ABSTRACT
Laccases are one of many groups of inducible enzymes produced by the filamentous fungus, Botrytis cinerea during colonisation of host plant tissues. While the processes involved in laccase induction are not fully understood, Cupric ions (e.g. CuSO4) and gallic acid (GA) have been reported as laccase inducers. This study investigates laccases activities and the expression of three laccase genes (BcLCC1, BcLCC2, BcLCC3) in three B. cinerea isolates grown in laccase-inducing medium (LIM) supplemented with CuSO4 and GA. Laccase activity in culture filtrates with CuSO4 increased after 48 h of growth in LIM at 24°C. The induction of BcLCC2 transcription was greatest at a concentration of 0.6 mM CuSO4, concentrations greater than 0.6 mM inhibited fungal growth. In contrast, no laccase induction was observed in the presence of GA. Liquid chromatography-mass spectroscopy (NanoLC ESI MS/MS) analysis confirmed the presence of a 63.4 kDa protein, the BcLCC2 isoform in the culture filtrate with 0.6 mM CuSO4. Analysis of mRNA transcripts further showed BcLCC3 was also inducible and the expression of BcLCC2 and BcLCC3 was isolate-dependent. In conclusion, CuSO4 induces a 63.4 kDa laccase in B. cinerea by induced transcription of the BcLCC2 gene.
KEYWORDS: Botrytis cinerea, laccases, inducers, copper, gene expression, enzyme activity
Introduction
Botrytis cinerea [anam.] Botryotinia fuckeliana [teleom.], Pers.: Fr- (grey mould) is a devastating fungal pathogen that is responsible for grey mould in many fruits, vegetables and horticultural crops, particularly in temperate regions (Dean et al. 2012). B. cinerea attacks many plant organs, including leaves, stems and fruits, resulting in severe losses even after the crop has been harvested (Lorenzini et al. 2013). At least 1400 plant species are reported to be affected by B. cinerea (Elad et al. 2016). The infection process typically involves extracellular enzymes and metabolites of the pathogen (Nakajima and Akutsu 2014), including cell wall-degrading enzymes, non-specific phytotoxic metabolites (botrydial and botcinolides), boosting compounds of reactive oxygen species (ROS) and the plants’ hypersensitive response (Williamson et al. 2007; Shah et al. 2009; Amselem et al. 2011). The extracellular enzymes include β-glucosidase, pectin methylesterases, polygalacturonases, aspartate proteinase, benzyl alcohol oxidase and laccases (Rha et al. 2001; Valette-Collet et al. 2003; Nakajima and Akutsu 2014).
Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) are found in vascular plants, fungi, bacteria and insects (Thurston 1994; Castilho et al. 2009), although the lignin-degrading white rot basidiomycetes are the main laccase producers (Rayner and Boddy 1988). Laccases belong to the largest subgroup of blue multi-copper oxidases (MCOs) and typically show phenoloxidase activity. During the oxidation process, laccases use oxygen as an electron acceptor to remove hydrogen radicals from a phenolic hydroxyl group and other similar molecules (Thurston 1994; Solomon et al. 1996). In addition, laccases oxidise a wide range of substituted aromatic amines, N-heterocycles, phenothiazines and thiol groups. Laccases contain two to four copper atoms per enzyme molecule (subunit), contributing to their oxidation properties (Thurston 1994; Giardina et al. 2010). Copper atoms are located in three types of copper centres that can be distinguished by their spectroscopic and paramagnetic properties (Thurston 1994). Cupric ions (Cu2+) are crucial for forming metal-active sites in laccases; hence, laccases are classified as copper-containing oxidases.
Fungal laccases are either inducible or constitutively expressed (Litvintseva and Henson 2002). Laccase production in lignin-degrading fungi and some ascomycete fungi have been investigated with phenolic inducers, different carbon sources and metal ions (Palmieri et al. 2000; Manavalan et al. 2013; Kim et al. 2014). Cu2+ is reportedly a strong metal-inducers for both laccase transcription and activity (Collins and Dobson 1997) and are vital in forming metal-active sites in copper-containing enzymes (Passarini et al. 2015). The interaction between Cu2+ and the fungal mycelium in culture medium has been shown to cause a substantial increase in total laccase activity in wood-rotting fungi, basidiomycetes (Guillén and Machuca 2008; Makela et al. 2013; Kuhar and Papinutti 2014) and the ascomycetes fungus, Aspergillus flavus (Gomaa and Momtaz 2015).
