Highlights
-
•
Hirschsprung’s disease or Aganglionosis should be suspected in patients with chronic constipation.
-
•
A good quality rectal biopsy is paramount for a correct diagnosis.
-
•
Early surgical referral can significantly improve the patient’s quality of life.
Keywords: Adult’s Hirschsprung’s disease, Colonic Hypoganglionosis, Constipation
Abstract
Hirschsprung’s disease (HD) is an uncommon condition in adulthood; the term adult HD denotes diagnosis after the age of ten. Patients suffer from constipation for many years before the diagnosis is established. They have very characteristic radiologic findings; however, the diagnosis is confirmed with full thickness biopsies. We describe the case of a 19-year-old Caucasian female patient from Southeast Missouri with a history of chronic constipation who was referred to the General Surgery Department by her primary care provider (PCP) due to massive colonic and rectal dilation in an abdominal CT scan. After rectal biopsies were performed the diagnosis of Hirschsprung’s disease was confirmed. She was referred to a tertiary center where she underwent a colo-anal pull through procedure. She has been doing well for three years. Diagnosis of this condition can be very challenging, hence the need for clinical suspicion, good quality biopsies and inter-specialty communication among PCPs, gastroenterologists, surgeons and pathologists. Surgery aiming to remove or bypass the aganglionic colonic or rectal segment is the standard of care; quality of life can be significantly improved after surgery.
