Abstract
Background.
Understanding the duration of human papillomavirus (HPV) infection may help find suitable end points for vaccine trials and testing intervals in screening studies. We studied genotype-specific infection duration among 2462 women enrolled in the Ludwig-McGill cohort study.
Methods.
Cervical specimens collected every 4–6 months were tested by a polymerase chain reaction protocol. Actuarial techniques were used to estimate the duration of HPV infection and to investigate the influence of age, number of sexual partners, and coinfection with multiple HPV types.
Results.
At enrollment, the prevalence of infection with high-risk HPV types was 10.6%, and the prevalence of infection with low-risk HPV types was 6.1%; incidence rates were 6.1 and 5.0 infections per 1000 women-months, respectively. Prevalent infections took longer to clear than incident infections (mean time to clearance, 18.6 months vs. 13.5 months). The mean duration of incident infection with high- and low-risk HPV varied according to the analytic approach used to measure this variable and showed considerable variation by HPV type (range, 5.1–15.4 months). Age and number of partners did not influence infection duration, whereas coinfection was associated with increased infection duration. The mean duration of HPV-16 monoinfection was 11.0 months, and the mean duration of HPV-16 coinfection was 15.4 months.
Conclusion.
There was considerable variation among HPV types with regard to the duration of infection. Coinfection with multiple types contributed to an increased infection duration.
Human papillomaviruses (HPVs) are among the most common sexually transmitted infectious agents. Approximately 13–18 mucosotropic HPV genotypes are considered of high oncogenic risk (HR-HPVs) and cause infections that can lead to cervical cancer [1–4]. The risk of cervical neoplasia is greatest among women with persistent HR-HPV infection [5–7]. Moreover, HR-HPV infection seems to persist longer than infection with HPVs considered to have a low oncogenic risk (LR-HPVs) [8–14]. However, little is known about the type-specific duration of HPV infection and its determinants. Some studies have estimated duration [9–12, 14–22] but used mostly grouped rather than type-specific HPV data, used data on prevalent rather than incident infections, or were based on short follow-up durations.
Knowledge of the duration of type-specific HPV infection will inform policy decisions concerning cervical cancer screening intervals using HPV tests and help identify realistic end points for assessing HPV vaccination efficacy. It can also be used to inform cost-effectiveness models of HPV screening and vaccination.
We analyzed data from a cohort study of women who were undergoing regular cervical cancer screening, to investigate the acquisition and clearance of HPV infection, by HPV type, oncogenic risk category, and phylogenetic relationship. We also studied the influence of age, number of sexual partners, and coinfection with multiple HPV types.
SUBJECTS, METHODS, AND MATERIALS
Subject recruitment.
Female subjects were enrolled into the Ludwig-McGill cohort study, a longitudinal investigation of the natural history of HPV and cervical cancer precursors [23]. In brief, women attending a maternal and child health program in a low-income neighborhood in São Paulo, Brazil, were recruited between 1993 and 1997 and followed for up to 10 years. Women were eligible to participate if they (1) were aged 18–60 years, (2) were São Paulo residents, (3) were not pregnant and had no intention of becoming pregnant during the next year, (4) had an intact uterus and no referral for hysterectomy, (5) reported no use of vaginal medication in the previous 2 days, and (6) had no treatment for cervical disease in the previous 6 months. Subjects gave written informed consent. The protocol was approved by institutional ethical and research review boards of the participating institutions in Canada and Brazil.
Follow-up consisted of 1 visit every 4 months for the first year and 2 visits per year thereafter. Cervical specimens were obtained for HPV testing at every visit. During most visits, subjects underwent an interview to provide information on sociodemographic, lifestyle, sexual, and reproductive characteristics. This analysis includes data from the first 12 follow-up visits over a period of 5 years.
HPV DNA testing.
An Accelon biosampler (Medscand) was used to collect ectocervical and endocervical samples. Samples were placed in tubes that contained Tris-EDTA buffer (pH 7.4). DNA was extracted, purified by spin-column chromatography, and amplified by polymerase chain reaction (PCR), using the MY09/11 and PGMY protocols [24, 25], for detection of HPV DNA. Typing of amplified products was performed by hybridization with individual oligonucleotide probes and by restriction fragment length polymorphism (RFLP) analysis, which identified >40 HPV genital types. Amplified products that hybridized only with a generic probe and were unidentifiable in RFLP analysis were classified as positive for unknown types. The types tested included HR-HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82 and LR-HPV types 6/11, 26, 32, 34, 40, 42, 44, 53, 54, 55, 57, 61, 62, 64, 67, 69, 70, 71, 72, 81, 83, 84, and 89 (unknown types were considered to be LR-HPVs) [3, 26]. We included >30 type-specific positive controls in hybridization membranes. DNA specimen quality was checked by amplification of a 268-bp human β-globin gene region [24]. Specimens were tested blindly, and precautions were taken to prevent contamination. Samples that were negative for both HPV and β–globin were considered inadequate for analysis.
Statistical analysis.
We used the Kaplan-Meier technique to obtain actuarial estimates of mean and median durations (with respective 95% confidence intervals [CIs]) of the first incident HPV infection, considering types individually and by their oncogenic risk. We also grouped HPV types according to their phylogenetic relationship within the genus alpha-papillomavirus (species 3, 5, 6, 7, 9, and 10) [27]. Women with prevalent infection involving a specific HPV type at enrollment were excluded from the analysis for that type. Duration was defined as the time to clearance of an incident infection and was measured from the first visit in which HPV was detected until the visit in which the same type was not detected or, for censored observations, the most recent recorded visit. Visits in which testing of the specimen was noninformative (i.e., test results were negative for both HPV and β–globin) were ignored; the next visit with an informative HPV test result was used. We assumed that, because of their randomness across visits, the patterns of missing data did not impact the duration of infection. The mean duration of infection was calculated for the entire cohort and was also stratified by age group (18–22, 23–27, 28–32, 33–37, 38–42, 43–47, and ⩾48 years), lifetime number of sexual partners (1, 2, 3, 4, and ⩾5 partners), and whether a given type was detected alone (monoinfection) or in coinfection with other HPV types during follow-up.
Infection duration was also estimated for prevalent infections via a similar approach, except that infection clearance was measured from enrollment. We used the log-rank test to assess the significance of differences in clearance times between prevalent and incident infections.
We estimated the mean duration of incident infections for grouped types in 2 ways. First, we calculated an unweighted group-specific duration, defined as the interval from the onset of the first infection with a type that belonged to a specified group to the end of the period during which the woman was still positive for a type of the same group, even if the latter type was different than the one detected at the onset. Because it was not uncommon for a subject to have different types of HPV detected on consecutive visits, this approach permitted the assessment of infections labeled as a group, as if detected by a single-probe cocktail test. We called this estimate the pooled-probe duration of positivity. Second, we calculated a weighted estimate of duration by considering only same-type infections within a group. The contribution of individual type-specific infections within a group to that group’s mean infection duration was weighted according to the infection incidence for each HPV type belonging to that group.
We also investigated the influence of gaps in a sequence of positive results for a given type on the estimate of mean duration for the group that included that type. First, we computed the above weighted (type-specific) estimates by considering each gap as indicative of clearance; that is, the episode was considered to have ended as soon as the first negative result for that type was encountered following a string of positive results (strict definition). Second, we interpreted a gap in type-specific positivity as a false-negative result that was possibly due to sampling error or to a low viral load in the specimen. This was done by ignoring the gap in the sequence of positive test results. We calculated these gap-ignored, weighted means by considering separately a gap of only 1 visit and a gap of 2 consecutive visits within the span of a type-specific episode.
