Abstract
Background
It is unclear whether asthma has an influence on contracting coronavirus disease 2019 (COVID-19) or having worse outcomes from COVID-19 disease.
Objective
To explore the prevalence of asthma in patients with COVID-19 and the relationship between asthma and patients with COVID-19 with poor outcomes.
Methods
The pooled prevalence of asthma in patients with COVID-19 and corresponding 95% confidence interval (CI) were estimated. The pooled effect size (ES) was used to evaluate the association between asthma and patients with COVID-19 with poor outcomes.
Results
The pooled prevalence of asthma in patients with COVID-19 worldwide was 8.3% (95% CI, 7.6-9.0) based on 116 articles (119 studies) with 403,392 cases. The pooled ES based on unadjusted effect estimates revealed that asthma was not associated with reduced risk of poor outcomes in patients with COVID-19 (ES, 0.91; 95% CI, 0.78-1.06). Similarly, the pooled ES based on unadjusted effect estimates revealed that asthma was not associated with the reduced risk of mortality in patients with COVID-19 (ES, 0.88; 95% CI, 0.73-1.05). However, the pooled ES based on adjusted effect estimates indicated that asthma was significantly associated with reduced risk of mortality in patients with COVID-19 (ES 0.80, 95% CI 0.74-0.86).
Conclusion
The pooled prevalence of asthma in patients with COVID-19 was similar to that in the general population, and asthma might be an independent protective factor for the death of patients with COVID-19, which suggests that we should pay high attention to patients co-infected asthma and COVID-19 and take locally tailored interventions and treatment. Further well-designed studies with large sample sizes are required to verify our findings.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel betacoronavirus, caused the coronavirus disease 2019 (COVID-19), which has posed huge challenges to global public health. To date (data as of September 28, 2020), more than 32.7 million confirmed cases and more than 991,000 deaths have been reported worldwide.1 The continuous increase of confirmed cases and related clinical studies has led to a greater understanding of COVID-19. Many comorbidities have been identified as risk factors for patients with COVID-19 with poor outcomes, such as diabetes, hypertension, malignancies, cardiovascular diseases, and chronic obstructive pulmonary disease, which can help clinicians identify patients with poor prognosis at an early stage and thus contribute to the control and prevention of COVID-19.2
Asthma, a common chronic disease, can be exacerbated by viral respiratory infections,3 which has recently attracted considerable attention of researchers focused on COVID-19. Nevertheless, the prevalence of asthma in patients with COVID-19 and the association between asthma and patients with COVID-19 with poor outcomes remains highly controversial. Zhang et al4 identified particularly low prevalence of asthma (0.3%) among 289 patients with COVID-19 in Wuhan, which was significantly lower than local population asthma prevalence (4.2%).5 Conversely, Latz et al6 pointed out that patients with asthma accounted for up to 26.9% of included patients with COVID-19 in the state of Massachusetts. In addition, the studies conducted by Yehia et al7 and Siso-Almirall et al8 indicated that asthma was not a predictive comorbidity for death of patients with COVID-19. However, Almazeedi et al9 reported that asthma was associated with an increased risk of death in patients with COVID-19, whereas Hernandez-Galdamez et al10 and Santos et al11 found that asthma was a protective factor of death.
In view of the above-mentioned studies, a systematic and quantitative meta-analysis to explore the prevalence of asthma in patients with COVID-19 and the relationship between asthma and patients with COVID-19 with poor outcomes would be of paramount importance.
Methods
Search Strategy and Selection Criteria
We conducted a systematical search of PubMed, Web of Science, and EMBASE databases to recognize eligible studies published from inception to September 18, 2020, using the following terms and keywords: “asthma” or “respiratory diseases” or “comorbidities” or “clinical” AND “novel coronavirus” or “nCoV” or “2019-nCoV” or “COVID-19” or “coronavirus” or “severe acute respiratory syndrome coronavirus 2” or “SARS-CoV-2.” The literature search was not restricted by language. The reference lists of all pertinent studies and reviews were sifted to identify other eligible studies. In addition, when publications with overlapping data were found, only the articles with the larger sample size or more complete analysis were included. EndNote (version X9.0, Thomson ResearchSoft, Stanford, Connecticut) was used for the management of literature. Our analyses were carried out on September 20, 2020, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (eTable 1).12
Inclusion criteria were the following: (1) all patients enrolled in articles were diagnosed as having COVID-19; and (2) articles clearly reported the number of patients with co-infection of asthma and COVID-19.
Exclusion criteria were as follows: (1) abstracts, reviews, meta-analysis, and errata; (2) studies with the sample size fewer than 100 patients; (3) articles with overlapping data; and (4) articles reporting unclear prevalence of asthma in patients with COVID-19.
Data Extraction and Quality Assessment
Notably, 2 researchers (Li Shi and Wenwei Xiao) respectively reviewed all literatures according to the inclusion and exclusion criteria and excerpted the following information: author, location or country, study design, total number of patients, age, sex, settings, the number of patients co-infected asthma and COVID-19, and the number of patients with asthma with poor outcomes (eg, patients diagnosed with having severe or critical COVID-19, or admitted to intensive care unit [ICU], or required mechanical ventilation [MV], or died). Any conflicts were resolved by group discussion.
The quality of the enrolled studies was evaluated by 2 independent researchers using the Agency for Healthcare Research and Quality score checklist.13 The quality of the studies was graded as low (0-3), moderate (4-7), or high (8-11), according to the corresponding range of scores.
Statistical Analysis
All statistical analyses were carried out using R (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria) and Stata (version SE 12.1, StataCorp, College Station, Texas). A meta-analysis of the included studies was done with the metaprop command in R to calculate the pooled prevalence of asthma in patients with COVID-19. Furthermore, a meta-analysis of the included studies was done with the metan command in Stata to evaluate the risk of having poor outcomes in patients with COVID-19 and asthma co-infection. Considering the influence of various factors such as sex, age, and other comorbidities on the risk of mortality in patients with COVID-19,2 the pooled effect size (ES) and corresponding 95% confidence interval (CI) were calculated on the basis of the studies reporting the adjusted effect estimates. The χ2-based Q test (represented as χ2 and P values) and I 2 statistic were applied to evaluate the heterogeneity among studies.14 If I 2 was less than 50% or P was greater than .05, we used the fixed-effects model. Otherwise, the random-effects model was chosen. Considering the obvious heterogeneity of our analysis, subgroup and meta-regression analyses were conducted to investigate possible factors that caused heterogeneity. The factors that we investigated were sample size, study design, region, settings, and quality score. Publication bias was examined by Begg test and Egger test.15 , 16 P values less than .05 were regarded as statistically significant.
Results
Study Selection
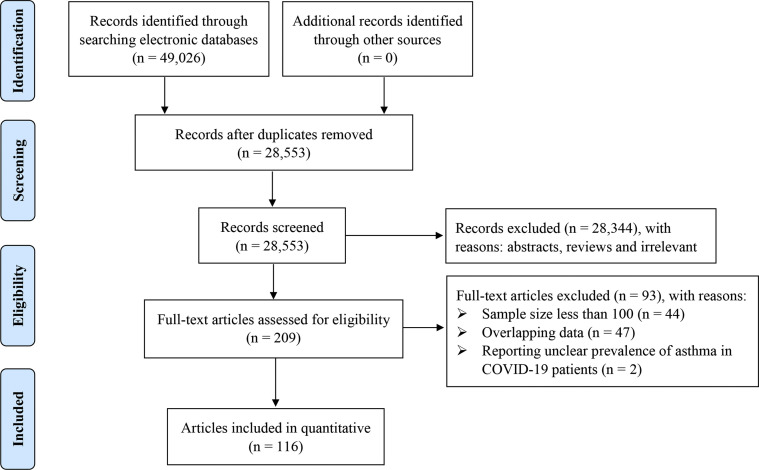
Initially, 49,026 records were retrieved by our search strategy. By deleting duplicates of original retrieved articles, 28,553 related articles were obtained. A total of 209 articles that reported the prevalence of asthma in patients with COVID-19 were yielded after reading the titles and abstracts. Subsequently, 44 articles were excluded because of a sample size less than 100, 47 articles were eliminated owing to the potential duplicate patients, and 2 articles were removed because they reported unclear prevalence of asthma in patients with COVID-19 (eTable 2). Ultimately, 116 articles (119 studies)4 , 6, 7, 8, 9, 10, 11 , 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125 with 403,392 patients with COVID-19 passed multiple screening (Fig 1 ).
Figure 1.
Study selection. COVID-19, coronavirus disease 2019.
