ABSTRACT
The plasma membrane NADPH Oxidase-derived ROS as signaling molecules play crucial roles in salt stress response. As the motor organelle of cells, mitochondria are also important for salt tolerance. However, the possible interaction between NADPH Oxidase-derived ROS and mitochondria is not well studied. Here, a transgenic Arabidopsis expressing mitochondrial matrix-targeted pH-sensitive indicator cpYFP was used to monitor the pH dynamics in root cells under salt stress. A significant alkalization in mitochondria was observed when the root was exposed to NaCl or KCl, but not osmotic stress such as isotonic mannitol. Interestingly, when pretreated with the NADPH Oxidase inhibitor DPI, the mitochondrial alkalization in root cells was largely abolished. Genetic evidence further showed that salt-induced mitochondrial alkalization was significantly reduced in the loss of function mutant atrbohF. Pretreatment with endocytosis-related inhibitor PAO or TyrA23, which inhibited the ROS accumulation under salt treatment, almost abolished this effect. Furthermore, [Ca2+]cyt increase might also play important roles by affecting ROS generation to mediate salt-induced mitochondrial alkalization as indicated by treatment with plasma membrane Ca2+ channel inhibitor LaCl3 and mitochondrial Ca2+ uniporter inhibitor Ruthenium Red. Together, these results suggest that the plasma membrane NADPH Oxidase-derived ROS promote the mitochondrial alkalization under salt treatment, providing a possible link between different cellular compartments under salt stress.
KEYWORDS: Salt, pH, mitochondria, NADPH Oxidase, ROS, Arabidopsis
Introduction
Soil salinity is a major environmental stress affecting crop production and food security worldwide and salt stress mainly causes ion toxicity, osmotic stress and oxidative damage to plants.1–3 It has been well documented that reactive oxygen species (ROS) induced by salt stress, acting as signaling molecules, play important roles on adaptive responses, although they are toxic at higher concentration.4
There are five potential sources for ROS generation under various stresses, the plasma membrane, chloroplasts, mitochondria, peroxisomes and apoplasts.5,6 As a major source of ROS, the plasma membrane NADPH Oxidase plays important roles in salt responses in Arabidopsis.7,8 ROS derived from AtRbohD and AtRbohF function as signaling molecules to increase proline synthesis,9 K+/Na+ ratio7,8 and antioxidative activities10 under salt stress in Arabidopsis, thus contributing to salt stress tolerance. Interestingly, salt treatment could also induce the endocytosis of NADPH Oxidase from the plasma membrane;11 it is suggested that the internalization of NADPH Oxidase might contribute to salt response.12 When the endocytosis pathway was inhibited, salt-induced ROS accumulation in roots was abolished.13
Salt stress also induces increases in cytosolic-free Ca2+ concentration ([Ca2+]cyt), which is mainly caused by Ca2+ influx through the plasma membrane Ca2+ channels. A recently identified plant cell-surface glycosyl inositol phosphorylceramide (GIPC) sphingolipids could sense Na+ to trigger Ca2+ influx,14 which acted as an important salt-sensing mechanism. Both as important second messengers, Ca2+ and ROS cross-talk is suggested in many processes including salt stress response.15
As selective uptake of essential ions such as K+ and selective exclusion or efflux of Na+ are all ATP-consuming processes. Plant mitochondria, the primary function of which is ATP generation via oxidative phosphorylation, are considered to play important roles in salinity stress response.16,17 In rice roots, high respiration rates can enhance salinity tolerance by facilitating ion exclusion.18 However, the underlying regulation mechanism for ATP generation is largely unknown.
The alkaline pH level in mitochondria (pHm) plays a central role in the formation of a proton gradient and electrochemical potential that drives the ATP synthesis. It has been shown that pHm is finely tuned and can be influenced by several bioactive species and environmental parameters.19,20 At present, the pHm is detected mostly by pH-sensitive fluorescent probes and green fluorescent protein-based pH indicators. In plants, due to their convenience for targeting specific parts of the cell, genetic-encoded pH-sensitive GFP derivatives were preferred. Different kinds of GFP-based pH sensors such as pHluorin have been constructed in plants.21 cpYFP (circular permuted yellow fluorescent protein), originally considered as a superoxide indicator, was recently identified as a high-sensitivity, high-pKa pH sensor.22,23
In the present study, the possible relationship and communication between the plasma membrane NADPH Oxidase-derived ROS and mitochondria under salt stress was studied and it is found that ROS derived from the NADPH Oxidase promoted mitochondrial alkalization under salt stress in Arabidopsis root cells, which might further contribute to the ATP generation.
