Abstract
Introduction
The highly infectious coronavirus disease 2019 (COVID-19) has now rapidly spread around the world. This meta-analysis was strictly focused on the influence of smoking history on the severe and critical outcomes on people with COVID-19 pneumonia.
Methods
A systematic literature search was conducted in eight online databases up to 1 February 2021. All studies meeting our selection criteria were included and evaluated. Stata 14.0 software was used to analyze the data.
Results
A total of 109 articles involving 517,020 patients were included in this meta-analysis. A statistically significant association was discovered between smoking history and COVID-19 severity, the pooled OR was 1.55 (95%CI: 1.41-1.71). Smoking was significantly associated with the risk of admission to intensive care unit (ICU) (OR=1.73, 95%CI: 1.36-2.19), increased mortality (OR=1.58, 95%CI: 1.38-1.81), and critical diseases composite endpoints (OR=1.61, 95%CI: 1.35-1.93), whereas there was no relationship with mechanical ventilation. The pooled prevalence of smoking using the random effects model (REM) was 15% (95%CI: 14%-16%). Meta-regression analysis showed that age (P=0.004), hypertension (P=0.007), diabetes (P=0.029), chronic obstructive pulmonary disease (COPD) (P=0.001) were covariates that affect the association.
Conclusions
Smoking was associated with severe or critical outcomes and increased the risk of admission to ICU and mortality in COVID-19 patients, but not associated with mechanical ventilation. This association was more significant for former smokers than in current smokers. Current smokers also had a higher risk of developing severe COVID-19 compared with non-smokers. More detailed data, which are representative of more countries, are needed to confirm these preliminary findings.
Keywords: COVID-19, Smoking, Hospital admission, Severity, Mortality, Systematic review
1. Introduction
Coronavirus disease 2019 (COVID-19) is a new type of severe acute respiratory syndrome (SARS) resulting from a novel strain of coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronaviruses, including strains considered to be the pathogens of Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), are a group of single-stranded RNA viruses [1]. Although COVID-19, MERS and SARS share some of the similar clinical manifestations and symptoms among, COVID-19 spreads more rapidly [2], [3]. Since being reported in China in late December 2019, COVID-19 has spread unprecedentedly around the world. On 12 March 2020, the World Health Organization (WHO) declared SARS-CoV-2 a global pandemic [4]. As of 4 February 2021, a total number of 103,448,210 cases of COVID-19 have been reported, including 2,236,453 deaths, most having been reported in America (46,270,502) and Europe (33,534,153) [5]. Initial study in China found that higher prevalence of current smokers among severe cases of COVID-19 [6].
More than 1.1 billion people worldwide smoke, and SARS-CoV-2 is a global epidemic affecting almost every country and region in the world, which highlights the significance of understanding the association between smoking and SARS-CoV-2 [7]. The main entrance route of the virus is through the mucosal tissue, such as mouth, nose, upper respiratory tract, and less frequently conjunctival mucosa. It is well known that tobacco smoke exposure leads to pulmonary inflammation, augmented mucosal inflammation, expression of tumor necrosis factor α and inflammatory cytokines, increased permeability of epithelial cells, excessive mucus production and impaired mucociliary clearance [8]. However, the correlation between smoking and SARS-CoV-2 infectiousness may be complicated. Some study suggested that smoking may increase the risk of COVID-19 by enhanced expression of angiotensin-converting enzyme 2 (ACE2) which is the receptor for SARS-Co-V-2 [9]. Conversely another study found that ACE2 among cigarette smokers might reduce this risk [10].
A large case series reporting the clinical features and results of COVID-19 patients from China found a higher prevalence of current smokers with severe COVID-19 [6]. Moreover, according to a recent meta-analysis [11], smoking is a risk factor for COVID-19 progression, and the probability of COVID-19 progression to more severe or critical conditions or death in smokers is greater than that in non-smokers. Interestingly, a cross-sectional study of COVID-19 outpatient and inpatient indicated that daily smokers have a greatly lower odds of progressing to symptomatic or serious SARS-CoV-2 infection as compared with the ordinary population [12]. A systematic review of the prevalence of current smokers in hospitalized COVID-19 patients in China suggested that pharmaceutical nicotine should be considered as an underlying treatment option in COVID-19 on account of the well-established immune regulation effects of nicotine [13]. Among numerous ingredients of cigarette smoke, carbon monoxide [14], several seasonings (such as menthol) [15] and nicotine [16] have been shown to have anti-inflammatory and positive immunomodulatory impact. Under the circumstances in which the outcomes of these studies are controversial, this meta-analysis addresses the discrepancy in consequence with the objective of assisting clinicians to evaluate the prognosis of COVID-19 infection in patients with smoking history.
2. Methods
2.1. Search strategy
A comprehensive systematic literature search was completed for relevant available articles from online databases: (1) EBSCO; (2) PubMed; (3) Web of Science; (4) EMBASE; (5) Science Direct; (6) China National Knowledge Infrastructure (CNKI); (7) Wanfang Data Knowledge Service Platform; and (8) China Science and Technology Journal VIP Database. The retrieval strategy was as follows: (“smoking” OR “cigarette” OR “smoke” OR “smoker” OR “characteristics” OR “risk factors” OR “outcomes” OR “Clinical”) AND (“coronavirus 2019” OR “COVID-19” OR “COVID19” OR “SARS-CoV-2” OR “Novel coronavirus” OR “2019 novel coronavirus” OR “2019-nCoV infection” OR “2019-nCoV disease” OR “Wuhan pneumonia” OR “Wuhan coronavirus” OR “2019-nCoV”). The search was restricted to studies on mankind published before 1 February 2021. In addition, we also reviewed the references of the interrelated articles to identify additional studies.
2.2. Eligibility criteria
2.2.1. Types of participants
The diagnosis of COVID-19 patients was based on WHO interim guidance or other accepted diagnostic criteria and treatment guideline, combined with epidemiological history, clinical manifestation, imaging detection, sequencing, and real-time reverse transcription polymerase chain reaction (real-time RT-PCR) of respiratory specimens which were used as tools to confirm positive cases.
2.2.2. Types of exposure
Current or former smokers before COVID-19 diagnosis.
2.2.3. Types of control
Never smoked before COVID-19 diagnosis.
2.2.4. Types of outcome measures
The outcome of the disease was divided into either a non-severe group (including mild type and ordinary type) or a severe group (including severe type and critical type), according to the guidance [17]. Critical COVID-19 disease was defined as having composite endpoints which consisted of admission to the ICU, acute respiratory distress syndrome (ARDS), invasive ventilation, or death [18,19]. These outcomes were adopted in a previous study to assess the severity of other infectious diseases [20].
2.2.5. Inclusion criteria
The included studies had to meet the criteria for participants, exposure, control and outcomes described above.
2.2.6. Exclusion criteria
(1) Article types including systematic reviews, meta-analyses, corrections, non-research letters, commentaries and qualitative studies. (2) The study was found to be a repeated publication. (3) The smoking data was not available, and the full text of the article could not be acquired. (4) The literature was classified to be of low quality by quality evaluation criteria. (5) Studies supported to any degree by the tobacco industry (TI).
