Supplemental Digital Content is Available in the Text.
We studied 46 eyes with Stickler syndrome using ultra-widefield fundus autofluorescence images. Abnormal autofluorescence lesions were found in 81% of the eyes that were associated with age-related alteration and visual field defects.
Key words: Stickler syndrome, ultra-wide field, fundus autofluorescence, retinal degeneration, retinal dystrophy
Purpose:
To determine the characteristics of fundus autofluorescence (FAF) images and visual functions in eyes with Stickler syndrome using ultra-widefield FAF images.
Methods:
Forty-six eyes of 26 patients with mutations in the COL2A1 gene underwent ultra-widefield FAF imaging. The eyes were categorized into three types; no signs of abnormal AF, predominantly hyperfluorescent AF (hyper-AF), and predominantly hypofluorescent AF (hypo-AF). Goldmann perimetry was performed on 34 eyes, and line-scan images of the abnormal AF lesions were obtained by swept-source optical coherence tomography in 4 eyes.
Results:
Abnormal AF lesions were found in 37 eyes of 21 (80.7%) of the 26 patients. Hyper-AF was found in 15 eyes and hypo-AF was found in 22 eyes. The FAF changes corresponded with the funduscopically observed radial paravascular retinal degeneration. The average age at the examination was significantly younger in patients who had eyes with hyper-AF or no abnormal AF than in those with hypo-AF (12.8 vs. 28.4 years; P = 0.009). Abnormal AF-associated visual field defects were found in 5/10 (50%) eyes with hyper-AF and 17/18 (94%) eyes with hypo-AF. Hyper-AF changes tended to appear before retinal changes were detectable by fluorescein angiography. An absence of the ellipsoid zone and the outer nuclear layer and a thinning of the overall retinal thickness were found corresponding to the hypo-AF lesions in the swept source optical coherence tomography images.
Conclusion:
Abnormal FAF is characteristic of eyes with Stickler syndrome. Age-related alterations of the FAF was associated with visual field defects and disruption of the photoreceptors and retinal pigment epithelial cells.
Stickler syndrome is an inherited systemic disorder that affects the eyes, ears, cartilage, and articular tissues.1 The disorder results from an insufficient expression of collagen due to mutations in the procollagen genes including the COL2A1, COL11A1, COL11A2, and COL9A1 genes. About 80% to 90% of the cases are caused by mutations in the COL2A1 gene.2–4 The ocular features of Stickler syndrome are characterized by high myopia, retinal detachments, vitreous degeneration, and presenile cataracts.1–4
Stickler syndrome is the major cause of pediatric retinal detachments and blindness,5–7 and a correct diagnosis in early childhood is critical. The important diagnostic clues of Stickler syndrome are the characteristic vitreous degeneration that can be seen by a slit-lamp microscopy, and the funduscopic alterations of the pigmentation along the retinal vessels, the so-called radial paravascular retinal degeneration (RPRD).3,6,7 However, the characteristics of the RPRDs have been investigated by only their funduscopic appearance.
Fundus autofluorescence (FAF) imaging is a noninvasive method that requires only a short acquisition time to obtain the AF images of the fundus. The AF changes result from an accumulation or the loss of products of retinol metabolism including pyridinium bis-retinoid (A2E) in the retinal pigment epithelial cells suggestive of the functional damages of the outer retina and choroid.8–10
An ultra-widefield (UW) fundus camera was recently developed that allowed clinicians to examine a greater extent of the posterior pole of the eye. The UW-FAF images have shown changes of the outer retina and choroid in several retinal disorders including retinitis pigmentosa, multiple evanescent white dot syndrome, and Vokt–Koyanagi–Harada disease.11–14 To the best of our knowledge, the FAF images in eyes with Stickler syndrome have not been examined.
Thus, the purpose of this study was to determine the characteristics of FAF images and associated visual function in eyes with Stickler syndrome. To accomplish this, UW-FAF images of 26 patients with mutations in the COL2A1 gene were examined.
