Abstract
Objectives
The impact of rheumatic diseases on COVID-19 infection remains poorly investigated. Here we performed a systematic review and meta-analysis to evaluate the outcomes of COVID-19 in patients with rheumatic diseases.
Methods
We systematically searched PubMed, Embase, Cochrane Library, Scopus and preprint database up to 29th August 2020, for publications with confirmed COVID-19 infection in patients with rheumatic diseases. The primary outcomes were the rates of hospitalization, oxygen support, intensive care unit (ICU) admission and death. A meta-analysis of effect sizes using the random-effects models was performed, and meta-regression analyses were performed to explore heterogeneity. The data from the COVID-19 Global Rheumatology Alliance physician registry (the COVID-19 GRA) was used as a reference.
Results
A total of 31 articles involving 1138 patients were included in this systematic review and meta-analysis. The publications were from Europe, Asia and North America, but none from other continents. The overall rates of hospitalization, oxygen support, ICU admission and fatality among COVID-19 infected patients with rheumatic diseases were 0.58 (95% confidence interval (CI) 0.48–0.67), 0.33 (95% CI 0.21–0.47), 0.09 (95% CI 0.05–0.15) and 0.07 (95% CI 0.03–0.11), respectively. The rate of oxygen support in Europe (0.48, 95% CI 0.4–0.57) was higher than that in other continents. Among all hospitalized patients, the rates of oxygen support, ICU admission and fatality were 0.61 (95% CI 0.48–0.73), 0.13 (95% CI 0.07–0.21) and 0.13 (95% CI 0.09–0.18), respectively. The fatality rate was highest in Europe (0.19, 95% CI 0.15–0.24). The fatality rate was higher both in this meta-analysis and the COVID-19 GRA (7.0% and 6.7%, respectively) than that (3.4%) in WHO database, although the age, gender and comorbidity were not matched.
Conclusion
Patients with rheumatic diseases remain vulnerable with substantial rates of severe outcomes and a geographic variation. More studies were urgently needed to elucidate the risk factors of severe outcomes in this population.
Keywords: Rheumatic diseases, COVID-19, Outcomes, SARS-CoV-2
1. Introduction
An outbreak of infection with the novel coronavirus (SARS-CoV2) since December 2019 has rapidly emerged as a pandemic [1]. Globally, more than 24 million confirmed cases of coronavirus disease 2019 (COVID-19) had been reported, with 833,951 confirmed death by 29th August 2020 [2]. COVID-19 does not only cause respiratory illness but also leads to uncontrolled inflammatory response and hypercoagulation through activating both the innate and adaptative immune system [3]. The presence of comorbidities, such as hypertension or diabetes mellitus, is a risk factor for disease severity and fatality [4]. It is imperative to evaluate the outcomes of COVID-19 in patients with rheumatic diseases. Firstly, patients with rheumatic diseases are vulnerable given the background of dysregulated immune response and the use of antirheumatic drugs with different degrees of immunosuppression. An exaggerated immune response may occur following an acute viral infection, for instance, the uncontrolled type I interferon activation in patients with systemic lupus erythematosus (SLE). Secondly, multiple cytokine has been involved in the pathogenesis of severe COVID-19 [[5], [6], [7]]. Anti-rheumatic drugs may dampen the hyperinflammatory state, becoming a potential treatment for COVID-19. Hydroxychloroquine (HCQ) and tocilizumab were promising candidates [8], but subsequent studies did not demonstrate their efficacy to improve clinical outcomes in COVID-19 infected patients or for post-exposure prophylaxis of close contacts [[9], [10], [11]]. Glucocorticoids, which are widely used in rheumatic diseases, has remained controversial in the treatment of COVID-19 infected patients [12]. The RECOVERY trial, however, demonstrated the beneficial effect of dexamethasone resulting in lower mortality among those who were receiving either invasive mechanical ventilation or oxygen alone [13]. Although a sustained elevation of interleukin (IL)-6 are associated with worse outcomes of COVID-19 [14], the result from a recent trial was disappointing [11]. A systematic review and meta-analysis was recently published and showed that the effect of most anti-rheumatic disease therapies in COVID-19 remained inconclusive [15].
At present, the impact of COVID-19 on patients with rheumatic diseases is poorly understood. Whether a poorer outcome due to the immunocompromised status among patients with rheumatic disease, or better outcomes with anti-rheumatic therapies that mitigate the hyperinflammation is unknown [16]. No specific rheumatic diseases was identified as risk factors for poor outcomes with COVID-19 in version 2 of American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic [17]. There is also a knowledge gap of the geographic differences in the severity and fatality rate among COVID-19 infected patients with rheumatic diseases. Recently, several studies reported the clinical and prognostic characteristics of COVID-19-infected patients with rheumatic diseases [[18], [19], [20], [21], [22], [23]]. However, there were concerns about the limitations of small sample sizes, heterogeneity of methodology, and a lack of generalizability to the overall population of COVID-19 patients with rheumatic diseases. The COVID-19 Global Rheumatology Alliance physician registry (The COVID-19 GRA) has been making a great effort to collect information pertinent to COVID19 infection in patients with rheumatic diseases, but it is voluntary and predominantly from western countries [24]. Therefore, we conducted this systematic review and meta-analysis of observational studies on COVID-19 patients with rheumatic disease, to summarize the published and preprint literature that described the clinical characteristics and outcomes of COVID-19 patients with rheumatic diseases, and to provide a higher level of evidence for better clinical decision making.
2. Methods
This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two reviewers (Ruyi Cai and Zixi Yi) undertook the literature search, assessment for eligibility and data extraction independently. A third reviewer (Chuanhui Xu) would adjudicate if there were any discordant findings between the two independent reviewers. The research was registered and approved in PROSPERO (CRD42020206279).
