ABSTRACT
Submergence and waterlogging lead to significant reductions in crop productivity and trigger dramatic changes in gene expression of plant biotic/abiotic stress response. Several of the host factors are involved in low-oxygen stress that is induced by endogenous reactive oxygen species (ROS) accumulation. Hypoxia-response unknown protein (HUP) has been found as a host factor of hypoxia screening but HUPs function largely is unknown. In this study, we found the Arabidopsis HUP26 gene which was conserved in different plant species and responded to various oxidative stress. HUP26 promoter analysis showed GUS activity in root and leaf tissues was significantly responsive to oxidative stress. HUP26-GFP is predominantly located in the cytoplasmic region. HUP26 overexpression results in altered enhanced pathogenesis-related gene 1 gene expression and reduced ion leakage levels compared with hup26 knockout and WT plants after inoculation with Pst DC3000. HUP26 overexpression transgenic plants showed improved resistance to Pst DC3000, but hup26 knockout plants exhibited increased susceptibility. Collectively, these results indicate that HUP26 plays important role in responses to various oxidative stress and confers biotic stress resistance. Engineering of HUP26 gene expression may represent a strategy to enhance biotic stress resistance of crops.
KEYWORDS: Hypoxia-response unknown protein, arabidopsis, biotic stress, ROS, crop development
Introduction
Based on the current climate model prediction, 450 million people will be exposed to flooding by 2050.1 Hypoxia occurs when the oxygen levels decrease in the critical oxygen pressures for respiration and flooding stress limits oxygen availability for plants.2 Submergence stress encounters in crop and contributes substantially to a decline in crop productivity. Plants adapt to hypoxia and low-oxygen stresses by regulating the expression of genes that respond to stresses via signal transduction and transcriptional regulation.3–6
Increased levels of reactive oxygen species (ROS) in plants are a consequence of various adverse biotic and abiotic conditions such as hypoxia, drought, salinity, wounding, and pathogen infection.7 The antioxidant system (AOS) maintains ROS concentrations to protect plants from ROS toxicity.7,8 In addition, ROS can also act as important signal molecules that modulate plant defense, plant growth, development, and responses to the environment.9
The effects of hypoxia on plant diseases have been reported that potential interplay between responses to flooding and plant–pathogen interaction.10,11 Transcript levels of Arabidopsis plant defense genes are strongly induced at 1 h after submergence. Moreover, submergence treatment for 2 h enhanced the plant tolerance to postsubmergence inoculation with Pseudomonas syringae. Interestingly, plant innate immunity is triggered by submergence in Arabidopsis via WRKY22, a response that might protect against pathogen infection upon flooding condition.11 Transcriptomic studies under submergence stress have found upregulated gene clusters associated with plant responses to different types of pathogens such as fungi and bacteria.10 It also reported that hypoxia-related gene markers Arabidopsis pyruvate decarboxylase 1 (PDC1), pyruvate decarboxylase 2 (PDC2), and alcohol dehydrogenase 1 (ADH1) were highly induced after challenged with Plasmodiophora brassicae. Clubroot symptoms were significantly resistant to the pdc1 and pdc2 mutants.12 These observations suggest that the hypoxia responses of Arabidopsis during P. brassicae infection is involved in pathogen infection.
Previously, Hypoxia-response unknown protein (HUP) genes were identified that responded to submergence in Arabidopsis, and these genes conferred seedling survival of low-oxygen stresses.13,14 However, many of the HUP genes showed largely conserved in other plant species but HUP proteins have no known protein domain or functional motif.13 One of them, similar transcripts of HUP26 are upregulated by low-oxygen condition and submergence in HUP26 orthologs such as poplar, rice, and cotton.13,14
Transcriptional controls play an important role in regulating submergence responses to plants. Although numerous genes are highly induced during hypoxia, their individual function of biotic/abiotic responses is still poorly understood. Here, we found that induction of HUP26 during Pst DC3000 infection may contribute to plant disease resistance via PR1 expression and ROS accumulation in cytosol.