Methods
This was a retrospective multicenter study of patients from 22 families with Stickler syndrome who had undergone UW-FAF imaging. The procedures used conformed to the tenets of the Declaration of Helsinki, and the study was approved by the Ethics Committee of the University of Occupational and Environmental Health Japan (H30-096), and Jikei University (24-231 6,997). A signed informed consent was obtained from all patients or parents to perform the examinations and present the findings in medical publications.
A diagnosis of Stickler syndrome was made for 25 families based on the criteria by Richard et al3 between Dec 2009 and Nov 2017. Genetic examinations were performed on all families, and 22 families (88%) were confirmed to have mutations in the COL2A1 gene. All mutations were localized except for exon 2. The clinical findings and mutations in the COL2A1 gene of 21 of the patients have been reported in detail.2,15–17 The remaining five patients were newly studied, and the associated mutations in the COL2A1 gene were c.3624del (predicted to p.Gly1209Valfs*18, NM_001844.4) for Patient 1, c.3188_3211delinsGT (p.Ala1063Glyfs*60) for Patient 6, c.2353C>T (p.Arg785*) for Patient 7, and c.1957C>T (p.Arg653*) for Patient 2. Patient 25 was a family member of Patient 24.
All patients underwent an ophthalmologic examination that included measurements of the refractive error, best-corrected visual acuity, perimetry by a Goldmann perimeter (Haag-Streit, Bern, Switzerland), slit-lamp examination, fundus examination, and b-mode scan of swept-source optical coherence tomography (SS-OCT; DRI OCT Triton, Topcon, Tokyo, Japan).
Ultra-widefield fundus photographs, fluorescein angiograms, and FAF images were obtained by the Optos 200Tx (Optos PLC; Dunfermline, Scotland, United Kingdom). Eyes were excluded if the quality of the FAF images was poor or if there was extensive retinal damage including phthisis with or without retinal detachments. In the end, 46 eyes of 26 patients (20 families) were studied, and an image of the central position of the FAF was analyzed. Abnormal AF patterns were found as hyperfluorescent AF or hypofluorescent AF lesions. The hypofluorescent AF lesions were surrounded by hyperfluorescent AF changes (Figure 1). These two AF patterns were often observed in the same eye, thus the eyes were categorized into three types; no signs of abnormal AF, predominantly hyperfluorescent AF, and predominantly hypofluorescent AF (Figure 1) by the classifications of the three retina specialists (K.F., T.N., H.K.).
Fig. 1.
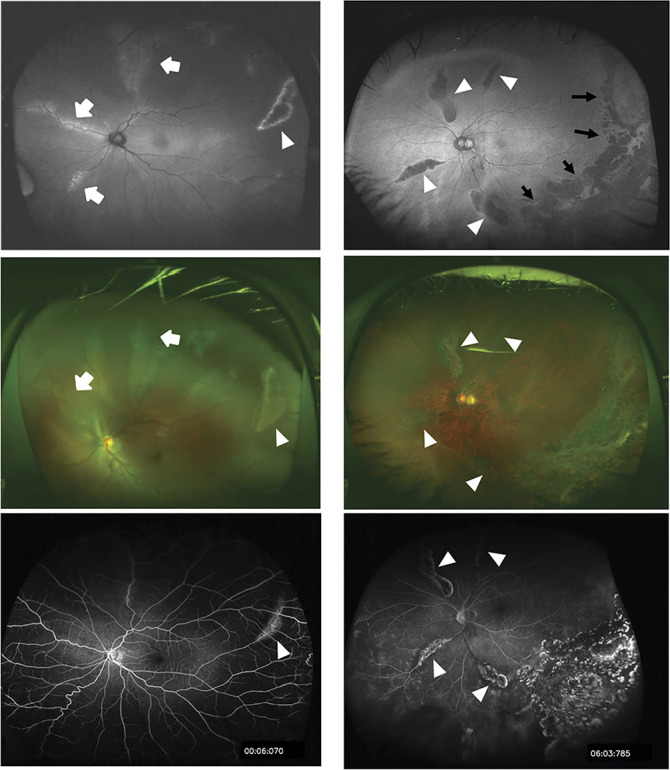
Ultra-widefield FAF (UW-FAF) images and corresponding color fundus and fluorescein angiographic images in eyes with stickler syndrome. The images in the left column are from the left eye of a 13-year-old boy (patient 14), and the images in the right column are from the left eye of a 49-year-old man (patient 26). Top: FAF images representing predominantly hyperfluorescent AF pattern (left) and predominantly hypofluorescent AF pattern (right). Abnormal AF lesions are categorized into hyperfluorescent AF (white arrows) or hypofluorescent AF lesions (white arrowheads). Photocoagulation scars can be seen as hypofluorescent AF spots (back arrows) on the posterior margin of the stickler syndrome-associated AF lesion. Middle: Color fundus photographs showing varying degree of pigmentary changes along the retinal vessels, the so-called radial paravascular retinal degeneration. The changes correspond to the hyper- or hypofluorescent AF changes in the FAF images are shown by identical arrows or arrowheads. Bottom: Fluorescein angiographic images showing window defects corresponding to the hypofluorescent AF lesions in the FAF images shown by the arrowheads. Note that the abnormalities are not shown corresponding to hyperfluorescent AF lesions in the FAF images.
