Abstract
Tendon/ligament-to-bone healing poses a formidable clinical challenge due to the complex structure, composition, cell population and mechanics of the interface. With rapid advances in tissue engineering, a variety of strategies including advanced biomaterials, bioactive growth factors and multiple stem cell lineages have been developed to facilitate the healing of this tissue interface. Given the important role of structure-function relationship, the review begins with a brief description of enthesis structure and composition. Next, the biomimetic biomaterials including decellularized extracellular matrix scaffolds and synthetic-/natural-origin scaffolds are critically examined. Then, the key roles of the combination, concentration and location of various growth factors in biomimetic application are emphasized. After that, the various stem cell sources and culture systems are described. At last, we discuss unmet needs and existing challenges in the ideal strategies for tendon/ligament-to-bone regeneration and highlight emerging strategies in the field.
Keywords: Tendon/ligament-to-bone interface, Tissue engineering, Biomaterial, Growth factor, Stem cell
Graphical abstract
Highlights
-
•
The interface tissue engineering for enthesis regeneration is promising.
-
•
The biomimetic scaffolds are examined.
-
•
The key issues in the biomimetic application of growth factors are emphasized.
-
•
The various stem cell sources and culture systems are described.
-
•
The challenges and emerging strategies for enthesis regeneration are presented.
1. The interfacial musculoskeletal diseases as a global burden
Tendons and ligaments connect muscles to bone or bone to bone, respectively, which enable locomotion, and the interface where tendon or ligament attaches to bone is known as the enthesis [1,2]. The enthesis displays gradients in tissue organization, composition and mechanical properties that have several functions, from effectively transferring mechanical stress between mechanically dissimilar tissues to sustaining heterotypic cellular communications for interface function and homeostasis [[3], [4], [5]]. The complexity of the enthesis enables musculoskeletal function but also imposes formidable challenges in tissue repair and regeneration.
Tendon and ligament injuries account for 30% of all musculoskeletal clinical cases with 4 million new incidences worldwide each year [6]. Two of the most common injury sites are rotator cuff tendon of the shoulder and anterior cruciate ligament (ACL) of the knee [7,8]. Within the shoulder, rotator cuff tendon consists of the supraspinatus infraspinatus, teres minor and subscapularis, and connects the muscles surrounding the scapula to the humerus, which in turn supports the rotation and stability of the humerus [9]. Rotator cuff tears have become increasingly common with more than half of adults >65 years being affected, which are contributed to significant levels of morbidity and shoulder pain [9,10]. More than 1.1 million rotator cuff tendon surgical procedures are performed around the world each year [11]. Since several factors influence the rate of retear, surgical therapy is extremely challenging, with the rate of retear ranging from 26% for small (<1 cm) and medium (1–3 cm) tears, and up to 94% for large (3–5 cm) and massive (>5 cm) tears [9,12]. In the knee, ACL is the primary static stabilizer in the anterior translation of the tibia in relation to the femur, which prevents extreme tibial rotations and plays an important role in enabling functional movements [13]. ACL rupture is a common sports injury that can be attended by a series of secondary symptoms, including meniscus and cartilage damage, movement dysfunction, knee laxity and even early post-traumatic osteoarthritis [14]. About 400000 ACL reconstructions are performed worldwide each year [13]. Collectively, the injury of the tendon/ligament-to-bone tissue has become a serious health problem, which significantly reduces the quality of life for millions of people around the world.
Therefore, the tendon/ligament-to-bone interface regeneration has increasingly become a subject of intense interest within the field of orthopedic research. Clinically, traditional conservative treatment or surgical repair cannot achieve enthesis healing and regeneration effectively to recapitulate the complex transition between tendon/ligament and bone. During the past decades, the important role of the enthesis and unsatisfactory results of current clinical treatment modalities have spurred the development of interface tissue engineering to facilitate the regeneration of the soft-to-hard tissues.
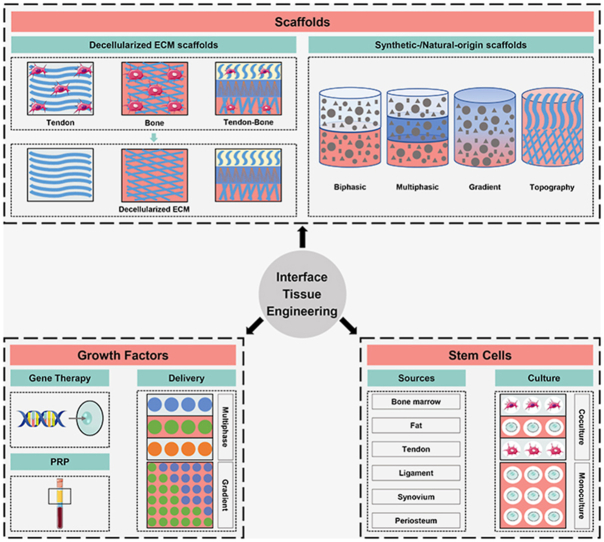
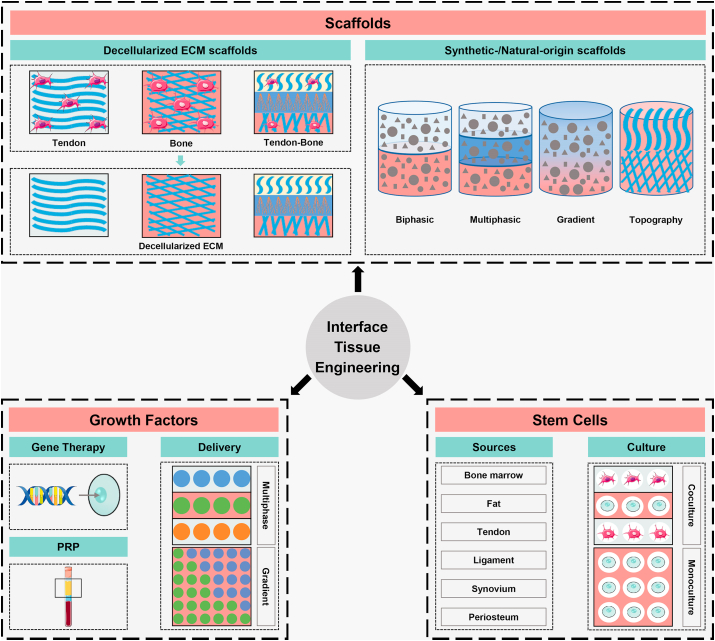
With greater understanding of enthesis structure and further technological advancement, utilizing biomaterial-based strategies, growth factor-based strategies and stem cell-based strategies alone or in combination have shown promising outcomes. In this review, given the important role of structure-function relationship, we will begin with a description of enthesis structure and composition. Next, we will examine biomimetic strategies, focusing on well-designed biomaterials, emphasizing key issues in the biomimetic utilization of growth factors, and describing potential stem cell sources and culture systems (Fig. 1). Finally, the present challenges and future development directions of enthesis tissue engineering will be highlighted.
Fig. 1.
The schematic of scaffolds, growth factors and stem cells as the biomimetic components for tendon/ligament-to-bone interface regeneration. ECM, extracellular matrix; PRP, platelet-rich plasma.
2. The structure and composition of enthesis
The enthesis can be broadly classified as direct and indirect attachment according to structure. Direct enthesis have a fibrocartilaginous region between the bone and the ligament/tendon, such as the insertion of achilles tendon, patellar tendon, anterior cruciate ligament and rotator cuff, as well as femoral insertion of medial collateral ligament [15]. Indirect enthesis are characterized by dense fibrous tissue that are attached to bone indirectly through the periosteum, such as the tibial insertion of the medial collateral ligament and the insertion of the deltoid tendon into the humerus [15]. Due to clinical relevance, this review will focus on the fibrocartilaginous enthesis.
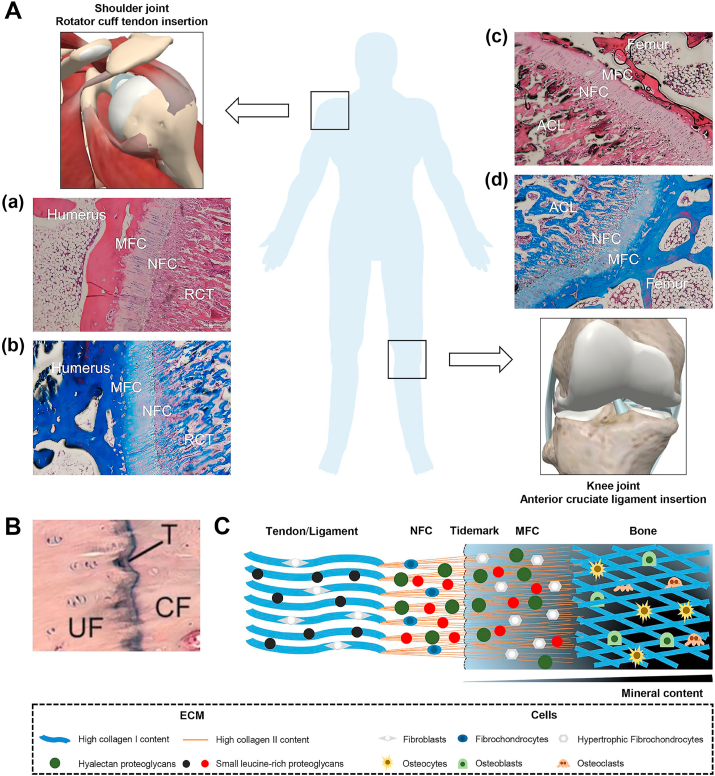
The enthesis is a specialized transitional region between tendon/ligament and bone for the proper transmission of forces, which exhibits gradients in cell phenotype, matrix composition, tissue organization, and mechanical properties. The histology of fibrocartilaginous enthesis consists of four distinct regions: ligament/tendon (I), non-mineralized fibrocartilage (II), mineralized fibrocartilage (III), and bone (IV) (Fig. 2A) [8]. The tidemark is a basophilic line that separates the uncalcified and calcified fibrocartilage zones (Fig. 2B) [16]. Tendon-to-bone insertion exhibited a gradual mechanical transition from tendon (200 MPa tensile modulus) to bone (20 GPa tensile modulus) [17]. The fibrocartilage regions span 300–800 μm in width on average, depending on the species [18]. To alleviate these concentrations of mechanical stress and allow for effective stress transfer at such scale, several mechanisms at various scale levels are involved.
Fig. 2.
The histological staining and schematic of tendon/ligament-to-bone insertion. (A) The H&E staining of rabbit supraspinatus tendon (a) and anterior cruciate ligament (c) insertion sites. The Masson staining of rabbit supraspinatus tendon (b) and anterior cruciate ligament (d) insertion sites. Reproduced with permission from Ref. [8]. Copyright 2017, Springer Nature. (B) The tidemark stained with H&E. Reproduced with permission from Ref. [32]. Copyright 2002, Elsevier Science Inc. (C) The schematic of tendon/ligament-to-bone insertion. The fibrocartilaginous enthesis is composed of four distinct zones: tendon/ligament, non-mineralized fibrocartilage, mineralized fibrocartilage, and bone. RCT, rotator cuff tendon; ACL, anterior cruciate ligament; NFC, non-mineralized fibrocartilage; MFC, mineralized fibrocartilage; UF, uncalcified fibrocartilage; CF, calcified fibrocartilage; T, tidemark; ECM, extracellular matrix.
At the nanometer scale, the mineral organization relative to collagen theoretically shifting the load-sharing mechanism among the various components of the enthesis [19,20]. There are various collagenous mineralized tissues in the human body, whose basic structural and functional element is mineralized collagen fibrils [21]. The precursor phase for mineral formation is amorphous calcium phosphate (ACP, amorphous phase) rather than hydroxyapatite (HA, crystal phase) [[22], [23], [24]]. The ACP stabilized by non-collagenous proteins enters the collagen fibers and bind to charged amino acid residues within collagen, particularly residues containing carboxyl groups [24]. Subsequently, the amorphous phase transforms into the crystalline phase and gradually becomes a hierarchical structure. At the micrometer scale, the tissue displays varying gradients in collagen orientation, mineral content and protein type across the interface [20]. In terms of fiber orientation, the collagen fibers are aligned and parallel at tendon/ligament region [25]. The tendon fibers unravel into thinner and smoother interface fibers and splay out symmetrically along the insertion before attaching to bone (Fig. 2C) [26]. These features might contribute to the durability of enthesis by reducing stress concentrations and increasing toughness [26]. In terms of the mineral content, there is a sharp increase in mineral content between the mineralized and non-mineralized region, and its content increases exponentially across the mineralized fibrocartilage toward bone (Fig. 2C) [27,28]. In terms of molecular composition, differential molecular composition across the interface are responsible for distinct structural and functional features. Tendon/Ligament is mostly composed of collagen I, which is very sparse in Zone II-III (Fig. 2C) [25,26]. Zone II consists mostly collagen II, and also has high levels of pericellular collagen III, together with small amounts of collagen I and X (Fig. 2C) [25]. Zone III is mainly composed of collagen II, together with significant amounts of collagen X (Fig. 2C) [25]. Bone consists mainly of collagen I and has a high mineral content (69%) of which 99% is HA (Fig. 2C) [25]. Moreover, negatively-charged hyalectan proteoglycans are upregulated in Zone II-III, which leads to a counteracting osmotic pressure (Fig. 2C) [26]. In addition, the different small leucine-rich proteoglycans that are highly-expressed in Zone I-III may be the prime cause of the splaying and subdividing of the fibers at the molecular level (Fig. 2C) [26]. In terms of the cell phenotypes, the tendon/ligament is populated by interspersed elongated fibroblasts organized in arrays (Fig. 2C) [18,25]. The Zone II is populated by fibrochondrocytes arranged in rows (Fig. 2C) [18,25]. The Zone III is populated by hypertrophic fibrochondrocytes arranged in columns (Fig. 2C) [18,25]. The bone is populated by osteoblasts, osteocytes and osteoclasts (Fig. 2C) [18,25]. Recently, Kuntz et al. presented a transcriptomic study at the tendon-to-bone interface and identified several interface biomarkers by combining transcriptomics with proteomics, which provides a bench-mark to study whether the differentiation of seeded MSCs towards a physiological phenotype [29]. However, the precise identification of the cell subpopulations at the interface remains unknown.
The healing of enthesis occurs in three phases: inflammatory phase (0–7 days), repair phase (5–14 days) and remodeling phase (>14 days) [16]. Tendon/ligament-to-bone healing typically occurs through the formation of disorganized fibrovascular scar tissue rather than the regeneration of natural gradient tissue. The disorganized fibrovascular scar can be due to abnormal or inadequate genes expression that direct insertion site formation, insufficient undifferentiated cells at the healing interface, and excessive mechanical loading on the healing tendon [30]. The disorganized scar tissue is mainly comprised of collagen ІІІ and gets gradually replaced with collagen І without collagen ІІ [31]. The mechanical strength of the neo-fibrovascular scar tissue is much lower than the native interface, due to lack of gradient mineral distribution and continuity of collagen fibers, thus resulting in the high failure rate [25].
In conclusion, a better understanding of the enthesis structure is the basis for guiding biomimetic strategies to achieve functional enthesis regeneration.
3. Biomaterials as biomimetic strategy
Among the three major components (biomaterials, growth factors, cells) of interface tissue engineering, the biomaterials play the most important role to facilitate tissue regeneration, which provide structural support, as well as appropriate biomechanical and biochemical properties that mimic the native microenvironment. In this section, we will focus on decellularized extracellular matrix (ECM) scaffolds and synthetic-/natural-origin scaffolds (Fig. 1).
3.1. Decellularized ECM scaffolds
It is extremely important to develop constructs with similar heterogeneous transition regions to achieve enthesis regeneration. However, it is greatly challenging to develop a scaffold to completely mimic the natural complex structure. Tissue engineering the native tissue which provides a naturally occurring three-dimensional scaffold with specific ECM could overcome the complications. During the past decades, scaffolds derived from decellularized allogeneic and xenogeneic ECM have been widely applied in surgical reinforcement of rotator cuff tears, which are mainly derived from mammalian ECM such as porcine small intestinal submucosa, human dermis and porcine dermis [4,12,33]. As is known, each tissue has a unique ECM with specific physical, topological, and biochemical composition, which provides the necessary physical scaffold for cell components, as well as initiates important biochemical and biomechanical clues for tissue morphogenesis, differentiation and homeostasis [34]. The varying ECM deriving from distinct tissues may exert tissue-specific regeneration functions [35]. For example, our previous study reported that tendon-derived decellularized matrix promoted the tenogenic differentiation while inhibited osteogenic differentiation of tendon stem/progenitor cells (TSPCs), even under osteogenic induction conditions [35]. Bone-derived decellularized matrix robustly induced osteogenic differentiation of TSPCs, while dermis-derived decellularized matrix did not have obvious effect on TSPCs differentiation [35]. Therefore, ECM derived from native tendon-to-bone tissue might be more appropriate for enthesis regeneration.