1. Introduction and importance
Hirschsprung’s disease (HD) is defined as the lack of ganglion cells in the distal section of the large intestine at the level of the Auerbach and Meissner plexus. Because it is an uncommon diagnosis in adulthood it is often ignored by primary care providers. More than 90% of the cases are diagnosed before the age of five. The disease prevalence is estimated at a level of 1:5000 live births and affects more males than females at a ratio of 4:1 [1,2]. Adult HD by definition occurs when the diagnosis is made after the age of ten. Patients usually suffer for many years before the diagnosis is established. They have a long-standing history of constipation and due to progressive dilation of the upstream colon present with very characteristic radiologic findings [3]. These patients manage their symptoms with laxatives and enemas; sometimes manual de-impaction is needed. Treatment consists of bypassing or removing the aganglionic segment. We report the case of a 19-year-old Caucasian female patient from Southeast Missouri (United States) who was referred to the General Surgery Department by her PCP with a long-standing history of abdominal pain, constipation and typical imaging findings. The patient underwent diagnostic testing in our small community hospital and she was referred for surgery to a tertiary care center. We also comment on specimen collection and processing techniques as well as HD allied disorders. Our aim is to raise awareness about this condition in rural populations. This article has been reported in line with the SCARE criteria [4].
2. Case presentation
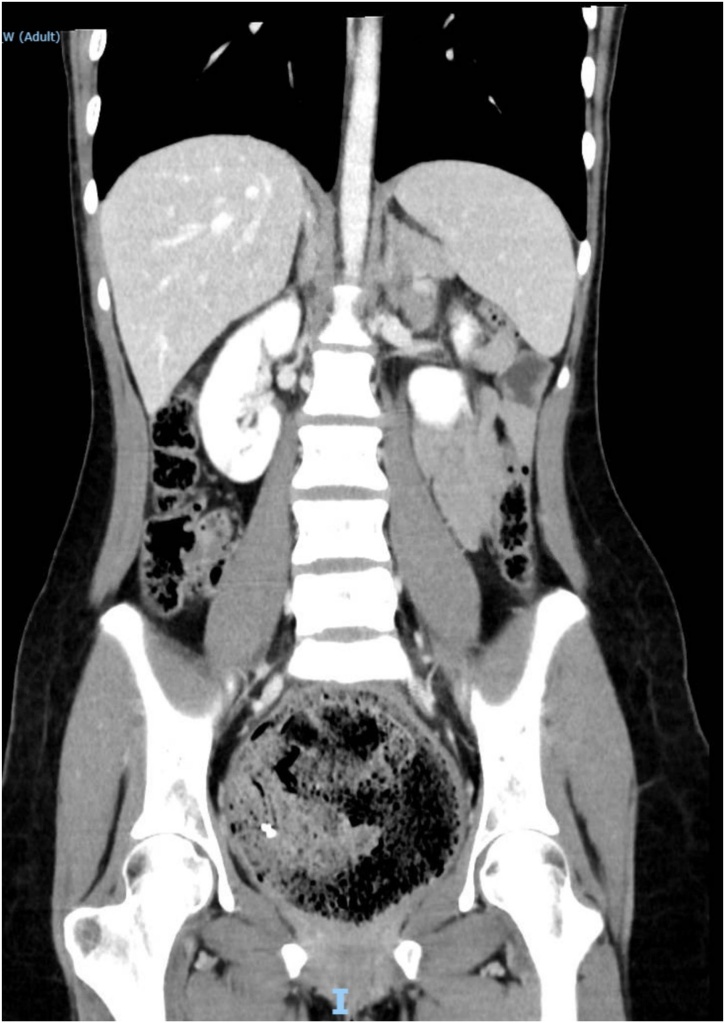
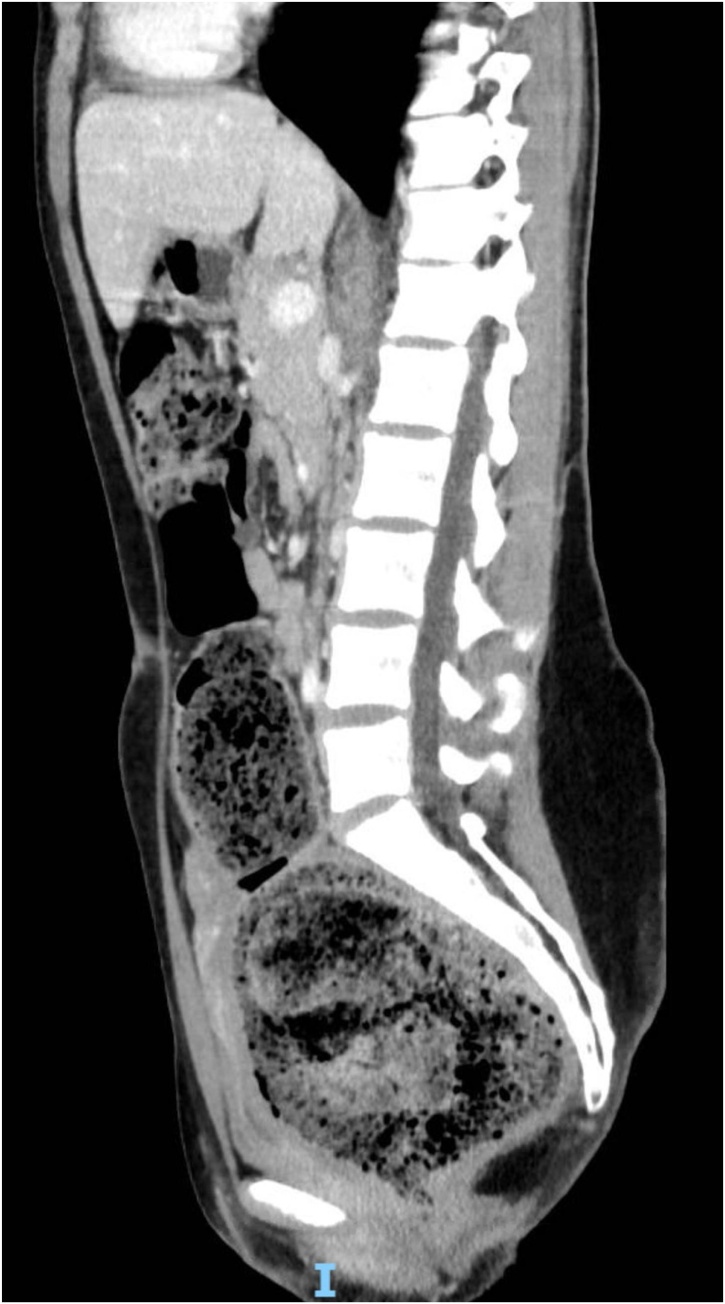
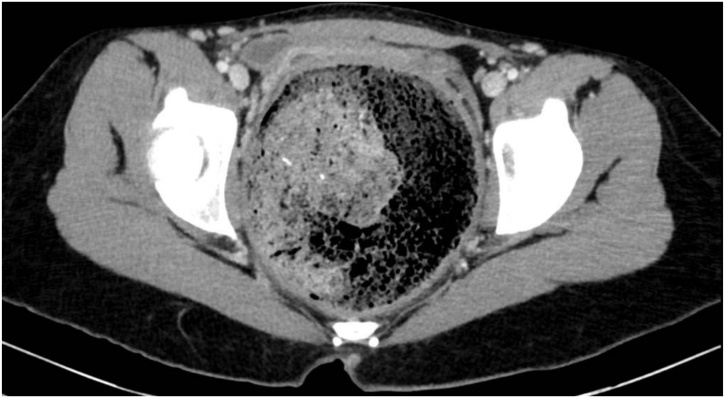
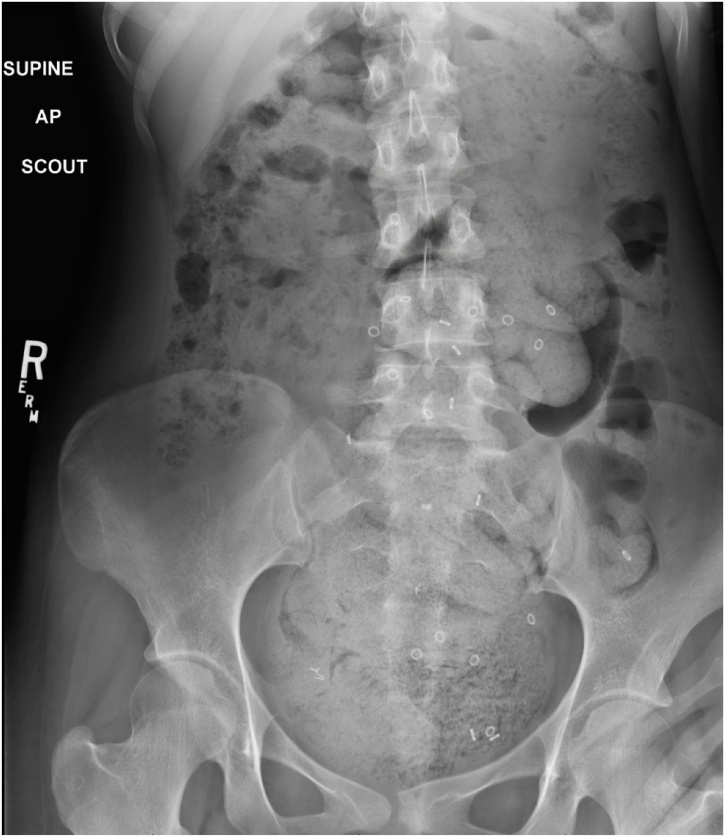
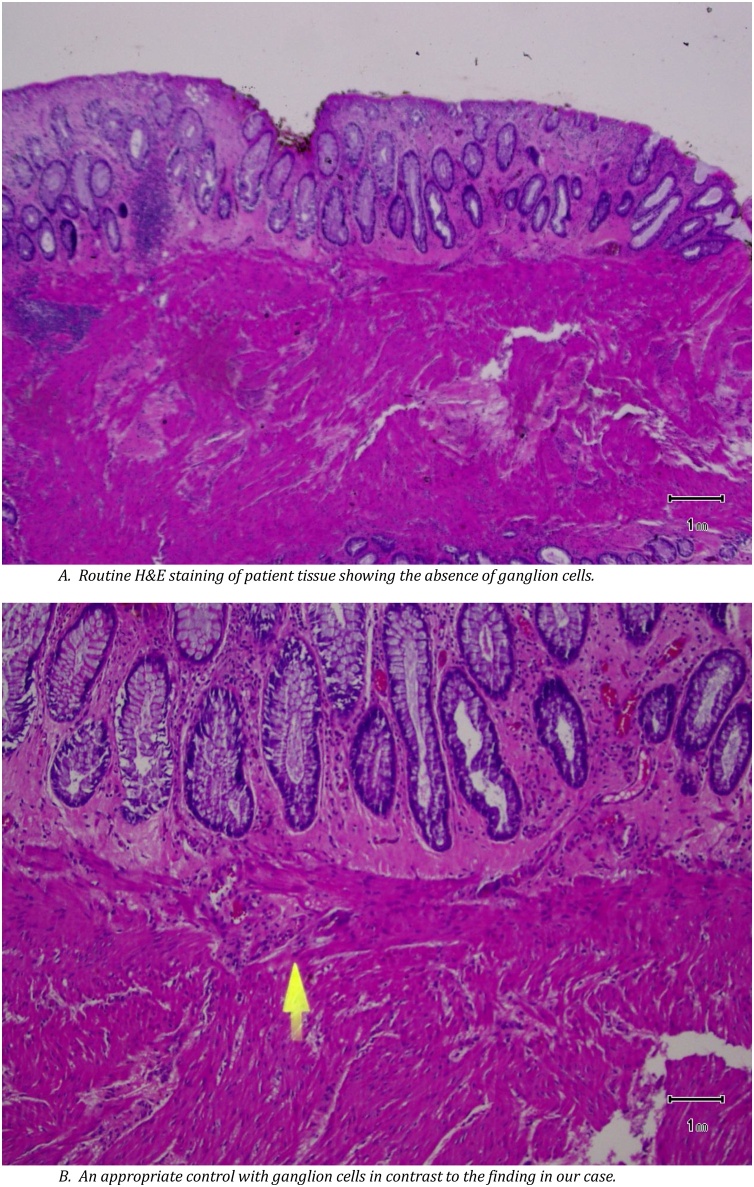
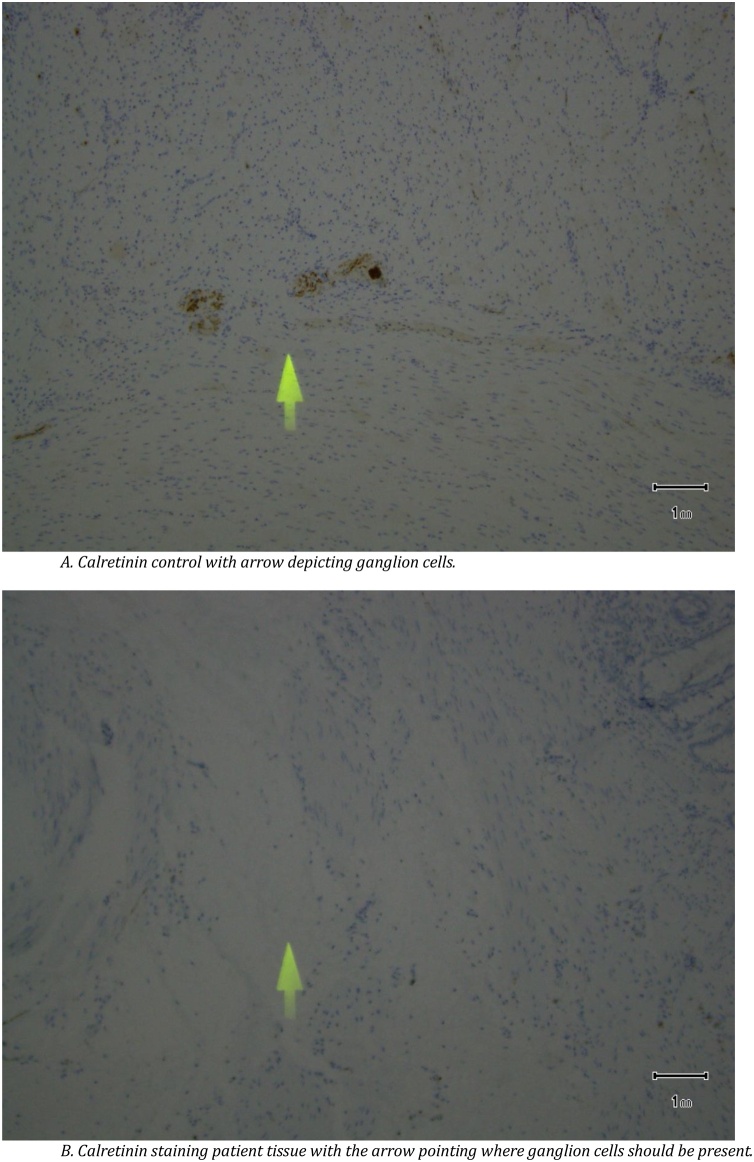
A 19-year-old Caucasian female patient was referred to General Surgery Department after her PCP found megacolon and megarectum in a computed tomography (CT) scan of the abdomen and pelvis (Fig. 1, Fig. 2, Fig. 3). Her evaluation and diagnostic procedures were performed by a board-certified general surgeon with colorectal surgery training. She had a lifelong history of intermittent abdominal pain as well as constipation requiring daily laxatives and enemas. Other than that, she did not have a history of medical or surgical problems. She did not smoke and her family history was unknown since she was raised by her aunt. Her abdominal exam was within normal limits. Her ano-rectal exam was normal on inspection, the digital rectal exam demonstrated a normal resting tone as well as hard stool in the rectal vault. The CT scan was significant for a severely distended sigmoid colon and rectum. Her lab work included a complete blood count and a comprehensive metabolic panel, these were within normal limits. Given her presentation the work up included: colonic