ABSTRACT
The tumor microenvironment includes a complex network of cytokines and chemokines that contribute to shaping the intratumoral immune reaction. Understanding the mechanisms leading to immune-hot (Immunoscore-high) altered (excluded and immunosuppressed) and cold tumors are of critical importance for successful anti-cancer therapies. Two essential mechanisms are highlighted. Specific chemokines and adhesion molecules appeared to target and attract immune effector T cells to the tumor microenvironment and to specific regions within the tumor. These mechanisms are dependent upon intratumoral IL-15 expression. Decreased IL15 expression also affected the local proliferation of B and T lymphocytes. A comprehensive analysis revealed a major contribution of IL15 in shaping the tumor immune contexture. Thus, an in situ lymphocytic infiltration is mediated through chemokines and attraction inside or around the tumor microenvironment, and an IL15-mediated in situ lymphocytic proliferation, which expand the local pool of intratumoral cytotoxic CD8 T-cells are key determinants of the immune contexture. Increased IL15 expression and local proliferation of T-cells were associated with decreased risk of tumor recurrence and prolonged survival of cancer patients. These data provide further mechanisms to prioritize research and help in designing better therapeutic interventions.
KEYWORDS: T-cells, proliferation, attraction, IL-15, chemokine, chromosomal instability, tumor microenvironment, colorectal cancer, prognosis, survival, immunity, immunoscore
Attraction and in situ lymphocyte proliferation to shape a proper immune contexture
Considerable progress has been made in the understanding of cancer and of intratumor immunity.1 Accumulating evidence suggests that tumor progression, invasion, metastatic dissemination and tumor recurrence are shaped and governed by the intratumoral immune landscape.1,2 From pre-cancer lesions to late metachronous metastases, the immune microenvironment plays a central role regarding cancer development and patients’ survival.1,2 The cytotoxic and memory T cells infiltrating primary tumors best predict survival of patients, notably in colorectal cancer.1–6 A powerful concept in oncology, the cancer immune contexture,1,2,7 came with the observation that the type, density, quality and location of immune cell within the tumor site predicted colorectal cancer (CRC) patients’ survival better than the classical TNM system, for the first time for any cancer.1,2 This led to the development and implementation of the Immunoscore. Immunoscore is a standardized consensus scoring-system based on densities of two lymphocyte populations (CD3, CD8) infiltrating the tumor (CT) and invasive margin (IM) that has a highly significant prognostic value, and that has been introduced into cancer classification (WHO classification of Digestive System Tumors) and into clinical guidelines (ESMO).1,2
The immune contexture parameters are associated with long-term survival and prediction of response to treatments.8 We first described three major immune coordination profiles (hot, altered, cold) observed within primary CRCs, thus classified according to the balance between tumor escape and immune coordination. The altered phenotype was further divided in two distinct patterns, “excluded” and “immunosuppressed”.8 This “excluded” phenotype reflects the ability of the host immune system to mount a T-cell-mediated immune response, and also the ability of the tumor to physically hindering T-cell infiltration. In contrast to these physical barriers, an “immunosuppressed” phenotype is characterized by suppressive cells, receptors and soluble factors, limiting further recruitment and expansion.8 Understanding the mechanisms leading to hot, altered (excluded and immunosuppressed) and cold tumors is of critical importance for successful anti-cancer therapies. Some mechanisms are related to host genetics, to microenvironmental characteristics, to intrinsic tumor cell features, but also to the tremendously complex interplay between the tumor and the immune system.
Given the major importance of the local immune reaction at the tumor site, it is critical to understand mechanisms resulting in high or low densities of specific immune cells within the tumors. Genomic alterations that occur in neoplastic cells during tumor development and progression could influence the tumor microenvironment. Our study presented a comprehensive analysis of all known cytokines and cytokine receptors, using comparative genomic hybridization combined with system biology approaches, functional network tailored pipeline and large-scale data analysis of the intratumoral immune reaction.9 We investigated mechanisms that may explain the difference in the intratumoral densities of particularly important immune cells?
The power of integrative cancer immunology approaches and functional network tailored pipeline allowed to define, in patients, relevant mechanisms resulting in high or low densities of specific immune cells within the tumor.
Our comprehensive analysis determined whether alterations of cytokine function contributed to tumor progression and how cytokines are shaping the intra-tumoral immune reaction. We highlighted several important findings (Figure 1).9
We revealed which chromosomal instability of cytokine genes is associated with tumor progression, lymph-node metastasis and distant metastasis.
Within the tumor microenvironment, IL-15 was produced by epithelial cells and activated myeloid cells.
We showed for the first time that copy number variation of the IL15 gene was a mechanism associated with different densities of intratumor immune cells in tumor regions.