Acellular tendon/ligament/fibrocartilage scaffolds are suitable to enhance tendon-to-bone healing owing to their good biodegradability, high biocompatibility, low immunogenicity and high similarity to the structure and composition of ECM [36]. Due to the native tendon/ligament/fibrocartilage being a dense and compact tissue, it is greatly challenging to completely remove internal cell, while mostly conserving the native ECM [37,38]. Therefore, several studies physically sectioned tissues into slices which could facilitate the infiltration of acellular agents into tissues, thus less exposure time is needed and more native ECM ingredients can be preserved [[37], [38], [39], [40]]. Ning et al. developed scaffolds based on decellularized tendon slices with excellent cytocompatibility, adequate mechanical strength and multiple growth factors, which supported proliferation and tenogenic differentiation of tendon-derived stem cells (TDSCs) and bone marrow-derived stem cells (BMSCs) [37,39]. In a further study, Liu et al. repaired rabbit large rotator cuff tears using decellularized tendon slices scaffold [40]. This scaffold promoted cell ingrowth and tissue integration, facilitated fibrocartilage, Sharpey fibers and new bone formation, and improved biomechanical properties. Inspired by book shape, Chen et al. sectioned the fibrocartilage to fabricate a book-shaped acellular scaffold [38]. The scaffold exhibited a superior cell-loading capacity and chondrogenic inducibility in vitro. The scaffold with or without adipose-derived stromal cells sheets improved fibrocartilage regeneration and mechanical properties in a rabbit partial patellectomy model. Recently, they synthesized a recombinant stromal cell‐derived factor‐1α capable of binding collagen and chemotactic activity, which was then tethered on the collagen fibers of a book‐shaped acellular fibrocartilage scaffold [41]. The scaffold enhanced the tendon-to-bone healing with larger cartilaginous metaplasia region, additional bone formation, better fibrocartilage regeneration, and improved biomechanical properties in a rabbit partial patellectomy model. Further, they proved that the scaffold could recruit postoperative injected CXCR4+ cells into the healing site to circumvent in-vitro loading of cells [42].
Demineralized bone matrix as an osteoinductive scaffold has been widely utilized for the tendon-to-bone repair [[43], [44], [45], [46], [47]]. Hexter et al. have systematically reviewed the utilization of demineralized bone matrix on tendon-to-bone healing [47]. The review indicated that the demineralized bone matrix could improve tendon-to-bone healing, while the application remains in the exploratory stage with no clinical research. As is known, acellular bone scaffold displays better osteoinductivity, whereas acellular fibrocartilage scaffold displays better chondrogenic inducibility. To compare the efficacy of the two scaffolds, Lu et al. implanted book‐type acellular bone scaffold and book‐type acellular fibrocartilage scaffold in a rabbit partial patellectomy model [36]. The implanted book‐type acellular fibrocartilage scaffold resulted in more new bone formation, more fibrocartilage regeneration, better failure load and stiffness, which thus suggested that the book‐type acellular fibrocartilage scaffold is a more promising scaffold for tendon-to-bone repair.
In some studies, decellularized tendon-bone composite tissues have been used to replace the injured interface, which avoids the challenges of mimicking the complex tendon-to-bone interface. For example, Farnebo et al. utilized decellularized Achilles tendon-calcaneus graft for tendon-bone reconstruction [48,49]. Decellularized grafts display superior biomechanical properties at earlier healing time points with decreased immune responses, as compared with untreated grafts [48]. Besides, the graft promoted more well-organized ECM and better biomechanical properties at early time points, as compared to conventional pullout repair [49]. Recently, Su et al. prepared decellularized bone-fibrocartilage-tendon composite scaffolds using differential decellularization [50]. The triphasic scaffold enabled BMSCs to achieve regional differentiation in vitro and enhanced bone formation along the bone tunnel within a femur-tibia defect model in vivo [50]. Based on book‐type design, Liu et al. and Tang et al. developed book-type acellular tendon-fibrocartilage-bone scaffold and they further combined acellular triphasic scaffold with BMSC cell sheet for rotator cuff tears repair and patella-patellar tendon interface injury repair, respectively [51,52]. These studies demonstrated that the combination of acellular composite tissue and BMSC cell sheet was an effective strategy for tendon-to-bone healing.
Considering mineral gradients of the natural enthesis, the bone could be demineralized or the tendon could be mineralized in a gradient manner to more accurately mimic the native enthesis. Recently, Boys et al. demineralized trabecular bone spatially using a top-down approach, exhibiting mineral gradients on the scale of 20–40 μm, which will be available for tendon-to-bone repair [53]. Besides, several studies have utilized calcium phosphate to mineralize the native tendon. For example, Mutsuzaki et al. hybridized tendon graft with calcium phosphate for ACL reconstruction, which could reduce bone-tunnel enlargement and the gap area associated with the cartilage layer and new bone formation [[54], [55], [56]]. Qu et al. achieved mineralization of the tendon by combining Na2HPO4 extraction and fetuin enhancement, to convert the tendon-to-bone healing into bone-to-bone healing, and a 200 μm-layer of bone was reported to be generated within the tendon [57]. Recently, Grue et al. developed decellularized bovine tendon sheets and mineralized tendon collagen sheets using an alternate soaking method that incorporates a polymer-induced liquid precursor process to promote intrafibrillar mineralization [58]. In the future, the tendon could be mineralized in a gradient manner, transforming the tendon to the tendon-bone tissue for interface regeneration.
3.2. Synthetic-/Natural-origin scaffolds
Stringent processing requirements as well as secondary wound site creation have limited the availability of acellular tissue scaffolds [59]. Instead of utilizing native tendon-bone tissues, advanced tissue engineering has applied various biomaterials for tendon-to-bone healing including synthetic polymers (e.g. polyethylene terephthalate (PET), poly-l-lactic acid (PLLA), polylactid-co-glycolid acid (PLGA), polyglycolic acid (PGA), and poly (ϵ-caprolactone) (PCL)), natural polymers (e.g. collagen and silk), and calcium phosphate biomaterials (e.g. calcium phosphate silicate ceramic (CPS), hydroxyapatite (HA) and tricalcium phosphate (TCP)). Table 1 has summarized the advantages and disadvantages of several different kinds of biomaterials. Synthetic polymers exhibit excellent mechanical properties, degradation rates and ease of processing, but their low bioactivity and acidic degradation products are limitations [12]. Natural polymers have been widely used for tissue engineering due to their intrinsic biocompatibility and biodegradability, but they lack sufficient mechanical properties [12]. Overall, the ideal biomaterial for enthesis tissue engineering not only needs to be biocompatible to ensure the growth and differentiation of transplanted and local cell populations and promote controlled matrix heterogeneity, but also needs to be biodegradable in order to be gradually replaced by newly-regenerated tissue [60].
Table 1.
The summary of advantages and disadvantages of common biomaterials for tendon/ligament-to-bone healing.
| Material | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Synthetic polymers | PLLA, PGA, PLGA | ∙Excellent mechanical properties ∙Biodegradability ∙Ease of processing |
∙Acidic degradation products | [12,60,[63], [64], [65]] |
| PET | ∙High mechanical property | ∙Non-degradable ∙Inferior biocompatibility |
[62] | |
| Natural polymers | Silk | ∙Biocompatibility ∙Slow degradability ∙Excellent mechanical properties |
∙Poor cell recruitment properties | [61,66,67] |
| Collagen | ∙Intrinsic biocompatibility ∙Biodegradability |
∙Insufficient mechanical strength | [61,66,67] | |
| Calcium phosphate biomaterials | HA | ∙Excellent osteoconductivity | ∙Slow biodegradation | [68] |
| CPS | ∙Excellent osteoconductivity ∙Superior biodegradability |
[68] | ||
| CaP cement | ∙Excellent osteoconductivity ∙Injectable |
∙Slow biodegradation | [69] |
PLLA, poly-l-lactic acid; PGA, polyglycolic acid; PLGA, poly (lactide-co-glycolid acid); PET, polyethylene terephthalate; HA, hydroxyapatite; CPS, calcium phosphate silicate ceramic; CaP, calcium phosphate.
To overcome the shortcomings of using a single type biomaterial in tendon-to-bone tissue engineering, the combination of different materials can exploit advantageous properties of each biomaterial and endow the composite scaffold with remarkable properties. For example, our previous study developed a knitted silk-collagen sponge scaffold, in which the silk provided excellent mechanical strength and the collagen increased the surface area and enhanced cell adhesion [61]. The knitted silk-collagen sponge scaffold promoted ACL and ligament-to-bone regeneration as well as prevented cartilage degeneration and osteoarthritis progression in both the short and long term. Recently, Zhang et al. fabricated a hybrid ligament combining biodegradable PCL nanofibrous membrane with non-degradable PET mesh fabric to overcome the limitations of conventional degradable and non-degradable scaffolds [62]. The PCL could promote new bone ingrowth and PET could maintain the biomechanical properties when PCL was degraded, which prevented reduction in ACL mechanical strength. Overall, the combination of varying biomaterials could meet the requirements of enthesis healing.
3.2.1. Biphasic/Multiphasic scaffolds
Given the graded composition and structure of the complex tendon/ligament-to-bone interface, it is generally believed that biphasic/multiphasic scaffolds are more suitable than single-phase scaffolds for interface tissue engineering. Because each layer can be made into different properties using distinct biomaterials so as to mimic the structural and compositional gradients of the enthesis, which enables phase-specific changes in supporting interface heterotypic cell populations and matrix and exhibits graded mechanical properties.
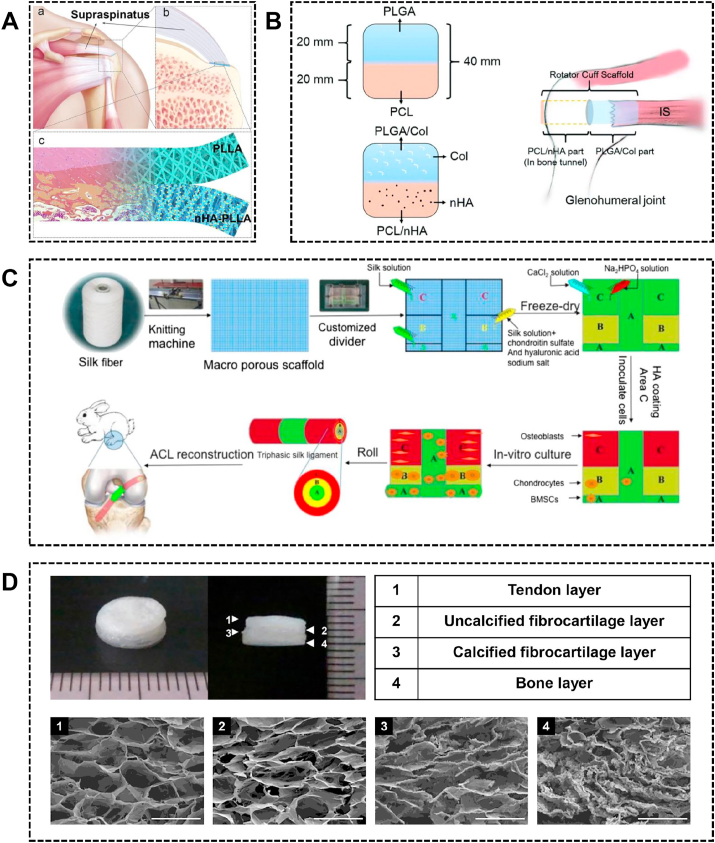
Inspired by the ECM composition of the enthesis, several studies have focused on the incorporation of inorganic calcium phosphate and/or natural biomaterials into specific regions of the scaffold to facilitate tendon/ligament-to-bone healing. To mimic non-mineralized and mineralized fibrocartilage, Li et al. fabricated integrated bipolar fibrous membranes, the upper and lower layer of which are PLLA fibrous membrane and nanohydroxyapatite (nHA)-PLLA fibrous membrane respectively (Fig. 3A) [70]. In a rabbit rotator cuff tear model, bipolar fibrous membrane increased glycosaminoglycan formation, improved collagen organization, induced bone formation and fibrillogenesis, and yielded a better ultimate load-to-failure and stiffness than the simplex PLLA fibrous membrane at 12 weeks. However, the better histological and mechanical performance was not observed at the early timepoint, which may result in re-tear in the first weeks after surgery. As the heterogeneity from soft tissue to hard tissue, the ideal scaffold should promote bone-to-scaffold ingrowth as well as tendon-to-scaffold ingrowth. For example, Sun et al. fabricated co-electrospun dual nano-scaffold of PLGA-PCL. Further, the collagen and nHA were incorporated into PLGA fibers and PCL fibers respectively to improve biocompatibility (Fig. 3B) [71]. The PLGA or the PLGA/collagen side was connected to the tendon stumps, whereas the PCL or PCL/nHA side was inserted into the bone tunnel. In vitro, fibroblasts seeded on PLGA/collagen scaffold showed better growth and collagen secretion compared with the PLGA scaffold. Osteoblasts seeded on the PCL/nHA scaffold showed better growth with higher mineralization compared with PCL scaffold. In a rabbit rotator cuff tear model, the PLGA/collagen scaffold induced better collagen ingrowth compared with PLGA scaffold, and the PCL/nHA scaffold induced more new bone versus PCL scaffold, and the PLGA/collagen-PCL/nHA showed better biomechanical properties versus PLGA-PCL scaffold.
Fig. 3.
The biphasic/multiphasic scaffolds for tendon/ligament-to-bone healing. (A) The schematic of the application of bipolar nanofibrous membrane. Reproduced with permission from Ref. [70]. Copyright 2017, Acta Materialia Inc. (B) The co-electrospun dual nano-scaffolds and the fixation technique. Reproduced with permission from Ref. [71]. Copyright 2016, The Royal Society of Chemistry. (C) The schematic of manufacturing triphasic silk graft used for restoration of osseointegration in the rabbit anterior cruciate ligament-defect model. Reproduced with permission from Ref. [72]. Copyright 2016, Elsevier Ltd. (D) Macroscopic images of a collagen-based four-layer scaffold and the cross-sectional images of the tendon layer, uncalcified fibrocartilage layer, calcified fibrocartilage layer, and bone layer. Reproduced with permission from Ref. [73]. Copyright 2014, Wiley Periodicals, Inc.
To better mimic the hierarchical structure of native enthesis, fabricating multiphasic scaffolds has attracted great interest. Li et al. developed a triphasic silk scaffold to mimic ligament-to-bone insertion (Fig. 3C) [72]. The different region of the silk scaffold was incorporated with various materials: the ligament region is coated with silk fibroin solution; the fibrocartilage region is coated with silk fibroin, chondroitin sulfate and hyaluronic acid sodium salt; the bone region is coated with silk fibroin solution and HA. In a rabbit ACL reconstruction model, the triphasic scaffold exhibited enhancement of osseointegration as demonstrated by robust mechanical properties together with multilayered tissue formation and corresponding matrix deposition. The ideal multiphasic scaffolds should mimic the enthesis as close as possible. Thus, generating a scaffold with four layers to better mimic the four distinct regions of the native enthesis is of great importance. Kim et al. developed a four-layer collagen scaffold with varying compositions (Fig. 3D) [73]. The four layers of the scaffold included the tendon layer (collagen), the fibrocartilage layer (collagen and chondroitin sulfate), the mineralized fibrocartilage layer (collagen and low calcification HA) and the bone layer (collagen and high calcification HA). The multilayer scaffold supported the adhesion and proliferation of fibroblasts, chondrocytes, and osteoblasts within each corresponding matrix layer. However, this study did not evaluate the efficacy of the scaffold in an animal model. Collectively, these studies demonstrated that the scaffold incorporated with specific biomaterials in each layer mimicking the native enthesis would better promote tendon/ligament-to-bone regeneration.
3.2.2. Gradient mineral scaffolds
In multiphase designs, scaffolds are segregated into two or more layers with abrupt transition from one layer to another. However, the native enthesis not only display region-specific properties but also exhibit gradient changes such as gradient mineral content. Consequently, several studies have developed gradient scaffolds, in which these changes are more gradual with no discrete segregation between layers [7]. Gradient scaffolds with continuous transition in composition and mechanical properties have been suggested to achieve a smoother integration of dissimilar tissues [74].