2.1. Search strategy
For a systematic and comprehensive search, we searched PubMed, Embase, Cochrane Library and Scopus for papers published up to 29th August 2020. Publications on preprint servers (medRxiv, bioRxiv and ChinaXiv) were also reviewed. Key/relevant MeSh terms and keywords included the following keywords: “2019-ncov”, “novel coronavirus”, “COVID-19”, “SARS-CoV-2”, “new coronavirus”, “coronavirus disease 2019”, “wuhan pneumonia”) AND (“rheumatic disease”, “rheumatic condition”, “autoimmune disease”, “autoimmune condition”, “connective tissue disease”, “musculoskeletal disease”, “muscle and skeletal disease”, “Arthritis”, “Systemic Lupus Erythematosus”, “Spondylarthritis”, “spondyloarthropathy”, “vasculitis”, “Sjogren's Syndrome”, “Scleroderma”, “systemic sclerosis”, “Osteoarthritis”, “Antiphospholipid Syndrome”, “myositis”, “gout”. Detailed literature search strategies are shown in Supplementary Table 1–5. Two investigators (Ruyi Cai and Zixi Yi) screened titles and abstracts of identified articles independently. Full text of identified studies was then further reviewed by the two reviewers.
2.2. Study selection and data extraction
All studies containing the epidemiological and clinical information of COVID-19-infected rheumatic patients were identified. Inclusion criteria included: (1) studies reporting data on COVID-19 confirmed patients, survivors, or COVID-19 related death; (2) studies containing available epidemiological or clinical data of COVID-19-infected patients with rheumatic disease; (3) studies limited to humans; (4) had one of the following outcomes: hospitalization, requirement for oxygen support, intensive care unit (ICU) admission, or death; (5) only the latest study to be included in the analysis if duplicated studies from same population or database were reported. The exclusion criteria were as follows: (1) duplicated studies; (2) reviews, conference proceedings, commentaries, quality of life studies, cost-effectiveness analyses; (3) those in which the rheumatic disease data or COVID-19 data could not be ascertained; (4) rheumatic diseases induced by COVID-19; (5) small sample size (≤3). The publications from the COVID-19 GRA were not included for the following reasons: (1) the data were voluntarily reported; (2) inability to ascertain whether the cases were also included in other studies; (3) lack of detail of the country/continent from which these data originated; (4) the data was used as a reference for this meta-analysis.
Two investigators (Ruyi Cai and Zixi Yi) independently reviewed potentially relevant articles, and disagreements were discussed and resolved with consensus and, if necessary, by involving the third reviewer (Chuanhui Xu).
The following information was extracted: country, continent, study design type, sample size, sex, age, rheumatological diagnosis, outcomes (hospitalization, oxygen support, ICU admission, death).
2.3. Endpoint setting and stratification strategy
Our primary outcomes were rates of hospitalization, oxygen support, ICU admission and fatality in COVID-19 infected patients with rheumatic diseases. The stratification strategy we adopted for the subgroup analysis was by continent: patient populations in Europe, Asia, and North America were analyzed respectively.
2.4. Data analysis
The pooled proportions with their corresponding 95% confidence intervals (CI) were calculated to estimate the rates of hospitalization, oxygen support, ICU admission and fatality in COVID-19 infected patients with rheumatic diseases. The Freeman-Tukey double arcsine transformation was used for pooled estimates to stabilize the variances. The I2 statistic and Q test were used to measure the between-study heterogeneity. If I2 > 50% and P < 0.1, the heterogeneity was considered high, and the summary rates were combined under with a random effects model; otherwise a fixed-effects model were used. The Z test was used to assess the statistical significance of pooled rates, and two-tailed P < 0.05 were considered significant. To explore potential sources of heterogeneity, subgroup analyses were performed based on the continent. Visual inspection of funnel plots and Egger's regression asymmetry test were applied to assess potential publication bias. STATA 14.0 (Stata Corporation, College Station, Texas, USA) was used for statistical analyses.
3. Results
3.1. Study selections
The initial search of databases yielded 1822 articles, which were narrowed down to 26 after removing 932 duplicated records, 716 unrelated publications by title and abstract screening, and 148 publications including reviews, case reports, data from Global Rheumatology Alliance registry and papers with small sample size (≤3), patients all admitted in ICU or without relevant outcome data. Five articles from preprint servers (medRxiv, bioRxiv and ChinaXiv) were added. The study selection flowchart was shown in Supplement Fig. 1.
3.2. Overall study characteristics
A total of 31 articles involving 1138 patients were assessed in this systematic review and meta-analysis. Characteristics of the included studies were shown in Table 1, Table 2 . There were 16 articles (n = 807) from Europe, 8 articles (n = 122) from Asia and 7 articles (n = 274) from North America (all from the US), respectively. No publication from South America, Africa or Oceania. There were ten studies which only included hospitalized patients (Table 2). About 64% of the patients were female. Various rheumatological diseases were reported, including 32.8% patients with rheumatoid arthritis (RA), 15.3% SLE, 22.3% spondyloarthritis, 6.07% vasculitis, 2.76% Sjogren syndrome, 5.85% inflammatory myopathy, 1.77% systemic sclerosis, 0% gout and 13.1% others (Supplementary Table 6). However, the outcomes were reported as aggregated outcomes for the respective cohort of patients without further classification by rheumatic disease type.
Table 1.