All analyses were performed using Stata software, version 9.0 (Stata). Significance tests were 2-sided.
RESULTS
The study enrolled 2528 women, corresponding to a 70% response rate. Sixty-six ineligible women were subsequently excluded. The remaining 2462 participants had 18,555 visits during which cervical specimens were obtained for HPV testing and typing. This included all visits (through the first 12) during the first 5 years of follow-up. The mean number of visits (±SD) was 7.7 ± 3.8, the mean follow-up time (±SD) was 46.7 ± 27.9 months, the mean age (±SD) was 32.7 ± 8.8 years (median, 32.0 years [range, 18–59 years]), and most women (64%) were white.
Table 1 shows the prevalence of HPV types at baseline, as well as the number and clearance rates of incident infections during the follow-up period by type, phylogenetic relationship, and oncogenic potential. HPV-16 was the most prevalent type (2.7% of women) and had the highest incidence of infection during follow-up (1.8 cases per 1000 women-months). HPV types 53, 51, 31, and 58 were also highly prevalent (1.7%, 1.3%, 1.2%, and 1.1% of women, respectively) and were among the types with the highest infection incidence during the follow-up period. The prevalence of any HPV type, HR-HPV types, and LR-HPV types was 16.8%, 10.6%, and 6.1%, respectively, and the infection incidence was 9.5, 6.1, and 5.0 cases per 1000 women-months, respectively. The clearance rate was higher for LR-HPVs than for HR-HPVs (94.7 vs. 75.3 infections per 1000 women-months). Alpha-papillomavirus species 9 had the greatest infection prevalence and incidence and the lowest clearance rate.
Table 1.
Characteristics of human papillomavirus (HPV) infection among women from São Paulo, Brazil, by HPV type, phylogenetic relationship, and oncogenic risk.
| HPV category | Prevalence at enrollment, no. (%) of women (n = 2351a) | Incident infections, no. of women | Follow-up duration, women-months | Incidence rate,b infections/1000 women-months (95% CI) | Cleared infection, no. of women | Follow-up duration, women-months | Clearance rate, infections/1000 women-months (95% CI) |
|---|---|---|---|---|---|---|---|
| Type | |||||||
| 6/11 | 26 (1.1) | 62 | 109,735 | 0.57 (0.44–0.72) | 53 | 506 | 104.8 (80.1–137.2) |
| 16 | 64 (2.7) | 189 | 104,559 | 1.81 (1.57–2.08) | 148 | 1795 | 82.4 (70.2–96.8) |
| 18 | 26 (1.1) | 52 | 109,960 | 0.47 (0.36–0.62) | 46 | 501 | 91.9 (68.8–122.7) |
| 26 | 3 (0.1) | 13 | 112,457 | 0.12 (0.07–0.20) | 9 | 82 | 109.3 (56.9–210.1) |
| 31 | 29 (1.2) | 56 | 109,718 | 0.51 (0.39–0.66) | 43 | 539 | 79.8 (59.2–107.6) |
| 32 | 1 (0.0) | 9 | 112,782 | 0.08 (0.04–0.15) | 4 | 27 | 146.2 (54.9–389.4) |
| 33 | 9 (0.4) | 31 | 111,271 | 0.28 (0.20–0.40) | 26 | 400 | 65.0 (44.3–95.5) |
| 34 | 2 (0.1) | 0 | 112,800 | NA | NA | NA | NA |
| 35 | 7 (0.3) | 59 | 111,048 | 0.53 (0.41–0.69) | 44 | 558 | 78.9 (58.7–106.0) |
| 39 | 3 (0.1) | 31 | 112,075 | 0.28 (0.19–0.39) | 22 | 298 | 73.9 (48.7–112.2) |
| 40 | 6 (0.3) | 30 | 111,857 | 0.27 (0.19–0.38) | 21 | 167 | 125.8 (82.1–193.0) |
| 42 | 1 (0.0) | 23 | 112,524 | 0.20 (0.14–0.31) | 13 | 159 | 81.8 (47.5–140.9) |
| 44 | 2 (0.1) | 7 | 112,540 | 0.06 (0.03–0.13) | 7 | 59 | 118.0 (56.3–247.6) |
| 45 | 13 (0.6) | 48 | 110,931 | 0.43 (0.33–0.57) | 38 | 414 | 91.9 (66.9–126.3) |
| 51 | 30 (1.3) | 106 | 107,976 | 0.98 (0.81–1.19) | 91 | 1045 | 87.0 (70.9–106.9) |
| 52 | 20 (0.9) | 65 | 109,872 | 0.59 (0.46–0.75) | 48 | 593 | 81.0 (61.0–107.4) |
| 53 | 39 (1.7) | 109 | 107,273 | 1.02 (0.84–1.23) | 91 | 1056 | 86.2 (70.2–105.8) |
| 54 | 8 (0.3) | 52 | 110,945 | 0.47 (0.36–0.62) | 42 | 439 | 95.6 (70.7–129.4) |
| 55 | 10 (0.4) | 62 | 110,952 | 0.56 (0.44–0.72) | 52 | 494 | 105.3 (80.3–138.2) |
| 56 | 13 (0.6) | 46 | 111,326 | 0.41 (0.31–0.55) | 32 | 410 | 78.1 (55.2–110.4) |
| 57 | 0 | 5 | 112,867 | 0.04 (0.02–0.11) | 4 | 29 | 136.3 (51.2–363.2) |
| 58 | 27 (1.1) | 68 | 109,536 | 0.62 (0.49–0.79) | 52 | 721 | 72.1 (55.0–94.7) |
| 59 | 13 (0.6) | 40 | 110,957 | 0.36 (0.26–0.49) | 33 | 319 | 103.5 (73.6–145.6) |
| 61 | 18 (0.8) | 32 | 110,842 | 0.29 (0.20–0.41) | 26 | 273 | 95.1 (64.7–139.6) |
| 62 | 2 (0.1) | 38 | 111,699 | 0.34 (0.25–0.47) | 32 | 305 | 104.9 (74.2–148.3) |
| 64 | 0 | 0 | 112,900 | NA | NA | NA | NA |
| 66 | 10 (0.4) | 33 | 111,600 | 0.30 (0.21–0.42) | 27 | 209 | 129.4 (88.8–188.8) |
| 67 | 0 | 6 | 112,570 | 0.05 (0.02–0.12) | 5 | 28 | 178.2 (74.2–428.1) |
| 68 | 15 (0.6) | 47 | 110,797 | 0.42 (0.32–0.56) | 41 | 368 | 111.3 (81.9–151.1) |
| 69 | 0 | 3 | 112,782 | 0.03 (0.01–0.08) | 2 | 10 | 197.4 (49.4–789.3) |
| 70 | 18 (0.8) | 23 | 111,380 | 0.21 (0.14–0.31) | 17 | 140 | 121.8 (75.7–195.9) |
