Abstract
Endotoxin exacerbates asthma. We designed the Louisa Environmental Intervention Project (LEIP) and assessed its effectiveness in reducing household endotoxin and improving asthma symptoms in rural Iowa children. Asthmatic schoolchildren (N=104 from 89 homes) of Louisa and Keokuk counties in Iowa (aged 5-14 years-old) were recruited and block-randomized to receive extensive (education + professional cleaning) or educational interventions. Environmental sampling collection and respiratory survey administration were done at baseline and during three follow-up visits. Mixed-model analyses were used to assess the effect of the intervention on endotoxin levels and asthma symptoms in the main analysis and of endotoxin reduction on asthma symptoms in exploratory analysis. In the extensive intervention group, dust endotoxin load was significantly reduced in post-intervention visits. The extensive compared to the educational intervention was associated with significantly decreased dust endotoxin load in farm homes and less frequent nighttime asthma symptoms. In exploratory analysis, dust endotoxin load reduction from baseline was associated with lower total asthma symptoms score (Odds ratio: 0.52, 95% confidence interval: 0.29-0.92). In conclusion, the LEIP intervention reduced household dust endotoxin and improved asthma symptoms. However, endotoxin reductions were not sustained post-intervention by residents.
Keywords: Air Sampling, Endotoxin, Asthma, Intervention, House dust, Reservoir Dust
INTRODUCTION
Asthma is a chronic respiratory disorder characterized bronchoconstriction, pulmonary inflammation, and airway remodeling with symptoms of wheezing, cough and shortness of breath.1 It affects 25 million people in the US, including more than 6 million children.2 Asthma is the most common chronic disease in children worldwide; it is responsible for 180,000 deaths every year around the globe and has an annual economic cost is estimated to over $56 billion in the US.3 Environmental exposures play a key role in the development and severity of asthma and there is strong evidence suggesting that endotoxin – a lipopolysaccharide from the outer membrane of the cell wall of Gram-negative bacteria – is associated with asthma exacerbations.4–11 Endotoxin induces airways inflammation and bronchoconstriction by acting through an amplifying cascade that leads to the production of pro-inflammatory cytokines/chemokines and to neutrophilic airway infiltration.12–18 Endotoxin also aggravates allergic sensitization through goblet cell hyperplasia and mucus hypersecretion, causing peri-bronchial inflammation in atopic people.19 We previously reported that endotoxin levels found in US households are associated with asthma outcomes and with chronic bronchitis or emphysema.6,20,21
Despite the well-known effects of endotoxin on the exacerbation of asthma and its ubiquity in our environment, no study has evaluated the effectiveness of environmental interventions in reducing household endotoxin exposure in children with asthma. Therefore, we designed the Louisa Environmental Intervention Project for Rural Childhood Asthma (LEIP). This project was a community–based, participatory, environmental intervention study in a cohort of asthmatic children who resided in two rural, medically-underserved, and ethnically-diverse Iowa counties. The LEIP recognized that interventions that are effective for urban residents do not apply to children living in rural communities where exposures include different allergens, agricultural bioaerosols, pesticides, irritant gases, and biomass burning.
METHODS
Participants
Study subjects were drawn from eleven rural school districts in the counties of Louisa and Keokuk, Iowa. These counties have a combined population of 21,898 and no cities with population over 2,100. Parents of all children 5 to 14 years-old who were enrolled in eleven school districts of Louisa and Keokuk counties in Iowa were sent asthma screening questionnaires. Details on the enrollment procedure and screening questionnaire are provided in the supplemental materials. The questionnaires were designed according to the format of the International Study of Asthma and Allergies in Childhood (ISAAC) to assess the prevalence and severity of asthma and allergic diseases in children.22 They were accompanied by a cover letter from the principal and the superintendent of the schools. After three weeks, parents who did not respond to the first mailed questionnaire were sent a postcard. Those who did not respond three weeks later were mailed a second questionnaire and they received phone calls to also administer the questionnaire over the phone. Of the 4,618 total questionnaires sent or administered by phone, 3,435 (74.4%) were completed and from these, 346 children were identified as having diagnosed symptomatic asthma. This was defined as doctor-diagnosed asthma ever AND either having wheezed in the past year or having prescription medication for wheeze during the past year. Children were eligible for inclusion in the LEIP if: 1) they had active asthma, 2) had lived in their house for at least two years, and 3) had no plans to move during the study period. Out of 346 eligible children, 104 children from 89 households were randomly selected for inclusion in the study. The study was approved by the Institutional Review Board of the University of Iowa.
Randomization and intervention
Households were block-randomized into educational intervention or extensive intervention (educational + professional cleaning). All interventions were supplied at no cost to participants. Both groups received the educational intervention, a high-efficiency vacuum cleaner (Hoover Supreme Windtunnel, 6-stage microfiltration, 99,98% pollen filtration, 100% dust mite filtration, Hoover), electrostatic filtration bags (Type Y, Allergen Filtration Bags, Hoover), and pest abatement supplies (mouse traps, roach motels, and miticides). Educational materials included instructions on monitoring for asthma triggers, reducing clutter, education on housekeeping and track-in control, and an Environmental Protection Agency (EPA) brochure on asthma and healthy homes (see supplemental material). Educational materials were offered in both English and Spanish. For the extensive intervention group, we hired contractors to come to the homes and provide professional cleaning of carpets, draperies, mattresses and bedding, furnace, air conditioners, and air ducts (the “Superclean”). This group also received a professional “Boosterclean” consisting of professional cleaning of carpets, draperies, and bedding several months later.
Study timeline
As illustrated in Figure 1, all participants received the home hygiene educational intervention at the first visit, 4 weeks after the enrollment. The extensive intervention group had their houses professionally cleaned at weeks 6 (SuperClean) and 18 (BoosterClean) after enrollment. Environmental samples from homes were collected at weeks 4 (Baseline or Visit 1), 7 (Visit 2), 14 (Visit 3), and 22 (Visit 4) after enrollment. The questionnaires assessing asthma symptoms and asthma medication use were administered at enrollment and on the weeks 7 (visit 2), 14 (Visit 3), and 22 (Visit 4). There was no contact with subjects between visits 3 and 4 except to schedule the last visit.