Laccase induction has been reported in B. cinerea in response to phenolic compounds, particularly gallic acid (GA). However, an increase in laccase activity in B. cinerea was reported when copper was added to the culture medium (Slomczynski et al. 1995; Sbaghi et al. 1996). Different isoforms of the enzymes, including a 36 kDa protein with 70% sugar and a 38 kDa protein with 80% sugar have been reported to be induced in liquid cultures of B. cinerea in the presence of GA and grape juice, respectively (Marbach et al. 1984). In addition, a 75 kDa protein has been purified from the liquid cultures of B. cinerea in the presence of Cu2+ (Slomczynski et al. 1995), respectively. Copper induction of B. cinerea laccases and the involvement of copper inducible laccase in disease development has not been fully elucidated, although the involvement of GA-inducible laccases in disease development has previously been reported (Gigi et al. 1980, 1981). However, the occurrence of 13 putative laccase encoding genes has been reported (Van Kan et al. 2017), although the biological basis of the induction of these genes, especially the enzyme-gene linkage and type of inducers remain unknown. Therefore, this study hypothesised that GA and Cu2+ are responsible for inducing a particular laccase gene, thereby increasing laccase activity. The current study investigates laccase activities, and the expression of laccase genes using RT-qPCR and RT-PCR methods in B. cinerea grown artificial media treated with Cu2+ and GA. To investigate this, three laccase genes (BcLCC1, BcLCC2, BcLCC3) were considered based on the previously published work of Schouten et al. (2002), which describes the potential involvement of BcLCC2 and BcLCC3 in disease development.
Materials and methods
Isolates
Three B. cinerea isolates (TN080, VRU0005, and TN077), representative of high, low and moderate virulence, respectively (data not shown), were used in the study. The isolates were taken from the culture collection stored at the National Wine and Grape Industry Centre, Charles Sturt University, Australia. The BcLCC2 gene from the three isolates was sequenced and the resulting sequences compared with NCBI GenBank sequences using BLAST. Sequences were deposited in GenBank with following accession numbers (TN080: MH789981, VRU0005: MH789982, and TN077: MH789980).
Culture conditions and preparation of laccase-inducing medium (LIM)
The basal media for liquid cultures were prepared by following the protocol described by Slomczynski et al. (1995). LIM was prepared by supplementing the basal medium with CuSO4 and GA at concentrations of 0.35 mM and 0.5 mM, respectively.
Induction of laccases by CuSO4 and GA
The three B. cinerea isolates were grown on potato dextrose agar (PDA) at 24°C. Sterile distilled water was added to the surface of 12-day-old sporulating cultures and spore suspensions were adjusted to 1 × 105 spores/ml using a haemocytometer. A 100 µl spore suspension (1 x 105 spores/ml) was inoculated into 30 ml of potato dextrose broth (PDB) in 100 ml conical flasks followed by incubation in a rotary shaker at 90 rpm at 24°C in the dark conditions. After 5 days, the mycelia were harvested using a sterilised Buchner funnel lined with sterilised filter paper. The mycelia were washed with sterile distilled water and transferred to LIM containing a mixture of CuSO4 and GA. Basal medium was used as a control. The cultures were incubated for 2 days at 24°C with agitation (240 rpm) in the dark. Aliquots of 2 ml were collected from the liquid cultures of inoculated LIM and the control flask within 12-h interval for up to 48 h and stored at −20°C for the determination of laccase activity and total protein. The experiment was performed in triplicates.
Expression analysis of laccase genes in the presence of CuSO4 and GA
Transcripts of laccase genes were quantified for the three isolates 48-h post-inoculation (hpi) in LIM. The LIM were treated separately with CuSO4 and GA. The experimental units consisted of PDB as the control, the basal medium as the control (NoI), LIM + CuSO4, LIM + GA, and LIM + CuSO4 + GA. LIM and control media were inoculated with three isolates separately as described in the aforementioned section. The experiment was performed in three independent replicates. Mycelia from all liquid cultures were collected 48-h post-inoculation (hpi) to analyse the expression profiling of laccase genes (BcLCC1, BcLCC2, BcLCC3). Culture filtrates were also collected at the same time to determine laccase activity. Mycelia collected from PDB were excluded from gene expression analysis as no laccase activity was detected in PDB.
Determination of the optimum copper concentration for the induction of laccase
B. cinerea isolate, TN077, in which the laccase genes were induced by both inducers was selected to determine the optimum concentration of CuSO4 for maximum laccase production. LIM supplemented with different concentrations of CuSO4 (0, 0.2, 0.4, 0.6, 0.8 mM) and inoculated with clean mycelia were prepared. The experiment was performed in triplicates. After 48-h incubation, mycelia were collected from each liquid culture using a Buchner funnel to determine fungal biomass and to investigate gene expression. Liquid filtrates were also collected from the same cultures at the same time to determine laccase activity and to perform polyacrylamide gel electrophoresis. The laccase protein was then purified from the liquid culture filtrate treated with 0.6 mM CuSO4 and followed by NanoLC ESI MS/MS analysis.
Enzyme assays
Measurement of enzyme activity was based on the oxidative dimerisation of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma). The reaction mixture contained 100 µl of ABTS (26 mg/ml), 800 µl of 0.1 M phosphate buffer (pH 6.0) and 100 µl of enzyme extract (culture filtrate) in a final volume of 1 ml. The reaction was incubated at room temperature (24°C) for 5 min, where the reaction was linear, and monitored by measuring absorbance at 436 nm (Guetsky et al. 2005) using a UV-visible spectrophotometer (Thermo Scientific Helios). One unit (U) of enzyme activity was defined as the amount of enzyme that converts one micromole of substrate per minute. Enzyme activities of all the samples were expressed as U ml−1. Total protein in culture filtrates was determined by the Bradford method (Bradford 1976).