Study Characteristics
All patients in enrolled articles were diagnosed with having COVID-19 (eTable 3). The main characteristics of the enrolled studies are found in Table 1 . The included studies were from different countries and regions around the world, of which 64 from the Americas, 28 from Europe, 22 from Asia, 1 from the Middle East, and 4 from other countries. In terms of the study design, 101 were retrospective studies, 7 prospective studies, 5 cross-sectional studies, and 3 each ambispective studies and randomized controlled trials. Through qualitative assessment, 47 studies were of high quality, 71 studies of moderate quality, and the remaining 1 study of low quality (eTable 4).
Table 1.
Baseline Characteristics of the Included Studies
| Author | Study design | Location or country | Sample size | Male (%) | Age (y) | Settings (%) | Asthma (%) | Poor outcomes (%)a | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| America | |||||||||
| Adrish et al17 | Retrospective | US | 469 | 279 (59.5) | N/R | Inpatient (100) | 83 (17.7) | N/R | 6 |
| Agarwal et al18 | Retrospective | US | 404 | 297 (73.5) | 61 (median) | Inpatient/Outpatient | 25 (6.2) | N/R | 7 |
| Argyropoulos et al19 | Retrospective | US | 205 | 108 (52.7) | N/R | Inpatient (19.5) Outpatient (80.5) |
26 (12.7) | N/R | 6 |
| Arshad et al20 | Retrospective | US | 2541 | 1298 (51.1) | 63.7 (mean) | Inpatient (100) | 251 (9.9) | N/R | 9 |
| Bajaj et al21 | Retrospective | US | 108 | 37 (34.3) | 61.3 (mean) | Inpatient (100) | 9 (8.3) | N/R | 6 |
| Broadhurst et al22 | Cross-sectional | US | 436 | 239 (50.1)b | 54.7 (mean) | Inpatient (100) | 53 (12.2) | 15 (10.8)b | 4 |
| Capone et al23 | Retrospective | US | 102 | 55 (53.9) | 63.3 (mean) | Inpatient (100) | 12 (11.8) | 12 (11.8) | 6 |
| Chachkhiani et al24 | Retrospective | US | 250 | 113 (45.2) | 60 (mean) | Inpatient (100) | 39 (15.6) | N/R | 7 |
| Chhiba et al25 | Retrospective | US | 1526 | 718 (47.1) | N/R | Inpatient (55.9) Outpatient (44.1) |
220 (14.4) | 8 (11.1) | 7 |
| Cummings et al26 | Prospective | US | 257 | 171 (66.5) | 62 (median) | Inpatient (100) | 21 (8.2) | 21 (8.2) | 7 |
| Enzmann et al27 | Retrospective | US | 150 | 85 (56.7) | 56 (median) | N/R | 27 (18.0) | N/R | 5 |
| Fox et al28 | Retrospective | US | 355 | 181 (51.0) | 66.2 (mean) | Inpatient (100) | 27 (7.6) | N/R | 7 |
| Garg et al29 | Retrospective | US | 178 | N/R | N/R | Inpatient (100) | 27 (17.0)b | N/R | 6 |
| Garibaldi et al30 | Retrospective | US | 832 | 443 (51.7) | 63 (median) | Inpatient (100) | 79 (9.5) | 24 (7.9) | 9 |
| Gavin et al31 | Retrospective | US | 140 | 72 (51.4) | 60 (mean) | Inpatient (100) | 15 (10.7) | 1 (5.6) | 8 |
| Gayam et al32 | Retrospective | US | 408 | 231 (56.6) | 67 (median) | Inpatient (100) | 54 (13.2) | 16 (12.1) | 7 |
| Gottlieb et al33 | Retrospective | US | 8673 | 4045 (46.6) | 41 (median) | Inpatient (17.1) Outpatient (82.9) |
736 (8.5) | N/R | 7 |
| Goyal et al34 | Retrospective | US | 1687 | 1004 (59.5) | 66.5 (median) | Inpatient (100) | 159 (9.4) | N/R | 7 |
| Gupta et al35 | Retrospective | US | 2215 | 1436 (64.8) | 60.5 (mean) | Inpatient (100) | 258 (11.6) | 70 (8.9) | 8 |
| Haberman et al36 | Prospective | US | 103 | 29 (28.2) | 52.7 (mean) | Inpatient (26.2) Outpatient (73.8) |
15 (14.6) | N/R | 6 |
| Hernandez-Galdamez et al10 | Cross-sectional | Mexico | 211003 | 115441 (54.7) | 45.7 (mean) | Inpatient (31.0) Outpatient (69.0) |
5854 (2.8) | 533 (2.1) | 7 |
| Jehi et al37 | Retrospective | US | 2852 | 1372 (48.1) | N/R | Inpatient (20.4) Outpatient (79.6) |
389 (13.6) | N/R | 6 |
| 1684 | 738 (43.8) | N/R | Inpatient (22.3) Outpatient (77.7) |
262 (15.6) | N/R | ||||
| Keller et al38 | Retrospective | US | 1806 | 965 (53.4) | 62.2 (mean) | Inpatient (100) | 344 (19.0) | N/R | 9 |
| Kim et al39 | Ambispective | US | 867 | 473 (54.6) | 56.9 (mean) | N/R | 91 (10.5) | 10 (8.3) | 9 |
| Ko et al40 | Retrospective | US | 5416 | 2847 (52.6) | N/R | N/R | 702 (13.0) | N/R | 8 |
| Krishnan et al41 | Retrospective | US | 152 | 95 (62.5) | 66 (mean) | Inpatient (100) | 25 (16.4) | 16 (17.4) | 6 |
| Lara et al42 | Retrospective | US | 121 | N/R | 64 (median) | Inpatient (54.5) Outpatient (45.5) |
10 (8.3) | 3 (15.0) | 6 |
| Latz et al6 | Retrospective | US | 1289 | 417 (32.4) | N/R | Inpatient (37.5) Outpatient (62.5) |
347 (26.9) | N/R | 7 |
| Lovinsky-Desir et al43 | Retrospective | US | 1298 | 762 (58.7) | N/R | Inpatient (100) | 163 (12.6) | 9 (8.2) | 8 |
| Maatman et al44 | Retrospective | US | 109 | 62 (56.9) | 61 (mean) | Inpatient (100) | 16 (14.7) | 16 (14.7) | 7 |
| Magagnoli et al45 | Retrospective | US | 807 | 772 (95.7) | N/R | Inpatient (100) | 40 (5.0) | N/R | 7 |
| Magleby et al46 | Retrospective | US | 678 | 414 (61.1) | N/R | Inpatient (100) | 62 (9.1) | N/R | 7 |
| McCarthy et al47 | Retrospective | US | 247 | 143 (57.9) | 61 (median) | Inpatient (100) | 29 (11.7) | 11 (9.8) | 7 |
| Mikami et al48 | Retrospective | US | 6493 | 3538 (54.5) | 59 (median) | Inpatient (55.1) Outpatient (42.9) |
271 (4.2) | 31 (3.8) | 6 |
| Moll et al49 | Retrospective | US | 210 | 101 (48.1) | 62.2 (mean) | Inpatient (100) | 35 (16.7) | 15 (14.7) | 6 |
| Mughal et al50 | Retrospective | US | 129 | 81 (62.8) | 63 (median) | Inpatient (100) | 3 (2.3) | 2 (6.7) | 6 |
| Mukherjee et al51 | Retrospective | US | 137 | 99 (72.3) | 59 (mean) | Inpatient (100) | 11 (8.0) | 11 (8.0) | 8 |
| Nakeshbandi et al52 | Retrospective | US | 504 | 263 (52.2) | 68 (median) | Inpatient (100) | 41 (8.1) | N/R | 8 |
