehydrogenase and is as involved as NDA1 in the oxidation of matrix NADH.5 We found that the level of expression of NDA2 was lower than that of NDA1 and increased gradually in all genotypes under HL conditions. NDA2 expression in A. thaliana has not been found to have distinct responses to HL and dark conditions.20 In addition, in comparison to NDA1, the NDA2 promoter had lower numbers in identical CAREs that were related to the AOX1a promoter. Interestingly, NDA2 belongs to another cluster tribe within the same large cluster as AOX1a.
Co-expression of AtNDB2 with AtAOX1a in treatment responses has often been reported.20,60 Both genes have identical functional stress-responsive CAREs, including I1, which is responsible for the MRR-dependent induction of their expression.17,18 Based on our analysis, promoters of NDB2 had, like NDA1, a large number of factors in common, especially stress-related CAREs, with the AOX1a promoter. However, expressions of NDB2 increased in all lines (Figure 1C). The co-expression of AOX1a and NDB2 in illuminated A. thaliana leaves has not always been observed (due to variations in light regimes, CO2 levels, or chemical treatments).20 According to our cluster analysis results, NBD2 is in the same cluster as AOX1a, but in a different sub-cluster (like NDA2). This is consistent with the idea that different signaling pathways regulate the expression of AOX1a and NDB2.17 It has been suggested that perturbations in the photosynthetic electron transport chain may be involved in the alteration of NDB2 expression.20
The expressions of UCP1 and UCP5 were similar to the observed patterns in NDB2 expression (Figure 1D). The expressions of these UCP genes have been shown to be highly induced under different stress conditions in A. thaliana, including HL.6,20,30,47 However, among other UCPs, the promoters of UCP1 and UCP5 showed the lowest and the highest numbers of observations, respectively, in CAREs in relation to the AOX1a promoter. In addition, all UCP genes were combined in the same AOX1d cluster. These data together indicate that UCP genes are relatively independent at the level of AOX1a expression under HL conditions. The UV-B-dependent expression of NDs genes has been found to be more cooperative with AOX1a at the mRNA level than the expression of UCPs.16
The expressions of NDB1, NDB3, NDC1, and UCP3 increased in the AS-12 and/or the WT lines but did not change or were down-regulated in the XX-2 line (Figure 1). This suggests that the expressions of these genes are induced in a complementary manner to AOX1a under the HL conditions. The promoters of these genes had higher or lower numbers in CAREs in relation to the AOX1a promoter. The expressions of NDC1, NDB4, and UCP4 were very low in all lines but their promoters contained different numbers of CAREs in common with the AOX1a promoter. In this experiment, the expression of NPhP genes under the different light conditions did not always depend on the number of CAREs in common with AOX1a in their promoters, but clearly depended on the level of AOX1a expression. In addition, the majority of NDs’ genes (five of the seven genes studied) belong to the same large cluster as AOX1a. Enhanced expressions of most genes in terms of NPhPs components in the antisense line suggest that these genes may respond to HL by compensating for the suppression of AOX1a. Altogether, these results indicate that there is an interaction between genes encoding mitochondrial non-phosphorylating proteins both at the level of the transcriptional response and at the level of the formation (convergence) of signaling pathways.17
A higher basal expression of certain genes (NDA2, NDB1, NDB3, UCP1, and UCP2) in AS-12 plants (0 h) was observed (Figure 1). This indicates that suppression of AOX can induce defenses that ‘overcompensate’ for the lack of AOX. Such ‘overcompensation’ has been observed in other studies ,16,29,31,32 as well as those involving the manipulation of components of the ROS network ,68 suggesting that this may be a common phenomenon. It is thought that AOX suppression enhances a mitochondrial stress-signaling pathway, which leads to an increase in the ROS-scavenging capacity of the cell.
Interestingly, both AOX1a-transformed lines had significantly lower ROS production levels compared with the WT, both in the beginning of the experiment and under light conditions (Figure 4). The response we observed of the XX-2 plants to HL conditions confirms that overexpression of AtAOX1a substantially reduces ROS production and mitigates stress impacts.16,23,26 In the AS-12 line, higher basal expressions of several NPhPs genes were observed. This can be termed an ‘overcompensation effect’ for AOX suppression in response to higher but not high stress-inducing irradiance conditions. It has been shown that AOX suppression enhances the mitochondrial stress-signaling pathway’s ability to increase the ROS-scavenging capacity of the cell, as well as, possibly, other acclimation responses, following stress.16,31,32 There is also evidence that the absence of AtAOX1a leads to a decrease in the ability of plants to withstand oxidative stress, especially under severe conditions.10,25,27,30 The responses of AOX antisense or knockdown lines are obviously determined by signaling pathways depending on the nature, duration, and intensity of the applied stress factor.