Figure 1:
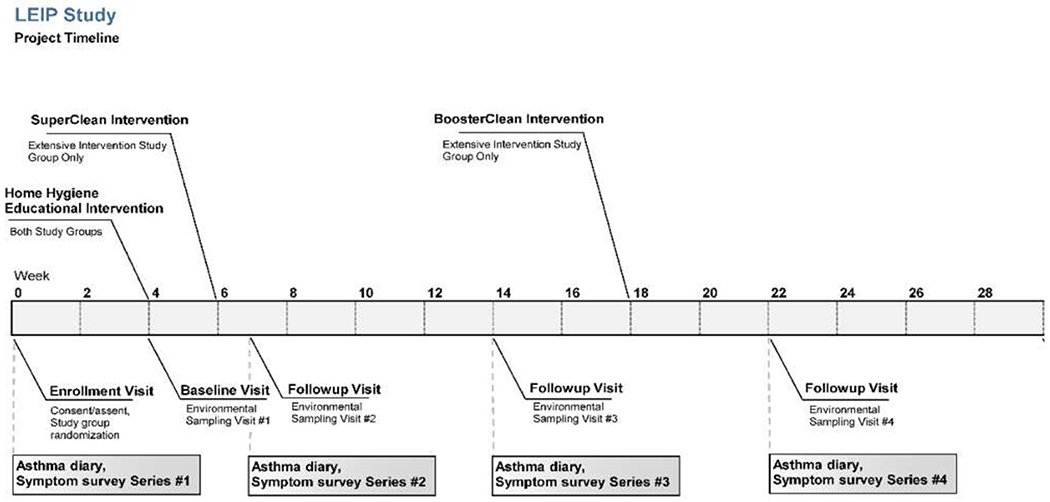
LEIP Study timeline of enrollment, intervention and environmental sampling
Air and dust endotoxin
Air and surface sampling for endotoxin analysis were performed both before the invention at Visit 1 and after the intervention at Visits 2, 3, and 4. Ambient air filters were collected from the houses for 24h at 4 L/min using a Button inhalable dust sampler placed in the main play area of the home. Binder-free glass fiber filters were pre- and post-weighed after equilibration in a gravimetrics laboratory using a microbalance (Mettler-Toledo MT-5) to determine particulate mass concentration. Filters were then eluted into 10 ml pyrogen-free water with 0.05% Tween-20 for the assessment of endotoxin as described below. Flooring type in each room was recorded by field staff as carpet or rug, hardwood/Pergo, vinyl/linoleum, ceramic tile, cement, or other. Where carpet was present it was further classified as closed loop or cut pile. Reservoir dust samples from homes were collected from the child’s bedroom bedding (CBB), the child’s bedroom floor (CBF), the main play area floor (PAF), and the kitchen floor (KNF) using special vacuums and endotoxin-free, high-efficiency particulate air socks. For these samples, 50mg sieved dust was eluted into 1ml of pyrogen-free water (Limulus amebocyte lysate water, Lonza, Inc.) with 0.05% Tween-20; shaken for 1hr; centrifuged at 600xg, 4° C for 20 minutes (Marathon 16KM, Fisher Scientific); after which the supernatant was transferred to a pyrogen-free polypropylene cryovials (Sarstedt), then diluted in Limulus amebocyte lysate water in heat-treated pyrogen-free borosilicate tube to assay for endotoxin.
Asthma symptoms
Asthma symptoms were assessed at three time-points using a questionnaire drawn from standard instruments. The questionnaires were administered in both English and Spanish and included nighttime cough or wheeze, daytime wheeze, daytime cough, as well as exercise-induced asthma. Based on frequency of each symptom, a daily score of 1 (no symptom), 2 (some symptoms), 3 (frequent symptoms), or 4 (continuous) was reported by participants and aggregated over a seven-day period to obtain a week-total score for each of the time-point. Because of the high proportion of missing data for exercise-induced asthma, the analysis was only performed on nighttime asthma symptoms, daytime wheeze, and daytime cough. Details on the scores for each symptom are reported in the supplemental materials.
Statistical analysis
Descriptive analyses were performed to compare the characteristics of participants screened, eligible, and enrolled for inclusion. We also described the participants included in the educational and extensive intervention groups and used a chi-square or Fisher exact test to calculate p-values for differences in characteristics. Endotoxin values were log10-transformed to improve the normality of their distribution for statistical analysis and geometric means along with geometric standard errors were used as measures of central tendency and variability. We examined the effectiveness of the extensive intervention (compared to the education) in reducing house endotoxin and the frequency of asthma symptoms using generalized linear mixed-models and generalized estimating equations.23 The analyses were adjusted for age and gender of the participants, the season of sampling, the farm or non-farm location of the house, family income, smoking in the house, presence of central air conditioning, presence of pets in the house, main heat source, and sampling location. We checked for multicollinearity between covariates in the model by calculating variance inflation factors. Multicollinearity was limited between the covariates included in our models and the diagnostic plots showed that model assumptions were reasonably met. We also stratified our analyses by whether the house was on a farm or not, due to known differences in endotoxin determinants between farm and non-farm homes. In exploratory analysis, generalized estimating equations were also used to examine the association of endotoxin reduction from baseline with the frequency of asthma symptoms. We used SAS (Version 9.4) for our analyses and STATA (Version 9.4) to check for collinearity and create graphs. P-values < 0.05 were considered statistically significant. A detailed description of the statistical analysis is reported in the online supplement.
RESULTS
Descriptive results
The characteristics of the children screened, eligible, and enrolled for inclusion are described in Table 1. Among the enrolled children, 20.2% lived in farm homes and 58.7% of all the enrollees were males. Wheeze in the past year was reported by 97.1% of them and 37.3% reported more than three wheeze attacks in the past year. Medication use for wheezing was reported by 93.1% of the children. Table 2 provides data on characteristics of the 104 participants at baseline for the overall cohort and by intervention arm. Those enrolled in the extensive intervention were more likely to have a cat alone or a cat and a dog, and to have refused to provide annual household income. Characterization of the flooring types in LEIP homes showed that 89.5% of the CBF and 88.8% of the PAF were carpeted or had at least one area rug. KNF were mostly smooth and cleanable floors (86.1%).
