Abstract
In the past 20 years, infections caused by coronaviruses SARS-CoV, MERS-CoV and SARS-CoV-2 have posed a threat to public health since they may cause severe acute respiratory syndrome (SARS) in humans. The Complement System is activated during viral infection, being a central protagonist of innate and acquired immunity. Here, we report some interactions between these three coronaviruses and the Complement System, highlighting the central role of C3 with the severity of these infections. Although it can be protective, its role during coronavirus infections seems to be contradictory. For example, during SARS-CoV-2 infection, Complement System can control the viral infection in asymptomatic or mild cases; however, it can also intensify local and systemic damage in some of severe COVID-19 patients, due to its potent proinflammatory effect. In this last condition, the activation of the Complement System also amplifies the cytokine storm and the pathogenicity of coronavirus infection. Experimental treatment with Complement inhibitors has been an enthusiastic field of intense investigation in search of a promising additional therapy in severe COVID-19 patients.
Keywords: Complement System, SARS-CoV-2, COVID-19, Inflammation, Complement inhibitors
Graphical abstract
1. Introduction
In the past two decades, we have been affected by outbreaks of three severe acute respiratory syndrome (SARS) infections caused by coronaviruses. The first outbreak of SARS emerged in China in late 2002 caused by the coronavirus SARS-CoV [1]. Later, a different coronavirus was first isolated in 2012 in Saudi Arabia [2]. This virus (MERS-CoV) causes the Middle East respiratory syndrome and has led to substantial morbidity and mortality since its emergence. With a mortality rate of approximately 34%, MERS-CoV continues to emerge and spread, having been detected in 27 countries to date [3]. In late December 2019, the outbreak of SARS (COVID-19) caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in a serious global health problem of rare proportions [4,5].
2. Structural aspects of coronavirus
Coronaviruses belong to the Coronaviridae family (order Nidovirales) and are enveloped positive-sense RNA viruses, containing four major structural proteins: (i) basic nucleocapsid (N) protein which complexes with RNA to form a helical capsid within the viral membrane; (ii) membrane spike (S) protein, a type I glycoprotein on the virion surface that gives the virus its crown-like morphology; (iii) membrane (M) protein, that spans the membrane three times and has a short N-terminal ectodomain and a cytoplasmic tail; and, (iv) a small highly hydrophobic membrane protein (E) [6].
S protein mediates the initial attachment of the virion to host cell receptors. SARS-CoV and SARS-CoV-2 employs angiotensin-converting enzyme 2 (ACE2) as receptor located on the surface of several cell types such as epithelial cells of alveoli, trachea, and bronchi, bronchial serous glands, and alveolar monocytes and macrophages. It is also expressed in the gastrointestinal tract and kidneys [7]. After membrane fusion and viral entry into susceptible target cells, the viral RNA genome is released into cytoplasm leading to translation of the two polyproteins, transcription of nested sub genomic RNAs and replication of the viral genome [8].
3. The Complement System and pathogen elimination
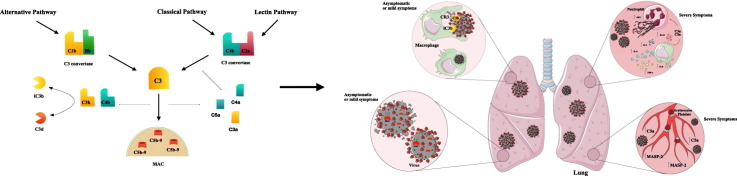
The Complement System is one of the central protagonists of innate and acquired immunity, playing an important role against viral, bacterial, fungal and protozoan pathogens. This system can be activated during innate (Alternative and Lectin Pathways) and acquired (Classical Pathway) immune responses (Fig. 1 ). Once activated, the Complement System generates several important functions (Fig. 2 ). Some of the most important related to pathogen killing are: (i) enhancement of microorganism phagocytosis and killing after interaction of pathogen-bound C3b/iC3b fragments with Complement Receptors (CR) CR1, CR3 and CR4 present on the surface of phagocytic neutrophils and macrophages [9]; (ii) inflammatory leukocytes infiltrate attracted by the presence of C3a and C5a fragments [10,11]; (iii) C3a and C5a fragments can trigger mast cell and basophil degranulation and release of inflammatory mediators which increase local vasodilation and vascular permeability [11]; (iv) activation of the inflammasome complex and inflammatory cytokine secretion [12]; (v) B lymphocytes activation after C3d-bound antigens are linked to CR2 which stimulates the production of specific antibodies [[13], [14], [15]]. C3d fragment is considered a molecular adjuvant to the innate immune response that strongly influences the production of antibodies [[16], [17], [18]]; (vi) lysis of several microorganisms after membrane attack complex (MAC; C5b-9n) is formed on their surface [9].
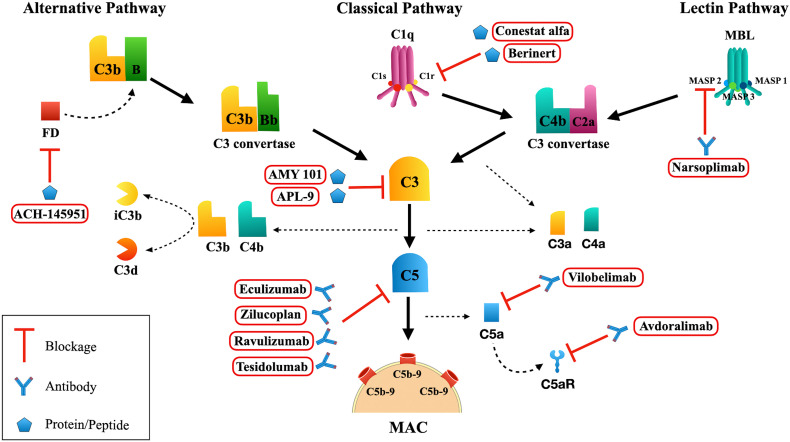
Fig. 1.