Statistical Analyses
Statistical analyses were performed with the JMP software (version 5.1; SAS Institute Inc, Cary, NC). For the demographic analysis, we divided the patients into two groups based on the FAF appearance: Group 1, both eyes had predominantly hyperfluorescent or no FAF changes; and Group 2, both eyes had predominantly hypofluorescent FAF. One patient (Patient 12) had predominantly hyperfluorescent AF in the left eye and predominantly hypofluorescent AF in the right eye, and was excluded. The right eye was used if both eyes were available. The characteristics of the two groups were compared by one-way analysis of variance. The statistical significance was set at P >0.05.
Results
Of the 26 patients, 13 were female and 13 were male patients. The average age at the time of examination was 21.3 years with a range from 4 to 49 years. For 46 eyes, the refractive error (spherical equivalent) ranged from −3.5 D to −18.0 D with an average of −9.3 D. Four eyes of four patients underwent pars plana vitrectomy with or without lens extraction, and five eyes of three patients underwent cataract extraction.
Abnormal AF lesions were found in 37 eyes of 21 patients (80.8%) of the 26 patients (Table 1). Predominantly hyperfluorescent AF was found in 15 eyes and predominantly hypofluorescent AF in 22 eyes. No sign of abnormal AF was found in nine eyes. The abnormal AF lesions were found along the retinal vessels corresponding to the funduscopic appearance of the so-called RPRD by the ultra-widefield color fundus photographs (Figure 1). These AF lesions were occasionally found to deviate from the retinal vessels and run circumferentially to the temporal equator and resembled peripheral lattice degeneration. The hyperfluorescent AF lesions of smaller size did not correspond to any changes in the color fundus photographs. Exceptional patterns of abnormal AF lesions were found including hypofluorescent AF circumferential to the nasal to temporal equator in Patient 4, and hyperfluorescent AF spots in the macula in Patients 4 and 5 (Table 1 and see Supplemental Figure 1, Supplemental Digital Content 1, http://links.lww.com/IAE/B260).
Table 1.