RNA extraction, cDNA synthesis and RT-qPCR
RNA was extracted from the fungal mycelia using the ISOLATE II RNA Plant Kit (Bioline) following the manufacturer’s guidelines. RNase-free DNase I was used to digest traces of contaminating genomic DNA. RNA quality and quantity were determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). cDNAs were synthesised from 1 µg of RNA using the High-Capacity cDNA Reverse Transcriptase Kit (Thermo Fisher Scientific, USA).
Laccase gene-specific primers were designed to measure the expression of laccase genes (supplementary Table). Aliquots of 2 µl from all cDNA samples were used for RT-qPCR in a 20 µl reaction mixture that contained 10 µl of 2x iTAQ Universal SYBR Green Supermix master mix (Bio-Rad), 0.5 µl of forward and reverse primers (Supplementary Table), and 7 µl of water. Two technical replicates were performed from each experimental unit. RT-qPCR was performed on a CFX96 real-time system (Bio-Rad) with cycling conditions of 3 min, initial denaturation at 95°C, 46 cycles for 10 s denaturation at 95°C, and 30 s annealing at 58°C, followed by melting curve analysis to confirm the specificity of PCR amplification. The relative mRNA expression levels (fold changes) of all laccases (BcLCC1, BcLCC2, and BcLCC3) were calculated using the ∆∆Ct method (Livak and Schmittgen 2001) and normalised to the expression of the ubiquitous housekeeping gene, ActinA (Simon et al. 2013; Kelloniemi et al. 2015). The mean gene expression in each treatment was normalised to the control (NoI).
Determination of fungal biomass
Fungal biomass of the mycelia was determined by measuring the constant dry weight. Fungal mycelia were harvested onto filter paper (45 mm diameter Whatman No 2) under vacuum and kept at 75°C in an oven until a constant weight was achieved.
Polyacrylamide gel electrophoresis
For purification of extracellular laccase, the collected liquid filtrates from the cultures of B. cinerea grown in different copper concentrations were thawed and centrifuged to remove precipitated polysaccharides. Each filtrate was concentrated using 30 kDa membrane filters (Merck Millipore, Amicon Ultra – 15 Centrifugal Filters, Castle Hill, NSW, Australia). Twenty microlitres of concentrated filtrate was mixed with 30 µl of NuPAGE LDS (4X) sample buffer (Novex, Life Technologies, USA) (pH 6.8), denatured at 95°C and 40 µl was loaded into each lane of a 12% precast gel (CriterionTM TGXTM, BIO-RAD), followed by electrophoresis at room temperature using Tris-glycine buffer (pH 8.3) at 300 V (Laemmli 1970). The polyacrylamide gels were stained with Coomassie Brilliant Blue R-250 (Castle Hill, NSW, Australia) and visualised using a Gel Doc system (Bio-Rad). A high range molecular marker (Bio-Rad) was used for the determination of 75 kDa protein.
Laccase purification from the fungal culture filtrate and identification
The liquid culture filtrate of TN077 collected from LIM supplemented with 0.6 mM CuSO4 was treated as described above in polyacrylamide gel electrophoresis analysis to remove precipitated polysaccharides followed by mixing with ammonium acetate in cold methanol. They were mixed in a ratio of one volume of the filtrate to five volumes of 0.1 M w/v ammonium acetate in cold methanol, kept at −20°C overnight (Vincent et al. (2006), and then centrifuged at 4000 rpm, for 20 min. The pellet was collected and washed with 5 ml cold acetone and dissolved in 30 µl of NuPAGE LDS (4X) sample buffer (Novex, Life Technologies, USA), followed by SDS/PAGE. The bands corresponding to 75 kDa were excised from the polyacrylamide gel and sent to the Australian proteome analysis facility (APAF; Macquarie University, Sydney) for identification using NanoLC ESI MS/MS. Detailed methods for NanoLC ESI MC/MC data acquisition are available in supplementary method.
Statistical analysis
Normality of the distribution of data from enzyme assays, transcriptomics analysis and fungal biomass, and the constancy of residuals were confirmed using the normality test. The data of laccase activity and relative expressions of each laccase gene in each isolate in the presence and absence of different inducers were subjected to one-way analysis of variance (ANOVA), and the means of each treatment, with inducers were compared with the control, without inducers (NoI) using Dunnett’s mean comparison test. Laccase activity data and the relative expression of each laccase gene in TN077 and in the presence of each copper concentration were subjected to one-way analysis of variance (ANOVA), and the means in each concentration were compared using Tukey’s mean comparison test. Statistical significance was attained at the 5% probability level and all statistical analyses were performed using MiniTab 15.0 (MiniTab, Inc., State College, PA).
Results
Laccase activity in B. cinerea increases 48 hpi in LIM
Laccase activity in the culture filtrates of all isolates in B. cinerea increased in response to a mixture of CuSO4 and GA in LIM 48 hpi (Figure 1).
