ABSTRACT
Extracellular vesicles (EVs) secreted by bone marrow mesenchymal stem cells (BMSCs) protect intervertebral disc degeneration (IDD) by regulating nucleus pulposus cell (NPC) apoptosis. But the mechanism of BMSCs-EVs-microRNA (miR)-199a in IDD remains unclear. In this study, after the acquisition and identification of BMSCs and BMSCs-EVs, IDD mouse model was established and treated with BMSCs-EVs. The pathological changes of NPCs, positive expression of MMP-2, MMP-6 and TIMP1, and the senescence and apoptosis of NPCs were evaluated. Microarray analysis was employed to analyze the differentially expressed miRs and genes after EV treatment. NPCs were treated with EVs/miR-199a/TGF-β agonist SRI-011381. The positive expression of col II and Aggrecan was assessed. The target gene and downstream pathway of miR-199a were analyzed. In vivo experiment, after BMSCs-EV treatment, MMP-2, MMP-6, TIMP1 and TUNEL-positive cells in IDD mice were decreased, and miR-199a was increased. In vitro experiments, the expression of col Ⅱ and Aggrecan, SA-β gal positive cells and apoptosis rate of NPCs were decreased after EV intervention. The protective effect of BMSCs-EVs on NPCs was impaired by reducing miR-199a carried by EVs. miR-199a could target GREM1 to inactivate the TGF-β pathway. miR-199a carried by BMSCs-EVs promotes IDD repair by targeting GREM1 and downregulating the TGF-β pathway. Our work confers a promising therapeutic strategy for IDD.
KEYWORDS: Intervertebral disc degeneration, extracellular vesicles, bone mesenchymal stem cells, nucleus pulposus cells, miR-199a, GREM1, TGF-β signaling pathway
1. Introduction
Intervertebral disc degeneration (IDD) is the leading cause of low back pain, leading to a great burden on global health-care system [1,2]. Drug therapy, vertebrectomy and decompression can relieve the pain to some degree [3,4]. However, these two therapies have the weakness of recurrent pain and disc degeneration of adjacent segments [5]. It is well known that intervertebral discs (IVD) consist of three anatomical sub-structures, including nucleus pulposus (NP), annulus fibrosus, and cartilaginous endplates [6]. When IDD occurs, the proliferation activity and number of NPCs in IVD decrease, followed by the decrease of extracellular matrix synthesis [7]. Mesenchymal stem cell (MSC) is one of the main cell sources of IVD tissue engineering because of its ability of multidirectional differentiation and self-renewal [8,9]. In addition, a prior study suggests that bone MSCs (BMSCs) differentiate into cells that create extracellular matrix and rebuild the disc and upregulate NPC viability and inhibit senescence and apoptosis [10]. Coculture of NPCs with MSCs has the potential to aid in repairing, delaying, or preventing IDD and regenerating the matrix [11]. In this context, we carried out this study with the purpose of finding out novel treatment method for IDD patients based on the understanding of MSCs in NPC behaviors and IDD progression.
MSCs could secrete several kinds of extracellular vesicles (EVs) to keep tissue homeostasis for regular tissue function [12]. EVs are a kind of membrane-bound vesicles released from cell surface, which have profound impacts by acting as mediators of intercellular communication on normal physiology and pathological processes, and EVs contain a broad range of nucleic acids such as mRNA and small non-coding RNAs [13]. EVs are important in information exchange between BMSCs and NPCs, which would be an optimal therapy for IDD alone or with specific genes [14]. microRNAs (miRNAs) play critical roles in cell differentiation, proliferation and survival by targeting mRNAs, and emerging evidence supports the role of miRNAs in IDD processes [15]. MSC-EVs carry a mass of nucleic acids, proteins and lipids, with abundant miRNAs [16], and deliver these cargos into recipient cells, rationalizing underlying applications of MSC-EVs to cell-free therapy [17]. As a potent mediator of MSC-EVs and NPC apoptosis, exosomal miRs deserve further exploration. In this study, microarray analyses were applied to identify differentially expressed miRs and genes in NP tissues between IDD models and normal controls. We also carried out in vivo and in vitro models to identify the molecular mechanism of BMSCs-EVs in IDD progression with potential miR and genes.
2. Materials and methods
2.1. Ethics statement
This study was performed with the approval of the Clinical Ethical Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. Informed consent was received from all subjects. All procedures were strictly conducted in accordance with the code of ethics. Significant efforts were made to minimize both the animals and their pain.
2.2. Isolation and identification of BMSCs
The Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin without EVs was prepared. The bone marrow cavities of the femur and tibia of the rats were washed with the prepared medium in a sterile environment. The obtained marrow cells were cultured in a CO2 cell incubator in a petri dish, and the medium was refreshed every 2 days to observe the morphology and growth of the cells under the microscope. When the cells grew to 80%-90% confluence, cells were detached with 0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) solution. When the BMSCs cultured to the third generation grew to about 80% confluence, they were detached with 0.125% trypsin-EDTA solution and suspended in phosphate buffer saline (PBS) containing 2% bovine serum albumin. Then, the cell suspension of about 1 × 105 cells/tube was incubated with monoclonal antibodies (CD54, CD44, CD90 and CD34) coupled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) (Biolegend, San Diego, CA, USA) at room temperature in dark for 30 minutes, while the control tube was incubated with the same type of control antibody. The BMSCs were washed and resuspended with PBS, the samples were analyzed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA), and the data were processed by flow Jo software 8.7. In addition, BMSCs were cultured in the lipid induction system (dexamethasone 1 um, indomethacin 200 um, isobutyl methyl yellow Heyin 0.5 mm, insulin 10 ug/mL) and osteogenesis induction system (dexamethasone 0.1 um, ascorbic acid phosphate 50 um, β-glycerophosphate 10 mm), and then oil red O staining and alizarin red staining were used to identify the BMSCs’ ability of lipogenesis and osteogenesis.