transit study (Fig. 4), barium enema (Fig. 5) and a full thickness rectal biopsy after rectal de-impaction. Anal manometry was not performed. The colonic transit study (Sitz Marker Test) displayed most of the markers in her sigmoid colon and rectum at day number five. The Barium enema showed similar findings to those of the CT scan which included a dilated rectum starting immediately above the anorectal ring. She was taken to the operating room for a rectal biopsy. Under general anaesthesia and in lithotomy position a manual de-impaction was performed followed by rectal irrigation and a full thickness rectal biopsy. An anoscope was used to identify the level of the target area. In order to include the submucosa 2 full thickness rectal biopsies at 1.5 and 3 cm from dentate line in the posterior aspect were taken. Distal and proximal to this location a 2-0 Vicryl full thickness traction stitch was placed. This allowed total control of the tissue to ensure full thickness specimens. Both defects were closed with running 2-0 Vicryl. The patient tolerated the procedure well; she was discharged home the same day on a soft diet and had no post-operative complications. Pathology results showed the lack of ganglion cells in the submucosa (Fig. 6a-b). Routine H&E staining, as well as Immunohistochemistry stains for S100, Smooth Muscle Actin (SMA) and Calretinin were performed. Both SMA and Calretinin were negative despite appropriate controls which confirmed diagnosis of Hirschsprung’s