| Ng et al53 | Retrospective | US | 10482 | 6239 (59.5) | N/R | Inpatient (100) | 859 (8.2) | N/R | 9 |
| Ortizz-Brizuela et al54 | Prospective | Mexico | 309 | 183 (59.2) | 43 (median) | Inpatient (45.3) Outpatient (54.7) |
9 (2.9) | 0 (0.0) | 9 |
| Ramachandran et al55 | Retrospective | US | 145 | 79 (54.5) | N/R | Inpatient (100) | 23 (15.9) | N/R | 8 |
| Richardson et al56 | Retrospective | US | 5700 | 3437 (60.3) | 63 (median) | Inpatient (100) | 479 (9.0) | N/R | 8 |
| Robilotti et al57 | Retrospective | US | 423 | 212 (50.1) | N/R | Inpatient (42.6) Outpatient (57.4) |
43 (10.2) | N/R | 6 |
| Santos et al11 | Retrospective | Brazil | 21408 | 12667 (59.2) | N/R | Inpatient (100) | 488 (5.7)b | 488 (5.7)b | 7 |
| Shady et al58 | Ambispective | US | 371 | 249 (67.1) | 57 (median) | Inpatient (100) | 42 (11.4)b | N/R | 6 |
| Shah et al59 | Retrospective | US | 522 | 218 (41.8) | 63 (median) | Inpatient (100) | 68 (13.0) | 11 (12.0) | 6 |
| Silver et al60 | Retrospective | US | 249 | 110 (44.2) | 59.6 (mean) | Inpatient (100) | 49 (20.0) | N/R | 8 |
| Singer et al61 | Retrospective | US | 1651 | 892 (54.0) | 50 (mean) | Inpatient (45.0) Outpatient (55.0) |
106 (6.4) | N/R | 6 |
| Sinha et al62 | Retrospective | US | 255 | 161 (63.1) | 59 (median) | Inpatient (100) | 29 (11.4) | N/R | 8 |
| Skipper et al63 | RCT | US and Canada | 212 | 89 (42.0) | 41 (median) | Outpatient (100) | 28 (13.2) | N/R | 9 |
| 211 | 96 (45.5) | 39 (median) | Outpatient (100) | 20 (9.5) | N/R | ||||
| Smith et al64 | Retrospective | US | 184 | 98 (53.3) | 64.4 (mean) | Inpatient (100) | 18 (9.8) | N/R | 6 |
| Somers et al65 | Retrospective | US | 154 | 102 (66.2) | 58 (mean) | Inpatient (100) | 31 (20.1) | 31 (20.1) | 9 |
| Souza et al66 | Cross-sectional | Brazil | 197 | 92 (46.7) | N/R | N/R | 1 (0.5) | 1 (0.5) | 5 |
| Suleyman et al67 | Retrospective | US | 463 | 204 (44.1) | 57.5 (mean) | Inpatient (76.7) Outpatient (23.3) |
73 (15.8) | 19 (13.5) | 8 |
| Tartof et al68 | Retrospective | US | 6916 | 3111 (45.0) | 49 (median) | N/R | 1273 (18.4) | 44 (21.4) | 8 |
| Tenforde et al69 | Cross-sectional | US | 350 | 165 (47.1) | 43 (median) | Inpatient (22.6) Outpatient (77.4) |
55 (15.7) | N/R | 7 |
| Twigg et al70 | Retrospective | US | 242 | 141 (58.3) | 59.6 (mean) | Inpatient (100) | 34 (14.0) | 34 (14.0) | 7 |
| Vaughn et al71 | Retrospective | US | 1705 | 885 (51.9) | 64.7 (median) | Inpatient (100) | 215 (12.6) | N/R | 7 |
| Yao et al72 | Retrospective | US | 242 | 138 (57.0) | N/R | Inpatient (100) | 28 (11.6) | N/R | 7 |
| Yehia et al7 | Retrospective | US | 11210 | 5583 (49.8) | 61 (median) | Inpatient (100) | 628 (5.6) | N/R | 8 |
| Zhao et al73 | Retrospective | US | 641 | 384 (59.9) | 60 (median) | Inpatient (100) | 41 (6.9)b | 16 (8.2) | 8 |
| Zuniga-Moya et al74 | Retrospective | Honduras | 877 | 538 (61.3) | N/R | Inpatient (25.1) Outpatient (74.9) |
31 (3.5) | 3 (7.9) | 10 |
| Asia | |||||||||
| Almazeedi et al9 | Retrospective | Kuwait | 1096 | 888 (81.0) | 41 (median) | Inpatient (100) | 43 (3.9) | 4 (21.1) | 9 |
| Alsofayan et al75 | Retrospective | Saudi Arabia | 1519 | 825 (54.3) | N/R | N/R | 54 (4.9)b | N/R | 5 |
| Asghar et al76 | Retrospective | Pakistan | 100 | 69 (69.0) | 52.6 (mean) | Inpatient (100) | 2 (2.0) | N/R | 6 |
| Gao et al77 | Retrospective | China | 2877 | 1470 (51.1) | N/R | Inpatient (100) | 22 (0.8) | N/R | 10 |
| Huang et al78 | Retrospective | China | 336 | 182 (54.2) | 43 (median) | Inpatient (100) | 5 (1.5) | N/R | 7 |
| Li et al79 | Ambispective | China | 548 | 279 (50.9) | 60 (median) | Inpatient (100) | 5 (0.9) | 3 (1.1) | 8 |
| Lian et al80 | Retrospective | China | 232 | 109 (47.0) | N/R | Inpatient (100) | 4 (1.7) | 3 (3.3) | 6 |
| Liu et al81 | Retrospective | China | 104 | 63 (60.6) | 42 (medina) | Inpatient (100) | 12 (11.5) | 6 (20.0) | 7 |
| Mao et al82 | Retrospective | China | 188 | 94 (50.0) | 46 (mean) | Inpatient (100) | 2 (1.1) | N/R | 9 |
| Ozger et al83 | Retrospective | Turkey | 175 | 74 (42.3) | N/R | Inpatient (100) | 9 (5.1) | N/R | 5 |
| Pan et al84 | Retrospective | China | 996 | 465 (46.7) | N/R | Inpatient (100) | 12 (1.2) | N/R | 7 |
| Satici et al85 | Retrospective | Turkey | 681 | 347 (51.0) | 56.9 (mean) | Inpatient (100) | 43 (6.3) | 1 (1.8) | 7 |
| Song et al86 | Retrospective | China | 961 | 500 (52.0) | 63 (median) | Inpatient (100) | 22 (2.3) | 1 (0.4) | 7 |
| Sy et al87 | Retrospective | Philippines | 530 | 373 (70.4) | 48.9 (mean) | N/R | 21 (4.0) | N/R | 8 |
| Tezcan et al88 | Retrospective | Turkey | 408 | 188 (46.1) | 54.3 (mean) | Inpatient (100) | 32 (7.8) | N/R | 5 |
| Trabulus et al89 | Retrospective | Turkey | 336 | 192 (57.1) | 55 (mean) | Inpatient (100) | 20 (6.0) | 1 (2.3) | 7 |
| Tsou et al90 | Retrospective | Taiwan | 100 | 44 (44.0) | 44 (median) | Inpatient (100) | 3 (3.0) | N/R | 5 |
| Wang et al91 | Retrospective | China | 123 | 60 (48.8) | 68 (median) | Inpatient (100) | 1 (0.8) | 0 (0.0) | 6 |
| Yang et al92 | Retrospective | Korea | 7340 | 2970 (40.5) | 47.1 (mean) | Inpatient (100) | 725 (9.9) | N/R | 8 |
| Yu et al93 | Retrospective | China | 142 | 81 (57.0) | 61.9 (mean) | Inpatient (100) | 1 (0.7) | N/R | 8 |
| Zhang et al4 | Retrospective | China | 289 | 154 (53.3) | 57 (median) | Inpatient (100) | 1 (0.3) | 1 (0.8) | 8 |
| Zhou et al94 | Retrospective | China | 110 | 60 (54.5) | 57.7 (mean) | Outpatient (100) | 1 (0.9) | N/R | 7 |
| Europe | |||||||||
| Alkundi et al95 | Retrospective | UK | 232 | 145 (62.5) | 70.5 (mean) | Inpatient (100) | 6 (2.6) | 0 (0.0) | 6 |
| Avdeev et al96 | Retrospective | Russia | 1307 | N/R | N/R | Inpatient (100) | 23 (1.8) | 23 (1.8) | 3 |