Complement System Activation. Alternative Pathway: Factor B (FB) bound to C3b is cleaved by Factor D (FD) into Ba and Bb fragments. The C3 convertase (C3bBb) cleaves C3 into C3a and C3b. The C5 convertase (C3bBbC3bn) cleaves C5 into C5a and C5b fragments. For simplicity, the C3 convertase C3(H2O)Bb is not represented here. Classical Pathway: once C1q binds to the immunocomplex, C1r is activated and subsequently activates C1s. Activated C1s cleaves C4 and C2, generating the C3 convertase (C4b2a) which cleaves C3 into C3a and C3b. When C3b fragments bind to the C4b2a, a C5 convertase (C3b2aC3bn) is formed. Lectin Pathway: once mannose binding lectin (MBL) or ficolins bind to targets, the MBL associated serine proteases (MASP)-1, MASP-2, MASP-3 are activated, which leads to the cleavage of C4 and C2, resulting in the formation of C3 convertase and later C5 convertase, similar to those generated in the Classical Pathway. Membrane Attack Complex (MAC; C5b-9n): C5 convertase cleaves C5 into C5a and C5b fragments. C5b binds to C6 and later to C7. C8 binds to C5b67 and several C9 molecules are incorporated (C5b6789n). The production of MAC can be blocked by Complement inhibitors such as AMY 101 and APL-6 that interact with C3 and prevent its cleavage into C3a and C3b. Eculizumab, Zilucoplan, Ravulizumab and Tesidolumab are antibodies that blockage the cleavage of C5. Vilobelimab: anti-C5a antibody. Avdoralimab: anti-C5aR antibody. This figure was created using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License: https://smart.servier.com. Adaptations from the original art were made on the cell membrane and macrophages.
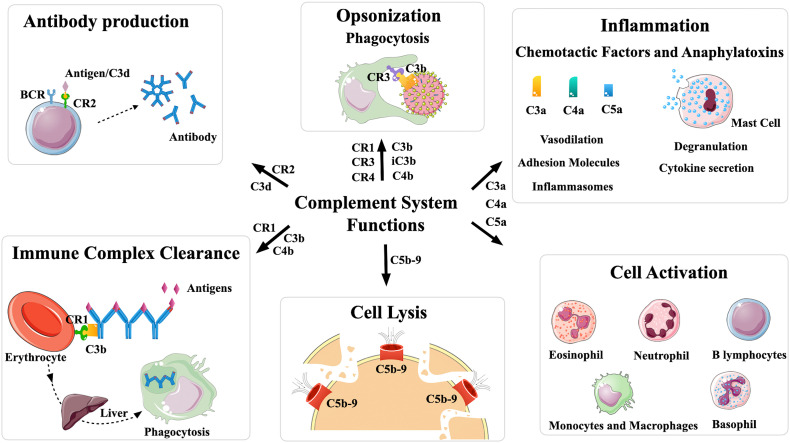
Fig. 2.
Biological functions of the Complement System. Antibody production: CR2 expressed in B lymphocytes and follicular dendritic cells interacts mainly with C3d fragments covalently bound to the pathogen surface, activating B lymphocytes, and increasing antibody production. Opsonization: C3b and iC3b are responsible for covalently attaching to the surface of different pathogens and they interact with Complement receptors CR1, CR3 and CR4 present on the surface of phagocytic cells such as neutrophils and macrophages, facilitating phagocytosis. Inflammation: the anaphylatoxins C3a and C5a are powerful chemoattractants to inflammatory cells and induce the release of mediators that amplify the inflammatory response. They perform different functions such as stimulation of mast cells and basophils, causing their degranulation, and release of inflammatory mediators, increased vasodilation, activation of complex inflammasomes and cytokine secretion. Cell activation: the anaphylatoxins are responsible for recruitment and activation of inflammatory cells, including eosinophils, neutrophils, basophils, monocytes and B lymphocytes. Cell Lysis: the formation of the Membrane Attack Complex (C5b-9n) causes cell lysis. Immune Complexes Clearance: immune complexes covalently attached to C3b or C4b fragments binds to CR1 present on the surface of blood cells and are removed from circulation. This figure was created using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License: https://smart.servier.com. Adaptations from the original art were made on the cell membrane and macrophages.
4. The role of the Complement System during viral infection
All three Complement Pathways are triggered in the presence of viruses [19]. Several viruses can trigger the Classical Pathway in the presence of natural or antigen-induced antibodies [20]. Direct C1q binding to several viruses have been observed resulting in viral neutralization and lysis [21]. Collectins such as MBL and collectin-11, and ficolins may recognize viruses and activate the Lectin Pathway [[22], [23], [24]]. In addition the activation of the Alternative Pathway depends on the presence of glycosaminoglycans on the viral or infected cell membrane [25].
Therefore, the functions triggered by Complement activation against virus include: (i) virus neutralization by loss of viral infectivity due to binding of Complement molecules and/or antibody on the virus surface [26]; (ii) Complement-mediated opsonization leading to virus aggregation and phagocytosis through CRs present in phagocytes [26,27]; (iii) decrease in the total number of viral particles due to aggregation-mediated neutralization of viruses [27]; (iv) phagocytosis-mediated neutralization [26]; (v) formation of the MAC that may lead to lysis of enveloped viruses [19,28]; and (vi) lysis of virus-infected cells [25,29,30].
5. The controversial role of the Complement System during coronaviruses infections
Although it can be protective, the role of the Complement System during coronavirus infections seems to be contradictory. Here, we report some interactions between SARS-CoV, MERS-CoV, and SARS-CoV-2 and the Complement System.
5.1. SARS-CoV
The role of Complement System during experimental infection by SARS-CoV was investigated using C57BL/6J wild type and C3 deficient mice [31]. After intranasal infection, C3 deficient mice presented less weight loss and less respiratory dysfunction when compared to wild type mice. Similar viral loads were observed in the lungs of both mouse groups. Furthermore, in the absence of C3, fewer neutrophils and inflammatory monocytes were observed in the lungs, followed by milder lung injury [31]. These results suggested that C3 modulates a systemic pro-inflammatory response during experimental SARS-CoV infection and additionally may contribute to disease progression.
The role of MBL in SARS-CoV infection still remains inconclusive. MBL binds to SARS-CoV in dose- and calcium-dependent manner [32]. Furthermore, MBL bound to the SARS-CoV enhanced C4 deposition and inhibited viral infectivity in vitro. Zhou et al. [33] showed that MBL selectively binds to retroviral particles pseudo typed with SARS-CoV S protein. MBL also mediated viral neutralization, inhibiting infection of susceptible cell lines at concentrations within or below the range observed in the serum of healthy individuals. The interaction between MBL and S protein also blocked viral binding to the dendritic cell membrane-bound calcium-dependent (C-type) lectin DC-SIGN. DC-SIGN has been shown to bind to, or facilitate infection by, a diverse array of viruses [34] by binding high-mannose carbohydrates and related surface glycans. Therefore, the ability of MBL to interfere with interactions between SARS-CoV and DC-SIGN could help to limit viral spread. Interestingly, the binding to MBL did not affect the interaction of S protein with ACE2. N-linked glycosylation site N330 in coronavirus S protein may be critical for the specific interaction with MBL since a mutation at this site significantly reduced both MBL binding and MBL-mediated viral neutralization [33].