Summary of Fundus Autofluorescent Features and Visual Field Defects in Patients With Stickler Syndrome
| Patient No. | Family | Kinship | Sex | Age at Examination (yo) | R/L | Refraction (Diopters) | BCVA | Predominant FAF Status | Visual Field Defect at Maximum Level of Isopter: Associated Findings* | Remarks | Patient No of Early Report (Reference) |
| 1 | 1 | Proband | F | 5 | R | −5 | 20/25 | None | None | Not listed | |
| L | −5 | 20/25 | None | None | |||||||
| 2 | 2 | Proband | F | 5 | R | −14.5 | 20/66 | None | NA | Not listed | |
| L | −13.0 | 20/40 | Hyperfluorescent | NA | |||||||
| 3 | 3 | Proband | M | 7 | R | −10 | 20/16 | None | NA | 22 (15) | |
| L | −13 | 20/33 | None | NA | |||||||
| 4 | 3 | Ant | F | 25 | R | −7.25† | 20/66† | Hypofluorescent | V4: O/O/O/U | R) Cat (27 yo), L) Cat (28 yo), FAF:B) Macular hyperfluorescent AF spot, B) circumferential hypoflurecent AF to the equator | 24 (15) |
| L | −14.5† | 20/28† | Hypofluorescent | V4: O | |||||||
| 5 | 3 | Mother | F | 29 | L | −15.87 | 20/25 | None | V4: Av | Cat (28 yo), FAF: Macular hyperfluorescent spot, FA: temporal avascular | 23 (15) |
| 6 | 4 | Proband | F | 9 | R | −12 | 20/66 | Hyperfluorescent | None | Not listed | |
| L | −12 | 20/40 | Hyperfluorescent | None | |||||||
| 7 | 5 | Proband | M | 10 | L | −6.75 | 20/25 | None | I4c: U | 23 (2) | |
| 8 | 6 | Proband | M | 10 | R | −14 | 20/33 | Hyperfluorescent | NA | 13 (15) | |
| L | −12.75 | 20/200 | Hyperfluorescent | NA | |||||||
| 9 | 7 | Proband | M | 10 | L | −14 | 20/22 | None | V4: Pc | Vit (9 yo) | 1 (15) |
| 10 | 7 | Mother | F | 35 | R | −4.5† | 20/20† | Hypofluorescent | V4: R/Pc | L) Vit (35 yo) | 2 (15) |
| L | −5.5† | 20/20† | Hypofluorescent | V4: O/Pc | |||||||
| 11 | 7 | Sister | F | 4 | R | −9 | 20/50 | Hyperfluorescent | NA | 3 (15) | |
| L | −9 | 20/20 | Hyperfluorescent | NA | |||||||
| 12 | 8 | Proband | F | 12 | R | −7.75 | 20/28 | Hypofluorescent | V4: R/O/O | 9 (15) | |
| L | −7.75 | 20/40 | Hyperfluorescent | V4: R/R | |||||||
| 13 | 8 | Mother | F | 35 | R | −6.25 | 20/20 | Hyperfluorescent | None | 11 (15) | |
| L | −3.75 | 20/40 | Hyperfluorescent | I4e: R | |||||||
| 14 | 9 | Proband | M | 13 | R | −5.25 | 20/20 | Hyperfluorescent | I4e: R/O | 27 (2) | |
| L | −4.75 | 20/16 | Hyperfluorescent | I4e: R/R | |||||||
| 15 | 10 | Proband | M | 14 | R | −7.5 | 20/20 | Hyperfluorescent | V4: U | L) Vit (10 yo) | 8 (15) |
| L | −6.25† | 20/16 | None | V4: Pc | |||||||
| 16 | 11 | Proband | M | 15 | R | −11 | 20/22 | Hyperfluorescent | None | 21 (15) | |
| L | −13.25 | 20/22 | Hyperfluorescent | V4: R | |||||||
| 17 | 12 | Proband | M | 15 | R | −8 | 20/16 | Hypofluorescent | V4: O/U | B) Cryo | 5 (15) |
| L | −7 | 20/22 | Hypofluorescent | V4: O/U | |||||||
| 18 | 13 | Proband | M | 17 | R | −14 | 20/33 | Hypofluorescent | NA | R) ENC/Vit/Cat (17 yo), L) ENC (14 yo) | 4 (15) |
| L | −10 | 20/16 | Hypofluorescent | NA | |||||||
| 19 | 14 | Proband | M | 18 | R | −3.5 | 20/66 | Hypofluorescent | V4: R/O | R) Vit (14 yo) | 6 (15) |