| Azoulay et al97 | Retrospective | France | 379 | 292 (77.0) | 66 (median) | Inpatient (100) | 23 (6.1)b | 23 (6.1)b | 7 |
| Barillari et al98 | Cross-sectional | Italy | 294 | 147 (50.0) | 42.1 (mean) | Inpatient (16.3) Outpatient (83.7) |
18 (6.1) | N/R | 4 |
| Barroso et al99 | Retrospective | Spain | 189 | N/R | N/R | Inpatient (100) | 11 (5.8) | N/R | 6 |
| Berenguer et al100 | Retrospective | Spain | 4035 | 2433 (61.0) | 70 (median) | Inpatient (100) | 299 (7.5)b | 69 (6.2)b | 10 |
| Beurnier et al101 | Prospective | France | 768 | N/R | N/R | Inpatient (100) | 37 (4.8) | N/R | 5 |
| Cellina et al102 | Retrospective | Italy | 246 | 170 (69.1) | 63 (mean) | Inpatient (100) | 10 (4.1) | N/R | 8 |
| Docherty et al103 | Prospective | UK | 20133 | 12068 (59.9) | 72.9 (median) | Inpatient (100) | 2540 (14.5)b | N/R | 9 |
| Fang et al104 | Retrospective | UK | 100 | 60 (60.0) | N/R | Inpatient (100) | 11 (11.0) | N/R | 9 |
| Ferrando et al105 | Prospective | Spain and Andorra | 742 | 504 (68.1)b | 64 (median) | Inpatient (100) | 19 (2.6) | 19 (2.6) | 10 |
| Fond et al106 | Retrospective | France | 1092 | 593 (54.3) | 62.5 (median) | Inpatient (100) | 71 (6.5) | N/R | 8 |
| Garcia-Pachon et al107 | Retrospective | Spain | 376 | 192 (51.1) | 54 (median) | Inpatient (42.0) Outpatient (58.0) |
10 (2.7) | N/R | 4 |
| Grandbastien et al108 | Retrospective | France | 106 | 66 (62.3) | 63.5 (median) | Inpatient (100) | 23 (21.7) | N/R | 7 |
| Helms et al109 | Prospective | France | 140 | 100 (71.4) | 62 (median) | Inpatient (100) | 5 (3.6) | 5 (3.6) | 10 |
| Ierardi et al110 | Retrospective | Italy | 234 | 70 (30.0) | 61.6 (mean) | Inpatient (100) | 10 (4.3) | N/R | 5 |
| Joseph et al111 | Retrospective | France | 100 | 70 (70.0) | 59 (median) | Inpatient (100) | 8 (8.0) | N/R | 7 |
| Lechien et al112 | Retrospective | Europec | 702 | 206 (29.3) | 40.3 (median) | N/R | 42 (6.0) | N/R | 6 |
| Lendorf et al113 | Retrospective | Denmark | 111 | 67 (60.4) | 68 (median) | Inpatient (100) | 12 (10.8) | 2 (10.0) | 8 |
| Lenti et al114 | Retrospective | Italy | 100 | 79 (79.0) | 70 (median) | Inpatient (100) | 6 (6.0) | N/R | 7 |
| Lombardi et al115 | Retrospective | Italy | 1043 | 704 (67.5) | N/R | Inpatient (100) | 20 (1.9) | N/R | 5 |
| Lund et al116 | Retrospective | Denmark | 9236 | 3892 (42.1) | 50 (median) | N/R | 629 (6.8) | N/R | 8 |
| Maguire et al117 | Retrospective | UK | 224 | 124 (55.4) | N/R | Inpatient (100) | 46 (20.5) | 4 (7.7) | 8 |
| Martinez-Del Rio et al118 | Retrospective | Spain | 921 | 500 (54.3) | 78 (mean) | Inpatient (100) | 39 (4.2) | 9 (3.6) | 8 |
| Perez-Guzman et al119 | Retrospective | UK | 614 | 382 (62.2) | 69 (median) | Inpatient (100) | 56 (9.1) | N/R | 7 |
| Poblador-Plou et al120 | Retrospective | Spain | 771 | 407 (52.8) | 84.2 (mean) | N/R | 25 (3.2) | 25 (3.2) | 6 |
| Sapey et al121 | Retrospective | UK | 2217 | 1290 (58.2) | 73 (median) | Inpatient (100) | 439 (19.8) | 143 (18.6) | 8 |
| Siso-Almirall et al8 | Retrospective | Spain | 322 | 161 (50.0) | 56.7 (mean) | Inpatient (49.1) Outpatient (50.9) |
13 (4.0) | 2 (3.6) | 7 |
| Middle East | |||||||||
| Jalili et al122 | Retrospective | Iran | 28981 | 16361 (56.5) | 57.3 (mean) | Inpatient (100) | 573 (2.0) | 141 (2.5) | 7 |
| Othersc | |||||||||
| COVIDSurg Collaborative123 | Retrospective | Countries | 1128 | 605 (53.6) | N/R | Inpatient (100) | 78 (7.0)b | 21 (7.8) | 9 |
| Mato et al124 | Retrospective | Countries | 198 | 125 (63.1) | 70.5 (median) | Inpatient (89.9) Outpatient (10.1) |
12 (6.1)b | 7 (10.8) | 7 |
| Olender et al125 | RCT | Countries | 298 | 182 (61.1) | N/R | Inpatient (100) | 42 (14.1) | N/R | 8 |
| Retrospective | Countries | 816 | 490 (60.0) | N/R | Inpatient (100) | 90 (11.0) | N/R |
Abbreviations: N/R, not (clearly) reported; RCT, randomized controlled trial; UK, United Kingdom; US, United States.
The prevalence of asthma in patients with coronavirus disease 2019 with poor outcomes.
Data missing for patients.
Patients were collected from multiple countries of different regions.
The Pooled Prevalence of Asthma in Patients With COVID-19
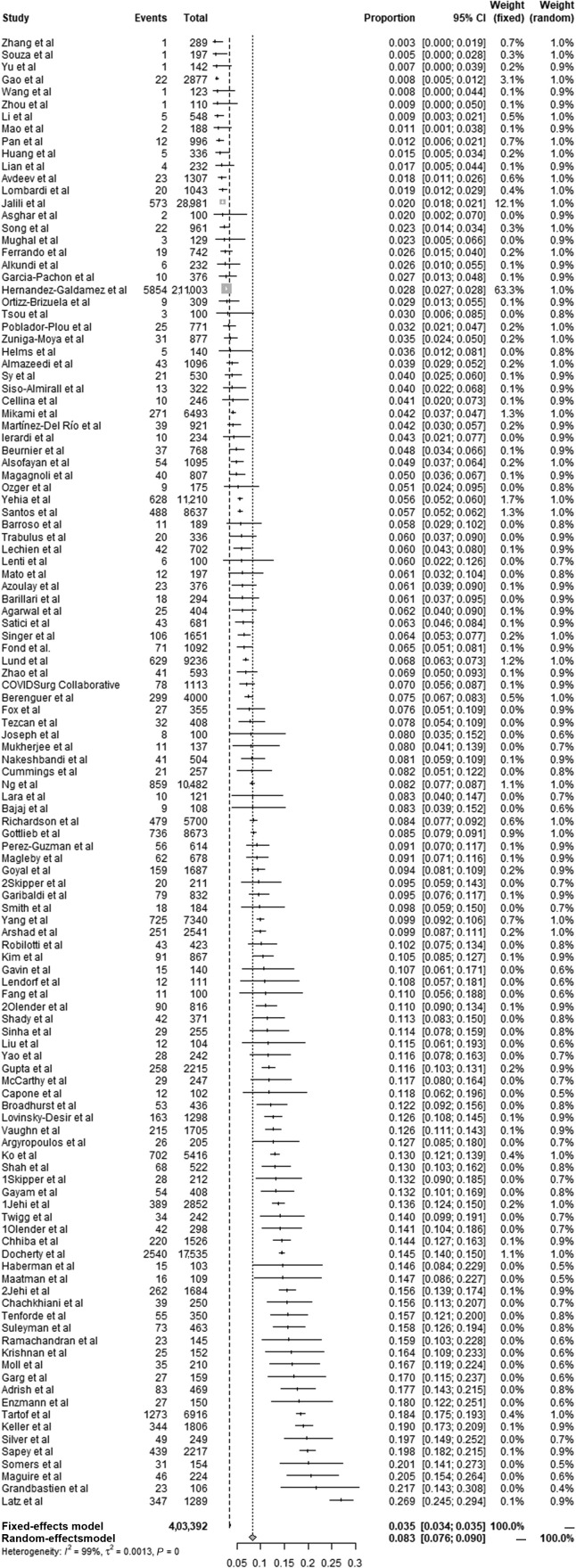
The estimated prevalence of asthma in patients with COVID-19 ranged from 0.3% to 26.9%. By combining 119 studies (a total of 403,392 patients) reporting the data of patients with co-infection of asthma and COVID-19, the pooled prevalence of asthma in patients with COVID-19 was 8.3% (95% CI, 7.6-9.0; random-effects model) and heterogeneity was obvious (χ2 = 9311.76; P < .01; I 2 = 98.7%) (Fig 2 ). Therefore, we conducted subgroup and meta-regression analyses to explore the possible factors that caused heterogeneity according to sample size, study design, region, settings, and quality score (Table 2 and eFigs 1-5). The pooled prevalence of asthma among patients with COVID-19 was 3.3% (95% CI, 1.9-4.6; χ2 = 712.56, P < .01; I 2 = 97.1%) in Asia, 11.1% (95% CI, 9.9-12.3; χ2 = 5466.42, P < .01; I 2 = 98.8%) in the Americas, 7.0% (95% CI, 5.0-9.0; χ2 = 1608.20, P < .01; I 2 = 98.3%) in Europe, and 9.4% (95% CI, 6.2-12.5; χ2 = 18.82, P < .01; I 2 = 84.1%) in other countries. Only 1 study was completed in the Middle East, and the prevalence of asthma in patients with COVID-19 was 2.0% (95% CI, 1.8-2.1). The results of univariate meta-regression revealed that region (P < .001) might be a factor caused by heterogeneity, whereas no significant differences were observed in sample size (P = .131), settings (P = .337), study design (P = .936), or quality score (P = .610).
