Abstract
Currently, there is an urgent need to find a treatment for the highly infectious coronavirus disease (COVID-19). However, the development of a new, effective, and safe vaccine or drug often requires years and poses great risks. At this critical stage, there is an advantage in using existing clinically approved drugs to treat COVID-19. In this study, in vitro severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike pseudotyped viral infection experiments indicated that histamine H1 antagonists loratadine (LOR) and desloratadine (DES) could prevent entry of the pseudotyped virus into ACE2-overexpressing HEK293T cells and showed that DES was more effective. Further binding experiments using cell membrane chromatography and surface plasmon resonance demonstrated that both antagonists could bind to ACE2 and that the binding affinity of DES was much stronger than that of LOR. Molecular docking results elucidated that LOR and DES could bind to ACE2 on the interface of the SARS-CoV-2-binding area. Additionally, DES could form one hydrogen bond with LYS31 but LOR binding relied on non-hydrogen bonds. To our knowledge, this study is the first to demonstrate the inhibitory effect of LOR and DES on SARS-CoV-2 spike pseudotyped virus viropexis by blocking spike protein–ACE2 interaction. This study may provide a new strategy for finding an effective therapeutic option for COVID-19.
Keywords: SARS-CoV-2, COVID-19, ACE2, Loratadine, Desloratadine
Abbreviations: COVID-19, Coronavirus disease 2019; LOR, loratadine; DES, desloratadine; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ACE2, angiotensin-converting enzyme 2; ACE2h, ACE2 overexpressing HEK293T cells; CMC, Cell membrane chromatography; SPR, Surface plasmon resonance; RBD, receptor binding domain
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious viral disease caused by a newly discovered, highly pathogenic coronavirus, namely severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1,2]. Since the outbreak of the disease in December 2019, this coronavirus has rapidly spread to many countries worldwide. Because of the rapid and global spreading of SARS-CoV-2, COVID-19 has been identified as a public health emergency of international concern [[3], [4], [5]]. As a virus belonging to the order Nidovirales and family Coronaviridae, SARS-CoV-2 requires the interaction of the viral spike (S) glycoprotein with angiotensin-converting enzyme 2 (ACE2) to enter cells [[6], [7], [8]]. The viral spike protein is a trimer, and each monomer contains two subunits, S1 and S2. During the infection period, S1 is responsible for viral attachment to the host cell surface through a receptor-binding domain, whereas S2 is required for the fusion of the viral and cellular membranes [9,10]. Recent studies have verified that binding to ACE2 is a critical initial step for the entry of SARS-CoV-2 into target cells [11,12]. Therefore, blocking or manipulating this binding process may prove very effective in defeating the virus.
To fight SARS-CoV-2, scientists all over the world have been attempting to employ different strategies [13]. In the past few months, government, academia, and industries have made huge efforts and spent several resources for the development of vaccines [14,15]. However, developing a new, effective, and safe vaccine often requires years and poses great risks [16]. At the same time, a lot of compounds obtained from natural products have been reported for their potential activity in preventing viral entry into cells using in silico analysis and in vitro inhibition experiments [17,18]. For example, curcumin has been reported to modulate the events of SARS-CoV-2 cellular entry and replication [19]. Additionally, andrographolide has been shown to inhibit the enzyme activity of SARS-CoV-2 main proteases by covalently linking to active cysteine [20]. However, the toxicology and bioavailability of these compounds remain unclear. Unfortunately, there is no effective medication for SARS-CoV-2 at present [21]; thus, the search for a suitable therapy to combat this virus still requires unremitting efforts globally.
Drug repurposing has gained enormous attention nowadays in treating various diseases, rather than the specific diseases for which they were originally developed. Compared with vaccine or new drug discovery, drug repurposing, an effective drug discovery strategy, can significantly reduce cost, time, and risks during drug development process [22,23]. For example, the antimalarial drugs chloroquine and hydroxychloroquine have been reported to shorten the duration of COVID-19 infection clinically [24,25]. Remdesivir, a broad-spectrum antiviral drug, has also been shown to be a promising direct-acting antiviral drug for SARS-CoV-2 in vivo and in vitro [25,26].