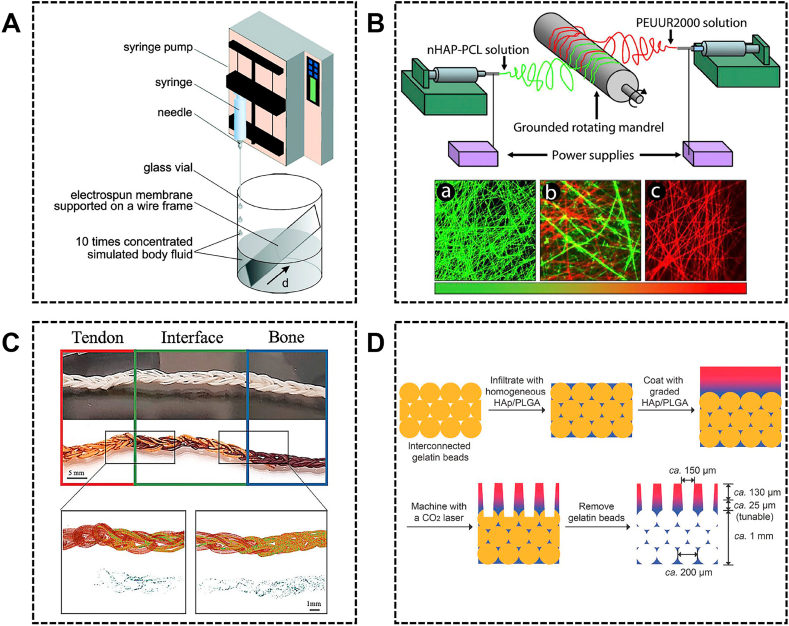
Given the gradient mineral distribution at the interface, several studies have modified the nanofibers scaffold with spatial control of mineral content, which not only mimics the mineral changes but also creates a mechanical gradient. There are varying methods to fabricate mineral gradient nanofibers scaffold. For example, Li et al. deposited gradient mineral content in PCL and PLGA nanofibers, by adding 10 x simulated body fluid at a constant rate to linearly reduce the deposition time from the bottom to the top end of the substrate (Fig. 4A) [75]. Their further study demonstrated that cell proliferation was negatively correlated with the mineral content, while the gradient mineral content could drive graded differentiation of adipose-derived mesenchymal stem cells (ASCs) toward osteoblasts [76]. In another method, PCL was doped with nHA and co-electrospun with poly(ester urethane) urea elastomer from offset spinnerets to form graded meshes (Fig. 4B). Subsequently, these meshes were treated with a 5 x simulated body fluid that selectively deposited mineral crystallites on nHA-PCL fibers to create mineral gradients [77]. The gradient mineral content could promote BMSCs to differentiate into a spatial gradient of varying osteoblastic phenotype [78]. Similarly, Ramalingam et al. developed a 2-spinnerette that can directly electrospun PCL and PCL/ACP together into a nonwoven mat in the form of a gradient [79]. Higher ACP content can enhance adhesion and proliferation of osteoblast, thus yielding a graded osteoblast response. However, these studies did not generate a 3D structure. Recently, Calejo et al. developed PCL/gelatin and PCL/gelatin/HA scaffolds using wet-spinning to mimic tendon and bone respectively [80]. Further, the PCL/gelatin and PCL/gelatin/HA scaffold were knitted together to form a gradient scaffold which displayed a gradient of mineral content along the scaffold mimicking the tendon-to-bone junction (Fig. 4C). Although the mineral gradient of these scaffolds mimics the trend in the enthesis, there was a mismatch in the length scale of the mineral gradients. Zhu et al. designed a hierarchically structured scaffold containing three regions for facilitating tendon-to-bone repair (Fig. 4D) [81]. In the transitional area, a series of PLGA solutions with varying HA concentrations were coated layer by layer to form a gradient calcium content in a thickness of ≈37 μm. The seeded ASCs exhibited graded tenogenic and osteogenic differentiation along the mineral gradient with monotonically changing mechanical properties. This hierarchically structured scaffold combined the multiphasic and the mineral gradient achieving a better biomimetic structure.
Fig. 4.
The gradient mineral scaffolds for tendon/ligament-to-bone healing. (A) The schematic of the procedure for generating a graded coating of calcium phosphate on a nonwoven mat of electrospun nanofibers. Reproduced with permission from Ref. [75]. Copyright 2009, American Chemical Society. (B) The diagram of electrospinning apparatus depicting offset spinnerets and the fluorescent images of nHAP-PCL fibers (green) (a), transition region (b) and PEUUR2000 fibers (red) (c). Reproduced with permission from Ref. [77]. Copyright 2011, Acta Materialia Inc. (C) The 3D scaffolds produced by crochet using PCL/gelatin and PCL/gelatin/HAP microfibers. Micro‐CT scans of different sections of the scaffold and HAP particles content. Reproduced with permission from Ref. [80]. Copyright 2019, WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (D) The schematic of the fabrication of a hierarchically structured scaffold for tendon-to-bone repair. Reproduced with permission from Ref. [81]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. PCL, polycaprolactone; HAP, hydroxyapatite; nHAP, nanohydroxyapatite; PLGA, poly (lactic-co-glycolic acid).
Overall, there have been remarkable advancements in design and material organization strategies, with current multiphasic or mineral gradient scaffolds showing great potential in enhancing interface healing. As is known, the difference in mineral content between mineralized and unmineralized region is sharp and increases across the mineralized fibrocartilage towards bone. Therefore, the combination of multiphasic and gradient mineral scaffolds can better mimic the native enthesis. In addition, the current studies only mimic the enthesis at the micrometer scale. It is still a great challenge to recreate the structure and composition of the natural interface at the nanometer scale, such as mimicking the interaction between mineralized deposits and collagen fiber. Recently, Lausch et al. fabricated multiphasic collagen scaffolds using an in-well mineralization system, which would be useful for tendon-to-bone healing [82]. Within the mineralized layer, the mineralized collagen fibrils have intrafibrillar oriented mineral resembling bone at the nano scale. In the future, we can have a greater breakthrough in scaffold design, only with a comprehensive understanding of the structure on a smaller scale.
3.2.3. Topographical cues
Topographical cues such as the fiber orientation and pore size of scaffolds, are also are key parameters that influence the healing process. The niche-mimicking features of physical topographic cues have profound effects on cellular behavior and tissue formation [83]. For instance, our previous study indicated that the aligned nanofiber promoted tenogenic differentiation of mesenchymal stem cells (MSCs) in vitro and induced more tendon-like tissue in vivo, while the random nanofiber promoted osteogenic differentiation of MSCs in vitro and induced more bone formation in vivo [83]. Similarly, Qian et al. seeded BMSCs onto random collagen scaffold and aligned collagen scaffold. The aligned scaffold promoted tenogenic induction while the random scaffold promoted osteogenic induction [66]. Among a variety of topographical cues, the fiber alignment at the enthesis has been widely studied in the interface tissue engineering.
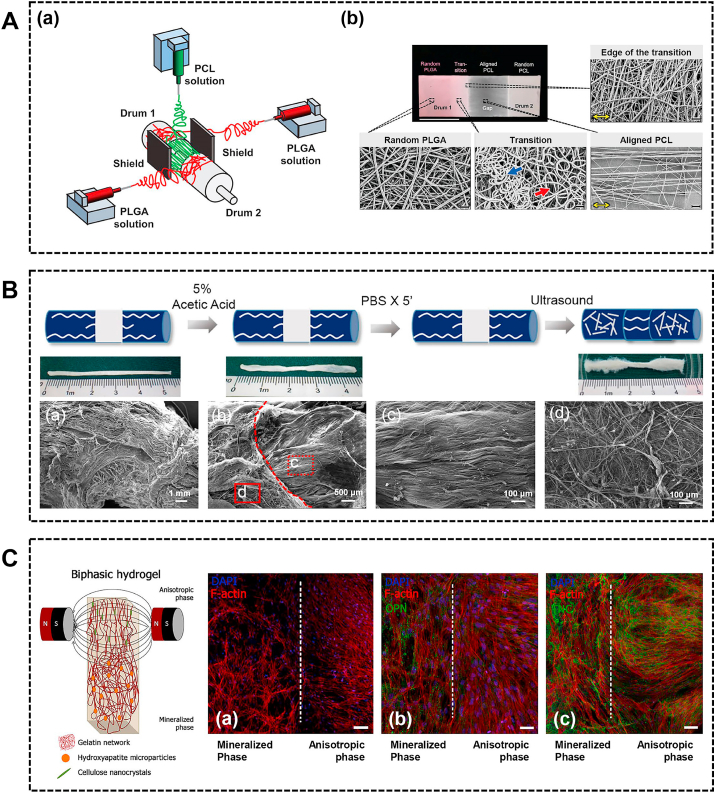
There are various methods of fabricating nanofibers. Electrospinning is the most popular method for fabricating nanofibers due to the ability to control the morphology, orientation, mechanical, electrical and chemical properties of the nanofibers, which could mimic the structure of the ECM [9,84]. Based on the graded fiber orientation from tendon to bone, several studies have designed aligned-to-random electrospun nanofiber scaffolds to mimic the change of collagen fiber orientation at the insertion site [[85], [86], [87]]. The aligned-to-random scaffold could direct the specific cell morphology and orientation along the scaffold. In addition to fabricating the scaffold from a single material, Samavedi et al. fabricated electrospun meshes by electrospinning two different polymers from offset spinnerets (Fig. 5A) [88]. The scaffold consists of a random PLGA region, a transition region, an aligned PCL region and a random PCL region (Fig. 5A). On the random PLGA region, BMSCs exhibited low aspect ratios and random orientations. On the aligned PCL region, BMSCs exhibited high aspect ratios and alignment. However, these studies did not apply the collagen fiber orientation-mimicking scaffold in vivo. Cai et al. fabricated a dual-layer aligned-random scaffold and investigated the effect of the scaffold in a rabbit extra-articular model [89]. The dual-layer scaffold facilitated fibrocartilage formation, improved collagen organization and maturation, and resulted in higher ultimate load-to-failure and stiffness compared to the random scaffold and the unwrapped control.
Fig. 5.
The topographical cues incorporated into the scaffold. (A) Three spinnerets were used to form two transition regions (a). The photograph and scanning electron microscopy micrographs of electrospun mesh comprising 4 regions: random PLGA, transition, aligned PCL, and random PCL (b). Reproduced with permission from Ref. [88]. Copyright 2014, Wiley Periodicals, Inc. (B) The preparation of random-aligned-random tendon extracellular matrix composite scaffold. The microstructure of the cross section of the random ends (a). Surface morphology of the transitional region (b) of the aligned (c) and random (d) portions. Reproduced with permission from Ref. [91]. Copyright 2017, Elsevier Ltd. (C) The schematic of developed gelatin-based hydrogels. The F-actin filaments (a), osteogenic differentiation-related marker OPN (b) and tendon tissue-related marker TNC (c) expression evaluation in each of the different phase. Reproduced with permission from Ref. [92]. Copyright 2019, American Chemical Society. PLGA, poly (lactide-co-glycolide); PCL, polycaprolactone.
In addition to electrospinning nanofiber, natural biomaterial was also able to be modified with specific topographical cues. For instance, Tellado et al. developed a biphasic silk fibroin scaffold with anisotropic at the tendon/ligament side and isotropic at the bone side using directional freezing, freeze-drying, and salt leaching [90]. The scaffold induced gene expression gradients of tendon/ligament and cartilage markers depending upon pore alignment along the scaffold. Besides, our previous study developed a random-aligned-random tendon ECM composite scaffold via ultrasound treatment for ligament-to-bone repair (Fig. 5B) [91]. BMSCs can spread along aligned collagen fibers with a spindle-shaped morphology, while it can spread unidirectionally on random fibers with a polygonal morphology. The random ECM promoted BMSCs differentiation into the chondrogenic and osteogenic lineage, unlike the aligned ECM. In a rabbit ACL reconstruction model, the random-aligned-random scaffold enhanced the bone and fibrocartilage formation.
To better mimic the physiology of the native enthesis, some studies have incorporated the biomimetic composition and topographical properties together into the composite. For example, Lin et al. fabricated an integrated random-aligned-random PCL nanofibrous scaffold, the random region of which was soaked in simulated body fluid to form a mineral layer [84]. The BMSCs displayed an up-regulation of tendon-specific markers in the aligned region and an up-regulation of bone-specific markers in the mineralized region [84]. Recently, Echave et al. fabricated a gelatin-based multiphasic hydrogel (Fig. 5C) [92]. For mineralized phase that mimics bone, HA particles were incorporated into hydrogel. For anisotropic phase that mimics tendon, cellulose nanocrystals were incorporated and exposed to magnetic fields to induce anisotropic structure. The randomly distributed cells were observed in the mineralized phase and the aligned cell growth was observed in anisotropic phase (Fig. 5C). Besides, the biphasic constructs could induce a distinct differentiation trend of ASCs as evidenced by a gradient of OPN and TNC deposition (Fig. 5C). Caliari et al. fabricated a biphasic collagen-glycosaminoglycan scaffold containing gradient mineralization and geometric anisotropy, which promoted spatially specific tenogenic and osteogenic differentiation of MSC within a single 3D scaffold [93]. Considering the graded extracellular matrix and collagen fiber alignment, Cong et al. fabricated multilayered co-electrospinning nanoscaffolds which contained aligned PCL-collagen I layer, nonaligned PCL-collagen II layer and nonaligned PCL-nHA layer to mimic tendon, fibrocartilage and bone layers respectively [94]. The enthesis-mimicking scaffold had more regularly arranged cells, more collagen organization, more fibrocartilage formation, higher tendon maturing score and better biomechanical performance in a rat massive rotator cuff tear model.
4. Growth factors as biomimetic strategy
In recent years, the application of growth factors to promote tendon/ligament-to-bone regeneration has attracted increasing attention. Growth factors are vital for cell proliferation, differentiation, recruitment and ECM synthesis during the healing process [10]. The enthesis healing process is a carefully-timed and organized event involving multiple factors [95]. To apply growth factors in tissue engineering, the most important challenge is the utilization of optimal factors and appropriate delivery vehicles that deliver factors to the repair site for a relevant period with suitable concentrations [16,30]. In this section, we emphasize the importance of utilizing the appropriate combination, concentration and location of growth factors for interface healing (Fig. 1).
4.1. The optimal combination of growth factors
Several individual growth factors have been examined independently for their capacity to facilitate tendon-to-bone healing. Table 2 summarizes several in vivo studies that applied growth factors for enthesis healing. However, single factor can not fully mimic the complex process of physiological events during the interface healing. To enhance the regeneration of tissues with heterogeneous cell types and matrix compositions, delivering multiple bioactive growth factors into the target region is essential [96]. For example, Kim et al. fabricated platelet-derived growth factor-BB (PDGF-BB)/bone morphogenetic protein-2 (BMP-2)-immobilized membrane to facilitate interface healing in a rat patellar tendon avulsion model [96]. The results demonstrated that delivery of single growth factor might be insufficient to reconstruct the tendon-to-bone interface with a multiphasic structure, whereas the delivery of both PDGF-BB and BMP-2 may provide a synergistic effect to generate a multiphasic structure similar to the native enthesis structure. Gene therapy can also be utilized to deliver combined growth factors and yield better results than single gene therapy. For instance, the BMSCs transfected with BMP-2 or basic fibroblast growth factor (bFGF) were injected into the tendon-to-bone interface in a rabbit ACL reconstruction model [97]. The implantation of BMSCs transfected with BMP-2 or bFGF induced better cellularity, new bone formation and greater mechanical properties. Furthermore, the co-transfection of these two genes induced the broadest zone resembling the native tendon-to-bone interface, the smallest bone tunnel area and the greatest mechanical strength, indicating the combination of these two growth factors exerted a much more potent effect than either single growth factor. Although the combination of multiple growth factors has shown a better potential to enhance enthesis healing, the optimal combination is still unknown, which should be further explored in the future.
Table 2.
The application of growth factors for tendon/ligament-to-bone repair in vivo.