Figure 1.

Effect of inducers on the laccase activity in three isolates of Botrytis cinerea (TN080, VRU0005, TN077) in laccase-inducing medium; kinetics of laccase activity was measured within 12-h incubation of Botrytis cinerea isolates in media supplemented with combination of inducers (GA and CuSO4)
Copper induces laccase activity and BcLCC2 and BcLCC3 gene expressions
In the presence of both inducers separately, copper induced the expression of laccase genes and increased the enzyme activity in B. cinerea. All isolates, except VRU0005, showed significantly (p < 0.05) increased laccase activity with CuSO4, whereas no significant increases in laccase activity in the presence of GA occurred for any of the isolates (Figure 2(a)). As a response to CuSO4, B. cinerea isolates showed different amounts of laccase activities, with TN080 the greatest (p < 0.05) (Figure 2(a)), and showed a significant (p < 0.05) increase in laccase activity when the medium contained a mixture of CuSO4 and GA (Figure 2(a)).
Figure 2.

Effect of inducers (CuSO4 and GA) apart on the laccase activity (a) and the normalised expression levels of BcLCC1, BcLCC2, BcLCC3 of Botrytis cinerea isolates (TN080), (b); VRU0005, (c); TN077, (d). The laccase-inducing medium was supplemented with the inducers, GA and CuSO4, and the control (NoI) was composed of basal medium without inducers. Error bars represent the standard error calculated from three biological replicates. Columns represent the three isolates in each inducer and columns with (*) are significantly different from the control of each isolate at p < 0.05 (Dunnett’s mean comparison test)
In terms of relative gene expression, all isolates showed significantly different expressions of BcLCC2 and BcLCC3 genes in the presence of CuSO4 with the greatest (p < 0.05) in TN080 (Figure 2(b)). Conversely, no expression of laccase genes was detected as a response to GA. However, in VRU0005, expression of only the BcLCC2 was evident (p < 0.05) with both inducers, whereas there was no significant difference in the expression of BcLCC1 and BcLCC13 (Figure 2(c)). However, in TN077, the expression of both BcLCC2 and BcLCC3 was significantly different (p < 0.001) in the presence of CuSO4, whereas a significant (p < 0.015) down-regulation was observed in BcLCC2 and BcLCC3 in the presence of GA (Figure 2(d)). For all isolates, the expression of BcLCC2 was induced by CuSO4. However, the expression of BcLCC1 was constant as observed in all isolates.
Copper increases laccase activity and induces BcLCC2 transcription in TN077 at an optimum concentration of 0.6 mM
Laccase activities in TN077 gradually increased with increasing concentration of copper, with the greatest (p < 0.001) at 0.6 Mm (Figure 3(a)). However, laccase activities were not significantly different between the highest, 0.8 mM and low concentrations (0 and 0.2 mM) (Figure 3(a)).
Figure 3.
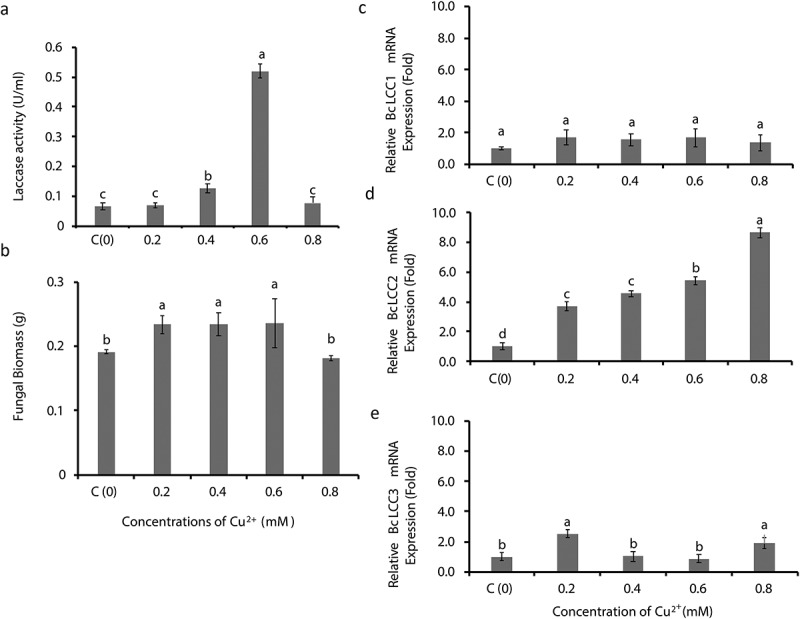
Copper-inducible laccase production in Botrytis cinerea (TN077) in the medium supplemented with different concentrations of CuSO4; (a) Effects of different concentrations of copper on laccase activity, (b) fungal biomass, and expression levels of laccase genes, (c) BcLCC1, D) BcLCC2 and E) BcLCC3 in Botrytis cinerea (TN077). The control medium was composed of basal medium with no inducers (NoI). Error bars represent the standard error calculated from three independent replicates and different letters (a,b,c,d) represent significant differences in mean value of each column at p < 0.05 (Tukey’s mean comparison test, n = 3)
Fungal biomass was significantly increased (p < 0.05) when the culture medium was supplemented with 0.2 mM CuSO4, and this remained the same with increasing concentrations up to 0.6 mM (Figure 3(b)). However, in the concentration between 0.8 mM and 0 mM (control), the fungal biomass was not significantly different (p > 0.05) and follows the same pattern of laccase activities as shown in the same concentration (Figure 3(a)).