The Arabidopsis HUP26 encodes evolutionally conserved protein among the plant spices and localizes mainly cytosol in plants
In previous studies, Hypoxia-responsive Unknown Protein (HUP) genes were identified as significantly upregulated genes in response to submergence.13,14 However, many of the HUPs function in abiotic and biotic stress still largely unknown (Table S1). To determine whether the HUP26 gene is conserved among plant species, we performed phylogenetic analysis indicated that HUP26 protein is evolutionally to be categorized into two groups – monocots and dicots (Figure 1a). Rice and Poplar HUP26 genes reported induced by low-oxygen stress which means that Arabidopsis HUP26 may have putative orthologs in monocot and dicot plants.15,16 We performed HUP26 domain analysis using the SMART (a Simple Modular Architecture Research Tool) and protein alignment (Figure 1b). HUP26 protein encodes 147 amino acids with a predicted molecular weight of 16.7 kD. It has no predicted domain and motif which is a poorly uncharacterized protein (Figure 1c). We generated a 35S::HUP26-GFP construct and transiently expressed it in N. benthamiana. HUP26-GFP showed predominantly cytosolic localization and partially existed in the nucleus outside (Figure 1c). These data indicate that HUP26 functions as the cytosolic protein.
Figure 1.
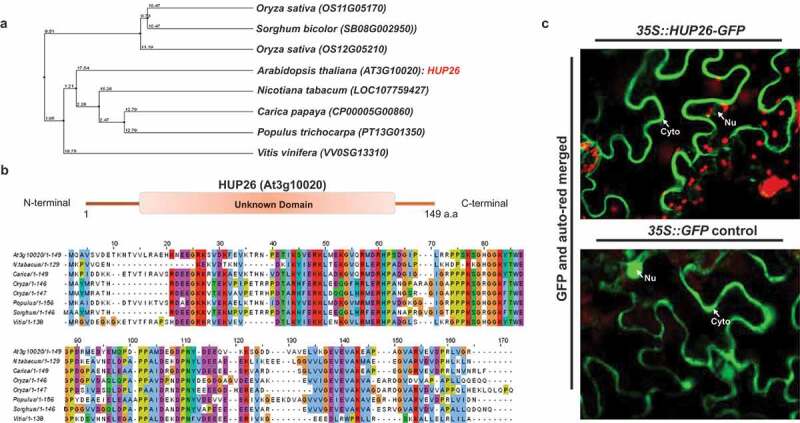
HUP26 has widespread homolog protein in plant and contains conserved unknown protein domain. (a) Phylogenetic tree analysis was performed through Clustal W using amino acid sequences of HUP26 and other plant species. (b) Amino acid alignments with other plant spices were performed with DNAStar software. Unknown domain of HUP26 is highly conserved among the homologs. (c) Subcellular localization of HUP26 in planta. Subcellular localization of 35::HUP26-GFP in N. benthamiana leaves transiently infiltrated by Agrobacterium-mediated method. For confocal laser scanning microscopy samples were taken 3 days. Red signal indicated chlorophyll fluorescence. 35S::GFP is used as a positive control
HUP26 rapidly responded to oxidative and biotic stresses
Plants are subjected to biotic and abiotic stresses and reactive oxygen species (ROS) which is signaling molecules play important roles in enhancing their tolerance.17,18 To explore the potential plant defense functions of HUP26, the expression profile following bacterial pathogen inoculation was analyzed by qRT-PCR. The results showed that the expression of HUP26 is very low without pathogen infection, but it was induced by Pst DC3000 inoculation although the mock control also showed a slightly increased HUP26 expression level (Figure 2a). Pathogenesis-related protein 1 (PR1) gene expression was induced in response to a pathogen inoculation but not mock control (Figure 2a). These results implied that the expression of the HUP26 gene can be induced in response to wounding-stress by syringe-infiltration. Moreover, we also checked HUP26 gene expression patterns after challenged with the fungal pathogen, B. cinerea. We observed HUP26 gene expression significantly increased during fungal inoculation (Figure S2) suggesting that HUP26 might function in biotic stress.
Figure 2.