Table 1:
Characteristics of the screened and eligible subject as well as the participants enrolled in the LEIP Study
| Characteristics | Screened % (n/N) | Eligible* % (n/N) | Enrolled % (n/N) |
|---|---|---|---|
| Location of the home | |||
| Farm | 19.4 (659/3435) | 14.8 (51/346) | 20.2 (21/104) |
| Non-farm | 80.6 (2,735/3435) | 85.2 (294/346) | 79.8 (83/104) |
| Sex | |||
| Female | 48.8 (1673/3435) | 54.9 (190/346) | 41.3 (43/104) |
| Male | 51.2 (1756/3435) | 45.1 (156/346) | 58.7 (61/104) |
| Age (years) | |||
| 5-9 | 48.0 (1644/3435) | 51.0 (176/346) | 47.1 (49/104) |
| 10-14 | 52.0 (1782/3435) | 49.0 (169/346) | 52.9 (55/104) |
| Asthma Diagnosis and symptoms | |||
| Wheeze ever | 28.5 (955/3435) | 99.4 (344/346) | 98.1 (102/104) |
| Asthma ever | 13.0 (441/3435) | 70.3 (239/346) | 66.0 (68/104) |
| Physician ever told you child has asthma | 12.8 (437/3435) | 70.8 (240/346) | 67.0 (69/104) |
| Wheeze in past year | 13.2 (452/3435) | 99.1 (343/346) | 97.1 (101/104) |
| Exercise wheeze in past year | 10.5 (359/3435) | 72.0 (247/346) | 69.9 (72/104) |
| Dry cough at night in past year | 17.8 (610/3435) | 55.9 (193/346) | 57.3 (59/104) |
| Wheeze attacks in past year | |||
| None | 53.8 (513/955) | 1.2 (4/344) | 2.0 (2/102) |
| 1-3 | 30.8 (293/955) | 60.9 (209/344) | 60.8 (62/102) |
| >3 | 15.4 (147/955) | 37.9 (130/344) | 37.3 (38/102) |
| Nocturnal wheeze in past year | |||
| None | 73.9 (703/955) | 40.5 (139/344) | 44.1 (45/102) |
| ≤2 nights/month | 18.8 (179/955) | 41.1 (141/344) | 37.3 (38/102) |
| >2nights/month | 7.3 (69/955) | 18.4 (63/344) | 18.6 (19/102) |
| Speech-limiting wheeze in past year | 7.3 (69/955) | 19.6 (67/344) | 17.7 (18/102) |
| Used medications for wheezing in past year | 33.5 (318/955) | 92.7 (318/344) | 93.1 (95/102) |
| Frequent symptoms in past year | 28.6 (273/955) | 53.9 (185/344) | 56.9 (58/102) |
| Severe symptoms in past year | 11.8 (113/955) | 30.0 (103/344) | 25.5 (26/102) |
Eligibility defined as doctor-diagnosed asthma ever AND either having wheezed in the past year or having prescription medication for wheeze during the past year.
Eligible subjects also include 4 children whose siblings screened positive for active asthma and had at least one of the following: wheezing in the past year; doctor diagnosis plus wheezing during exercise; or inhaled bronchodilator use.
Table 2:
Characteristics of the LEIP Study participants at baseline
| Characteristics | Overall cohort (N=104) | Educational Intervention (N=52) | Extensive Intervention (N=52) | P-value |
|---|---|---|---|---|
| Location of the home | 0.46 | |||
| Farm | 20.2 (21/104) | 17.3 (9/52) | 23.1 (12/52) | |
| Non-farm | 79.8 (83/104) | 82.7 (43/52) | 76.9 (40/52) | |
| Sex | 0.84 | |||
| Female | 41.3 (43/104) | 40.4 (21/52) | 42.3 (22/52) | |
| Male | 58.7 (61/104) | 59.6 (31/52) | 57.7 (30/52) | |
| Age (years) | 0.84 | |||
| 5-9 | 47.1 (49/104) | 48.1 (25/52) | 46.2 (24/52) | |
| 10-14 | 52.9 (55/104) | 51.9 (27/52) | 53.8 (28/52) | |
| Annual household income | 0.007 | |||
| Missing | 28.8 (30/104) | 15.4 (8/52) | 42.3 (22/52) | |
| < $30,000 | 20.2 (21/104) | 26.9 (14/52) | 13.5 (7/52) | |
| ≥ $30,000 | 51.0 (58/102) | 57.7 (30/52) | 44.2 (23/52) | |
| Main heat source | 0.36 | |||
| Electric | 8.7 (9/104) | 9.6 (5/52) | 7.7 (4/52) | |
| Gas | 77.9 (81/104) | 71.2 (37/52) | 84.6 (44/52) | |
| Radiator | 5.8 (6/104) | 7.7 (4/52) | 3.8 (2/52) | |
| Wood | 7.7 (8/104) | 11.5 (6/52) | 3.8 (2/52) | |
| Kind of A/C system in home | 0.23 | |||
| Central A/C only | 65.4 (68/104) | 63.5 (33/52) | 67.3 (35/52) | |
| Both central air and window unit(s) | 5.8 (6/104) | 3.8 (2/52) | 7.7 (4/52) | |
| Window unit(s) only | 24.0 (25/104) | 30.8 (16/52) | 17.3 (9/52) | |
| No A/C | 4.8 (5/104) | 1.9 (1/52) | 7.7 (4/52) | |
| Pets or animals currently living in home | 0.02 | |||
| Cat(s) only | 17.3 (18/104) | 13.5 (7/52) | 21.2 (11/52) | |
| Dog(s) only | 23.1 (24/104) | 34.6 (18/52) | 11.5 (6/52) | |
| Cat(s) and dog(s) | 19.2 (20/104) | 17.3 (9/52) | 21.2 (11/52) | |
| Other | 3.9 (4/104) | 0.0 (0/52) | 7.7 (4/52) | |
| None | 36.5 (38/104) | 34.6 (18/52) | 38.5 (20/52) |
Abbreviations: A/C, air conditioning; LEIP, Louisa Environmental Intervention Project. P-values for difference in characteristics calculated using chi-square for location of the home, sex, age, and income. Fisher exact test used to calculate P-values for differences in main heat source, type of A/C in the home and the type of pet because of small cell sizes (<5 participants)
At baseline, the geometric mean (standard error) of air endotoxin in the educational and extensive intervention groups were 7.2 (1.7) EU/m3 and 6.2 (1.7) EU/m3 respectively. In reservoir dust, baseline geometric mean (standard error) of endotoxin load ranged from 8.4 (3.7) x 103 EU/m2 in CBB to 13.0 (7.3) x 103 EU/m2 in PAF in the educational group and from 7.7 (2.7) x 103 EU/m2 in CBB to 13.6 (29.2) x 103 EU/m2 in PAF in the extensive intervention group. In KNF where total sampled endotoxin was reported, the geometric mean (standard error) was 40.6 (19.4) x 103 EU and 29.7 (11.8) x 103 EU in the educational and extensive intervention groups respectively. The crude geometric mean (standard error) for air and dust endotoxin by intervention groups and visits are reported in Table 3.