| L | −5.25 | 20/20 | Hypofluorescent | I4e: O | |||||||
| 20 | 15 | Proband | M | 27 | R | −8 | 20/16 | Hypofluorescent | V4: O | 12 (15) | |
| 21 | 16 | Proband | F | 30 | R | −9 | 20/22 | Hypofluorescent | V4: O/O/O/O/O/U | Not listed | |
| L | −10 | 20/20 | Hypofluorescent | V4: O/O/O/O/U/U | |||||||
| 22 | 17 | Proband | M | 36 | L | −18 | 20/16 | Hypofluorescent | V4: O | 6 (15) | |
| 23 | 18 | Proband | F | 37 | R | −11 | 20/133 | Hypofluorescent | NA | 19 (2) | |
| L | −12.25 | 20/40 | Hypofluorescent | NA | |||||||
| 24 | 19 | Proband | F | 39 | R | −14† | 20/66† | Hypofluorescent | V4: O/O/O/O/O/O/O | B) Cat (40 yo) | 15 (15) |
| L | −10† | 20/33† | Hypofluorescent | V4: O/O/O/O/O/O/O | |||||||
| 25 | 19 | Daughter | F | 13 | R | −8 | 20/66 | Hypofluorescent | V4: O/O/O/O/O/Pc | Not listed | |
| L | −12 | 20/20 | Hypofluorescent | V4: R/U | |||||||
| 26 | 20 | Father | M | 49 | L | −10.25 | 20/22 | Hypofluorescent | V4: Pc | 20 (15) |
Note that “V4: O/O/O/U” indicates four visual field defects found at an isopter level of V4 consisting of three hypofluorescent AF lesions (O) and one lesion due to undetermined cause (U).
Status before surgery.
Av, avascularization; B, both eyes; BCVA, best-corrected visual acuity; Cat, cataract surgery; Cryo, cryotherapy; ENC, encircling; NA, not analyzed; O, hypofluorescent; Pc, photocoagulation; R, hyperfluorescent; U, undetermined; Vit, vitrectomy, yo, year-old.
Thirteen patients were placed in Group 1 and 12 patients in Group 2. The average refractive errors and average best-corrected visual acuities were not significantly different between patients in Groups 1 and 2; −10.1 D versus −9.6 D, (P = 0.77) and 0.15 logarithm of the minimum angle of resolution (logMAR) units versus 0.24 logMAR units, (P = 0.41). However, the average age at the time of the examination was significantly younger in the patients in Group 1 than in Group 2 (12.8 vs. 28.4 years; P = 0.009).
Goldmann perimetry was performed on 34 eyes, and visual field defects were detected in 28 eyes (82%). At least one visual field defect was associated with the abnormal AF changes in 24 of the 28 eyes; however, the visual field defects were not associated with the abnormal AF in 4 eyes and were probably attributable to retinal photocoagulation and retinal avascular changes in the periphery (Table 1 and Figure 2). Thus, abnormal AF-associated with visual field defects were found in 5/10 (50%) eyes with predominantly hyperfluorescent AF and 17/18 (94%) eyes with predominantly hypofluorescent AF.
Fig. 2.
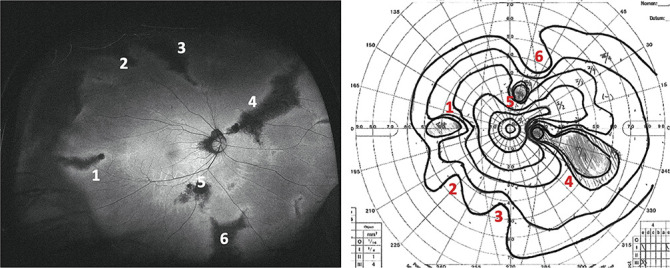
Ultra-widefield FAF (UW-FAF) image and visual field defects detected by Goldmann perimetry of the right eye of a 39-year-old woman with stickler syndrome (patient 24). Left: UW-FAF image showing a predominantly hypofluorescent AF pattern. Right: Goldmann perimetry showing visual field defects that correspond to hypofluorescent AF lesions (numbers one through 6).