The summary of characteristics of studies recruiting patients from community.
| First author | Country | Continent | Study design | No. of patients | Age (years) mean ± sd,/median(range) | Sex |
|
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Wallace et al. [48] | America | North America | cohort | 31 | 61 (28–82) | 9 | 22 |
| Haberman et al. [49] | America | North America | case series | 59 | 50 (25–73) | 7 | 7 |
| Gartshteyn et al. [50] | America | North America | case series | 10 | 44.3 | 1 | 9 |
| Fernandez-Ruiz et al. [34] | America | North America | cohort | 41 | 47 ± 17.19 | 3 | 38 |
| Veenstra et al. [35] | America | North America | cohort | 77 | – | – | – |
| D'silva et al. [51] | America | North America | cohort | 52 | 62.5 ± 15.1 | 16 | 36 |
| Mathian et al. [52] | France | Europe | cohort | 17 | 53.5 (26.6–69.2) | 4 | 13 |
| Aries et al. [44] | Germany | Europe | cross-sectional | 30 | – | – | – |
| Ansarin et al. [20] | Iran. | Asia | cross-sectional | 30 | 55.1 ± 13.6 | 7 | 23 |
| Scirè et al. [53] | Italy | Europe | cross-sectional | 232 | 62.2 ± 13.9 | 83 | 149 |
| Bozzalla Cassione et al. [54] | Italy | Europe | case series | 4 | 52.5 (27–53) | 0 | 4 |
| Fredi et al. [19] | Italy | Europe | case-control | 65 | 68 (55–76) | 24 | 41 |
| Favalli et al. [55] | Italy | Europe | cohort | 6 | 49 ± 20.42 | 2 | 4 |
| Monti et al. [56] | Italy | Europe | case series | 4 | 58 ± 5 | 0 | 4 |
| Quartuccio et al. [57] | Italy | Europe | cross-sectional | 4 | 60.25 ± 12.6 | 2 | 2 |
| Santos et al. [43] | Spain | Europe | cross-sectional | 30 | Female 61.8 (46.5–75) male 68 (48.5–72) |
12 | 18 |
| Pablos et al. [23] | Spain | Europe | cohort | 228 | 63 (54–78) | 87 | 141 |
| Queiro Silva et al. [58] | Spain | Europe | cross-sectional | 7 | 49.2 ± 6.8 (37–56) | 4 | 3 |
| Espinosa et al. [33] | Spain | Europe | cross-sectional | 4 | 43.75 ± 5.54 | 0 | 4 |
| Michelena et al. [59] | Spain | Europe | cross-sectional | 11 | 45 (30,63) | 6 | 5 |
| Freites Nuñez et al. [60] | Spain | Europe | cohort | 123 | 59.88 (14.90) | 37 | 86 |
Table 2.
The summary of characteristics of studies only recruiting hospitalized patients.
| First author | Country | Continent | Study design | No. of patients | Age (years) mean + −sd,/ median(range) | Sex |
|
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Sharmeen et al. [37] | America | North America | case series | 4 | Male (78,49) Female (76,27) |
2 | 2 |
| Cheng et al. [36] | China | Asia | cross-sectional | 5 | 66 (61–72) | 1 | 4 |
| Ye et al. [18] | China | Asia | case series | 21 | – | ||
| Lin et al. [40] | China | Asia | cohort | 11 | 55 (25,71) | 1 | 10 |
| Zhao et al. [61] | China | Asia | cross-sectional | 29 | Median 61 | 4 | 25 |
| Huang et al. [41] | China | Asia | cross-sectional | 17 | 64.0 (60.5–71.5) | 3 | 14 |
| Benucci et al. [62] | Italy | Europe | cross-sectional | 4 | 60 ± 9.5 | 1 | 3 |
| Teh et al. [39] | Malaysia | Asia | cross-sectional | 5 | 42.8 ± 18.3 | 0 | 5 |
| Wan et al. [42] | Malaysia | Asia | cross-sectional | 4 | – | 0 | 4 |