| 71 | 5 (0.2) | 19 | 112,094 | 0.17 (0.11–0.27) | 15 | 162 | 92.7 (55.9–153.7) |
| 72 | 6 (0.3) | 6 | 112,360 | 0.05 (0.02–0.12) | 5 | 39 | 127.4 (53.0–306.1) |
| 73 | 10 (0.4) | 40 | 110,957 | 0.36 (0.26–0.49) | 35 | 326 | 107.5 (77.2–149.7) |
| 81 | 9 (0.4) | 20 | 111,794 | 0.18 (0.12–0.28) | 16 | 159 | 100.6 (61.6–164.2) |
| 82 | 3 (0.1) | 19 | 111,962 | 0.17 (0.11–0.27) | 15 | 152 | 98.7 (59.5–163.7) |
| 83 | 0 | 34 | 111,805 | 0.30 (0.22–0.43) | 29 | 297 | 97.6 (67.8–140.4) |
| 84 | 14 (0.6) | 72 | 110,396 | 0.65 (0.52–0.82) | 54 | 588 | 91.9 (70.4–119.9) |
| 89 | 1 (0.0) | 16 | 112,360 | 0.14 (0.09–0.23) | 16 | 127 | 126.5 (77.5–206.4) |
| Phylogenetic relationshipc | |||||||
| Species 3 | 54 (2.3) | 194 | 104,346 | 1.86 (1.62–2.14) | 161 | 1655 | 97.3 (83.3–113.5) |
| Species 5 | 34 (1.5) | 132 | 106,770 | 1.24 (1.04–1.47) | 109 | 1247 | 87.4 (72.5–105.5) |
| Species 6 | 62 (2.6) | 172 | 104,814 | 1.64 (1.41–1.91) | 138 | 1598 | 86.4 (73.1–102.1) |
| Species 7 | 85 (3.6) | 199 | 102,683 | 1.94 (1.69–2.23) | 164 | 1742 | 94.1 (80.8–109.7) |
| Species 9 | 144 (6.1) | 354 | 94,387 | 3.75 (3.38–4.16) | 280 | 3661 | 76.5 (68.0–86.0) |
| Species 10 | 36 (1.5) | 122 | 107,612 | 1.13 (0.95–1.35) | 104 | 1045 | 99.5 (82.1–120.6) |
| Oncogenic riskd | |||||||
| Any | 394 (16.8) | 666 | 70,150 | 9.49 (8.80–10.24) | 548 | 7368 | 74.4 (68.4–80.9) |
| High | |||||||
| Any | 250 (10.6) | 509 | 83,575 | 6.09 (5.58–6.64) | 411 | 5459 | 75.3 (68.4–82.9) |
| Except type 16 | 200 (8.5) | 432 | 88,586 | 4.88 (4.44–5.36) | 348 | 4516 | 77.1 (69.4–85.6) |
| Except types 16 and 18 | 176 (7.5) | 416 | 90,414 | 4.60 (4.18–5.07) | 330 | 4232 | 78.0 (70.0–86.9) |
| Low | 144 (6.1) | 450 | 90,893 | 4.95 (4.51–5.43) | 379 | 4002 | 94.7 (85.6–104.7) |
NOTE. CI, confidence interval; NA, not applicable.
Data exclude women who tested negative for both β-globin and HPV and women who were not tested.
Data exclude women who tested positive for HPV at baseline.
Data are for alpha-papillomavirus species. HPV types 61, 62, 72, 81, 83, 84, and 89 are classified as alpha-papillomavirus species 3; HPV types 26, 51, 69, and 82 as species 5; HPV types 53, 56, and 66 as species 6; HPV types 18, 39, 45, 59, 68, and 70 as species 7; HPV types 16, 31, 33, 35, 52, 58, and 67 as species 9; and HPV types 6/11, 44, and 55 as species 10.
See the text for descriptions of high-risk and low-risk HPV types.
Table 2 shows the duration of the first incident infections for HPV types and groups and the duration of prevalent infections for HPV groups. HPV-33 infection had the longest mean and median duration (15.4 and 11.8 months, respectively). Infections due to HPV types 58, 39, 35, 42, 56, 16, 52, 31, and 53 were also appreciably longer than those due to the remaining types. The mean infection duration for individual types ranged from 5.1 to 15.4 months, whereas the median duration ranged from 3.9 to 11.8 months. The mean pooled-probe positivity duration of incident infection for any HPVs, HR-HPVs, HR-HPVs except HPV-16, and HR-HPVs except types 16 and 18 were approximately the same (slightly longer than 13 months), and the mean duration of LR-HPV infection was significantly shorter (10.5 months). Alpha-papillomavirus species 9 had the longest pooled-probe positivity mean for a phylogenetically defined group (13.3 months).
Table 2.
Mean and median duration of the first incident human papillomavirus (HPV) infection and prevalent HPV infection among women from São Paulo, Brazil, by HPV type, phylogenetic relationship, and oncogenic risk.
| Infection type, HPV category | Subject, no. | Duration, months (95% confidence interval) | |||
|---|---|---|---|---|---|
| Mean | Median | ||||
| Incidenta | |||||
| Type | |||||
| 6/11 | 53 | 9.5 (6.9–12.1) | 6.0 (5.7–6.9) | ||
| 16 | 162 | 11.9 (10.3–13.5) | 7.3 (6.3–10.7) | ||
| 18 | 47 | 10.9 (8.3–13.4) | 6.9 (6.0–12.0) | ||
| 26 | 9 | 9.1 (6.4–11.9) | 10.4 (3.6–12.2) | ||
| 31 | 50 | 11.5 (9.3–13.7) | 11.1 (5.9–12.2) | ||
| 32 | 4 | 6.8 (4.1–9.6) | 5.6 (4.1)b | ||
| 33 | 29 | 15.4 (9.6–21.2) | 11.8 (6.5–16.1) | ||
| 35 | 53 | 12.8 (8.6–17.1) | 6.2 (6.0–8.4) | ||
| 39 | 24 | 13.2 (9.7–16.8) | 6.5 (6.0–19.4) | ||
| 40 | 21 | 7.9 (6.1–9.8) | 6.0 (5.4–7.9) | ||
| 42 | 16 | 12.0 (4.1–19.9) | 6.0 (5.5–12.7) | ||
| 44 | 7 | 8.5 (5.9–11.1) | 6.0 (5.1–12.1) | ||
| 45 | 44 | 10.0 (8.3–11.8) | 6.4 (6.0–12.0) | ||
| 51 | 100 | 11.4 (9.4–13.3) | 6.3 (6.0–11.7) | ||
| 52 | 55 | 11.8 (9.4–14.2) | 11.7 (6.1–12.0) | ||
| 53 | 95 | 11.5 (9.7–13.3) | 10.0 (6.1–11.7) | ||
| 54 | 46 | 10.4 (7.4–13.4) | 6.0 (5.9–6.4) | ||
| 55 | 58 | 9.0 (7.2–10.8) | 6.1 (6.0–6.5) | ||