Significant expression of BcLCC2 (p < 0.001) (Figure 3(d)) and BcLCC3 (p < 0.001) (Figure 3(e)) was observed along with different concentrations of CuSO4, whereas there was no significant expression of BcLCC1 (p > 0.05 (Figure 3(c)). However, BcLCC2 expression was concentration-dependent with a five-fold increase at 0.6 mM CuSO4, and BcLCC2 showed a significant (p < 0.001) up-regulation relative to BcLCC3. The greatest laccase activity with a 40-fold increase of BcLCC2 expression and fungal biomass was observed at 1 mM concentration (data not shown).
NanoLC ESI MS/MS confirms BcLCC2 as copper inducible laccase with the molecular mass 63.4 kDa
SDS/PAGE resulted in a single protein band of ≈75 kDa in that supplemented with 0.6 mM CuSO4 (Figure 4). ESI/MS/MS confirmed the molecular mass of this band as 63.4 kDa, and the peptides matched with LAC2-BOTFU Laccase-2 OS = Botryotinia fuckeliana GN = LAC2, and the score sequence coverage was 994 (individual ions scores > 37 indicate identity or extensive homology (p < 0.0059)).
Figure 4.

SDS-PAGE analysis for the purified protein from the two separate liquid culture filtrates (T1, T2) of Botrytis cinerea supplemented with 0.6 mM CuSO4. The basal medium with 0 mM CuSO4 and PDB as control media and M = protein molecular weight marker
Discussion
The lack of knowledge of the biological basis of the induction and expression of laccase genes in B. cinerea limits our understanding of their role in grey mould development. This study examined the expression of laccase genes (BcLCC1, BcLCC2 and BcLCC3) and enzyme activity in B. cinerea with respect to inducers, GA and CuSO4. Laccase activities of B. cinerea isolates peaked after 48-h incubation in LIM, when the culture medium was supplemented with a mixture of CuSO4 and GA. The expression of laccases genes when tested in the presence of each inducer alone, CuSO4 rather than GA induced laccase activity and the expression patterns of BcLCC2 depended on the isolate. The synthesis and secretion of laccases in response to Cu2+ have been reported to be species and isolate dependent (Piscitelli et al. 2011; Park et al. (2015). The concentration of Cu2+ required for laccase induction varied between 0.01 and 1 mM, and that this depended on the fungal species (Passarini et al. 2015) as demonstrated in white rot fungi (Palmieri et al. 2000; Fonseca et al. 2010) and ascomycetes such as Aspergillus flavus (Gomaa and Momtaz 2015) and G. graminis (Litvintseva and Henson 2002). The induction of laccase in Dichomitus squalens has an optimum Cu2+concentration as low as 0.06 mM, whereas the marine filamentous non-heterocystous cyanobacterium, Phormidium valderianum, is negatively affected when the concentration of Cu2+ is greater than 0.01 mM (Kannaiyan et al. 2012). However, this is not always the case. The growth of some isolates of wood rotting fungi is stimulated with low concentrations of Cu2+, while others may be inhibited by even the lowest concentration (Guillén and Machuca 2008). From our study, it appears that a copper concentration of 0.6 mM is the optimum concentration for laccase induction in B. cinerea.
B. cinerea stimulated fungal growth in a dose depended manner up to 0.6 mM Cu2+. However, concentrations beyond this become inhibitory to fungal growth as a result of oxidative stress. This can be explained as Cu2+ mediated oxidative stress, which is extremely toxic to fungal cells (Fernández-Larrea and Stahl 1996; Manzl et al. 2004; Kannaiyan et al. 2012). As Cu2+stimulates fungal growth at concentrations below 0.6 mM, it is reasonable to suggest that there is a correlation between laccase activity and fungal biomass, unless the fungal growth is affected by the copper toxicity. Although 1 mM is toxic to fungal biomass, the dramatic increase of laccase activity and the induction of BcLCC2 at this concentration could imply the over-expression of laccase to alleviate copper toxicity. It has been reported that fungi produce laccases to scavenge ROS to protect themselves from oxidative stresses caused by free Cu2+ (Stohs and Bagchi 1995) and, as a defence mechanism against copper (Viswanath et al. 2008). This scenario suggests that BcLCC2 might be the stress-regulated laccase in B. cinerea as previously suggested by Schouten et al. (2002), although further investigation into this is required.