| Santos et al. [38] | Spain | Europe | cohort | 38 | Survivors 75.1 (69.3–75.8), Deceased 78.4 (74.5–83.5) | 18 | 20 |
3.3. Outcomes of COVID-19 infected patients with rheumatic diseases
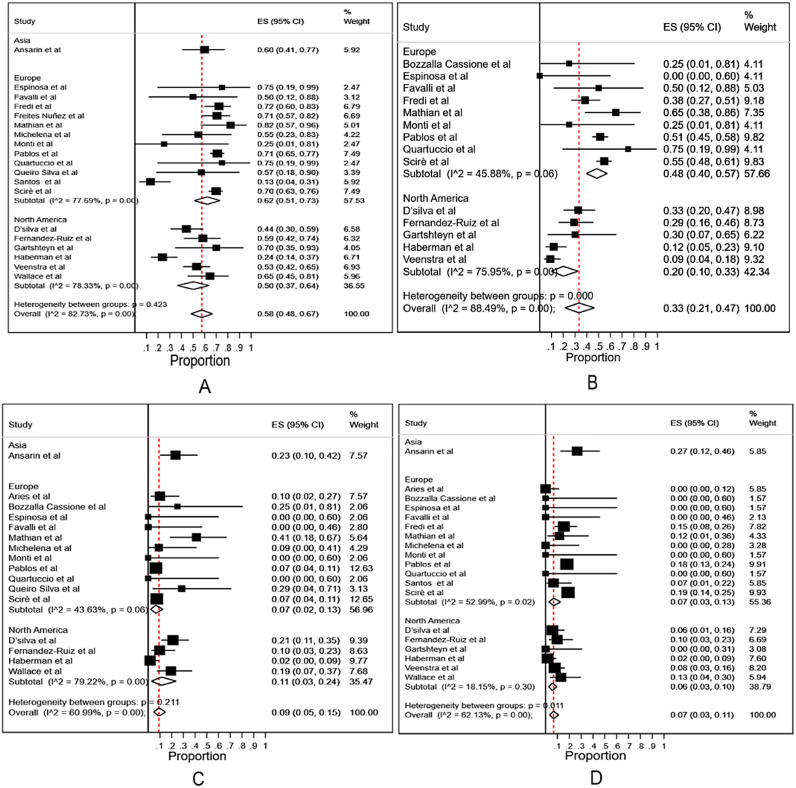
The outcomes would be different between the patients recruited from the community and those only from hospitalized patients. We first analyzed all the data excluding the studies which only recruited hospitalized patients (data from Table 1, excluded data from Table 2 that only recruited hospitalized patients). The overall hospitalization rate among COVID-19 infected patients with rheumatic diseases was 0.58 (95% CI 0.48–0.67, Fig. 1A). The rates of oxygen support, ICU admission and fatality were 0.33 (95% CI 0.21–0.47), 0.09 (95% CI 0.05–0.15) and 0.07 (95% CI 0.03–0.11), respectively (Fig. 1B - D). Subgroup analysis among different continents showed no differences with regards to the rates of hospitalization(0.62 (95% CI 0.51–0.73) in Europe, 0.5 (95% CI 0.37–0.64) in North America and 0.60 (95% CI 0.41–0.77) in Asia) and ICU admission(0.07 (95% CI 0.02–0.13) in Europe, 0.11 (95% CI 0.03–0.24) in North America and 0.23 (95% CI 0.1–0.42) in Asia) (Fig. 1A and Fig. 1C). The rate of oxygen support was higher in Europe (0.48, 95% CI 0.4–0.57) than that in North American (0.20, 95% CI 0.10–0.33) (Fig. 1B). The fatality rate was higher in Asia(0.27, 95% CI 0.12–0.46) (Fig. 1D), but only one study was available from Asia (Iran, n = 30) [20] for subgroup analysis among different continents.
Fig. 1.
Meta-analysis and subgroup analysis of the rates of hospitalization, oxygen support, ICU admission and fatality in COVID-19 infected patients with rheumatic diseases in different continents, excluding the studies only recruiting hospitalized patients.
A: the rates of hospitalization. B: the rates of oxygen support. C: the rates of ICU admission. D: the rates of fatality.
Overall: meta-analysis of the rates in Asia, Europe and North America.
Subtotal: subgroup meta-analysis of the rates in different continents.
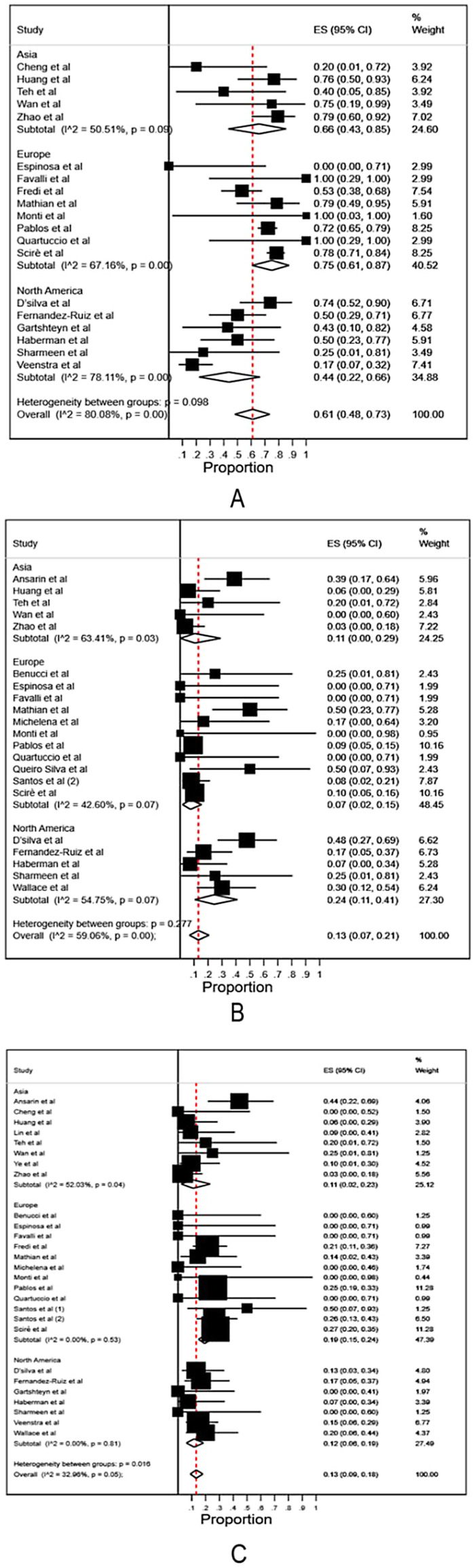
We then anlyzed the data among all hospitalized patients (data of hospitalized patients from Table 1 and all data from Table 2). The rates of oxygen support, ICU admission and fatality among all hospitalized patients were 0.61 (95% CI 0.48–0.73), 0.13 (95% CI 0.07–0.21) and 0.13 (95% CI 0.09–0.18), respectively (Fig. 2A - C). Subgroup analysis among different continents showed the rate of oxygen support was 0.66 (95% CI 0.43–0.85) in Asia, 0.75 (95% CI 0.61–0.87) in Europe, and 0.44 (95% CI 0.22–0.66) in North America, respectively, without significant difference (Fig. 2A). The rate of ICU admission was 0.11 (95% CI 0.00–0.29) in Asia, 0.07 (95% CI 0.02–0.15) in Europe, and 0.24 (95% CI 0.11–0.41) in North America, respectively, without significant difference (Fig. 2B). The fatality rate was higher in Europe (0.19, 95% CI 0.15–0.24) than that in North America (0.12, 95% CI 0.06–0.19) and Asia (0.11, 95% CI 0.02–0.23) (Fig. 2C).