2.3. Data extraction and quality assessment
The literature retrieval, selection of research, and extraction of data was conducted independently by two reviewers. Disagreements were resolved through discussion with the third investigator. In the process of extracting data, the severe COVID-19 patients were considered as the case group, and the non-severe COVID-19 patients were considered as the control group in the original articles. The exposure factor was the history of smoking. The extracted data from the included studies were as follows: first author, publication year, location, type of study design, characteristics of included patients, sample size, age, sex, disease outcome, number of patients in the severe and non-severe group.
The methodological quality of retrospective and observational studies was evaluated with the improved Newcastle-Ottawa scale (NOS) [[21], [22]], which is composed of three elements: patient selection, comparability between the two study groups and assessment of outcome. For NOS, studies scored 0-3, 3-6, and 6-9 could be identified as low, moderate and high quality, respectively [22].
2.4. Statistical analysis
All data statistical analyses were conducted using Stata (version 14.0; Stata Corp, College Station, TX) software. The results were displayed using forest plots. The raw data of extraction were used to compute the pooled odds ratio (OR) estimates with 95% confidence interval (CI) to assess the strength of association between smoking exposure and COVID-19 severity. The heterogeneity across the included studies was measured by Cochran's Q test and I square (I 2) statistic. I² values of 25%, 50% and 75% represent low, moderate and high heterogeneity, respectively [23]. The random effects model attempts to evaluate the distribution of the mean effect size in the light of the assumption that each primary study has a different population, while the fixed effect model estimates a single effect that is deemed to be common in each study [24]. If I²<50%, the fixed effects model was used for analysis, and if I² ≥50%, the random effects model was used for analysis. Sensitivity analysis was performed to explore whether a study had a significant effect on the results [25]. Subgroup analysis was carried out to detect the source of heterogeneity. Furthermore, to estimate the contribution of study characteristics to the overall heterogeneity, we adopted a meta- regression analysis with restricted maximum likelihood method for age, gender, sample size, hypertension, cardiovascular disease, diabetes and COPD. On behalf of evaluating the significance of publication bias, we employed Egger's and Begg's tests combined with trim and fill method funnel plots for quantitative determination and P < 0.05 was considered to be the evidence of publication bias.
3. Results
3.1. Study selection and characteristics
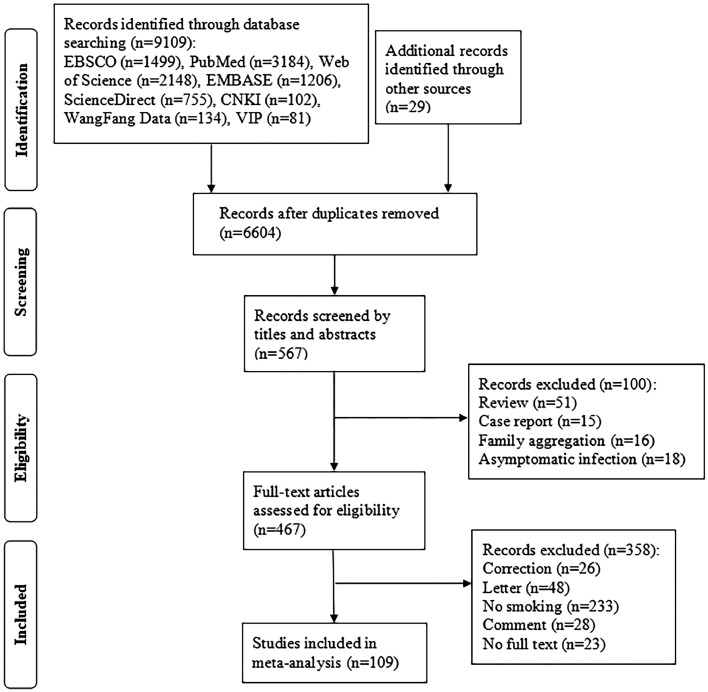
Based on the pre-formulated search strategy, a total of 9,138 potentially relevant papers were generated from the online databases and the references of the relevant articles, as described above. After screening of abstracts, eventually 517,020 patients in 109 articles were able to satisfy the eligibility criteria and were included in this meta-analysis. The flowchart of literature search is shown in Fig. 1 . Among the included studies, 50 studies were conducted in China [6,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75]], 27 in the United States [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], 32 in other nations [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134]. In terms of disease outcomes in patients who smoke or not, 109 reported disease severity, 85 reported critical illness. The detailed patient characteristics and demographic data of the included studies are shown in Supplementary Table 1.
Fig. 1.
Flow diagram of the study search and selection process.
3.2. Meta-analysis results
3.2.1. Smoking history and disease severity (including severe and critical type)
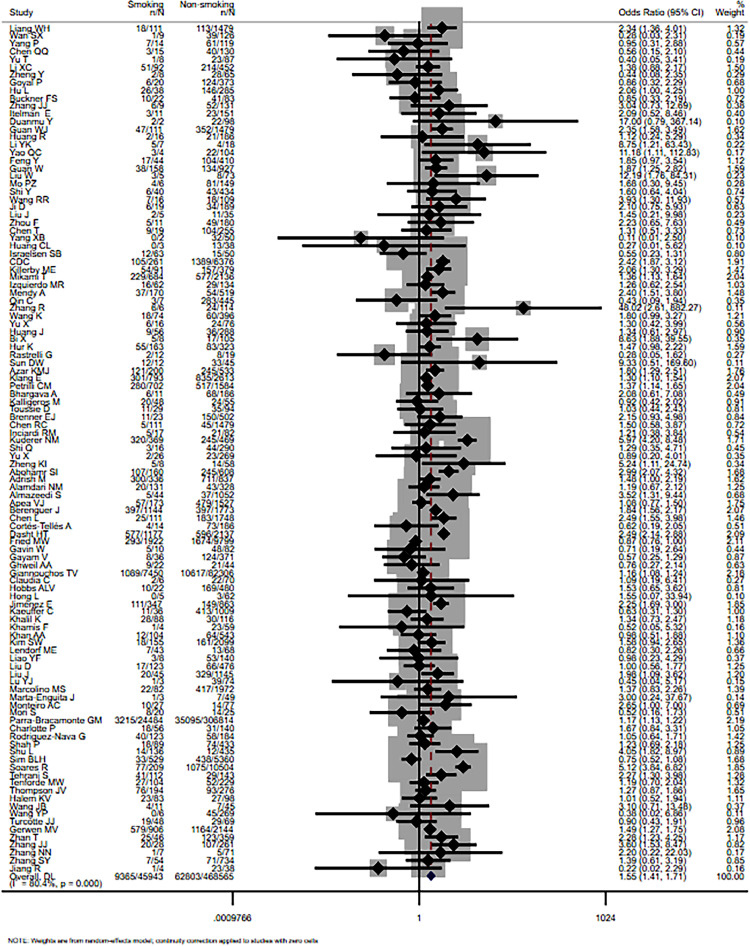
Overall, unadjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated for respective study using the number of smokers (former and current) and never smokers with severe and non-severe COVID-19 patients. A significant association was discovered statistically between smoking and COVID-19 severity, the pooled OR was 1.55 (95% CI: 1.41-1.71, I 2=80.4%, P<0.001). The main result is shown in Fig. 2 .
Fig. 2.
Forest plot for the association of smoking history and COVID-19 disease severity.