The consistent expression of BcLCC2 along with different concentrations of Cu2+ indicates that BcLCC2 is the copper-responsive gene. Laccase 2 has been reported to be the copper-inducible gene in Gaeumannomyces graminis (Litvintseva and Henson 2002), whereas Pleurotus ostreatus has more than one copper-responsive gene (Palmieri et al. 2000). BcLCC3 was also found to be inducible, although the isozyme was not found. BcLCC2 isozyme was identified from the liquid culture filtrate treated with 0.6 mM copper. The molecular mass of BcLCC2 was confirmed to be 63 kDa in size, although 75 kDa laccase protein has been reported before (Slomczynski et al. 1995). The present study, however, demonstrated the molecular mass75 kDa before ESI/MS/MS analysis, which appears to concur with the findings of Slomczynski et al. (1995). This difference of the molecular mass could be due to the nature of the glycoprotein (Morozova et al. 2007; Maestre-Reyna et al. 2015), the function is presumably to confer thermostability of laccases up to 70°C (Yaropolov et al. 1994).
Most fungal laccases are inducible, although at least one laccase isozyme can be produced constitutively (Baldrian 2006). We observed the expression of BcLCC1 to be constitutive, which explains why minor laccase activity was observed with no inducers. Besides the type of inducers, in-vitro fungal laccase production is dependent on culture conditions, nutrient levels, medium composition, fungal isolates or the strain, and developmental stage and the time of incubation of the fungus (Viswanath et al. 2014; Garrido-Bazán et al. 2016). Despite the fact that Cu2+ induced increased laccase activity, CuSO4 in conjunction with GA resulted in the greatest laccase activity. Similarly, increased laccase activities in Ganoderma lucidum (Manavalan et al. 2013), Trametes spp. (Jang et al. 2006) and Pycnoporus sanguineus (Ramirez-Cavazos et al. 2014) have been reported in the presence of combinations of different inducers, although the reason for this is not yet known.
The gene induction and the expression of laccase protein could be attributed to internal regulation mechanisms such as micro RNAs (miRNAs). Copper-miRNA is a systemic regulator of copper enzymes as microRNA expression patterns have shown negatively correlate with the accumulation of copper protein transcripts as reported in Arabidopsis (Abdel-Ghany and Pilon 2008; Delye 2013). Jin and Wu (2015) observed in B. cinerea that up-regulated miRNAs inhibited the expression of their target genes involving the post-transcriptional modifications. In B. cinerea, copper-miRNA might have been involved in the regulation of the transcription of BcLCC2 in the presence of copper. However, this concept is yet to be investigated.
In conclusion, this study confirms that BcLCC2 and BcLCC3 in B. cinerea are induced in the presence of Cu2+. BcLCC2 induction is a maximum at a Cu2+ concentration of 0.6 mM, and at higher concentrations, fungal growth is inhibited. However, the expression of the target gene apparent to be induced by a Cu2+ dependent internally regulated mechanism resulting in different laccase activities and expression patterns depending on the isolate. Copper induction of BcLCC2 transcripts encodes a 63.4 kDa isoform of the enzyme in B. cinerea at the early stage of growth in a liquid culture. This study provides novel insight into BcLCC2 induction in which Cu2+ as the inducer for the expression of BcLCC2-63.4 kDa enzyme, hence establishes an enzyme-gene linkage relevant to the type of inducer. However, such a link for BcLCC3 and its induction as a response to a relevant inducer remains to be elucidated in future research and discovery of specific inducers for individual laccases may provide a better platform for understanding the biological basis of laccase expression during different stages of the disease development.
Supplementary Material
Acknowledgements
The authors thank Prof Leigh Schmidtke, National Wine and Grape Industry Centre, Charles Sturt University for valuable comments and suggestions, Dr Padraig Strappe, Central Queensland University, Australia for providing his valuable suggestions and Dr Nadeeka Bandara St. Vincent’s Institute of Medical Research, Melbourne, Australia for his technical support and guidance in the lab. This research project was funded by a CSU Postgraduate Research Scholarship awarded to U.V.A. Buddhika. Protein identification was undertaken at the Australian Proteome Analysis Facility with infrastructure provided by the Australian Government through the National Collaborative Research Infrastructure Strategy (NCRIS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Supplementary material
The supplementary data for this article can be accessed here.