Fig. 2.
Meta-analysis and subgroup analysis of the rates of oxygen support, ICU admission and fatality in COVID-19 infected patients with rheumatic diseases in different continents, including the studies only recruiting hospitalized patients.
A: the rates of oxygen support. B: the rates of ICU admission. C: the rates of fatality.
Overall: meta-analysis of the rates in Asia, Europe and North America.
Subtotal: subgroup meta-analysis of the rates in different continents.
3.4. Comparison with data from the COVID-19 GRA and WHO
There could be differences between this meta-analysis and the data from the COVID-19 GRA as the latter was voluntarily reported. As shown in Table 3 , there was a difference in specific diseases but largely comparable. There were fewer female patients (64%) in this meta-analysis compared with that (75.9%) in the COVID-19 GRA (Table 3). The rate of hospitalization and death were higher in this meta-analysis than that in the COVID-19 GRA (58% vs 32.8% and 7.0% vs 6.7%, respectively) (Table 3) [25]. It was not possible to compare the rates of oxygen support and ICU admission because there were 11.9% of patients who required ventilation, but the type of ventilation used was unknown in the COVID-19 GRA [25]. The fatality rate was higher both in this meta-analysis and the COVID-19 GRA (7.0% and 6.7%, respectively) than that (3.4%) in WHO database [2], but the age, gender and comorbidities were not matched.
Table 3.
Comparison with data from The COVID-19 Global Rheumatology Alliance Global Registry.
| Meta-analysis | Global Registry | |
|---|---|---|
| Female % | 64% | 76% |
| Rheumatoid arthritis | 33.7% | 38.4% |
| Spondyloarthritisa | 22.0% | 15.5% |
| Systemic Lupus Erythematosus | 14.3% | 20.7% |
| Vasculitis | 5.5% | 5.6% |
| Inflammatory myopathy | 5.2% | 2.4% |
| Sjogren syndrome | 3.3% | 3.8% |
| Systemic sclerosis | 1.7% | 3.4% |
| Gout | 0% | 2.4% |
| Othersb | 14.4% | 5.7% |
| Hospitalized | 56% | 33% |
| Death | 7.0% | 6.7% |
Merged data of spondyloarthritis and psoriatic arthritis in the COVID-19 Global Rheumatology Alliance Global Registry.
: merged data of other inflammatory arthritis, sarcoidosis and undifferentiated connective tissue disease in the COVID-19 Global Rheumatology Alliance Global Registry (accessed on 13rd November 2020).
3.5. Publication bias analysis
Visual inspection of funnel plots and Egger's test were used to evaluate the publication bias. The funnel plot was displayed in Supplementary Fig. 2 and Supplementary Fig. 3. The statistical results showed there was no publication bias in our study (Egger's test P = 0.439 for hospitalization, P = 0.661 for oxygen support, P = 0.446 for ICU admission and P = 0.172 for fatality in COVID-19 infected patients with rheumatic diseases, excluding the studies only recruiting hospitalized patients; Egger's test P = 0.295 for oxygen support, P = 0.394 for ICU admission and P = 0.141 for fatality in COVID-19 infected patients with rheumatic diseases among all hospitalized patients, including the studies only recruiting hospitalized patients).
4. Discussion
This systematic review and meta-analysis of global data summarized the clinical outcomes of COVID-19 in patients with rheumatic diseases, including 1138 patients with various rheumatic diseases from 31 studies. Overall, the rates of hospitalization, oxygen support, ICU admission and fatality among COVID-19 infected patients with rheumatic diseases was 0.58 (95% CI 0.48–0.67), 0.33 (95% CI 0.21–0.47), 0.09 (95% CI 0.05–0.15) and 0.07 (95% CI 0.03–0.11), respectively. Among the patients hospitalized, the rates of oxygen support, ICU admission and fatality were 0.61 (95% CI 0.48–0.73), 0.13 (95% CI 0.07–0.21) and 0.13 (95% CI 0.09–0.18), respectively. The results imply that rheumatic disease is a risk factor for poor outcomes in patients with COVID-19. Of note, the fatality was higher in Europe among hospitalized patients.
We noted the heterogeneity of publications. Most cases were reported from Europe with a higher fatality. Fewer cases were reported from Asia, most of which only recruited hospitalized patients. The heterogeneity among different studies and different continents may be due to several reasons. Firstly, the capacity for screening and diagnosis of COVID-19 cases evolved over the course of the pandemic, limited initially by access to test kits and reagents, manpower and laboratory infrastructure. Over time, testing was gradually scaled up to include community testing with the increasing sophistication of test kits with rapid turnaround times, improving the accuracy of reporting. The cases may be under or not reported in some developing countries with challenges in access to testing and consolidated reporting of cases. Secondly, many countries implemented different policies during different stages of the pandemic. Some countries, such as China, South Korea, Singapore and New Zealand, adopted early intensive measures to contain COVID-19 with strict contact tracing, mandatory mask-wearing, safe social distancing, early lockdowns and implementation of infectious disease legislations. In contrast, the restriction may have been more lenient among some countries in Europe and North America during the early days of the pandemic. Containment of milder cases in the form of home or community quarantine facilities, hospitalization only for more severe cases, and palliative care for nursing home residents without hospital transfers would also have impacted the profile and outcomes of non-hospitalized vs hospitalized patients. Notably, 13 out of 15 studies were from Spain and Italy (except 1 from Germany and one from France. Table 1) where were most severely affected by COVID-19 in Europe [26] Thirdly, the genotypes of SARS-CoV2 due to mutation may vary in different regions, or may have evolved over time, both for imported and community transmitted cases, which may explain the different severities of the disease. A previous study showed that the virus genomes in Europe and America were different from those in Asia [27]. Fourthly, the ethnic disparities of outcomes in COVID-19 patients may contribute to the heterogeneity. For instance, the mortality is lower among South Asian compared to White British patients from the study in Bradford [28]. Similarly, the disparities in the risk and outcomes of COVID-19 were also reported by Public Health England [29].