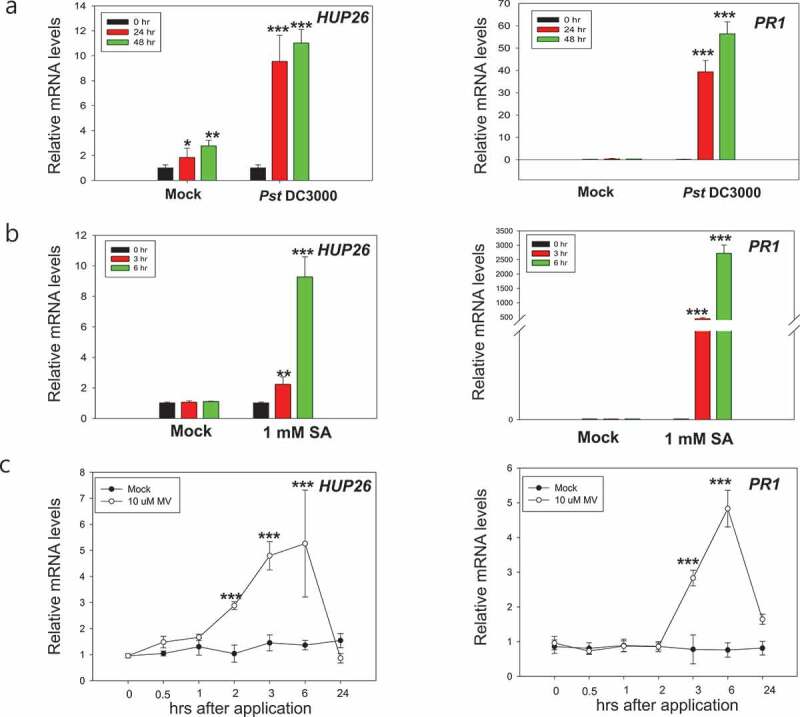
Expression analysis of HUP26 by qRT-PCR upon Pst DC3000 inoculation, salicylic acid, and oxidative stress treatments. (a) Expression patterns of HUP26 upon Pst DC3000 inoculation. Rosette leaves of 4-week-old Col-0 plants were syringe infiltrated with Pst DC3000 suspensions (OD600 = 0.001 in 10 mM MgCl2). Expression patterns of PR1 were used as a positive control. (b and c) Leaves of 4-week-old Col-0 plants were sprayed salicylic acid (1 mM) or methyl viologen (10 µM). Total RNA extracted plants was used for qRT-PCR analysis to detect the expression of HUP26 and PR1. Error bars indicate standard deviations (n = 3). Student’s t-test; *P < .01, **P < .05, ***P < .0001
SA as a signal molecule plays a critical role in plant immunity via transcriptional reprograming and interplays with ROS.19,20 Therefore, next we investigated whether the HUP26 gene expression can be induced by SA and oxidative stress inducer methyl viologen (MV) treatment at the early time points.3 To eliminate wounding stress, 4-week-old plants were sprayed with 1 mM SA and 10 µM MV or ddH2O as a control. As expected, HUP26 mRNA levels were increased markedly by 3 and 6 h after SA and MV treatments (Figure 2b and c). These gene expression patterns supported that HUP26 is involved in oxidative stress response and maybe playing a role in both biotic and abiotic stress.
GUS of HUP26 promoter showed inducible activity in root and leaf
In order to identify the cis-acting elements involved in the response to biotic and abiotic stress, we analyzed cis-element using PLANTCARE tool. The 500 bp promoter region upstream of the HUP26 start codon contained at least four putative cis-elements (Figure 3a). ABRE is a cis-element that is involved in the abscisic acid (ABA) responsive element and most of the PR gene promoters contain ABRE sequence.21,22 MYC cis-element showed a response to drought, ABA, and cold signals.22 ACE is a number of cis-elements associated with light stress.21 STRE was known as a binding site for the transcriptional activator. Tomato HsfA1a conferred heat stress tolerance and pathogen resistance via binding to the heat shock proteins (HSPS) or respiratory burst oxidase homolog (RBOH) gene promoter to regulate ROS.23,24 This suggested that HUP26 expression might be regulated by Hsf proteins. We also tested whether HUP26 response to heat stress. The transcript level of HUP26 gene was highly induced by heat stress (Figure S1).