| Animal model | Growth factor | Delivery | Dose | Release | Major results | Ref. |
|---|---|---|---|---|---|---|
| Rat supraspinatus tendon repair | TGF-β1 | Fibrin glue | 5, 10 ng/mL | High concentrations of TGF-β1 resulted in more collagen type III proportion, stronger load and stiffness. | [105] | |
| TGF-β1 | HA | 0.2 μg | The HA-TGF-β1 improved novel bone formation, collagen organization, fibrocartilage formation and load-to-failure force. | [106] | ||
| TGF-β3 | Heparin/fibrin-based gel | 100 ng | Gels without the heparin released 99% of TGF-β3 by day 10, whereas the gels with the heparin at ratios of 1:100, 1:1,000, and 1:10,000 released 88%, 74%, and 52%, respectively. | The TGF-β3 increased inflammation, cellularity, vascularity, cell proliferation, ultimate force and stiffness at early timepoint, and increased extracellular matrix remodeling, ultimate stress and modulus at late timepoint. | [107] | |
| TGF-β3 | Injectable calcium phosphate matrix | 2.75 μg | The TGF-β3 improved biomechanical strength at 4 weeks and resulted in an increased collagen I/III ratio. | [108] | ||
| BMP-7 | Gelatin hydrogel sheet | 500 ng | The BMP-7 released in a sustained manner over 3 weeks. | The gelatin hydrogel sheet with BMP-7 induced the favorable cartilage matrix production and tendon orientation and the highest tendon-to-bone maturing score and ultimate force-to-failure at 8 weeks. | [109] | |
| FGF-2 | PLGA membrane | 2 μg | The cumulative releasing percentage was 62.2% during 24 days. | The membrane loaded with FGF-2 improved fibrocartilage formation and collagen organization, and induced the highest ultimate load-to-failure, stiffness and ultimate stress. | [63] | |
| FGF-2 | Gelatin hydrogel sheet | 3, 30 μg | The FGF-2 released within 1 day up to about 30% and the remaining FGF-2 was persistently released for approximately 2 weeks. | The FGF-2 treatment resulted in histologic and biomechanical improvements at 6 and 12 weeks and ectopic calcification formation in some specimens from each group. | [110] | |
| FGF-2 | Fibrin sealant | 100 μg/kg | The FGF-2 treatment resulted in higher tendon-to-bone insertion maturing scores and mechanical strength than untreatment at 2 weeks and exhibited similar maturing scores and strength at 4 and 6 weeks. | [111] | ||
| PDGF-BB | Gelatin hydrogel sheets | 0.5 μg | The PDGF-BB impregnated hydrogel induced greater collagen fiber orientation, ultimate load to failure, stiffness, and ultimate load to stress at 12 weeks. | [112] | ||
| PDGF-BB | Type I collagen | 0.6, 2, 6 μg | The rhPDGF-BB enhanced cellular proliferation and angiogenesis in a dose-dependent response at 5 days, but had no effect on fibrocartilage formation, collagen fiber maturity and mechanical properties at 28 days. | [113] | ||
| Rat supraspinatus tendon chronic repair | FGF-2 | Gelatin hydrogel | 5 μg | The FGF-2 increased the mesenchymal progenitors and influenced genes expression at 2 weeks. The FGF-2 enhanced the formation of tough tendon-like tissues and tendon marker genes expression at 12 weeks. | [114] | |
| Rat infraspinatus tendon repair | PDGF-BB | PCL/Pluronic F127 membrane | PCL (123.01 ± 13.13 ng); PCL/Pluronic F127 (140.31 ± 8.68 ng), PCL/Pluronic F127/heparin (276.07 ± 5.49 ng) | The heparin-bound membrane showed a moderate initial burst release of PDGF-BB and then sustainably released up to ~90% of the initial loading over 42 days. | The PDGF-BB-immobilized membrane group showed greater regeneration of rotator cuff tendon in histological and biomechanical analyses compared with the suturing groups and membrane without PDGF-BB immobilization group. | [115] |
| Sheep infraspinatus tendon repair | PDGF-BB | Type I collagen matrix | 75, 150, 500 μg | The 75-μg and 150-μg rhPDGF-BB groups showed higher ultimate load to failure and increased tendon-to-bone interdigitation compared with suture only group and 500-μg rhPDGF-BB group. | [116] | |
| Rabbit infraspinatus tendon repair | BMP-2 | β-tricalcium phosphate | 10 μg | The rhBMP-2 treatment resulted in a more abundant organized fibrocartilage and improved biomechanical properties at 4 weeks. | [117] | |
| Rabbit ACL reconstruction | BMP | Injected calcium phosphate Cement; Injected fibrin sealant |
4 mg BMP per 2.5 g calcium phosphate cement, 2 mg BMP per 0.5 ml injected fibrin sealant | The calcium phosphate cement composite improved the new bone formation gradually during the whole process, while the fibrin sealant composite had a burst effect on enhancing healing at 2 and 6 weeks. | [69] | |
| BMP-2 | Injectable calcium phosphate matrix | 11.5, 50, 115 μg | The rhBMP-2 had positive dose-dependent effect on osteointegration. The rhBMP-2 increased bone formation and decrease tunnel diameters with increasing time, and increased stiffness at 8 weeks. | [118] | ||
| FGF-1 | Collagen solution | 1, 4 μg | Both treatment groups formed Sharpey-like fibers at 8 weeks and fibrocartilage transition zone at 12 weeks and showed higher elastic modulus and stiffness at 8 and 12 weeks. | [119] | ||
| Rabbit patellar tendon repair | BMP-2 | Fibrin glue, collagen gel | 1 μg | The BMP-2 improved more abundant new bone, mature bone and organized fibrocartilage formation at 4 and 8 weeks. | [120] | |
| Rabbit extra-articular bone tunnel model | BMP-2 | Collagen gel | 10 μg | The release of rhBMP-2 was maintained for over 28 days. | The rhBMP-2 induced new bone and fibrocartilage formation at the interface and higher ultimate failure load at 3 and 6 weeks. | [121] |
| Rabbit Achilles tendon repair | TGF-β3/BMP-2 | Fibrin glue | 10 ng | The BMP-2 accelerated tendon-bone healing and improved the histological and biomechanical properties. The TGF-β3 did not significantly improve the biomechanical properties. | [122] |
Since delivery of multiple growth factors yields much better repair effects than single growth factor, platelet-rich plasma (PRP) as a rich storage vehicle of several growth factors, including platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor-β (TGF-β), fibroblast growth factor and insulin growth factor, may also address the limitations of single factor therapy such as inducing only one biological aspect of interface healing [[98], [99], [100]]. Moreover, PRP also offers many other benefits such as being safe, inexpensive, easy to extract and simple to process, compared to isolating purified individual growth factors [101]. However, the beneficial role of PRP remains controversial. In vivo, lots of studies showed that PRP could enhance the tendon-to-bone healing at early process but failed to improve long-term healing [[102], [103], [104]]. In the clinic, Cai et al. carried out a meta-analysis to investigate the efficacy of PRP therapy in arthroscopic rotator cuff repair [100]. The study found that the PRP therapy would not improve the clinical outcome scores in full-thickness rotator cuff repairs but significantly decreased the failure-to-heal rate in small-to-moderately sized tears, suggesting that PRP therapy may improve tendon-to-bone healing in patients with small or moderate rotator cuff tears.
TGF-β1/β3, transforming growth factor-β1/β3; BMP-2/7, bone morphogenetic protein-2/7; rhBMP-2, recombinant human bone morphogenetic protein-2; PDGF-BB, platelet-derived growth factor-BB; rhPDGF-BB, recombinant human platelet-derived growth factor-BB; FGF-1/2, fibroblasts growth factor-1/2; ACL, anterior cruciate ligament; HA, hydroxyapatite; PLGA, poly(lactic-co-glycolic acid); PCL, polycaprolactone.
4.2. The optimal concentration of growth factors
In a large number of studies, varying doses of different growth factors were delivered to repair sites (Table 2). Among these investigations, several studies have demonstrated that the growth factors could enhance tendon/ligament-to-bone healing in a dose-dependent manner. For example, Ma et al. reported a rabbit ACL reconstruction with injectable calcium phosphate matrix-recombinant human BMP-2 (rhBMP-2) and found that rhBMP-2 induced new bone formation at the tendon-bone interface in a dose-dependent manner [118]. Zhang et al. investigated the efficacy of 5 ng/ml versus 10 ng/ml TGF-β1 on tendon-to-bone reconstruction in a rat rotator cuff tears model [105]. The 10 ng/ml treatment exerted positive effects with more collagen type III formation and better biomechanical properties on tendon healing compared with 5 ng/ml and no treatment. Although these growth factors showed positive dose-dependent effects, an overhigh concentration of growth factors may exert adverse effects on the healing process, and the optimal concentration should be in the appropriate range. For instance, the 75 μg and 150 μg of recombinant human PDGF-BB (rhPDGF-BB) combined with collagen matrix significantly increased the mechanical properties and improved the morphological appearance compared with high-dose (500 μg) rhPDGF-BB treatment in a rotator cuff repair model [116]. Another study reported that excessive activation of TGF-β in bone after partial injury to the Achilles tendon contributes to pathogenesis of enthesopathy [123]. However, if the concentration of the delivered growth factor is too low, it would not be able to promote repair effectively. In a rat rotator cuff repair model, rhPDGF-BB delivered by collagen scaffold enhanced cellular proliferation and angiogenesis in a dose-dependent manner during the early stage, but the treatment did not induce a more structurally organized or stronger attachment site during the late stage [113]. This might be attributed to insufficient doses and inadequate temporal availability of rhPDGF-BB. Hence, there is requirement for an effective delivery vehicle that is able to retain growth factors, as well as exhibit continued controlled release to mimic the physiological growth factors release of natural ECM.
Currently, growth factors can be delivered by injectable vehicles (fibrin glue, collagen gel, hydrogel, calcium phosphate matrix) or implanted nanofibers for tendon-bone healing. In addition to delivering growth factors by injection, synthetic nanofiber scaffolds with appropriate degradation properties could also be utilized for growth factor delivery. Some studies have encapsulated growth factors within electrospun nanofibers to achieve the continuous release of growth factors. Besides, several studies have applied heparin to improve the efficiency of delivery systems, which can protect their biological activity and slow their diffusion from the matrix [124]. For example, Kim et al. immobilized PDGF-BB/BMP-2 on PCL/Pluronic F127 membrane via heparin-mediated binding, which exhibited continuous release of over 80% of the initial loading amount after 5 weeks without a notable initial burst [96]. Lee et al. fabricated poly(l-lactide-co-ϵ-caprolactone) (PLCL) scaffold containing fibrocartilage and ligament regions, and delivered BMP-2 to the fibrocartilage region using heparin-based hydrogel [125]. The heparin-based hydrogel and the hydrogel-containing PLCL scaffold released BMP-2 up to 60 days without an initial burst. In addition to the important role of sustained release, delivering growth factors with appropriate dosage at the proper time should be considered. Previous studies have demonstrated that different growth factors exhibit unique functions at specific stages during the healing process [[126], [127], [128]]. Therefore, stimuli-responsive polymers can be incorporated into the scaffold or combining different delivery systems to achieve programmed release. For example, Silva et al. incorporated magnetic nanoparticles in a hydrogel that can be further manipulated by an external magnetic field, which could modulate the release of platelet lysate-derived growth factors [129].
Because the effects of growth factors were dose- and time-dependent, gene therapy based on stem cells has been introduced to achieve the continuous and stable concentration of these factors at the interface. Table 3 summarizes the main results of in vivo studies applying gene therapy for enthesis healing. Overall, gene therapy based on stem cells exerts a strong, positive, continuous and stable expression of growth factors that effectively promote tendon-bone healing. As the proliferation and differentiation of transfected stem cells, the efficacy of the growth factors secretion could be enhanced [14]. Even though gene therapy ensures a steady and continuous delivery of growth factors, a decrease of growth factors with time may lead to a loss of the desired therapeutic effects after several weeks [14]. Furthermore, the safety of gene therapy in vivo is not guaranteed due to mutagenesis, development of malignancy and other uncontrollable side effects [14,99,130].
Table 3.
The application of gene therapy for tendon/ligament-to-bone repair in vivo.
| Animal model | Cell | Vehicle | Major results | Ref. | |
|---|---|---|---|---|---|
| TGF-β | Rabbit ACL reconstruction | BMSCs | Injection | The over-expression group exhibited tighter tendon-bone interface, increased number of chondrocyte-like cells and fibrochondrocytes, more collagen fibers, better biomechanical properties and greater bone formation than the inhibition, empty vector and untreated groups. | [131] |
| TGF-β3, BMP-2 | Rabbit ACL reconstruction | BMSCs | Genes-immobilized triphasic silk scaffold | The gene-modified silk scaffold seeded with BMSCs induced approximately complete osseointegration as a result of multilayered tissue formation and robust mechanics as early as 12 weeks. | [132] |
| BMP-2 | Rat supraspinatus tendon repair | ASCs | Aligned nanofibrous PLGA scaffold | The transduced cell group showed evident bone loss at 28 days, decreased strength and modulus at 28 and 56 days. | [64] |
| Rat ACL reconstruction | ACL-derived CD34+ cells | Cell sheet | The cells transduced with BMP-2 had the smallest cross-sectional areas of bone tunnels. Tensile strength was highest in the cells transduced with BMP-2 (100%) group at 4 weeks, and in cells transduced with BMP-2 (25%) group at 8 weeks. Graft-bone integration occurred most rapidly in the cells transduced with BMP-2 (25%) group. | [133] | |
| Rabbit ACL reconstruction | BMSCs | Gastrocnemius tendons wrapped by BMSCs | The infected BMSCs with BMP-2 virus or control virus groups exhibited better biomechanical properties, and an increased perpendicular collagen fibers formation. The cartilage-like cells proliferation and the fibrocartilage-like tissue formation were highest in the infected BMSCs with BMP-2 virus group. | [134] | |
| Rabbit ACL reconstruction | Normal rat kidney cells | Fibrin scaffold | The transfected cell group showed no significant difference of bone mineral density, better contact between tendon and bone, higher failure load and maximal graft tension, more bone tissue and less fibrous tissue, enhanced new vessel formation, cell activity, and remodeling compared with the control group. | [135] | |
| BMP-12 | Rabbit supraspinatus tendon repair | BMSCs | PLGA scaffold | The BMP-12-overexpressing promoted the tendon-to-bone healing, improved collagen fiber organization and fibrocartilage formation at the interface. | [136] |
| BMP-13 | Rat supraspinatus tendon repair | BMSCs | Fibrin glue | The transduced cell group showed no differences in the amount of new cartilage formation, collagen fiber organization, and biomechanical strength compared with untransduced cell group. | [137] |
| FGF-2 | Rabbit extra-articular model | Human amniotic mesenchymal stem cells | Human acellular amniotic membrane | The scaffold loaded with transfected cell group had the narrowest bone tunnel, higher macroscopic and histological scores, the best mechanical strength. | [138] |
TGF-β, transforming growth factor-β; TGF-β3, transforming growth factor-β3; BMP-2/12/13, bone morphogenetic protein-2/12/13; FGF-2, fibroblasts growth factor-2; ACL, anterior cruciate ligament; BMSCs, bone marrow-derived stem cells; PLGA, poly(lactic-co-glycolic acid).
4.3. The optimal location of growth factors
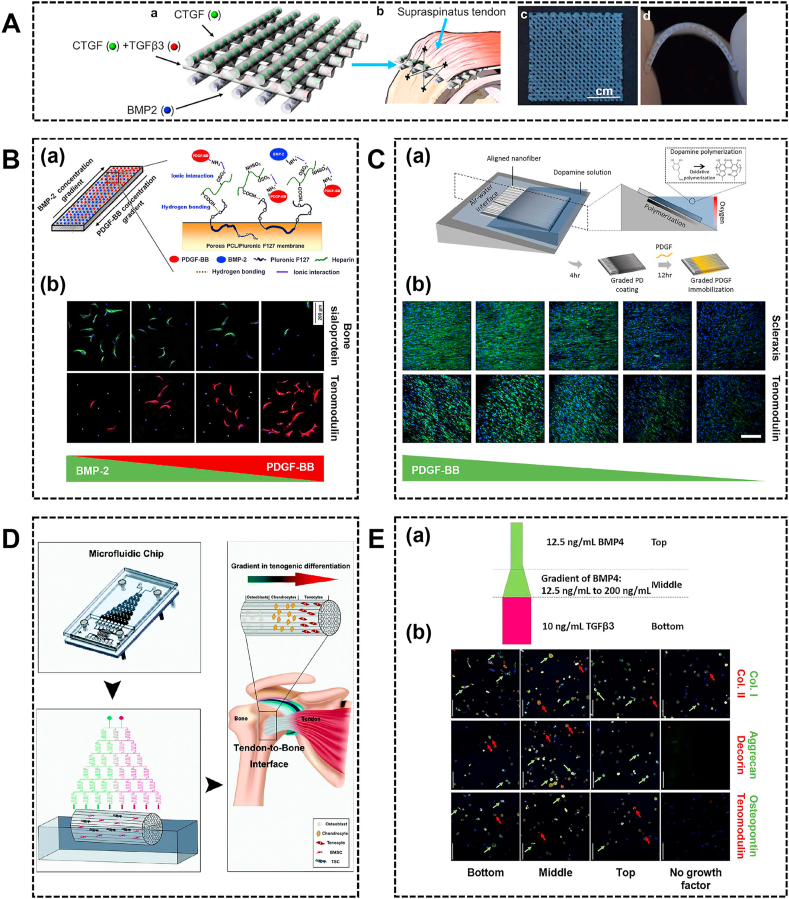
The natural enthesis exhibits different growth factors requirements in each tissue region. To mimic the native enthesis and induce stem cells to differentiate into specific target cells, the proper spatial distribution of growth factors along the scaffold is essential for interface region regeneration. In recent years, scaffolds presenting graded growth factors have been developed. For example, Harris et al. engineered a bi-layered alginate-fibrin scaffold mimicking the ligament-to-bone interface [139]. The alginate region was maintained in medium supplemented with TGF-β3, while the fibrin region was maintained in medium supplemented with connective tissue growth factor (CTGF). The alginate region induced cartilaginous phenotype with increased GAG and collagen type II staining, and the fibrin region induced ligamentous phenotype with more intense collagen type I staining in the ligamentous portion. Recently, Tarafder et al. fabricated a three-layered scaffold by 3D printing for spatiotemporal delivery of multiple growth factors to the tendon-to-bone interface (Fig. 6A) [140]. In the 3D scaffold, CTGF and BMP-2 were separately encapsulated within PLGA microspheres and respectively embedded in PCL microstrands at the top and bottom layer to facilitate tendon and bone formation, while both CTGF and TGF-β3 encapsulated within PLGA microspheres were embedded in PCL on the middle layer to promote fibrocartilage formation (Fig. 6A). The spatially delivered growth factors promoted the recruitment of endogenous tendon progenitor cells, gradient fibrocartilaginous matrix and densely-aligned tendon collagen fibers formation at the tendon-to-bone insertion site, and induced more bone volume and higher tensile maximum load and stiffness.
Fig. 6.
The region-specific growth factors incorporated into the scaffold. (A) Design of 3D-printed scaffolds with spatiotemporal delivery of CTGF, TGFβ3 and BMP2 (a) and implantation site (b). The 3D-printed, growth factors embedded three-layered PCL scaffolds were prepared as sheets (c) with high flexibility to fit to anatomical contour of humeral heads (d). Reproduced with permission from Ref. [140]. Copyright 2019, IOP Publishing Ltd. (B) The schematic of the formation of PCL/Pluronic F127 membrane with reverse gradients of PDGF-BB and BMP-2 (a). The immunohistochemical images for tenomodulin (red) and bone sialoprotein (green) of ASCs cultured on the membrane with reverse PDGF-BB and BMP-2 concentration gradients (b). Reproduced with permission from Ref. [142]. Copyright 2013, Acta Materialia Inc. (C) The polydopamine gradient on substrate by spatially controlling oxygen availability could be used as template for graded immobilization of PDGF (a). The immunofluorescence images for scleraxis and tenomodulin of ASCs on PDGF gradient aligned nanofiber (b). Reproduced with permission from Ref. [143]. Copyright 2018, Elsevier Ltd. (D) The generation of a concentration gradient of osteogenic induction medium to promote the cultured stem cells in the scaffold differentiation into different cell types to mimic the tendon-to-bone interface. Reproduced with permission from Ref. [144]. Copyright 2020, The Royal Society of Chemistry. (E) The schematic of growth factor layout (a). The immunofluorescence images for aggrecan (green) and decorin (red), collagen I (green) and collagen II (red), and osteopontin (green) and tenomodulin (red) (b). Reproduced with permission from Ref. [145]. Copyright 2020, IOP Publishing Ltd. PCL, polycaprolactone; ASCs, adipose-derived stem cells.