3.2.2. Smoking history and critical illness (including admission to ICU, mortality, mechanical ventilation and composite endpoints)
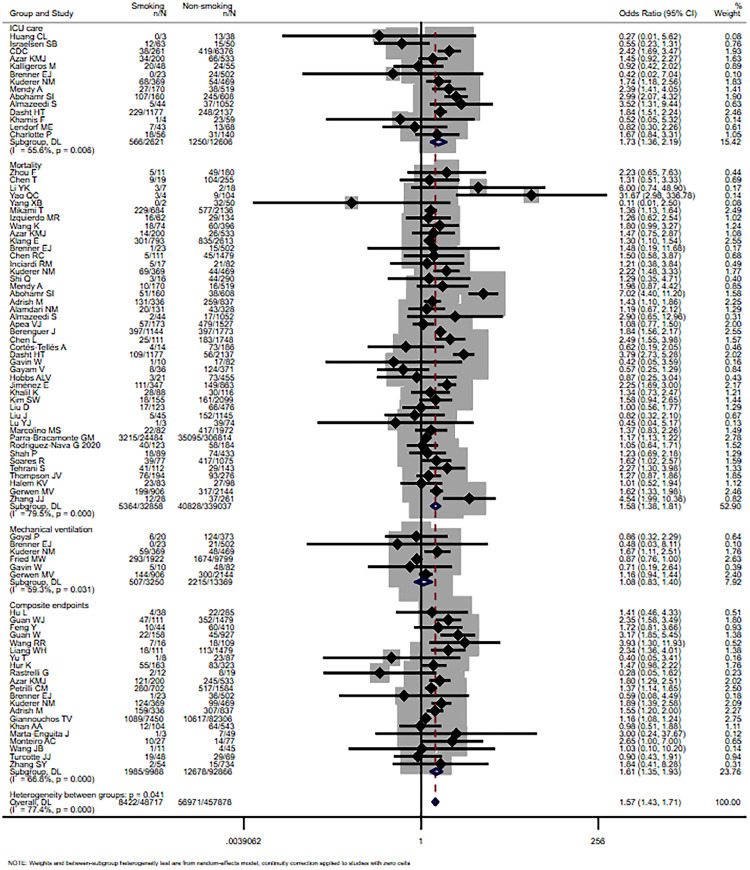
Smoking (former and current) was not significantly associated with mechanical ventilation, with the pooled OR of 1.08 (95% CI: 0.83-1.40, I 2=59.3%, P=0.031). Whereas for the risk of admission to ICU, mortality and composite endpoints, it remained significant (OR=1.73, 95% CI: 1.36-2.19, I 2=55.6%, P=0.006; OR=1.58, 95% CI: 1.38-1.81, I 2=79.5%, P<0.001; OR=1.61, 95% CI: 1.35-1.93, I 2=66.8%, P<0.001). The result is shown in Fig. 3 .
Fig. 3.
Forest plot for the association of smoking history and critical outcome (including ICU care, mortality, mechanical ventilation and composite endpoints) in patients with COVID-19.
3.2.3. Differences between current, former and never smokers on disease severity
25 articles reported the number of former smokers and current smokers respectively. The result suggested that there was a significant relationship between former smokers and the risk of severe COVID-19, compared to the current smokers, and the pooled OR was 1.85(95% CI: 1.33-2.55, I 2 =67.7%, P<0.001). However, there were no significant effects neither in the comparison between current smokers and never smokers, nor in current smokers and never or former smokers.
Furthermore, we extracted data of only current smokers from 26 articles, a statistically significant association was also found, the pooled OR was 1.19 (95% CI: 1.11-1.26, I 2=44.3%, P=0.009). The detailed results are shown in Supplementary Figs. 1-4.
3.2.4. Prevalence of smokers among COVID-19 patients
The prevalence of smokers among COVID-19 patients were assessed using meta-analysis of proportions. The smoking prevalence ranged from 2% to 47%. The pooled prevalence of smoking for the random effects model was 9% (95% CI: 8%-10%, I 2 =89.9%) in China, 20% (95% CI: 16%-24%, I 2=99.3%) in the United States of America (USA) and 19% (95% CI: 17%-20%, I 2=99.3%) in other areas. Findings from the meta-analysis of the random effects model are presented in Supplementary Fig. 5.
3.2.5. Subgroup analysis and meta-regression
To evaluate whether specific study characteristics had an impact on the association between smoking and the risk of COVID-19 disease progression, we performed a predesigned subgroup analysis stratified by the location where the study was conducted, median age (years), male proportion, sample size, prevalence of hypertension, diabetes, cardiovascular disease (excluding hypertension) and COPD.
The pooled ORs of the association between smoking and severity of patients with COVID-19, on the basis of different geographical locations of the research, indicated that smoking was statistically significantly associated with COVID-19 severity in China (OR=1.81, 95% CI: 1.52-2.15, I 2=30.1%), USA (OR=1.43, 95% CI: 1.21-1.70, I 2=82.8%) and other areas (OR=1.47, 95% CI: 1.24-1.74, I 2=88.9%). Subgroup analysis based on median age groups showed a statistically significant association among those whose age ≥40 years (P<0.001) but not in the age group of <40 years. There was a statistically significant correlation for the proportion of males in the subgroups. In regard to sample size, there was no statistically significant association for subgroups with sample size <100 while it was significant for those with sample size ≥100.
Due to the limitations in the original data, we divided the subgroups of underlying comorbidities according to percentage. Considering the prevalence of hypertension, diabetes and COPD [[135], [136]–137], there was statistical significance in all subgroups (≤30% and >30%; ≤10% and >10%; ≤5% and >5%). In terms of prevalence of cardiovascular disease [138], a statistically significant association was found in studies with prevalence of cardiovascular disease ≤40% whereas no relevance found in studies with prevalence of cardiovascular disease >40%. The detailed heterogeneity values and major results of subgroup analysis are displayed in Table 1 .
Table 1.
The subgroup analysis of COVID-19 outcome between smokers and non-smokers.
| Subgroups | Number of studies | OR(95%CI) | Z | P | Heterogeneity |
|
|---|---|---|---|---|---|---|
| I²(%) | P | |||||
| Location | ||||||
| USA | 27 | 1.43(1.21,1.70) | 4.14 | 0.000 | 82.8 | 0.000 |
| China | 50 | 1.81(1.52,2.15) | 6.71 | 0.000 | 30.1 | 0.025 |
| Other | 32 | 1.47(1.24,1.74) | 4.54 | 0.000 | 88.9 | 0.000 |
| Median age (years) | ||||||
| <40 | 5 | 1.45(0.55,3.32) | 0.91 | 0.362 | 73.8 | 0.004 |
| 40-49 | 28 | 1.82(1.40,2.37) | 4.44 | 0.000 | 70.3 | 0.000 |
| 50-59 | 31 | 1.75(1.48,2.07) | 6.62 | 0.000 | 48.9 | 0.001 |
| ≥60 | 40 | 1.34(1.15,1.56) | 3.81 | 0.000 | 69.7 | 0.000 |
| Male proportion(%) | ||||||
| <50% | 17 | 2.10(1.55,2.85) | 8.53 | 0.000 | 76.2 | 0.000 |
| ≥50% | 89 | 1.45(1.32,1.59) | 7.79 | 0.000 | 71.9 | 0.000 |
| Sample size | ||||||
| <100 | 22 | 1.17(0.73,1.88) | 0.65 | 0.516 | 32.6 | 0.071 |
| 100-199 | 24 | 1.41(1.04,1.93) | 2.20 | 0.028 | 41.7 | 0.018 |
| 200-599 | 31 | 1.46(1.27,1.68) | 5.12 | 0.000 | 22.6 | 0.131 |
| ≥600 | 32 | 1.71(1.49,1.96) | 7.62 | 0.000 | 92.9 | 0.000 |
| Prevalence of hypertension | ||||||
| ≤30% | 43 | 1.59(1.37,1.86) | 5.96 | 0.000 | 77.5 | 0.000 |
| >30% | 51 | 1.44(1.30,1.59) | 7.17 | 0.000 | 42.6 | 0.001 |
| Prevalence of diabetes | ||||||
| ≤10% | 24 | 1.78(1.30,2.43) | 3.63 | 0.000 | 61.1 | 0.000 |
| >10% | 75 | 1.47(1.32,1.62) | 7.39 | 0.000 | 81.1 | 0.000 |
| Prevalence of cardiovascular disease | ||||||
| ≤40% | 61 | 1.51(1.34,1.69) | 7.00 | 0.000 | 78.4 | 0.000 |
| >40% | 3 | 1.15(0.42,3.12) | 0.27 | 0.790 | 79.1 | 0.008 |
| Prevalence of COPD | ||||||
| ≤5% | 33 | 1.55(1.38,1.74) | 7.39 | 0.000 | 64.4 | 0.000 |
| >5% | 27 | 1.35(1.17,1.56) | 4.08 | 0.000 | 39.1 | 0.021 |
USA: the United States of America; COPD: chronic obstructive pulmonary disease.