disease (Fig. 7a-b). The patient was then referred to a tertiary care center. The chief of Pediatric Surgery took over her case. She underwent manual fecal de-impaction, a confirmatory rectal biopsy, laparoscopic seromuscular sigmoid biopsies for levelling and a diverting loop ileostomy for decompression of the dilated colon and rectum. The distal sigmoid colon biopsy showed few ganglion cells; the proximal colon had a normal amount. Seven months later she underwent an uneventful laparoscopic proctectomy with perianal colo-anal pull through procedure of which she recovered well. The operation was uneventful. Two months later her loop ileostomy was closed. She has been doing well. She is having two bowel movements a day with very good control and no longer suffers from constipation.
Fig. 1.
CT scan showing megarectum and fecaloma in a coronal section.
Fig. 2.
CT scan showing megarectum and fecaloma in a sagittal section.
Fig. 3.
CT scan showing megarectum and fecaloma in the axial section.
Fig. 4.
Colonic transit study with most of the markers in the sigmoid colon and rectum.
Fig. 5.
Barium enema showing dilated rectum and sigmoid colon.
Fig. 6.
A. Routine H&E staining of patient tissue showing the absence of ganglion cells. B. An appropriate control with ganglion cells in contrast to the finding in our case.
Fig. 7.
A. Calretinin control with arrow depicting ganglion cells. B. Calretinin staining patient tissue with the arrow pointing where ganglion cells should be present.