Fluorescein angiography was performed on 14 eyes of 8 patients. Of these, eight eyes had a predominantly hypofluorescent FAF pattern and four eyes had a predominantly hyperfluorescent FAF pattern (two eyes had normal AF). In eight eyes with a predominantly hypofluorescent FAF pattern, the window defects corresponded with the hypofluorescent AF spots (Figure 1 right). However, in four eyes with predominantly hyperfluorescent FAF pattern, the window defects were limited to the part of the hypofluorescent AF areas and no angiographic changes were detected corresponding to the hyperfluorescent AF areas (Figure 1 left and see Supplemental Figure 2, Supplemental Digital Content 1, http://links.lww.com/IAE/B260).
In four eyes, line-scan images of the RPRD lesions were obtained by SS-OCT. An absence of the ellipsoid zone and the outer nuclear layer were found, and the sites of these lesions corresponded with the hypofluorescent AF lesions (Figure 3). The inner retinal layers were also thinner to varying degrees over the disrupted outer retinal layers resulting in a severe reduction of the total retinal thickness.
Fig. 3.
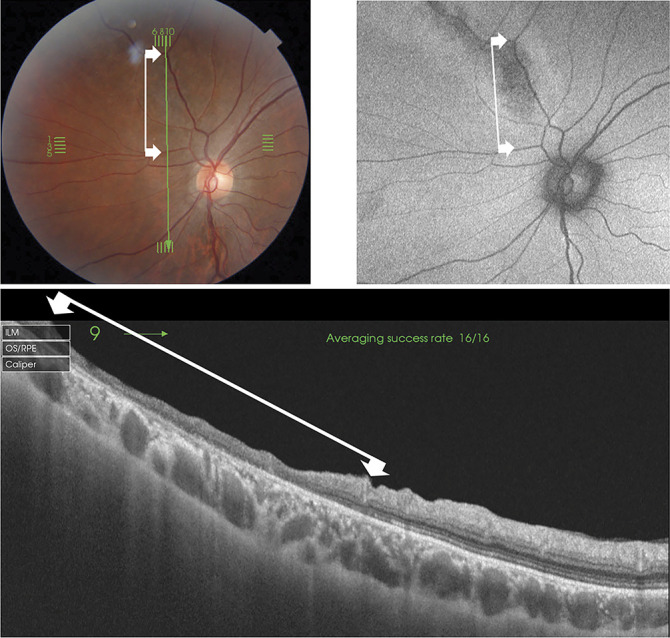
Swept-source optical coherence tomographic (SS-OCT) and ultra-widefield FAF (UW-FAF) images showing changes in the left eye of an 18-year-old young man (patient 19). Top left: Fundus photograph nasal to the posterior pole showing mild pigmentary changes along with an upper nasal vessel. Top right: Part of UW-FAF image superimposed on the fundus photograph showing hypofluorescent AF. Bottom: B-scan SS-OCT image along the lesion of the hypofluorescent AF designated by the green vertical line of the fundus photograph. This photograph demonstrates the regional absence of the ellipsoid zone and the outer nuclear layer and a thinning of the inner retinal layers. The corresponding extent is shown by the lines between the arrows in the three images.
Discussion
Our results showed that abnormal AF was present in 81% of eyes with Stickler syndrome. Younger patients tended to show no abnormal AF or predominantly the hyperfluorescent AF type, and the older patients had hypofluorescent AF surrounding the hyperfluorescent AF. It is possible that the abnormal AF patterns transition from hyperfluorescent to hypofluorescent AF during the course of the disease process. Typical abnormal AF changes were consistent with the funduscopic RPRD appearance and window defects by fluorescein angiography. Eyes with predominantly hypofluorescent AF were more likely to be associated with visual field defects. The presence or absence of visual field defect was not correlated with degree of myopia.