Materials and methods
Plant materials and growth conditions
The mitochondrial-targeted cpYFP transgenic Arabidopsis seeds were kindly provided by Dr. Qu Lijia (Peking University).24 The rbohF mutant line (SALK_034674) was kindly provided by Dr. Hao Fushun (Henan University) which was originally obtained from the Arabidopsis Biological Resource Center (ABRC).8
Seeds were surface sterilized with 0.1% HgCl2 for about 5 min and rinsed with deionized water for at least four times, and then the seeds were sown on solid 1/2 Murashige and Skoog medium containing 1% (w/v) agar and 1% sucrose (pH was adjusted to about 5.9 with KOH), and incubated for 2 d at 4°C and then petri dishes were placed vertically in a plant growth chamber under 120 μmol photons m−2 s−1 with a 16 h/8 h (day/night) photoperiod at 22 ± 1°C and a relative humidity at 60%. Five-day-old Arabidopsis seedlings were used for imaging in this study.
Identification of homozygous mutants with the mito-cpYFP indicator
The rbohF was crossed to the mito-cpYFP in the wild type (ecotype Columbia (Col-0)); then, the homozygous mutants with mito-cpYFP indicator were identified by confocal microscope and PCR analysis as indicated by Ma et al.8
Salt treatment and pharmacological assays
Five-day-old seedlings were put on the dish and then exposed to solutions of 140 mM NaCl, 300 mM mannitol or mock for time-course imaging. For pharmacological assays, the seedlings were incubated in DPI, PAO, TyrA23, LaCl3 and Ruthenium Red at indicated concentrations for 1 h before salt treatment.
Imaging of mito-cpYFP with Laser confocal scanning microscopy (LCSM)
All microscopic observations were performed using a Laser confocal scanning microscopy (Zeiss, LSM710, Germany). The cpYFP signal was visualized with excitation at 488 nm and emission at 525 nm.
H2O2 levels detection in roots under different treatments
The H2O2 levels in roots under different treatments were detected according to He et al. (2012) with some modification.24 Briefly, roots of five-day-old seedlings were incubated in 50 μΜ H2DCFDA for 15 minutes in darkness, after washed for three times with the buffer, the roots were exposed to salt stimuli or pretreated with inhibitors such as LaCl3. The imaging was carried out at the same condition of mito-cpYFP.
Quantitative analysis
Microscope images were analyzed with ImageJ for calculating the average fluorescent intensity. More than three independent experiments were analyzed. The results were expressed as means ± standard divisions of biological replicates.
Results
Exogenous NaCl treatment induced mitochondrial alkalization in Arabidopsis root cells
To directly monitor the dynamic changes of pH in mitochondria under salt stimuli, mitochondrial-targeted cpYFP, a genetic-encoded pH-sensitive indicator, transgenic plants were used.23 When the Arabidopsis root was exposed to 140 mM NaCl (140 mM was chosen as this concentration of salt was previously shown to substantially inhibit root growth but does not cause seedling death25), the fluorescence increased rapidly (Figure 1). After treatment for about 30 minutes, more than two-fold increase of fluorescence was observed, indicating the rapid alkalization in root cell mitochondria under salt stimuli.
Figure 1.