Figure 4.

Levels of lipid peroxidation presented as TBARS content (A), superoxide production (B), and hydrogen peroxide concentrations (C) in leaves of wild type (Col-0), XX-2, and AS-12 Arabidopsis thaliana plants grown in control (at 90 μmol m−2 s−1, i.e. 0 h) and in higher light conditions (at 400 μmol m−2 s−1, i.e. 2–8 h). Data are presented as mean values ± SE (n = 4–6) taken during three independent experiments. Significant differences between mean values (ANOVA, Duncan’s test, P < 0.05) are indicated by different letters
Clearly, changes in the level of AOX1a expression affected the expression of other AOX genes and genes encoding NPhPs components and, ultimately, determined the level of oxidative reactions. The AOX1a antisense line displayed a clear compensatory effect by induction of NPhPs genes expression showing cooperative and complementarily functioning of the NPhPs components. All NPhPs genes share, in common with AOX1a, light- and stress-related CAREs in their promoters and the majority of NDs’ genes belong to the same large cluster as AOX1a. However, the expression of respiratory genes does not always depend on the level of AOX1a expression. This suggests multiple combinations of signaling pathways involved in NPhPs gene expression. In this way, applying genome engineering techniques to understand the role of certain predicted CAREs in the AtAOX1a promoter in relation to light-dependent regulation of the expressions of AOX1a and genes of other NPhPs components should be the focus of further research.
Supplementary Material
Acknowledgments
The authors would like to thank Vladislav Tarasenko and Olga Grabelnych for seeds of mutant A. thaliana lines, Siberian Institute of Plant Physiology and Biochemistry, Siberian Branch RAS. The work is done using the equipment of the Collective Usage Center (CUC) “Molecular Biology”, Institute of Biology, Komi Science Centre, Ural Branch RAS. We also would like to thank Ksenia V. Ermolina and Maria V. Kyrnysheva for their technical assistance with Western blot analysis, and Ruslan V. Malyshev for his support throughout the experiment, Institute of Biology, Komi Science Centre, Ural Branch RAS.
Funding Statement
This reported study was funded by RFBR (Russian Foundation for Basic Research) according to the research project №19-04-00476 А and partially supported by the grant from The Ministry of Science and Higher Education of the Russian Federation (No. AAAA-A17-117033010038-7).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Noguchi K, Yoshida K.. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion. 2008;8:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Raghavendra AS, Padmasree K.. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003;8:546–553. [DOI] [PubMed] [Google Scholar]
- 3.Tcherkez G, Boex-Fontvieille E, Mahe A, Hodges M. Respiratory carbon fluxes in leaves. Curr Opin Plant Biol. 2012;15:308–314. [DOI] [PubMed] [Google Scholar]
- 4.Garmash EV. Mitochondrial respiration of the photosynthesizing cell. Russ J Plant Physiol. 2016;63:13–25. [Google Scholar]
- 5.Rasmusson AG, Geisler DA, Møller IM. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion. 2008;8:47–60. [DOI] [PubMed] [Google Scholar]
- 6.Borecký J, Nogueira FT, de Oliveira KA, Maia IG, Vercesi AE, Arruda P. The plant energy-dissipating mitochondrial systems: depicting the genomic structure and the expression profiles of the gene families of uncoupling proteins and alternative oxidase in monocots and dicots. J Exp Bot. 2006;57:849–864. [DOI] [PubMed] [Google Scholar]
- 7.Vanlerberghe GC, Dahal K, Alber NA, Chadee A. Photosynthesis, respiration and growth: a carbon and energy balancing act for alternative oxidase. Mitochondrion. 2020;52:197–211. [DOI] [PubMed] [Google Scholar]
- 8.Del-Saz NF, Ribas-Carbo M, McDonald AE, Lambers H, Fernie AR, Florez-Sarasa I. An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci. 2018;23:206–219. [DOI] [PubMed] [Google Scholar]
- 9.Dinakar C, Raghavendra AS, Padmasree K. Importance of AOX pathway in optimizing photosynthesis under high light stress: role of pyruvate and malate in activating of AOX. Physiol Plant. 2010;139:13–26. [DOI] [PubMed] [Google Scholar]