3. Clinical discussion
Hirschsprung’s disease is rare in adults; it is usually diagnosed during infancy. The first case was reported in 1887 and the first case in adults in 1950 [5,6]. It is defined as the complete absence of ganglion cells within the colonic wall and an absent recto-anal inhibitory reflex. It is the most common of the gastrointestinal neuromuscular disorders (GINMD); a group of disorders that also include Hypoganglionosis, Ganglioneuromatosis, intestinal neuronal dysplasia, myopathies and abnormalities of the interstitial cells of Cajal [7,8]. The incidence rate of adult HD is hard to establish since the condition gets typically overlooked in adults; its known incidence is about 1 in 1700 live births and it affects males more than females with the ratio of 2-4:1. It is believed that 2% of patients with chronic constipation are affected by the disease. These patients outgrow the disease most likely due to a short aganglionic segment of less than 10 cm in length [9]. Most commonly these patients initially present to their PCP, hence the importance of recognizing the condition so proper referrals can be arranged.
Once the diagnosis is clinically suspected work up usually includes imaging studies, anal manometry and full thickness rectal biopsies. HD is mostly confined to the rectum (79.8%), followed by the recto-sigmoid junction (12.5%) and the descending colon (0.8%). A markedly dilated proximal colonic segment with a transition zone and a narrowed distal colonic segment on CT and double-contrast barium enema suggests adult HD [10]. If available anorectal manometry should be performed; our hospital however does not offer this diagnostic test and the closest facility performing it is more than 4 h way. Anorectal manometry’s sensitivity (97%) is similar to that of a full thickness rectal biopsy [11]. The latter is however the gold standard; accuracy depends on: the biopsy site, the representativeness of the tissue, the number of specimens and pathologist’s skill. The specimen can be obtained via suction, endoscopy or open full thickness surgical rectal wall excision in the operating room. An adequate rectal biopsy has been defined as measuring at least 3 mm in diameter with at least one- third of its thickness being submucosa, ideally one-half submucosa [12]. The diagnosis of HD requires full thickness pathology biopsies that are judged to be adequate and align properly. Multiple biopsies are on occasion necessary to prove the absence of ganglion cells. Immunostains can be adjunctive, as they were in this case, with Calretinin, S100 and SMA [13]. The S100 and Calretinin were noted to be negative for ganglion cells in this case. The slides underwent external review and consultation at the Mayo Clinic where they concurred with the diagnosis.
The final treatment of Hirschsprung’s disease is surgical. It involves bypassing or removing the aganglionic segment of the rectum. Operations include: The Duhamel technique, described in 1956 consists of a retro-rectal transanal pull-through and does not require transection of the rectum. The Swenson procedure includes a levelling procedure where extra mucosal biopsies are taken along the antimesenteric border of the sigmoid to determine the level of ganglionated bowel. The rectum is mobilized and affected bowel and rectum excised, followed by a colo-anal anastomosis. The Soave procedure consists of stripping the rectal mucosa and the muscular cuff of the rectum is maintained; a ganglionic segment of colon is brought down and a colo-anal anastomosis is performed [14]. Our patient after a life long history of constipation finally was diagnosed and underwent a successful Swenson procedure. After three years her quality of life has dramatically improved. We want to raise awareness of this treatable condition; it is important for primary care physicians to suspect this entity in patients with a history of chronic constipation and abnormal dilated colon or rectum with a narrowing or transition zone.
4. Conclusion
Adults Hirschsprung’s disease is a rarely seen condition. PCPs should particularly be aware of this condition. Unnoticed cases can progress to adulthood. Team work and good communication among primary care physicians, gastroenterologists, surgeons and pathologists is essential for its diagnosis and management. Treatment of this condition can dramatically improve quality of life.
Conflicts of interest
None.
Funding
None.
Ethical approval
No ethical approval needed.
Consent
“Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Author contribution
All authors contributed to the following:
Study concept or design.
Data collection.
Data analysis or interpretation.
Writing the paper.
Registration of research studies
Not applicable.