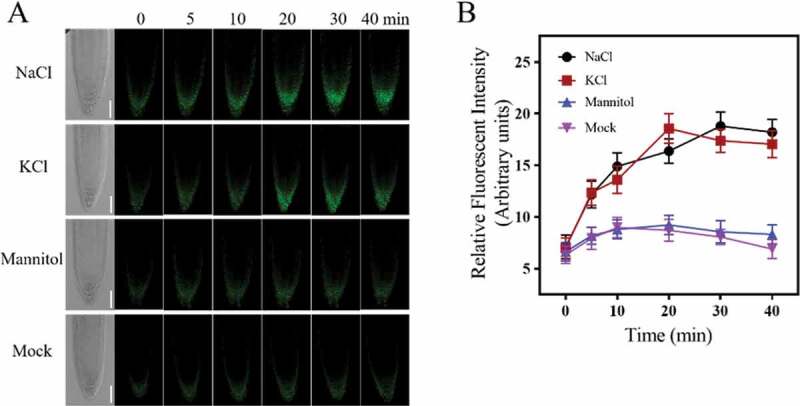
Salt treatment-induced mitochondrial alkalization in Arabidopsis root tip cells. (a) LCSM images of mito-cpYFP in Arabidopsis root tip cells, which were treated with 140 mM NaCl, 140 mM KCl, 300 mM mannitol or mock (1/2 MS). The figure showed a representative layer of a 1 μm optical section along the z-axis. Figures at different time points were chosen at almost the same position according to the transmitted images. Scale bars= 50 μm. (b) The kinetics of the relative fluorescent intensity of root tip cells under different treatments. Data represent means ± standard divisions from at least three independent experiments (n≥5)
Consistent with previous studies, the alterations in mitochondrial morphology under salt treatment were also observed. After 10 minutes of NaCl treatment, mitochondria began to show an aggregated distribution, by about 30–40 min, mitochondria showed a significant clustered morphology within the cytoplasm, which was similar to that under phytohormone SA treatment.26 Furthermore, when treated with isotonic mannitol or even higher osmotic potential such as 300 mM, no significant fluorescence increase or mitochondrial morphology change was observed in 60 min treatment, indicating that salt-induced mitochondrial alkalization and morphology changes in mitochondria were mainly caused by ionic stress but not osmotic stress.
Pretreatment with NADPH Oxidase inhibitor DPI or impairment in AtRbohF reduced NaCl-induced mitochondrial alkalization
Previous study showed that salt but not mannitol treatment could induce the accumulation of ROS in Arabidopsis roots,12 we hypothesized that salt treatment activated the NADPH Oxidase activity and led to increases in cytosolic ROS, which further affected the mitochondria behavior. As a first try, pharmacological assays were taken. As shown in Figure 2, when pretreated with 15 μM NADPH Oxidase inhibitor DPI (diphenyleneiodonium chloride), at which concentration salt-induced ROS accumulation in root cells was significantly reduced (FigureS1), salt-induced mito-cpYFP fluorescence increase was significantly reduced compared with that in the wild type. When treated with higher concentration of DPI at 30 μM, the basal fluorescence was reduced to a lower level and almost no fluorescence increase was observed under NaCl treatment in 40 min observation. These results indicated that inhibition of the NADPH oxidase activity with DPI could significantly reduce salt-induced mitochondrial alkalization.
Figure 2.
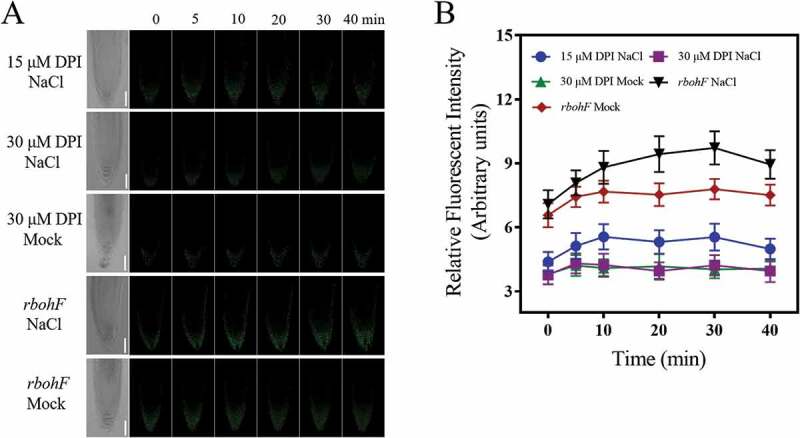
Pretreatment with NADPH Oxidase inhibitor DPI blocked salt-induced mitochondrial alkalization. (a) LCSM images of mito-cpYFP in root tip cells under NaCl or mock (1/2 MS) treatment when pretreated with 15 μM and 30 μM DPI in wild type or in rbohF mutant. Scale bars= 50 μm. (b) The kinetics of the fluorescence intensities of root tip cells under different treatments. Data represent means ± standard divisions from at least three independent experiments (n≥5)
Considering the relative higher transcription level of AtrbohF in root tips in the control and salt stress condition,27,28 to further study the genetic basis of ROS-induced mitochondrial alkalization, homozygous atrbohF with the mito-cpYFP was constructed. Under NaCl treatment, the mito-cpYFP fluorescence in atrbohF mutant was significantly weakened when compared with that in the wild type (Figure 2), suggesting that AtRbohF might act as an important source of ROS to mediate salt-induced mitochondrial alkalization at least in root tip cells.