In our earlier electroretinographic (ERG) studies, eyes with Stickler syndrome were found to have reduced ERGs which worsened with increasing age progressing to a cone dominant impairment.17 Although this tendency was correlated with age, the reduced ERG amplitudes under scotopic condition was not significantly correlated with the degree of myopia. From this and the results of our earlier studies, the retinal degenerative changes seem to be age-dependent. However, the extent of the RPRD was not directly correlated with the ERG changes.17
The RPRDs have been believed to be one of the characteristic findings in eyes with Stickler syndrome.6,7 Hagler et al6 analyzed 33 patients with Stickler syndrome and reported that RPRDs were detected in all eyes during a long-term follow-up study. Our cross-sectional study showed that nearly 50% of the Stickler patients (Group 1) had limited or no signs of RPRD. Although the RPRD is an essential sign in eyes with Stickler syndrome, it is worth noting that RPRD is not a requisite for the diagnosis. Ultra-widefield FAF is useful because all funduscopic RPRD changes can be detected more clearly by this noninvasive method. Moreover, UW-FAF is useful for an earlier diagnosis of Stickler syndrome because UW-FAF can show small hyperfluorescent AF lesions before obvious RPRD lesions appear in the ophthalmoscopic images. Hyperfluorescent AF lesions are likely to appear before the corresponding retinal changes are detectable by fluorescein angiography.
The RPRD changes possibly originate from the retinal vessels which are located in the inner retinal layers. Our earlier study showed that the b-wave/a-wave amplitude ratio of the dark-adapted ERGs was significantly smaller, and thus the inner retinal layers including the bipolar cells are more severely affected than the photoreceptors.17 Therefore, we assume that damages of the neurons in the inner retinal layers precede those of the photoreceptors and the retinal pigment epithelial cells. Nevertheless, a breakdown of the inner blood–retinal barrier along the RPRD lesions was not evident by fluorescein angiography.
The SS-OCT images of eyes with Stickler syndrome showed a disruption of the photoreceptors and retinal pigment epithelial layers that resulted in a thinning of the retina at the areas of the RPRD. The degenerative areas can be superimposed on the hypofluorescent AF lesions associated with visual field defects. The combination of the anatomical and functional changes detected by an OCT, FAF, and perimetry, are consistent with other retinal dystrophies including retinitis pigmentosa, macular dystrophy, and pigmented paravenous retinochoroidal atrophy.18–20 However, in contrast with these retinal dystrophies in which the degenerative changes are restricted to the outer retina, eyes with Stickler syndrome had a retinal thinning involving the inner retina.17 No mechanism has been suggested to explain how the retinal degeneration progresses in Stickler syndrome. A strong adhesion is inherent between the vitreous and retinal vessels,21 and a pathologic vitreoretinal interface is known to exert traction on the retina in eyes with Stickler syndrome.22 One possibility is that trauma due to the traction on the retinal vessels leads to secondary retinal degeneration. Further studies are needed to understand the mechanism for the retinal degeneration in Stickler syndrome.
This study has several limitations. First, this was a retrospective, cross-sectional study. Thus, we were not able to conclude whether the abnormal AF lesions were progressive. Second, we used UW-FAF images at the central position. Using additional images away from the center can detect further retinal changes in the periphery. Third, the number of the patients was limited and only patients with mutations in the COL2A1 gene were examined. Moreover, we cannot assess the ocular-only type of Stickler syndrome that is caused by mutations of exon two of the COL2A1 gene.23 Fourth, Goldmann perimetry measurements may be influenced by the proficiency of examiners. Nonetheless, we believe the clinical significance of this study were not altered by these limitations.