Figure 2.
Forest plot of the pooled prevalence of asthma in patients with COVID-19 on a basis of 119 studies. CI, confidence interval; COVID-19, coronavirus disease 2019.
Table 2.
Subgroup Analysis and Meta-Regression
| Variables | No. of studies | Meta-regression |
Subgroup analysis |
Heterogeneity |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Tau2 | t value | P value | Pooled ES (95% CI) | P value | I2 (%) | χ2 | P value | ||
| Sample size (continuous) | 0.0018 | −1.52 | .131 | ||||||
| ≥500 | 53 | 0.081 (0.072-0.091) | <.01 | 99.4 | 8339.83 | <.01 | |||
| <500 | 66 | 0.088 (0.075-0.100) | <.01 | 93.2 | 962.03 | <.01 | |||
| Settings (continuous) | 0.0018 | −0.96 | .337 | ||||||
| Inpatient | 85 | 0.082 (0.073-0.092) | <.01 | 98.5 | 5633.00 | <.01 | |||
| Outpatient | 3 | 0.077 (0.000-0.157) | <.01 | 94.2 | 34.41 | <.01 | |||
| Others | 31 | 0.090 (0.073-0.107) | <.01 | 99.1 | 3475.58 | <.01 | |||
| Region | 0.0015 | — | <.001 | ||||||
| Asia | 22 | — | 0.45 | .656 | 0.033 (0.019-0.046) | <.01 | 97.1 | 712.56 | <.01 |
| Americas | 64 | — | 2.22 | .029 | 0.111 (0.099-0.123) | <.01 | 98.8 | 5466.42 | <.01 |
| Europe | 28 | — | 1.30 | .197 | 0.070 (0.050-0.090) | <.01 | 98.3 | 1608.20 | <.01 |
| Middle East | 1 | — | — | — | 0.020 (0.018-0.021) | <.01 | — | — | — |
| Others | 4 | — | 1.52 | .132 | 0.094 (0.062-0.125) | <.01 | 84.1 | 18.82 | <.01 |
| Study design | 0.0019 | −0.08 | .936 | ||||||
| Prospective/RCT | 10 | 0.086 (0.042-0.130) | <.01 | 98.4 | 549.08 | <.01 | |||
| Others | 109 | 0.082 (0.076-0.089) | <.01 | 98.6 | 7491.52 | <.01 | |||
| Quality score | 0.0019 | −0.51 | .610 | ||||||
| High | 46 | 0.088 (0.073-0.103) | <.01 | 98.9 | 4017.89 | <.01 | |||
| Moderate/low | 73 | 0.079 (0.072-0.086) | <.01 | 98.1 | 3855.35 | <.01 | |||
Abbreviations: CI, confidence interval; ES, effect sizes; RCT, randomized controlled trial.
Italic value indicates statistical significance.
The Association Between Asthma and the Poor Outcomes of Patients With COVID-19
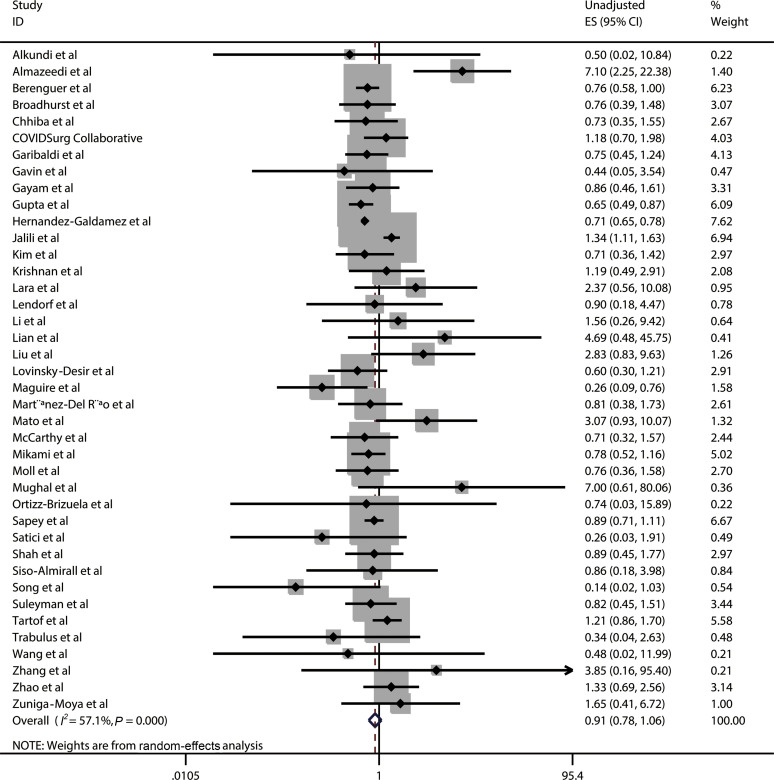
Poor outcomes included severe or critical illness, ICU admission, requirement of MV, or death. A total of 40 studies comprising 274,395 patients reported the data on asthma in patients with COVID-19 with poor outcomes and patients with COVID-19 without poor outcomes (eTable 5). The pooled results revealed that asthma was not significantly associated with the reduced risk of poor outcomes in COVID-19 (ES, 0.91; 95% CI, 0.78-1.06; χ2 = 90.97, P < .001; I 2 = 57.1%; random-effects model) based on unadjusted effect estimates (Fig 3 ).
Figure 3.
Forest plot of unadjusted ES for the association between asthma and the poor outcomes of patients with COVID-19 on a basis of 40 studies. CI, confidence interval; COVID-19, coronavirus disease 2019; ES, effect size; ID, identification.
The Association Between Asthma and the Risk of Mortality in Patients With COVID-19
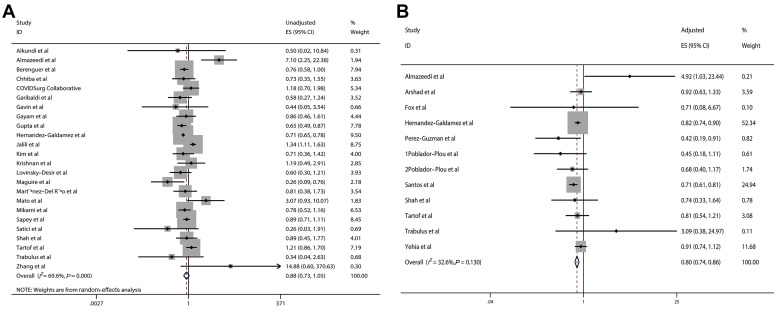
A meta-analysis of 24 studies reporting the unadjusted ES (eTable 5) and a meta-analysis of 12 studies reporting the adjusted ES (eTable 6) were conducted to evaluate the association between asthma and the risk of mortality in patients with COVID-19, respectively. The pooled results of unadjusted effect estimates revealed that asthma was not significantly associated with the reduced risk of mortality in patients with COVID-19 (ES, 0.88; 95% CI, 0.73-1.05; χ2 = 75.65, P < .001; I 2 = 69.6%; random-effects model) (Fig 4A). However, the pooled results of adjusted effect estimates indicated that asthma was significantly associated with the reduced risk of mortality in patients with COVID-19 (ES, 0.80; 95% CI, 0.74-0.86; χ2 = 16.31; P = .13; I 2 = 32.6%; fixed-effects model) (Fig 4B).
Figure 4.
Forest plots of the pooled ES for the relationship between asthma and the risk of mortality in patients with COVID-19. A, The unadjusted ES on a basis of 24 studies. B, The adjusted ES on a basis of 12 studies. CI, confidence interval; COVID-19, coronavirus disease 2019; ES, effect size; ID, identification.
Publication Bias
Significant publication bias was found by Begg test (P = .038) and Egger test (P < .001) within our analysis (eFig 6).