The endocytosis of NADPH Oxidase facilitated salt-induced mitochondrial alkalization
Previous studies showed that salt stress could stimulate the endocytosis of NADPH Oxidase from the plasma membrane, which might contribute to the maintaining of the activities of plasma membrane localized NADPH Oxidase.10 To test the role of internalization of NADPH Oxidase on salt-induced mitochondrial alkalization, we analyzed the effects of PAO and TyrA23, two inhibitors of clathrin-mediated endocytosis (CME) pathway.29–31 TyrA23 was previously considered as a tyrosine kinase inhibitor to block CME; however, a recent study suggested that it might inhibit CME pathway largely through cytosolic acidification.31 As is shown in Figure 3, pretreatment with both inhibitors strongly suppressed salt-induced mito-cpYFP fluorescence increase. Consistent with previous studies,13 PAO or TyrA23 pretreatment significantly reduced NaCl-induced ROS accumulation in root cells as indicated by the fluorescent probe H2DCFDA (Figure S2). These results indicated that the CME pathway facilitated the NADPH Oxidase-derived ROS generation, which further contributed to ROS-induced mitochondrial alkalization under salt treatment.
Figure 3.
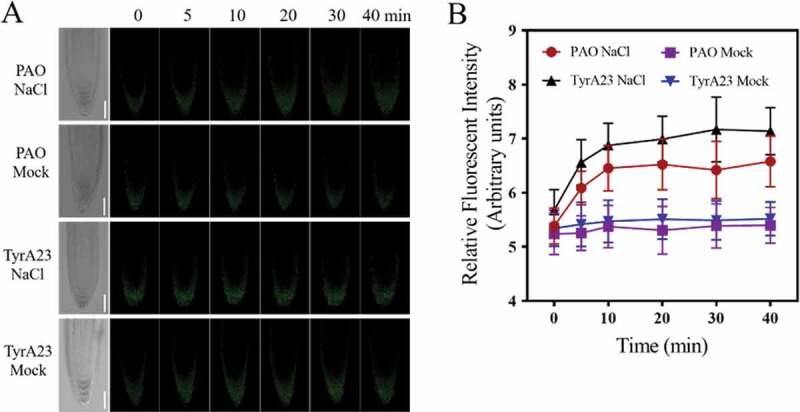
Pretreatment with endocytosis inhibitor PAO reduced salt-induced mitochondrial alkalization. (a) LCSM images of mito-cpYFP in root tip cells under NaCl or mock (1/2 MS) treatment when pretreated with 10 μM PAO or 50 μM TyrA23. Scale bars= 50 μm. (b) Changes in the relative fluorescent intensity of root tip cells under different treatments at indicated time points. Data represent means ± standard divisions from at least three independent experiments (n≥5)
Role of [Ca2+]cyt increase in NADPH Oxidase-derived ROS-mediated mitochondrial alkalization under salt stress
Considering the importance of Ca2+ in ROS signaling and salt tolerance for plants,15 we further tested whether the salt-stimulated increase of [Ca2+]cyt played a role in regulating salt-induced mitochondrial alkalization by pharmacological assays. The plasma membrane Ca2+ channel inhibitor LaCl3, which could significantly reduce the salt-induced increase of [Ca2+]cyt (Figure S3), was used. When pretreated with 50 μM LaCl3 before salt exposure, to some extent the salt-induced mitochondrial alkalization was abolished (Figure 4), indicating an important role for Ca2+ influx mediated by plasma membrane Ca2+ channels in this process. At the same time, LaCl3 treatment significantly reduced salt-induced ROS accumulation in root cells (Figure S4), indicating that [Ca2+]cyt increase might regulate salt-induced mitochondrial alkalization through affecting salt-induced ROS accumulation in root cells. On the other hand, considering the reciprocal regulation between ROS and Ca2+ under salt stress,15 it is possible that salt-induced accumulation of ROS especially through the plasma membrane NADPH Oxidase might further induce the increase of [Ca2+]cyt. However, whether [Ca2+]cyt increase regulates ROS-induced mitochondrial alkalization is still obscure and further study is needed. Interestingly, mitochondrial Ca2+ uniporter inhibitor Ruthenium Red (RR) treatment had no significant effects on the salt-induced mitochondrial alkalization (Figure 4), which further indicated the important roles of [Ca2+]cyt increase in salt-induced and NADPH Oxidase-derived ROS-mediated mitochondrial alkalization.