2.3. Extraction and identification of BMSCs-EVs
EVs were isolated by ultracentrifugation according to previous reports [18]. Briefly, the fetal bovine serum (FBS) was ultra-centrifuged for 18 h at 100,000 × g, 4°C to remove EVs. The BMSCs were cultured with EV-free FBS for 72 hours in a CO2 incubator at 37°C. The supernatant (90 mL) of BMSCs was centrifuged at 4°C and 300 × g for 10 minutes, at 2000 × g for 10 minutes, at 10,000 × g for 30 minutes, and another centrifugation at 10,000 × g and 4°C for 2 hours. Then, the supernatant was removed and the pellet was BMSCs-EVs, which was resuspended in 100 μL PBS for identification. Then, 10 μL EV suspension was dripped onto the copper net covered by carbon membrane, standing at 25°C for 10 minutes. After removal of the excess liquid with filter papers, EVs were dyed with 3% phosphotungstic acid solution for 5 minutes, and observed under a transmission electron microscope running at 100 kV. The extracted EVs were diluted to the appropriate concentration with double distilled water, and the particle size and distribution of EVs were detected by nanosight nanoparticle tracking analyzer (Malvern Panalytical Co., Ltd, Malvern, Worcestershire, UK). In addition, Western blot was used to detect the levels of CD68 (0.5 μg/mL, ab125212), CD81 (1/1000, ab109201), tumor susceptibility gene 101 (TSG101) (1/1000, ab125011) and Calnexin (1 μg/mL, ab22595) (all from Abcam Inc., Cambridge, MA, USA).
2.4. Establishment of IDD models in mice
Forty male 2-month-old Balc/c mice with complete bone structure were purchased from the experimental animal center of Tongji Medical College of Huazhong University of Science and Technology (Wuhan, Hubei, China). Then, the mice were numbered according to their weight and randomized into Non group (only slitting mouse skin), IDD group (IDD modeling mice as previously described [19]), IDD + PBS group (IDD mice injected with equal volume of PBS), and IDD + EVs group (IDD mice injected with 100 μg/mL extracted EVs into the spine [20], once a week for totally 12 weeks), with 10 mice in each group.
After 12 weeks of treatment, all mice except those in the Non group were identified with disc damage by X-ray. The mice were euthanized by injection of excess pentobarbital sodium. The IVD tissues of L2-3 were extracted and sectioned for hematoxylin and eosin (HE) and TUNEL staining and immunohistochemistry; the IVD tissues of L3-4 were extracted for the separation of NPCs; the IVD of L4-5 were extracted for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Enzyme-linked immunosorbent assay (ELISA).
2.5. HE staining
After IVD tissues of L2-3 were extracted, fixed with 10% formalin for 24 hours, decalcified with 10% EDTA for 30 minutes, dehydrated, embedded in paraffin, stained with HE (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), and histopathological changes were observed under an optical microscope (200 ×) (Olympus Optical Co., Ltd, Tokyo, Japan).
2.6. Immunohistochemistry
IVD tissue sections at 5 μm of L2-3 were incubated antibodies against tissue inhibitor of matrix metalloproteinase1 (TIMP1) (1:1,000; ab34712), matrix metalloproteinase (MMP)2 (1:200; ab48394) and MMP6 (1:50; ab109012) overnight at 4°C. Then, IDD tissue sections were incubated with antibody against immunoglobulin G (IgG, 1:1,000; ab6721) for 30 minutes. Next, 3,3-diaminobenzidine (DAB, DA1010, Solarbio) was used for visualization. Five fields of 200 × magnification were randomly captured for each repetition using an inverted microscope (Nikon, Tokyo, Japan).
2.7. TUNEL staining
Seven micrometer sections of L2-3 tissue or NPCs were fixed in 4% paraformaldehyde for 10 min and then treated with 0.5% TritonX-100 for 10 minutes. After PBS washing, Sections and NPCs were incubated with the cell death detection kit (Roche, Basel, Switzerland). In addition, in vitro NPCs were counterstained for 5 minutes with 5 μg/mL 4ʹ,6-diamidino-2-phenylindole (DAPI) (Beyotime Biotechnology Co., Ltd, Shanghai, China), and images were captured under a microscope (Olympus, BX53).
2.8. RT-qPCR
Total RNA in tissues and cells was extracted by RNAiso Plus (Takara, Otsu, Shiga, Japan) and TRIzol LS reagent (Takara), respectively. Then, the reliability of RNA was verified by formaldehyde denaturing electrophoresis. Subsequently, the primescriptTM RT kit (Takara) was used to conduct RT-qPCR in strict accordance with the instructions. SYBR Premier Ex Taq (Takara) was used to quantify mRNA expression by standard real-time qPCR with U6 as a reference gene. The forward sequence of miR-199a primer was 5ʹ-GTCACAGTAGTCTGCACAT-3ʹ and the reverse sequence was 5ʹ-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAACCA-3ʹ. The forward sequence of U6 primer was 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and the reverse sequence was 5ʹ-AACG-CTTCACGAATTTGCGT-3ʹ.
2.9. mRNA microarray analysis
GSE34095 chip was downloaded from GEO (Gene Expression Omnibus) database, and Limma Rstudio package was used to filter chip data with LogFC >1.5 and P value <0.05. Subsequently, the differentially expressed genes were enriched and analyzed on the website of DAVID (https://david.ncifcrf.gov/) bioinformatics, and PPI (protein–protein interaction) was analyzed on STRIN (http://string-db.org/cgi/input.pl?sessionId=KaJYaJ1842HK&input_page_show_search=off), and Cytoscape was used for mapping.
2.10. miR microarray analysis
Microarray analysis was performed as described earlier [21]. In short, total RNA was extracted from the lumbar disc tissues of three healthy controls and three IDD patients. Then, 0.5 μg total RNA was used to synthesize cDNA by GeneChip 3ʹinvitro transcript express kit (Thermo Fisher Scientific, 902,789, Waltham, MA, USA). Subsequently, the cDNA was segmented and hybridized with human miR expression array V4.0 (miRCURY LNA™ Universal RT miR PCR Human panel). After hybridization, the microarray was washed and scanned with GeneChipTM Scanner 3000 7 G system (Thermo Fisher Scientific, 000213).