Discussion
Our quantitative meta-analysis suggested that the pooled prevalence of asthma in patients with COVID-19 worldwide was 8.3%, which was contained in a range (4.3%-8.6%) of the global prevalence rates of asthma.126 The pooled prevalence of asthma in patients with COVID-19 worldwide (8.3%) was more similar to the global prevalence of wheezing (8.6%) using the least stringent definition of asthma.126 Considering the obvious heterogeneity of our analysis, we subsequently performed subgroup analysis and meta-regression according to sample size, study design, region, settings, and quality score. The univariate meta-regression implied that region (P < .001) might be a potential source of heterogeneity. According to the results of subgroup analysis, the pooled prevalence of asthma among patients with COVID-19 was 3.3%, 11.1%, 7.0%, 2.0%, and 9.4% in Asia, the Americas, Europe, the Middle East, and other countries, respectively, which highlighted the demand for locally tailored interventions and initiatives. Interestingly, Gibson et al127 reported that the prevalence of asthma in the European population was 4% to 7%. Huang et al5 identified that the overall prevalence of asthma in 57,779 participants of China was 4.2%. Furthermore, the US Centers for Disease Control and Prevention pointed out that adult self-reported asthma prevalence was 9.2%.128 All of these evidences indicate that the prevalence of asthma among patients with COVID-19 in different regions and countries seemed to be similar to that of asthma in the general population.
To explore the relationship between asthma and patients with COVID-19 with poor outcomes (including severe or critical illness, ICU admission, requirement of MV, or death), we calculated the pooled unadjusted ES based on 40 studies comprising 274,395 patients. The pooled unadjusted ES was less than 1, which revealed that asthma might be associated with the reduced risk of poor outcomes in patients with COVID-19, although the corresponding 95% CI crossed 1 (ES, 0.91; 95% CI, 0.78-1.06). We hypothesized that the different poor outcomes reported in the included articles and the known factors (such as sex, age, and other comorbidities) influencing the risk of poor outcomes in patients with COVID-19 might contribute to the results.2 , 129 , 130 Therefore, we specifically explored the association between asthma and the risk of mortality in patients with COVID-19 based on the limited data reported by the included articles. Similarly, the pooled unadjusted ES was less than 1, which also revealed that asthma might be significantly associated with the reduced risk of mortality in patients with COVID-19 (ES, 0.88; 95% CI, 0.73-1.05). Considering that this result might be because of the influence of various factors on the risk of mortality in patients with COVID-19, we subsequently calculated the pooled ES on the basis of adjusted effect estimates. The corresponding results suggested that asthma was significantly associated with the reduced risk of mortality in patients with COVID-19 (ES, 0.80; 95% CI, 0.74-0.86). In summary, asthma might be an independent protective factor for death of patients with COVID-19. There are some complicated and multifactorial reasons. One reason is immune response triggered by asthma. Li et al79 speculated that TH2 immune response in patients with asthma may counter the inflammation process induced by SARS-CoV-2 infection. Another is the use of inhaled corticosteroids or bronchodilators, which can suppress viral replication and decrease the impact of the inflammatory storm.131 , 132
Several limitations inevitably exist in our meta-analysis. First, most studies we included were retrospective; therefore, the interpretation of our results should be taken with caution because of their inherent limitations. Further well-designed prospective studies with large sample sizes are required to verify our findings. Second, the substantial heterogeneity across the studies should not be ignored, which was why we conducted subgroup analysis and meta-regression, and thus identified the region as a potential source of heterogeneity. Third, in the included studies, the definitions of asthma were not uniform and relatively diverse, including patients' self-report, which might lead to a certain bias. Fourth, we did not carry out statistics and analysis on the use of corticosteroids because of insufficient data provided in the original publications. Fifth, different poor outcomes including severe illness, critical illness, ICU admission, MV, and death were reported in the selected studies; we only specifically explored the association between asthma and the risk of mortality in patients with COVID-19 based on the limited data reported by the included articles. Further subgroup analysis on the relationship between asthma and certain outcomes of patients with COVID-19 should be performed when sufficient data are available. Finally, obvious publication bias was observed in our study, which might be because of the unrecognized duplicate population.
The pooled prevalence of asthma in patients with COVID-19 was similar to that in the general population. Asthma was not associated with the reduced risk of poor outcomes in patients with COVID-19. Interestingly, asthma might be an independent protective factor for the death of patients with COVID-19, which suggests that we should pay high attention to patients with co-infection of COVID-19 and asthma and take locally tailored interventions and treatment. Further well-designed studies with large sample sizes are required to verify our findings.
Acknowledgments
We thank Xuan Liang, Peihua Zhang, and Jian Wu (all are from the Department of Epidemiology, College of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data and valuable suggestions for data analysis.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number 81973105), Key Scientific Research Project of Henan Institution of Higher Education (grant number 21A330008), the National Science and Technology Major Projects of China (grant number 2018ZX10301407), and Joint Construction Project of Henan Medical Science and Technology Research Plan (grant number LHGJ20190679). The funders have no role in the data collection, data analysis, preparation of manuscript, and decision to submit the article for publication.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2021.02.013.
Supplementary Data
References
- 1.World Health Organization Coronavirus disease (COVID-2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at:
- 2.Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies [e-pub ahead of print]. Aging Male. https://doi.org/10.1080/13685538.2020.1774748, accessed September 28, 2020. [DOI] [PubMed]
- 3.Papadopoulos N.G., Christodoulou I., Rohde G. Viruses and bacteria in acute asthma exacerbations—a GA (2) LEN-DARE systematic review. Allergy. 2011;66(4):458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J.J., Cao Y.Y., Tan G. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021;76(2):533–550. doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang K., Yang T., Xu J. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 6.Latz C.A., DeCarlo C., Boitano L. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99(9):2113–2118. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yehia B.R., Winegar A., Fogel R. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siso-Almirall A., Kostov B., Mas-Heredia M. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almazeedi S., Al-Youha S., Jamal M.H. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Galdamez D.R., Gonzalez-Block M.A., Romo-Duenas D.K. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51(7):683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos M.M., Lucena E.E.S., Lima K.C., Brito A.A.C., Bay M.B., Bonfada D. Survival and predictors of deaths of patients hospitalised due to COVID-19 from a retrospective and multicentre cohort study in Brazil. Epidemiol Infect. 2020;148:e198. doi: 10.1017/S0950268820002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West S., King V., Carey T.S. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) 2002;(47):1–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 16.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adrish M., Chilimuri S., Sun H., Mantri N., Yugay A., Zahid M. The association of renin-angiotensin-aldosterone system inhibitors with outcomes among a predominantly ethnic minority patient population hospitalized with COVID-19: the Bronx experience. Cureus. 2020;12(9) doi: 10.7759/cureus.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal P, Ray S, Madan A, Tyson B. Neurological manifestations in 404 COVID-19 patients in Washington State [e-pub ahead of print]. J Neurol. 10.1007/s00415-020-10087-z, accessed September 6, 2020. [DOI] [PMC free article] [PubMed]