Gene transfer strategy is also able to induce specific stem cell phenotypes. Sun et al. developed a chondroitin sulfate-hyaluronate acid-silk fibroin hybrid scaffold with each end being modified with lentiviral-transfected TGF-β3 gene, which could induce the chondrogenic differentiation of MSCs [141]. Fan et al. developed a transgenic vector-immobilized triphasic silk scaffold, which contained ligament, fibrocartilage and bone tissue regions [132]. The fibrocartilage and bone tissue regions were immobilized with customized lentiviral vectors encoding TGF-β3 and BMP-2 respectively. The scaffold had the ability to induce the seeded BMSCs to differentiate into the chondrogenic and osteogenic lineages in vitro and in vivo.
Instead of delivering the different growth factors in each phase, incorporated growth factors within the scaffold could be distributed in a gradient manner. For example, Min et al. developed reverse PDGF-BB and BMP-2 concentration gradients on porous membrane using a diffusion method and investigated the differentiation of ASCs on the membrane (Fig. 6B) [142]. The higher PDGF-BB and lower BMP-2 concentrations could better promote tenogenic differentiation of ASCs, while higher BMP-2 and lower PDGF-BB concentrations could better promote osteogenic differentiation of ASCs (Fig. 6B). In another method, PDGF-BB was immobilized on an aligned nanofiber in a gradient manner by controlling oxidative polymerization of dopamine, which can spatially control tenogenic differentiation of ASCs (Fig. 6C) [143]. However, these studies only incorporated one or two growth factors that might not function the optimal regulatory effect of multiple growth factors. Recently, Lyu et al. modified decellularized tendon with gradient concentration of the osteogenic induction medium containing multiple essential growth factors across the scaffold as controlled by the microfluidic chip (Fig. 6D) [144]. In an achilles tendon model, the seeded BMSCs and TDSCs performed differentiation into desired cell types in response to the concentration gradient and presented a similar three-layer structure to normal tendon-to-bone interface at 8 weeks. In another study, a multi-phasic hydrogel construct that combined a gradient of BMP-4 and a region of TGF-β3 was developed (Fig. 6E) [145]. The top region was cast with BMP-4 which induced MSCs towards osteogenesis tendency, the middle region was cast with an increasing concentration of BMP-4 which induced the combination of chondrogenic, osteogenic, and tenogenic marker expression, and the bottom was cast with TGF-β3. Altogether, region-specific growth factor concentrations are necessary to induce cell differentiation for tendon/ligament-to-bone healing.
Collectively, abundant investigations have confirmed the capacity of growth factors to enhance enthesis healing. In the future, it is highly desirable to fabricate more sophisticated systems incorporated with multiple growth factors, with optimal doses to be delivered to the repair sites at specific stages controllably. In addition, enthesis healing requires varying biophysical, biochemical and biological cues to achieve functional tissue regeneration. Thus, the enthesis-mimicking scaffold should incorporate multiple cues. For example, Madhurakkat Perikamana et al. fabricated a bone-patella tendon-bone mimicking graft, in which the gradient PDGF was immobilized on a continuous random-aligned-random oriented nanofiber and both ends of the nanofiber were mineralized [143]. Tellado et al. designed a biphasic silk scaffold with integrated anisotropic and isotropic pore alignment as described above and they further loaded TGF-β2 and GDF5 on the scaffold [146]. The combined effect of chemical (growth factors) and biophysical (pore alignment) cues could enhance tenogenic/ligamentogenic, fibrochondogenic, or chondrogenic differentiation pathways in the different zones of the scaffold. Future studies should consider combining multiple biomimetic cues at the same time to develop an all-in-one system.
5. Stem cells as biomimetic strategy
Cells play a pivotal role in the regeneration, composition and homeostasis of the graded interface tissue [25]. Various stem cell types have shown promising prospects and have drawn increasing attention to enthesis healing. In this section, we will describe the different stem cell sources from widely used BMSCs and ASCs to tendon/ligament/synovium/periosteum-derived stem/progenitor cells, followed by the culture systems to mimic the native cell populations (Fig. 1).
5.1. Stem cell sources
The generally used cell types of enthesis tissue engineering include terminally differentiated cells and MSCs. Instead of differentiated cells, MSCs are more suitable than differentiated cells for interface tissue engineering owing to their nonimmunogenicity, ease of isolation, high responsiveness to distinct environmental cues and capacity for self-renewal and differentiation [147]. For example, Liu et al. compared the cellular responses of rabbit ACL fibroblasts and BMSCs on combined silk scaffolds, and observed that BMSCs displayed better cell proliferation, GAG excretion, gene and protein expression of ligament-related ECM markers and in vivo survivability than ACL fibroblasts [148]. Compared to the local injection of chondrocytes versus MSCs in the Achilles tendon, a new enthesis was clearly produced in both groups, but only the MSC-injected rats exhibited uniform collagen II distribution at the tendon-bone junction and an organized enthesis with columnar chondrocytes [149].
For enthesis healing, the most widely utilized stem cells are BMSCs and ASCs, which could be differentiated into almost every connective tissue lineage, including bone, cartilage and tendon [150]. Compared to BMSCs, ASCs can be readily isolated from an abundant autologous cell source from adipose tissues with relatively lower donor site morbidity and pain. Besides, ASCs display higher in vitro proliferative capacity, lower senescence and higher capacity for generating tendon, bone and cartilage-like tissues [[151], [152], [153], [154]]. Although MSCs of different origins share similar biological potential, MSCs may display some tissue-specific properties and hence functional differences, which indicate that certain MSC populations are better than others for specific tissue regeneration [147,155]. Apart from widely-used BMSCs and ASCs, MSCs isolated from other tissues, such as tendon, ligament, synovium and periosteum, have shown the potential to enhance enthesis healing.
TDSCs as a cell source have shown much potential for interface tissue engineering. Compared with BMSCs, TDSCs exhibit higher clonogenicity and proliferative capacity, as well as display higher expression of osteogenic, chondrogenic, tenogenic and adipogenic markers and pose higher differentiation potential to these lineages, thus making it a much better alternative cell source than BMSCs for musculoskeletal tissue repair [147]. In a rat ACL reconstruction model, the tendon graft wrapped with TDSCs sheet induced better graft osteointegration, higher intra articular graft integrity, and better biomechanical properties during the early stages [156].
Additionally, ACL-derived cells isolated at the site of ACL rupture have shown to be capable of differentiating into multiple lineages in vitro including osteogenic, chondrogenic, adipogenic and endothelial cell lineages [157]. Upon assessing the efficacy of transplanted ACL-derived CD34+ cells in an ACL reconstruction model, it was found that ACL-derived CD34+ promoted greater collagen fiber formation, enhanced angiogenesis and osteogenesis, increased perigraft bone mass and exhibited the greatest failure load of tensile [158]. Additionally, ACL-derived CD34+ cell sheet wrapped grafts was further developed to deliver cell more efficiently, which increased proprioceptive recovery, graft maturation and biomechanical strength in a rat ACL reconstruction model [159]. Recently, our study incorporated ligament stem/progenitor cell sheet within knitted silk-collagen sponge scaffold [67]. The scaffold with cell sheet resulted in higher ligament repair markers expression, better collagen fibril formation, good integration of bone and ligament, and no obvious cartilage and meniscus degeneration in a rabbit ACL reconstruction model.
It has been reported that MSCs derived from the synovium have a higher proliferation and differentiation potential than other mesenchymal tissue-derived MSCs from both human and rat sources [160]. Furthermore, the efficacy of the synovial MSCs on tendon-to-bone healing was investigated [160]. The implantation of synovial MSCs increased collagen fibers production at 1 week, and formed more oblique collagen fibers connecting the bone to tendon, which resembled Sharpey's fibers at 2 weeks, but the effect was not observed at 4 weeks, indicating the potential to promote early tendon-to-bone healing.
In a few studies, the periosteum was selected as a source of multipotent cells for tendon-to-bone regeneration. There is an outer fibrous layer and an inner cambium layer, while the cambium layer consists of chondroprogenitor and osteoprogenitor cells that can differentiate into cartilage and bone [161]. Chang et al. evaluated periosteal progenitor cell sheets for promoting tendon-to-bone healing in a rabbit extra-articular bone tunnel model [161]. The periosteal progenitor cell sheets promoted collagen fiber and fibrocartilage formation at the tendon-bone junction. In another study, Chen et al. developed injectable hydrogel seeded with periosteal progenitor cells and incorporated with BMP-2 to enhance tendon-bone healing [162].
5.2. Culture systems
Since the interfacial regions typically have a mix of different cell types, specific cell populations need to be incorporated into the scaffold to reestablish normal formation, homeostasis and insertion sites repair effectively [5]. Tissue engineering strategies have developed different culture systems to mimic the native cell populations including co-culture of differentiated cells, culture of stem cells alone and co-culture of differentiated cells with stem cells.
5.2.1. Coculture of differentiated cells
Several studies have co-cultured relevant differentiated cells including fibroblasts, osteoblasts, chondrocytes within different regions of the scaffolds to mimic the native cell phenotype and investigated the interaction between these different cell types [[163], [164], [165], [166]]. To determine the effects of heterotypic interactions on cell phenotype, Wang et al. used a co-culture fibroblast and osteoblast model [164]. Co-culture of fibroblasts and osteoblasts leads to changes in their respective phenotypes as well as the increased expression of interface-relevant markers. The study suggested that the osteoblast-fibroblast interactions may lead to transdifferentiation and initiate fibrocartilage formation. Spalazzi et al. performed a in vivo study using tri-cultured fibroblasts, chondrocytes, and osteoblasts on triphasic scaffold and found phase-specific matrix deposition with distinct mineral and fibrocartilage-like tissue formation [165]. Taken together, coculture of differentiated cells could simply mimic the native cell populations. However, the lower regeneration ability of mature fibroblasts, chondrocytes and osteoblasts has limited the application. Besides, isolating mature fibroblasts, chondrocytes, and osteoblasts from separate sites involves complicated procedures and considerable cost and pain. Therefore, applying coculture of differentiated cells exhibited limited clinical translation potential for interface regeneration.
5.2.2. Monoculture of stem cells
An enormous amount of research cultured stem cells alone on the scaffold. Controlling cellular response is the critical strategy to ensure the stem cell efficiency for enthesis regeneration. However, this strategy should explore the appropriate biophysical or biochemical cues to induce MSCs differentiation. For instance, a study reported that the addition of BMSCs for rotator cuff repair could not improve the composition, structure, or biomechanical strength, which might due to the lack of necessary molecular or cellular signals to induce appropriate differentiation of MSCs [167]. In interface tissue engineering, a variety of biomimetic signals have shown the ability to promote the stem cells towards differentiation into targeted cells.
The biophysical properties, such as surface topography and mechanical stimulation, play a vital role in regulating stem cell fate [168]. Among various biophysical properties, the surface topography, especially the fiber orientation, was evaluated extensively in interface tissue engineering. As discussed above, it is evident that the aligned fiber could promote tenogenic differentiation of MSCs, while the random fiber could promote osteogenic differentiation of MSCs. Besides, stem cells have also been shown to be highly mechanosensitive and mechanical stimulation is able to induce or enhance stem cells differentiation into a wide variety of tissue specific cells including tenogenesis and osteogenesis [169]. However, few studies have applied mechanical stimulation to induce stem cell differentiation for interface healing. Liu et al. fabricated a decellularized tendon-fibrocartilage-bone composite scaffold seeded with BMSCs sheets, which was subjected to mechanical stimulation for up to 7 days [170]. The mechanical stimulation significantly upregulated early tenogenesis maker scleraxis gene expression [170]. Recently, Grier et al. applied cyclic tensile strain to the collagen-glycosaminoglycan scaffold [171]. The mineral compartment on its own has shown to robustly promote MSCs osteogenic differentiation, the cyclic tensile strain had limited effects on the osteogenic capacity of MSCs in the mineralized region of the scaffold, while cyclic tensile strain promoted significant upregulation of tenogenic markers and cellular metabolic activity in the anisotropic nonmineral region. In the future, the precise control of mechanical stimulation to direct stem cell differentiation for interface healing is promising.
The biochemical properties, such as the characteristics of the material itself, small molecules, growth factors, oxygen concentration, could also modulate stem cell fate and induce differentiation. In terms of material itself, mineral gradient scaffolds have the ability to induce graded osteogenic differentiation due to the release of calcium and phosphate ions in aqueous environments [[78], [79]]. In addition, growth factors are often used as inducers of differentiation. As discussed above, the spatial distribution of growth factors through immobilization or encapsulation along the scaffold has demonstrated good ability to induce stem cell differentiation toward different phenotypes in a single system for interface repair. Besides, oxygen tension in the culture environment is also an important regulator determining stem cells differentiation [172]. For example, hypoxia promotes the chondrogenic differentiation of MSCs, while hypoxia promotes osteogenic differentiation with less than 3 h preconditioning and inhibits osteogenic differentiation with more than 4 h preconditioning [173]. Soft-to-hard tissue interfaces present a gradual transition in oxygen concentrations [174]. Thus, the spatial control of oxygen concentration in a single structure could enable control over stem cell differentiation toward different lineages for interface healing.
5.2.3. Co-culture of stem cells with differentiated cells
Instead of co-cultured differentiated cells or culture of stem cells alone, co-culture of stem cells with differentiated cells is possible to mimic the tendon-to-bone insertion and promote the stem cell differentiation. To investigate the influence of cell interaction on BMSCs differentiation, He et al. seeded fibroblasts, BMSCs and osteoblasts on hybrid silk scaffolds, with the osteoblast section being coated with HA [175]. In this tri-culture system, osteoblasts and fibroblasts maintained their cell lineage phenotype, while BMSCs differentiated towards the fibrocartilage lineage. In another study, Wang et al. investigated the effects of co-culture with fibroblasts and osteoblasts on either ACL fibroblasts or BMSCs response [176]. In the tri-culture model, fibroblasts and osteoblasts were seeded on each side, while BMSCs or fibroblasts were seeded in the middle. BMSCs exhibited greater fibrochondrogenic potential than ACL fibroblasts. Besides, the effects of fibroblast-osteoblast physical contact and paracrine interactions were investigated. The paracrine factors from different conditioned media model regulated BMSCs response, and fibroblast-osteoblast contact promoted proteoglycan and TGF-β1 production and SOX9 upregulation in BMSCs indicating the fibrochondrogenic differentiation. This study demonstrated that fibroblast-osteoblast interactions play a vital role in BMSC differentiation, which are contributed to both paracrine factors and cell physical contact [176]. However, these studies only investigated the cell interactions in vitro without the application in vivo. Recently, Cao et al. developed a 3D printed biomimetic multiphasic PCL/TCP-PCL/TCP porous scaffold [177]. Fibroblasts, BMSCs and osteoblasts were separately encapsulated in gelatin methacrylate and seeded on PCL phase, intermediate phase and TCP phase respectively. After subcutaneous implantation in mice, the gene expression of tenogenic, chondrogenic and osteogenic markers showed an overall increasing pattern during 8 weeks. Particularly, the collagen type X increased drastically indicating regenerative potential of mineralized fibrocartilage. However, the scaffold was implanted subcutaneously but not evaluated in an enthesis repair model. Altogether, these investigations suggested that cellular communication plays a key role in influencing stem cells fate and co-culture of stem cells with differentiated cells has the potential to promote fibrocartilage formation.
6. Conclusions and perspectives
During the past decades, the regenerative strategies including the application of biomaterials, growth factors and stem cells have shown the capacity to promote tendon/ligament-to-bone healing. Successful biomimetic strategy for enthesis tissue engineering requires a complete understanding of the structure and composition of native enthesis. However, the structure-function relationship and enthesis healing process are not completely understood at present. It's still a long way to find the structural correlations between cells, ECM and mineral at the nano-scale. Recently, researchers have decoded the nano-structure of the bone [178]. Similarly, there will be a demand for more high-definition description of the tendon/ligament-to-bone insertion site in the near future, which will undoubtedly greatly promote the advance of biomimetic strategy for enthesis tissue engineering.
Advances in manufacturing techniques enable the development of scaffolds with specific regional cues to effectively mimic the microenvironment of native enthesis. Currently, well-designed scaffolds have the ability to enhance the interface healing, from inducing organized collagen fibers to new bone formation. Despite the positive achievements of interface tissue engineering, current biomimetic scaffolds could not fully regenerate the functional interface with similar mechanical properties to native tissue. This might be due to most of the scaffolds mimicking native enthesis at the micro-scale, while it remains challenging to recreate functionally native enthesis at the nano-scale.
