Abstract
Introduction
The management of chronic kidney disease (CKD) costs in excess of $114 billion in the USA and £1.45 billion in the UK annually and is projected to increase alongside the increasing disease prevalence. The aim of this review was to evaluate the risks of cardiovascular (CV) morbidity, CV mortality or all-cause mortality based on KDIGO (Kidney Disease: Improving Global Outcomes) 2012 categorisations and estimate the additional costs and healthcare resource utilisation associated with CV morbidity linked to CKD severity in US and UK settings.
Methods
A systematic literature review was conducted of studies reporting on the risk of CV morbidity, CV mortality or all-cause mortality characterised by CKD severity (published between January 2000 and September 2018). Additional costs and bed days associated with CKD severity in the USA and UK were estimated on the basis of median hazard ratios for CV morbidity risk at each CKD and albuminuria stage.
Results
Twenty-nine studies reported risk of adverse clinical outcomes based on KDIGO categorisations. Compared to stage 1 (or without) CKD, patients with stage 5 CKD and macroalbuminuria experienced a relative risk increase of 11.77–12.46 across all outcomes. Additional costs and bed days associated with stage 5 CKD and macroalbuminuria (versus stage 1 (or without) CKD) per 1000 patient years were US$3.93 million and 803 bed days and £435,000 and 1017 bed days, in the USA and UK, respectively.
Conclusion
Risks of adverse clinical outcomes increase with CKD and albuminuria severity and are associated with substantial additional costs and resource utilisation. Thus, early diagnosis and proactive management of CKD and its complications should be a priority for healthcare providers to alleviate the burden of CV morbidity and its management on healthcare resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-020-01607-4.
Keywords: Albuminuria, Cardiovascular morbidity, Chronic kidney disease, Healthcare resource utilisation, Systematic literature review
Key Summary Points
| Costs associated with the management of CKD are significant and are projected to rise alongside the increasing prevalence of CKD due to ageing populations and an increased prevalence of comorbidities |
| The aim of this study was to quantify the costs and impact on service delivery that CV morbidity associated with CKD—as categorised by KDIGO classification—imposes on healthcare providers in both US and UK settings |
| The risk of CV morbidity and the costs and bed days associated with its management increased substantially with CKD severity |
| Albuminuria was a significant driver of the health economic burden associated with the management of CKD-related CV morbidity; costs and bed days associated with managing a patient with macroalbuminuria are four to eight times greater than for a patient with normoalbuminuria at the same CKD stage |
| The results highlight the clinical and economic importance of early diagnosis and proactive management of CKD to reduce the risk of adverse clinical outcomes and their associated burden |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.13360040.
Introduction
The prevalence of chronic kidney disease (CKD) is estimated to be 8–16% worldwide and continues to grow as a result of an ageing population and an increasing prevalence of comorbidities, including type 2 diabetes and hypertension [1–3]. Consequently, costs associated with the management of CKD are significant. US Medicare expenditure for combined CKD and end-stage renal disease (ESRD) exceeded US$114 billion (£86 billion) in 2016 [4], and the National Health Service (NHS) in England spent an estimated US$1.95 billion (£1.45 billion) on CKD in 2009–2010 [5]. The healthcare burden of CKD is projected to increase alongside increasing disease prevalence and, when combined with a shortage of kidney specialists [6], the need exists to prioritise services and manage resource utilisation efficiently.
CKD severity has traditionally been evaluated on the basis of estimated glomerular filtration rate (eGFR) decline; however, in 2012 KDIGO (Kidney Disease: Improving Global Outcomes) highlighted the importance of albuminuria in the evaluation and prognostication of CKD [7]. It is relatively well established in the literature that more advanced CKD is associated with poor outcomes [8, 9]; however, it remains uncertain how the magnitude of the risks of CV morbidity and mortality (CV and all-cause mortality) change as CKD progresses according to the KDIGO categorisation of both eGFR and albuminuria.
Although the cost and service delivery implications of the direct management of CKD are well characterised [5, 10], especially in patients with ESRD, there is a need to update costs and healthcare resource utilisation in line with KDIGO CKD categorisation. A better understanding of the costs associated with the management of CV morbidity as a result of CKD will better support healthcare resource planning according to level of risk and therefore aid overall service prioritisation.