There were differences in terms of proportions of specific rheumatic diseases, gender and outcomes when this study was compared with the COVID-19 GRA data. The COVID-19 GRA was a commendable effort from the global rheumatology community. The results were mutually corroborated between our meta-analysis and the COVID-19 GRA, although differences exist. Furthermore, our meta-analysis added the data from Asia, which were scant in the COVID-19 GRA with only <5 out of 600 from the Asian region [24]. Our meta-analysis included eight studies (n = 122) from Asia, mainly from China and Malaysia. There were studies that showed no case of rheumatic disease contracted COVID-19 in Hong Kong [30], and the incidence of COVID-19 infection was low in the Asia Pacific Lupus Collaboration patient cohort [31]. Reporting on COVID-19 in patients with rheumatic diseases should be encouraged from other countries heavily burdened by COVID-19 infection, such as India, Indonesia and Philippine.
A meta-analysis on the prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases was published by Akiyama et al. [32]. However, the results should be interpreted with cautions. The fatality rate was higher (0.113, 95% CI 0.098 to 0.13) than our study (0.07, 95% CI 0.03–0.11) and the COVID-19 GRA data (0.067). There were total 16 publications included in our meta-analysis but not in the study by Akiyama et al. Three publications were missed [[33], [34], [35]], eight were excluded because only hospitalized patients were recruited [18,[36], [37], [38], [39], [40], [41], [42]], four published after 31st July 2020 when is the updated time of literature search by Akiyama et al. [19,20,43,44]. Furthermore, we were not certain whether the cases were duplicated in published data and the COVID-19 GRA data. Therefore, it would not have been appropriate to include COVID-19 GRA data for the meta-analysis.
The strengths of our systematic review and meta-analysis were the rigorous application of systematic review methodology and a comprehensive search of the literature, which included published and preprint archives. We distinguished whether the studies only recruited hospitalized patients, and compared with the COVID-19 GRA data. A few limitations existed in this study. Firstly, most publications included were small case series and cohort studies. Secondly, the criteria for hospitalization and ICU admission may differ in different countries, or different centres in the same country due to resource limitations, differences in healthcare and financing models of care. Nevertheless, it would have reflected the severity of the disease. Thirdly, the details of the outcomes were mostly unavailable for specific rheumatic disease and specific treatments. The immunomodulatory effects of conventional synthetic disease modifying anti-rheumatic drugs (DMARDs) would be different from biologic synthetic DMARDs versus targeted therapies and immunosuppressants. Even though no robust data has shown that anti-rheumatic drugs were effective for the treatment of COVID-19 [15], including the most recently published randomized control trial of tocilizumab [11]. The use of anti-rheumatic drugs before the onset of COVID-19 infection may also have had an impact on COVID-19 rheumatology patients with bad prognostic factors and may be context-dependent [45]. For instance, accumulating data showed that methotrexate reduced the risk of cardiovascular events in patients with rheumatoid arthritis [46], but not in the general population with a high risk of cardiovascular events [47]. Therefore, researchers and the COVID-19 GRA are strongly encouraged to report the outcomes in specific rheumatic disease and with a specific treatment, which may shed light on the safety and effectiveness of anti-rheumatic drugs in COVID-19 patients with rheumatic diseases. We appreciate that the heterogeneity of treatments even among a certain rheumatic disease, let alone the less common diseases may still make interpretation of aggregated data challenging.
This systematic review and meta-analysis inform a comprehensive picture of the clinical outcomes of COVID-19 patients with rheumatic diseases. These patients remain vulnerable, given the significant rates of ICU admission with a high risk of fatality. This study emphasized the urgent need for more data with a larger sample size, more detailed treatment and disease-specific outcomes, longer-term follow-up, and sociodemographic and clinicopathological variables.
Declaration of Competing Interest
This work is not funded by any organization. The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.autrev.2021.102778.
Appendix A. Supplementary data
Supplementary Fig. 1: Study Exclusion and Inclusion Flowchart.
Supplementary Fig. 2: The funnel plot of hospitalization, oxygen support, ICU admission and fatality in all COVID-19 infected patients with rheumatic diseases, excluding the studies only recruiting hospitalized patients.
Supplementary Fig. 3: The funnel plot of oxygen support, ICU admission and fatality in hospitalized COVID-19 infected patients with rheumatic diseases, including the studies only recruiting hospitalized patient.
References
- 1.Gates B. Responding to Covid-19 - a once-in-a-century pandemic? N Engl J Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 2.Coronavirus disease (COVID-19) pandemic view dashboard. World Health Organisation. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed on 29th August 2020.