2.11. Isolation and culture of NPCs
IDD tissues from L3-4 of mice in the IDD and Non groups were extracted, treated with 0.05% trypsin and 0.25% collagenase at 37°C for 2 hours, centrifuged at 1000 g/min for 15 minutes, and the supernatant was removed. Cells were cultured in DMEM containing 10% fetal bovine serum (Gibco, Grand Island, USA), and then passaged when cell confluence reached 80%. Well-grown NPCs at the 3rd generation were assigned into IDD-NPC group; NPCs from IDD mice were treated with PBS buffer. IDD-NPC + EVs group; EVs were isolated by ultracentrifugation from BMSCs. NPCs were treated for 24 hours with 20 μM EVs. EVs/Mock group; EVs were isolated by ultracentrifugation from BMSCs, which were transfected for 48 hours with 50 nM negative control inhibitor (Mock). NPCs were treated for 24 hours with 20 μM EVs/Mock. EVs/Inhibitor group; EVs were isolated by ultracentrifugation from BMSCs, which were transfected for 24 hours with 50 nM miR-199 inhibitor. NPCs were treated for 24 hours with 20 μM EVs/Inhibitor. EVs + DMSO group; EVs were isolated by ultracentrifugation from BMSCs. NPCs were treated for 24 hours with 20 μM EVs and DMSO buffer. EVs + Agonist group; EVs were isolated by ultracentrifugation from BMSCs. NPCs were treated for 24 hours with 20 μM EVs and 10 μM TGFβ agonist (SRI-01138, HY-100347A, MedChemExpress, MCE) [22]. The non-degenerated disc derived NPCs (ID-NPC) at the 3rd generation were treated with PBS as the control group (ID-NPC + PBS).
2.12. Safranine O staining
The primary NPCs were seeded at 1 × 104 cells mL into 6-well culture plates containing a cover glass for cell slide climbing. When the cells grew into a single layer, take out the cover glass was taken out and the general morphology of NPCs was observed under the light microscope. After washing with PBS, the cover glass was fixed with 4% paraformaldehyde for 30 min, and washed with PBS 3 times. Then, the glass was stained with hematoxylin for 10 min, washed in running water, stained with ponceau 2 R-brilliant green for 10 min and washed with PBS 3 times. Next, the glass was stained with safranine O for 10 min and differentiated in 1% hydrochloric alcohol for 3 seconds after 3 PBS washes. After drying, the glass was sealed by resin and observed and photographed under the microscope.
2.13. Toluidine blue staining
After PBS washes, the cell slides were fixed with 4% paraformaldehyde for 30 minutes and washed in PBS 3 times again. Then, slides were stained with 1% toluidine blue for 10 minutes and washed 3 times in PBS. After drying, the slides were sealed by resin and observe and photographed under the light microscope.
2.14. Uptake of EVs by NPCs
Purified BMSCs-EVs were incubated with PKH26 (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 5 minutes. After PBS washes and centrifugation at 110,000 g for 90 min, EVs were suspended in basal medium and incubated with NPCs for 12 hours at 37°C. Then, EVs were stained by 0.1 g/mL DAPI (Beyotime) for 5 minutes, and NPCs were observed and captured under a fluorescence microscope (Olympus, BX53).
2.15. Immunofluorescence
NPCs of each group were rinsed on the glass cover slides, washed with PBS 3 times, and fixed with 4% paraformaldehyde at 4°C for 15 minutes. NPCs were treated with 0.5% Triton-100 X for 20 minutes. NPCs were blocked for 1 h with 5% goat serum at room temperature. And then cells were incubated with primary antibodies Aggrecan (1:100; AB1031) or Col II (1:80; AB2031, Sigma-Aldrich) at 4°C overnight, respectively. Subsequently, NPCs were washed with PBS and incubated with goat anti-rabbit secondary antibody (Alexa Fluor® 488, 1:1000, ab150077; Alexa Fluor® 647, 1:1000, ab150079) at 37°C for 1 h. Then, NPCs were counterstained by DAPI staining and observed.
2.16. Senescence-associated β-galactosidase (Saβ-G) staining
The activity of galactosidase in NPs was detected by Saβ-G staining kit (Beyotime). All experimental operations were strictly in accordance with the instructions. After staining, five fields were randomly selected for observation under the inverted microscope, and the senescent cells were dyed blue-green.
2.17. Flow cytometry
The treated cells were collected and centrifuged at 37°C for 5 minutes with the supernatant removed. The cells were washed with PBS and resuspended in 500 μL buffer solution. Then, cells were incubated with 5 μL annexin V-FITC for 15 minutes, and then with 5 μL PI staining solution at room temperature for 5 minutes in dark. Cell apoptosis was detected by a flow cytometer (Beckman Coulter, Chaska, MN, USA).
2.18. Dual-luciferase reporter gene assay
Online prediction of Starbase (http://starbase.sysu.edu.cn/) predicted the 3ʹuntranslated region (3ʹUTR) binding sequence of miR-199a and Gremlin 1 (GREM1), the wild-type (WT) of GREM1 and mutant (MT) of 3ʹUTR binding sequence were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China) and inserted into pMIR REPORTTM (Thermo Fisher Scientific) [23] luciferase reporter vector. The binding relationship between miR-199a and GREM1 was verified by double luciferase kit (Dual Glo ® dual-luciferase reporter gene detection system, E2920, Promega Corp., Madison, Wisconsin, USA) [24].
2.19. ELISA
The levels of TGF-β, Smad1, p-Smad1, Smad3 and p-Smad3 in IDD tissues of L4-5 and NPCs of each group were detected by ELISA kits (Shanghai Bogoo Biotechnology Co., Ltd., Shanghai, China).
2.20. Statistical analysis
Statistical analysis was conducted by SPSS 21.0 (IBM Corp. Armonk, NY, USA). All the data were in normality distribution checked by the Kolmogorov–Smirnov test. Measurement data were expressed as mean ± standard deviation (s.d). One-way or two-way analysis of variance (ANOVA) was utilized for comparisons among multiple groups, and Tukey’s multiple comparisons test for post hoc test. The p value was obtained by a two-tailed test and p < 0.05 indicated statistically significant.