| 56 | 36 | 12.3 (9.3–15.2) | 11.3 (6.5–12.2) | ||
| 57 | 4 | 7.3 (4.4–10.2) | 6.1 (4.3)b | ||
| 58 | 61 | 14.5 (9.7–19.3) | 6.3 (6.0–10.8) | ||
| 59 | 34 | 9.5 (7.8–11.2) | 7.4 (6.0–11.8) | ||
| 61 | 27 | 10.4 (7.6–13.2) | 6.5 (5.8–11.5) | ||
| 62 | 33 | 9.4 (7.5–11.3) | 8.1 (6.0–11.8) | ||
| 66 | 27 | 7.7 (6.5–8.9) | 6.1 (6.0–8.0) | ||
| 67 | 5 | 5.6 (2.0–9.2) | 3.9 (3.1)b | ||
| 68 | 42 | 8.8 (7.0–10.5) | 6.3 (5.9–7.8) | ||
| 69 | 2 | 5.1 (3.8–6.3) | 4.1 (4.1)b | ||
| 70 | 18 | 8.1 (6.0–10.1) | 6.0 (4.7–10.5) | ||
| 71 | 16 | 10.6 (7.1–14.1) | 11.7 (4.1–13.4) | ||
| 72 | 6 | 7.4 (4.4–10.4) | 5.3 (3.7)b | ||
| 73 | 36 | 9.4 (7.0–11.8) | 6.2 (5.9–7.1) | ||
| 81 | 17 | 9.7 (6.4–13.0) | 6.8 (4.3–12.0) | ||
| 82 | 15 | 10.1 (5.0–15.3) | 6.2 (4.0–10.3) | ||
| 83 | 32 | 10.1 (7.7–12.5) | 6.3 (6.0–11.8) | ||
| 84 | 59 | 10.6 (8.6–12.5) | 7.1 (6.0–11.0) | ||
| 89 | 16 | 7.9 (5.8–10.1) | 6.0 (4.9–9.7) | ||
| Phylogenetic relationshipc | |||||
| Species 3 | 174 | 10.0 (9.0–11.1) | 6.3 (6.1–9.5) | ||
| Species 5 | 118 | 11.3 (9.5–13.2) | 6.3 (6.0–10.4) | ||
| Species 6 | 146 | 11.5 (9.8–13.2) | 9.0 (6.2–11.3) | ||
| Species 7 | 176 | 10.5 (9.3–11.8) | 6.4 (6.0–9.7) | ||
| Species 9 | 313 | 13.3 (11.6–14.9) | 6.7 (6.2–9.1) | ||
| Species 10 | 111 | 10.3 (8.2–12.5) | 6.1 (6.0–6.5) | ||
| Oncogenic riskd | |||||
| Any | 605 | 13.5 (12.4–14.7) | 7.1 (6.3–9.7) | ||
| High | |||||
| Any | 459 | 13.4 (12.1–14.8) | 6.5 (6.2–8.6) | ||
| Except type 16 | 393 | 13.2 (11.8–14.7) | 6.5 (6.2–8.4) | ||
| Except types 16 and 18 | 375 | 13.1 (11.6–14.6) | 6.5 (6.2–8.4) | ||
| Low | 396 | 10.5 (9.6–11.5) | 6.2 (6.0–6.7) | ||
| Prevalente | |||||
| Any | 342 | 18.6 (16.0–21.1) | 8.2 (8.0–9.2) | ||
| High oncogenic risk | 216 | 15.2 (12.5–18.0) | 8.0 (6.9–8.2) | ||
| Low oncogenic risk | 126 | 12.2 (9.4–15.1) | 6.2 (4.5–8.0) | ||
| HPV-16 | 54 | 15.5 (10.8–20.2) | 8.5 (6.9–11.9) | ||
Number of subjects does not include women with incident infection detected at the last visit.
Upper limit of the confidence interval was not determined.
Data are for alpha-papillomavirus species. HPV types 61, 62, 72, 81, 83, 84, and 89 are classified as alpha-papillomavirus species 3; HPV types 26, 51, 69, and 82 as species 5; HPV types 53, 56, and 66 as species 6; HPV types 18, 39, 45, 59, 68, and 70 as species 7; HPV types 16, 31, 33, 35, 52, 58, and 67 as species 9; and HPV types 6/11, 44, and 55 as species 10.
Data are pooled-probe positivity estimates (from onset of positivity to the end of positivity for any of the HPV types that comprised a given group). See the text for descriptions of high-risk and low-risk HPV types.
Does not include women with prevalent infection who did not return for subsequent visits.
Figure 1 shows the Kaplan-Meier curves for time to clearance of prevalent and incident infections. For any HPV, the median and mean times to clearance of prevalent infection were 8.2 months (95% CI, 8.0–9.2 months) and 18.6 months (95% CI, 16.0–21.1 months), respectively, whereas the times to clearance of incident infection were 7.1 months (95% CI, 6.3–9.7 months) and 13.5 months (95% CI, 12.4–14.7 months), respectively (P = .009). For both HPV groups and for HPV-16, prevalent infections took longer to clear than incident infections, although differences were not statistically significant (P = .989 for HR-HPVs, P = .834 for LR-HPVs, and P = .232 for HPV-16).
Figure 1.
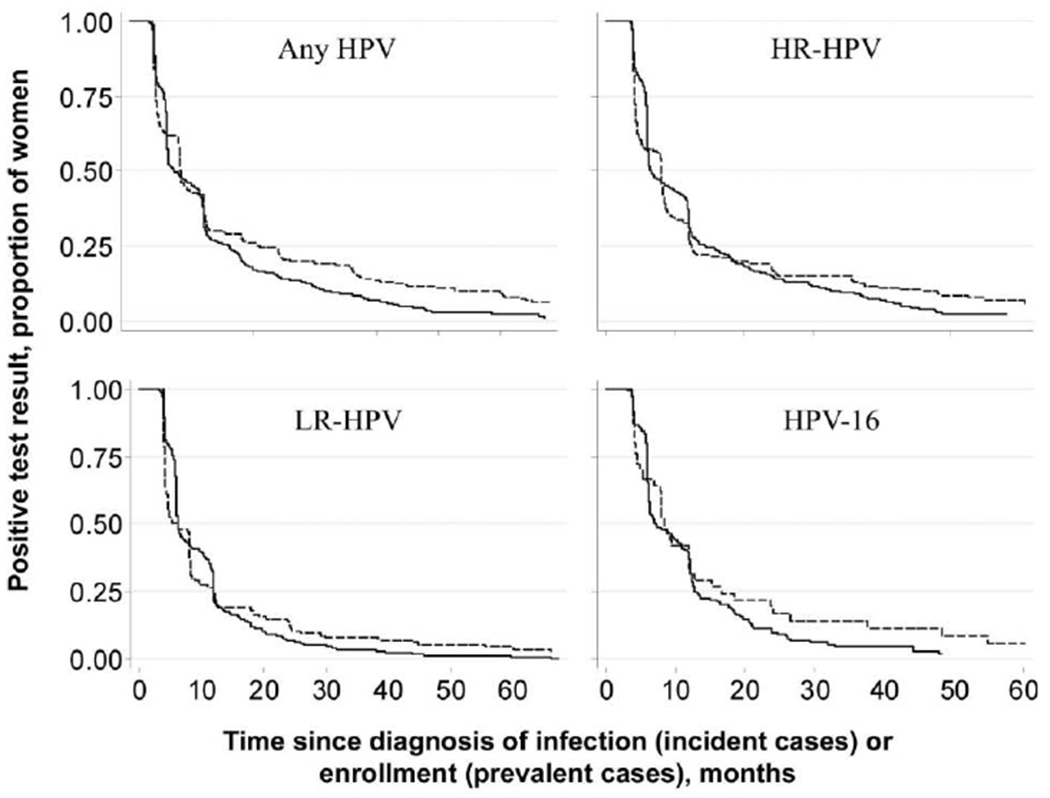
Kaplan-Meier curves for time to clearance of prevalent and incident human papillomavirus (HPV) infections. See the text for descriptions of HPVs with a high oncogenic risk (HR) and those with a low oncogenic risk (LR). Solid lines denote incident infection, and dashed lines denote prevalent infection.
For each HPV group, weighted (type-specific) estimates of infection duration (table 3) were shorter than the respective pooled-probe positivity durations (table 2). On average, same-type HR-HPV infections lasted 1.6 months longer than LR-HPV infections, based on the strict definition. The mean duration of incident infection due to alpha-papillomavirus species 9 was also longer than that for other species, although the difference was significant only in comparison with species 3 and 10. When the gap in a sequence of positive test results was ignored, the average infection duration was longer than that estimated via the strict definition. Although this maneuver indicated that the underestimation in infection duration due to sampling or other errors was not substantial, it tended to eliminate the differences in duration between HR-HPVs and LR-HPVs.
Table 3.