Multiple growth factors participate in the complexity of enthesis regeneration process at different time points. Although the growth factors-based strategy has shown the capacity to improve tendon/ligament-to-bone healing in animal models, the optimal delivery combination, dosage, distribution and timing of multiple growth factors that interact with the tissue during the healing process are still unknown. In the future, a more comprehensive understanding of the complex mechanisms between the signal molecules and physiology could provide guidance to precisely mimic the native environment for effective treatment. Furthermore, to achieve accurate spatiotemporal control of growth factors delivery for enthesis repair, there is a pressing need to develop more sophisticated delivery systems based on the functional materials and advanced fabrication.
Stem cell transplantation has become a promising approach and acts a pivotal role in tissue engineering due to their capacity for differentiation into the targeted lineage and for improving the microenvironment at the repair sites. The appropriate signals regulate the stem cells to differentiate into targeted cells are critically important. The various signals, such as scaffold composition, structural topographies, chemical cues, have shown the capacity to induce stem cell differentiation, achieving graded cell phenotype, ensuring region-specific ECM deposition, and supporting tissue regeneration. The ideal microenvironment requires a combination of various cues to mimic the ECM as close as possible to guide stem cell differentiation more efficiently. To regenerate the functional interface, the precise cell subpopulations in native enthesis and interface regeneration process need to be identified. However, there have yet been no studies on enthesis cell sub-populations to date. In recent years, single-cell RNA sequencing has become an established and powerful method to reveal new cell types, study developmental processes and investigate transcriptomic cell-to-cell variation [179]. In the future, it is possible to dissect cell subpopulations and find the most suitable cell population for enthesis regeneration via single-cell RNA sequencing, achieving enthesis regeneration more safely and efficiently.
The damaged interface lacks the sufficient endogenous signal cues to regenerate a functional tissue, thus the addition of exogenous biomimetic components to stimulate the body's endogenous repair is necessary. To supply sufficient signals and achieve precise recapitulation, the biomimetic strategy should integrate the biomaterial-, growth factor- and stem cell-based strategies to develop an all-in-one system. In this ideal system, the interplay between scaffolds, growth factors and cells are rather critical to create a biomimetic environment for the regeneration of functional tissues. In recent years, 3D printing has attracted much interest and may provide a powerful strategy for producing graded scaffolds, and enable incorporation of multiple cellular, biophysical, biochemical, biological gradients into scaffolds with high resolution, which will recapitulate the heterogeneous microenvironment of native enthesis with high spatiotemporal control.
In addition, understanding the interactions between biomimetic systems and biological systems is also important for interface tissue engineering. Although the biomimetic components have presented excellent performance, most of the current studies focused on the repair outcomes in animal models and lacks the in-depth mechanism research, such as the cell tracking of stem cells, the involved signal pathway, the cell interaction and the critical role of immune cells. Future studies should pay more attention on exploring how the biomimetic components interact with enthesis regeneration to clarify the potential mechanism.
Although these biomimetic strategies are advanced, translating these innovations into clinical practice is of great challenge. For clinical translation, the assurance of safety and effectiveness is critically important. In terms of the side effects and safety, the potential health risks of novel treatment such as nanomaterials, gene therapy, supra-physiological level dosage of growth factors should be examined before clinical trials. In terms of the efficacy, large animals are required to test the treatment outcomes of the promising strategy as compared to available counterparts. However, most of the investigations have been conducted in small animal models, which limits its potential for clinical translation. Besides, there are great differences between varying animal models and the human, such as the intrinsic healing capabilities, pathological condition, chronic degenerative changes, underlying diseases, biomechanical loading, age, etc. Therefore, there is an urgent need to establish standardized animal models to evaluate the clinical translation potential. More recently, the emphasis in the field of orthopedic tissue engineering has shifted from tissue formation to tissue function. Successful clinical translation will also require comprehensive measurement to determine the performance of interface regeneration. Therefore, it is necessary to uniform quantitative measurements to evaluate treatment efficacy. Other issues such as commercial manufacturing, regulatory, economic, and ethical challenges related to the innovative strategies must be taken into consideration.
Collectively, a biomimetic scaffold system with appropriate cell types, combined together with specific biophysical and biochemical cues would be required to achieve functional tendon/ligament-to-bone regeneration. There is still a long way to go to realize the translation from theoretical tissue engineering to clinical application technology.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFC1105100), NSFC grants (81871764, 82072463, 81772418, 81972099), Zhejiang Provincial Natural Science Foundation of China (LR20H060001).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Benjamin M., Toumi H., Ralphs J.R., Bydder G., Best T.M., Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calejo I., Costa-Almeida R., Gomes M.E. Cellular complexity at the interface: challenges in enthesis tissue engineering. Adv. Exp. Med. Biol. 2019;1144:71–90. doi: 10.1007/5584_2018_307. [DOI] [PubMed] [Google Scholar]
- 3.Lu H.H., Spalazzi J.P. Biomimetic stratified scaffold design for ligament-to-bone interface tissue engineering. Comb. Chem. High Throughput Screen. 2009;12:589–597. doi: 10.2174/138620709788681925. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Bogdanowicz D., Erisken C., Lee N.M., Lu H.H. Biomimetic scaffold design for functional and integrative tendon repair. J. Shoulder Elbow Surg. 2012;21:266–277. doi: 10.1016/j.jse.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H.H., Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim W.L., Liau L.L., Ng M.H., Chowdhury S.R., Law J.X. Current progress in tendon and ligament tissue engineering. Tissue Eng Regen Med. 2019;16:549–571. doi: 10.1007/s13770-019-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atesok K., Doral M.N., Karlsson J., Egol K.A., Jazrawi L.M., Coelho P.G., Martinez A., Matsumoto T., Owens B.D., Ochi M., Hurwitz S.R., Atala A., Fu F.H., Lu H.H., Rodeo S.A. Multilayer scaffolds in orthopaedic tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:2365–2373. doi: 10.1007/s00167-014-3453-z. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y., Dong Y., Jiang J., Li H., Zhu T., Chen S. Evaluation of the Bone-ligament and tendon insertions based on Raman spectrum and its PCA and CLS analysis. Sci. Rep. 2017;7:38706. doi: 10.1038/srep38706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saveh-Shemshaki N., Nair L.S., Laurencin C.T. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater. 2019;94:64–81. doi: 10.1016/j.actbio.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Prabhath A., Vernekar V.N., Sanchez E., Laurencin C.T. Growth factor delivery strategies for rotator cuff repair and regeneration. Int. J. Pharm. 2018;544:358–371. doi: 10.1016/j.ijpharm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickerson D.A., Misk T.N., Van Sickle D.C., Breur G.J., Nauman E.A. In vitro and in vivo evaluation of orthopedic interface repair using a tissue scaffold with a continuous hard tissue-soft tissue transition. J. Orthop. Surg. Res. 2013;8:18. doi: 10.1186/1749-799X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S., Su W., Shah V., Hobson D., Yildirimer L., Yeung K., Zhao J., Cui W., Zhao X. Biomaterials based strategies for rotator cuff repair. Colloids Surf. B Biointerfaces. 2017;157:407–416. doi: 10.1016/j.colsurfb.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Rayan F., Nanjayan S.K., Quah C., Ramoutar D., Konan S., Haddad F.S. Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J. Orthoped. 2015;6:252–262. doi: 10.5312/wjo.v6.i2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Z.C., Wang S.Z., Zhang X.J., Lu J. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49:154–162. doi: 10.1111/cpr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui P., Zhang P., Chan K., Qin L. Biology and augmentation of tendon-bone insertion repair. J. Orthop. Surg. Res. 2010;5:59. doi: 10.1186/1749-799X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apostolakos J., Durant T.J., Dwyer C.R., Russell R.P., Weinreb J.H., Alaee F., Beitzel K., McCarthy M.B., Cote M.P., Mazzocca A.D. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–342. [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrasekaran S., Pankow M., Peters K., Huang H.S. Composition and structure of porcine digital flexor tendon-bone insertion tissues. J. Biomed. Mater. Res. 2017;105:3050–3058. doi: 10.1002/jbm.a.36162. [DOI] [PubMed] [Google Scholar]
- 18.Patel S., Caldwell J.M., Doty S.B., Levine W.N., Rodeo S., Soslowsky L.J., Thomopoulos S., Lu H.H. Integrating soft and hard tissues via interface tissue engineering. J. Orthop. Res. 2018;36:1069–1077. doi: 10.1002/jor.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander B., Daulton T.L., Genin G.M., Lipner J., Pasteris J.D., Wopenka B., Thomopoulos S. The nanometre-scale physiology of bone: steric modelling and scanning transmission electron microscopy of collagen-mineral structure. J. R. Soc. Interface. 2012;9:1774–1786. doi: 10.1098/rsif.2011.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deymier A.C., An Y., Boyle J.J., Schwartz A.G., Birman V., Genin G.M., Thomopoulos S., Barber A.H. Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater. 2017;56:25–35. doi: 10.1016/j.actbio.2017.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshpande A.S., Beniash E. Bio-inspired synthesis of mineralized collagen fibrils. Cryst. Growth Des. 2008;8:3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahamid J., Sharir A., Addadi L., Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boonrungsiman S., Gentleman E., Carzaniga R., Evans N.D., McComb D.W., Porter A.E., Stevens M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14170–14175. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao C., Zhao R., Jiang S., Yao S., Wu Z., Jin B., Yang Y., Pan H., Tang R. Citrate improves collagen mineralization via interface wetting: a physicochemical understanding of biomineralization control. Adv. Mater. 2018;30:1704876. doi: 10.1002/adma.201704876. [DOI] [PubMed] [Google Scholar]
- 25.Font Tellado S., Balmayor E.R., Van Griensven M. Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015;94:126–140. doi: 10.1016/j.addr.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Rossetti L., Kuntz L.A., Kunold E., Schock J., Müller K.W., Grabmayr H., Stolberg-Stolberg J., Pfeiffer F., Sieber S.A., Burgkart R., Bausch A.R. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017;16:664–670. doi: 10.1038/nmat4863. [DOI] [PubMed] [Google Scholar]
- 27.Spalazzi J.P., Boskey A.L., Pleshko N., Lu H.H. Quantitative mapping of matrix content and distribution across the ligament-to-bone insertion. PloS One. 2013;8 doi: 10.1371/journal.pone.0074349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu D., Subramony S.D., Boskey A.L., Pleshko N., Doty S.B., Lu H.H. Compositional mapping of the mature anterior cruciate ligament-to-bone insertion. J. Orthop. Res. 2017;35:2513–2523. doi: 10.1002/jor.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuntz L.A., Rossetti L., Kunold E., Schmitt A., von Eisenhart-Rothe R., Bausch A.R., Burgkart R.H. Biomarkers for tissue engineering of the tendon-bone interface. PloS One. 2018;13 doi: 10.1371/journal.pone.0189668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacevic D., Rodeo S.A. Biological augmentation of rotator cuff tendon repair. Clin. Orthop. Relat. Res. 2008;466:622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabuchi K., Soejima T., Kanazawa T., Noguchi K., Nagata K. Chronological changes in the collagen type composition at tendon-bone interface in rabbits. Bone Joint Res. 2012;1:218–224. doi: 10.1302/2046-3758.19.2000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin M., Kumai T., Milz S., Boszczyk B.M., Boszczyk A.A., Ralphs J.R. The skeletal attachment of tendons-tendon "entheses. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 33.Hakimi O., Mouthuy P.A., Carr A. Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int. J. Exp. Pathol. 2013;94:287–292. doi: 10.1111/iep.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Z., Chen X., Zhu T., Hu J.J., Song H.X., Shen W.L., Jiang L.Y., Heng B.C., Ji J.F., Ouyang H.W. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9:9317–9329. doi: 10.1016/j.actbio.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Lu H., Tang Y., Liu F., Xie S., Qu J., Chen C. Comparative evaluation of the book-type Acellular bone scaffold and fibrocartilage scaffold for bone-tendon healing. J. Orthop. Res. 2019;37:1709–1722. doi: 10.1002/jor.24301. [DOI] [PubMed] [Google Scholar]
- 37.Ning L.J., Zhang Y., Chen X.H., Luo J.C., Li X.Q., Yang Z.M., Qin T.W. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J. Biomed. Mater. Res. 2012;100:1448–1456. doi: 10.1002/jbm.a.34083. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Liu F., Tang Y., Qu J., Cao Y., Zheng C., Chen Y., Li M., Zhao C., Sun L., Hu J., Lu H. Book-shaped acellular fibrocartilage scaffold with cell-loading capability and chondrogenic inducibility for tissue-engineered fibrocartilage and bone-tendon healing. ACS Appl. Mater. Interfaces. 2019;11:2891–2907. doi: 10.1021/acsami.8b20563. [DOI] [PubMed] [Google Scholar]
- 39.Ning L.J., Zhang Y.J., Zhang Y., Qing Q., Jiang Y.L., Yang J.L., Luo J.C., Qin T.W. The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials. 2015;52:539–550. doi: 10.1016/j.biomaterials.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 40.Liu G.M., Pan J., Zhang Y., Ning L.J., Luo J.C., Huang F.G., Qin T.W. Bridging repair of large rotator cuff tears using a multilayer decellularized tendon slices graft in a rabbit model. Arthroscopy. 2018;34:2569–2578. doi: 10.1016/j.arthro.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Li M., Chen Y., Hu J., Shi Q., Li X., Zhao C., Chen C., Lu H. Sustained release of collagen-affinity SDF-1α from book-shaped acellular fibrocartilage scaffold enhanced bone-tendon healing in a rabbit model. J. Orthop. Res. 2020:1–13. doi: 10.1002/jor.24687. [DOI] [PubMed] [Google Scholar]
- 42.Chen C., Chen Y., Li M., Xiao H., Shi Q., Zhang T., Li X., Zhao C., Hu J., Lu H. Functional decellularized fibrocartilaginous matrix graft for rotator cuff enthesis regeneration: a novel technique to avoid in-vitro loading of cells. Biomaterials. 2020;250:119996. doi: 10.1016/j.biomaterials.2020.119996. [DOI] [PubMed] [Google Scholar]
- 43.Sundar S., Pendegrass C.J., Blunn G.W. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J. Biomed. Mater. Res. B Appl. Biomater. 2009;88:115–122. doi: 10.1002/jbm.b.31157. [DOI] [PubMed] [Google Scholar]
- 44.Kiliçoğlu Ö.İ., Dikmen G., Koyuncu Ö., Bilgiç B., Alturfan A.K. Effects of demineralized bone matrix on tendon-bone healing: an in vivo, experimental study on rabbits. Acta Orthop. Traumatol. Turcica. 2012;46:443–448. doi: 10.3944/aott.2012.2748. [DOI] [PubMed] [Google Scholar]
- 45.Lovric V., Chen D., Yu Y., Oliver R.A., Genin F., Walsh W.R. Effects of demineralized bone matrix on tendon-bone healing in an intra-articular rodent model. Am. J. Sports Med. 2012;40:2365–2374. doi: 10.1177/0363546512457648. [DOI] [PubMed] [Google Scholar]
- 46.Thangarajah T., Shahbazi S., Pendegrass C.J., Lambert S., Alexander S., Blunn G.W. Tendon reattachment to bone in an ovine tendon defect model of retraction using allogenic and xenogenic demineralised bone matrix incorporated with mesenchymal stem cells. PloS One. 2016;11 doi: 10.1371/journal.pone.0161473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hexter A.T., Pendegrass C., Haddad F., Blunn G. Demineralized bone matrix to augment tendon-bone healing: a systematic review. Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117734517. 2325967117734517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farnebo S., Woon C.Y., Bronstein J.A., Schmitt T., Lindsey D.P., Pham H., Castillo A.B., Chang J. Decellularized tendon-bone composite grafts for extremity reconstruction: an experimental study. Plast. Reconstr. Surg. 2014;133:79–89. doi: 10.1097/01.prs.0000436823.64827.a0. [DOI] [PubMed] [Google Scholar]