Paradoxically, recombinant S protein was not recognized by serum MBL in in vitro studies, while lung surfactant protein D (SP-D), a collectin found in lung alveoli effectively bound S protein in a dose- and carbohydrate moieties-dependent manner. This impairment in recognition of S protein could be caused by different sugar specificities between MBL and SP-D [35].
Wang et al. [36] observed that erythrocyte-bound CR1 could be involved in the immunopathogenesis of SARS- CoV infection. In a small cohort of 54 patients, CR1 expression in erythrocytes decreased as long as disease progressed and gradually returned close to normal levels in the convalescent phase. The magnitude of this decrease was consistent with the severity of SARS.
5.2. MERS-CoV
Several studies have shown that MERS-CoV can modulate the immune response to the same degree as that observed for SARS-CoV [37], including a decrease in the presentation of antigens by MHC molecules of both Classes I and II, resulting in reduced activation of lymphocytes [38].
Patients with severe cases of MERS present fever and lung damage as aggravating factors with Complement System proteins participating in an exacerbated inflammatory response. An increase in the deposition of Complement proteins and an increase in circulating C5a have been observed in patients infected with MERS-CoV [39]. To investigate the effects of these fragments on MERS-CoV infection, a monoclonal antibody to block the C5a cell receptor (C5aR) was used resulting in decreased secretion of several cytokines and viral load in mice [39].
Tissue damage caused by cell death known as pyroptosis has also been observed [40], and the inhibition of C5aR1 by monoclonal antibodies in mice and human monocytes and macrophage lineage cells (THP-1) resulted in a reduction in the expression of activated caspase 1, and thus a decrease in cell death due to pyroptosis. This suggests that inhibition of anaphylatoxins may represent a possible therapeutic target for controlling MERS-CoV infection [40].
5.3. SARS-CoV-2
The Complement System plays an ambiguous role during SARS-CoV-2 infection. While it may effectively contribute to the control of this infection in many asymptomatic individuals or even in patients with mild symptoms, Complement activation may also contribute to several pathologies observed in some of severe COVID-19 patients, due to its potent proinflammatory effect [41].
In a retrospective study, Ramlall et al. [42] observed that in response to SARS-CoV-2 infected patients presented a strong induction of transcription of Complement genes (C1QA, C1QB, C1QC, SERPING1, C4B, C4A, C2, CFB, CFH, C3), although other Complement genes were shown to be down-regulated (C5AR1, C5AR2, CD59). In the same study [42], 102 genes with known roles in regulating Complement and coagulation cascades were analyzed to evaluate whether genetic variations are associated with poor SARS-CoV-2 clinical outcomes. Three SNPs in the C3 gene (rs1047286, rs2230203 and rs2230199) were identified that seem to confer protection against SARS-CoV-2 infection. Two other SNPs (rs61821114 and rs61821041) that mapped to Expression Quantitative Trait Loci (eQTLs) associated with CD55 were shown to negatively affect expression levels of this complement regulatory protein. These eQTLs were associated with increased risk of adverse clinical outcome following SARS-CoV-2 infection, probably due to an increase of sensitivity to Complement-mediated injury.
However, there was no difference in C3 and C4 serum levels between intensive care unit (ICU) and non-ICU patients in a retrospective study with 57 COVID-19 positive patients at the moment of hospital admission [43]. In a different study, plasma C3a, C3c and MAC were significantly elevated in COVID-19 patients when compared to healthy controls. In addition, the plasma levels of C3a and MAC were higher in ICU when compared to non-ICU patients. However, similar plasma C3c levels were observed in both groups of COVID-19 patients [44]. In a similar way, Holter et al. [45] observed a systemic activation of the Complement System in COVID-19 patients with increased levels of C4d, C3bBbP, C3bc, C5a, and sC5b-9. The concentration of sC5b-9 was particularly associated with respiratory failure and systemic inflammation.
On the other hand, dimers of N-protein from three coronaviruses (SARS-CoV, MERS-CoV and SARS-CoV-2) bind MASP-2. This association potentiates MBL interaction, MASP-2 auto-activation and subsequently Lectin Pathway activation, leading to uncontrolled activation of the Complement cascade. This mechanism could be behind the inflammation and pathogenesis observed in COVID-19 patients [46].
During the COVID-19 pandemic, special attention is being given to the procoagulant state observed in patients frequently associated with disease severity and a fatal outcome [42]. COVID-19 regularly manifests as a hyper-coagulable inflammatory condition, presenting high levels of inflammatory cytokines, D-dimers [47], fibrinogen [48,49] and mild thrombocytopenia [50]. Post-mortem pathology studies have confirmed high incidence of venous thromboembolism [51,52]; and micro vascular thrombi [53,54] in the lungs and kidneys with endothelial swelling, consistent with a thrombotic microangiopathy. In parallel, strong Complement activation has been observed on endothelial cells [55].
The Complement and coagulation systems exhibit cross-talk between them and some evidence that Complement System activation is associated with thrombosis in COVID-19 patients. During the course of this infection, the Complement System can be activated via multiple pathways by the virus itself and by damaged tissues. The generation of C5a, whose levels are highly elevated in symptomatic COVID-19 patients (accompanied by a strong expression of C5aR in monocytes and neutrophils) [56], increases tissue factor activity both in circulation [57] and on endothelial cells [58]. The induction of endothelial P-selectin by C5a [59] is important for the recruitment and aggregation of platelets. It has been shown that C5a stimulates excess production of neutrophil extracellular traps (NET) [60] which is pro-coagulant and traps platelets resulting in platelet aggregation, coagulation and thrombus formation. This is consistent with the fact that patients with severe COVID-19 have elevated serum markers of neutrophil activation and NET formation [61]. Endothelial cells are induced by MAC to secrete von Willebrand factor [62] which seems to be accompanied by an increase in prothrombinase activity. This may contribute to fibrin deposition and endothelial injury. It has been shown that MASP-1 and MASP-2 can cleave prothrombin [63] and activate fibrinogen and factor XIII [64]. In a recent study, Magro et al. [55] observed strong deposition of C4d, MASP-2 and MAC in the lung and in the dermal microvasculature from COVID-19 patients with acute respiratory distress syndrome (ARDS). Complement can also inhibit fibrinolysis through C1-INH that in its native state inhibits plasmin [65] leading to decreased fibrinolysis and increased thrombus formation. On the other hand, fibrinolysis factors are able to modify Complement components. For example plasmin can activate C3 and C5 independently of convertases [66,67]. The interplay between the Complement System and Coagulation Systems could induce, at least in part, the thrombi-inflammatory state in COVID-19 patients.