Footnotes
Supported by Grants-in-Aid for Scientific Research, grant numbers 17K11441, 2017-2019 (H. Kondo). None of the authors has any financial/conflicting interests to disclose. The authors thank Professor Duco Hamasaki, Professor Emeritus, Bascom Palmer Eye Institute, University of Miami, Miami, Florida, for his critical comments and valuable assistance.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Stickler GB, Belau PG, Farrell FJ, et al. Hereditary progressive arthro-ophthalmopathy. Mayo Clin Proc 1965;40:433–455. [PubMed] [Google Scholar]
- 2.Kondo H, Matsushita I, Nagata T, et al. Novel mutations in the COL2A1 gene in Japanese patients with Stickler syndrome. Hum Genome 2016;3:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards AJ, Baguley DM, Yates JR, et al. Variation in the vitreous phenotype of Stickler syndrome can be caused by different amino acid substitutions in the X position of the type II collagen Gly-X-Y triple helix. Am J Hum Genet 2000;67:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards AJ, McNinch A, Martin H, et al. Stickler syndrome and the vitreous phenotype: mutations in COL2A1 and COL11A1. Hum Mutat 2010;31:E1461–E1471. [DOI] [PubMed] [Google Scholar]
- 5.Fincham GS, Pasea L, Carroll C, et al. Prevention of retinal detachment in stickler syndrome: the cambridge prophylactic cryotherapy protocol. Ophthalmology 2014;121:1588–1597. [DOI] [PubMed] [Google Scholar]
- 6.Hagler WS, Crosswell HH., Jr Radial perivascular chorioretinal degeneration and retinal detachment. Trans Am Acad Ophthalmol Otolaryngol 1968;72:203–216. [PubMed] [Google Scholar]
- 7.Parma ES, Korkko J, Hagler WS, Ala-Kokko L. Radial perivascular retinal degeneration: a key to the clinical diagnosis of an ocular variant of Stickler syndrome with minimal or no systemic manifestations. Am J Ophthalmol 2002;134:728–734. [DOI] [PubMed] [Google Scholar]
- 8.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 1995;36:718–729. [PubMed] [Google Scholar]
- 9.Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res 2012;31:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparrow JR, Yoon KD, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci 2010;51:4351–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heussen FM, Vasconcelos-Santos DV, Pappuru RR, et al. Ultra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging 2011;42:272–277. [DOI] [PubMed] [Google Scholar]
- 12.Ogura S, Yasukawa T, Kato A, et al. Wide-field fundus autofluorescence imaging to evaluate retinal function in patients with retinitis pigmentosa. Am J Ophthalmol 2014;158:1093–1098. [DOI] [PubMed] [Google Scholar]
- 13.Robson AG, Saihan Z, Jenkins SA, et al. Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol 2006;90:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto H, Kishi S. Ultra-wide-field fundus autofluorescence in multiple evanescent white dot syndrome. Am J Ophthalmol 2015;159:698–706. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita I, Nagata T, Hayashi T, et al. Foveal hypoplasia in patients with stickler syndrome. Ophthalmology 2017;124:896–902. [DOI] [PubMed] [Google Scholar]
- 16.Kondo H. Corrigendum Ophthalmol 2018;125:786. [DOI] [PubMed] [Google Scholar]
- 17.Kondo H, Fujimoto K, Imagawa M, et al. Electroretinograms of eyes with Stickler syndrome. Doc Ophthalmol 2020;140:233–243. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa S, Mitamura Y, Hagiwara A, et al. Changes of fundus autofluorescence, photoreceptor inner and outer segment junction line, and visual function in patients with retinitis pigmentosa. Clin Exp Ophthalmol 2010;38:597–604. [DOI] [PubMed] [Google Scholar]
- 19.Fleckenstein M, Charbel Issa P, Helb HM, et al. Correlation of lines of increased autofluorescence in macular dystrophy and pigmented paravenous retinochoroidal atrophy by optical coherence tomography. Arch Ophthalmol 2008;126:1461–1463. [DOI] [PubMed] [Google Scholar]
- 20.Murray AT, Kirkby GR. Pigmented paravenous retinochoroidal atrophy: a literature review supported by a unique case and insight. Eye 2000;14:711–716. [DOI] [PubMed] [Google Scholar]
- 21.Sebag J. Structure of the Vitreous. The Vitreous. Structure, Function, and Pathology. New York, NY: Springer-Verlag; 1989. [Google Scholar]
- 22.Yokoi T, Koide R, Matsuoka K, et al. Analysis of the vitreous membrane in a case of type 1 Stickler syndrome. Graefes Arch Clin Exp Ophthalmol 2009;247:715–718. [DOI] [PubMed] [Google Scholar]
- 23.Richards AJ, Martin S, Yates JR, et al. COL2A1 exon 2 mutations: relevance to the Stickler and Wagner syndromes. Br J Ophthalmol 2000;84:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]