3. Results
3.1. Identification of BMSCs-EVs
BMSCs began to adhere to the wall gradually after several hours of culture, with the extension of time, the adherent BMSCs showed clonal and aggregative growth (Figure 1(a)). To confirm that the cells we cultured were BMSCs, we used commercial medium Adipogenic and osteogenic induction of BMSCs at P3 generation, and detected the ability of lipogenesis and osteogenic differentiation by oil red O staining and alizarin red staining. There were red lipid droplets in BMSCs after oil red O staining (Figure 1(b)) and bright red lumps after alizarin red staining, indicating a large number of red nodules in calcium deposition inside and outside cells (Figure 1(c)). In addition, BMSCs surface markers were detected by flow cytometry. The results showed that CD54, CD44 and CD90 were positive and CD34 was negative (Figure 1(d)). In conclusion, the cells used in our experiment are in line with the definition of BMSCs.
Figure 1.

Identification of BMSCs-EVs. (a), representative image of primary BMSCs (× 200); (b,c), Oil red staining and Alizarin red staining were performed to validate the lipogenesis and osteogenic differentiation ability of BMSCs (× 200); (d), BMSCs surface markers (CD44, CD54, CD34 and CD90) were detected by flow cytometry; (e), transmission electron microscope of EVs at 5000 demonstrating homogenous, cup‐shaped vesicles with size in 80 nm. Scale bar represents 200 nm in both panels; (f), Nanoparticle tracking analysis of BMSCs-EVs; (g), western blot analysis assessed expression of EV markers in BMSCs
After that, we identified the EVs by nanoparticle tracker, transmission electron microscope and western blot analysis. The results showed that the EVs were round or ellipsoid (Figure 1(e)), the size of the EVs was about 80 nm (Figure 1(f)). CD68, CD81 and TSG101 were all positive, while Calnexin was negative (Figure 1(g)).
3.2. BMSCs-EVs promote the repair of IDD in mice
Firstly, HE staining was used to observe the status of the NP in the IVD of mice. The results showed that compared with mice in the Non group, the chondrocytes in the NP of IDD mice were necrotic in a large amount, and compared with the IDD + PBS group, the chondrocyte necrosis in the NP of mice in the IDD + EVs group were improved in some extent (Figure 2(a)). Then, we used immunohistochemistry to evaluate the positive expression of MMP-2, MMP-6 and TIMP1 in the NP of the IVD of mice in each group. The results showed that compared with the Non group, IDD group presented elevated positive expression of MMP-2 (Figure 2(b)), MMP-6 (Figure 2(c)) and TIMP1 (Figure 2(d)); compared with the IDD + PBS group, the IDD + EVs group showed significantly decreased expression of MMP-2, MMP-6 and TIMP1 (all p < 0.05). In addition, TUNEL staining evaluated the apoptosis of NPCs in each group. Compared with the Non group, NPC apoptosis in the IDD group was significantly increased, and compared with the IDD + PBS group, the apoptosis of NPCs s in the IDD + EVs group was significantly reduced (all p < 0.05) (Figure 2(e)).
Figure 2.

BMSCs-EVs promote the repair of IDD in mice. (a), HE staining was performed to represent for intervertebral disc pathology observation (× 200);(b–d), immunohistochemical staining to evaluate the positive expression of MMP-2 (b), MMP-6 (c) and TIMP1 (d) in the nucleus pulposus of the intervertebral disc of mice in each group; E, TUNEL staining were performed to measure apoptosis index of the intervertebral disc (× 200). Three independent experiments were performed, and each group contained at least 8 mice. Data are expressed as mean ± s.d. One-way ANOVA and Tukey’s multiple comparisons test were used to determine statistical significance. **p < 0.01
3.3. BMSCs-EVs promote proliferation of NPCs and inhibit apoptosis
In vivo experiments, we have confirmed the protective effect of EVs derived from BMMSCs on disc degeneration, but the effect of BMSCs-EVs in vitro is not clear. Therefore, we isolated and cultured mouse NPCs. It can be seen that the cells around the NP tissues crawled out in 2–3 days, and the primary NPCs showed a long spindle-like shape, with a relatively slow growth rate. After 5 days of culture, some of NPCs grew close to the tissue mass, which were clonal and polygonal or spindle-shaped (Figure 3(a)). To confirm that the cells we cultured were NPCs, safranine O staining and toluidine blue staining were performed. We found that the cells were positive in safranine O staining and pink in cytoplasm with red granular substance around the nucleus (Figure 3(b)); the cells were strong positive in toluidine blue staining, blue in cytoplasm, mostly in long fusiform and irregular in morphology, with obvious staining around the nucleus and in endocrinic granules (Figure 3(c)). In addition, the positive expression of col Ⅱ and Aggrecan was found by immunofluorescence staining (Figure 3(d)), indicating we have successfully isolated the NPCs. After that, we observed the absorption of EVs by NPCs under the fluorescence microscope. The results showed that NPCs treated with EVs had red fluorescence (PKH26), suggesting EVs could be absorbed by NPCs (Figure 3(e)).
Figure 3.

BMSCs-EVs promote proliferation of NPCs and inhibit apoptosis. (a), representative image of primary NPCs (× 200); (b), representative image of safranine O staining (× 200); (c), representative image of toluidine blue staining (× 200); (d), immunofluorescence of col II and Aggrecan represented for typical NPCs marker (× 200); (e), fluorescence microscopy showing the uptake of EVs labeled with the red fluorescent dye PKH26 (× 200); (f,g), Immunofluorescence of col II and Aggrecan (× 200); H, SA-βgal staining was utilized for NPC senescence measurement; and blue represented senescent cells; (i), TUNEL staining were performed to measure apoptosis index; green indicates TUNEL-positive cells, blue indicates nucleus (× 200). Three independent experiments were performed. Data are expressed as mean ± s.d. One-way ANOVA and Tukey’s multiple comparisons test were used to determine statistical significance. **p < 0.05
To confirm the effect of EVs on the function of NPCs, we detected the expression of col Ⅱ and Aggrecan in degenerative NPCs by immunofluorescence staining. It found that col Ⅱ (Figure 3(f)) and Aggrecan (Figure 3(g)) in the IDD-NPC + EVs group were higher than those in the IDD-NPC group (both p < 0.05). SAβ-gal and TUNEL staining showed that SAβ-gal positive cells (Figure 3(h)) and TUNEL positive cells (Figure 3(i)) in the IDD-NPC + EVs group were lower than those in the IDD-NPC group.