The primary aim of this study was to undertake a systematic literature review and meta-analysis to quantify the combined associations between eGFR and albuminuria and the risks of CV morbidity, CV mortality and all-cause mortality in patients with CKD. We then aimed to utilise results from the meta-analysis to inform economic analyses to estimate the additional costs and bed days associated with the management of CV morbidity linked to advancing CKD and albuminuria in both US and UK settings.
Methods
Systematic Literature Review
A systematic literature review was designed and conducted according to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) checklist [11]. Inclusion criteria were defined prior to undertaking the literature searches. Searches were conducted across PubMed, Embase, The Cochrane Library and relevant conference proceedings from January 2000 to September 2018. Electronic search strategies for each database and a list of congresses are included in the Supplementary Material (Tables S1–S3). In addition, a grey literature search was performed to identify further studies that may have met the eligibility criteria and included relevant conference proceedings (Table S4), a free-text internet search and reference lists from relevant studies. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Bibliographic details and abstracts of all citations retrieved by the literature search were downloaded into Endnote version X7. Titles and abstracts were independently assessed for eligibility by two reviewers [CD and Sukhvir Rai (Health Economics and Outcomes Research Ltd)]. Full texts of all potentially eligible studies (both open access and paywall-restricted) were retrieved and assessed against the Population-Intervention-Comparators-Outcomes-Study (PICOS) eligibility criteria (Table S5) independently by two reviewers. Any discrepancies between the two reviewers concerning eligibility were resolved by consensus.
Studies were included if they comprised adult patients with stages 2–5 CKD and reported the risk of CV morbidity, CV mortality or all-cause mortality characterised by both CKD and albuminuria stage. Advancing CKD was defined as worsening of either eGFR or albuminuria, or the worsening of combined eGFR and albuminuria. To align with KDIGO 2012 criteria, only studies that reported advancing CKD based on combined eGFR and albuminuria data were included in the main analysis; risks of CV morbidity, CV mortality and all-cause mortality based on the worsening of eGFR alone and albuminuria alone are included in the Supplementary Material (Tables S8 and S9). CV morbidity included CV disease (e.g. coronary heart disease and heart failure), cardiac or ischaemic events (e.g. myocardial infarction) and stroke. Patients receiving haemodialysis or peritoneal dialysis were not included in the analysis; studies specifically in patients with prior renal transplantation were not included; however, patients with prior transplantation may have been included in cohorts of included studies. Relative measures of risk [hazard ratios (HR) odds ratios (OR), incidence rate ratios (IRR), or relative risk (RR)] for CV morbidity, CV mortality and all-cause mortality were extracted to enable comparability of risk based on disease progression across studies. Meta-analyses were identified in the systematic review but were excluded from our meta-analysis. Studies that only assessed the impact of CKD of any severity in comparison with no CKD were excluded as this study aimed to characterise the impact of advancing CKD on outcomes. Studies conducted in child or adolescent patients and studies reported in languages other than English were also excluded, and searches were limited to publications from 2000 onwards to only capture contemporary data.
For analysis, categorical eGFR data were aligned with the corresponding CKD stage, where possible, as per KDIGO classification [7]. Albuminuria data were categorised as normoalbuminuria (normal to mildly increased), microalbuminuria (moderately increased) and macroalbuminuria (severely increased), based on the KDIGO 2012 classifications of urine albumin-to-creatinine ratio (UACR) less than 30 mg/g, 30–300 mg/g, and greater than 300 mg/g respectively. Those that reported data in alternative units (e.g. milligrams per millimole) were converted to milligrams per gram where possible. Extracted HRs and ORs were grouped according to eGFR and/or albuminuria category (based on KDIGO criteria) and further aligned as per the reference group. Associations were most frequently reported in terms of HR, and as such studies reporting the HR of CV morbidity, mortality or all-cause mortality associated with CKD stages 2, 3a, 3b, 4 and 5 in comparison with stage 1 or no CKD extracted. Extracted data were combined using a random-effects meta-analysis (R version 4.0.3) of log HRs with inverse variance weighting used for pooling, and all data are reported as mean HR (95% confidence interval), unless stated otherwise. To assess the impact of publication bias on the results of the meta-analysis, sensitivity analysis was conducted comparing the results of random- and fixed-effects meta-analysis and the exclusion of studies including fewer than 10,000 patients, and results displayed in a funnel plot.