- 49.Farnebo S., Woon C.Y., Kim M., Pham H., Chang J. Reconstruction of the tendon-bone insertion with decellularized tendon-bone composite grafts: comparison with conventional repair. J Hand Surg Am. 2014;39:65–74. doi: 10.1016/j.jhsa.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Su M., Zhang Q., Zhu Y., Wang S., Lv J., Sun J., Qiu P., Fan S., Jin K., Chen L., Lin X. Preparation of decellularized triphasic hierarchical bone-fibrocartilage-tendon composite extracellular matrix for enthesis regeneration. Adv Healthc Mater. 2019;8:1900831. doi: 10.1002/adhm.201900831. [DOI] [PubMed] [Google Scholar]
- 51.Liu Q., Yu Y., Reisdorf R.L., Qi J., Lu C.K., Berglund L.J., Amadio P.C., Moran S.L., Steinmann S.P., An K.N., Gingery A., Zhao C. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 2019;192:189–198. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Tang Y., Chen C., Liu F., Xie S., Qu J., Li M., Li Z., Li X., Shi Q., Li S., Li X., Hu J., Lu H. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials. 2020;241:119837. doi: 10.1016/j.biomaterials.2020.119837. [DOI] [PubMed] [Google Scholar]
- 53.Boys A.J., Zhou H., Harrod J.B., McCorry M.C., Estroff L.A., Bonassar L.J. Top-down fabrication of spatially controlled mineral-gradient scaffolds for interfacial tissue engineering. ACS Biomater. Sci. Eng. 2019;5:2988–2997. doi: 10.1021/acsbiomaterials.9b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutsuzaki H., Sakane M. Calcium phosphate-hybridized tendon graft to enhance tendon-bone healing two years after ACL reconstruction in goats. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011;3:31. doi: 10.1186/1758-2555-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mutsuzaki H., Sakane M., Fujie H., Hattori S., Kobayashi H., Ochiai N. Effect of calcium phosphate-hybridized tendon graft on biomechanical behavior in anterior cruciate ligament reconstruction in a goat model: novel technique for improving tendon-bone healing. Am. J. Sports Med. 2011;39:1059–1066. doi: 10.1177/0363546510390427. [DOI] [PubMed] [Google Scholar]
- 56.Mutsuzaki H., Sakane M., Nakajima H., Ochiai N. Calcium phosphate-hybridised tendon graft to reduce bone-tunnel enlargement after ACL reconstruction in goats. Knee. 2011;19:455–460. doi: 10.1016/j.knee.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Qu J., Thoreson A.R., Chen Q., An K.N., Amadio P.C., Zhao C. Tendon gradient mineralization for tendon to bone interface integration. J. Orthop. Res. 2013;31:1713–1719. doi: 10.1002/jor.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grue B.H., Veres S.P. Use of tendon to produce decellularized sheets of mineralized collagen fibrils for bone tissue repair and regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2019;108:845–856. doi: 10.1002/jbm.b.34438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caliari S.R., Harley B.A. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials. 2011;32:5330–5340. doi: 10.1016/j.biomaterials.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo J., Su W., Jiang J., Ning C., Zhao J., Liu X. Enhanced tendon to bone healing in rotator cuff tear by PLLA/CPS composite films prepared by a simple melt-pressing method: an in vitro and in vivo study. Compos. B Eng. 2019;165:526–536. [Google Scholar]
- 61.Shen W., Chen X., Hu Y., Yin Z., Zhu T., Hu J., Chen J., Zheng Z., Zhang W., Ran J., Heng B.C., Ji J., Chen W., Ouyang H.W. Long-term effects of knitted silk-collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials. 2014;35:8154–8163. doi: 10.1016/j.biomaterials.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P., Han F., Chen T., Wu Z., Chen S. Swiss roll"-like bioactive hybrid scaffolds for promoting bone tissue ingrowth and tendon-bone healing after anterior cruciate ligament reconstruction. Biomater Sci. 2020;8:871–883. doi: 10.1039/c9bm01703h. [DOI] [PubMed] [Google Scholar]
- 63.Zhao S., Zhao J., Dong S., Huangfu X., Li B., Yang H., Zhao J., Cui W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 2014;9:2373–2385. doi: 10.2147/IJN.S59536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lipner J., Shen H., Cavinatto L., Liu W., Havlioglu N., Xia Y., Galatz L.M., Thomopoulos S. In vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-bone repair. Tissue Eng. 2015;21:2766–2774. doi: 10.1089/ten.tea.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoya S., Mochizuki Y., Natsu K., Omae H., Nagata Y., Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am. J. Sports Med. 2012;40:1259–1268. doi: 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]
- 66.Qian S., Wang Z., Zheng Z., Ran J., Zhu J., Chen W. A collagen and silk scaffold for improved healing of the tendon and bone interface in a rabbit model. Med Sci Monit. 2019;25:269–278. doi: 10.12659/MSM.912038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruan D., Zhu T., Huang J., Le H., Hu Y., Zheng Z., Tang C., Chen Y., Ran J., Chen X., Yin Z., Qian S., Pioletti D., Heng B.C., Chen W., Shen W., Ouyang H. Knitted silk-collagen scaffold incorporated with ligament stem/progenitor cells sheet for anterior cruciate ligament reconstruction and osteoarthritis prevention. ACS Biomater. Sci. Eng. 2019;5:5412–5421. doi: 10.1021/acsbiomaterials.9b01041. [DOI] [PubMed] [Google Scholar]
- 68.Zhao S., Peng L., Xie G., Li D., Zhao J., Ning C. Effect of the interposition of calcium phosphate materials on tendon-bone healing during repair of chronic rotator cuff tear. Am. J. Sports Med. 2014;42:1920–1929. doi: 10.1177/0363546514532781. [DOI] [PubMed] [Google Scholar]
- 69.Pan W., Wei Y., Zhou L., Li D. Comparative in vivo study of injectable biomaterials combined with BMP for enhancing tendon graft osteointegration for anterior cruciate ligament reconstruction. J. Orthop. Res. 2011;29:1015–1021. doi: 10.1002/jor.21351. [DOI] [PubMed] [Google Scholar]
- 70.Li X., Cheng R., Sun Z., Su W., Pan G., Zhao S., Zhao J., Cui W. Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater. 2017;61:204–216. doi: 10.1016/j.actbio.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y., Han F., Zhang P., Zhi Y., Yang J., Yao X., Wang H., Lin C., Wen X., Chen J., Zhao P. A synthetic bridging patch of modified co-electrospun dual nano-scaffolds for massive rotator cuff tear. J. Mater. Chem. B. 2016;4:7259–7269. doi: 10.1039/c6tb01674j. [DOI] [PubMed] [Google Scholar]
- 72.Li H., Fan J., Sun L., Liu X., Cheng P., Fan H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials. 2016;106:180–192. doi: 10.1016/j.biomaterials.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Kim B.S., Kim E.J., Choi J.S., Jeong J.H., Jo C.H., Cho Y.W. Human collagen-based multilayer scaffolds for tendon-to-bone interface tissue engineering. J. Biomed. Mater. Res. 2014;102:4044–4054. doi: 10.1002/jbm.a.35057. [DOI] [PubMed] [Google Scholar]
- 74.Criscenti G., Longoni A., Luca A.D., De Maria C., van Blitterswijk C.A., Vozzi G., Moroni L. Triphasic scaffolds for the regeneration of the bone-ligament interface. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/1/015009. [DOI] [PubMed] [Google Scholar]
- 75.Li X., Xie J., Lipner J., Yuan X., Thomopoulos S., Xia Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009;9:2763–2768. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu W., Lipner J., Xie J., Manning C.N., Thomopoulos S., Xia Y. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl. Mater. Interfaces. 2014;6:2842–2849. doi: 10.1021/am405418g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samavedi S., Horton C.O., Guelcher S.A., Goldstein A.S., Whittington A.R. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament-bone interface. Acta Biomater. 2011;7:4131–4138. doi: 10.1016/j.actbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 78.Samavedi S., Guelcher S.A., Goldstein A.S., Whittington A.R. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament-bone interface. Biomaterials. 2012;33:7727–7735. doi: 10.1016/j.biomaterials.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 79.Ramalingam M., Young M.F., Thomas V., Sun L., Chow L.C., Tison C.K., Chatterjee K., Miles W.C., Simon C.G., Jr. Nanofiber scaffold gradients for interfacial tissue engineering. J. Biomater. Appl. 2013;27:695–705. doi: 10.1177/0885328211423783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calejo I., Costa-Almeida R., Reis R.L., Gomes M.E. A textile platform using continuous aligned and textured composite microfibers to engineer tendon-to-bone interface gradient scaffolds. Adv Healthc Mater. 2019;8:1900200. doi: 10.1002/adhm.201900200. [DOI] [PubMed] [Google Scholar]
- 81.Zhu C., Pongkitwitoon S., Qiu J., Thomopoulos S., Xia Y. Design and fabrication of a hierarchically structured scaffold for tendon-to-bone repair. Adv. Mater. 2018;30:1707306. doi: 10.1002/adma.201707306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lausch A.J., Chong L.C., Uludag H., Sone E.D. Multiphasic collagen scaffolds for engineered tissue interfaces. Adv. Funct. Mater. 2018;28:1804730. [Google Scholar]
- 83.Yin Z., Chen X., Song H.X., Hu J.J., Tang Q.M., Zhu T., Shen W.L., Chen J.L., Liu H., Heng B.C., Ouyang H.W. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials. 2015;44:173–185. doi: 10.1016/j.biomaterials.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 84.Lin Z., Zhao X., Chen S., Du C. Osteogenic and tenogenic induction of hBMSCs by an integrated nanofibrous scaffold with chemical and structural mimicry of the bone-ligament connection. J. Mater. Chem. B. 2017;5:1015–1027. doi: 10.1039/c6tb02156e. [DOI] [PubMed] [Google Scholar]
- 85.Xie J., Li X., Lipner J., Manning C.N., Schwartz A.G., Thomopoulos S., Xia Y. Aligned-to-random" nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale. 2010;2:923–926. doi: 10.1039/c0nr00192a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie J., Ma B., Michael P.L., Shuler F.D. Fabrication of nanofiber scaffolds with gradations in fiber organization and their potential applications. Macromol. Biosci. 2012;12:1336–1341. doi: 10.1002/mabi.201200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowlin J., Bismi M.A., Delpech B., Dumas P., Zhou Y., Tan G.Z. vol. 5. 2018. (Engineering the Hard-Soft Tissue Interface with Random-To-Aligned Nanofiber Scaffolds). 1849543518803538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samavedi S., Vaidya P., Gaddam P., Whittington A.R., Goldstein A.S. Electrospun meshes possessing region-wise differences in fiber orientation, diameter, chemistry and mechanical properties for engineering bone-ligament-bone tissues. Biotechnol. Bioeng. 2014;111:2549–2559. doi: 10.1002/bit.25299. [DOI] [PubMed] [Google Scholar]
- 89.Cai J., Wang J., Ye K., Li D., Ai C., Sheng D., Jin W., Liu X., Zhi Y., Jiang J., Chen J., Mo X., Chen S. Dual-layer aligned-random nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int. J. Nanomed. 2018;13:3481–3492. doi: 10.2147/IJN.S165633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Font Tellado S., Bonani W., Balmayor E.R., Foehr P., Motta A., Migliaresi C., van Griensven M. Fabrication and characterization of biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Tissue Eng. 2017;23:859–872. doi: 10.1089/ten.TEA.2016.0460. [DOI] [PubMed] [Google Scholar]
- 91.Liu H., Yang L., Zhang E., Zhang R., Cai D., Zhu S., Ran J., Bunpetch V., Cai Y., Heng B.C., Hu Y., Dai X., Chen X., Ouyang H. Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 2017;56:129–140. doi: 10.1016/j.actbio.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 92.Echave M.C., Domingues R., Gómez-Florit M., Pedraz J.L., Reis R.L., Orive G., Gomes M.E. Biphasic hydrogels integrating mineralized and anisotropic features for interfacial tissue engineering. ACS Appl. Mater. Interfaces. 2019;11:47771–47784. doi: 10.1021/acsami.9b17826. [DOI] [PubMed] [Google Scholar]
- 93.Caliari S.R., Weisgerber D.W., Grier W.K., Mahmassani Z., Boppart M.D., Harley B.A. Collagen scaffolds incorporating coincident gradations of instructive structural and biochemical cues for osteotendinous junction engineering. Adv Healthc Mater. 2015;4:831–837. doi: 10.1002/adhm.201400809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cong S., Sun Y., Lin J., Liu S., Chen J. A synthetic graft with multilayered Co-electrospinning nanoscaffolds for bridging massive rotator cuff tear in a rat model. Am. J. Sports Med. 2020;48:1826–1836. doi: 10.1177/0363546520917684. [DOI] [PubMed] [Google Scholar]
- 95.Bedi A., Maak T., Walsh C., Rodeo S.A., Grande D., Dines D.M., Dines J.S. Cytokines in rotator cuff degeneration and repair. J. Shoulder Elbow Surg. 2012;21:218–227. doi: 10.1016/j.jse.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 96.Kim J.H., Oh S.H., Min H.K., Lee J.H. Dual growth factor-immobilized a symmetrically porous membrane for bone-to-tendon interface regeneration on rat patellar tendon avulsion model. J. Biomed. Mater. Res. 2018;106:115–125. doi: 10.1002/jbm.a.36212. [DOI] [PubMed] [Google Scholar]
- 97.Chen B., Li B., Qi Y.J., Ni Q.B., Pan Z.Q., Wang H., Chen L.B. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci. Rep. 2016;6:25940. doi: 10.1038/srep25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Longo U.G., Rizzello G., Berton A., Maltese L., Fumo C., Khan W.S., Denaro V. Biological strategies to enhance rotator cuff healing. Curr. Stem Cell Res. Ther. 2013;8:464–470. doi: 10.2174/1574888x113086660065. [DOI] [PubMed] [Google Scholar]
- 99.Atesok K., Fu F.H., Wolf M.R., Ochi M., Jazrawi L.M., Doral M.N., Lubowitz J.H., Rodeo S.A. Augmentation of tendon-to-bone healing. J Bone Joint Surg Am. 2014;96:513–521. doi: 10.2106/JBJS.M.00009. [DOI] [PubMed] [Google Scholar]
- 100.Cai Y.Z., Zhang C., Lin X.J. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J. Shoulder Elbow Surg. 2015;24:1852–1859. doi: 10.1016/j.jse.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 101.Ahmad Z., Henson F., Wardale J., Noorani A., Tytherleigh-Strong G., Rushton N. Review article: regenerative techniques for repair of rotator cuff tears. J Orthop Surg (Hong Kong) 2013;21:226–231. doi: 10.1177/230949901302100223. [DOI] [PubMed] [Google Scholar]
- 102.Hapa O., Cakıcı H., Kükner A., Aygün H., Sarkalan N., Baysal G. Effect of platelet-rich plasma on tendon-to-bone healing after rotator cuff repair in rats: an in vivo experimental study. Acta Orthop. Traumatol. Turcica. 2012;46:301–307. doi: 10.3944/aott.2012.2664. [DOI] [PubMed] [Google Scholar]
- 103.Hasan S., Weinberg M., Khatib O., Jazrawi L., Strauss E.J. The effect of platelet-rich fibrin matrix on rotator cuff healing in a rat model. Int. J. Sports Med. 2016;37:36–42. doi: 10.1055/s-0035-1554637. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M., Zhen J., Zhang X., Yang Z., Zhang L., Hao D., Ren B. Effect of autologous platelet-rich plasma and gelatin sponge for tendon-to-bone healing after rabbit anterior cruciate ligament reconstruction. Arthroscopy. 2019;35:1486–1497. doi: 10.1016/j.arthro.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 105.Zhang C., Liu Y.J. Biomechanic and histologic analysis of fibroblastic effects of tendon-to-bone healing by transforming growth factor β1 (TGF-β1) in rotator cuff tears. Acta Cir. Bras. 2017;32:1045–1055. doi: 10.1590/s0102-865020170120000006. [DOI] [PubMed] [Google Scholar]
- 106.You X., Shen Y., Yu W., He Y. Enhancement of tendon-bone healing following rotator cuff repair using hydroxyapatite with TGFβ1. Mol. Med. Rep. 2018;17:4981–4988. doi: 10.3892/mmr.2018.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manning C.N., Kim H.M., Sakiyama-Elbert S., Galatz L.M., Havlioglu N., Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011;29:1099–1105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 108.Kovacevic D., Fox A.J., Bedi A., Ying L., Deng X.H., Warren R.F., Rodeo S.A. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am. J. Sports Med. 2011;39:811–819. doi: 10.1177/0363546511399378. [DOI] [PubMed] [Google Scholar]
- 109.Kabuto Y., Morihara T., Sukenari T., Kida Y., Oda R., Arai Y., Sawada K., Matsuda K., Kawata M., Tabata Y., Fujiwara H., Kubo T. Stimulation of rotator cuff repair by sustained release of bone morphogenetic protein-7 using a gelatin hydrogel sheet. Tissue Eng. 2015;21:2025–2033. doi: 10.1089/ten.tea.2014.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tokunaga T., Karasugi T., Arimura H., Yonemitsu R., Sakamoto H., Ide J., Mizuta H. Enhancement of rotator cuff tendon-bone healing with fibroblast growth factor 2 impregnated in gelatin hydrogel sheets in a rabbit model. J. Shoulder Elbow Surg. 2017;26:1708–1717. doi: 10.1016/j.jse.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 111.Ide J., Kikukawa K., Hirose J., Iyama K., Sakamoto H., Fujimoto T., Mizuta H. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J. Shoulder Elbow Surg. 2009;18:391–398. doi: 10.1016/j.jse.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Tokunaga T., Ide J., Arimura H., Nakamura T., Uehara Y., Sakamoto H., Mizuta H. Local application of gelatin hydrogel sheets impregnated with platelet-derived growth factor BB promotes tendon-to-bone healing after rotator cuff repair in rats. Arthroscopy. 2015;31:1482–1491. doi: 10.1016/j.arthro.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 113.Kovacevic D., Gulotta L.V., Ying L., Ehteshami J.R., Deng X.H., Rodeo S.A. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin. Orthop. Relat. Res. 2015;473:1644–1654. doi: 10.1007/s11999-014-4020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yonemitsu R., Tokunaga T., Shukunami C., Ideo K., Arimura H., Karasugi T., Nakamura E., Ide J., Hiraki Y., Mizuta H. Fibroblast growth factor 2 enhances tendon-to-bone healing in a rat rotator cuff repair of chronic tears. Am. J. Sports Med. 2019;47:1701–1712. doi: 10.1177/0363546519836959. [DOI] [PubMed] [Google Scholar]