A parallel among the pathogenesis of COVID-19, atypical Hemolytic Uremic Syndrome (aHUS) and Paroxysmal Nocturnal Hemoglobinuria (PNH) has been established. The latter two are rare but lethal human diseases that result from defects in the Alternative Pathway, in the case of aHUS [68]; and absence of GPI-linked Complement regulatory proteins due to rare mutations, in the case of PNH [69]. Consequently, both are associated with Complement overactivation, dysfunctional coagulation and thrombotic microangiopathy [70] as seen in COVID-19. Both disorders are successfully treated with Eculizumab or Ravulizumab [71,72]. In fact, patients who were on anti-Complement therapy to treat PNH at the time they became infected with SARS-CoV-2 had relatively milder symptoms [73,74].
Although the precise mechanisms underlying the acute lung injury induced during SARS-CoV-2 infection are still undefined, inflammatory mediators of the cytokine storm have been recognized to cause damage to the host cells [75]. The term cytokine storm has been used to refer to dysregulation of the immune system, with a substantial release of pro-inflammatory cytokines (mainly IL-6, IL-1, TNF-α and interferon) by several immune cells, such as macrophages, dendritic cells, natural killer cells, T and B lymphocytes [76]. In SARS-CoV-2 infection, it has been proposed that four distinct axes might cross-talk with each other in multiple known and unknown interfaces to induce the cytokine storm. The axes are: (i) ACE/AngII/AT1R; (ii) ACE2/MasR; (iii) ACE2/DABK/BradykininB1 receptor; and, (iv) activation of the Complement [77]. In this last axis, while C5a through C5aR contributes to release of pro-inflammatory cytokines, neutrophil recruitment, smooth muscle contraction and vascular permeability, MAC complex induces cell lysis, apoptosis and cytokine production such as IL-6, monocyte chemoattractant protein-1 (MCP-1), IL-1, IL-17, IL-21, IFN-γ, IL-22, granulocyte-macrophage colony-stimulating factor (GM-CSF) [77].
6. The role of Complement in the pathogenicity of coronavirus infection
6.1. Lung pathology
Studies of the lungs of patients with COVID-19 [55,78] detected the presence of diffuse alveolar damage, formation of hyaline membranes, inflammation, edema and fibrosis - typical characteristics of the ARDS. Advanced age, smoking, and cardiovascular diseases are some of the risk factors for ARDS and COVID-19.
It is known that alveolar macrophages and pulmonary alveolar type II can secrete several proteins of the Complement System. C5a can attract neutrophils, eosinophils, lymphocytes, and monocytes to the local of infection. Additionally, C5a can induce the formation of NETs and induce monocytes and neutrophils to release reactive oxygen species (ROS) [60,79]. In the lungs, excessive production of NETs and ROS can damage the lung epithelium, increasing the permeability of the alveolar-capillary barrier, causing edema and hemorrhage [79].
Increased levels of C5a can lead to a cytokine storm, characterized by IL-1β, IL-7, IL-8, IL-9, TNF-α and many other factors [79,80]. C5a plasma levels are elevated in COVID-19 patients, especially those with pneumonia and ARDS. The C5aR1 molecule had its expression stabilized or increased for circulating monocytes and neutrophils, depending on the severity of disease. In the bronchi-alveolar lavage fluid of ARDS COVID-19 patients, C5a was detected, along with inflammatory cytokines and chemokines, such as CCL2, CXCL8, CXCL9, IL-6, TNF-α and IL-1β. Monocytes and neutrophils expressing C5aR1 were also detected in the lavage [56].
Magro and colleagues [55] analyzed skin and lung tissues from 5 patients affected with severe symptoms of COVID-19. MAC deposition was observed in the lung capillaries of 2 deceased patients, as well as C4d deposition within the lung parenchyma. Vascular MAC deposits were also seen on the skin of 3 other patients, all affected by retiform purpura, indicating vascular compromised blood vessels and ischemia of the affected region [81].
Furthermore, COVID-19 patients with pneumonia presented increased CR3 levels in blood monocytes and granulocytes, distinguishing them from those patients with mild symptoms. These results suggest that CR3 in these cells of patients with respiratory failure may be a candidate effector of both the thrombotic and inflammatory features of COVID-19 pneumonia, especially since CR3 is important for leukocyte activation and cytokine release, as well functioning as a fibrinogen receptor [82].
6.2. Kidney pathology
Similar to lungs, kidneys also express the ACE2 protein [83] and can be a target for SARS-CoV-2 infection [84,85]. A recent study investigated the correlation between 634 patients with ARDS and the development of acute kidney injury (AKI) [86]. 68% of these patients developed AKI. The common risk factors were old age, obesity, diabetes and history of heart failure. Interestingly, Diao and colleagues [84] also analyzed six kidney autopsy samples from patients with COVID-19. They observed lymphocyte infiltration in five of those and varying degrees of acute tubular necrosis, as well as strong MAC deposition in tubules. The latter was not observed in normal kidney samples, suggesting that SARS-CoV-2 is triggering Complement activation and deposition in the kidneys.
C5a can favor platelet aggregation and adhesion [58] and MAC can induce the production of procoagulant conditions [87]. It is possible that the renal condition of COVID-19 patients is caused by the release of Complement bioactive molecules, especially C5a and MAC formation [88], and the subsequent cytokine storm. These molecules, together with newcomer inflammatory cells, can damage the kidney tubules, leading to AKI.
6.3. Other pathologies
Other organs, such as heart and liver, and their respective endothelial cells can also express ACE2, and are susceptible targets for SARS-CoV-2 infection. Consequently, they are affected by Complement activation, which generates bioactive molecules such as C3a and C5a, attracting immune cells, and the MAC complex, causing a procoagulant state of the endothelium, resulting in ischemia and endotheliitis [89]. Recent reports showed an endothelial dysfunction of these organs, along with accumulation of immune cells and presence of apoptotic bodies in the heart and liver cell necrosis [90]. Blood clots were also common findings at these locations in COVID-19 patients [89].
7. Blockage of Complement System activation
Numerous lines of evidence point to an uncontrolled activation of the Complement System in severe COVID-19 cases. The intense inflammatory response and the deposition of proteins such as C3 and C5 observed in the lung tissues and in the patients' serum open the possibility for a supplementary treatment with Complement inhibitors and several of them are being tested for the treatment of patients with COVID-19 (Table 1 ).