Histamine H1 antagonists are a class of drugs commonly used in the treatment of allergic diseases. In addition to their antihistaminic effects, it is now recognized that H1 receptor antagonists possess other pharmacologic properties, like anti-inflammatory effect [27]. Since the outbreak of COVID-19, a large number of virtual screening efforts have developed rapidly. Azelastine, clemastine, loratadine (LOR), desloratadine (DES) and other antihistamines have been reported for the potential ability of preventing the infection of SARS-CoV-2 [28]. An online interactive web server of Xu's systemic in silico screening ranks histamine H1 antagonists LOR and DES are on the top of these SARS-CoV-2-inhibiting drugs [29]. Therefore, in this study, we evaluated the inhibitory effects and preliminary mechanism of action of LOR and DES on SARS-CoV-2 viropexis, hoping to find effective agents in against SARS-CoV-2 infection.
2. Materials and methods
2.1. Materials and reagents
LOR and DES were from Dalian meilun Co. Ltd. (Dalian, China) with the purity of 99%. Dulbecco's Modification of Eagle's Medium (DMEM) with high glucose and fetal bovine serum (FBS) was obtained from HyClone (Logan, UT, USA). Penicillin–streptomycin solution was purchased from Xi'an Hat Biotechnology Co., Ltd (Xi'an, China). Cell Counting Kit was purchased from 7Sea Pharmatech Co., Ltd (Shanghai, China).
2.2. Cell lines
ACE2 overexpressing HEK293T cells (ACE2h) were built by the preliminary work of our group [30]. The cells were cultured in DMEM containing 10% FBS, 1% penicillin-streptomycin and 4 μg/mL puromycin at 37 °C in a 5% CO2 incubator.
2.3. Cytotoxicity assay
Cell viability was determined using Cell Counting Kit assays. ACE2h cells were seeded into 96‐well plates at a density of 5 × 103 cells per well. After cultured the plate for 24 h, cells were treated with different concentrations of LOR or DES (0, 1, 2.5, 5, 10, 20, 40 and 80 μM) for 24 h. 10 μL of Cell Counting Kit solution was then added to each well followed by incubation for 2 h. Further, the relative cell viability was assessed by detecting the absorbance at 450 nm using a microplate reader (Bio‐Rad, Carlsbad, CA, USA).
2.4. The detection of SARS-CoV-2 spike pseudotyped virus entry into ACE2h cells
The SARS-CoV-2 Spike pseudotyped virus was purchased from Sino Biological Inc. (PSC001). The copy number of pseudotyped virus was 1010 virus copies/mL, and the content of SARS-CoV-2 Spike protein was 860 ng/mL. Firstly, 5 × 104 of ACE2h cells in 100 μL DMEM per well were seeded into white 96‐well plates. The cells were cultured in a 37 °C incubator containing 5% CO2 for 24 h. Then 50 μL of medium was carefully aspirated from wells followed by adding another 50 μL of medium containing corresponding dose of LOR or DES and incubating for 2 h. 10 μL of SARS-CoV-2 Spike pseudotyped virus was then added. After infection at 37 °C with 5% CO2 in incubator for 10–12 h, the culture medium containing the virus was sucked away and replaced by 200 μL of fresh DMEM, and the cells were incubated continuously at 37 °C for 48 h. After that, the culture medium was aspirated. 20 μL of cell lysate and 100 μL of luminescence solution were added to each well before the luciferase luminescence detection by a microplate reader under 560 nm with exposure time as 1 s.
2.5. ACE2-HEK293T/cell membrane chromatography (ACE2-HEK293T/CMC)
ACE2-HEK293T/CMC columns were prepared using ACE2h cells and according to a published procedure [31,32]. The CMC assay was performed via LC-40A high performance liquid chromatography instrument. The CMC column were pre-equilibrated for 1 h before sample injection. Then, LOR and DES were analyzed with the CMC columns, respectively.
2.6. Surface plasmon resonance (SPR)
ACE2 protein (30 μg/mL) was fixed on the COOH sensor chip (Nicoya, Canada) by capture-coupling. LOR and DES were configured with DMSO as 100 mM first, then diluted with PBS to 40, 20, 10 or 5 μM before the experiment. After that, they were sequentially injected into the chamber in PBS running buffer. PBS containing 0.04% DMSO was used as a control. The interaction of ACE2 with the small molecules was detected by OpenSPRTM (Nicoya Lifesciences, Waterloo, Canada) at 25 °C. The binding time and disassociation time were both 250 s, the flow rate was 20 μL/s. A one to one diffusion corrected model was fitted to the wavelength shifts corresponding to the varied drug concentration. The data was retrieved and analyzed with TraceDrawer software.