Weighted (type-specific) mean duration of incident human papillomavirus (HPV) infection among women from São Paulo, Brazil, by HPV phylogenetic relationship and oncogenic risk.
| HPV category | Subjects, no. | Weighted mean infection duration (95% CI) | ||
|---|---|---|---|---|
| Strict definitiona | Gap of 1 negative result ignoredb | Gap of 2 negative results ignoredc | ||
| Phylogenetic relationshipd | ||||
| Species 3 | 190 | 9.8 (9.0–10.7) | 10.8 (9.4–12.2) | 11.5 (10.0–13.0) |
| Species 5 | 126 | 11.0 (9.1–12.8) | 11.7 (9.3–14.1) | 11..7 (9.3–14.1) |
| Species 6 | 158 | 11.0 (6.3–15.7) | 11.9 (6.8–17.0) | 12.2 (6.8–17.6) |
| Species 7 | 209 | 10.1 (8.5–11.7) | 10.4 (8.6–12.2) | 10.4 (8.6–12.2) |
| Species 9 | 415 | 12.5 (11.1–14.0) | 13.5 (12.1–15.0) | 14.4 (12.5–16.2) |
| Species 10 | 118 | 9.2 (8.3–10.1) | 11.0 (6.8–15.2) | 11.4 (6.8–15.2) |
| Oncogenic riske | ||||
| Any | 1359 | 10.9 (10.3–11.5) | 11.8 (11.1–12.5) | 12.3 (11.5–13.0) |
| High | ||||
| Any | 815 | 11.5 (10.6–12.5) | 12.3 (11.2–13.4) | 12.8 (11.5–14.1) |
| Except type 16 | 653 | 11.4 (10.4–12.5) | 12.1 (10.8–13.3) | 12.4 (11.0–13.9) |
| Except types 16 and 18 | 606 | 11.5 (10.3–12.7) | 12.2 (10.9–13.5) | 12.6 (11.0–14.1) |
| Low | 544 | 9.9 (9.3–10.5) | 11.1 (10.3–11.7) | 11.5 (10.6–12.3) |
NOTE. CI, confidence interval.
Average of the actuarial mean of each implicated HPV type weighted by the frequency of incident episodes. For each type, the first negative test result following a string of positive test results was arbitrarily taken as indicative of clearance. See the text for details.
Same as above, except that a gap of 1 negative test result in a sequence of positive results for a given HPV type was ignored. For example, women who tested positive for the same type at visits 2, 3, and 5 were considered to have been infected from visits 2 to 5; the gap in positivity at visit 4 was ignored.
Same as above, except that a gap of up to 2 consecutive negative test results for a given HPV type were ignored. For example, women who tested positive for the same type at visits 2, 3, and 5 or women who tested positive for the same type at visits 2 and 5 were considered to have been continuously infected from visits 2 through 5.
Data are for alpha-papillomavirus species. HPV types 61, 62, 72, 81, 83, 84, and 89 are classified as alpha-papillomavirus species 3; HPV types 26, 51, 69, and 82 as species 5; HPV types 53, 56, and 66 as species 6; HPV types 18, 39, 45, 59, 68, and 70 as species 7; HPV types 16, 31, 33, 35, 52, 58, and 67 as species 9; and HPV types 6/11, 44, and 55 as species 10.
See the text for descriptions of high-risk and low-risk HPV types.
Figures 2 and 3 show the mean duration of incident infections for HPV groups and for HPV-16 according to age and lifetime number of sexual partners. No significant trends were found between duration and age or lifetime number of sexual partners (P > .05 by the test for trend, for both weighted [type-specific] and pooled-probe positivity means for all HPV groups and for HPV-16).
Figure 2.
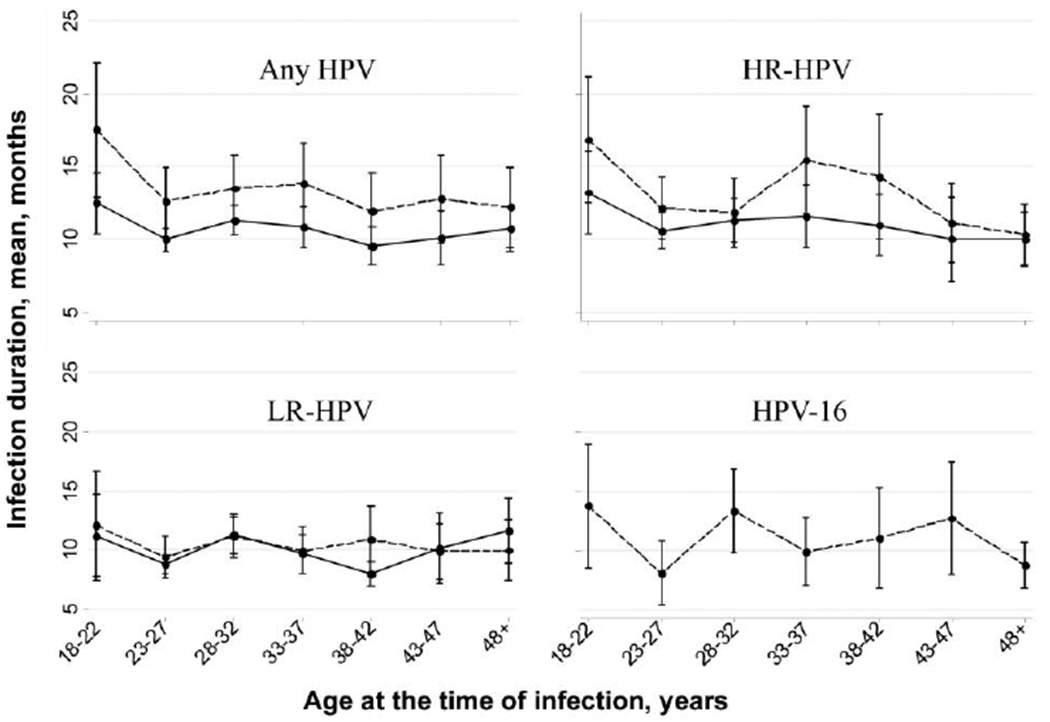
Duration of human papillomavirus (HPV) infection, by age. See the text for descriptions of HPVs with a high oncogenic risk (HR) and those with a low oncogenic risk (LR). Bars denote 95% confidence intervals, dashed lines denote pooled-probe positivity values, and solid lines denote weighted (type-specific) values.
Figure 3.