We pinpointed IL15 as a pivotal cytokine in human tumors, and shown that IL15 is involved into the local proliferation of T and B cells within the tumor, and particularly CD8 cytotoxic T-cells.
We demonstrated that actively proliferating lymphocytes are correlating with the survival of the patients.
Figure 1.
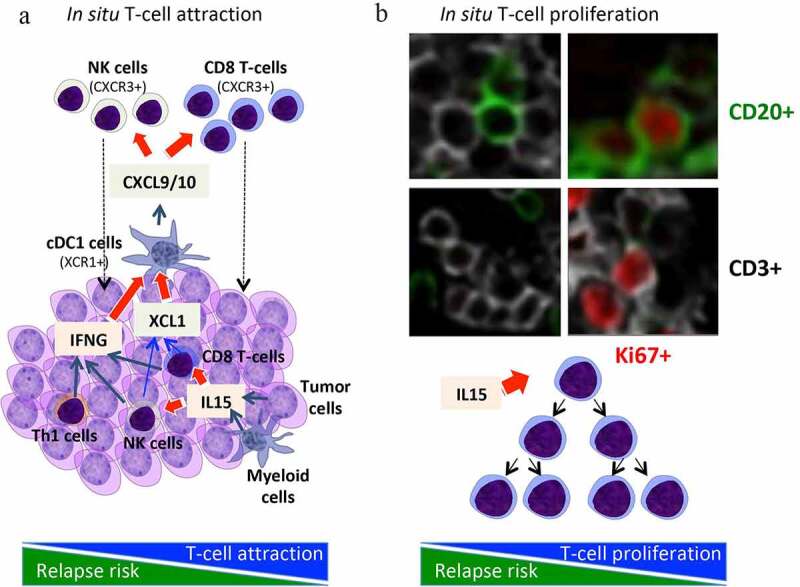
Intrametastatic immune landscape. (a) an in situ lymphocytic infiltration is mediated through chemokines and attraction inside or around the tumor microenvironment. IL15, produced mainly by activated myeloid cells and tumor cells, activates cytotoxic CD8 T-cells and NK-cells, which together with Th1 cells produce IFNG, a major cytokine involved together with XCL1 in shaping a good immune contexture and in activating cDC1 cells. These activated cDC1 cells, by producing chemokines such as CXCL9/10 are attracting CXCR3+ cells, notably memory CD45RO+ T-cells, cytotoxic CD8 T-cells and NK-cells. (b) In parallel, IL15 is also inducing a direct in situ lymphocytic proliferation, which expand the local pool of intratumoral cytotoxic CD8 T-cells. IL15 and these proliferating CD8 T-cells were associated with prolonged survival in cancer patients
We also revealed mechanisms determining the densities (high or low) of specific immune cells in colorectal tumors (Figure 1).10 Chemokines and adhesion molecules appeared to target and attract immune effector T cells to the tumor microenvironment and to specific regions within the tumor. The majority of cells attracted by CX3CL1 were TH1 and effector-activated cytotoxic T cells, whereas CXCL9 and CXCL10 attracted mostly memory CD45RO T cells.10
In mouse models, multiple and concomitant effects, combining both expansion of tumor-resident lymphocytes and recruitment of new immune cells via chemokine gradients led to the increased tumor accumulation of CD8+ T and NK cells observed in hetIL-15 treated animals (Figure 1). Activated lymphocytes also secrete XCL1 in response to hetIL-15 treatment, a chemokine involved in the enhancement of intratumoral influx of cDC1. Importantly, CXCL9 and CXCL10 secretion by myeloid cells require exposure to IFN-γ, suggesting a positive feedback loop whereby the hetIL-15-dependent increase in intratumoral XCL-1 and IFN-γ–producing lymphocytes enhances cDC1 recruitment, amplifying the CXCL9/10-mediated intratumoral infiltration by CXCR3+ effector lymphocytes. Therapy with hetIL-15 stimulated trafficking of CD8+ T and NK cells into the tumors, and promoted proliferation (increased Ki67 levels) and survival (increased Bcl-2) of the intratumoral lymphocytes.11 Overall, mouse model results showed that hetIL-15 administration, may be a general method to enhance T and cDC1 cell entry in tumors, increasing the success rate of immunotherapeutic interventions.