2.7. Molecular docking
Molecular docking studies were carried out with SYBYL-X 2.0 version. The small molecules and the X-ray crystal structure of protein (PDB code: 6M0J) were imported. Water molecules were removed and hydrogen was added. Tripos force field and Pullman charge were applied to minimize. LOR and DES was depicted by Sybyl/Sketch module (Tripos Inc.), optimized by Powell's method with Tripos force field with convergence criterion at 0.005 kcal/(Å mol), and assigned with Gasteiger–Hückel method. The active residues of ACE2 used in the docking was referred to the binding area of SARS-CoV-2 spike protein, which was used as a ligand of ACE2 to model a standard ligand binding activity cavity.
2.8. Statistical analysis
All statistical analyses of the data were carried out using GraphPad 5.0 Prism software. All of the results were represented as the mean ± standard derivation (SD) from three independent experiments. * represents p-value <0.05; ** represents p-value <0.01; *** represents p-value <0.001 by Student's t-test.
3. Results
3.1. Viability of LOR and DES on ACE2h cells
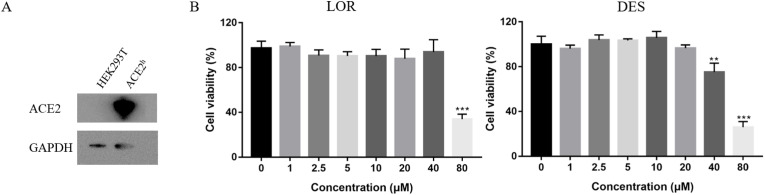
The expression of ACE2 protein in ACE2h cells was significantly higher than that in HEK293T cells, indicating a successful construction of ACE2h cells (Fig. 1 A). The effects of LOR and DES on the viability of ACE2h cells were then evaluated. As shown in Fig. 1B, LOR (<40 μM) and DES (<20 μM) were non-toxic to the ACE2h cells. However, at 80 μM concentration, cell viability reduced to 35.57% and 25.30% for LOR and DES, respectively, revealing that the effect of the toxicity of DES on the viability of ACE2h cells was higher than that of LOR. Thus, to ensure accuracy in further experiments, both antagonists were tested at ≤20 μM concentrations.
Fig. 1.
The effect of cell viability on ACE2h cells treated with LOR and DES for 24 h. (A) The protein expression level of ACE2 in HEK293T cell and ACE2-HEK293T cell. (B) The cell viability of LOR and DES on ACE2h cells. n = 5, *p < 0.05, **p < 0.01, ***p < 0.001, compared with concentration was 0 μM.
3.2. Effects of LOR and DES on the entry of SARS-CoV-2 spike pseudotyped virus into ACE2h cells
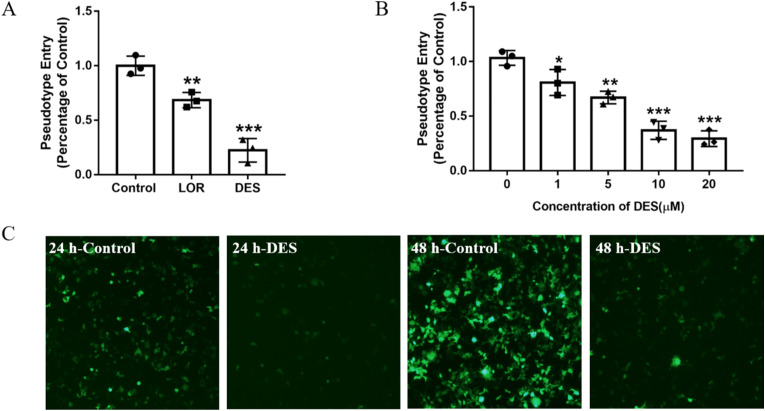
Considering the highly pathogenic and infectious nature of SARS-CoV-2, the handling of live viruses is faced with high risk, long cycle, strict experimental conditions, and strict requirements in laboratories. Currently, pseudotyped virus technology is a very effective research method for investigating potential inhibitors [33]. Here, ACE2h cells infected by the SARS-CoV-2 spike pseudotyped virus were considered controls, and the SARS-CoV-2 spike pseudotyped viral entry ratio was defined as 1 with luciferase luminescence value. After treatment with 20 μM LOR or DES, the ratios were reduced to 0.68 ± 0.07 or 0.23 ± 0.10, respectively (Fig. 2 A). Although both inhibitors exhibited significant inhibitory effect on the pseudotyped viral entry, DES was better than LOR at the same concentration. Different concentrations of DES were then evaluated, and the results are shown in Fig. 2B. When treated with 1, 5, 10, and 20 μM DES, the viral entry ratios were reduced to 0.81 ± 0.12, 0.67 ± 0.06, 0.37 ± 0.09, and 0.29 ± 0.08, respectively, in a dose-dependent manner. Moreover, this inhibitory effect of DES could be intuitively reflected by the decrease in the fluorescence intensity observed in the cells after infection with SARS-CoV-2 spike pseudotyped virus at 24 and 48 h (Fig. 2C).