Figure 3.
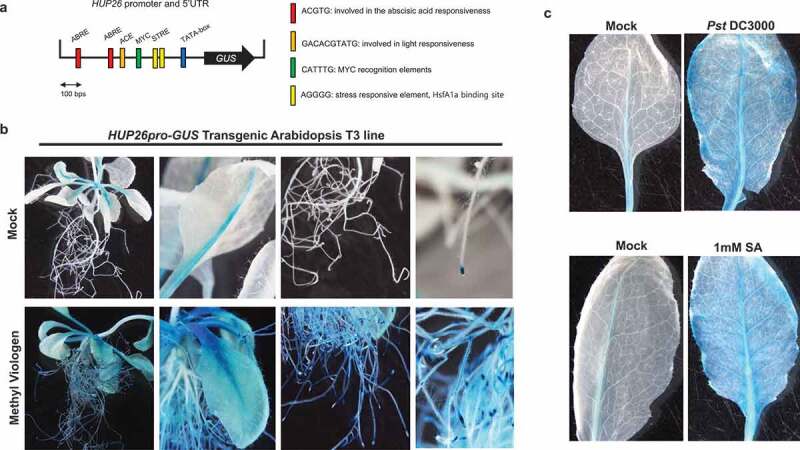
HUP26 promoter assays upon various stresses. (a) Schematic representation of the HUP26pro-GUS constructs used to GUS. Construct of the HUP26 promoter was fused to the GUS reporter gene in the vector pCAMBIA1301. Each cis-acting regulatory elements (CAREs) were predicted by PlantCARE tool. (b) The promoter activity of HUP26 was highly induced by oxidative stress treatment. HUP26pro-GUS transgenic plant shows normal expression of vascular tissue and root tip region. (c) After 24 h upon Pst DC3000 (dipping inoculation with 1 × 108 CFU/ml) or 6 h upon 1 mM SA spraying treatment, GUS activity of HUP26 promoter was highly induced in the leaf
To determine HUP26 promoter activity in the whole plant, in response to oxidative stress, HUP26pro-GUS transgenic plants were exposed to MV. GUS activity in the HUP26pro-GUS transgenic plant was detected at a low level in the root tip and leaf vascular tissues before MV treatment. GUS activity was induced in the whole root and leaf tissues after 6 h of MV treatment (Figure 3b). GUS activity was strongly observed at 48 h after Pst DC3000 inoculation compared with mock control (Figure 3c). And similar results were obtained for strong GUS activity in the SA sprayed HUP26pro-GUS transgenic plant leaves (Figure 3c). Therefore, the expression of HUP26 can be induced by pathogens in root and leaf tissues.
HUP26 confers disease resistance to bacterial pathogen
To analyze the potential role of HUP26 in the plant defense against the pathogen, we generated transgenic Arabidopsis plants overexpressing (OE) HUP26 by CaMV-35S-promoter and obtained hup26 knockout (KO) plants (SALK_078717 and SALK_025845) whose HUP26 expression levels were confirmed by semiquantitative RT-PCR (Figure 4a and b). These transgenic plants were not observed in developmental phenotype (Figure 4b). Leaves from the Col-0, HUP26 OE, and hup26 KO plants were inoculated with Pst DC3000 by dip inoculation method. Disease symptoms of HUP26 OE transgenic plants were observed much less than Col-0 and hup26 KO plants (Figure 4c), suggesting that the overexpression of HUP26 is involved in bacterial resistance. Bacterial growth of Pst DC3000 was monitored to evaluate whether the lack of disease symptom reflected the restriction of pathogen growth in the HUP26 OE plants. As expected, bacterial growth in HUP26 OE transgenic plants showed that the growth of Pst DC3000 significantly was reduced compared with Col-0 and hup26 KO plants, whereas the growth of bacterial pathogens in the hup26 KO plants was extremely enhanced bacterial growth (Figure 4d). We also checked ion leakage and PR1 gene expression level in Pst DC3000 inoculated plants. Ion leakage was significantly increased in Pst DC3000 inoculated hup26 KO plants and HUP26 OE transgenic plant showed reduced ion leakage (Figure 4e). To determine the role of HUP26 in the ROS burst in response to flg22 elicitor, we examined ROS levels in flg22 elicitor treated Col-0, HUP26 OE, and hup26 KO plants via luminol-based ROS generation detection method.25 The results showed significantly increased levels of ROS were detected in HUP26 OE plants compared with Col-0. On the other hand, lower ROS bursts were observed in hsp26 KO plants (Figure S3). Therefore, the results presented above indicated that HUP26 is required for pathogen resistance in Arabidopsis via ROS accumulation.