Figure 4.
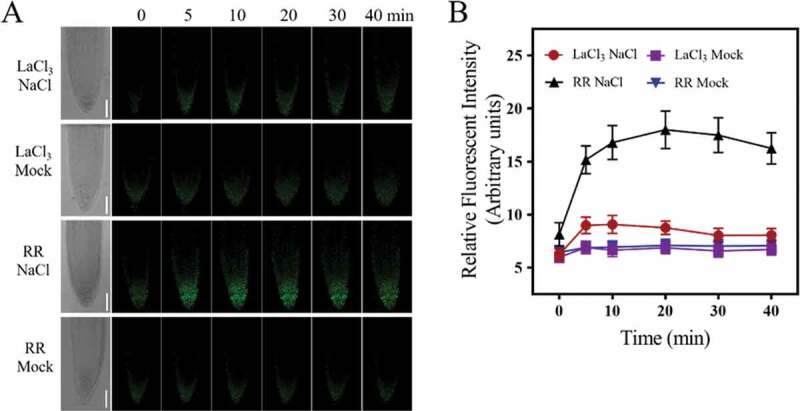
Effects of interfering with salt-induced plasma membrane Ca2+ influx on salt-induced mitochondrial alkalization. (a) LCSM images of mito-cpYFP in root tip cells under NaCl or mock (1/2 MS) treatment when pretreated with 50 μM LaCl3 or 10 μM RR. Scale bars= 50 μm. (b) Changes in the relative fluorescent intensity of root tip cells under different treatments at indicated time points. Data represent means ± standard divisions from three independent experiments (n≥5)
Discussion
As a semi-autonomous organelle, mitochondrion plays important roles in plant development and stress responses.32 Signaling or communication between mitochondria and other organelles or cytoplasm is essential for proper cellular function. The alkaline pH level in mitochondria is closely related with many biological processes, but how it is regulated and the dynamic changes under different stimuli is not precisely understood.33
In this study, we demonstrated that the NADPH Oxidase-derived ROS promoted mitochondrial alkalization under salt stress, based mainly on the following results. Firstly, salt stress could induce mitochondrial alkalization in root cells as indicated by the pH-sensitive indicator cpYFP (Figure 1).34 Secondly, pretreatment with the NADPH Oxidase inhibitor DPI significantly reduced this effect (Figure 2). Thirdly, in the NADPH Oxidase F loss of function mutant rbohF, salt-induced mitochondrial alkalization was impaired (Figure 2), indicating that the NADPH Oxidase F contributed to this process at least in the root tip cells. Fourthly, when the ROS generation-related endocytosis pathway was inhibited by PAO or TyrA23, this effect was also abolished (Figure 3). These results strongly indicated a role for NADPH Oxidase-derived ROS in promoting the mitochondrial alkalization under salt stress and presented a possible link between different cellular compartments under salt stress condition. Interestingly, a similar mechanism seemed to exist also in animal cells. For example, mitochondrial alkalization was observed under exogenous H2O2 stimuli in animal cell lines indicated by a mitochondrial pH-sensitive fluorescent indicator Mito-pH-1.33 In diabetic nephropathy, advanced glycation end-products (AGEs) induced cytosolic ROS generation mainly through the NADPH Oxidase, cytosolic ROS further facilitated the production of mitochondrial superoxide and promoted diabetic kidney disease.33 These results indicated a possible conserved function for ROS to mediate the communication between the plasma membrane and mitochondria.