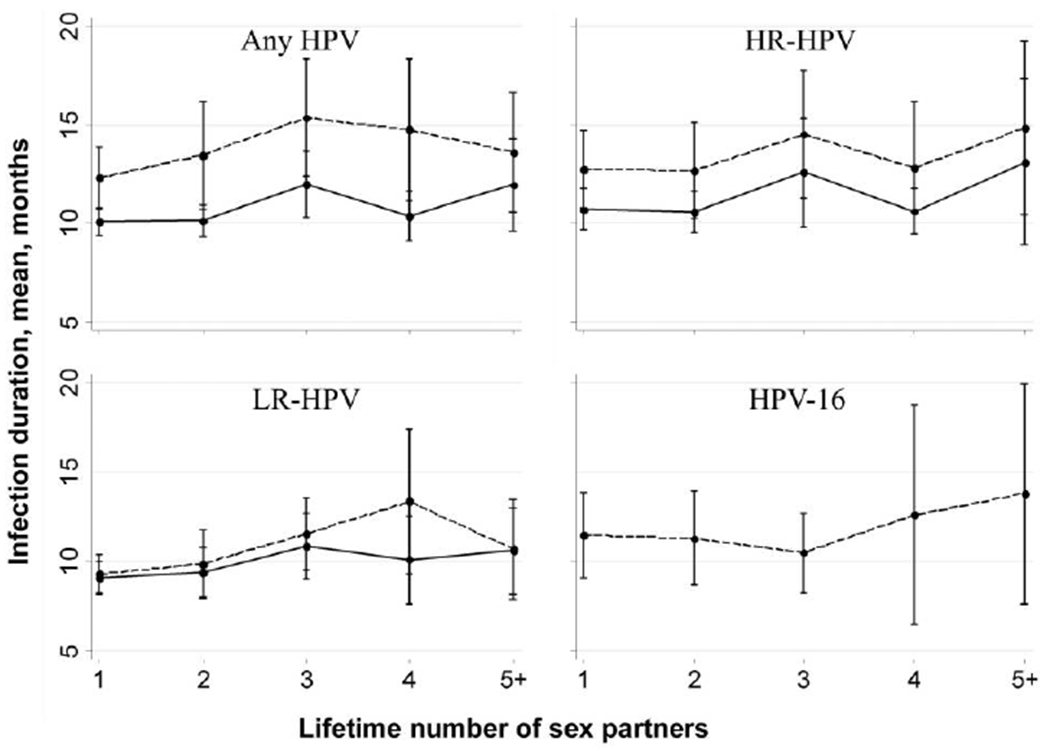
Duration of human papillomavirus (HPV) infection, by lifetime number of sex partners. See the text for descriptions of HPVs with a high oncogenic risk (HR) and those with a low oncogenic risk (LR). Bars denote 95% confidence intervals, dashed lines denote pooled-probe positivity values, and solid lines denote weighted (type-specific) values.
Mean durations of monoinfection and coinfection were calculated for individual HPV types and groups (table 4). The mean duration of infection due to HPV-16 alone was 11.0 months, but the duration of coinfection involving HPV-16 and ⩾1 HPV type was 15.4 months. For HPV-58, mean infection durations were 6.6 months for monoinfection and 17.2 months for coinfection. For most types the estimate for coinfection duration exceeded the estimate for monoinfection, and for some HPV types (i.e., types 42, 57, 69, 72, and 82) infections always occurred with ⩾1 additional type (table 4). The mean duration of coinfection involving multiple HPV types was ~33 months, whereas the mean duration of coinfection involving multiple HR-HPV types was ~24 months (pooled-probe positivity values are specified in table 4). The weighted average duration of monoinfection due to any HPV type (a situation in which the pooled-probe positivity estimate is similar to the weighted estimate) was 10.2 months, whereas the weighted average duration of coinfection involving multiple HPV types was 12.7 months; for HR-HPVs, these estimates were 10.1 and 13.5 months, respectively. For monoinfections, no differences in duration were seen between infections due to LR-HPVs and those due to HR-HPVs.
Table 4.
Characteristics of human papillomavirus (HPV) monoinfection and coinfection among women from São Paulo, Brazil, by HPV type and oncogenic risk.
| HPV category | Subjects, no. | Duration, mean, monthsa (95% CI) | ||
|---|---|---|---|---|
| Monoinfection | Coinfection | Monoinfection | Coinfection | |
| Type | ||||
| 6/11 | 7 | 46 | 16.5 (1.1–31.9) | 8.9 (7.1–10.6) |
| 16 | 47 | 115 | 11.0 (7.8–14.3) | 15.4 (12.9–17.8) |
| 18 | 13 | 34 | 9.7 (5.1–14.3) | 11.3 (8.2–14.4) |
| 26 | 2 | 7 | 7.8 (2.0–13.6) | 9.5 (6.5–12.6) |
| 31 | 14 | 36 | 8.7 (6.0–11.5) | 12.8 (9.9–15.7) |
| 32 | 2 | 2 | 7.8 (2.7–12.9) | 5.9 (5.5–6.3) |
| 33 | 2 | 27 | 10.4 (5.1–15.8) | 15.8 (9.6–22.0) |
| 35 | 11 | 42 | 11.2 (4.0–18.4) | 14.3 (9.5–19.1) |
| 39 | 2 | 22 | 9.2 (4.4–14.1) | 14.1 (10.3–17.9) |
| 40 | 4 | 17 | 10.0 (6.5–13.4) | 8.2 (5.9–10.6) |
| 42 | 0 | 15 | NA | 13.3 (5.3–21.2) |
| 44 | 2 | 5 | 5.4 (5.0–5.8) | 9.7 (6.7–12.7) |
| 45 | 8 | 36 | 9.0 (5.6–12.4) | 10.3 (8.3–12.3) |
| 51 | 20 | 80 | 10.5 (5.6–15.4) | 12.7 (10.3–15.1) |
| 52 | 6 | 49 | 7.1 (5.2–8.9) | 18.6 (13.8–23.3) |
| 53 | 18 | 77 | 10.8 (7.1–14.5) | 13.5 (10.9–16.0) |
| 54 | 13 | 33 | 14.2 (4.8–23.6) | 12.5 (8.1–17.0) |
| 55 | 15 | 43 | 8.4 (5.5–11.3) | 13.3 (8.8–17.9) |
| 56 | 5 | 31 | 8.7 (6.3–11.1) | 13.5 (10.2–16.8) |
| 57 | 0 | 4 | NA | 7.3 (4.4–10.2) |
| 58 | 12 | 49 | 6.6 (5.0–8.1) | 17.2 (11.6–22.8) |
| 59 | 7 | 27 | 12.4 (5.5–19.4) | 9.9 (7.8–11.9) |
| 61 | 9 | 18 | 8.4 (5.3–11.5) | 12.9 (8.3–17.5) |
| 62 | 4 | 29 | 15.8 (9.8–21.8) | 11.7 (8.1–15.2) |
| 66 | 6 | 21 | 7.9 (5.8–10.1) | 8.4 (6.6–10.2) |
| 67 | 1 | 4 | 13.8 (13.8–13.8) | 3.6 (3.2–3.9) |
| 68 | 10 | 32 | 11.3 (6.5–16.2) | 8.3 (6.6–10.0) |
| 69 | 0 | 2 | NA | 5.1 (3.8–6.3) |
| 70 | 3 | 15 | 6.8 (3.8–9.8) | 8.3 (6.0–10.7) |
| 71 | 2 | 14 | 4.6 (3.8–5.3) | 14.8 (7.3–22.2) |
| 72 | 5 | 8.9 (5.1–12.8) | ||
| 73 | 6 | 30 | 6.6 (5.1–8.1) | 10.9 (7.3–14.6) |
| 81 | 4 | 13 | 10.2 (6.9–13.6) | 11.9 (6.7–17.2) |
| 82 | 0 | 15 | NA | 10.1 (5.0–15.3) |
| 83 | 9 | 23 | 12.0 (6.8–17.3) | 8.6 (7.0–10.2) |
| 84 | 11 | 48 | 12.0 (7.2–16.9) | 13.1 (9.3–17.0) |
| 89 | 4 | 12 | 6.7 (3.6–9.8) | 8.3 (5.7–10.9) |
| Pooled-probe positivity, by oncogenic risk | ||||
| Any | 320 | 285 | 10.5 (9.3–11.7) | 32.8 (29.7–35.9) |
| High | ||||
| Any | 169 | 290 | 10.1 (8.6–11.5) | 23.7 (21.1–26.2) |
| Except type 16 | 122 | 271 | 9.7 (8.2–11.3) | 20.7 (18.3–23.1) |
| Except types 16 and 18 | 109 | 266 | 9.7 (8.1–11.4) | 20.2 (17.8–22.5) |
| Low | 151 | 245 | 10.7 (8.9–12.5) | 17.9 (15.4–20.4) |
| Weighted (type-specific), by oncogenic risk | ||||
| Anyb | 279 | 1078 | 10.2 (9.4–11.1) | 12.7 (11.7–13.6) |
| High | ||||
| Any | 169 | 646 | 10.1 (8.6–11.5) | 13.5 (11.9–15.0) |
| Except type 16 | 122 | 531 | 9.7 (8.2–11.3) | 13.1 (11.3–14.8) |
| Except types 16 and 18 | 109 | 497 | 9.7 (8.1–11.4) | 13.2 (11.3–15.0) |
| Lowb | 110 | 432 | 10.9 (9.3–12.4) | 11.5 (10.4–12.6) |
NOTE. See the text for descriptions of high-risk and low-risk HPV types. CI, confidence interval; NA, not applicable.