Figure 4.
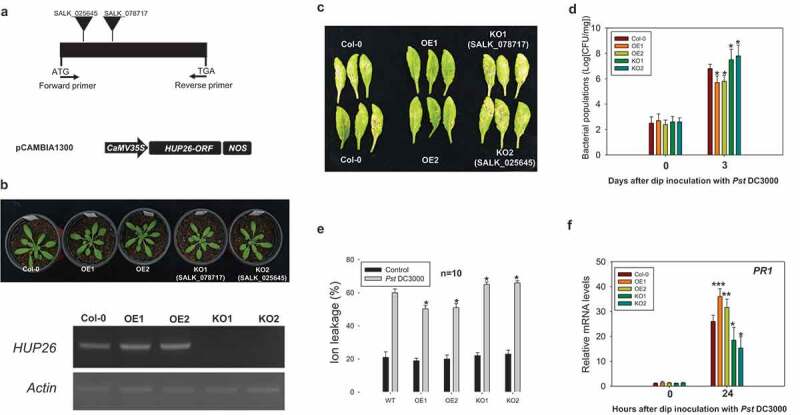
Overexpression of HUP26 leads to enhanced resistance to virulent Pst DC3000 inoculation via PR1 gene expression. (a) Schematic representation of hup26 knockout T-DNA lines (KO1: SALK_078717 and KO2: SALK_025645) regions and the 35S::HUP26 overexpression construct. (b) 4-week-old transgenic plants showed no developmental phenotype. Identification of homozygous hup26 mutants. Semi-quantitative analysis demonstrates that HUP26 is knocked-out in the hup26 mutants and confirms HUP26 overexpression transgenic lines. (c) Disease symptoms of Col-0, HUP26 OE, hup26 KO plants in 4 day after dip inoculation with Pst DC3000 at 1 × 108 CFU/ml. Pictures were taken 4 days post inoculation. (d) Col-0, HUP26 OE, hup26 KO plants were dip inoculated with Pst DC3000 at 1 × 108 CFU/ml. Bacterial growth was counted at 0 and 3 dpi. Error bars indicate standard deviations (n = 5). Student’s t-test; *P < .01. (e) The ion electrolyte leakage measurements were performed with Col-0, HUP26 OE, and hup26 KO plant leaf discs at 1 day after inoculated with Pst DC3000 at 1 × 108 CFU/ml. Bars represent the average ion leakage measured for triplicates of ten leaf disks each (n = 10). Student’s t-test; *P < .01. (f) PR1 gene expression pattern analysis by qRT-PCR after bacterial pathogen inoculation. Total RNA was isolated from leaves of 4-week-old Col-0, HUP26 OE, and hup26 KO lines plants in response to Pst DC3000 after dip inoculation with Pst DC3000 at 1 × 108 CFU/ml. Relative transcript levels of PR1 were determined by RT-qPCR. Error bars indicate standard deviations (n = 3). Student’s t-test; *P < .01, **P < .05, ***P < .0001
In this report, we presented evidence that HUP26 is an oxidative response gene and is involved in the biotic stress resistance against pathogen infection. The overexpression of HUP26 exhibited enhanced PR1 gene expression and reduced ion leakage upon pathogen inoculation. These results are consistent with the hup26 KO plant upon Pst DC3000 inoculation. Thus, we provided the function of Arabidopsis HUP26 in plant immunity via the oxidative stress response.