- 19.Argyropoulos K.V., Serrano A., Hu J. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol. 2020;190(9):1881–1887. doi: 10.1016/j.ajpath.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arshad S., Kilgore P., Chaudhry Z.S. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj J.S., Garcia-Tsao G., Biggins S.W. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broadhurst R., Peterson R., Wisnivesky J.P. Asthma in COVID-19 hospitalizations: an overestimated risk factor? Ann Am Thorac Soc. 2020;17(12):1645–1648. doi: 10.1513/AnnalsATS.202006-613RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capone S., Abramyan S., Ross B. Characterization of critically ill COVID-19 patients at a Brooklyn Safety-Net Hospital. Cureus. 2020;12(8) doi: 10.7759/cureus.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chachkhiani D., Soliman M.Y., Barua D. Neurological complications in a predominantly African American sample of COVID-19 predict worse outcomes during hospitalization. Clin Neurol Neurosurg. 2020;197:106173. doi: 10.1016/j.clineuro.2020.106173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhiba K.D., Patel G.B., Vu T.H.T. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enzmann M.O., Erickson M.P., Grindeland C.J., Lopez S.M.C., Hoover S.E., Leedahl D.D. Treatment and preliminary outcomes of 150 acute care patients with COVID-19 in a rural health system in the Dakotas. Epidemiol Infect. 2020;148:e124. doi: 10.1017/S0950268820001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox T., Ruddiman K., Lo K.B. The relationship between diabetes and clinical outcomes in COVID-19: a single-center retrospective analysis. Acta Diabetol. 2021;58(1):33–38. doi: 10.1007/s00592-020-01592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garibaldi B.T., Fiksel J., Muschelli J. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33–41. doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavin W., Campbell E., Zaidi S.A. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. 2021;49(2):158–165. doi: 10.1016/j.ajic.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gayam V., Chobufo M.D., Merghani M.A., Lamichanne S., Garlapati P.R., Adler M.K. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2021;93(2):812–819. doi: 10.1002/jmv.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb M., Sansom S., Frankenberger C., Ward E., Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med. 2020;27(10):963–973. doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goyal P., Ringel J.B., Rajan M. Obesity and COVID-19 in New York City: a retrospective cohort study. Ann Intern Med. 2020;173(10):855–858. doi: 10.7326/M20-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US [e-pub ahead of print]. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.3596, accessed July 25, 2020. [DOI] [PMC free article] [PubMed]
- 36.Haberman R.H., Castillo R., Chen A. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and DMARDs on clinical outcomes. Arthritis Rheumatol. 2020;72(12):1981–1989. doi: 10.1002/art.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jehi L., Ji X., Milinovich A. Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller M.J., Kitsis E.A., Arora S. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489–493. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Adeniji N, Latt N, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study [e-pub ahead of print]. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2020.09.027, accessed September 18, 2020. [DOI] [PMC free article] [PubMed]
- 40.Ko JY, Danielson ML, Town M, et al. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1419, accessed September 18, 2020. [DOI] [PMC free article] [PubMed]
- 41.Krishnan S., Patel K., Desai R. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020;67:110005. doi: 10.1016/j.jclinane.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lara O.D., O’Cearbhaill R.E., Smith M.J. COVID-19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126(19):4294–4303. doi: 10.1002/cncr.33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovinsky-Desir S., Deshpande D.R., De A. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146(5):1027–1034.e4. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maatman T.K., Jalali F., Feizpour C. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48(9):e783–e790. doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magagnoli J., Narendran S., Pereira F. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med (N Y) 2020;1(1):114–127.e3. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magleby R, Westblade LF, Trzebucki A, et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019 [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa851, accessed July 3, 2020. [DOI] [PMC free article] [PubMed]
- 47.McCarthy C.P., Murphy S., Jones-O’Connor M. Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine. 2020;26:100504. doi: 10.1016/j.eclinm.2020.100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikami T., Miyashita H., Yamada T. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2020;36(1):17–26. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moll M., Zon R.L., Sylvester K.W. VTE in ICU patients with COVID-19. Chest. 2020;158(5):2130–2135. doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mughal M.S., Kaur I.P., Jaffery A.R. COVID-19 patients in a tertiary US hospital: assessment of clinical course and predictors of the disease severity. Respir Med. 2020;172:106130. doi: 10.1016/j.rmed.2020.106130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukherjee V., Toth A.T., Fenianos M. Clinical outcomes in critically ill coronavirus disease 2019 patients: a unique New York City public hospital experience. Crit Care Explor. 2020;2(8) doi: 10.1097/CCE.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakeshbandi M., Maini R., Daniel P. The impact of obesity on COVID-19 complications: a retrospective cohort study. Int J Obes (Lond) 2020;44(9):1832–1837. doi: 10.1038/s41366-020-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng J.H., Hirsch J.S., Wanchoo R. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98(6):1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ortiz-Brizuela E., Villanueva-Reza M., Gonzalez-Lara M.F. Clinical and epidemiological characteristics of patients diagnosed with COVID-19 in a tertiary care center in Mexico City: a prospective cohort study. Rev Investig Clin. 2020;72:165–177. doi: 10.24875/RIC.20000211. [DOI] [PubMed] [Google Scholar]
- 55.Ramachandran P., Perisetti A., Gajendran M., Chakraborti A., Narh J.T., Goyal H. Increased serum aminotransferase activity and clinical outcomes in coronavirus disease 2019. J Clin Exp Hepatol. 2020;10(6):533–539. doi: 10.1016/j.jceh.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robilotti E.V., Babady N.E., Mead P.A. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shady A., Singh A.P., Gbaje E. Characterization of patients with COVID-19 admitted to a community hospital of East Harlem in New York City. Cureus. 2020;12(8) doi: 10.7759/cureus.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah P., Owens J., Franklin J. Demographics, comorbidities and outcomes in hospitalized COVID-19 patients in rural southwest Georgia. Ann Med. 2020;52(7):354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silver V., Chapple A.G., Feibus A.H. Clinical characteristics and outcomes based on race of hospitalized patients with COVID-19 in a New Orleans cohort. Open Forum Infect Dis. 2020;7(9):ofaa339. doi: 10.1093/ofid/ofaa339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singer A.J., Morley E.J., Meyers K. Cohort of four thousand four hundred four persons under investigation for COVID-19 in a New York hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020;76(4):394–404. doi: 10.1016/j.annemergmed.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinha P., Mostaghim A., Bielick C.G. Early administration of interleukin-6 inhibitors for patients with severe COVID-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. Int J Infect Dis. 2020;99:28–33. doi: 10.1016/j.ijid.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skipper C.P., Pastick K.A., Engen N.W. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173(8):623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19 [e-pub ahead of print]. J Med Virol. https://doi.org/10.1002/jmv.26227, accessed July 20, 2020. [DOI] [PMC free article] [PubMed]
- 65.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19 [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa954, accessed July 20, 2020. [DOI] [PMC free article] [PubMed]
- 66.Souza C.D.F., Leal T.C., Santos L.G. Circulatory system diseases in patients with COVID-19: description of clinical and epidemiological profile of 197 deaths. Arq Bras Cardiol. 2020;115(2):281–283. doi: 10.36660/abc.20200453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suleyman G., Fadel R.A., Malette K.M. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tartof S.Y., Qian L., Hong V. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tenforde M.W., Billig Rose E., Lindsell C.J. Characteristics of adult outpatients and inpatients with COVID-19—11 academic medical centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(26):841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Twigg H.L., 3rd, Khan S.H., Perkins A.J. Mortality rates in a diverse cohort of mechanically ventilated patients with novel coronavirus in the urban midwest. Crit Care Explor. 2020;2(8) doi: 10.1097/CCE.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaughn VM, Gandhi T, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1239, accessed August 23, 2020. [DOI] [PMC free article] [PubMed]
- 72.Yao J.S., Paguio J.A., Dee E.C. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Z., Chen A., Hou W. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuniga-Moya JC, Norwood DA, Romero Reyes LE, et al. Epidemiology, outcomes and associated factors of COVID-19 RT-PCR confirmed cases in the San Pedro Sula Metropolitan Area, Honduras [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1188, accessed August 23, 2020. [DOI] [PMC free article] [PubMed]
- 75.Alsofayan Y.M., Althunayyan S.M., Khan A.A., Hakawi A.M., Assiri A.M. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13(7):920–925. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asghar M.S., Haider Kazmi S.J., Ahmed Khan N. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi. Cureus. 2020;12(6) doi: 10.7759/cureus.8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao C., Cai Y., Zhang K. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang D., Wang T., Chen Z., Yang H., Yao R., Liang Z. A novel risk score to predict diagnosis with coronavirus disease 2019 (COVID-19) in suspected patients: a retrospective, multicenter, and observational study. J Med Virol. 2020;92(11):2709–2717. doi: 10.1002/jmv.26143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lian J., Jin C., Hao S. High neutrophil-to-lymphocyte ratio associated with progression to critical illness in older patients with COVID-19: a multicenter retrospective study. Aging (Albany NY) 2020;12(14):13849–13859. doi: 10.18632/aging.103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X., Yue X., Liu F. Analysis of clinical features and early warning signs in patients with severe COVID-19: a retrospective cohort study. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0235459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao B., Liu Y., Chai Y.H. Assessing risk factors for SARS-CoV-2 infection in patients presenting with symptoms in Shanghai, China: a multicentre, observational cohort study. Lancet Digit Health. 2020;2(6):e323–e330. doi: 10.1016/S2589-7500(20)30109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ozger H.S., Aysert Yildiz P., Gaygisiz U. The factors predicting pneumonia in COVID-19 patients: preliminary results of a university hospital in Turkey. Turk J Med Sci. 2020;50(8):1810–1816. doi: 10.3906/sag-2005-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pan W., Zhang J., Wang M. Clinical features of COVID-19 in patients with essential hypertension and the impacts of renin-angiotensin-aldosterone system inhibitors on the prognosis of COVID-19 patients. Hypertension. 2020;76(3):732–741. doi: 10.1161/HYPERTENSIONAHA.120.15289. [DOI] [PubMed] [Google Scholar]