Table 1.
Complement System inhibitors in clinical trials for COVID-19 treatment.
| Target | Inhibitors | Status/phase | Type of study | Responsible party/sponsor | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| C1 esterase | Conestat alfa (Ruconest) | Recruiting Phase 2 |
Interventional (clinical trial) | University Hospital, Basel, Switzerland Pharming Technologies B.V. |
NCT04414631 |
| Not yet recruiting | Interventional (clinical trial) | Pharming Technologies B.V. | NCT04530136 | ||
| C3 | AMY-101 | Not yet recruiting Phase 2 |
Interventional (clinical trial) | Amyndas Pharmaceuticals S.A. | NCT04395456 |
| APL-9 | Recruiting Phase 1 Phase 2 |
Interventional (clinical trial) | Apellis Pharmaceuticals, Inc. | NCT04402060 | |
| C5 | Eculizumab (Soliris) | Available | Interventional (clinical trial) | Alexion Pharmaceuticals | NCT04355494 |
| Available | Interventional (clinical trial) | Hudson Medical Thomas Pitts, M.D. |
NCT04288713 | ||
| Recruiting | Observational | University Hospital, Basel, Switzerland | NCT04351503 | ||
| Recruiting Phase 2 |
Interventional (clinical trial) | Assistance Publique - Hôpitaux de Paris | NCT04346797 | ||
| Zilucoplan (RA101495) | Recruiting Phase 2 |
Interventional (clinical trial) | University Hospital, Ghent Bart N. Lambrecht UCB Pharma |
NCT04382755 | |
| Ravulizumab (Ultomiris) | Not yet recruiting Phase 3 |
Interventional (clinical trial) | Brigham and Women's Hospital Andrew Michael Siedlecki |
NCT04570397 | |
| Recruiting Phase 4 |
Interventional (clinical trial) | Cambridge University Hospitals NHS Foundation Trust Frances Hall |
NCT04390464 | ||
| Recruiting Phase 3 |
Interventional (clinical trial) | Alexion Pharmaceuticals |
NCT04369469 | ||
| C5a | Vilobelimab (IFX-1) | Recruiting Phase 2 Phase 3 |
Interventional (clinical trial) | InflaRx GmbH | NCT04333420 |
| C5aR | Avdoralimab (IPH5401) | Recruiting Phase 2 |
Interventional (clinical trial) | Assistance Publique Hopitaux De Marseille Innate Pharma |
NCT04371367 |
| Suspended Phase 2 |
Interventional (clinical trial) | Centre Leon Berard | NCT04333914 |
Eculizumab, a monoclonal anti-C5 antibody, was the first approved Complement inhibitor and is used in patients with PNH [72,91], aHUS [92] and myasthenia gravis [93]. The deposition of C5 and C5a in the lungs of patients makes the use of anti-C5 interesting. Clinical trials with Eculizumab and Ravulizumab (another C5 inhibitor) are being carried out to evaluate the possible effectiveness of the treatment in COVID-19 patients.
Some studies have demonstrated an intense correlation between the production of C5 fragments, such as C5a, with the exacerbated proinflammatory response and lung tissue injuries of patients with ARDS [79], similar to that observed in patients with severe SARS-CoV-2 infections. Blocking C5a could prevent an excessive inflammatory response without preventing the formation of MAC and a possible defense against the virus by the Complement System. Clinical trials to assess the efficacy of IFX-1, an anti-C5a monoclonal antibody, and Avdoralimab, a C5a receptor blocking monoclonal antibody, anti-C5aR1, are also being performed as possible treatment in patients with COVID-19. Zelek et al. [94] observed that LFG316 (Tesidolumab), a C5-blocking monoclonal antibody that prevents generation of C5a and membrane attack complex, was efficient to decrease the excessive inflammatory response favoring the clinical recovery in severe COVID-19 patients.
Although inhibitors related to the C5 protein present themselves as important possibilities, studies have pointed to the attenuation of ARDS, and lung lesions, in C3 deficient mice infected with SARS-CoV, indicating that an elevated activation of the Complement System could cause a worsening of the condition [31]. As a result, the use of C3 inhibitors may result in more effective mediation of inflammation than C5 inhibitors [95]. Clinical trials with C3 blocking peptides, such as AMY-101 are in the process of elucidating a possible control in the exacerbated inflammatory response, lung injuries and even multiple organ failure, observed in some COVID-19 cases [96]. The cyclic peptide C3 inhibitor AMY-101 was first used as an experimental treatment in a patient with severe ARDS due to COVID-19. A remarkable resolution of the inflammatory response was observed 48 h after the initiation of treatment.
A comparative study by Mastellos et al. [97] of COVID-19 patients that were treated with two distinct Complement blockers, Eculizumab or AMY-101, showed promising results especially for C3 inhibitors. As Eculizumab treated patients exhibited high levels of C3a, it confirms that C5 inhibition did not affect C3 activation in SARS-CoV-2 infection. More complete C3 inhibition enables better inflammatory control of leukocyte recruitment, cytokine release, lymphocyte hyperactivation and NET-driven thrombi-inflammatory pathways. In two other studies, patients showed a substantial improvement of clinical parameters after treatment with Eculizumab [96,98]. However, the concomitant use of antiviral, anticoagulant and antibiotic therapies could limit the attribution of the observed effect to Eculizumab.
Regarding the Alternative Pathway activation by SARS-CoV-2, it was demonstrated that both subunit 1 and 2 from SARS-CoV-2 S protein directly activates this pathway [98]. Factor D inhibitor (ACH-145951) was able to block Complement activation in vitro and prevented both C3c and C5b-9 accumulation induced by S protein. On the other hand, Ravulizumab prevented only the accumulation of C5b-9 [99].
As mentioned above, severe cases of COVID-19 commonly show arterial thrombosis and pulmonary endothelial damage and dysfunction which can lead to Lectin Pathway activation and inflammatory damage. Narsoplimab, a monoclonal MASP-2 antibody, is under study as an inhibitor of microvascular thrombosis and is being tested for the treatment of transplant-associated thrombotic microangiopathy and so may be effective in the treatment of patients with COVID-19 [100].