Rice ERF-VII transcription factor gene, SUB1A, is known as the master regulator of submergence.26 For example, RAP2.12 binds to LOB domain-containing protein 41 (LBD41) and plant cysteine oxidase 1 (PCO1) promoters in Hypoxia-Responsive Promoter Element (HRPE) regions which are quite similar to the ARE in sequence and function.27 Generally, HUP gene expressions were also observed as a core hypoxia response gene.27,28 RAP2.2 and RAP2.12 redundantly function and directly bind to HUP29 and 43 promoter which contain the HRPE-containing 33bp sequence.29 Similarly, HUP6, HUP9, HUP39, HUP40, HUP44, and HUP54 promoter also contained the HRPE motif.29 However, the HUP26 promoter has no HRPE motif in the promoter (Figure 3a) but still responses to oxidative, hypoxia, and biotic stresses (Figures 2 and 3). Interestingly, the HUP26 promoter contained two stress-responsive element (STRE) for HsfA1a, and transcript levels of HUP26 were significantly induced by heat stress (Figure 3a and S1). A correlation between heat shock-related gene induction by low-oxygen tolerance has been demonstrated.30 Thus, it is still possible that plant Hsf and ERF transcription factor might regulate HUP26 gene expression in response to oxidative stress.
In the GUS activity assay, HUP26pro-GUS was only observed in the root tip and leaf vascular tissue (Figure 4b). In root tip, GUS signals spread over the whole root tissues after oxidative stress (Figure 4b) suggesting that HUP26 might be involved in the low-oxygen response to the root although HUP26 transgenic seedlings had no oxidative stress tolerance after paraquat treatment.20 This phenotype might be different abiotic and biotic stress conditions. In the leaf, pathogen inoculation, SA, and MV treatments strongly activated HUP26pro-GUS (Figure 4c). Furthermore, HUP26 OE transgenic plants exhibit a pathogen resistance phenotype which is a ROS-dependent manner and hup26 KO is consistent with these results (Figure 4d). These results provide that HUP26 is an oxidative response gene, which plays a positive role in plant stress resistance and might be involved in the ROS regulation pathway in response to biotic stress.
In this study, we have characterized in detail the HUP26, an unknown cytosolic protein. Molecular characterization of the HUP26 OE and hup26 KO lines revealed that HUP26 is a novel biotic and abiotic response gene that enhanced resistance to bacteria pathogen. Our study suggests that HUP26 is an associated hypoxia-response gene to counteract biotic stress through the regulation of PR1 and ROS accumulation. Thus, this work might provide a novel strategy for improving the agricultural traits of rice, poplar, and cotton to enhance the defense response.
Experimental procedures
Plant materials, growth conditions, and transgenic plants
Arabidopsis thaliana Columbia-0 (Col-0) was used as wild-type backgrounds and was grown in soil under a 16 h-light/8 h-dark photoperiod at 23°C. T-DNA insertion mutants SALK_025645 and SALK_078717 were obtained from the Arabidopsis Biological Resource Center. For constitutive expression of HUP26 (At3g10020) in Arabidopsis, HUP26 open reading frame was amplified using Pfu-polymerase (Promega, Madison, WI, USA) and cloned into pCAMBIA2300. To prepare the HUP26pro-GUS construct, the 500 bp promoter region was amplified and the fragment was then cloned into the pBI101 vector system (Clontech, Mountain View, CA, USA). To prepare the 35S::HUP26-GFP construct, the HUP26 ORF region was amplified. The amplified fragment was then cloned into the GATEWAY Binary Vector (pGWB5). Arabidopsis Col-0 plants were transformed according to the floral dip method.31
Manipulation of RNA and quantitative gene expression analysis upon the various stress conditions
Arabidopsis total RNA was isolated using a phenol extraction protocol.32 Total RNA extracted from rosette leaves of 4-week-old Arabidopsis wild-type plants was used for qRT-PCR analysis upon 1 mM salicylic acid (SA), 10 µM methyl viologen (MV), 42°C heat shock, Pst DC3000 suspensions (OD600 = 0.001 in 10 mM MgCl2), or Botrytis cinerea spore suspension (5 × 104 spores/mL in potato dextrose media). All qRT-PCRs were performed with SYBR Green (KAPABIOSYSTEMS, Liang Seah, Singapore) and the expression data were normalized to the Atactin7. The other primers used for real-time PCR reactions are listed in Table S2.