3.4. BMSCs-EVs promote miR-199a expression in IVD
As mentioned in the Introduction, EVs can encapsulate miRNAs and release it into cells. To further explore the mechanism of IDD repair by BMSCs-EVs, miR microarray was used to analyze the differentially expressed miRNAs after EVs treatment. It was found that multiple miRNAs were differentially expressed (miR-7, miR-155, miR-129-5p, miR-199a, miR-1237-3p, miR-140) (Figure 4(a)). In addition, the previous literature reported that miR-199 upregulation inhibited the injury of human NPCs [25]. Therefore, RT-qPCR detected the expression of miR-199a in EVs-treated IVD tissues and NPCs and showed the expression of miR-199a in EVs-treated IVD tissue and NPCs was significantly increased (Figure 4(b)). In conclusion, BMSCs-EVs treatment improved the expression of miR-199a in mouse IVD. To confirm whether BMSCs-EVs delivered the miR-199a into the NPCs, we successfully inhibited the expression of miR-199a in BMSCs by transfecting miR-199a inhibitor (Figure 4(c)). Subsequently, we found that miR-199a expression was also significantly reduced in the BMSCs-EVs and BMSCs-EVs-treated NPCs (Figure 4(c)), indicating that miR-199a may be carried by BMSCs-EVs into the NPCs. Finally, to exclude the interference effect of nonspecific precipitation substances, we treated BMSCs using RNase A or RNase A in combination with Triton X-100, and confirmed that miR-199a was wrapped by the BMSCs-EVs (Figure 4(d)), which meant that BMSCs-EVs can deliver the miR-199a into the NPCs.
Figure 4.
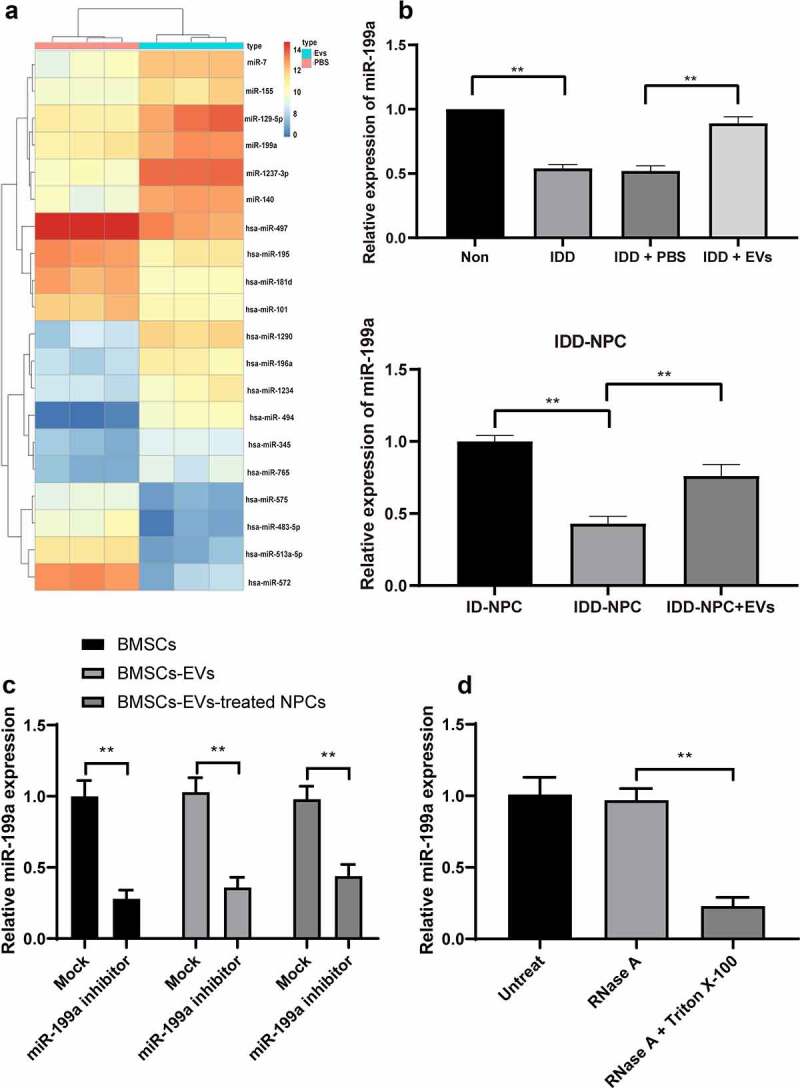
BMSCs-EVs promote miR-199a expression in intervertebral disc. (a), microarray was performed to determine dysregulated miRs after EV treatment; (b), intervertebral disc miR-199a expression was measured by RT-qPCR after EV treatment. (c) RT-qPCR analysis of the expression of miR-199a in miR-199a inhibitor-transfected-BMSCs, BMSCs-EVs and BMSCs-EVs-treated NPCs. (d) BMSCs were treated with RNase A or RNase A in combination with Triton X-100. RT-qPCR analysis of the expression of miR-199a. Three independent experiments were performed, and each group contained at least 8 mice.). Data are expressed as mean ± s.d. One-way ANOVA and Tukey’s multiple comparisons test were used to determine statistical significance. **p < 0.01
3.5. Reduction of miR-199a in BMSCs-EVs weakens the protection of EVs on mouse NPCs
In order to test our hypothesis, miR-199a was knocked down in BMMSCs-EVs-treated degenerative NPCs, and assigned into the EVs/Inhibitor group, with the EVs/Mock group as a control. Immunofluorescence staining displayed that the expression of col II (Figure 5(a)) and Aggrecan (Figure 5(b)) in degenerative NPCs in the EVs/inhibitor group was significantly lower than that in the EVs/Mock group (both p < 0.05). SAβ-gal staining and flow cytometry showed that compared with the EVs/Mock group, SAβ-gal positive cells (Figure 5(c)) and apoptosis rate (Figure 5(d)) in the EVs/Inhibitor group were increased significantly (both p < 0.05). In conclusion, after reducing miR-199a carried by EVs, the protective effect of EVs on mouse NPCs was weakened.