Estimated by ignoring the gap between 2 consecutive visits with negative test results (see the text and table 3 for details).
Excludes infections with unknown HPV types.
DISCUSSION
Several cohort studies have provided insights concerning the natural history of HPV infection [7, 9, 10, 12, 13, 17, 28–32]. In our analysis, the incidence rates for HR-HPV and LR-HPV infections were 6.1 and 4.9 episodes per 1000 women-months, respectively. Incidence rates in a cohort of Canadian female university students (a younger cohort than the cohort in this study) were 14.0 and 12.4 infections per 1000 women-months for HR-HPV and LR-HPV, respectively [12], and were 4.2 and 1.7 infections per 1000 women-months, respectively, in a cohort of Colombian women whose age was comparable to that of women in our cohort [13]. HPV-16 is invariably the most common HPV type, irrespective of study design and geographical area. HPV-18 is also a common type. However, the prevalence and incidence of HPV-18 infection is more variable across populations, and other types are sometimes more frequent [33]. In this study, HPV-16 was the most common type, followed by HPV-53 and HPV-51. The clearance rate was significantly higher for LR-HPVs than for HR-HPVs, which is in line with findings of another South American study [19]. In the interest of being explicit, we presented both median and mean infection durations. The median value may be more useful to design clinical management algorithms and follow-up intervals in HPV-based screening programs. On the other hand, the mean duration provides a more complete description of the distribution of HPV clearance times for an entire group of women and, thus, would have greater value in modeling the impact of interventions and cost-effectiveness.
Measurement of HPV persistence was influenced by our ability to identify the onset of infection. Prevalent infection took longer to clear than incident infection. We would have expected the opposite because a fraction of the infection period had already elapsed for women who tested positive for HPV at enrollment. Prevalent infections may take longer to clear because they tend to overrepresent the most “severe” infections (e.g., those with a high viral load). As the duration of infection increases, its chance of detection as a prevalent case increases. It has been shown that the likelihood that an infection will not clear also increases as the infection duration increases [17, 34]. Our reasoning for analyzing incident infections was pragmatic: cohort studies can better define the time boundaries of such episodes and reveal the entire set of acquisition, persistence, and clearance events.
There was considerable variation among types with regard to the duration of incident infection. The mean duration for individual types ranged from 5.1 to 15.4 months, which is consistent with findings from other studies (range, 4–20 months [34]). HR-HPV infection has been shown to usually last longer than LR-HPV infection, and among the former, HPV-16 infection tends to have the longest duration [10, 12, 13, 19, 35, 36]. In this study, the mean duration (weighted or pooled-probe positivity) was higher for HR-HPVs than for LR-HPVs. However, the pooled-probe positivity estimate for grouped HPVs (i.e., any HPVs, LR-HPVs, and HR-HPVs) is useful only to the extent that it represents positivity via a probe-cocktail assay. It does not account for the variation in infection duration for individual types. The weighted (type-specific) estimate provides an unbiased summary of the typical durations of infection due to “member” HPV types in a particular group, thus controlling for the overlap in positivity spans for individual episodes. This procedure tends to reduce the difference in duration between HR-HPVs and LR-HPVs because HR-HPVs were detected more often in coinfections than were LR-HPVs, which led to longer intervals of positivity detected via a hypothetical probe-cocktail assay. Moreover, misclassification of infection status may further bias the estimation. We addressed this issue by ignoring the gaps in a sequence of type-specific positive test results. Although the effect of ignoring the gaps was small, it tended to eliminate the differences between HR-HPVs and LR-HPVs. The percentage of episodes with gaps was somewhat higher among LR-HPVs (5.0%) than HR-HPVs (3.3%). This may have occurred because infection with LR-HPV may have a lower viral load than infection with HR-HPV, which may have caused more-frequent false-negative test results among visits during which the viral load was below the detection threshold. This helped make the differences between group durations disappear in analyses that ignored the gaps between visits with positive test results.
It is important to consider this study’s limitations. An important caveat is that incident infections could not be ascertained as true new infections. Because most women had been sexually active for several years, it was impossible to distinguish a new infection from a recurrent infection or a reactivated previous infection. Also, despite the PCR assay’s high sensitivity, the true clearance rate could not be measured. Whether infection clears completely or the virus remains latent and undetectable in basal cells is debatable and cannot be verified empirically. Another study design influence that may have biased the estimation of infection duration was the need to treat women with high-grade lesions. Because HR-HPVs, and HPV-16 in particular, tend to cause such lesions, the observation period for some infections due to these types was truncated, and thus censored, from analysis as a result of the need for clinical management outside of the study environment. In such cases, the duration likely exceeded the time we documented. This issue is common to all prospective cohort studies of cervical HPV infection, because of the ethical requirement to provide timely treatment of all clinically relevant lesions. The above caveats notwithstanding, this study presents advantages inherent to a molecular epidemiologic investigation that uses a validated HPV typing assay to study infection dynamics over multiple visits and long follow-up durations and with high retention rates.
Our findings concerning pooled-probe positivity and weighted estimates of infection duration for grouped HPVs have important implications for the clinical use of HPV detection to ascertain infection persistence. Clinical use of HPV testing currently involves a commercial probe-cocktail assay that simultaneously tests for 13 types of HR-HPVs. Our findings reveal that this practice overestimates the duration of an infection episode. Distinct episodes of infection involving different HR-HPVs will result in a longer period of positivity. Although not desirable from the perspective of understanding the behavior of a single HPV infection episode, this overestimation is in the interest of conservative clinical management. For practical purposes, if a woman has 2 overlapping episodes of infection with different HR-HPV types, it is clinically relevant that the period of prolonged positivity be documented.
No association was observed between age and infection duration, although longer durations were usually observed among women aged 18–22 years, compared with older women. To date, only a few studies have reported on this relationship, with inconsistent results. Some have shown that HPV persistence is more common among older women [15, 17, 37], whereas others failed to replicate this finding [38–40]. Ahdied et al. [39] showed that the odds ratio of HPV persistence among HIV-negative women was 0.87 for women aged ⩾35 years, compared with those <35 years old. Syrjanen et al. [40] observed that women with persistent HR-HPV infection were younger than those who cleared their infection. Likewise, Richardson et al. [38] observed no relationship between age and clearance. Differences in study design and analysis may partially explain the discrepancies. Most studies of this relationship have used data on prevalent HPV infection. Prevalent infection at enrollment in older women may be of longer duration than that in young women [37]. Also, most studies of the relationship between age and persistence have not used survival analyses. Typically, logistic regression models were used to estimate the risk of a persistent prevalent infection, defined as the detection of the same HPV type at the enrollment and follow-up visits (with intervals of 6 months, 12 months, or longer), compared with the risk of a transient prevalent infection, defined as an infection that clears on follow-up [15, 17, 37, 39]. Compliance with attendance at follow-up visits may have varied by age, which could have biased the association with age. To assess whether this could explain our findings, we examined the median time between visits across age groups and found no differences (data not shown). We focused on incident infection and analyzed persistence as an interval-scaled variable without arbitrary commitment to a specific duration threshold to define persistence. We used actuarial analysis, which controlled for the potential biasing effect of age-dependent right censoring. Finally, the lack of an association between infection duration and age may have been due to specific characteristics of our cohort of women who attended screening in Brazil.