- 115.Min H.K., Kwon O.S., Oh S.H., Lee J.H. Platelet-derived growth factor-BB-immobilized a symmetrically porous membrane for enhanced rotator cuff tendon healing. Tissue Eng Regen Med. 2016;13:568–578. doi: 10.1007/s13770-016-9120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hee C.K., Dines J.S., Dines D.M., Roden C.M., Wisner-Lynch L.A., Turner A.S., McGilvray K.C., Lyons A.S., Puttlitz C.M., Santoni B.G. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am. J. Sports Med. 2011;39:1630–1639. doi: 10.1177/0363546511404942. [DOI] [PubMed] [Google Scholar]
- 117.Hirakawa Y., Manaka T., Orita K., Ito Y., Ichikawa K., Nakamura H. The accelerated effect of recombinant human bone morphogenetic protein 2 delivered by β-tricalcium phosphate on tendon-to-bone repair process in rabbit models. J. Shoulder Elbow Surg. 2018;27:894–902. doi: 10.1016/j.jse.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 118.Ma C.B., Kawamura S., Deng X.H., Ying L., Schneidkraut J., Hays P., Rodeo S.A. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am. J. Sports Med. 2007;35:597–604. doi: 10.1177/0363546506296312. [DOI] [PubMed] [Google Scholar]
- 119.Lu D., Yang C., Zhang Z., Xiao M. Enhanced tendon-bone healing with acidic fibroblast growth factor delivered in collagen in a rabbit anterior cruciate ligament reconstruction model. J. Orthop. Surg. Res. 2018;13:301. doi: 10.1186/s13018-018-0984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim J.G., Kim H.J., Kim S.E., Bae J.H., Ko Y.J., Park J.H. Enhancement of tendon-bone healing with the use of bone morphogenetic protein-2 inserted into the suture anchor hole in a rabbit patellar tendon model. Cytotherapy. 2014;16:857–867. doi: 10.1016/j.jcyt.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 121.Lee K.W., Lee J.S., Jang J.W., Shim Y.B., Lee K.I. Tendon-bone interface healing using an injectable rhBMP-2-containing collagen gel in a rabbit extra-articular bone tunnel model. J Tissue Eng Regen Med. 2017;11:1435–1441. doi: 10.1002/term.2041. [DOI] [PubMed] [Google Scholar]
- 122.Kim H.J., Kang S.W., Lim H.C., Han S.B., Lee J.S., Prasad L., Kim Y.J., Kim B.S., Park J.H. The role of transforming growth factor-beta and bone morphogenetic protein with fibrin glue in healing of bone tendon junction injury. Connect. Tissue Res. 2007;48:309–315. doi: 10.1080/03008200701692610. [DOI] [PubMed] [Google Scholar]
- 123.Wang X., Xie L., Crane J., Zhen G., Li F., Yang P., Gao M., Deng R., Wang Y., Jia X., Fan C., Wan M., Cao X. Aberrant TGF-β activation in bone tendon insertion induces enthesopathy-like disease. J. Clin. Invest. 2018;128:846–860. doi: 10.1172/JCI96186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakiyama-Elbert S.E. Incorporation of heparin into biomaterials. Acta Biomater. 2014;10:1581–1587. doi: 10.1016/j.actbio.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee J., Choi W.I., Tae G., Kim Y.H., Kang S.S., Kim S.E., Kim S.H., Jung Y., Kim S.H. Enhanced regeneration of the ligament-bone interface using a poly(L-lactide-co-ε-caprolactone) scaffold with local delivery of cells/BMP-2 using a heparin-based hydrogel. Acta Biomater. 2011;7:244–257. doi: 10.1016/j.actbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 126.Galatz L.M., Sandell L.J., Rothermich S.Y., Das R., Mastny A., Havlioglu N., Silva M.J., Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J. Orthop. Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 127.Kobayashi M., Itoi E., Minagawa H., Miyakoshi N., Takahashi S., Tuoheti Y., Okada K., Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J. Shoulder Elbow Surg. 2006;15:371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 128.Würgler-Hauri C.C., Dourte L.M., Baradet T.C., Williams G.R., Soslowsky L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J. Shoulder Elbow Surg. 2007;16:S198–S203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Silva E.D., Babo P.S., Costa-Almeida R., Domingues R., Mendes B.B., Paz E., Freitas P., Rodrigues M.T., Granja P.L., Gomes M.E. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomedicine. 2018;14:2375–2385. doi: 10.1016/j.nano.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 130.Calejo I., Costa-Almeida R., Reis R.L., Gomes M.E. Enthesis tissue engineering: biological requirements meet at the interface. Tissue Eng. B Rev. 2019;25:330–356. doi: 10.1089/ten.TEB.2018.0383. [DOI] [PubMed] [Google Scholar]
- 131.Wang R., Xu B., Xu H.G. Up-regulation of TGF-β promotes tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells through the TGF-β/MAPK signaling pathway in a New Zealand white rabbit model. Cell. Physiol. Biochem. 2017;41:213–226. doi: 10.1159/000456046. [DOI] [PubMed] [Google Scholar]
- 132.Fan J., Sun L., Chen X., Qu L., Li H., Liu X., Zhang Y., Cheng P., Fan H. Implementation of stratified approach and gene immobilization to enhance the osseointegration of silk-based ligament graft. J. Mater. Chem. B. 2017;5:7035–7050. doi: 10.1039/c7tb01579h. [DOI] [PubMed] [Google Scholar]
- 133.Kawakami Y., Takayama K., Matsumoto T., Tang Y., Wang B., Mifune Y., Cummins J.H., Warth R.J., Kuroda R., Kurosaka M., Fu F.H., Huard J. Anterior cruciate ligament-derived stem cells transduced with BMP2 accelerate graft-bone integration after ACL reconstruction. Am. J. Sports Med. 2017;45:584–597. doi: 10.1177/0363546516671707. [DOI] [PubMed] [Google Scholar]
- 134.Dong Y., Zhang Q., Li Y., Jiang J., Chen S. Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int. J. Mol. Sci. 2012;13:13605–13620. doi: 10.3390/ijms131013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang C.J., Weng L.H., Hsu S.L., Sun Y.C., Yang Y.J., Chan Y.S., Yang Y.L. pCMV-BMP-2-transfected cell-mediated gene therapy in anterior cruciate ligament reconstruction in rabbits. Arthroscopy. 2010;26:968–976. doi: 10.1016/j.arthro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 136.Chen P., Cui L., Chen G., You T., Li W., Zuo J., Wang C., Zhang W., Jiang C. The application of BMP-12-overexpressing mesenchymal stem cells loaded 3D-printed PLGA scaffolds in rabbit rotator cuff repair. Int. J. Biol. Macromol. 2019;138:79–88. doi: 10.1016/j.ijbiomac.2019.07.041. [DOI] [PubMed] [Google Scholar]
- 137.Gulotta L.V., Kovacevic D., Packer J.D., Ehteshami J.R., Rodeo S.A. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am. J. Sports Med. 2011;39:180–187. doi: 10.1177/0363546510379339. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J., Liu Z., Li Y., You Q., Yang J., Jin Y., Zou G., Tang J., Ge Z., Liu Y. FGF-2-Induced human amniotic mesenchymal stem cells seeded on a human acellular amniotic membrane scaffold accelerated tendon-to-bone healing in a rabbit extra-articular model. Stem Cell. Int. 2020;2020:4701476. doi: 10.1155/2020/4701476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harris E., Liu Y., Cunniffe G., Morrissey D., Carroll S., Mulhall K., Kelly D.J. Biofabrication of soft tissue templates for engineering the bone-ligament interface. Biotechnol. Bioeng. 2017;114:2400–2411. doi: 10.1002/bit.26362. [DOI] [PubMed] [Google Scholar]
- 140.Tarafder S., Brito J.A., Minhas S., Effiong L., Thomopoulos S., Lee C.H. In situ tissue engineering of the tendon-to-bone interface by endogenous stem/progenitor cells. Biofabrication. 2019;12 doi: 10.1088/1758-5090/ab48ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun L., Li H., Qu L., Zhu R., Fan X., Xue Y., Xie Z., Fan H. Immobilized lentivirus vector on chondroitin sulfate-hyaluronate acid-silk fibroin hybrid scaffold for tissue-engineered ligament-bone junction. BioMed Res. Int. 2014;2014:816979. doi: 10.1155/2014/816979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Min H.K., Oh S.H., Lee J.M., Im G.I., Lee J.H. Porous membrane with reverse gradients of PDGF-BB and BMP-2 for tendon-to-bone repair: in vitro evaluation on adipose-derived stem cell differentiation. Acta Biomater. 2014;10:1272–1279. doi: 10.1016/j.actbio.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 143.Madhurakkat Perikamana S.K., Lee J., Ahmad T., Kim E.M., Byun H., Lee S., Shin H. Harnessing biochemical and structural cues for tenogenic differentiation of adipose derived stem cells (ADSCs) and development of an in vitro tissue interface mimicking tendon-bone insertion graft. Biomaterials. 2018;165:79–93. doi: 10.1016/j.biomaterials.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 144.Lyu J., Chen L., Zhang J., Kang X., Wang Y., Wu W., Tang H., Wu J., He Z., Tang K. A microfluidics-derived growth factor gradient in a scaffold regulates stem cell activities for tendon-to-bone interface healing. Biomater Sci. 2020;8:3649–3663. doi: 10.1039/d0bm00229a. [DOI] [PubMed] [Google Scholar]
- 145.Hurley-Novatny A., Arumugasaamy N., Kimicata M., Baker H., Mikos A.G., Fisher J.P. Concurrent multi-lineage differentiation of mesenchymal stem cells through spatial presentation of growth factors. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/ab9bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Font Tellado S., Chiera S., Bonani W., Poh P., Migliaresi C., Motta A., Balmayor E.R., van Griensven M. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018;72:150–166. doi: 10.1016/j.actbio.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 147.Tan Q., Lui P.P., Rui Y.F., Wong Y.M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng. 2012;18:840–851. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu H., Fan H., Toh S.L., Goh J.C. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008;29:1443–1453. doi: 10.1016/j.biomaterials.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 149.Nourissat G., Diop A., Maurel N., Salvat C., Dumont S., Pigenet A., Gosset M., Houard X., Berenbaum F. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PloS One. 2010;5:e12248. doi: 10.1371/journal.pone.0012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bianco S.T., Moser H.L., Galatz L.M., Huang A.H. Biologics and stem cell-based therapies for rotator cuff repair. Ann. N. Y. Acad. Sci. 2019;1442:35–47. doi: 10.1111/nyas.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith L., Xia Y., Galatz L.M., Genin G.M., Thomopoulos S. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect. Tissue Res. 2012;53:95–105. doi: 10.3109/03008207.2011.650804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang X., Ma Y., Fu X., Liu Q., Shao Z., Dai L., Pi Y., Hu X., Zhang J., Duan X., Chen W., Chen P., Zhou C., Ao Y. Runx2-Modified adipose-derived stem cells promote tendon graft integration in anterior cruciate ligament reconstruction. Sci. Rep. 2016;6:19073. doi: 10.1038/srep19073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Calejo I., Costa-Almeida R., Gonçalves A.I., Berdecka D., Reis R.L., Gomes M.E. Bi-directional modulation of cellular interactions in an in vitro co-culture model of tendon-to-bone interface. Cell Prolif. 2018;51:e12493. doi: 10.1111/cpr.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen P., Ouyang J., Xiao J., Han Z., Yu Q., Tian J., Zhang L. Co-injection of human adipose stromal cells and rhBMP-2/fbrin gel enhances tendon graft osteointegration in a rabbit anterior cruciate ligament-reconstruction model. Am J Transl Res. 2018;10:535–544. [PMC free article] [PubMed] [Google Scholar]
- 155.Mora M.V., Ibán M.A.R., Heredia J.D., Laakso R.B., Cuéllar R., Arranz M.G. Stem cell therapy in the management of shoulder rotator cuff disorders. World J. Stem Cell. 2015;7:691–699. doi: 10.4252/wjsc.v7.i4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lui P.P., Wong O.T., Lee Y.W. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am. J. Sports Med. 2014;42:681–689. doi: 10.1177/0363546513517539. [DOI] [PubMed] [Google Scholar]
- 157.Matsumoto T., Ingham S.M., Mifune Y., Osawa A., Logar A., Usas A., Kuroda R., Kurosaka M., Fu F.H., Huard J. Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cell. Dev. 2012;21:859–872. doi: 10.1089/scd.2010.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mifune Y., Matsumoto T., Ota S., Nishimori M., Usas A., Kopf S., Kuroda R., Kurosaka M., Fu F.H., Huard J. Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant. 2012;21:1651–1665. doi: 10.3727/096368912X647234. [DOI] [PubMed] [Google Scholar]
- 159.Mifune Y., Matsumoto T., Takayama K., Terada S., Sekiya N., Kuroda R., Kurosaka M., Fu F.H., Huard J. Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials. 2013;34:5476–5487. doi: 10.1016/j.biomaterials.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 160.Ju Y.J., Muneta T., Yoshimura H., Koga H., Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469–478. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 161.Chang C.H., Chen C.H., Liu H.W., Whu S.W., Chen S.H., Tsai C.L., Hsiue G.H. Bioengineered periosteal progenitor cell sheets to enhance tendon-bone healing in a bone tunnel. Biomed. J. 2012;35:473–480. doi: 10.4103/2319-4170.104412. [DOI] [PubMed] [Google Scholar]
- 162.Chen C.H., Chang C.H., Wang K.C., Su C.I., Liu H.T., Yu C.M., Wong C.B., Wang I.C., Whu S.W., Liu H.W. Enhancement of rotator cuff tendon-bone healing with injectable periosteum progenitor cells-BMP-2 hydrogel in vivo. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:1597–1607. doi: 10.1007/s00167-010-1373-0. [DOI] [PubMed] [Google Scholar]
- 163.Spalazzi J.P., Doty S.B., Moffat K.L., Levine W.N., Lu H.H. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497–3508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 164.Wang I.E., Shan J., Choi R., Oh S., Kepler C.K., Chen F.H., Lu H.H. Role of osteoblast-fibroblast interactions in the formation of the ligament-to-bone interface. J. Orthop. Res. 2007;25:1609–1620. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 165.Spalazzi J.P., Dagher E., Doty S.B., Guo X.E., Rodeo S.A., Lu H.H. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J. Biomed. Mater. Res. 2008;86:1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 166.Cooper J.O., Bumgardner J.D., Cole J.A., Smith R.A., Haggard W.O. Co-cultured tissue-specific scaffolds for tendon/bone interface engineering. J. Tissue Eng. 2014;5 doi: 10.1177/2041731414542294. 2041731414542294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gulotta L.V., Kovacevic D., Ehteshami J.R., Dagher E., Packer J.D., Rodeo S.A. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am. J. Sports Med. 2009;37:2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 168.Zhang H., Liu M.F., Liu R.C., Shen W.L., Yin Z., Chen X. Physical microenvironment-based inducible scaffold for stem cell differentiation and tendon regeneration. Tissue Eng. B Rev. 2018;24:443–453. doi: 10.1089/ten.TEB.2018.0018. [DOI] [PubMed] [Google Scholar]
- 169.Delaine-Smith R.M., Reilly G.C. Mesenchymal stem cell responses to mechanical stimuli. 2012;2:169–180. [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Q., Hatta T., Qi J., Liu H., Thoreson A.R., Amadio P.C., Moran S.L., Steinmann S.P., Gingery A., Zhao C. Novel engineered tendon-fibrocartilage-bone composite with cyclic tension for rotator cuff repair. J Tissue Eng Regen Med. 2018;12:1690–1701. doi: 10.1002/term.2696. [DOI] [PubMed] [Google Scholar]
- 171.Grier W.K., Sun Han, Chang R.A., Ramsey M.D., Harley B. The influence of cyclic tensile strain on multi-compartment collagen-GAG scaffolds for tendon-bone junction repair. Connect. Tissue Res. 2019;60:530–543. doi: 10.1080/03008207.2019.1601183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 173.Chen W., Zhuo Y., Duan D., Lu M. Effects of hypoxia on differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2020;15:332–339. doi: 10.2174/1574888X14666190823144928. [DOI] [PubMed] [Google Scholar]
- 174.Calejo I., Costa-Almeida R., Reis R.L., Gomes M.E. A physiology-inspired multifactorial toolbox in soft-to-hard musculoskeletal interface tissue engineering. Trends Biotechnol. 2020;38:83–98. doi: 10.1016/j.tibtech.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 175.He P., Ng K.S., Toh S.L., Goh J.C.H. In vitro ligament-bone interface regeneration using a trilineage coculture system on a hybrid silk scaffold. Biomacromolecules. 2012;13:2692–2703. doi: 10.1021/bm300651q. [DOI] [PubMed] [Google Scholar]
- 176.Wang I.E., Bogdanowicz D.R., Mitroo S., Shan J., Kala S., Lu H.H. Cellular interactions regulate stem cell differentiation in tri-culture. Connect. Tissue Res. 2016;57:476–487. doi: 10.1080/03008207.2016.1230106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cao Y., Yang S., Zhao D., Li Y., Cheong S.S., Han D., Li Q. Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering. J Orthop Translat. 2020;23:89–100. doi: 10.1016/j.jot.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reznikov N., Bilton M., Lari L., Stevens M.M., Kröger R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science. 2018;360:507. doi: 10.1126/science.aao2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Svensson V., Natarajan K.N., Ly L.H., Miragaia R.J., Labalette C., Macaulay I.C., Cvejic A., Teichmann S.A. Power analysis of single-cell RNA-sequencing experiments. Nat. Methods. 2017;14:381–387. doi: 10.1038/nmeth.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]