Subcellular localization of HUP26
Agro-mediated transient assay was performed in Nicotiana benthamiana. After 3 days of agro-infiltration, GFP and chlorophyll auto red signals were detected by LSM 700 confocal microscopy (Zeiss, Axioskop, Germany).
Determination of electrolyte leakage
The electrolyte leakage assay was conducted with leaves of HUP26 OE, hup26 KO, and WT plants which were Pst DC3000 dipping inoculation. Five leaf discs were taken from each plant and washed with 5 ml ddH2O. The leaf discs were transferred to the new tubes with 5 ml ddH2O and measured using a portable conductivity meter.
GUS Staining
For histochemical analysis of the GUS activity, the HUP26pro-GUS transgenic seedlings or plants under the normal or stress conditions were vacuum-infiltrated with the X-Gluc staining solution (0.5 M sodium phosphate buffer, pH7.0, 10% Triton X-100, 0.1 M K3Fe(CN)6, 0.1 M K4Fe(CN)6, 2 mM X-GlcA), incubated at 37°C 12 h, and eliminated chlorophyll with 70% EtOH.
Pathogen inoculation
P. syringae pv. tomato DC3000 was cultured in King’s B medium and resuspended in 10 mM MgCl2 for bacterial pathogen inoculation. Leaves of the 4-week-old plant were dipping inoculation with Pst DC3000 suspensions (1 × 108 CFU/ml). Leaf discs were collected from plant leaves at 0 and 3 days post-inoculation (dpi) to detect bacterial growth. B. cinerea was grown on potato dextrose agar for the fungal pathogen inoculation. A spore suspension (5 × 104 spores/mL in potato dextrose media) was dropped on the leaves of plants.
Supplementary Material
Funding Statement
This work was supported by the Next-Generation BioGreen 21 Program (Project No. PJ01365301), Rural Development Administration, Republic of Korea.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Giang PQ, Toshiki K, Sakata M, Kunikane S, Vinh TQ.. Modelling climate change impacts on the seasonality of water resources in the Upper Ca River Watershed in Southeast Asia. ScientificWorldJournal. 2014;2014:1. doi: 10.1155/2014/279135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, Fukao T, Geigenberger P, Hebelstrup KH, Hill RD, et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017;214(4):1403–7. doi: 10.1111/nph.14519. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Ma Q, Wang R, Wang P, Liu Y, Mao T.. Submergence stress-induced hypocotyl elongation through ethylene signaling-mediated regulation of cortical microtubules in Arabidopsis. J Exp Bot. 2020;71:1067–1077. doi: 10.1093/jxb/erz453. [DOI] [PubMed] [Google Scholar]
- 4.Liew SY, Stanbridge EJ, Yusoff K, Shafee N.. Hypoxia affects cellular responses to plant extracts. J Ethnopharmacol. 2012;144:453–456. doi: 10.1016/j.jep.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Fukao T, Bailey-Serres J. Plant responses to hypoxia–is survival a balancing act? Trends Plant Sci. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Fukao T, Barrera-Figueroa BE, Juntawong P, Pena-Castro JM. Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Front Plant Sci. 2019;10:340. doi: 10.3389/fpls.2019.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 8.Fichman Y, Mittler R. Rapid systemic signaling during abiotic and biotic stresses: is the ROS wave master of all trades? Plant J. 2020;102:887–896. doi: 10.1111/tpj.14685. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RR, Weits DA, Feulner CFJ, van Dongen JT, Sensing O. Integrative stress signaling in plants. Plant Physiol. 2018;176:1131–1142. doi: 10.1104/pp.17.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera-Contreras IK, Zamora-Hernandez T, Huerta-Heredia AA, Capataz-Tafur J, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM. Transcriptomic analysis of submergence-tolerant and sensitive Brachypodium distachyon ecotypes reveals oxidative stress as a major tolerance factor. Sci Rep. 2016;6:27686. doi: 10.1038/srep27686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu FC, Chou MY, Chou SJ, Li YR, Peng HP, Shih MC. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell. 2013;25:2699–2713. doi: 10.1105/tpc.113.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravot A, Richard G, Lime T, Lemarie S, Jubault M, Lariagon C, Lemoine J, Vicente J, Robert-Seilaniantz A, Holdsworth MJ, et al. Hypoxia response in Arabidopsis roots infected by Plasmodiophora brassicae supports the development of clubroot. BMC Plant Biol. 2016;16:251. doi: 10.1186/s12870-016-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, Voesenek LACJ, Bailey-Serres J. Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol. 2011;190(2):457–471. doi: 10.1111/j.1469-8137.2010.03590.x. [DOI] [PubMed] [Google Scholar]