Table 3:
Geometric mean (GM) and geometric standard error (GSE) for inhalable airborne dust endotoxin concentration (EU/m3) and endotoxin load in reservoir dust (EU/m2) by intervention group and over visits for the LEIP Study households
| Air endotoxin (EU/m3) | Endotoxin load in dust (x 103 EU/m2) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CBB | CBF | KNF a | PAF | ||||||||
| Study Group | Visit | N | GM (GSE) | N | GM (GSE) | N | GM (GSE) | N | GM (GSE) | N | GM (GSE) |
| Educational Intervention | 1 | 45 | 7.2 (1.7) | 45 | 8.4 (3.7) | 46 | 13.1 (11.7) | 45 | 40.6 (19.4) | 46 | 13.0 (7.3) |
| 2 | 43 | 6.6 (1.8) | 45 | 6.1 (4.9) | 46 | 10.3 (5.7) | 44 | 34.5 (18.1) | 46 | 12.5 (12.7) | |
| 3 | 45 | 5.4 (2.2) | 45 | 8.1 (3.5) | 46 | 14.9 (8.3) | 45 | 46.0 (23.3) | 46 | 9.5 (4.3) | |
| 4 | 43 | 7.2 (1.7) | 43 | 8.9 (3.0) | 44 | 17.0 (8.7) | 43 | 55.1 (21.9) | 43 | 12.6 (4.7) | |
| Extensive Intervention | 1 | 38 | 6.2 (1.7) | 40 | 7.7 (2.7) | 40 | 12.2 (7.1) | 39 | 29.7 (11.8) | 40 | 13.6 (29.2) |
| 2 | 37 | 4.3 (1.0) | 39 | 6.0 (3.7) | 40 | 8.6 (5.7) | 39 | 28.4 (8.0) | 40 | 7.4 (3.3) | |
| 3 | 37 | 4.6 (1.0) | 40 | 7.3 (2.6) | 40 | 8.4 (2.6) | 39 | 33.4 (18.4) | 40 | 10.6 (26.1) | |
| 4 | 36 | 6.8 (1.7) | 38 | 10.9 (2.1) | 38 | 9.8 (2.7) | 37 | 41.1 (11.1) | 38 | 10.6 (10.1) | |
Abbreviations: LEIP, Louisa Environmental Intervention Project; GM, geometric mean; GSE, geometric standard error; EU, endotoxin units; CBB, child bedroom bedding; CBF, child bedroom floor; PAF, play area floor; KNF, kitchen floor.
For KNF, endotoxin load was expressed as EU per sample.
Change in endotoxin over visits by intervention group
Regarding airborne endotoxin, there was a trend toward a decrease in least squared mean endotoxin in both the educational and extensive interventions at Visits 2 and 3 compared to Visit 1 as shown in Figure 2a. However, the airborne endotoxin reduction did not reach significance. This was mostly due a decreasing trend in endotoxin in non-farm homes, where the decrease in airborne endotoxin reached significance in educational intervention at Visit 3 (P=0.049) (Figure 2b). Outdoor air on farms may have contributed to indoor airborne endotoxin concentrations, especially at Visit 4 (Figure 2c).
Figure 2:
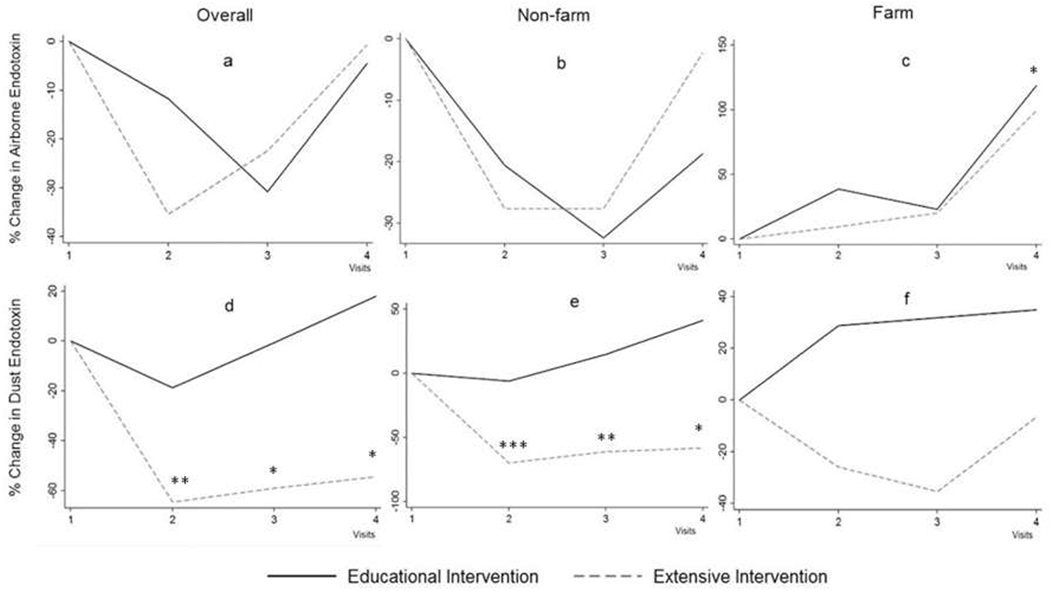
Change in airborne inhalable endotoxin concentration in the overall sample (a), in participants living in non-farm areas (b), and in participants living in farm areas (c). Change in reservoir dust endotoxin load in the overall sample (d), in participants living in non-farm areas (e), and in participants living in farm areas (f). Changes in endotoxin calculated using least square means derived from generalized linear mixed-effects modeling adjusted for age and gender of participants, season of sampling, place sampled in the home, location of the house (farm or non-farm for overall, except in stratified analysis by this variable), family income, smoking in the house, presence of central air conditioning, presence of pets in the house, and main heat source.