Fig. 2.
The effect of LOR and DES on the entrance of SARS-CoV-2 Spike pseudotyped virus into ACE2h cells. (A) The entrance of SARS-CoV-2 Spike pseudotyped virus into ACE2h cells after treated with 20 μM LOR and DES. (B) The effect of different concentration of DES on the entrance of SARS-CoV-2 Spike pseudotyped virus into ACE2h cells. (C) The fluorescence image of SARS-CoV-2 Spike pseudotyped virus infected ACE2h cells in 24 h and 48 h after treated with 20 μM DES. Data are presented as mean ± S.D. n = 3, *p<0.05, **p < 0.01, ***p < 0.001 compared with group 0 or control.
3.3. Detection of the binding affinity of LOR and DES with ACE2 receptor
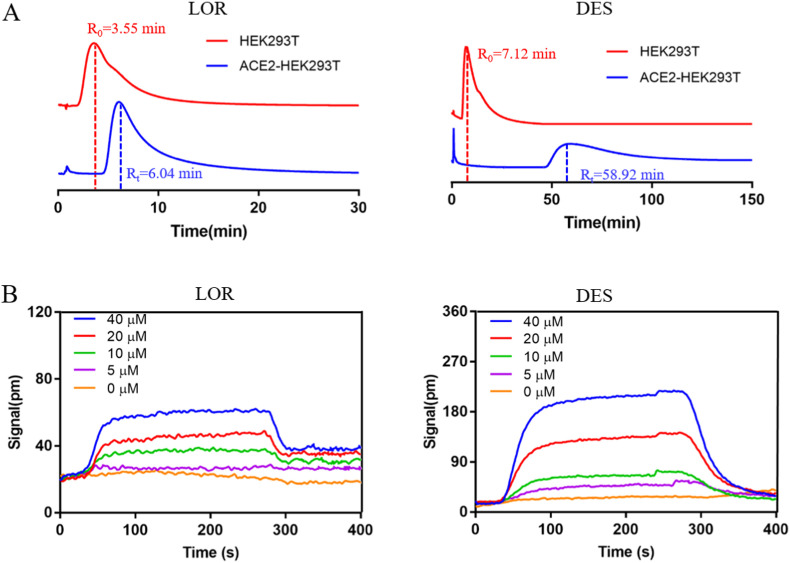
It is well known that SARS-CoV-2 can initiate infection by binding spike protein functional subunits to ACE2 on host cells. Therefore, in this experiment, we focused on whether LOR or DES could bind to ACE2. Cell membrane chromatography (CMC) is a new receptor kinetics method for the research of drug-receptor interactions. This method has high accuracy and reliability for analyzing these interactions because it guarantees the structural integrity of the membrane receptor [32]. Our CMC results showed that LOR and DES have obvious retention characteristics on the ACE2-HEK293T/CMC column with retention times of 6.04 and 58.92 min, respectively. HEK293T/CMC was also used to evaluate the nonspecific binding durations of LOR (3.55 min) and DES (7.12 min). Further, the specific binding durations of LOR and DES to ACE2 receptor were 2.49 and 51.8 min, respectively, indicating that DES had much better binding to the ACE2 receptor than LOR (Fig. 3 A). Additionally, surface plasmon resonance (SPR) method was used to detect the binding affinity of LOR and DES with ACE2. The response value of DES was easily observed to be higher than that of LOR at the same concentration, with binding constants of (9.13 ± 0.67) × 10−6 M and (1.02 ± 0.38) × 10−7 M, respectively (Fig. 3B). This result revealed that the binding affinity between DES and ACE2 was much stronger than that between LOR and ACE2, which is in accordance with the CMC results.