- 14.Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010;152(3):1484–1500. doi: 10.1104/pp.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christianson JA, Llewellyn DJ, Dennis ES, Wilson IW. Comparisons of early transcriptome responses to low-oxygen environments in three dicotyledonous plant species. Plant Signal Behav. 2010;5:1006–1009. doi: 10.4161/psb.5.8.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christianson JA, Llewellyn DJ, Dennis ES, Wilson IW. Global gene expression responses to waterlogging in roots and leaves of cotton (Gossypium hirsutum L. Plant Cell Physiol. 2010;51:21–37. doi: 10.1093/pcp/pcp163. [DOI] [PubMed] [Google Scholar]
- 17.Nadarajah KK. ROS Homeostasis in abiotic stress tolerance in plants. Int J Mol Sci. 2020;21. doi: 10.3390/ijms21155208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi J, Song CP, Wang B, Zhou J, Kangasjarvi J, Zhu JK, Gong Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol. 2018;60:805–826. doi: 10.1111/jipb.12654. [DOI] [PubMed] [Google Scholar]
- 19.Herrera-Vasquez A, Salinas P, Holuigue L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front Plant Sci. 2015;6:171. doi: 10.3389/fpls.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luhua S, Ciftci-Yilmaz S, Harper J, Cushman J, Mittler R. Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiol. 2008;148:280–292. doi: 10.1104/pp.108.124875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur A, Pati PK, Pati AM, Nagpal AK. In-silico analysis of cis-acting regulatory elements of pathogenesis-related proteins of Arabidopsis thaliana and Oryza sativa. PLoS One. 2017;12:e0184523. doi: 10.1371/journal.pone.0184523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama K, Todaka D, Mizoi J, Yoshida T, Kidokoro S, Matsukura S, Takasaki H, Sakurai T, Yamamoto YY, Yoshiwara K, et al. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Res. 2012;19:37–49. doi: 10.1093/dnares/dsr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Xu XC, Cao JJ, Yin LL, Xia XJ, Shi K, Zhou Y-H, Yu J-Q. Heat shock factor HsfA1a is essential for R gene-mediated nematode resistance and triggers H2O2 Production(1). Plant Physiol. 2018;176:2456–2471. doi: 10.1104/pp.17.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo M, Liu JH, Ma X, Luo DX, Gong ZH, Lu MH. The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JM, Heese A. Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods. 2014;10:6. doi: 10.1186/1746-4811-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ, et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- 27.Gasch P, Fundinger M, Muller JT, Lee T, Bailey-Serres J, Mustroph A. Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in arabidopsis. Plant Cell. 2016;28:160–180. doi: 10.1105/tpc.15.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 2011;479:419–422. doi: 10.1038/nature10536. [DOI] [PubMed] [Google Scholar]
- 29.Weits DA, Giuntoli B, Kosmacz M, Parlanti S, Hubberten HM, Riegler H, Hoefgen R, Perata P, van Dongen JT, Licausi F, et al. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat Commun. 2014;5:3425. doi: 10.1038/ncomms4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P. Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ. 2008;31:1029–1037. doi: 10.1111/j.1365-3040.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1:641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Han R, Yu N, Zhang W, Xing L, Xie D, Peng D. A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS One. 2018;13:e0196592. doi: 10.1371/journal.pone.0196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