C1-INH Berinert, an inhibitor of C1 esterase derived from human plasma, used in patients with Hereditary or Acquired Angioedema, acts in the regulation of the kinin-bradykinin system, coagulation and thrombolysis. Since all of these mechanisms are affected in patients with ARDS, C1-INH Berinert could also be considered as a possible regulator of pro-inflammatory responses triggered by coronavirus infection [101]. Heparin, an anticoagulant that is being used to treat some cases of COVID-19, has inhibitory effects on the Complement System very similar to those of C1-INH Berinert [102]. Furthermore, Urwyler et al. [103] investigated another human C1 esterase inhibitor (Conestat alfa). This treatment reduced fever, levels of serum inflammatory markers and oxygen supplementation. In addition, the products of Complement activation, C4d and C5a, decreased after treatment. Encouraged by the clinical improvements observed in the majority of severe COVID-19 patients treated with Conestat alfa, a randomized controlled trial has been initiated to more deeply investigate this Complement inhibitor.
To date, only Eculizumab and C1-INH Berinert are approved inhibitors for treatment, but there is intense research for other Complement inhibitors [94]. Important considerations, such as the risk of opportunistic infections, especially by Neisseria meningitidis and even the high cost of drugs like Eculizumab, are limitations. The development of new drugs that block excessive Complement activation is necessary and at this moment is under investigation by several groups.
8. Final considerations
Over the past two decades, coronaviruses have emerged and regressed; however, the SARS-CoV-2 pandemic resulted in a serious global health problem in all continents, causing, so far, more than two million deaths.
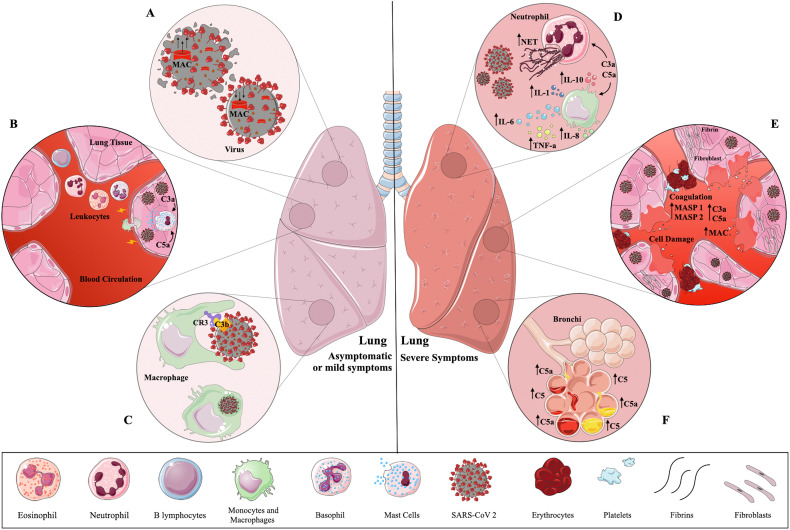
Once SARS-CoV-2 enters the human host, the Complement System is immediately activated in order to control and mitigate the infection. However, as observed for other pathogens, the Complement activation can be circumvented and the infection is established. Along with this, the role of the Complement System in SARS-CoV-2 infection was pointed out as a double-edged sword. Although Complement activation is crucial to control infection in asymptomatic or mild cases, exacerbated activation can also contribute to several pathologies such as cytokine storm and tissue damage seen in patients with severe COVID-19 (Fig. 3 ).
Fig. 3.
A double-edged sword – The Complement System during SARS-CoV-2 infection. During early infection (left panel) the Complement System is immediately activated in lung after SARS-CoV-2 invasion, triggering membrane attack complex (MAC) formation which may cause virus lysis (A). Circulating leukocytes migrate to the inflammatory site attracted by the presence of C3a and C5a. These fragments also trigger the release of inflammatory mediators by mast cells (B). SARS-CoV-2 coated with C3b or iC3b bind to Complement Receptor (CR) 3 present in phagocytic cells. As a consequence, they can be internalized and killed by local macrophages (C). In most cases, these three steps effectively control infection, which is observed in asymptomatic individuals or patients with mild symptoms. In severe patients (right panel) an intense inflammatory response is observed locally followed by cytokine storm and intense production of neutrophil extracellular traps (NET) induced by C3a and C5a (D). In the presence of MAC, endothelial cells secrete von Willebrand factor which increases prothrombinase activity. This may contribute to fibrin deposition and endothelial injury (cell swelling and damage). Mannose binding lectin-associated serine protease (MASP)-1 and MASP-2 can cleave prothrombin and activate fibrinogen and factor XIII. The induction of endothelial P-selectin by C5a is important for the recruitment and aggregation of platelets. The intense NET production stimulated by C3a and C5a is pro-coagulant, resulting in platelet aggregation, coagulation and thrombi formation (E). This exacerbated inflammatory condition leads to bronchial infiltration, followed by C5 activation. In this way, hyperactivation of the Complement System can contribute to several pathologies such as cytokine storm and tissue damage experienced by patients with severe COVID-19. This figure was created using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License: https://smart.servier.com. Adaptations from the original art were made on the cell membrane and macrophages.
Considering that the unregulated activation of the Complement System is an important trigger for systemic inflammation and tissue damage, this system becomes an interesting target for the development of activation inhibitors. This field of investigation is extremely relevant, because at its center is the concern to help patients with severe conditions by the use of Complement inhibitors. At least ten clinical trials with Complement inhibitors are in progress and they are promising tools to mitigate the severity of symptoms during SARS-CoV-2 infection.
Funding sources
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant numbers 141874/2012-0, 420553/2017-7]; the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant numbers 2017/12924-3, 2019/19800-3, 2019/01435-7, 2018/26574-7, 2017/10208-9]; and the Programa de Excelência Acadêmica da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PROEX/CAPES) [grant number 88882.327978/2019-01].
CRediT authorship contribution statement
Lazara Elena Santiesteban-Lores: writing-original draft preparation. Thais Akemi Amamura: writing-original draft preparation. Tiago Francisco da Silva: writing-original draft preparation. Leonardo Moura Midon: writing-original draft preparation. Milena Carvalho Carneiro: writing-original draft preparation, figures. Lourdes Isaac: conceptualization, writing-review and editing, supervision. Lorena Bavia: writing-review and editing, supervision.
Acknowledgements
We thank Prof. Dr. Skaker Chuck Farah from Institute of Chemistry, University of Sao Paulo, for the critical reading of this text. We also thank Servier Medical Art (https://smart.servier.com) for permission to use images to prepare the three figures.