In addition, it should be noted that heterogeneity decreased when the included studies were divided into subgroups based on median age, sample size and prevalence of underlying comorbidities, suggesting that these factors may be the source of heterogeneity in the process of pooling effect size.
Meta-regression analysis indicated that the association between smoking history and increased COVID-19 severity was influenced by age (P=0.004), hypertension (P=0.007), diabetes (P=0.029), COPD (P=0.001), but not by gender (P=0.078), sample size (P=0.148), cardiovascular diseases (P=0.289) and . The bubble plot is shown in Supplementary Fig. 6.
3.3. Sensitivity analysis and publication bias analysis
Sensitivity analysis excluded any of the included studies, and the variation observed in combined OR was insignificant, which demonstrated the stabilization of the meta-analysis consequences. The results of Begg's and Egger's tests suggested that there were publication bias across the studies (P<0.001). However, after adding 33 points to eliminating the influence of publication bias, the center of the funnel chart moved to the left. The 95% confidence interval before and after trim and fill method was not statistically significant, indicating that the result was stable. The trim and fill method funnel plot is shown in Supplementary Fig. 7.
4. Discussion
Based on the results of this meta-analysis, smoking history was shown to be associated with increased severity of disease in COVID-19 patients. In addition, this association also existed for critical illness of patients with COVID-19 in ICU care, mortality and composite endpoints subgroup but was not significant for the mechanical ventilation subgroup. It is still unclear whether people with smoking history are more likely to be infected with COVID-19 disease. However, individuals with smoking history tend to be more severely influenced by COVID-19, with angiotensin converting enzyme 2 (ACE2) being a potential explanation. This enzyme has been identified as a cell entry receptor for SARS-CoV-2 [9]. Exposure to cigarette smoke increased the expression of coronavirus receptor ACE2 in respiratory tissues of rodents and humans, which was potentially reversible, according to a study [139]. This up-regulation is possibly mediated by the expansion of ACE2+ secretory cells resulted from chronic smoke exposure. Some inflammatory cytokines also trigger the up-regulation of ACE2, which may further affect the expression of ACE2 due to smoking-related pulmonary inflammation. The excessive burden of ACE2 in the lungs of smokers may partly explain why smokers are more likely to develop severe SARS-CoV-2 infection, which requires more aggressive medical intervention [139]. Furthermore, it is well known that smoking alone can increase risks of cardiovascular diseases, cerebral vascular accidents, Chronic Obstructive Pulmonary Disease and other comorbidities that might contribute to develop severe outcomes of smokers contracting COVID-19. Besides, in vivo and clinical data suggested that cigarette smoke increased the expression of Cathepsin B, which enhanced the possibility of increased susceptibility among smokers towards COVID-19 infection [140].
In terms of the stratification of smoking history, the results of this meta-analysis found a significant association between smoking and the severity of COVID-19 in former smokers, compared with current smokers. This may be due to the fact that former smokers are likely to be older and have smoked for longer time than current smokers. Despite the relative lower risk compared with former smokers, current smokers also have a higher risk of developing severe COVID-19 compared with non-smokers. There is sufficient scientific data to indicate that the positive effects of smoking on COVID-19 is likely to be mediated by nicotine. It is known that to SARS-CoV-2 enters cells via ACE2 receptor [141,142], and there is evidence that nicotine regulates the expression of ACE2 [143], which could in turn regulates the nicotinic acetylcholine receptor. Since patients are usually forced to quit smoking and nicotine during hospitalization, tobacco (nicotine) cessation could give rise to the release of nicotine receptors, which are increased in smokers, as well as to a deteriorating of disease observed in former smokers owe to the “rebound effect” [12]. Additionally, the benefits of improving pulmonary function of smoking cessation are less among older smokers as a result of the cumulative lung damage over a prolonged period of time [144].
Regarding smoking prevalence and COVID-19 infection, the results showed a pooled prevalence of smoking was 9% (95% CI: 8%-10%) among the COVID-19 patients in China, which was lower than 27.7% smoking prevalence among general population in 2015 [145]. Reported pooled smoking prevalence in USA was 20% (95% CI: 16%-24%) among the COVID-19 patients, higher than the 13.7% reported in 2018 [146]. In view of the circumstances of the pandemic, the quality of data in China, the United States and other nations is uncertain. However, even if a better data collection method was used to report smoking conditions, a similarly low smoking prevalence was also found among hospitalized COVID-19 patients [147]. Although these findings are confusing, the results deserve serious scientific consideration in the search for new treatments to prevent the spread of the novel coronavirus pandemic and restrict morbidity and mortality of COVID-19.
The results of subgroup analysis suggested that a statistically significant association between smoking and COVID-19 disease severity was found in China, USA and other nations. In terms of median age, the results showed a statistically significant relationship in median age ≥40 years whereas not in <40 years subgroup. The epidemic situation of COVID-19 seems to more severely affect the middle-aged and elderly. A single-center study that analyzed the complete clinical data of hospitalized patients with COVID-19 found that elderly patients with confirmed COVID-19 were more likely to develop ARDS and cardiac injury than younger patients and were more likely to be admitted to the ICU [148]. Based on gender subgroups, the results showed that statistically significant correlation was found in both subgroups. Although some evidence of gender differences in the severity of COVID-19 disease appeared in China, where the number of hospitalizations and mortality were higher in males than females [149,150], which could be explained by more circulating ACE2 in males [151]. However, another study showed that the expression of ACE2 was equivalent between males and females [139]. Considering smoking may increase the expression of ACE2 [139,152], the different expressions of ACE2 between male and female could be further reduced. Therefore, male and female smokers may face similar risk of COVID-19 infection, as shown in our study. Nevertheless, more studies are needed to explore the effects of smoking on ACE2, and whether there is a difference between male and female smokers.