- 10.Vishwakarma A, Tetali SD, Selinski J, Scheibe R, Padmasree K. Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway in Arabidopsis thaliana. Ann Bot. 2015;116:555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garmash EV, Grabelhych OI, Velegzhaninov IO, Borovik OA, Dalke IV, Voinikov VK, Golovko TK. Light regulation of AOX pathway during greening of etiolated wheat seedlings. J Plant Physiol. 2015;174:75–84. [DOI] [PubMed] [Google Scholar]
- 12.Garmash EV, Velegzhaninov IO, Grabelhych OI, Borovik OA, Silina EV, Voinikov VK, Golovko TK. Expression profiles of genes for mitochondrial respiratory energy-dissipating systems and antioxidant enzymes in wheat leaves during de-etiolation. J Plant Physiol. 2017;215:110–121. [DOI] [PubMed] [Google Scholar]
- 13.Yamada S, Ozaki H, Noguchi K. The mitochondrial respiratory chain maintains the photosynthetic electron flow in Arabidopsis thaliana leaves under high-light stress. Plant Cell Physiol. 2020;61:283–295. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D-W, Xu F, Zhang Z-W, Chen Y-E, Du J-B, Jia S-D, Yuan S, Lin H-H. Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ. 2010;33:2121–2131. [DOI] [PubMed] [Google Scholar]
- 15.Xu F, Yuan S, Lin -H-H. Response of mitochondrial alternative oxidase (AOX) to light signals. Plant Signal Behav. 2011;6:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garmash EV, Velegzhaninov IO, Ermolina KV, Rybak AV, Malyshev RV. Altered levels of AOX1a expression result in changes in metabolic pathways in Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation. Plant Sci. 2020;291:110332. doi: 10.1016/j.plantsci. [DOI] [PubMed] [Google Scholar]
- 17.Ho LHM, Giraud E, Uggalla V, Lister R, Clifton R, Glen A, Thirkettle-Watts D, Van Aken O, Whelan J. Identification of regulatory pathways controlling gene expression of stress responsive mitochondrial proteins in Arabidopsis. Plant Physiol. 2008;147:1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dojcinovic D, Krosting J, Harris AJ, Wagner DJ, Rhoads DM. Identification of a region of the Arabidopsis AtAOX1a promoter necessary for mitochondrial retrograde regulation of expression. Plant Mol Biol. 2005;58:159–175. [DOI] [PubMed] [Google Scholar]
- 19.Gray GR, Maxwell DP, Villarimo AR, McIntosh L. Mitochondria/nuclear signaling of alternative oxidase gene expression occurs through distinct pathways involving organic acids and reactive oxygen species. Plant Cell Rep. 2004;23:497–503. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Noguchi K. Differential gene expression profiles of the mitochondrial respiratory components in illuminated Arabidopsis leaves. Plant Cell Physiol. 2009;50:1449–1462. [DOI] [PubMed] [Google Scholar]
- 21.Dahal K, Martyn GD, Alber NA, Vanlerberghe GC. Coordinated regulation of photosynthetic and respiratory components is necessary to maintain chloroplast energy balance in varied growth conditions. J Exp Bot. 2017;68:657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clifton R, Millar AH, Whelan J. Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochim Biophys Acta. 2006;1757:730–741. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D-W, Yuan S, Xu F, Zhu F, Yuan M, Ye H-X, Guo H-Q, Lv X, Yin Y, Lin H-H. Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant Cell Environ. 2016;39:12–25. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe CKA, Yamori W, Takahashi S, Terashima I, Noguchi K. Mitochondrial alternative pathway-associated photoprotection of photosystem II is related to the photorespiratory pathway. Plant Cell Physiol. 2016;57:1426–1431. [DOI] [PubMed] [Google Scholar]
- 25.Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008;147:595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florez-Sarasa I, Flexas J, Rasmusson AG, Umbach AL, Siedow JN, Ribas-Carbo M. In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions. Plant Cell Environ. 2011;34:1373–1383. [DOI] [PubMed] [Google Scholar]
- 27.Strodtkötter I, Padmasree K, Dinakar C, Speth B, Niazi PS, Wojtera J, Voss I, Do PT, Nunes-Nesi A, Fernie AR, et al. Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol Plant. 2009;2:284–297. [DOI] [PubMed] [Google Scholar]