HPV infection duration was also not influenced by the number of sexual partners, which is in agreement with the findings of Wang et al. [41]. We observed that the duration of type-specific episodes was influenced by coinfection with other HPVs, which is in agreement with findings from previous studies [10, 17, 42]. Some studies found no relationship between coinfection and persistence [34, 43], but they used logistic regression to measure persistence by coinfection status or analyzed only women with abnormal cytologic findings, for whom the dynamics of HPV clearance likely reflects the high viral load present in the underlying lesions and the effect of treatment. Coinfection with multiple types may be an indication that the woman’s immune system may have responded poorly to the virus and permitted individual infection foci to reach viral loads that were sufficiently high to be detected. This would in turn lead to continued detection of each episode over longer periods. Because women with multiple infections are also at a higher risk of having cervical lesions [44], our findings have implications in terms of how HPV testing and typing may be used in the future to guide management decisions.
Acknowledgments
We are grateful to Maria L. Baggio, Lenice Galan, and Silvaneide Ferreira for managing patients and specimens and to Romulo Myamura for DNA extraction work.
Financial support: Ludwig Institute for Cancer Research (intramural grant to L.L.V. and E.L.F.), US National Cancer Institute (grant CA70269 to E.L.F.), and Canadian Institutes of Health Research (Distinguished Scientist Award and grant MA13647 to E.L.F.).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 23rd International Papillomavirus Conference and Clinical Workshop, 1–7 September 2006, Prague, Czech Republic (abstract PS2–3).
References
- 1.International Agency for Research on Cancer (IARC) Working Group. Human papillomaviruses. Lyon, France: IARC, 1995:277–82. IARC monographs on the evaluation of carcinogenic risks to human, vol. 64. [Google Scholar]
- 2.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003; 88:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 2003; 127:930–4. [DOI] [PubMed] [Google Scholar]
- 5.Liaw KL, Glass AG, Manos MM, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions. J Natl Cancer Inst 1999; 91:954–60. [DOI] [PubMed] [Google Scholar]
- 6.Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–14. [DOI] [PubMed] [Google Scholar]
- 7.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325(7364):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–84. [DOI] [PubMed] [Google Scholar]
- 9.Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 1999; 180:1415–23. [DOI] [PubMed] [Google Scholar]
- 10.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet 2001; 357:1831–6. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AR, Harris R, Sedjo RL, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: the Young Women’s Health Study. J Infect Dis 2002; 186:462–9. [DOI] [PubMed] [Google Scholar]
- 12.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev 2003; 12:485–90. [PubMed] [Google Scholar]
- 13.Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis 2004; 190:2077–87. [DOI] [PubMed] [Google Scholar]
- 14.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005; 191:182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis 1994; 169:235–40. [DOI] [PubMed] [Google Scholar]
- 16.Kotloff KL, Wasserman SS, Russ K, et al. Detection of genital human papillomavirus and associated cytological abnormalities among college women. Sex Transm Dis 1998; 25:243–50. [DOI] [PubMed] [Google Scholar]
- 17.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–8. [DOI] [PubMed] [Google Scholar]
- 18.Xi LF, Carter JJ, Galloway DA, et al. Acquisition and natural history of human papillomavirus type 16 variant infection among a cohort of female university students. Cancer Epidemiol Biomarkers Prev 2002; 11:343–51. [PubMed] [Google Scholar]
- 19.Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology:apopulation-based, 5-year follow-up study. Am J Epidemiol 2003; 158:486–94. [DOI] [PubMed] [Google Scholar]
- 20.Koshiol JE, Schroeder JC, Jamieson DJ, et al. Time to clearance of human papillomavirus infection by type and human immunodeficiency virus serostatus. Int J Cancer 2006; 119:1623–9. [DOI] [PubMed] [Google Scholar]
- 21.Syrjanen S, ShabalovaI, Petrovichev N, et al. Age-specific incidence and clearance of high-risk human papillomavirus infections in women in the former Soviet Union. Int J STD AIDS 2005; 16:217–23. [DOI] [PubMed] [Google Scholar]
- 22.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–15. [DOI] [PubMed] [Google Scholar]
- 23.Franco E, Villa L, Rohan T, Ferenczy A, Petzl-Erler M, Matlashewski G. Design and methods of the Ludwig-McGill longitudinal study of the natural history of human papillomavirus infection and cervical neoplasia in Brazil. Rev Panam Salud Publica 1999; 6:223–33. [DOI] [PubMed] [Google Scholar]
- 24.Bauer HM, Ting Y, Greer CE, et al. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA 1991; 265:472–7. [PubMed] [Google Scholar]
- 25.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol 2000; 38:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Agency for Research on Cancer (IARC). Human papillomaviruses Lyon, France: IARC, 2007. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 90. [Google Scholar]
- 27.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324:17–27. [DOI] [PubMed] [Google Scholar]
- 28.Castle PE, Wacholder S, Lorincz AT, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst 2002; 94:1406–14. [DOI] [PubMed] [Google Scholar]
- 29.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis 2005; 191:1796–807.15871111 [Google Scholar]
- 30.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001; 285:2995–3002. [DOI] [PubMed] [Google Scholar]
- 31.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis 2004; 190:37–45. [DOI] [PubMed] [Google Scholar]
- 32.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003; 157:218–26. [DOI] [PubMed] [Google Scholar]
- 33.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24(Suppl 1):S1–15. [DOI] [PubMed] [Google Scholar]
- 34.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis 2007; 195:1582–9. [DOI] [PubMed] [Google Scholar]
- 35.Brisson J, Bairati I, Morin C, et al. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. J Infect Dis 1996; 173:794–9. [DOI] [PubMed] [Google Scholar]
- 36.Elfgren K, Kalantari M, Moberger B, Hagmar B, Dillner J. A population-based five-year follow-up study of cervical human papillomavirus infection. Am J Obstet Gynecol 2000; 183:561–7. [DOI] [PubMed] [Google Scholar]
- 37.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis 2005; 191:1808–16.15871112 [Google Scholar]
- 38.Richardson H, Abrahamowicz M, Tellier PP, et al. Modifiable risk factors associated with clearance of type-specific cervical human papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev 2005; 14:1149–56. [DOI] [PubMed] [Google Scholar]
- 39.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis 2001; 184:682–90. [DOI] [PubMed] [Google Scholar]
- 40.Syrjanen S, Shabalova I, Petrovichev N, et al. Factors predicting persistence of high-risk human papillomavirus (HPV) infections in women prospectively followed-up in three New Independent States (NIS) of the former Soviet Union. Eur J Gynaecol Oncol 2005; 26:491–8. [PubMed] [Google Scholar]
- 41.Wang SS, Schiffman M, Herrero R, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10000 women in Costa Rica.Br J Cancer 2004; 91:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrons C, Jelley R, Kleter B, Quint W, Brink N. Detection of persistent high risk human papillomavirus infections with hybrid capture II and SPF10/LiPA. J Clin Virol 2005; 32:278–85. [DOI] [PubMed] [Google Scholar]
- 43.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis 2001; 183:8–15. [DOI] [PubMed] [Google Scholar]
- 44.Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2006; 15:1274–80. [DOI] [PubMed] [Google Scholar]