Cigarette smoke is composed of over 7,000 different compounds, most of which have adverse effects on respiratory tract cells. In addition to more than 50 known human carcinogens, tobacco smoke also contains toxins, chemical reactions of solid substances and oxidants [153,154]. Smoking can lead to oxidative stress and inflammation in the lung, which make smokers more vulnerable to bacterial or viral infections [155,156]. Oxidative stress has negative influence on epithelial permeability and the expression of ACE2, which may have a serious effect on smokers with COVID-19 disease [157,158]. However, the implication of cigarette smoke on the immune system is diverse, with dual properties-pro-inflammatory and immunosuppressive. Due to different ways of smoking (how to smoke, number of puffs, puff duration, puff volume, etc.), age, gender, source and socioeconomic status, the impact of smoking on the immune system may also vary between smokers and passively exposed people [159]. Experimental evidence demonstrates that smoking may exert both beneficial and adverse influences on the regulation of the immune system [36]. It is worth noting that individual components found in cigarettes, such as nicotine, may have a different impact on ACE2 than whole cigarettes, and that smoking itself may change ACE2 levels in non-respiratory organs in different modes [160]. Nicotine, one of the primary components of tobacco cigarette smoke, has been found to prevent acute lung damage in an animal model of ARDS and to restrain the expression of TNF in airway epithelial cells in vitro [161,162]. It also showed anti-inflammatory properties in humans exposed to endotoxin [163]. Besides, nicotine is an agonist of cholinergic anti-inflammatory pathway that modulates host immune and inflammatory reaction [164]. Although the results of meta-analysis indicated that smoking increased the risk of developing severe illness of COVID-19, it is not clear whether the harm comes from nicotine itself or from other toxic ingredient of tobacco smoke. Consequently, according to current research, the positive or neutral effect of nicotine alone on the severity of the disease cannot be ruled out. Furthermore, the World Health Organization (WHO) lists nicotine replacement therapy as an essential medicine. As a matter of fact, the Addiction Prevention Network in France (RESPADD) usually advises smokers to use nicotine replacement therapy when hospitalized for any disease [165].
Our meta-analysis has several limitations. First, in view of the urgency of the epidemic, it may be that the smoking situation of patients was inaccurately recorded or some patients were unable to report their smoking condition. It is also possible that some patients were already in critical condition when they were hospitalized, which would affect their ability to communicate and report their smoking status. Additionally, smoking duration was not reported in the majority of included studies. The majority of included studies did not classify former smoking and current smoking. This may have result in an underestimation of the prevalence of smoking, and some former smokers may have been misclassified as never smokers. Pack-year of cigarette smoking history was also not reported. Second, since all included studies did not statistically adjust the effect of smoking for baseline confounding variables, and the subjects were patients diagnosed with COVID-19, the risk assessment we reported did not represent the influence of smoking on the risk of COVID-19 infection in the general population. And only symptomatic patients, but not asymptomatic carriers, were included. Third, for some articles the full text could not be retrieved during the search of the literature. In addition, due to the limitation of extractable data in the original article, we were unable to analyze the relationship between the treatment success (or recovery) and smoking history. Finally, none of these studies evaluated electronic cigarette use.
The effects of smoking history on COVID-19 severity has been previously reported in several meta-analysis, which were limited by small included sample size and incomplete analytical method. As far as we know, our meta-analysis is the largest meta-analysis in the peer-reviewed literature to assess the impact of smoking on the severity of COVID-19. It strictly and specifically refined the influence of smoking history on the severity of COVID-19. We conducted more detailed subgroup analysis and meta regression to explore the factors that may affect this association. We also made a distinction of never, former and current smokers in COVID-19 patients. And our classification of COVID-19 disease outcomes is more detailed.
In conclusion, smoking is associated with severe and critical outcome in COVID-19 patients, and increased the risk of admission to ICU and mortality, while not with mechanical ventilation. This association is more significant in former smokers than in current smokers. And the current smokers also have a higher risk of developing severe COVID-19 compared with non-smokers. We ought to advocate public awareness campaigns focusing on how to achieve smoking cessation among smokers. The beneficial effects of nicotine replacement in treating patients with COVID-19 still remains controversial. More detailed data, which are more representative of the global population, are needed to confirm these preliminary findings.
Author contribution
Huimei Zhang: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. Shaodi Ma: Investigation, Software, Writing - review & editing. Tiantian Han: Resources, Data curation, Writing - review & editing. Guangbo Qu: Software, Writing - review & editing. Ce Cheng: Visualization, Writing - review & editing. John Patrick Uy: Supervision, Writing - review & editing. Mohammad Baseem Shaikh: Project administration. Qin Zhou: Supervision. Evelyn J. Song: Writing - review & editing. Chenyu Sun: Conceptualization, Resources, Formal analysis, Supervision, Writing - review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Data availability
Data and any supplementary material related to this article can be obtained from the corresponding author on request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eujim.2021.101313.
Appendix. Supplementary materials
References
- 1.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeri N.C., Shrestha N., Rahman M.S., et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020 doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Who Director-General’s Opening Remarks at the Media Briefing on Covid-19-11. https://www.who.int/dg/speeches/detail/who-director-general-sopening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 Accessed 10.04.2020, 2020. [Google Scholar]
- 5.ECDC . 2021. COVID-19 Situation Update Worldwide, as of Week 4.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases updated. Accessed February 4, 2021. [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18) doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global Health Observatory (GHO) Data. https://www.who.int/gho/tobacco/use/en/.
- 8.Strzelak A., Ratajczak A., Adamiec A., Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int. J. Environ. Res. Public Health. 2018;15(5):1033. doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Luo Q., Chen R., Chen T., Li J. Susceptibility analysis of COVID-19 in smokers based on ACE2. Prereprints. 2020 doi: 10.20944/preprints202003.0078.v. [DOI] [Google Scholar]
- 10.Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315(5):R895–R906. doi: 10.1152/ajpregu.00099.2018. .5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roengrudee P., Glantz SA. Smoking is associated with COVID-19 Progression: a meta- analysis. Nicotine Tob. Res. 2020:1–4. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyara M., Tubach F., Pourcher V., et al. Low Incidence of Daily Active Tobacco Smoking in Patients With Symptomatic COVID-19. Qeios ID: WPP19W.3. https://doi.org/10.32388/WPP19W.3.