*P < 0.05 **P < 0.01 ***P < 0.001
Regarding reservoir dust endotoxin, least squared mean endotoxin in the extensive intervention group significantly decreased in Visit 2 (P=0.003), Visit 3 (P=0.01), and Visit 4 (P=0.03) compared to Visit 1 (Figure 2d). In subgroup analysis by non-farm or farm location of the homes, the decrease in least squared mean endotoxin was mainly significant in the non-farm homes (Figure 2e) but failed to reach significance in farm homes (Figure 2f). Adjustment for type of flooring (carpeted vs. smooth) had no impact on the effectiveness of the interventions.
Effect of intervention on endotoxin levels and asthma symptoms
In mixed-model analysis, extensive compared to educational intervention was not associated with a decrease in endotoxin. However, there was a significant interaction between the farm or non-farm location of the homes and the place of the home sampled (P= 0.002). Only in farm homes, the extensive intervention was associated with significant reductions in overall dust endotoxin by 81.3% (P<0.001) and in dust collected in CBB by 81.5% (P<0.001), in CBF by 89.1% (P=0.002), in PAF by 89.1% (P=0.008), or in KNF by 90.8% (P<0.001) (Table 4).
Table 4:
Mixed model with repeated measures to assess the effect of extensive (education + cleaning) compared to educational intervention on house endotoxin in the LEIP Study
| Extensive versus educational Intervention |
||
|---|---|---|
| % change | P-value | |
| All homes | ||
| Air endotoxin (EU/m3) | −14.1 | 0.54 |
| Endotoxin load (EU/m2) | ||
| Overall b | −16.6 | 0.38 |
| CBB | +8.8 | 0.69 |
| CBF | −28.0 | 0.16 |
| PAF | −22.4 | 0.38 |
| KNF c | −7.1 | 0.78 |
| Non-farm homes | ||
| Air endotoxin (EU/m3) | −11.2 | 0.51 |
| Endotoxin load (EU/m2) | ||
| Overall b | −22.9 | 0.40 |
| CBB | +11.9 | 0.64 |
| CBF | −32.4 | 0.10 |
| PAF | −21.5 | 0.43 |
| KNF c | −15.9 | 0.52 |
| Farm homes | ||
| Air endotoxin (EU/m3) | −22.6 | 0.58 |
| Endotoxin load (EU/m2) | ||
| Overall b | −81.3 | <0.001 |
| CBB | −81.5 | <0.001 |
| CBF | −89.1 | 0.002 |
| PAF | −89.1 | 0.008 |
| KNF c | −90.8 | <0.001 |
Models adjusted for age and gender of the participants, visits, season of sampling, place sampled in the home (for overall), location of the house (farm or non-farm, except in stratified analysis by this variable), family income, smoking in the house, presence of central air conditioning, presence of pets in the house, and main heat source.
Abbreviations: df, degree of freedom; EU, endotoxin units; CBB, child bedroom bedding; CBF, child bedroom floor; PAF, play area floor; KNF, kitchen floor. Bold indicates significant changes (p-value < 0.05).
The extensive compared to educational intervention was also associated with reduced frequency of night wheeze and cough with a significant intervention-by-visit interaction (P=0.049).
Endotoxin reduction and asthma symptoms
Overall house dust endotoxin reduction from Visit 1 significantly decreased the frequency of night wheeze or cough (OR: 0.62, 95% CI: 0.42-0.91), daytime wheeze (OR: 0.58, 95% CI: 0.37-0.92), daytime cough (OR: 0.62, 95% CI: 0.40-0.95), and overall asthma symptom (OR: 0.52, 95% CI: 0.29-0.92). By place of the home sampled, the house dust endotoxin reduction in CBB was associated with a decrease in daytime wheeze (OR: 0.13, 95% CI: 0.04-0.46), daytime cough (OR: 0.26, 95% CI: 0.11-0.71), and overall asthma symptoms (OR: 0.14, 95% CI: 0.04-0.46). House dust endotoxin reduction in CBF was associated with less frequent night wheeze or cough (OR: 0.29, 95% CI: 0.09-0.92), daytime cough (OR: 0.23, 95% CI: 0.10-0.57), and overall asthma symptom (OR: 0.15, 95% CI: 0.05-0.48) (Table 5).
Table 5:
Odds ratio for the relationship between intervention, visits and asthma symptoms in the LEIP Study.
| Night wheeze or cough |
Daytime Wheeze |
Daytime Cough |
Total Symptom Score |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Visit | − a | - | 0.65 (0.29, 1.47) | 0.30 | 1.12 (0.67, 1.87) | 0.64 | 0.69 (0.34, 1.39) | 0.30 |
| Intervention | − a | - | 2.05 (0.69, 6.04) | 0.20 | 2.48 (0.94, 6.57) | 0.07 | 1.75 (0.58, 5.22) | 0.32 |
| Air Endotoxin Reduction | 0.72 (0.20, 2.50) | 0.61 | 1.57 (0.48, 5.18) | 0.46 | 1.12 (0.32, 3.88) | 0.86 | 0.84 (0.19, 3.75) | 0.82 |
| Endotoxin Load Reduction | ||||||||
| Overall | 0.62 (0.42, 0.91) | 0.014 | 0.58 (0.37, 0.92) | 0.02 | 0.62 (0.40, 0.95) | 0.03 | 0.52 (0.29, 0.92) | 0.02 |
| CBB | 0.20 (0.03, 1.18) | 0.22 | 0.13 (0.04, 0.46) | 0.002 | 0.26 (0.11, 0.71) | 0.002 | 0.14 (0.04, 0.46) | 0.001 |
| CBF | 0.29 (0.09, 0.92) | 0.036 | 0.45 (0.19, 1.06) | 0.07 | 0.23 (0.10, 0.57) | 0.001 | 0.15 (0.05, 0.48) | 0.001 |
| PAF | 0.51 (0.26, 1.01) | 0.053 | 0.60 (0.26, 1.38) | 0.23 | 0.74 (0.43, 1.26) | 0.27 | 0.59 (0.30, 1.15) | 0.12 |
| KNF | 1.72 (0.59, 4.99) | 0.32 | 1.25 (0.35, 4.46) | 0.73 | 1.29 (0.49, 3.35) | 0.61 | 1.16 (0.43, 3.15) | 0.77 |
Models adjusted for age and gender of the participants, season of sampling, place sampled in the home, location of the house (farm, non-farm), family income, smoking in the house, presence of central air conditioning, presence of pets in the house, and main heat source.