Figure 5.
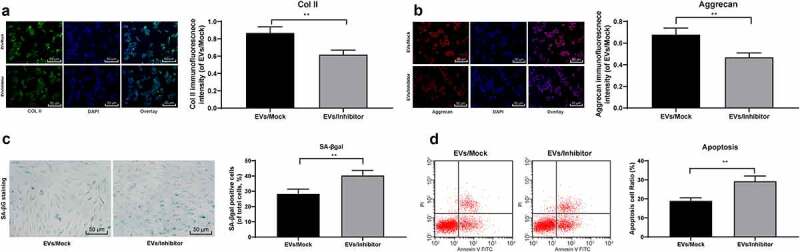
Reduction of miR-199a in BMSCs-EVs weakens the protection of EVs on mouse NPCs. A/B, Immunofluorescence of col II and Aggrecan (×200); C, SA-βgal staining was utilized for NPC senescence measurement (×200); D: PI/Annexin labeled cells were performed to measure apoptosis index by flow cytometry. Three independent experiments were performed. Data are expressed as mean ± s.d. One-way ANOVA and Tukey’s multiple comparisons test were used to determine statistical significance
3.6. miR-199a targets GREM1 to downregulate the TGF-β pathway
To explore the downstream mechanism of miR-199a in IDD repair, mRNA microarray was performed, and it found that GREM1 in NPCs was significantly downregulated after EV treatment (Figure 6(a)). Then, we use StarBase and miRSearch to predict that GREM1 is the target gene of miR-199a (Figure 6(b)). Dual-luciferase reporter gene assay confirmed the binding relationship between miR-199a and GREM1 (Figure 6(c)). STRING bioinformatics website found that GREM1 could bind to TGF-β1 protein (Figure 6(d)), and TGF-β1 signaling pathway was enriched by KEGG enrichment analysis of differentially expressed genes by using bioinformatics website David (Figure 6(e)). So we conjectured that miR-199a affected the TGF-β signaling pathway by targeting GREM1. We detected the activation of TGF-β signaling pathway in each group of IVD tissues and NPCs by ELISA. The results showed that in the degenerative IVD tissue and NPCs, after EV treatment, the activation level of TGF-β was decreased significantly (p < 0.05). After treatment of the degenerative NPCs with miR-199a inhibitor, the TGF-β activation level was partially recovered (Figure 6(f)).
Figure 6.
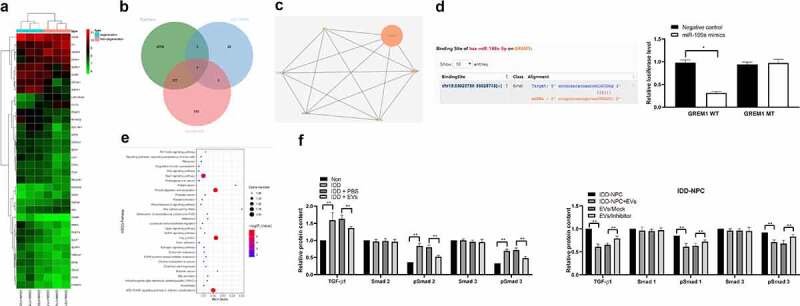
miR-199a targets GREM1 to downregulate the TGF-β pathway. (a), clustering analysis was performed using the limma Rstudio based on GSE34095 using FC ≥2.0 and P < 0.05 for mRNA different analysis; (b), 36 dysregulated mRNAs, miR-199a target mRNAs predicted by Starbase and TargetScan took intersection and GREM1 was filtered; (c), TargetScan predicts GREM1 target miRNAs and validated by dual luciferase assays; (d), PPI (protein – protein interaction) analysis by STRING; (e), clustering analysis was performed using the limma Rstudio based on GSE37075 using FC ≥2.0 and P < 0.05 for KEGG pathway; (f), ELISA were performed to determine TGF-β, Smad1, pSmad1, Smad3 and pSmad3 protein content. Three independent experiments were performed. Data are expressed as mean ± s.d. Two-way ANOVA and Tukey’s multiple comparisons test were used to determine statistical significance
3.7. TGF-β agonist counteracts the protective effect of EVs on NPCs
To confirm the effect of TGF-β signaling pathway on IDD repair, we added TGF-β signaling pathway agonist SRI-011381 to the degenerative NPCs treated by EVs. Immunofluorescence images indicated that the relative expressions of Aggrecan and col Ⅱ were significantly decreased in the EVs ± agonist group compared with those in the EVs ± DMSO group (Figure 7(a,b)), while SA-β gal staining and flow cytometry showed that SA-β gal positive cells and apoptosis rate were obviously increased (Figure 7(c,d)). In conclusion, TGF-β signaling pathway agonist can counteract the protective effect of EVs on NPCs.
Figure 7.

TGF-β agonist counteracts the protective effect of EVs on NPCs. (a,b), Immunofluorescence of col II and Aggrecan (×200); (c), SA-βgal staining was utilized for NPC senescence measurement (×200); (d), PI/Annexin labeled cells were performed to measure apoptosis index by flow cytometry. Three independent experiments were performed. Data are expressed as mean ± s.d. One-way ANOVA and Tukey’s multiple comparisons test were used to determine statistical significance
4. Discussion
In recent years, MSCs-based regenerative medicine offers a promising approach to treat IDD through anti-inflammatory effects, NP tissue regeneration and amelioration of disc degradation [26]. EVs are important bioactive components of MSCs and an alternative to MSCs-based therapies [27]. In this study, we investigated the mechanism of BMSCs-EVs in the course of IDD and screened out the differentially expressed miRs and genes in IDD.