- 28.Selinski J, Hartmann A, Deckers-Hebestreit G, Day DA, Whelan J, Scheibe R. Alternative oxidase isoforms are differentially activated by tricarboxylic acid cycle intermediates. Plant Physiol. 2018;76:1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amirsadeghi S, Robson CA, McDonald AE, Vanlerberghe GC. Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol. 2006;47:1509–1519. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe CKA, Hachiya T, Terashima I, Noguchi K. The lack of alternative oxidase at low temperature leads to a disruption of the balance in carbon and nitrogen metabolism, and to an up-regulation of antioxidant defence systems in Arabidopsis thaliana leaves. Plant Cell Environ. 2008;31:1190–1202. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Rajakulendran N, Amirsadeghi S, Vanlerberghe GC. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol Plant. 2011;142:339–351. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Vanlerberghe GC. A lack of mitochondrial alternative oxidase compromisescapacity to recover from severe drought stress. Physiol Plant. 2013;149:461–473. [DOI] [PubMed] [Google Scholar]
- 33.Tepperman JM, Hudson ME, Khanna R, Zhu T, Chang SH, Wang X, Quail PH. Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 2004;38:725–739. [DOI] [PubMed] [Google Scholar]
- 34.Garmash EV. Role of mitochondrial alternative oxidase in the regulation of cellular homeostasis during development of photosynthetic function in greening leaves. Plant Biology. 2020. doi: 10.1111/plb.13217. [DOI] [PubMed] [Google Scholar]
- 35.Van Aken O, Giraud E, Clifton R, Whelan J. Alternative oxidase: a target and regulator of stress response. Physiol Plant. 2009;137:354–361. [DOI] [PubMed] [Google Scholar]
- 36.Arnholdt-Schmitt B, Costa JH, de Melo DF. AO X - a functional marker for efficient cell reprogramming under stress? Trends Plant Sci. 2006;11:281–287. [DOI] [PubMed] [Google Scholar]
- 37.Umbach AL, Fiorani F, Siedow JN. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol. 2005;139:1806–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarasenko VI, Garnik EY, Shmakov VN, Konstantinov YM. Modified alternative oxidase expression results in different reactive oxygen species content in Arabidopsis cell culture but not in whole plants. Biologia Plantarum. 2012;56:635–640. [Google Scholar]
- 39.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST - a tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaitanya KSK, Naithani SC. Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn. New Phytol. 1994;126:623–627. [Google Scholar]
- 42.Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxinregulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000;122:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22(12):1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 45.R Core Team R . A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org/ [Google Scholar]
- 46.Keunen E, Schellingen K, Van Der Straeten D, Remans T, Colpaert J, Vangronsveld J, Cuypers A. ALTERNATIVE OXIDASE1a modulates the oxidative challenge during moderate Cd exposure in Arabidopsis thaliana leaves. J Exp Bot. 2015;66:2967–2977. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe CK, Hachiya T, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, Terashima I, Noguchi K. Effects of AOX1a deficiency on plant growth, gene expression of respiratory components and metabolic profile under low-nitrogen stress in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:810–822. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Huang J, Liang X, Bi Y. Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis callus. Planta. 2012;235:53–67. [DOI] [PubMed] [Google Scholar]
- 49.Waters MT, Langdale JA. The making of a chloroplast. The EMBO J. 2009;28:2861–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harter K, Kircher S, Frohnmeyer H, Krenz M, Nagy F, Schafer E. Light regulated modification and nuclear translocation of cytosolic G-box binding factors in parsley. Plant Cell. 1994;6:545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menkens AE, Schindler U, Cashmore AR. The G-box: a ubiquitous regulatory DNA element in plants bound by the GBF family of bZIP proteins. Trends Biochem Sci. 1995;20:506–510. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z-Y, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EMA. Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson M, Quail P. Identification of promoter motifs involved in the network of phytochrome A-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol. 2003;133:1605–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol. 2013;16:307–314. [DOI] [PubMed] [Google Scholar]