Immunostimulatory cytokines are often employed as immunological adjuvants, in order to unleash the immunogenic potential of other immunotherapeutic agents, such as tumor-targeting vaccines and checkpoint inhibitors. There are preclinical and clinical advances for the use of cytokines as immunostimulatory factors, including IL-15, in oncological indications.12–16
In translation of many results, combination immunotherapy clinical trials were initiated with IL-15.16 A phase I trial was initiated involving recombinant human IL-15 (rhIL-15), nivolumab and ipilimumab in patients with malignancy (NCT03388632). A clinical trial is being initiated for patients with cancer using an intralesional anti-CD40 in combination with rhIL-15. Combination therapy of IL-15 with anticancer monoclonal antibodies including rituximab (anti-CD20) or alemtuzumab (anti-CD52), showed clinical benefit in mouse models. IL-15 enhanced the ADCC and therapeutic efficacy of both antibodies. These results provided the scientific basis for trials of IL-15 combined with alemtuzumab (anti-CD52) for patients with ATL (NCT02689453), with obinutuzumab (anti-CD20) for patients with CLL (NCT03759184), and with avelumab (anti-PD-L1) in patients with T-cell lymphoma (NCT03905135) and renal cancer (NCT04150562).16
Our data highlight the power of integrative cancer immunology approaches to understand the challenging issues of tumor progression and tumor recurrence in human cancer. This knowledge can be used to prioritize research and therapeutic interventions.
Funding Statement
The work was supported by INSERM, the LabEx Immuno-oncology, the Transcan ERAnet European project, the Society for Immunotherapy of Cancer (SITC), Association pour la Recherche contre le Cancer (ARC), Site de Recherche intégrée sur le Cancer (SIRIC) CAncer Research for PErsonalized Medicine (CARPEM), MedImmune, AstraZeneca, La Ligue contre le Cancer, The Qatar National Research Fund (QNRF) grant number NPRP11S-0121-180351, Assistance publique – Hôpitaux de Paris (AP-HP), HalioDx, Agence Nationale de la Recherche (ANR Grant TERMM ANR-20-CE92-0001), and Institut National du Cancer, France (INCa).
Disclosure of potential conflicts of interest
JG and BM have patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx biotech company. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM) licensed to HalioDx.
References
- 1.Galon J, Bruni D.. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–3. doi: 10.1016/j.immuni.2019.12.018. PMID: 31940273. [DOI] [PubMed] [Google Scholar]
- 2.Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020. doi: 10.1038/s41568-020-0285-7. PMID: 32753728. [DOI] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33:335–340. doi: 10.1007/s00281-011-0264-x. PMID: 21461991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. doi: 10.1186/s12967-016-1029-z. PMID: 27650038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. doi: 10.1093/intimm/dxw021. PMID: 27121213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlecnik B, Bifulco C, Bindea G, Marliot F, Lugli A, Lee JJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, et al. Multicenter international society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020;38:JCO1903205. doi: 10.1200/jco.19.03205. PMID: 32897827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987. doi: 10.1189/jlb.1107773. PMID: 18559950; [pii] 10.1189/jlb.1107773. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. PMID: 30610226. [DOI] [PubMed] [Google Scholar]
- 9.Mlecnik B, Bindea G, Angell HK, Sasso MS, Obenauf AC, Fredriksen T, Lafontaine L, Bilocq AM, Kirilovsky A, Tosolini M, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med. 2014;6:228ra237. doi: 10.1126/scitranslmed.3007240. PMID: 24648340. [DOI] [PubMed] [Google Scholar]
- 10.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–1440. doi: 10.1053/j.gastro.2009.10.057. PMID: 19909745. [DOI] [PubMed] [Google Scholar]
- 11.Bergamaschi C, Pandit H, Nagy BA, Stellas D, Jensen SM, Bear J, Cam M, Valentin A, Fox BA, Felber BK, et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J Immunother Cancer. 2020;8:e000599. doi: 10.1136/jitc-2020-000599. PMID: 32461349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vacchelli E, Aranda F, Bloy N, Buqué A, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Spisek R, et al. Trial Watch-Immunostimulation with cytokines in cancer therapy. Oncoimmunology. 2016;5:e1115942. doi: 10.1080/2162402x.2015.1115942. PMID: 27057468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vacchelli E, Aranda F, Obrist F, Eggermont A, Galon J, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunostimulatory cytokines in cancer therapy. Oncoimmunology. 2014;3:e29030. doi: 10.4161/onci.29030. PMID: 25083328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacchelli E, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunostimulatory cytokines. Oncoimmunology. 2013;2:e24850. doi: 10.4161/onci.24850. PMID: 24073369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vacchelli E, Galluzzi L, Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G. Trial watch: immunostimulatory cytokines. Oncoimmunology. 2012;1:493–506. doi: 10.4161/onci.20459. PMID: 22754768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. IL-15 in the combination immunotherapy of cancer. Front Immunol. 2020;11:868. doi: 10.3389/fimmu.2020.00868. PMID: 32508818. [DOI] [PMC free article] [PubMed] [Google Scholar]