References
- Abdel-Ghany SE, Pilon M.. 2008. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem. 283:15932–15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amselem J, Cuomo CA, van Kan JAL, Viaud M, Benito EP, Couloux A, Coutinho PM, de Vries RP, Dyer PS, Fillinger S, et al. 2011. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7:e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian P. 2006. Fungal laccases - occurrence and properties. Fems Microbiol Rev. 30:215––242.. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. [DOI] [PubMed] [Google Scholar]
- Castilho FJ, Torres RA, Barbosa AM, Dekker RF, Garcia JE. 2009. On the diversity of the laccase gene: a phylogenetic perspective from Botryosphaeria rhodina (Ascomycota: fungi) and other related taxa. Biochem Genet. 47:80–91. [DOI] [PubMed] [Google Scholar]
- Collins PJ, Dobson A. 1997. Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol. 63:3444–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, et al. 2012. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 13:414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delye C. 2013. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Manag Sci. 69:176–187. [DOI] [PubMed] [Google Scholar]
- Elad Y, Pertot I, Prado AMC, Stewart A. 2016. Plant hosts of Botrytis spp. In: Fillinger S, Elad Y, editors. Botrytis – the Fungus, the pathogen and its management in agricultural systems. Cham: Springer International Publishing; p. 413–486. [Google Scholar]
- Fernández-Larrea J, Stahl U. 1996. Isolation and characterization of a laccase gene from Podospora anserina. Mol Gen Genet MGG. 252:539–551. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Shimizu E, Zapata PD, Villalba LL. 2010. Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina). Enzyme Microb Technol. 46:534–539. [DOI] [PubMed] [Google Scholar]
- Garrido-Bazán V, Téllez-Téllez M, Herrera-Estrella A, Díaz-Godínez G, Nava-Galicia S, Villalobos-López MÁ, Arroyo-Becerr A, Bibbins-Martínez M. 2016. Effect of textile dyes on activity and differential regulation of laccase genes from Pleurotus ostreatus grown in submerged fermentation. AMB Express. 6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G. 2010. Laccases: a never-ending story. Cell Mol Life Sci. 67:369–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigi O, Marbach I, Mayer AM. 1980. Induction of laccase formation in Botrytis. Phytochemistry. 19:2273–2275. [Google Scholar]
- Gigi O, Marbach I, Mayer AM. 1981. Properties of gallic acid-induced extracellular laccase of Botrytis cinerea. Phytochemistry. 20:1211–1213. [Google Scholar]
- Gomaa OM, Momtaz OA. 2015. Copper induction and differential expression of laccase in Aspergillus flavus. Braz J Microbiol. 46:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetsky R, Kobiler I, Wang X, Perlman N, Gollop N, Avila-Quezada G, Hadar I, Prusky D. 2005. Metabolism of the flavonoid epicatechin by laccase of Colletotrichum gloeosporioides and its effect on pathogenicity on avocado fruits. Phytopathology. 95:1341–1348. [DOI] [PubMed] [Google Scholar]
- Guillén Y, Machuca Á. 2008. The effect of copper on the growth of wood-rotting fungi and a blue-stain fungus. World J Microbiol Biotechnol. 24:31–37. [Google Scholar]
- Jang MY, Ryu WY, Cho MH. 2006. Enhanced production of laccase fromTrametes sp. by combination of various inducers. Biotechnol Bioprocess Eng. 11:96–99. [Google Scholar]
- Jin W, Wu F. 2015. Characterization of miRNAs associated with Botrytis cinerea infection of tomato leaves. BMC Plant Biol. 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannaiyan R, Mahinpey N, Mani T, Martinuzzi RJ, Kostenko V. 2012. Enhancement of Dichomitus squalens tolerance to copper and copper-associated laccase activity by carbon and nitrogen sources. Biochem Eng J. 67:140–147. [Google Scholar]
- Kelloniemi J, Trouvelot S, Heloir MC, Simon A, Dalmais B, Frettinger P, Cimerman A, Fermaud M, Roudet J, Baulande S, et al. 2015. Analysis of the molecular dialogue between gray mold (Botrytis cinerea) and grapevine (Vitis vinifera) reveals a clear shift in defense mechanisms during berry ripening. Mol Plant-Microbe Interact. 28:1167–1180. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee HS, Kwon HJ, Patnaik BB, Nam KW, Han YS, Bang IS, Han MD. 2014. Effects of different selenium levels on growth and regulation of laccase and versatile peroxidase in white-rot fungus, Pleurotus eryngii. World J Microbiol Biotechnol. 30:2101–2109. [DOI] [PubMed] [Google Scholar]
- Kuhar F, Papinutti L. 2014. Optimization of laccase production by two strains of Ganoderma lucidum using phenolic and metallic inducers. Rev Argent Microbiol. 46:144–149. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Litvintseva AP, Henson JM. 2002. Cloning, characterization, and transcription of three laccase genes from Gaeumannomyces graminis var. tritici, the take-all fungus. Appl Environ Microbiol. 68:1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lorenzini M, Azzolini M, Tosi E, Zapparoli G. 2013. Postharvest grape infection of Botrytis cinerea and its interactions with other moulds under withering conditions to produce noble-rotten grapes. J Appl Microbiol. 114:762–770. [DOI] [PubMed] [Google Scholar]