- 13.Farsalinos K., Barbouni A., Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern. Emerg. Med. 2020;(4):1–8. doi: 10.1007/s11739-020-02355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sopori Mohan. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002;2(5):372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 15.Fagone P., Mazzon E., Bramanti P., Bendtzen K., Nicoletti F. Gasotransmitters and the immune system: mode of action and novel therapeutic targets. Eur. J. Pharmacol. 2018;5(834):92–102. doi: 10.1016/j.ejphar.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Bastaki S.M., Adeghate E., Amir N., Ojha S., Oz M. Menthol inhibits oxidative stress and infammation in acetic acid-induced colitis in rat colonic mucosa. Am. J. Transl. Res. 2018;10(12):4210–4222. [PMC free article] [PubMed] [Google Scholar]
- 17.Liang X.F., Feng Z.J., Li L.M. BeiJing: People's Medical Publishing House; 2020. Guidance for Corona Virus Disease 2019: Prevention, Control, Diagnosis and Management; pp. 14–17. [Google Scholar]
- 18.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay J.P., Waterer G.W., Long A.C., et al. Diagnosis and treatment of adults with community- acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67.. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao H.N., Lu H.Z., Cao B., et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 21.Taggart D.P., D’Amico R., Altman D.G. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–875. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 22.Wells G., Shea B., O’Connell D., et al. Ottawa Hospital Research Institute Web site; 2012. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed. [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borenstein M., Hedges L., Higgins J., Rothstein H. Vol. 13. John Wiley & Sons; 2009. pp. 77–86. (Fixed-Effect Versus Random-Effects Models. Introduction to Meta-Analysis). [DOI] [PubMed] [Google Scholar]
- 25.Haidich A.B. Meta-analysis in medical research. Hippokratia. 2010;14:29–37. [PMC free article] [PubMed] [Google Scholar]
- 26.Liang W.H., Liang H.R., Ou M.L., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang P., Wang P., Song Y., et al. A retrospective study on the epidemiological characteristics and establishment of an early warning system of severe COVID patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q.Q., Zheng Z.C., Zhang C., et al. Infection; Taizhou, Zhejiang, China: 2020. Clinical Characteristics of 145 Patients with Corona Virus Disease 2019 (COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu T., Cai S.H., Zheng Z.D., et al. Association between clinical manifestations and prognosis in patients with COVID-19. Clin. Ther. 2020;42(6):964–972. doi: 10.1016/j.clinthera.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Xu S., Yu M., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y., Xiong C., Liu Y., et al. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229) doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu L., Chen S., Fu Y., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J.J., Dong X., Cao Y., et al. Clinical characteristics of 140 patients infected with SARS- CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 37.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan W.-jj., Liang W.-hh., Zhao Y., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang R., Zhu L., Xue L., et al. A Retrospective, Multi-Center Study. PLoS Negl Trop Dis.; Jiangsu Province, China: 2019. Clinical findings of patients with coronavirus disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Peng S., Li L., et al. Clinical and transmission characteristics of covid-19-a retrospective study of 25 cases from a single thoracic surgery department. Curr. Med. Sci. 2020;40(2):295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Q., Wang P., Wang X., et al. Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Pol. Arch. Intern. Med. 2020 doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 42.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severity: a multi-center study of clinical features. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W., Tao Z., Lei W., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mo P., Xing Y., Xiao Y., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020;16:16. [Google Scholar]
- 45.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit. Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R., Pan M., Zhang X., et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji D., Zhang D., Xu J., et al. Prediction for progression risk in patients with COVID-19 Pneumonia: the CALL score. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J., Li S., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Y., Chen W., Wu X., et al. Clinical characteristics of COVID-19 patients in three consecutive generations of spread in Zhejiang, China. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.2139/ssrn.3541136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang R., Ouyang H., Fu L., et al. CT features of SARS-CoV-2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang K., Zhang Z., Yu M., Tao Y., Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46(7):1472–1474. doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu X., Sun S., Shi Y., Wang H., Zhao R., Sheng J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-02893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang J., Cheng A., Lin S., Zhu Y., Chen G. Individualized prediction nomograms for disease progression in mild COVID. J. Med. Virol. 2020 doi: 10.1002/jmv.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bi X., Su Z., Yan H., et al. Prediction of severe illness due to COVID-19 based on an analysis of initial fibrinogen to albumin ratio and platelet count. Platelets. 2020;31(5):1–6. doi: 10.1080/09537104.2020.1760230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun D.W., Zhang D., Tian R.H., et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: a sentinel? Clin. Chim. Acta. 2020;508 doi: 10.1016/j.cca.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen R.C., Liang W., Jiang M., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Q., Zhang X., Jiang F., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 60.Yu X., Sun X., Cui P., et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound. Emerg. Dis. 2020;67(4):1697–1707. doi: 10.1111/tbed.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng K.I., Gao F., Wang X.B., et al. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L., Yu J., He W., et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34(8):2173–2183. doi: 10.1038/s41375-020-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong L., Ye E., Sun G., et al. Clinical and radiographic characteristics, management and short-term outcomes of patients with COVID-19 in Wenzhou, China. BMC Infect. Dis. 2020;20(1):841. doi: 10.1186/s12879-020-05528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao Y.F., Feng Y., Wang B., et al. Clinical characteristics and prognostic factors of COVID-19 patients progression to severe: a retrospective, observational study. Aging (Albany NY) 2020;12(19):18853–18865. doi: 10.18632/aging.103931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu D., Wang Y.Y., Wang J., et al. Characteristics and outcomes of a sample of patients with COVID-19 identified through social media in Wuhan, China: observational study. J. Med. Internet Res. 2020;22(8):e20108. doi: 10.2196/20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J., Zhang S., Wu Z.X., et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann. Intensive Care. 2020;10(1):99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Y.J., Huang Z.L., Wang M.J., et al. Clinical characteristics and predictors of mortality in young adults with severe COVID-19: a retrospective observational study. Ann. Clin. Microbiol. Antimicrob. 2021;20(1):3. doi: 10.1186/s12941-020-00412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shu L., Wang X., Li M., et al. Clinical characteristics of moderate COVID-19 patients aggravation in Wuhan Stadium Cabin Hospital: A 571 cases of retrospective cohort study. J. Med. Virol. 2021;93(2):1133–1140. doi: 10.1002/jmv.26414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J.B., Wang H.T., Wang L.S., et al. Epidemiological and clinical characteristics of fifty-six cases of COVID-19 in Liaoning Province, China. World J. Clin. Cases. 2020;8(21):5188–5202. doi: 10.12998/wjcc.v8.i21.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y.P., Liao B.L., Guo Y., et al. Clinical characteristics of patients infected with the novel 2019 coronavirus (SARS-Cov-2) in Guangzhou, China. Open Forum Infect. Dis. 2020;7(6):ofaa187. doi: 10.1093/ofid/ofaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhan T., Liu M., Tang Y., et al. Retrospective analysis of clinical characteristics of 405 patients with COVID-19. J. Int. Med. Res. 2020;48(8) doi: 10.1177/0300060520949039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J.J., Cao Y.Y., Tan G., et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2020 doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang N.N., Zhang H.R., Tang Y.H., et al. Risk factors for illness severity in patients with COVID-19 pneumonia: a prospective cohort study. Int. J. Med. Sci. 2021;18(4):921–928. doi: 10.7150/ijms.51205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S.Y., Lian J.S., Hu J.H., et al. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China. Infect. Dis. Poverty. 2020;9(1):85. doi: 10.1186/s40249-020-00710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang R., Duan Y., Kang W.X., Wei D.G., Wu Y.Z., Zhao A.B. Analysis of clinical features and glucocrticod application of 42 cases of COVID-19. CHin. J. Diffic. Compl. Cas. 2021;20(1):53–57. [Google Scholar]
- 76.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of covid-19 in New York City. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buckner F.S., Mcculloch D.J., Vidya A., et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin. Infect. Dis. doi:10.109 3/cid/ciaa632. [DOI] [PMC free article] [PubMed]
- 78.Duanmu Y., Brown I.P., Gibb W.R., et al. Characteristics of emergency department patients with COVID-19 at a single site in Northern California: clinical observations and public health implications. Acad. Emerg. Med. 2020;00:1–5. doi: 10.1111/acem.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019-United States, February 12-March 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Killerby M.E., Link-Gelles R., Haight S.C., et al. Characteristics associated with hospitalization among patients with covid-19 — metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb. Mortal Wkly. RepMMWR. Morbidity and mortality weekly report. 2020;69(25):790–794. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mikami T., Miyashita H., Yamada T., et al. Risk factors for mortality in patients with COVID-19 in New York City. J. Gen. Intern. Med. 2020 doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mendy A., Apewokin S., Wells A.A., Morrow A.L. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.06.25.20137323. [DOI] [Google Scholar]
- 83.Hur K., Price C.P.E., Gray E.L., et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol. Head Neck Surg. 2020;163(1) doi: 10.1177/0194599820929640. 2092964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azar K.M.J., Shen Z., Romanelli R.J., et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff. 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 85.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity. 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ashish B., Akagi F.E., Miriam L., et al. Predictors for severe COVID-19 infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalligeros M., Shehadeh F., Mylona E.K., et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toussie D., Voutsinas N., Finkelstein M., et al. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adrish M., Chilimuri S., Mantri N., et al. Association of smoking status with outcomes in hospitalised patients with COVID-19. BMJ Open Respir. Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dashti H.T., Bates D., Fiskio J.M., Roche E.C., Mora S., Demler O. Clinical Characteristics and Severity of COVID-19 Disease in Patients from Boston Area Hospitals. medRxiv [Preprint]. 2020:2020.07.27.20163071. doi: 10.1101/2020.07.27.20163071.