- 55.Ambawat S, Sharma P, Yadav NR, Yadav RC. MYB transcription factor genes as regulators for plant responses: an overview. Physiol Mol Biol Plants. 2013;19:307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juszczuk IM, Ostaszewska M. Respiratory activity, energy and redox status in sulphur-deficient bean plants. Env Exp Bot. 2011;74:245–254. [Google Scholar]
- 57.Sánchez-Guerrero A, Fernández del-Saz N, Florez-Sarasa I, Ribas-Carbó M, Ferni AR, Jiménez A, Sevilla F. Coordinated responses of mitochondrial antioxidative enzymes, respiratory pathways and metabolism in Arabidopsis thaliana thioredoxin trxo1 mutants under salinity. Env Exp Bot. 2019:162:212–222. doi: 10.1016/j.envexpbot.2019.02.026. [DOI] [Google Scholar]
- 58.Kerbler SM, Taylor N, Millar AH. Cold sensitivity of mitochondrial ATP synthase restricts oxidative phosphorylation in Arabidopsis thaliana. New Phytol. 2019;221:1776–1788. [DOI] [PubMed] [Google Scholar]
- 59.Saisho D, Nambara E, Naito S, Tsutsumi N, Hirai A, Nakazono M. Characterization of the gene family for alternative oxidase from Arabidopsis thaliana. Plant Mol Biol. 1997;35:585–596. [DOI] [PubMed] [Google Scholar]
- 60.Rasmusson AG, Fernie AR, van Dongen JT. Alternative oxidase: a defence against metabolic fluctuations? Physiol Plant. 2009;137:371–382. [DOI] [PubMed] [Google Scholar]
- 61.Grabelnych OI, Borovik OA, Tauson EL, Pobezhimova TP, Katyshev AI, Pavlovskaya NS, Koroleva NA, Lyubushkina IV, Bashmakov V, Popov VN, et al. Mitochondrial energy-dissipating systems (alternative oxidase, uncoupling proteins, and external NADH dehydrogenase) are involved in development of frost-resistance of winter wheat seedlings. Biochemistry (Moscow). 2014;79:647–662. [DOI] [PubMed] [Google Scholar]
- 62.Wallström SV, Aidemark M, Escobar MA, Rasmusson AG. An alternatively spliced domain of the NDC1 NAD(P)H dehydrogenase gene strongly influences the expression of the ACTIN2 reference gene in Arabidopsis thaliana. Plant Sci. 2012;183:190–196. [DOI] [PubMed] [Google Scholar]
- 63.Wallström SV, Florez-Sarasa I, Araújo WL, Aidemark M, Fernandez-Fernandez M, Fernie AR, Ribas-Carbo M, Rasmusson AG. Suppression of the external mitochondrial NADPH dehydrogenase, NDB1, in Arabidopsis thaliana affects central metabolism and vegetative growth. Mol Plant. 2014a;7:356–368. [DOI] [PubMed] [Google Scholar]
- 64.Wallström SV, Florez-Sarasa I, Araújo WL, Escobar MA, Geisler DA, Aidemark M, Lager I, Fernie AR, Ribas-Carbo M, Rasmusson AG. Suppression of NDA-type alternative mitochondrial NAD(P)H dehydrogenases in Arabidopsis thaliana modifies growth and metabolism, but not high light stimulation of mitochondrial electron transport. Plant Cell Physiol. 2014b;55:881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweetman C, Waterman CD, Rainbird BM, Smith PMC, Jenkins CD, Day DA, Soole KL. AtNDB2 is the main external NADH dehydrogenase in mitochondria and is Important for tolerance to environmental stress. Plant Physiol. 2019;181:774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michalecka AM, Svensson ÅS, Johansson FI, Agius SC, Johanson U, Brennicke A, Binder S, Rasmusson AG. Arabidopsis genes encoding mitochondrial type II NAD(P)H dehydrogenases have different evolutionary origin and show distinct responses to light. Plant Physiol. 2003;133:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elhafez D, Murcha MW, Clifton R, Soole KL, Day DA, Whelan J. Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: intraorganelle location and expression. Plant Cell Physiol. 2006;47:43–54. [DOI] [PubMed] [Google Scholar]
- 68.Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inze D, Mittler R. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 2002;32:329–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