References
- 1.Zhong N.S., Zheng B.J., Li Y.M., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki A.M., van Boheemen S., Bestebroer T.M., et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Mobaraki K., Ahmadzadeh J. Current epidemiological status of Middle East respiratory syndrome coronavirus in the world from 1.1.2017 to 17.1.2018: a cross-sectional study. BMC Infect. Dis. 2019;19(1) doi: 10.1186/s12879-019-3987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Available from.
- 5.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond C.W., Leibowitz J.L., Robb J.A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D., Hajishengallis G., Yang K., et al. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugli T.E. Biochemistry and biology of anaphylatoxins. Complement. 1986;3(3):111–127. doi: 10.1159/000467889. [DOI] [PubMed] [Google Scholar]
- 11.Haas P.J., van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol. Res. 2007;37(3):161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- 12.Triantafilou M., Hughes T.R., Morgan B.P., et al. Complementing the inflammasome. Immunology. 2016;147(2):152–164. doi: 10.1111/imm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepys M.B. Role of complement in induction of the allergic response. Nat New Biol. 1972;237(74):157–159. doi: 10.1038/newbio237157a0. [DOI] [PubMed] [Google Scholar]
- 14.Carroll M.C. The role of complement in B cell activation and tolerance. Adv. Immunol. 2000;74:61–88. doi: 10.1016/s0065-2776(08)60908-6. [DOI] [PubMed] [Google Scholar]
- 15.Thornton B.P., Vetvicka V., Ross G.D. Function of C3 in a humoral response: iC3b/C3dg bound to an immune complex generated with natural antibody and a primary antigen promotes antigen uptake and the expression of co-stimulatory molecules by all B cells, but only stimulates immunoglobulin synthesis by antigen-specific B cells. Clin. Exp. Immunol. 1996;104(3):531–537. doi: 10.1046/j.1365-2249.1996.57761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey P.W., Allison M.E., Akkaraju S., et al. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 17.Fang Y., Xu C., Fu Y.X., et al. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 1998;160(11):5273–5279. [PubMed] [Google Scholar]
- 18.Carroll M.C., Isenman D.E. Regulation of humoral immunity by complement. Immunity. 2012;37(2):199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal P., Sharma S., Pal P., et al. The imitation game: a viral strategy to subvert the complement system. FEBS Lett. 2020;594(16):2518–2542. doi: 10.1002/1873-3468.13856. [DOI] [PubMed] [Google Scholar]
- 20.Toussaint A.J., Muschel L.H. Studies on the bacteriophage neutralizing activity of serums. I. An assay procedure for normal antibody and complement. J. Immunol. 1962;89:27–34. [PubMed] [Google Scholar]
- 21.Bartholomew R.M., Esser A.F., Müller-Eberhard H.J. Lysis of oncornaviruses by human serum. Isolation of the viral complement (C1) receptor and identification as p15E. J. Exp. Med. 1978;147(3):844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reading P.C., Hartley C.A., Ezekowitz R.A., et al. A serum mannose-binding lectin mediates complement-dependent lysis of influenza virus-infected cells. Biochem. Biophys. Res. Commun. 1995;217(3):1128–1136. doi: 10.1006/bbrc.1995.2886. [DOI] [PubMed] [Google Scholar]
- 23.Favier A.L., Gout E., Reynard O., et al. Enhancement of Ebola virus infection via ficolin-1 interaction with the mucin domain of GP glycoprotein. J. Virol. 2016;90(11):5256–5269. doi: 10.1128/JVI.00232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Ali M.A., Shi Y., et al. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell Mol Immunol. 2009;6(4):235–244. doi: 10.1038/cmi.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyaram K., Yadav V.N., Reza M.J., et al. Virus-complement interactions: an assiduous struggle for dominance. Future Virol. 2010;5:709–730. [Google Scholar]
- 26.Van Strijp J.A., Van Kessel K.P., van der Tol M.E., et al. Complement-mediated phagocytosis of herpes simplex virus by granulocytes. Binding or ingestion. J. Clin. Invest. 1989;84:107–112. doi: 10.1172/JCI114129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldstone M.B., Cooper N.R., Larson D.L. Formation and biologic role of polyoma virus-antibody complexes. A critical role for complement. J. Exp. Med. 1974;140:549–565. doi: 10.1084/jem.140.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal P., Nawadkar R., Ojha H., et al. Complement evasion strategies of viruses: an overview. Front. Microbiol. 2017;8:1117. doi: 10.3389/fmicb.2017.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper N.R., Nemerow G.R. Complement, viruses, and virus infected cells. Springer Semin. Immunopathol. 1983;6:327–347. doi: 10.1007/BF02116278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernet J., Mullick J., Singh A.K., et al. Viral mimicry of the complement system. J. Biosci. 2003;28(3):249–264. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gralinski L.E., Sheahan T.P., Morrison T.E., et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ip W.K., Chan K.H., Law H.K., et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Lu K., Pfefferle S., et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010;84(17):8753–8764. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geijtenbeek T.B., den Dunnen J., Gringhuis S.I. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 2009;4(7):879–890. doi: 10.2217/fmb.09.51. [DOI] [PubMed] [Google Scholar]
- 35.Leth-Larsen R., Zhong F., Chow V.T., et al. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007;212(3):201–211. doi: 10.1016/j.imbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F.S., Chu F.L., Jin L., et al. Acquired but reversible loss of erythrocyte complement receptor 1 (CR1, CD35) and its longitudinal alteration in patients with severe acute respiratory syndrome. Clin. Exp. Immunol. 2005;139(1):112–119. doi: 10.1111/j.1365-2249.2005.02681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J., Chu H., Li C., et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josset L., Menachery V.D., Gralinski L.E., et al. Cell host response to infection with novel human coronavirus EMC predictsR potential antivirals and important differences with SARS coronavirus. mBio. 2013;4(3) doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y., Zhao G., Song N., et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7(1):77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y., Li J., Teng Y., et al. Complement receptor C5aR1 inhibition reduces pyroptosis in hDPP4-transgenic mice infected with MERS-CoV. Viruses. 2019;11(1):39. doi: 10.3390/v11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jodele S., Köhl J. Tackling COVID-19 infection through complement-targeted immunotherapy. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15187. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramlall V., Thangaraj P.M., Meydan C., et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020;26(10):1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dheir H., Sipahi S., Yaylaci S., et al. Is there relationship between SARS-CoV 2 and the complement C3 and C4? Turk J Med Sci. 2020;50(4):687–688. doi: 10.3906/sag-2004-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Nooijer A.H., Grondman I., Janssen N.A.F., et al. Complement activation in the disease course of COVID-19 and its effects on clinical outcomes. J. Infect. Dis. 2020;223(2):214–224. doi: 10.1093/infdis/jiaa646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holter J.C., Pischke S.E., de Boer E., et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(40):25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao M., Hu X., Zhang H., et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020 doi: 10.1101/2020.03.29.20041962. [DOI] [Google Scholar]