Several studies have demonstrated that pHm controls the rate of oxidative phosphorylation, so salt-induced mitochondrial alkalization can ensure the uptake of essential substances for oxidative metabolisms and elevate ATP synthetic rates, which is important for salt tolerance in plants.16 On the other hand, pHm is associated directly or indirectly with several important biological processes occurring in mitochondria, such as the ROS generation, Ca2+ homeostasis and programmed cell death, salt-induced pHm elevation might also affect these processes as an early response to salt stress. Further studies are expected to monitor the pHm dynamics under long-term salt exposure and respiration dynamics in both roots and shoots to explore the role of pHm alternation and ATP synthesis in salt tolerance. The mechanism by which NADPH Oxidase-derived ROS induce the alkalization in mitochondria is still obscure, and further research is needed to uncover the detailed underlying mechanism including whether Ca2+ signaling mediates this process.
In response to cellular and environmental stresses, mitochondria undergo morphology transitions regulated by dynamic processes of membrane fusion and fission and mitochondrial dynamics are important for cellular activity regulation.35 Besides salt stress, similar results for mitochondrial morphology changes were observed in many other conditions such as phytohormone salicylic acid-treated leaf tissues.26,35 It has been shown that mitochondrial ROS (mtROS) were closely related to this mitochondrial morphology transition.26 It is possible to speculate that pHm might be involved in this process. In fact, previous study showed that the pHm is involved in the regulation of the ROS generation in mitochondria.36 It will be interesting to explore whether mitochondrial alkalization induce mitochondrial morphology transition by promoting mtROS generation under salt stress.
Supplementary Material
Acknowledgments
We thank Dr. Lijia Qu (Peking University) for kindly providing the mito-cpYFP Arabidopsis seeds, and Dr. Fushun Hao (Henan University) for kindly providing the atrbohF seeds, which were originally provided by ABRC.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 31170252).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:1–6. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munns R, Tester M.. Mechanism of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018;217:523–539. doi: 10.1111/nph.14920. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Zhang H, Song C, Zhu JK, Shabala S. Mechanisms of plant responses and adaptation to soil salinity. Innovation. 2020. doi: 10.1016/j.xinn.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 6.Pottosin I, Shabala S. Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci. 2014;5:154. doi: 10.3389/fpls.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang C, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Smith JAC, Harberd NP. ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. Embo J. 2012;31:4359–4370. doi: 10.1038/emboj.2012.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺ homeostasis in Arabidopsis under salt stress. J Exp Bot. 2012;63:305–317. doi: 10.1093/jxb/err280. [DOI] [PubMed] [Google Scholar]
- 9.Ben Rejeb K, Lefebvre-De Vos D, Le Disquet I, Leprince AS, Bordenave M, Maldiney R, Jdey A, Abdelly C, Savouré A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015;208:1138–1148. doi: 10.1111/nph.13550. [DOI] [PubMed] [Google Scholar]
- 10.Ben Rejeb K, Benzarti M, Debez A, Bailly C, Savouré A, Abdelly C. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J Plant Physiol. 2015;174:5–15. doi: 10.1016/j.jplph.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Hao H, Fan L, Chen T, Li R, Li X, He Q, Botella MA, Lin J. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in arabidopsis. Plant Cell. 2014;26:1729–1745. doi: 10.1105/tpc.113.122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007;51:185–197. doi: 10.1111/j.1365-313x.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- 13.Golani Y, Kaye Y, Gilhar O, Ercetin M, Gillaspy G, Levine A. Inositol polyphosphate phosphatidylinositol 5-phosphatase9 (At5ptase9) controls plant salt tolerance by regulating endocytosis. Mol Plant. 2013;6:1781–1794. doi: 10.1093/mp/sst072. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Zhou X, Tao M, Yuan F, Liu L, Wu F, Wu X, Xiang Y, Niu Y, Liu F, et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature. 2019;572:341–346. doi: 10.1038/s41586-019-1449-z. [DOI] [PubMed] [Google Scholar]