Fig. 3.
The binding character of LOR and DES with ACE2. (A) Chromatogram of LOR or DES on the HEK293T/CMC and ACE2-HEK293T/CMC model. (B) SPR analysis of LOR or DES and ACE2 protein.
3.4. Binding character of LOR and DES with ACE2 receptor via docking
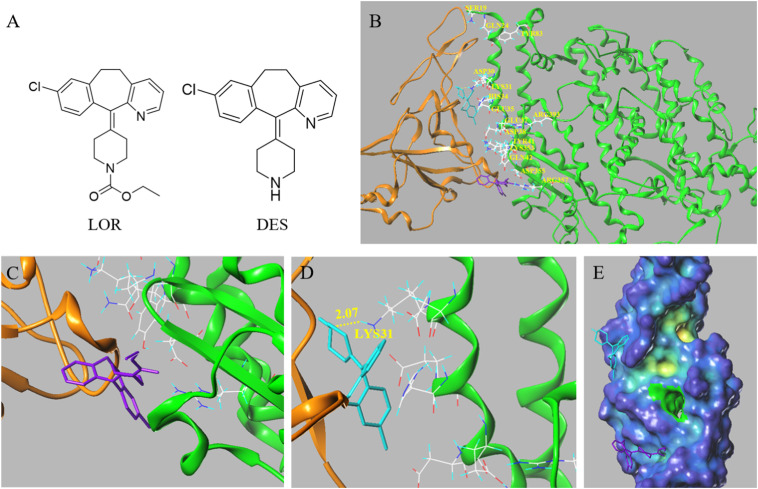
To explain the different activities of LOR and DES, molecular docking was used for the virtual evaluation of their binding character with ACE2 (PDB code: 6M0J). The contacting residues of ACE2 and SARS-CoV-2 spike proteins were labeled at the ACE2 interfaces (Fig. 4 B). Docking results showed that the position of LOR was far away from the active site of ACE2 and their interaction was mainly based on non-hydrogen bonds (Fig. 4C). However, after removing the substituent group in piperidine, compared with LOR, DES could form one hydrogen bond with LYS31 at a distance of 2.07 Å, and the position also moved to the center of the active site, indicating a better interaction with ACE2 (Fig. 4D).
Fig. 4.
The molecular docking of LOR and DES with ACE2 receptor. (A) The chemical structural of LOR and DES. (B) The docking mode of ACE2 and SARS-CoV-2 spike protein, in which the contacting residues are shown as sticks at the SARS-CoV-2 RBD–ACE2 interfaces. (C) The docking mode of LOR with ACE2. (D) The docking mode of DES withACE2. (E) The docking mode of LOR and DES with ACE2 represented in cavity depth maps. ACE2 is shown in green, and the SARS-CoV-2 RBD core is shown in orange. DES is shown in blue, and LOR shown in purple. (PDB ID: 6M0J).
4. Discussion
The COVID-19 pandemic presents an immense challenge globally that has been met with a remarkable response through the rapid production of pharmacological research and clinical trials.Although many studies and clinical trials with small-molecule drugs and vaccines have been performed or are ongoing, there are still no specific drugs against COVID-19. Therefore, finding the treatment of COVID-19 infection is still an urgent matter.
Histamine H1 antagonists have a long history of clinical efficacy in a variety of allergic inflammatory disorders. Besides of that, they also have been reported for the potent antiviral activity against Ebola, Marburg, and a broad spectrum of influenza viruses [34,35]. Recently, molecular docking and simulation studies have also reported that azelastine has optimal features for binding with the main protease on SARS-CoV-2 [28]. Antihistamines trimeprazine, azelastine, and clemastine have been identified as SARS-CoV-2 entry inhibitors following an antiviral test using native SARS-CoV-2 virus in Vero E6 cells [36]. This suggests that antihistamines have the potential to inhibit SARS-CoV-2 entry into cells.
In this study, to evaluate the antiviral effect of LOR and DES on SARS-CoV-2, a cytotoxicity assay was carried out that indicated nearly no cytotoxicity of them on ACE2h cells under 20 μM concentration. Then they were tested for the subsequent antiviral evaluations at 20 μM. It was observed that both of them inhibited virus entry into ACE2h cells, and DES was more obvious. Further test verified the effective concentration of DES as low as 1 μM. In order to evaluate the differences in their antiviral effect, CMC and SPR experiments was conducted. We not only found both of them can bind with ACE2 protein, but also revealed that the binding affinity between DES and ACE2 was much stronger than that between LOR and ACE2. Hence, our results indicted that antihistamines LOR and DES can inhibit SARS-CoV-2 entry into cells, which confirmed the antiviral effect of LOR and DES at the experimental level.