- 47.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb. Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klok F.A., Boon G.J.A.M., Barco S., et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020;56(1) doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lippi G., Sanchis-Gomar F., Henry B.M. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8(7):497. doi: 10.21037/atm.2020.03.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodigiani C., Iapichino G., Carenzo L., et al. Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T., Chen R., Liu C., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):362–363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvelli J., Demaria O., Vély F., et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritis K., Doumas M., Mastellos D., et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006;177(7):4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda K., Nagasawa K., Horiuchi T., et al. C5a induces tissue factor activity on endothelial cells. Thromb. Haemost. 1997;77(2):394–398. [PubMed] [Google Scholar]
- 59.Conde I., Crúz M.A., Zhang H., et al. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005;201(6):871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo R.F., Ward P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 61.Zuo Y., Yalavarthi S., Shi H., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hattori R., Hamilton K.K., McEver R.P., et al. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989;264(15):9053–9060. [PubMed] [Google Scholar]
- 63.Krarup A., Wallis R., Presanis J.S., et al. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2(7):623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hess K., Ajjan R., Phoenix F., et al. Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation. PLoS One. 2012;7(4):35690. doi: 10.1371/journal.pone.0035690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown E.W., Ravindran S., Patston P.A. The reaction between plasmin and C1-inhibitor results in plasmin inhibition by the serpin mechanism. Blood Coagul. Fibrinolysis. 2002;13(8):711–714. doi: 10.1097/00001721-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Huber-Lang M., Sarma J.V., Zetoune F.S., et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 67.Amara U., Flierl M.A., Rittirsch D., et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang K., Lu Y., Harley K.T., et al. Atypical hemolytic uremic syndrome: a brief review. Hematol. Rep. 2017;9(2):7053. doi: 10.4081/hr.2017.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill A., De Zern A.E., Kinoshita T., et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bajic G., Degn S.E., Thiel S., et al. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34(22):2735–2757. doi: 10.15252/embj.201591881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cofiell R., Kukreja A., Bedard K., et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125(21):3253–3262. doi: 10.1182/blood-2014-09-600411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hillmen P., Young N.S., Schubert J., et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006;355(12):1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 73.Araten D.J., Belmont H.M., Schaefer-Cutillo J., et al. Mild clinical course of COVID-19 in 3 patients receiving therapeutic monoclonal antibodies targeting C5 complement for hematologic disorders. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulasekararaj A.G., Lazana I., Large J., et al. Terminal complement inhibition dampens the inflammation during COVID-19. Br. J. Haematol. 2020;190(3):141–143. doi: 10.1111/bjh.16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chatterjee S.K., Saha S., Munoz M.N.M. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front. Mol. Biosci. 2020;7:196. doi: 10.3389/fmolb.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ragab D., Salah Eldin H., Taeimah M., et al. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahmudpour M., Roozbeh J., Keshavarz M., et al. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H., Wang C.Y., Zhou P., et al. Histopathologic changes and SARS-CoV-2 Immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;173(4):324. doi: 10.7326/L20-0895. [DOI] [PubMed] [Google Scholar]
- 79.Wang R., Xiao H., Guo R., et al. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4(5):28. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Georgesen C., Fox L.P., Harp J. Retiform purpura: a diagnostic approach. J. Am. Acad. Dermatol. 2020;82(4):783–796. doi: 10.1016/j.jaad.2019.07.112. [DOI] [PubMed] [Google Scholar]
- 82.Gupta R., Gant V.A., Williams B., et al. Increased Complement Receptor-3 levels in monocytes and granulocytes distinguish COVID-19 patients with pneumonia from those with mild symptoms. Int. J. Infect. Dis. 2020;99:381–385. doi: 10.1016/j.ijid.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caibin F., Kai L., Yanhong D., et al. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. MedRxiv. 2020 doi: 10.1101/2020.02.12.20022418. [DOI] [Google Scholar]
- 84.Diao B., Wang C., Wang R., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panitchote A., Mehkri O., Hastings A., et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann. Intensive Care. 2019;9(1):74. doi: 10.1186/s13613-019-0552-5. (Erratum in: Ann Intensive Care 2019; 9(1):84) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sims P.J., Faioni E.M., Wiedmer T., et al. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988;263(34):18205–18212. [PubMed] [Google Scholar]
- 88.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vinayagam S., Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rother R.P., Rollins S.A., Mojcik C.F., et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007;25(11):1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 92.Noris M., Galbusera M., Gastoldi S., et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uzawa A., Kuwabara S. Eculizumab treatment for refractory generalized myasthenia gravis. Brain Nerve. 2019;71(6):565–570. doi: 10.11477/mf.1416201317. [DOI] [PubMed] [Google Scholar]
- 94.Zelek W.M., Cole J., Ponsford M.J., et al. Complement inhibition with the C5 blocker LFG316 in severe COVID-19. Am. J. Respir. Crit. Care Med. 2020;202(9):1304–1308. doi: 10.1164/rccm.202007-2778LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Risitano A.M., Mastellos D.C., Huber-Lang M., et al. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. (Erratum in: Nat Rev Immunol 2020; 20(7):448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mastaglio S., Ruggeri A., Risitano A.M., et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215:108450. doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mastellos D.C., Pires da Silva B.G.P., Fonseca B.A.L., et al. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. ClinImmunol. 2020;108598:220. doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diurno F., Numis F.G., Porta G., et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 99.Yu J., Yuan X., Chen H., et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rambaldi A., Gritti G., Micò M.C., et al. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;9 doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomson T.M., Toscano E., Casis E., et al. C1-INH and the contact system in COVID-19-associated coagulopathy. Br. J. Haematol. 2020;225(6) doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schoenfeld A.K., Lahrsen E., Alban S. Regulation of complement and contact system activation via C1 inhibitor potentiation and factor XIIa activity modulation by sulfated glycans - structure-activity relationships. PLoS One. 2016;11(10):0165493. doi: 10.1371/journal.pone.0165493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urwyler P., Moser S., Charitos P., et al. Treatment of COVID-19 with conestat alfa, a regulator of the complement, contact activation and kallikrein-kinin system. Front. Immunol. 2020;11:2072. doi: 10.3389/fimmu.2020.02072. [DOI] [PMC free article] [PubMed] [Google Scholar]