- Maestre-Reyna M, Liu WC, Jeng WY, Lee CC, Hsu CA, Wen TN, Wang AHJ, Shyur L. 2015. Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS One. 10(4):e0120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela MR, Lundell T, Hatakka A, Hildén K. 2013. Effect of copper, nutrient nitrogen, and wood-supplement on the production of lignin-modifying enzymes by the white-rot fungus phlebia radiata. Fungal Biol. 117:62––70.. [DOI] [PubMed] [Google Scholar]
- Manavalan T, Manavalan A, Thangavelu KP, Heese K. 2013. Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem Eng J. 70:106–114. [Google Scholar]
- Manzl C, Enrich J, Ebner H, Dallinger R, Krumschnabel G. 2004. Copper-induced formation of reactive oxygen species causes cell death and disruption of calcium homeostasis in trout hepatocytes. Toxicology. 196:57–64. [DOI] [PubMed] [Google Scholar]
- Marbach I, Harel E, Mayer AM. 1984. Molecular properties of extracellular Botrytis cinerea laccase. Phytochemistry. 23:2713–2717. [Google Scholar]
- Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI. 2007. “Blue” laccases. Biochemistry (Mosc). 72:1136–1150. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Akutsu K. 2014. Virulence factors of Botrytis cinerea. J Gen Plant Pathol. 80:15–23. [Google Scholar]
- Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G. 2000. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol. 66:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Kang HW, Ha BS, Kim SI, Kim S, Ro HS. 2015. Strain-dependent response to Cu2+ in the expression of laccase in Pycnoporus coccineus. Arch Microbiol. 197:589–596. [DOI] [PubMed] [Google Scholar]
- Passarini MR, Ottoni CA, Santos C, Lima N, Sette LD. 2015. Induction, expression and characterisation of laccase genes from the marine-derived fungal strains Nigrospora sp. CBMAI 1328 and Arthopyrenia sp. CBMAI 1330. AMB Express. 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V. 2011. Induction and transcriptional regulation of laccases in fungi. Curr Genomics. 12:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Cavazos LI, Junghanns C, Nair R, Cardenas-Chavez DL, Hernandez-Luna C, Agathos SN, Parra R. 2014. Enhanced production of thermostable laccases from a native strain of Pycnoporus sanguineus using central composite design. J Zhejiang Univ Sci B. 15:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner ADM, Boddy L. 1988. Fungal decomposition of wood: its biology and ecology. Chichester, Sussex, UK: John Wiley & Sons Ltd. [Google Scholar]
- Rha E, Park HJ, Kim MO, Chung YR, Lee CW, Kim JW. 2001. Expression of exo-polygalacturonases in Botrytis cinerea. FEMS Microbiol Lett. 201:105–109. [DOI] [PubMed] [Google Scholar]
- Sbaghi M, Jeandet P, Bessis R, Leroux P. 1996. Degradation of stilbene-type phytoalexins in relation to the pathogenicity of Botrytis cinerea to grapevines. Plant Pathol. 45:139–144. [Google Scholar]
- Schouten A, Wagemakers L, Stefanato FL, van der Kaaij RM, van Kan JAL. 2002. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol Microbiol. 43:883–894. [DOI] [PubMed] [Google Scholar]
- Shah P, Gutierrez-Sanchez G, Orlando R, Bergmann C. 2009. A proteomic study of pectin-degrading enzymes secreted by Botrytis cinerea grown in liquid culture. Proteomics. 9:3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Dalmais B, Morgant G, Viaud M. 2013. Screening of a Botrytis cinerea one-hybrid library reveals a Cys2His2 transcription factor involved in the regulation of secondary metabolism gene clusters. Fungal Genet Biol. 52:9–19. [DOI] [PubMed] [Google Scholar]
- Slomczynski D, Nakas JP, Tanenbaum SW. 1995. Production and characterization of laccase from Botrytis cinerea 61-34. Appl Environ Microbiol. 61:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon EI, Sundaram UM, Machonkin TE. 1996. Multicopper oxidases and oxygenases. Chem Rev. 96:2563––2606.. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 18:321–336. [DOI] [PubMed] [Google Scholar]
- Thurston CF. 1994. The structure and function of fungal laccases. Microbiology-Sgm. 140:19–26. [Google Scholar]
- Valette-Collet O, Cimerman A, Reignault P, Levis C, Boccara M. 2003. Disruption of Botrytis cinerea pectin methylesterase gene Bcpme1 reduces virulence on several host plants. Mol Plant Microbe Interact. 16:360–367. [DOI] [PubMed] [Google Scholar]
- Van Kan JA, Stassen JH, Mosbach A, Van Der Lee TA, Faino L, Farmer AD, Papasotiriou DG, Zhou S, Seidl MF, Cottam E., et al. 2017. A gapless genome sequence of the fungus botrytis cinerea. Mol Plant Pathol. 18:75––89.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent D, Wheatley MD, Cramer GR. 2006. Optimization of protein extraction and solubilization for mature grape berry clusters. Electrophoresis. 27:1853–1865. [DOI] [PubMed] [Google Scholar]
- Viswanath B, Chandra MS, Kumar KP, Pallavi H, Reddy BR. 2008. Fungal laccases and their biotechnological applications with special reference to bioremediation. Dyn Biochem Process Biotech Mol Biol. 2:1–13. [Google Scholar]
- Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G. 2014. Fungal laccases and their applications in bioremediation. Enzyme Res. 2014:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson B, Tudzynski B, Tudzynski P, van Kan JA. 2007. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 8:561–580. [DOI] [PubMed] [Google Scholar]
- Yaropolov AI, Skorobogat’ko OV, Vartanov SS, Varfolomeyev SD. 1994. Laccase. Appl Biochem Biotechnol. 49:257–280. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.