Interaction term for Intervention-by-visit was significant (P=0.049).
Bold indicates significant associations with the asthma symptoms (p-value < 0.05)
DISCUSSION
The present study was performed in a cohort of rural asthmatic children to investigate the effect of an environmental intervention on household endotoxin levels and asthma symptoms. Residents of Keokuk and Louisa Counties are low income, medically underserved, and reside amid intensive agricultural activity, primarily row crops (corn and soybeans) and industrialized swine production. The results suggest that dust endotoxin significantly decreased in the visits after the extensive intervention in the overall sample and in non-farm homes. The extensive intervention compared to education alone was associated with a significant reduction in dust endotoxin in homes located on a farm and in the frequency of nighttime asthma symptoms. Our results also demonstrated that lowering dust endotoxin, especially in the bedroom is effective in reducing the frequency of asthma symptoms. To our knowledge, this is the first randomized interventional study evaluating measures to reduce endotoxin in the households of rural asthmatic children.
The level of endotoxin found in the homes of rural asthmatic children from our study were higher than we reported in the two previous US representative studies of mostly urban households.6,20 In the National Survey of Endotoxin in United States Housing, we analyzed endotoxin concentration and load in reservoir house dust sampled from bedroom bed and floor, family room floor, kitchen floors, as well as sofa upholstery, and found levels 50% lower than endotoxin levels in the present study.6 Likewise, our estimates in the present study are higher than endotoxin levels in inner city New York sampled in the bedroom floor of the houses of children of Dominican and African-American descent, though our lab performed the endotoxin measurement for both studies.24 In the ISAAC Phase Two study, endotoxin was measured in living room floor dust and levels were also generally lower than the levels from our present study (between 684 EU/m2 and 3,602 EU/m2 depending on the ISAAC centers).25 The ISAAC study included 840 children aged 9–12 years from five countries (Albania, Italy, New Zealand, Sweden and the United Kingdom) and it is worth noting that carpeting is rare in these countries compared to the US, which could explain the difference in endotoxin load (endotoxin per unit of sampled surface area).25 Similarly, Lawson et al. measured endotoxin in the homes of 6- to 18-year-old rural children in Saskatchewan, Canada and reported lesser endotoxin loads in play area and mattress, respectively up to 1,032 EU/m2 (versus ≥ 10,000 EU/m2 in our study) and up to 308.8 EU/m2 (vs ≥ 8,000 EU/m2 in our study).26 However, they extracted the dust samples in phosphate-buffered saline and then diluted in water without adding a surfactant. Extraction of house dust into water without including a surfactant has been shown to markedly reduce endotoxin extraction efficiency.27
Few studies have measured airborne endotoxin and they have also reported lower levels in total dust sampled from US urban home or in inhalable dust from European rural areas.28,29 Our results are consistent with airborne endotoxin concentrations reported outside of Iowa hoop-structured and conventional animal feeding operations. Thirty meters upwind of the animal feeding operations, endotoxin concentration was 2.7 and 5.3 EU/m3 for the hoop and conventional animal feeding operations, respectively. Downwind from the animal feeding operations, outdoor endotoxin levels averaged 190 EU/m3 (hoop animal feeding operations) and 60 EU/m3 (conventional animal feeding operations) at 30 meters and approximately 27 EU/m3 at 160 meters (for both). Wind speeds during sampling ranged from 0 to 5.4 m/s.30 Comparisons between the endotoxin values reported in the present study with those from other investigations must be interpreted cautiously due to very impactful laboratory differences in sample collection, storage, extraction and assay techniques across studies.27,31,32
Several smaller studies have evaluated the impact of environmental interventions on the levels of endotoxin in homes.33–36 In China, Wu et al. recruited 20 volunteers who vacuumed their mattress daily for 8 weeks and found that the total house endotoxin dropped by 75%. However, they did not see a change in endotoxin concentration per amount of dust.37 In metropolitan Washington, DC, the effectiveness of vacuuming in reducing dust endotoxin and other allergens was examined in 20 homes over a 5-week period.38 The study reported that weekly and monthly vacuuming periods resulted in an increase in the concentration of endotoxin in the majority of homes.38 To possibly explain these results, the authors speculated that vacuuming could release organisms from the base of rugs and subsequently stimulate bacterial growth.36,38,39 Studying predictors of endotoxin concentrations in house dust of German homes for the Indoor Exposure and Genetics in Asthma study, Bischof et al. observed that vacuuming weekly versus monthly as well as dry dust cleaning was predictive of lower endotoxin concentration in living room dust.40 Likewise, frequent dusting significantly reduced airborne endotoxin in inner city Baltimore homes.41 Yet, in some studies, cleaning practices (vacuum, sweep or mop) were unrelated to house dust endotoxin 42,43 We noted that endotoxin levels in reservoir dust generally increased from visit 3 to visit 4 after an initial drop observed during active intervention around visits 2 and 3. We can speculate that perhaps cleaning procedures in the study mobilized dust or that participants reduced their housekeeping during the intervention thinking that it was supplanted by LEIP staff efforts.