LOR and DES are new-generation antihistamines that lack central nervous system and cardiovascular side effects. Besides blocking airway inflammation and bronchoconstriction provoked by histamine release from mast cells, LOR and DES possess a number of anti-inflammatory activities [37,38]. Their inhibitory effects on the rhinovirus-induced upregulation of intercellular adhesion molecule 1 and promoter activation in respiratory epithelial cells have been documented recently, which might be important for the therapeutic control of virus-induced asthma exacerbations [39]. Therefore, we inferred that in addition to the inhibitory effects of LOR and DES against SARS-CoV-2, their anti-inflammatory properties are attractive research options to ease the symptoms of COVID-19 and could be promising new agents for COVID-19 treatment.
SARS-CoV-2, a beta coronavirus, similar to SARS-CoV and Middle East respiratory syndrome-CoV, binds to ACE2 to enter human cells [40]. The spike glycoprotein of SARS-CoV-2 is essential for attachment, fusion, and viral invasion [7]. Before the virus binds to ACE2, blocking or manipulating the SARS-CoV-2 receptor binding domain (RBD) and ACE2 binding interface may be a very effective strategy against the virus. Here, by combining CMC and SPR method, we demonstrated for the first time that histamine H1 antagonists LOR and DES can bind with ACE2, which may be why they exhibit the antiviral effects.
A study on the crystal structure of the binding between SARS-CoV-2 RBD and ACE2 showed that the overall interface is similar to that between SARS-CoV and ACE2, mediated mainly through polar interactions. With a cut-off distance of 4 Å, a total of 17 residues of the SARS-CoV-2 RBD are in contact with 20 residues of ACE2, and most of the contacting residues are located at the N-terminal helix [41,42].
In this study, the contacting residues of SARS-CoV-2 RBD and ACE2 are labeled on the interfaces of ACE2, as shown in Fig. 4B. Further, our docking results revealed that removing the substituent group in piperidine of LOR led to differences in location and binding forces. The ethoxycarbonyl group in LOR has strong electronegativity, which reduces the electron cloud density of nitrogen atoms in piperidine, resulting in difficulty in forming hydrogen bonds with amino acid residues. Thus, DES could form one hydrogen bond with LYS31, but LOR binding to ACE2 relied on non-hydrogen bonds. This may explain why the binding affinity between DES and ACE2 is much stronger than that between LOR and ACE2. Notably, LEU455 of SARS-CoV-2 RBD can interact with LYS31 of ACE2 [42]. However, the binding of DES to LYS31 can block the interaction between SARS-CoV-2 RBD and ACE2. This may result in better inhibitory effect of DES on SARS-CoV-2 spike pseudotyped virus than that of LOR.
Because of the highly pathogenic and infectious nature of SARS-CoV-2, the handling of live viruses needs to achieve a laboratory safety level of 3 or above [43]. Limited by these strict experimental conditions, this study could only be conducted using a pseudotyped virus technology. The anti-SARS-CoV-2 activities of LOR and DES would be examined in a native virus and animal models in further studies. We believe that these drugs could be effective in the clinical treatment of COVID-19 as well as act as novel targets and lead compounds for anti-SARS-CoV-2 drug development.
5. Conclusion
Our study revealed that LOR and DES could prevent the entry of SARS-CoV-2 spike pseudotyped virus into cells by blocking the binding of SARS-CoV-2 with ACE2. For the treatment of COVID-19, DES may be a more effective novel drug than LOR. Limited by the strict experimental conditions, this study can only confirm that LOR and DES prevent the invasion of pseudotyped virus. In further studies, the anti-SARS-CoV-2 activities of these drugs would be examined in a native virus and animal models as soon as possible. Considering the stress on healthcare systems in most countries to combat this virus, we hope that this study may provide a new strategy for finding an effective clinical treatment for COVID-19.
Author statement
Yajing Hou: Conceptualization, Formal analysis, Writing - original draft Data curation. Shuai Ge: Conceptualization, Formal analysis, Writing - original draft. Xiaowei Li: Formal analysis, Data curation. Cheng Wang: Data curation. Huaizhen He: Data curation. Langchong He: Supervision, Funding acquisition, Writing - review and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was cofounded by National Natural Science Foundation of China (Grant number: 81930096, 81903573), the Postdoctoral Research Foundation of China (No. 2018M643682), and the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2020JQ-089).