- 93.Fried M.W., Crawford J.M., Mospan A.R., et al. Patient characteristics and outcomes of 11,721 patients with COVID19 hospitalized across the United States. Clin. Infect. Dis. 2020:ciaa1268. doi: 10.1093/cid/ciaa1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gavin W., Campbell E., Zaidi S.A., et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am. J. Infect. Control. 2021;49(2):158–165. doi: 10.1016/j.ajic.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gayam V., Chobufo M.D., Merghani M.A., Lamichhane S., Garlapati P.R., Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J. Med. Virol. 2021;93(2):812–819. doi: 10.1002/jmv.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hobbs A.L.V., Turner N., Omer, et al. Risk factors for mortality and progression to severe COVID-19 disease in the Southeast United States (US): a report from the SEUS Study Group. Infect. Control Hosp. Epidemiol. 2021:1–33. doi: 10.1017/ice.2020.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monteiro A.C., Suri R., Emeruwa I.O., et al. 2020. Obesity and Smoking as Risk Factors for Invasive Mechanical Ventilation in COVID-19: a Retrospective, Observational Cohort Study. medRxiv [Preprint]2020.08.12.20173849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodriguez-Nava G., Yanez-Bello M.A., Trelles-Garcia D.P., et al. Clinical characteristics and risk factors for mortality of hospitalized patients with COVID-19 in a community hospital: a retrospective cohort study. Mayo Clin. Proc. Innov. Qual. Outcomes. 2020 doi: 10.1016/j.mayocpiqo.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah P., Owens J., Franklin J., et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann. Med. 2020;52(7):354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tenforde M.W., Billig Rose E., Lindsell C.J., et al. Characteristics of adult outpatients and inpatients with COVID-19 - 11 academic medical centers, United States, March-May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(26):841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turcotte J.J., Meisenberg B.R., MacDonald J.H., et al. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gerwen M.V., Alsen M., Little C., et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J. Med. Virol. 2021;93(2):907–915. doi: 10.1002/jmv.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Itelman E., Wasserstrum Y., Segev A., et al. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr. Med. Assoc. J. 2020;22:271–274. [PubMed] [Google Scholar]
- 104.Israelsen S.B., Kristiansen K.T., Hindsberger B., et al. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March-April 2020. Dan Med. J. 2020;67(6):A052 00313. [PubMed] [Google Scholar]
- 105.Izquierdo M.R., Valero-Ubierna M.C., R-delAmo J.L., et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: a retrospective observational study. PLoS One. 2020 doi: 10.1371/journal.pone.0235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rastrelli G., Stasi V.D., Inglese F., et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2020 doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inciardi R.M., Adamo M., Lupi L., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abohamr S.I., Abazid R.M., Aldossari M.A., et al. Clinical characteristics and in-hospital mortality of COVID-19 adult patients in Saudi Arabia. Saudi Med. J. 2020;41(11):1217–1226. doi: 10.15537/smj.2020.11.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alamdari N.M., Afaghi S., Rahimi F.S., et al. Mortality risk factors among hospitalized COVID-19 Patients in a major referral center in Iran. Tohoku J. Exp. Med. 2020;252(1):73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 111.Almazeedi S., Al-Youha S., Jamal M.H., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. E Clin. Med. 2020;24 doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Apea V.J., Wan Y.I., Dhairyawan R., et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berenguer J., Ryan P., Rodríguez-Baño J., et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin. Microbiol. Infect. 2020;26(11):1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cortés-Tellés A., López-Romero S., Mancilla-Ceballos R., Ortíz-Farías D.L., Núñez-Caamal N., Figueroa-Hurtado E. Risk factors for mortality in hospitalized patients with COVID-19: an overview in a Mexican population. Tuberc. Respir. Dis. (Seoul) 2020;83(Supple 1):S46–S54. doi: 10.4046/trd.2020.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghweil A.A., Hassan M.H., Khodeary A., et al. Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA quarantine hospital’s patients, Egypt: a retrospective study. Infect. Drug Resist. 2020;13:2375–2383. doi: 10.2147/IDR.S263489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannouchos T.V., Sussman R.A., Mier J.M., Poulas K., Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases. Eur. Respir. J. 2020 doi: 10.1183/13993003.02144-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gregoriano C., Koch D., Haubitz S., et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med. Wkly. 2020;150:w20316. doi: 10.4414/smw.2020.20316. [DOI] [PubMed] [Google Scholar]
- 118.Jiménez E., Fontán-Vela M., Valencia J., et al. Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaeuffer C., Le Hyaric C., Fabacher T., et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill. 2020;25(48) doi: 10.2807/1560-7917.ES.2020.25.48.2000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khalil K., Agbontaen K., McNally D., et al. Clinical characteristics and 28-day mortality of medical patients admitted with COVID-19 to a central London teaching hospital. J. Infect. 2020;81(3):e85–e89. doi: 10.1016/j.jinf.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khamis F., Al-Zakwani I., Al Naamani H., et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J. Infect. Public Health. 2020;13(7):906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khan A.A., Althunayyan S.M., Alsofayan Y.M., et al. Risk factors associated with worse outcomes in COVID-19: a retrospective study in Saudi Arabia. East Mediterr. Health J. 2020;26(11):1371–1380. doi: 10.26719/emhj.20.130. [DOI] [PubMed] [Google Scholar]
- 123.Kim S.W., Kim S.M., Kim Y.K., et al. Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu metropolitan city outbreak in 2020. J. Korean Med. Sci. 2021;36(1):e12. doi: 10.3346/jkms.2021.36.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lendorf M.E., Boisen M.K., Kristensen P.L., et al. Characteristics and early outcomes of patients hospitalised for COVID-19 in North Zealand, Denmark. Dan Med. J. 2020;67(9) [PubMed] [Google Scholar]
- 125.Marcolino M.S., Ziegelmann P.K., Souza-Silva M.V.R., et al. Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: results from the Brazilian COVID-19 Registry. Int. J. Infect. Dis. 2021;S1201-9712(21) doi: 10.1016/j.ijid.2021.01.019. 00030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marta-Enguita J., Corroza-Laviñeta J., Ostolaza A. Risk factors and severity predictors in COVID-19 hospitalized patients: analysis of 52 patients: Factores de riesgo y predictores de gravedad en pacientes hospitalizados por COVID-19: análisis de 52 casos. Med. Clin. (Engl Ed) 2020;155(8):360–361. doi: 10.1016/j.medcle.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mori S., Ai T., Otomo Y. Characteristics, laboratories, and prognosis of severe COVID-19 in the Tokyo metropolitan area: a retrospective case series. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parra-Bracamonte G.M., Lopez-Villalobos N., Parra-Bracamonte F.E. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann. Epidemiol. 2020;52:93–98. doi: 10.1016/j.annepidem.2020.08.005. .e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Charlotte P., Gaël G., Pham Huu T.H.P., et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area - a retrospective cohort study. Swiss Med. Wkly. 2020;150:w20314. doi: 10.4414/smw.2020.20314. [DOI] [PubMed] [Google Scholar]