For analysis exploring associations between renal impairment and adverse patient outcomes within comorbid subpopulations, HR and OR were pooled independently by CKD and albuminuria stage and the median and interquartile range (IQR) calculated to provide indicative measures of risk for each KDIGO category. All comparisons were based on data where stage 1 (or no) CKD and normoalbuminuria formed the reference groups. A subgroup analysis was also performed on patients according to baseline comorbidity.
A quality assessment was performed using the National Institute for Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Quality assessment was performed by one reviewer (CD) and checked by a second reviewer [Sukhvir Rai (Health Economics and Outcomes Research Ltd)]. Disagreements were resolved by consensus. Studies were not categorised by quality and no studies were excluded from the meta-analysis on the basis of the outcome of the quality assessment.
Health Economic Analyses
The summarised risks of CV morbidity at each CKD and albuminuria stage obtained from the conducted systematic literature review were used to estimate the additional costs and hospitalisation requirements of CV morbidity associated with each CKD and albuminuria stage compared to stage 1 (or without) CKD. Relative risks derived from the results of the systematic review were used to estimate the additional risk of CV events with advancing CKD, which were then scaled to CKD population level figures based on prevalence data from the US Renal Data System [12], NHS England [13] and the UK Renal Registry [14], and population data reported by the US Census Bureau [15] and the UK Office for National Statistics [16]. Total costs and median length of hospital stay estimates associated with CV events were sourced from published literature for analysis conducted from a US perspective [17], and from NHS data [18] for UK analysis (Table S10 presents input costs and healthcare resource utilisation for each setting).
A baseline risk of coronary heart disease (CHD), ischaemic stroke or transient ischaemic attack (TIA) in patients without CKD was derived using the QRISK-3 risk equation, a validated risk equation developed on a large cohort of primary care patients in England to predict the incidence of CV disease [19]. Patient characteristics were aligned to the population of the Chronic Renal Insufficiency Cohort [20], an observational study that examined risk factors for the progression of CKD and CV disease to provide patient characteristics representative of a CKD population and allow the estimation of marginal risk associated with CKD alone. Any patient characteristics not reported were assumed to be equal to the QRISK-3 development cohort.
Results
Systematic Literature Review
Study Characteristics
The literature search identified 4168 studies after the removal of duplicates. After titles and abstracts were reviewed, 3396 studies were excluded and the full texts for the remaining 772 studies were retrieved and reviewed in full (Fig. S1). A total of 314 studies met the inclusion criteria and reported associations between eGFR and/or albuminuria and the risk of CV morbidity, CV mortality or all-cause mortality, or included risk equations that included eGFR and/or albuminuria as predictive covariates for CV morbidity or mortality. Whilst the majority of included studies evaluated the risk of CV morbidity, CV mortality or all-cause mortality by either eGFR or albuminuria worsening alone, the primary focus of this analysis was on the 29 studies that assessed the risk of adverse clinical outcomes based on the combined associations between eGFR and albuminuria (Table 1).
Table 1.