- 3.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z.J., McGoogan M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Raucci F., Mansour A.A., Casillo G.M., Saviano A., Caso F., Scarpa R., et al. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun Rev. 2020;19(7):102572. doi: 10.1016/j.autrev.2020.102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brikman S., Bieber A., Dori G. The hyper-inflammatory response in adults with severe COVID-19 pneumonia differs from the cytokine storm of hemophagocytic syndrome. Isr Med Assoc J. 2020;8(22):439–447. [PubMed] [Google Scholar]
- 7.Ruscitti P., Giacomelli R. Ferritin and severe COVID-19, from clinical observations to pathogenic implications and therapeutic perspectives. Isr Med Assoc J. 2020;8(22):450–452. [PubMed] [Google Scholar]
- 8.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. New Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 Jul 17 (NEJMoa2021436) [Google Scholar]
- 14.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putman M., Chock Y.P.E., Tam H., Kim A.H.J., Sattui S.E., Berenbaum F., et al. Antirheumatic disease therapies for the treatment of COVID-19: A systematic review and meta-analysis. Arthritis Rheumatol. 2020 Aug 2 doi: 10.1002/art.41469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikuls T.R., Johnson S.R., Fraenkel L., Arasaratnam R.J., Baden L.R., Bermas B.L., et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 2. Arthritis Rheumatol. 2020 Sep;72(9):e1–e12. doi: 10.1002/art.41437. [DOI] [PubMed] [Google Scholar]
- 18.Ye C., Cai S., Shen G., Guan H., Zhou L., Hu Y., et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis. 2020;79(8):1007–1013. doi: 10.1136/annrheumdis-2020-217627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredi M., Cavazzana I., Moschetti L., AndreoliF L. Franceschini, COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case-control study. Lancet Rheumatol. 2020;2(9):e549–e556. doi: 10.1016/S2665-9913(20)30169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansarin K., Taghizadieh A., Safiri S., Malek Mahdavi A., Ranjbar S., Teymouri S., et al. COVID-19 outcomes in patients with systemic autoimmune diseases treated with immunomodulatory drugs. Ann Rheum Dis. 2020 Aug 5 doi: 10.1136/annrheumdis-2020-218737. (annrheumdis-2020-218737) [DOI] [PubMed] [Google Scholar]
- 21.Montero F., Martínez-Barrio J., Serrano-Benavente B., González T., Rivera J., Collada J.M., et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. 2020:1–6. doi: 10.1007/s00296-020-04676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong J., Shen G., Yang H., Huang A., Chen X., Dong L., et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2(9):e557–e564. doi: 10.1016/S2665-9913(20)30227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pablos J.L., Galindo M., Carmona L., Lledó A., Retuerto M., Blanco R., et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 24.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Data from The COVID-19 Global Rheumatology Alliance Global Registry. https://rheum-covid.org/updates/combined-data.html Accessed on November 13th, 2020.
- 26.Coronavirus (COVID-19) death rate in countries with confirmed deaths and over 1000 reported cases as of April 3, 2020, by country. Statista. https://www.statista.com/statistics/1105914/coronavirus-death-rates-worldwide/. Accessed Apr 2020.
- 27.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santorelli G., Sheldon T., West J., Cartwright C., Wright J. COVID-19 in-patient hospital mortality by ethnicity. Wellcome Open Res. 2020;5:86. doi: 10.12688/wellcomeopenres.15913.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi K. Ethnic disparities in COVID-19 mortality: are comorbidities to blame? The Lancet. 2020;396(10243):22. doi: 10.1016/S0140-6736(20)31423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.So H., Mak J.W.Y., Tam L.-S. No systemic lupus erythematosus with COVID-19 in Hong Kong: the effect of masking? J Rheumatol. 2020;47(10):1591. doi: 10.3899/jrheum.200605. [DOI] [PubMed] [Google Scholar]
- 31.Cho J., Kandane-Rathnayake R., Louthrenoo W., Hoi A., Golder V., Chen Y.H., et al. COVID-19 infection in patients with systemic lupus erythematosus: data from the Asia Pacific lupus collaboration. Int J Rheum Dis. 2020;23(9):1255–1257. doi: 10.1111/1756-185X.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheumatic Dis. 2020 Oct 13 doi: 10.1136/annrheumdis-2020-218946. annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 33.Espinosa G., Araujo O., Amaro S., Bodro M., Moreno P.J., Moreno R., et al. COVID-19 and Behçet's disease: clinical case series. Ann Rheum Dis. 2020 Jul 21 doi: 10.1136/annrheumdis-2020-217778. (annrheumdis-2020-217778) [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Ruiz R., Masson M., Kim M.Y., Myers B., Haberman R.H., Castillo R., et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2020 Dec;72(12):1971–1980. doi: 10.1002/art.41450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veenstra J., Buechler C.R., Robinson G., Chapman S., Adelman M., Tisack A., et al. Antecedent immunosuppressive therapy for immune-mediated inflammatory diseases in the setting of a COVID-19 outbreak. J Am Acad Dermatol. 2020;83(6):1696–1703. doi: 10.1016/j.jaad.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng C., Li C., Zhao T., Yue J., Yang F., Yan Y., et al. COVID-19 with rheumatic diseases: a report of 5 cases. Clin Rheumatol. 2020;39(7):2025–2029. doi: 10.1007/s10067-020-05160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharmeen S., Elghawy A., Zarlasht F., Yao Q. COVID-19 in rheumatic disease patients on immunosuppressive agents. Semin Arthritis Rheum. 2020;50(4):680–686. doi: 10.1016/j.semarthrit.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos C.S., Morales C.M., Álvarez E.D., Castro C., Robles A.L., Sandoval T.P. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. 2020;39(9):2789–2796. doi: 10.1007/s10067-020-05301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teh C.L., Cheong Y.K., Wan Musa W.R., Akbar S.A. Wan Mohd, Husin N. Mat, Gun S.C. COVID-19 among Malaysian patients with systemic lupus erythematosus on hydroxychloroquine. Ann Rheum Dis. 2020 Jul 31 doi: 10.1136/annrheumdis-2020-218154. (annrheumdis-2020-218154) [DOI] [PubMed] [Google Scholar]