MSC transplantation is an ideal way for IVD regeneration because MSCs can prevent IVD cell death through the paracrine action [28]. More and more studies have shown that EVs play a mediating role in intercellular communication [29]. MSC-derived exosomes are recognized as new candidate for treating critical-sized bone defects [30]. miR-126-modified MSC-EVs can reduce the volume of spinal cord injury, retain neurons, promote axon regeneration, and promote the recovery of limb motor function [31]. Initially, we found the chondrocyte necrosis in the NP of IDD mice treated with BMSCs-EVs was improved to some extent. Lumbar IDD is closely associated with cell apoptosis, abnormal endplate calcification, vascular morphology, inflammatory cytokines and MMP production, which induce the degeneration of chondrocyte of IVD [32]. EVs treatment in this study significantly decreased expression of MMP-2, MMP-6 and TIMP1, and the apoptosis of NPCs. MMPs and TIMPs maintain balance in healthy IVD for normal metabolism [14]. MMPs in the NP tissue extracts of human IVD fibroid ring lead to the disorder of matrix composition synthesis and degradation, resulting in the change of extracellular matrix composition, which is one of the direct causes of the loss of mechanical characteristics of IVD [33]. At the beginning of the course of IDD, the IVD transfers to a catabolic mode, with reduced expression of Aggrecan, col II, prostaglandin and TIMPs, and increased activity of MMPs and TGF-β [34]. Consistently, levels of aggrecan and col II were enhanced when NPCs were stimulated by BMSCs-EVs, and MMP-1 and MMP-3 were repressed [14]. In conclusion, BMSCs-EVs promote the repair of IDD in mice.
Moreover, we observed that the expression of col II and Aggrecan, SA-β-gal-positive cells and TUNEL-positive cells in NPCs after EV treatment was significantly lower. Aggrecan is the most abundant proteoglycan secreted by NPCs, which in turn makes NPCs have compressive capacity [35]. Healthy NP is gelatinous and mainly composed of proteoglycans in the loose network of col II [34]. BMSCs present similar phenotypic activity to NPCs, including upregulation of Aggrecan and col II with inhibited expression of proinflammatory cytokines, suggesting that BMSCs can enhance matrix homeostasis in the disc [36]. The IVD is in cartilage structure which shows degenerative and age-related changes, and IDD occurs when its cells are senescent, dead or dysfunctional [37]. Thus, the improvement of cell senescence and apoptosis might be beneficial to prevent IDD progression. Likewise, human umbilical cord MSCs-EV treatment restored the expression of extracellular matrix components (col II and Aggrecan) and protected NP-MSCs from cell senescence [26]. To sum up, BMSCs-EVs promote the proliferation of NPCs and inhibit apoptosis.
To further explore the molecular mechanism of BMSCs-EVs in IDD process, we performed microarray analyses to screen out the differentially expressed miRs and genes. The expression of miR-199a in EVs-treated IVD tissues and NPCs was significantly increased, while GREM1 was mostly downregulated. A recent study demonstrated that miR-199a-5p, the most significant determinant of the IDD process, is commonly upregulated in IDDs and is associated with extracellular matrix degeneration [38]. miR-199 was obviously decreased in NPCs, and miR-199 overexpression significantly reduced NPC apoptosis [25]. Similarly, MSCs-EVs-carried miR-21 prevented NPCs from apoptosis and alleviated IDD progression [39]. Reduction of miR-199a in BMSCs-EVs weakens the protection of EVs on mouse NPCs.
In addition, miR-199a targets GREM1 to downregulate the TGF-β pathway. GREM1, a BMP antagonist, plays a crucial role in dormant stem cell population that is able to differentiate into cartilage and bone especially important for skeletal patterning and homeostasis, and even has a reticular stromal potential to improve spinal fusion therapy [40]. IVD cells secrete bone morphogenetic protein (BMP)-binding proteins, like GREM1, to block BMP that originates from vertebral bodies to provide a BMP-reduced zone and prevent disc ossification [41]. In a senescence-accelerated mice, the expression of TGF-b decreased with age in IDD models, and activation of TGF-β partially reversed the proinflammatory cytokines-induced matrix-degrading enzymes upregulation [42]. TGF-β stimulates MSCs to differentiate into chondrocytes with NP-like phenotype and changes the phenotype of NPCs, resulting in an increase in Aggrecan synthesis [43]. TGF-β pathway plays a critical role in IDD process and can be determined specifically by upregulating miR-199a-5p [38]. Taken together, TGF-β agonist counteracts the protective effect of EVs on NPCs.
As tissue remodeling and turnover is critical for maintenance of structural integrity and healing of injury even in mature tissue, any attempt to limit MMP activity must keep this delicate balance in mind. In addition, in approaching therapeutic consideration, treatment of the patients’ symptoms must be paramount, and identifying the clinical application value of the BMSCs-EVs and miR-199a might provide a clearer picture of where and how to intervene to improve treatment of individuals suffering from IDD-related disorders.
Funding Statement
This study was supported by General project of Guangzhou scientific research program (No. 201904010421), Guangdong Provincial Hospital of Chinese Medicine science and technology research project (No. YN2019MJ08).
Author contributions
TW is the guarantor of integrity of the entire study; TW contributed to the study concepts, study design, and definition of intellectual content, HSW contributed to the literature research, YJL contributed to the manuscript preparation and YPL contributed to the manuscript editing and review; TW contributed to the clinical studies; SZ and JGL contributed to the experimental studies and data acquisition; BLC contributed to the data analysis and statistical analysis. All authors read and approved the final manuscript.
Disclosure statement
The authors declared that they have no competing interests.
Data accessibility statement
All the data generated or analyzed during this study are included in this published article.