- 130.Sim B.L.H., Chidambaram S.K., Wong X.C., et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg. Health West Pac. 2020;4 doi: 10.1016/j.lanwpc.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soares R.C.M., Mattos L.R., Raposo L.M. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espírito Santo State, Brazil. Am. J. Trop. Med. Hyg. 2020;103(3):1184–1190. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tehrani S., Killander A., Åstrand P., Jakobsson J., Gille-Johnson P. Risk factors for death in adult COVID-19 patients: frailty predicts fatal outcome in older patients. Int. J. Infect. Dis. 2021;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thompson J.V., Meghani N.J., Powell B.M., et al. Patient characteristics and predictors of mortality in 470 adults admitted to a district general hospital in England with Covid-19. Epidemiol. Infect. 2020;148:e285. doi: 10.1017/S0950268820002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Halem K.V., Bruyndonckx R., van der Hilst J., et al. Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: a retrospective cohort study. BMC Infect. Dis. 2020;20(1):897. doi: 10.1186/s12879-020-05605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.International Diabetes Federation . Ninth edition. 2019. IDF Diabetes Atlas.https://www.diabetesatlas.org/en/resources/ [Google Scholar]
- 136.World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs. https://www.who.int/gho/publications/world_health_statistics/2020/en/.
- 137.World Health Organization . 2017. Chronic Obstructive Pulmonary Disease (COPD)https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd Key facts. [Google Scholar]
- 138.National Center for Cardiovascular Diseases Summary of the 2018 report on cardiovascular diseases in China. Chin. Circ. J. 2019;34(3):209–220. [Google Scholar]
- 139.Smith J.C., Sausville E.L., Girish V., et al. 2020. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith J.C., Sheltzer J.M. 2020. Cigarette Smoke Triggers the Expansion of a Subpopulation of Respiratory Epithelial Cells that Express the SARS-CoV-2 Receptor ACE2. bioRxiv. [DOI] [Google Scholar]
- 141.Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 142.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burns D.M. Cigarette smoking among the elderly: disease consequences and the benefits of cessation. Am. J. Health Promot. 2000;14(6):357–361. doi: 10.4278/0890-1171-14.6.357. [DOI] [PubMed] [Google Scholar]
- 145.World Health Organization . World Health Organization; England and Denmark: 2019. WHO Report on the Global Tobacco Epidemic, 2019: Country Profile. Republic of China.https://www.who.int/tobacco/surveillance/policy/country_profile/kor.pdf?ua=1 [Google Scholar]
- 146.Centers for Disease Control and Prevention . 2019. Current Cigarette Smoking Among Adults in the United States.https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/ [Google Scholar]
- 147.Polosa R., Caci G. COVID-19: counter-intuitive data on smoking prevalence and therapeutic implications for nicotine. Intern. Emerg. Med. 2020 doi: 10.1007/s11739-020-02361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song J., Hu W., Yu Y., et al. A comparison of clinical characteristics and outcomes in elderly and younger patients with COVID-19. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.925047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu Z., McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 150.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sama I.E., Ravera A., Santema B.T., et al. Circulating plasma concentrations of angiotensin- converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin- aldosterone inhibitors. Eur. Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cai G. 2020. Bulk and Single-Cell Transcriptomics Identify Tobacco-Use Disparity in Lung Gene Expression of ACE2, the Receptor of 2019-nCov. medRxiv. [DOI] [Google Scholar]
- 153.Smith C.J., Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem. Toxicol. 2000;38(7):637–646. doi: 10.1016/s0278-6915(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 154.Proulx L.I., Castonguay A., Bissonnette E.Y. Cytokine production by alveolar macrophages is down regulated by the alpha-methylhydroxylation pathway of 4-(methylnitrosamino)-1-(3-pyridyl) -1-butanone (NNK) Carcinogenesis. 2004;25:997–1003. doi: 10.1093/carcin/bgh103. [DOI] [PubMed] [Google Scholar]
- 155.Bauer C.M.T., Morissette M.C., Stämpfli M.R. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest. 2013;143(1):196–206. doi: 10.1378/chest.12-0930. [DOI] [PubMed] [Google Scholar]
- 156.Yao H., Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol. Appl. Pharmacol. 2011;254(2):72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wiener R.S., Cao Y.X., Hinds A., Ramirez M.I., Williams M.C. Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung. J. Cell. Biochem. 2007;101(5):1278–1291. doi: 10.1002/jcb.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meng Y., Li T., Zhou G.S., et al. The angiotensin-converting enzyme 2/angiotensin (1-7)/mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/rho kinase pathway. Antioxid. Redox. Signal. 2015;22(3):241–258. doi: 10.1089/ars.2013.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Agnieszka S., Aleksandra R., Aleksander A., et al. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int. J. Environ. Res. Public Health. 2018;15(5):1033. doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ferrari M.F.R., Raizada M.K., Fior-Chadi D.R. Differential regulation of the renin-angiotensin system by nicotine in WKY and SHR Glia. J. Mol. Neurosci. 2008;35(2):151–160. doi: 10.1007/s12031-007-9025-7. [DOI] [PubMed] [Google Scholar]
- 161.Mabley J., Gordon S., Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 2011;34(4):231–237. doi: 10.1007/s10753-010-9228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Q., Zhou X.D., Kolosov V.P., Perelman J.M. Nicotine reduces TNF-α expression through a α7 nAChR/MyD88/NF-ĸB pathway in HBE16 airway epithelial cells. Cell. Physiol. Biochem. 2011;27(5):605–612. doi: 10.1159/000329982. [DOI] [PubMed] [Google Scholar]
- 163.Wittebole X., Hahm S., Coyle S.M., Kumar A., Calvano S.E., Lowry S.F. Nicotine exposure alters in vivo human responses to endotoxin. Clin. Exp. Immunol. 2007;147:28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tracey K.J. Physiology and immunology of the cholinergic antiinfammatory pathway. J. Clin. Investig. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.RESPADD. Prendre en charge les fumeurs dans les lieux de santé. Un livret d'aide à la pratique pour les professionnels. https://www.respadd.org/wp-content/uploads/2019/09/Livret-Prise-en-charg.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and any supplementary material related to this article can be obtained from the corresponding author on request.