References
- 1.Lai C., Liu Y.H., Wang C., Wang Y., Hsueh S., Yen M., Ko W., Hsueh P.R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J. Microbiol. Immunol. Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He F., Deng Y., Li W. Coronavirus Disease 2019 (COVID-19): what we know? J. Med. Virol. 2020;92(7):719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable batorigin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F., Zhao S., Yu B., Chen Y.M., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D.Y., Wang W.L., Li X.W., Yang B., Song J.D., Zhao X., Huang B.Y., Shi W.F., Lu R.J., Niu P.H., Zhan F.X., Ma X.J., Wang D.Y., Xu W.B., Wu G.Z., Gao G. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin M., Sorour M.K., Kasry A. Comparing the binding interactions in the receptor binding domains of SARS-CoV-2 and SARS-CoV. J. Phys. Chem. Lett. 2020;11:4897–4900. doi: 10.1021/acs.jpclett.0c01064. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud I.S., Jarrar Y.B., Alshaer W., Ismail S. SARS-CoV-2 entry in host cellsmultiple targets for treatment and prevention. Biochimie. 2020;175:93–98. doi: 10.1016/j.biochi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42:3–11. [PubMed] [Google Scholar]
- 9.Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madu I.G., Roth S.L., Belouzard S., Whittaker G.R. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J. Virol. 2009;83:7411–7421. doi: 10.1128/JVI.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharifkashani S., Bafrani M.A., Khaboushan A.S., Pirzadeh M., Rezaei N. Angiotensin-converting enzyme 2 (ace2) receptor and sars-cov-2: potential therapeutic targeting. Eur. J. Pharmacol. 2020;884:173455. doi: 10.1016/j.ejphar.2020.173455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng W.H., Liu X., Mahalingam S. Development of vaccines for sars-cov-2, F1000. Research. 2020;9:991. doi: 10.12688/f1000research.25998.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S. Timely development of vaccines against sars-cov-2, Emerg. Microb. Infect. 2020;9(1):542–544. doi: 10.1080/22221751.2020.1737580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padron-Regalado E. Vaccines for sars-cov-2: lessons from other coronavirus strains. Infect. Dis. Ther. 2020;9(2):255–274. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi T., Joshi T., Sharma P., Mathpal S., Chandra S. In silico screening of natural compounds against covid-19 by targeting mpro and ace2 using molecular docking. Eur. Rev. Med. Pharmaco. 2020;24:4529–4536. doi: 10.26355/eurrev_202004_21036. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande R.R., Tiwari A.P., Nyayanit N., Modak M. In silico molecular docking analysis for repurposing therapeutics against multiple proteins from sars-cov-2. Eur. J. Pharmacol. 2020;886:173430. doi: 10.1016/j.ejphar.2020.173430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soni V.K., Mehta A., Ratre Y.K., Tiwari A.K., Amit A., Singh R.P., Sonkar S.C., Chaturvedi N., Shukla D., Vishvakarma N.K. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur. J. Pharmacol. 2020;886:173551. doi: 10.1016/j.ejphar.2020.173551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi T.H., Huang Y.L., Chen C.C., Pi W.C., Lin C.H. Biochem. Bioph. Res. Co.; 2020. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-ncov and sars-cov through covalent linkage. Published on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 23.Muralidharan N., Sakthivel R., Velmurugan D., Gromiha M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with sars-cov-2 protease against covid-19. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1752802. Published on line. [DOI] [PubMed] [Google Scholar]
- 24.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzunova K., Filipova E., Pavlova V., Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new sars-cov-2, Biomed. Pharmacother. 2020;131:110668. doi: 10.1016/j.biopha.2020.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gtte M. Remdesivir is a direct-acting antiviral that inhibits rna-dependent rna polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelfand E.W., Appajosyula S., Meeves S. Anti-inflammatory activity of H1-receptor antagonists: review of recent experimental research. Curr. Med. Res. Opin. 2008;20(1):73–81. doi: 10.1185/030079903125002586. [DOI] [PubMed] [Google Scholar]