References
- [1].Priyadarshani P, Li Y, Yao L.. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthritis Cartilage. 2016;24:206–212. [DOI] [PubMed] [Google Scholar]
- [2].Rider SM, Mizuno S, Kang JD. Molecular mechanisms of intervertebral disc degeneration. Spine Surg Relat Res. 2019;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190:E786–E793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sclafani J, Leong M, Desai MJ, et al. Conventional versus high-frequency neuromodulation in the treatment of low back pain following spine surgery. Pm R. 2019;11:1346–1353. [DOI] [PubMed] [Google Scholar]
- [5].Forozeshfard M, Jahan E, Amirsadat J, et al. Incidence and factors contributing to low back pain in the nonobstetrical patients operated under spinal anesthesia: a prospective 1-year follow-up study. J Perianesth Nurs. 2020;35:34–37. [DOI] [PubMed] [Google Scholar]
- [6].Zhang F, Zhao X, Shen H, et al. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med. 2016;37:1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Centeno C, Markle J, Dodson E, et al. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017;15:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Binch ALA, Richardson SM, Hoyland JA, et al. Combinatorialconditioning of adipose derived-mesenchymal stem cells enhances their neurovascular potential: implications for intervertebral disc degeneration. JOR Spine. 2019;2:e1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang YT, Wu XT, Wang F. Regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration. J Orthop Sci. 2010;15:707–719. [DOI] [PubMed] [Google Scholar]
- [11].Drazin D, Rosner J, Avalos P, et al. Stem cell therapy for degenerative disc disease. Adv Orthop. 2012;2012:961052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- [13].D’Souza-Schorey C, Schorey JS. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018;62:125–133. [DOI] [PubMed] [Google Scholar]
- [14].Lu K, Li HY, Yang K, et al. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ji ML, Jiang H, Zhang XJ, et al. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9:5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rani S, Ryan AE, Griffin MD, et al. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shi L, Wang Z, Geng X, et al. Exosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells in vitro and in vivo. Aging (Albany NY). 2020;12:8549–8564. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [19].Yuan W, Che W, Jiang YQ, et al. Establishment of intervertebral disc degeneration model induced by ischemic sub-endplate in rat tail. Spine J. 2015;15:1050–1059. [DOI] [PubMed] [Google Scholar]
- [20].Liao Z, Luo R, Li G, et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang K, Song Y, Liu W, et al. The noncoding RNA linc-ADAMTS5 cooperates with RREB1 to protect from intervertebral disc degeneration through inhibiting ADAMTS5 expression. Clin Sci (Lond). 2017;131:965–979. [DOI] [PubMed] [Google Scholar]
- [22].Liu Y, Gao L, Zhao X, et al. Saikosaponin a protects from pressure overload-induced cardiac fibrosis via inhibiting fibroblast activation or endothelial cell EndMT. Int J Biol Sci. 2018;14:1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jiang C, Zhu W, Xu J, et al. MicroRNA-26a negatively regulates toll-like receptor 3 expression of rat macrophages and ameliorates pristane induced arthritis in rats. Arthritis Res Ther. 2014;16:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu T, Niu C, Zhang X, et al. beta-Ecdysterone protects SH-SY5Y cells against beta-amyloid-induced apoptosis via c-Jun N-terminal kinase- and Akt-associated complementary pathways. Lab Invest. 2018;98:489–499. [DOI] [PubMed] [Google Scholar]
- [25].Wang W, Guo Z, Yang S, et al. Upregulation of miR-199 attenuates TNF-alpha-induced Human nucleus pulposus cell apoptosis by downregulating MAP3K5. Biochem Biophys Res Commun. 2018;505:917–924. [DOI] [PubMed] [Google Scholar]
- [26].Qi L, Wang R, Shi Q, et al. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J Bone Miner Metab. 2019;37:455–466. [DOI] [PubMed] [Google Scholar]
- [27].Liu H, Liang Z, Wang F, et al. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4. DOI: 10.1172/jci.insight.131273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brossa A, Fonsato V, Grange C, et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int J Cancer. 2020. DOI: 10.1002/ijc.32925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ratajczak MZ, Ratajczak D, Pedziwiatr D. Extracellular microvesicles (ExMVs) in cell to cell communication: a role of telocytes. Adv Exp Med Biol. 2016;913:41–49. [DOI] [PubMed] [Google Scholar]
- [30].Liang B, Liang JM, Ding JN, et al. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yuan B, Pan S, Dong YQ, et al. Effect of exosomes derived from mir-126-modified mesenchymal stem cells on the repair process of spinal cord injury in rats. Eur Rev Med Pharmacol Sci. 2020;24:483–490. [DOI] [PubMed] [Google Scholar]
- [32].Yang L, Sun X, Geng X. Effects of psoralen on chondrocyte degeneration in lumbar intervertebral disc of rats. Pak J Pharm Sci. 2015;28:667–670. [PubMed] [Google Scholar]
- [33].Vo NV, Hartman RA, Yurube T, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kadow T, Sowa G, Vo N, et al. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473:1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Molladavoodi S, McMorran J, Gregory D. Mechanobiology of annulus fibrosus and nucleus pulposus cells in intervertebral discs. Cell Tissue Res. 2020;379:429–444. [DOI] [PubMed] [Google Scholar]
- [36].Le Maitre CL, Baird P, Freemont AJ, et al. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res Ther. 2009;11:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen K, Wu D, Zhu X, et al. Gene expression profile analysis of human intervertebral disc degeneration. Genet Mol Biol. 2013;36:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sherafatian M, Abdollahpour HR, Ghaffarpasand F, et al. MicroRNA expression profiles, target genes, and pathways in intervertebral disk degeneration: a meta-analysis of 3 microarray studies. World Neurosurg. 2019;126:389–397. [DOI] [PubMed] [Google Scholar]
- [39].Cheng X, Zhang G, Zhang L, et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chan SC, Tekari A, Benneker LM, et al. Osteogenic differentiation of bone marrow stromal cells is hindered by the presence of intervertebral disc cells. Arthritis Res Ther. 2015;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen S, Liu S, Ma K, et al. TGF-beta signaling in intervertebral disc health and disease. Osteoarthritis Cartilage. 2019;27:1109–1117. [DOI] [PubMed] [Google Scholar]
- [43].Lehmann TP, Jakub G, Harasymczuk J, et al. Transforming growth factor beta mediates communication of co-cultured human nucleus pulposus cells and mesenchymal stem cells. J Orthop Res. 2018;36:3023–3032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.