- 40.Lin C., Wang Z., Li J., Xia X., Liu Y., Wang Q. Implications of SARS-CoV-2 infection for patients with rheumatic disease. Ann Rheum Dis. 2020 Aug 13 doi: 10.1136/annrheumdis-2020-218050. (annrheumdis-2020-218050) [DOI] [PubMed] [Google Scholar]
- 41.Huang Y., Chen Z., Wang Y., Han L., Qin K., Huang W., et al. Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study. Ann Rheum Dis. 2020;79(9):1163–1169. doi: 10.1136/annrheumdis-2020-217425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan S.A., Teh C.L., Singh B. Sachdev Manjit, Cheong Y.K., Chuah S.L., Jobli A.T. Clinical features of patients with rheumatic diseases and COVID-19 infection in Sarawak, Malaysia. Ann Rheum Dis. 2020 Jul 24 doi: 10.1136/annrheumdis-2020-218425. annrheumdis-2020-218425. [DOI] [PubMed] [Google Scholar]
- 43.Sieiro Santos C., Fernandez X. Casas, Morales C. Moriano, Diez E. Alvarez, Castro C. Alvarez, Robles A. Lopez, et al. 2020. Biologic agents for rheumatic diseases in the break of COVID-19: friend or foe? 2020.2009.2001.20184333. [Google Scholar]
- 44.Aries P., Iking-Konert C. No increased rate of SARS-CoV-2 infection for patients with inflammatory rheumatic diseases compared with the general population in the city of Hamburg (Germany) Ann Rheum Dis. 2020 Aug 7 doi: 10.1136/annrheumdis-2020-218400. (annrheumdis-2020-218400) [DOI] [PubMed] [Google Scholar]
- 45.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roubille C., Richer V., Starnino T., McCourt C., McFarlane A., Fleming P., et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker P.M., Everett B.M., Pradhan A., Macfadyen J.G., Solomon D.H., Zaharris E., et al. Low-dose methotrexate for the prevention of atherosclerotic events. New Engl J Med. 2019;380(8):752–762. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace B., Washer L., Marder W., Kahlenberg J.M. Patients with lupus with COVID-19: University of Michigan experience. Ann Rheum Dis. 2020 May 31 doi: 10.1136/annrheumdis-2020-217794. (annrheumdis-2020-217794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haberman R., Axelrad J., Chen A., Castillo R., Yan D., Izmirly P., et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med. 2020;383(1):85–88. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gartshteyn Y., Askanase A.D., Schmidt N.M., Bernstein E.J., Khalili L., Drolet R., et al. COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol. 2020;2(8):e452–e454. doi: 10.1016/S2665-9913(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Silva K.M., Serling-Boyd N., Wallwork R., Hsu T., Fu X., Gravallese E.M., et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis. 2020;79(9):1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathian A., Mahevas M., Rohmer J., Roumier M., Cohen-Aubart F., Amador-Borrero B., et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79(6):837–839. doi: 10.1136/annrheumdis-2020-217566. [DOI] [PubMed] [Google Scholar]
- 53.Scirè C.A., Carrara G., Zanetti A., Landolfi G., Chighizola C., Alunno A., et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19) Clin Exp Rheumatol. 2020;38(4):748–753. [PubMed] [Google Scholar]
- 54.Bozzalla Cassione E., Zanframundo G., Biglia A., Codullo V., Montecucco C., Cavagna L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis. 2020;79(10):1382–1383. doi: 10.1136/annrheumdis-2020-217717. [DOI] [PubMed] [Google Scholar]
- 55.Favalli E.G., Monti S., Ingegnoli F., Balduzzi S., Caporali R., Montecucco C. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: What can we learn from observational data? Arthritis Rheumatol. 2020 Oct;72(10):1600–1606. doi: 10.1002/art.41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrelli V.S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(5):667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quartuccio L., Valent F., Pasut E., Tascini C., De Vita S. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. 2020;87(5):439–443. doi: 10.1016/j.jbspin.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Queiro Silva R., Armesto S., González Vela C., Fernández C. Naharro, González-Gay M.A. COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in North Spain. Dermatol Ther. 2020 Nov;33(6) doi: 10.1111/dth.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michelena X., Borrell H., López-Corbeto M., López-Lasanta M., Moreno E., Pascual-Pastor M., et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2020;50(4):564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freites Nuñez D.D., Leon L., Mucientes A., Rodriguez-Rodriguez L., Urgelles J. Font, García A. Madrid, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(11):1393–1399. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 61.Zhao J., Pang R., Wu J., Guo Y., Yang Y., Zhang L., et al. Clinical characteristics and outcomes of patients with COVID-19 and rheumatic disease in China 'hot spot' versus in US 'hot spot': similarities and differences. Ann Rheum Dis. 2020 Jun 16 doi: 10.1136/annrheumdis-2020-218183. (annrheumdis-2020-218183) [DOI] [PubMed] [Google Scholar]
- 62.Benucci M., Damiani A., Giannasi G., Li Gobbi F., Quartuccio L., Grossi V., et al. Serological tests confirm the low incidence of COVID-19 in chronic rheumatic inflammatory diseases treated with biological DMARD. Ann Rheum Dis. 2020 Jul 6 doi: 10.1136/annrheumdis-2020-218214. (annrheumdis-2020-218214) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Study Exclusion and Inclusion Flowchart.
Supplementary Fig. 2: The funnel plot of hospitalization, oxygen support, ICU admission and fatality in all COVID-19 infected patients with rheumatic diseases, excluding the studies only recruiting hospitalized patients.
Supplementary Fig. 3: The funnel plot of oxygen support, ICU admission and fatality in hospitalized COVID-19 infected patients with rheumatic diseases, including the studies only recruiting hospitalized patient.