- 28.Odhar H.A., Ahjel S.W., Albeer A., Hashim A.F., Rayshan A.M., Humadi S.S. Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation. 2020;16(3):236–244. doi: 10.6026/97320630016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu C., Ke Z., Liu C., Wang Z., Liu D., Zhang L., Wang J., He W., Xu Z., Li Y., Yang Y., Huang Z., Lv P., Wang X., Han D., Li Y., Qiao N., Liu B. Systemic in silico screening in drug discovery for coronavirus disease (covid-19) with an online interactive web server. J. Chem. Inf. Model. 2020 doi: 10.1021/acs.jcim.0c00821. [DOI] [PubMed] [Google Scholar]
- 30.Wang N., Han S., Liu R., Meng L., He H., Zhang Y., Wang C., Lv Y., Wang J., Li X., Ding Y., Fu J., Hou Y., Lu W., Ma W., Zhan Y., Dai B., Zhang J., Pan X., Hu S., Gao J., Jia Q., Zhang L., Ge S., Wang S., Liang P., Hu T., Lu J., Wang X., Zhou H., Ta W., Wang Y., Lu S., He L. Chloroquine and hydroxychloroquine as ace2 blockers to inhibit viropexis of 2019-ncov spike pseudotyped virus. Phytomedicine. 2020;79:153333. doi: 10.1016/j.phymed.2020.153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou X., Zhou M., Jiang Q., Wang S., He L. A vascular smooth muscle/cell membrane chromatography-offline-gas chromatography/mass spectrometry method for recognition, separation and identification of active components from traditional Chinese medicines. J. Chromatogr., A. 2009;1216:7081–7087. doi: 10.1016/j.chroma.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 32.He L., Wang S., Geng X. Coating and fusing cell membranes onto a silica surface and their chromatographic characteristics. Chromatographia. 2001;54:71–76. [Google Scholar]
- 33.Lei C., Qian K., Li T., Zhang S., Hu S. Neutralization of sars-cov-2 spike pseudotyped virus by recombinant ace2-ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W., Xia S., Pu J., Wang Q., Li P.Y., Lu L., Jiang S.B. The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses. Front. Microbiol. 2018;9:2643. doi: 10.3389/fmicb.2018.02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng H., Lear-Rooney C.M., Johansen L., Varhegyi E., Rong L. Inhibition of ebola and marburg virus entry by g protein-coupled receptor antagonists. J. Virol. 2015;89(19):9932–9938. doi: 10.1128/JVI.01337-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L., Pei R., Li H., Ma X., Zhou Y., Zhu F., He P., Tang W., Zhang Y., Xiong J., Xiao S., Tong X., Zhang B., Zuo J. Identification of SARS-CoV-2 entry inhibitors among already approved drugs. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-00556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agrawal K. Devendra, Pharmacology and clinical efficacy of desloratadine as an anti-allergic and anti-inflammatory drug. Expet Opin. Invest. Drugs. 2001;10:547. doi: 10.1517/13543784.10.3.547. [DOI] [PubMed] [Google Scholar]
- 38.Traidl-Hoffmann C., Mü I., Nster, Ring J., Behrendt H. Impact of desloratadine and loratadine on the crosstalk between human keratinocytes and leukocytes: implications for anti-inflammatory activity of antihistamines. Int. Arch. Allergy Immunol. 2006;140:315–320. doi: 10.1159/000093709. [DOI] [PubMed] [Google Scholar]
- 39.Papi A., Papadopoulos N.G., Stanciu L.A., Degitz K., Holgate S.T., Johnston S.L. Effect of desloratadine and loratadine on rhinovirus-induced intercellular adhesion molecule 1 upregulation and promoter activation in respiratory epithelial cells. J. Allergy Clin. Immunol. 2001;108:221–228. doi: 10.1067/mai.2001.116861. [DOI] [PubMed] [Google Scholar]
- 40.P. Zhou, X.L. Yang, X.G. Wang, B. Hu, L. Zhang, W. Zhang, H.R. Si, Y. Zhu, B. Li, C.L. Huang, Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin, BioRxiv. doi: 10.1101/2020.01.22.914952. [DOI]
- 41.Shang J., Ye G., Shi K., Wan Y., Li F. Structural basis of receptor recognition by sars-cov-2. Nature. 2020;581:7807. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 43.Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Fan C., Huang W., Xu M., Wang Y. Establishment and validation of a pseudovirus neutralization assay for sars-cov-2, Emerg. Microb. Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]