Many reports have examined the influence of asthma education and environmental interventions on asthma symptoms.44–46 About 15 years ago, Cote et al. prospectively followed 188 asthma patients to evaluate the effect of an asthma education program on airway responsiveness, asthma symptoms, patient quality of life and environmental control. One year later, they found asthma symptoms, quality of life and exposure to house dust mites had significantly improved. However, they did not see an effect of removal of domestic pets.44 Other studies, including meta-analyses subsequently confirmed modest benefits of educational intervention on asthma control.45,46 There are also reports that environmental interventions have more beneficial effects on asthma control than education, especially in children.33 In one study, education about environmental interventions was assessed. The intervention group received education applying social learning theory to provide knowledge, skills, motivation, equipment, and supplies necessary to perform comprehensive environmental remediation for a year, while controls received family visits only for evaluation.47 During the intervention year and afterwards, the intervention group had significantly lower levels of allergens in the bed and bedroom floor. This decline was also correlated with reduced asthma severity.47 Studies that evaluated the efficacy of asthma educational interventions in rural pediatric populations are few. In rural counties of Maryland, educational workshops for both children and parents significantly improved asthma knowledge and reports of asthma symptoms, but not quality of life.48 Despite an extensive literature search, we found no study that compared education with and without environmental interventions. We found the decrease in house dust endotoxin, mainly in the bedroom bed and bedroom floor associated with a significant improvement in asthma symptoms. This is also consistent with previous findings of stronger relationships between bedroom floor and bedding dust endotoxin and asthma prevalence and symptoms.6
Our study had several limitations. Three of the 89 households had more than 10 days elapse between super clean and visit 2, the first follow-up visit; therefore, they were excluded from these models. Only one child per household was used for the models assessing the effectiveness of education with or without cleaning in reducing endotoxin. There were not enough households with multiple children to add another random factor to account for a household effect. Some diary questions about asthma severity and inhaled bronchodilator or corticosteroids use had response rates between 40 and 70%. With such high levels of missing data, we questioned the reliability of these data and therefore excluded them. We also did not have enough data on the symptoms to perform subgroup analyses of the relationship of the intervention and the endotoxin reduction on asthma symptoms by the location of the home in a farm or not. The association between endotoxin reduction and asthma symptoms was not adjusted for medication use in our sample. Nonetheless, aside from these limitations, this study has major advantages. It is the first intervention conducted in rural asthmatic children in the US. Also, among the parents of school children initially contacted, more than 70% responded with completed questionnaires. Participants were contacted by mail up to 3 times and by telephone to increase participation. Endotoxin was measured in air and in reservoir dust sampled in multiple locations within each household and four times over the course of the study.
In conclusion, overall and in non-farm homes, reservoir dust endotoxin was significantly decreased during the visits following the extensive intervention compared to the visit before the intervention. Extensive intervention (cleaning + education) compared to educational intervention was associated with reduced reservoir dust endotoxin in the homes located in farms and with less frequent nighttime asthma symptoms. Effective reduction of endotoxin levels mainly in the bedroom floor or the bedroom bed was associated with improved respiratory symptoms in children with asthma.
Supplementary Material
PRACTICAL IMPLICATIONS.
The Louisa Environmental Intervention Project (LEIP) was designed to evaluate the effectiveness of environmental interventions in reducing household endotoxin and improving respiratory symptoms in children with asthma.
LEIP demonstrated that cleaning measures such as cleaning of carpets, draperies, mattresses and bedding, furnace, air conditioners, and air ducts can effectively reduce settled dust endotoxin, especially in farm homes.
In LEIP, professional cleaning and endotoxin reduction were associated with fewer asthma symptoms.
However, Cleaning efforts should be sustained over time to maintain reduced levels of endotoxin and ultimately improve asthma symptoms.
Acknowledgements:
The authors acknowledge the contributions to the study of the coordinator Angela K.W. Kuehl and the field staff: Linda M. Harris, Marsha E. O’Neill, Alicia K. Quella, Alba Quinones and Ruth E. Walker. Thanks also go to Kevin M. Kelly and Yufang Zhang who helped with database management. This project was supported by NIH R01 ES11392 and NIH P30 ES005605.
Abbreviations
- CBB
Child’s bedroom bed
- CBF
Child’s bedroom floor
- EPA
Environmental Protection Agency
- ISAAC
International Study of Asthma and Allergies in Childhood
- KNF
Kitchen floor
- LEIP
Louisa Environmental Intervention Project
- PAF
Play area floor
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose
REFERENCES
- 1.Mendy A, Forno E, Niyonsenga T, Carnahan R, Gasana J. Prevalence and features of asthma-COPD overlap in the United States 2007–2012. Clin Respir J. 2018;12(8):2369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milligan KL, Matsui E, Sharma H. Asthma in urban children: epidemiology, environmental risk factors, and the public health domain. Curr Allergy Asthma Rep 2016;16(4):33. [DOI] [PubMed] [Google Scholar]
- 3.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel O, Ginanni R, Duchateau J, Vertongen F, Bon B, Sergysels R. Domestic endotoxin exposure and clinical severity of asthma. Clin Exp Allergy 1991;21(4):441–448. [DOI] [PubMed] [Google Scholar]
- 5.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res 2005;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med 2005;172(11):1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorne PS. Inhalation toxicology models of endotoxin-and bioaerosol-induced inflammation. Toxicology 2000;152(1):13–23. [DOI] [PubMed] [Google Scholar]
- 8.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 2000;25(2):187–191. [DOI] [PubMed] [Google Scholar]
- 9.Jagielo PJ, Thorne PS, Watt JL, Frees KL, Quinn TJ, Schwartz DA. Grain dust and endotoxin inhalation challenges produce similar inflammatory responses in normal subjects. Chest 1996;110(1):263–270. [DOI] [PubMed] [Google Scholar]
- 10.Kline JN, Cowden JD, Hunninghake GW, Schutte BC, Watt JL, Wohlford-Lenane CL, et al. Variable airway responsiveness to inhaled lipopolysaccharide. Am J Respir Crit Care Med 1999;160(1):297–303. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz DA, Thorne PS, Jagielo PJ, White GE, Bleuer SA, Frees KL. Endotoxin responsiveness and grain dust-induced inflammation in the lower respiratory tract. Am J Physiol 1994;267(5 Pt 1):L609–17. [DOI] [PubMed] [Google Scholar]