- 15.Kurusu T, Kuchitsu K, Tada Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front Plant Sci. 2015;6:427. doi: 10.3389/fpls.2015.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacoby RP, Taylor NL, Millar AH. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011;16:614–623. doi: 10.1016/j.tplants.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Jacoby RP, Li L, Huang S, Lee CP, Millar AH, Taylor NL. Mitochondrial composition, function and stress response in plants. J Integr Plant Biol. 2012;54:887–906. doi: 10.1111/j.1744-7909.2012.01177.x. [DOI] [PubMed] [Google Scholar]
- 18.Malagoli P, Britto DT, Schulze LM, Kronzucker HJ. Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance. J Exp Bot. 2008;59:4109–4117. doi: 10.1093/jxb/ern249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abad MF, Di Benedetto G, Magalhaes PJ, Filippin L, Pozzan T. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J Biol Chem. 2004;279:11521–11529. doi: 10.1074/jbc.m306766200. [DOI] [PubMed] [Google Scholar]
- 20.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 21.Martinière A, Desbrosses G, Sentenac H, Paris N. Development and properties of genetically encoded pH sensors in plants. Front Plant Sci. 2013;4:523. doi: 10.3389/fpls.2013.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behera S, Xu Z, Luoni L, Bonza MC, Doccula FG, Michelis MID, Morris RJ, Schwarzländer M, Costa A. Cellular Ca 2+ signals generate defined pH signatures in plants. Plant Cell. 2018;30:2704–2719. doi: 10.1105/tpc.18.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu L-J, Gong Z. DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell. 2012;24:1815–1833. doi: 10.1105/tpc.112.098707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–945. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- 26.Nie S, Yue H, Zhou J, Xing D. Mitochondrial-derived reactive oxygen species play a vital role in the salicylic acid signaling pathway in Arabidopsis thaliana. PloS One. 2015;10:e0119853. doi: 10.1371/journal.pone.0119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Xie Y, Gu Q, Zhao G, Zhang Y, Cui W, Xu S, Wang R, Shen W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic Biol Med. 2017;108:465–477. doi: 10.1016/j.freeradbiomed.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Toufighi K, Brady SM, Austin R, Ly E, Provart NJ. The botany array resource: e-Northerns, expression angling, and promoter analyses. Plant J. 2005;43:153–163. doi: 10.1111/j.1365-313x.2005.02437.x. [DOI] [PubMed] [Google Scholar]
- 29.Aniento F, Robinson DG. Testing for endocytosis in plants. Protoplasma. 2005;226:3–11. doi: 10.1007/s00709-005-0101-y. [DOI] [PubMed] [Google Scholar]
- 30.Robinson DG, Jiang L, Schumacher K. The endosomal system of plants: charting new and familiar territories. Plant Physiol. 2008;147:1482–1492. doi: 10.1104/pp.108.120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dejonghe W, Kuenen S, Mylle E, Vasileva M, Keech O, Viotti C, Swerts J, Fendrych M, Ortiz-Morea FA, Mishev K, et al. Mitochondrial uncouplers inhibit clathrin-mediated endocytosis largely through cytoplasmic acidification. Nat Commun. 2016;7:11710. doi: 10.1038/ncomms11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberatore KL, Dukowic-Schulze S, Miller ME, Chen C, Kianian SF. The role of mitochondria in plant development and stress tolerance. Free Radic Biol Med. 2016;100:238–256. doi: 10.1016/j.freeradbiomed.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Cao L, Zhao Z, Zhang T, Guo X, Wang S, Li S, Li Y, Yang G. In vivo observation of the pH alternation in mitochondria for various external stimuli. Chem Comm. 2015;51:17324–17327. doi: 10.1039/c5cc07118f. [DOI] [PubMed] [Google Scholar]
- 34.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan ALY, Fukami K, Thallas-Bonke V, Nawroth PP, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. doi: 10.1681/asn.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol. 2013;304:R393–R406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert AJ, Brand MD. Superoxide production by NADH: ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