Guarantor
Cesar Reategui MD FACS.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.De Lorijn F., Boeckxstaens G.E., Benninga M.A. Symptomatology, pathophysiology, diagnostic work-up, and treatment of Hirschsprung disease in infancy and childhood. Curr. Gastroenterol. Rep. 2007;9(June):245–253. doi: 10.1007/s11894-007-0026-z. PubMed PMID: 17511924. [DOI] [PubMed] [Google Scholar]
- 2.Martucciello G. Hirschsprung’s disease, one of the most difficult diagnoses in pediatric surgery: a review of the problems from clinical practice to the bench. Eur. J. Pediatr. Surg. 2008;18(June):140–149. doi: 10.1055/s-2008-1038625. PubMed PMID: 18493886. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P.R., Lennard-Jones J.E., Howley P.R., Todd I.P. Hirschprung’s disease and idiopathic mega- colon in adults and adolescents. Gut. 1986;27(May):534–541. doi: 10.1136/gut.27.5.534. PubMed PMID: 3699562: PubMed Central PMCID: PMC1433503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(December):226–230. doi: 10.1016/j.ijsu.2020.10.034. Epub 2020 Nov 9. PMID: 33181358. [DOI] [PubMed] [Google Scholar]
- 5.Hirschsprung H. Stuhlträgheit neugeborener in folge von dilatation and hypertrophic des colons. Jahrb Kinderh. 1887;27:1–3. [Google Scholar]
- 6.Rosin J.D., Bargen J.A., Waugh J.M. Congenital megacolon of a man 54 years of age: report of case. Proc. Staff Meet. Mayo Clin. 1950;25(December (26)):710–715. PubMed PMID: 14797838. [PubMed] [Google Scholar]
- 7.Pescatori M., Mattana C., Castiglioni G.C. Adult megacolon due to total hypoganglionosis. Br. J. Surg. 1986;73(September (9)):765. doi: 10.1002/bjs.1800730930. PubMed PMID: 3756447. [DOI] [PubMed] [Google Scholar]
- 8.Russell M.B., Russell C.A., Niebuhr E. An epidemiological study of Hirschsprung’s disease and additional anomalies. Acta Paediatr. 1994;83(January (1)):68–71. doi: 10.1111/j.1651-2227.1994.tb12955.x. PubMed PMID: 8193476. [DOI] [PubMed] [Google Scholar]
- 9.Doodnath R., Puri P. A systematic review and meta-analysis of Hirschsprung’s disease presenting after childhood. Pediatr. Surg. Int. 2010;26(November (11)):1107–1110. doi: 10.1007/s00383-010-2694-2. PubMed PMID: 20725836. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.J. Hirschsprung disease and hypoganglionosis in adults: radiologic findings and differentiation. Radiology. 2008;247(2):428–434. doi: 10.1148/radiol.2472070182. [DOI] [PubMed] [Google Scholar]
- 11.Meinds R.J., Trzpis M., Broens P.M.A. Anorectal manometry may reduce the number of rectal suction biopsy procedures needed to diagnose hirschsprung disease. J. Pediatr. Gastroenterol. Nutr. 2018;67(September (3)):322–327. doi: 10.1097/MPG.0000000000002000. PubMed PMID: 29652729. [DOI] [PubMed] [Google Scholar]
- 12.Muise E.D., Cowles R.A. Rectal biopsy for Hirschsprung’s disease: a review of techniques, pathology, and complications. World J. Pediatr. 2016;12(May (2)):135–141. doi: 10.1007/s12519-015-0068-5. PubMed PMID: 26684314. [DOI] [PubMed] [Google Scholar]
- 13.Szylberg L., Marszałek A. Diagnosis of Hirschsprung’s disease with particular emphasis on histopathology. A systematic review of current literature. Prz. Gastroenterol. 2014;9(5):264–269. doi: 10.5114/pg.2014.46160. Published online 2014 Oct 18. doi: 10.5114/pg.2014.46160. PubMed PMID: 25395999; PubMed Central PMCID: PMC4223113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holschneider A.M., Puri P. 2nd ed. Harwood Academic Publishers; Amsterdam: 2000. Hirschsprung’s Disease and Allied Disorders. [Google Scholar]