- 12.Michel O, Duchateau J, Sergysels R. Effect of inhaled endotoxin on bronchial reactivity in asthmatic and normal subjects. J Appl Physiol 1989;66(3):1059–1064. [DOI] [PubMed] [Google Scholar]
- 13.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med 1996;154(6 Pt 1):1641–1646. [DOI] [PubMed] [Google Scholar]
- 14.Hađina S, Weiss JP, McCray PB Jr, Kulhankova K, Thorne PS. MD-2–dependent pulmonary immune responses to inhaled lipooligosaccharides: Effect of acylation state. Am J Respir Cell Mol Biol. 2008;38(6):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gioannini TL, Teghanemt A, Zhang D, Esparza G, Yu L, Weiss J. Purified monomeric ligand. MD-2 complexes reveal molecular and structural requirements for activation and antagonism of TLR4 by Gram-negative bacterial endotoxins. Immunol Res 2014;59(1–3):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doreswamy V, Peden DB. Modulation of asthma by endotoxin. Clin Exp Allergy 2011;41(1):9–19. [DOI] [PubMed] [Google Scholar]
- 17.Nightingale JA, Rogers DF, Hart LA, Kharitonov SA, Chung KF, Barnes PJ. Effect of inhaled endotoxin on induced sputum in normal, atopic, and atopic asthmatic subjects. Thorax 1998;53(7):563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt LW, Gleich GJ, Ohnishi T, Weiler DA, Mansfield ES, Kita H, et al. Endotoxin contamination causes neutrophilia following pulmonary allergen challenge. Am J Respir Crit Care Med 1994;149(6):1471–1475. [DOI] [PubMed] [Google Scholar]
- 19.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med 2001;163(2):511–516. [DOI] [PubMed] [Google Scholar]
- 20.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, Rose KM, Zeldin DC. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the US. Am J Respir Crit Care Med 2015;192(11):1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendy A, Salo PM, Cohn RD, Wilkerson J, Zeldin DC, Thorne PS. House Dust Endotoxin Association with Chronic Bronchitis and Emphysema. Environ Health Perspect. 2018;126(3):037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. The Lancet 2006;368(9537):733–743. [DOI] [PubMed] [Google Scholar]
- 23.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons; 2012. [Google Scholar]
- 24.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006;117(5):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gehring U, Strikwold M, Schram-Bijkerk D, Weinmayr G, Genuneit J, Nagel G, et al. Asthma and allergic symptoms in relation to house dust endotoxin: Phase Two of the International Study on Asthma and Allergies in Childhood (ISAAC II). Clin Exp Allergy 2008;38(12):1911–1920. [DOI] [PubMed] [Google Scholar]
- 26.Lawson JA, Dosman JA, Rennie DC, Beach J, Newman SC, Senthilselvan A. Relationship between indoor environment and asthma and wheeze severity among rural children and adolescents. J Agromedicine 2009;14(2):277–285. [DOI] [PubMed] [Google Scholar]
- 27.Hoppe Parr KA, Hađina S, Kilburg-Basnyat B, Wang Y, Chavez D, Thorne PS, et al. Modification of sample processing for the Limulus amebocyte lysate assay enhances detection of inflammogenic endotoxin in intact bacteria and organic dust. Innate immun 2017;23(3):307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Spiegelman DL, Gold DR, Burge HA, Milton DK. Predictors of airborne endotoxin in the home. Environ Health Perspect 2001;109(8):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mutius V Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy 2000;30(9):1230–1234. [DOI] [PubMed] [Google Scholar]
- 30.Thorne PS, Ansley AC, Perry SS. Concentrations of bioaerosols, odors, and hydrogen sulfide inside and downwind from two types of swine livestock operations. J Occup Environ Hyg. 2009;6(4):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaan S, Doekes G, Heederik D, Thorne PS, Wouters IM. Effect of extraction and assay media on analysis of airborne endotoxin. Appl Environ Microbiol 2008;74(12):3804–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaan S, Heederik DJ, Thorne PS, Wouters IM. Optimization of airborne endotoxin exposure assessment: effects of filter type, transport conditions, extraction solutions, and storage of samples and extracts. Appl Environ Microbiol 2007;73(19):6134–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, et al. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. Am J Prev Med 2011;41(2):S5–S32. [DOI] [PubMed] [Google Scholar]
- 34.Takaro TK, Krieger JW, Song L. Effect of environmental interventions to reduce exposure to asthma triggers in homes of low-income children in Seattle. J Expo Anal Environ Epidemiol. 2004;14:S133–S143. [DOI] [PubMed] [Google Scholar]
- 35.Krieger J, Jacobs DE, Ashley PJ, Baeder A, Chew GL, Dearborn D, et al. Housing interventions and control of asthma-related indoor biologic agents: a review of the evidence. J Public Health Manag Pract 2010;16(5 Suppl):S11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, et al. Mold and endotoxin levels in the aftermath of Hurricane Katrina: a pilot project of homes in New Orleans undergoing renovation. Environ Health Perspect 2006:1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu FF, Wu M, Pierse N, Crane J, Siebers R. Daily vacuuming of mattresses significantly reduces house dust mite allergens, bacterial endotoxin, and fungal β-glucan. J Asthma 2012;49(2):139–143. [DOI] [PubMed] [Google Scholar]
- 38.Bellanti JA, Zeligs BJ, MacDowell-Carneiro AL, Abaci AS, Genuardi JA. Study of the effects of vacuuming on the concentration of dust mite antigen and endotoxin. Ann Allergy Asthma Immunol 2000;84(2):249–254. [DOI] [PubMed] [Google Scholar]
- 39.Hoppe KA, Metwali N, Perry SS, Hart T, Kostle PA, Thorne PS. Assessment of airborne exposures and health in flooded homes undergoing renovation. Indoor Air 2012;22(6):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bischof W, Koch A, Gehring U, Fahlbusch B, Wichmann H, Heinrich J. Predictors of high endotoxin concentrations in the settled dust of German homes. Indoor Air 2002;12(1):2–9. [DOI] [PubMed] [Google Scholar]
- 41.Mazique D, Diette G, Breysse P, Matsui E, McCormack M, Curtin-Brosnan J, et al. Predictors of airborne endotoxin concentrations in inner city homes. Environ Res 2011;111(4):614–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gehring U, Bischof W, Borte M, Herbarth O, Wichmann H, Heinrich J. Levels and predictors of endotoxin in mattress dust samples from East and West German homes. Indoor Air 2004;14(4):284–292. [DOI] [PubMed] [Google Scholar]
- 43.Gereda JE, Klinnert MD, Price MR, Leung DY, Liu AH. Metropolitan home living conditions associated with indoor endotoxin levels. J Allergy Clin Immunol 2001;107(5):790–796. [DOI] [PubMed] [Google Scholar]
- 44.Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence of asthma education on asthma severity, quality of life and environmental control. Can Respir J 2000;7(5):395–400. [DOI] [PubMed] [Google Scholar]
- 45.Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self-management of asthma in children and adolescents: systematic review and meta-analysis. BMJ 2003;326(7402):1308–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coffman JM, Cabana MD, Halpin HA, Yelin EH. Effects of asthma education on children’s use of acute care services: a meta-analysis. Pediatrics 2008;121(3):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004;351(11):1068–1080. [DOI] [PubMed] [Google Scholar]
- 48.Butz A, Pham L, Lewis L, Lewis C, Hill K, Walker J, et al. Rural children with asthma: impact of a parent and child asthma education program. J Asthma 2005;42(10):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