- 85.Satici C., Demirkol M.A., Sargin Altunok E. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84–89. doi: 10.1016/j.ijid.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song J., Zeng M., Wang H. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2021;76(2):483–496. doi: 10.1111/all.14517. [DOI] [PubMed] [Google Scholar]
- 87.Sy K.T.L., Haw N.J.L., Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond) 2020;52(12):902–907. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 88.Tezcan M.E., Dogan Gokce G., Sen N., Zorlutuna Kaymak N., Ozer R.S. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microbes New Infect. 2020;37:100753. doi: 10.1016/j.nmni.2020.100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trabulus S., Karaca C., Balkan Kidney function on admission predicts in-hospital mortality in COVID-19. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsou T.P., Chen W.C., Huang A.S., Chang S.C., Taiwan C.-O.I.T. Epidemiology of the first 100 cases of COVID-19 in Taiwan and its implications on outbreak control. J Formos Med Assoc. 2020;119(11):1601–1607. doi: 10.1016/j.jfma.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W., Zhao Z., Liu X. Clinical features and potential risk factors for discerning the critical cases and predicting the outcome of patients with COVID-19. J Clin Lab Anal. 2020 doi: 10.1002/jcla.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang J.M., Koh H.Y., Moon S.Y. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Y., Tu J., Lei B. Incidence and risk factors of deep vein thrombosis in hospitalized COVID-19 patients. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620953217. 1076029620953217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou X., Zhu J., Xu T. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients with hypertension on renin-angiotensin system inhibitors. Clin Exp Hypertens. 2020;42(7):656–660. doi: 10.1080/10641963.2020.1764018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alkundi A., Mahmoud I., Musa A., Naveed S., Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: a retrospective single centre study. Diabetes Res Clin Pract. 2020;165:108263. doi: 10.1016/j.diabres.2020.108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Avdeev S., Moiseev S., Brovko M. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19. Allergy. 2020;75(10):2703–2704. doi: 10.1111/all.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Azoulay E., Fartoukh M., Darmon M. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020;46(9):1714–1722. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barillari M.R., Bastiani L., Lechien J.R. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: a multicenter Italian study. J Med Virol. 2021;93(2):983–994. doi: 10.1002/jmv.26354. [DOI] [PubMed] [Google Scholar]
- 99.Barroso B., Valverde-Monge M., Canas J.A. Presenting prevalence, characteristics and outcome of asthmatic patients with T2 diseases in hospitalized subjects with COVID-19 in Madrid, Spain. J Investig Allergol Clin Immunol. 2020;30(5):382–384. doi: 10.18176/jiaci.0627. [DOI] [PubMed] [Google Scholar]
- 100.Berenguer J., Ryan P., Rodriguez-Bano J. Characteristics and predictors of death among 4,035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beurnier A., Jutant E.M., Jevnikar M. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56(5):2001875. doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cellina M., Gibelli D., Valenti Pittino C., Toluian T., Marino P., Oliva G. Risk factors of fatal outcome in patients with COVID-19 pneumonia. Disaster Med Public Health Prep. 2020:1–30. doi: 10.1017/dmp.2020.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang C., Garzillo G., Batohi B. Extent of pulmonary thromboembolic disease in patients with COVID-19 on CT: relationship with pulmonary parenchymal disease. Clin Radiol. 2020;75(10):780–788. doi: 10.1016/j.crad.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrando C., Suarez-Sipmann F., Mellado-Artigas R. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fond G, Pauly V, Orleans V, et al. Increased in-hospital mortality from COVID-19 in patients with schizophrenia [e-pub ahead of print]. Encéphale. https://doi.org/10.1016/j.encep.2020.07.003, accessed August 3, 2020. [DOI] [PMC free article] [PubMed]
- 107.Garcia-Pachon E., Zamora-Molina L., Soler-Sempere M.J. Asthma prevalence in patients with SARS-CoV-2 infection detected by RT-PCR not requiring hospitalization. Respir Med. 2020:171. doi: 10.1016/j.rmed.2020.106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grandbastien M., Piotin A., Godet J. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. 2020;8(8):2600–2607. doi: 10.1016/j.jaip.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helms J., Kremer S., Merdji H. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ierardi AM, Coppola A, Fusco S, et al. Early detection of deep vein thrombosis in patients with coronavirus disease 2019: who to screen and who not to with Doppler ultrasound? [e-pub ahead of print] J Ultrasound. https://doi.org/10.1007/s40477-020-00515-1, accessed August 23, 2020. [DOI] [PMC free article] [PubMed]
- 111.Joseph A., Zafrani L., Mabrouki A., Azoulay E., Darmon M. Acute kidney injury in patients with SARS-CoV-2 infection. Ann Intensive Care. 2020;10(1):117. doi: 10.1186/s13613-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lechien JR, Chiesa-Estomba CM, Cabaraux P, et al. Features of mild-to-moderate COVID-19 patients with dysphonia [e-pub ahead of print]. J Voice. https://doi.org/10.1016/j.jvoice.2020.05.012, accessed July 20, 2020. [DOI] [PMC free article] [PubMed]
- 113.Lendorf M.E., Boisen M.K., Kristensen P.L. Characteristics and early outcomes of patients hospitalised for COVID-19 in North Zealand, Denmark. Dan Med J. 2020;67(9) [PubMed] [Google Scholar]
- 114.Lenti M.V., Borrelli de Andreis F., Pellegrino I. Impact of COVID-19 on liver function: results from an internal medicine unit in Northern Italy. Intern Emerg Med. 2020;15(8):1399–1407. doi: 10.1007/s11739-020-02425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lombardi C., Roca E., Bigni B., Cottini M., Passalacqua G. Clinical course and outcomes of patients with asthma hospitalized for severe acute respiratory syndrome coronavirus 2pneumonia: a single-center, retrospective study. Ann Allergy Asthma Immunol. 2020;125(6):707–709. doi: 10.1016/j.anai.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lund L.C., Kristensen K.B., Reilev M. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maguire D., Woods M., Richards C. Prognostic factors in patients admitted to an urban teaching hospital with COVID-19 infection. J Transl Med. 2020;18(1):354. doi: 10.1186/s12967-020-02524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martinez-Del Rio J., Piqueras-Flores J., N-SM de la Sierra P. Comparative analysis between the use of renin-angiotensin system antagonists and clinical outcomes of hospitalized patients with COVID-19 respiratory infection. Med Clin (Barc) 2020;155(11):473–481. doi: 10.1016/j.medcle.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perez-Guzman PN, Daunt A, Mukherjee S, et al. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1091, accessed July 20, 2020.
- 120.Poblador-Plou B., Carmona-Pirez J., Ioakeim-Skoufa I. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int J Environ Res Public Health. 2020;17(14) doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sapey E., Gallier S., Mainey C. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: an observational cohort study in an urban catchment area. BMJ Open Respir Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jalili M., Payandemehr P., Saghaei A., Sari H.N., Safikhani H., Kolivand P. Characteristics and mortality of hospitalized patients with COVID-19 in Iran: a national retrospective cohort study. Ann Intern Med. 2021;174(1):125–127. doi: 10.7326/M20-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mato A.R., Roeker L.E., Lamanna N. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olender SA, Perez KK, Go AS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1041, accessed July 28, 2020. [DOI] [PMC free article] [PubMed]
- 126.To T., Stanojevic S., Moores G. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gibson G.J., Loddenkemper R., Lundback B., Sibille Y. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J. 2013;42(3):559–563. doi: 10.1183/09031936.00105513. [DOI] [PubMed] [Google Scholar]
- 128.Centers for Disease Control and Prevention. Adult asthma data: BRFSS prevalence tables and maps. 2018. Available at: https://www.cdc.gov/asthma/brfss/2018/current_C1-H.pdf. Accessed September 28, 2020.
- 129.Wang Y., Shi L., Wang Y., Duan G., Yang H. Cerebrovascular disease is associated with the risk of mortality in coronavirus disease 2019. Neurol Sci. 2020;41(8):2017–2019. doi: 10.1007/s10072-020-04542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang X., Shi L., Wang Y. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J Infectol. 2020;81(3):e44–e47. doi: 10.1016/j.jinf.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamaya M., Nishimura H., Deng X. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58(3):155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen Y., Li Y., Wang X., Zou P. Montelukast, an anti-asthmatic drug, inhibits Zika virus infection by disrupting viral integrity. Front Microbiol. 2020;10:3079. doi: 10.3389/fmicb.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.