Study characteristics of the subgroup of 29 studies that met the inclusion criteria and reported combined associations between eGFR, albuminuria and the risk of adverse clinical outcomes
| Author | Year | Country | Study design | Setting | Sample size | Median eGFR (mL/min/1.73 m2) |
CKD stages included (% of cohort)* |
Follow-up (years) | Outcome definition | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3a | 3b | 4 | 5 | |||||||||
| Alonso et al. [28] | 2011 | USA | Prospective observational cohort | Population | 10,328 | NR | 59 | 5.5 | 5.5 | 1 | NR | 10.1 | Atrial fibrillation |
| Amin et al. [29] | 2013 | USA | Prospective observational cohort | Population | 42,761 | NR | 45 | 13 | 6 | 1 | 1 | 4.0 | All-cause mortality |
| Berhane et al. [30] | 2011 | USA | Prospective observational cohort | Population | 2420 | 110 | 8.8 | 1.3 | 1.3 | 0.6 | NR | 10.2 | All-cause mortality |
| Blecker et al. [31] | 2011 | USA | Prospective observational cohort | Population | 10,975 | NR | 62.4 | 8.1 | 1.3 | NR | NR | 8.3 | Heart failure |
| Deo et al. [32] | 2017 | USA | Prospective observational cohort | Population | 27,296 | NR | 44 | 7.3 | 1.2 | 1.2 | 1.2 | 6.1 | Sudden cardiac death |
| Kovesdy et al. [33] | 2013 | USA | Prospective observational cohort | Database | 298,875 | NR | NR | NR | NR | NR | NR | 5.0 | All-cause mortality |
| Muntner et al. [34] | 2011 | USA | Prospective observational cohort | Population | 24,350 | NR | NR | NR | NR | NR | NR | 4.5 | All-cause mortality |
| Yuyun et al. [35] | 2004 | UK | Prospective observational cohort | Population | 20,911 | NR | NR | NR | NR | NR | NR | 6.3 | All-cause mortality, CV-specific mortality, CV events |
| Bruno et al. [36] | 2007 | Italy | Prospective observational cohort | Population | 1565 | NR | 57.4 | 25.6 | 6.8 | 1.5 | NR | 11.0 | CV-specific and all-cause mortality |
| Garofolo et al. [37] | 2018 | Italy | Prospective observational cohort | Secondary care (Outpatient) | 774 | 95.2 | 3.4 | 0.94 | 0.94 | 0.94 | 0.94 | 8.3 | All-cause mortality |
| Nerpin et al. [38] | 2011 | Sweden | Prospective observational cohort | Population | 1113 | 75 | NR | NR | NR | NR | NR | 12.9 | All-cause mortality |
| Sasso et al. [39] | 2012 | Italy | Prospective observational cohort | Secondary care (outpatient) | 742 | 66 | 43.5 | 17.2 | 17.2 | 5.1 | 0.8 | 4.6 | CV event |
| Solini et al. [40] | 2012 | Italy | Prospective observational cohort | Secondary care (outpatient) | 15,773 | NR | 45 | 7.5 | 7.5 | 1 | 1 | NR | CV disease |
| van der Velde et al. [41] | 2010 | The Netherlands | Prospective observational cohort | Population | 8047 | 81 | NR | NR | NR | NR | NR | 7.0 | CV event |
| Vlek et al. [42] | 2008 | The Netherlands | Prospective observational cohort | Primary and secondary care | 2600 | NR | NR | NR | NR | NR | NR | 4.0 | CV-specific mortality, all-cause mortality, CV event |
| Lim et al. [43] | 2015 | Singapore | Prospective observational cohort | Population | 7098 | NR | 87.84 | 8.49 | NR | NR | NR | 4.3 | Incident CV disease and all-cause mortality |
| So et al. [44] | 2006 | Hong Kong | Prospective observational cohort | Secondary care (outpatient) | 4421 | 91 | 37 | 5.2 | 5.2 | 1.6 | 0 | 3.3 | Composite of CV mortality, angina, MI, stroke, revascularization, or HF |
| Wada et al. [45] | 2014 | Japan | Retrospective observational cohort | Secondary care (outpatient) | 4,328 | 77 | 47.4 | 14.8 | 7.2 | 2.7 | 0.1 | 7.0 | All-cause mortality and CV event |
| Wang et al. [46] | 2017 | China | Prospective observational cohort | Population | 47,204 | 92 | 40.1 | NR | NR | NR | NR | 6.1 | CV-specific and all-cause mortality |
| Wen et al. [47] | 2008 | Taiwan | Prospective observational cohort | Population | 56,977 | 69 | 37.7 | 22.5 | 22.5 | 1.3 | 0.7 | 13.0 | CV-specific and all-cause mortality |
| Yokoyama et al. [48] | 2008 | Japan | Cross-sectional | Primary and secondary care | 3002 | NR | NR | NR | 5.8 | 5.8 | 5.8 | NR | CV disease |
| Zhang et al. [49] | 2015 | China | Prospective observational cohort | Population | 20,702 | 94 | 29.4 | NR | NR | NR | NR | 4.5 | Stroke |
| Molnar et al. [50] | 2017 | Canada | Retrospective observational cohort | Database | 736,666 | 84 | NR | 5.75 | 5.75 | 1.2 | 0.2 | 6.0 | Incident atrial fibrillation |
| Salles et al. [51] | 2011 | Brazil | Prospective observational cohort | Secondary care (outpatient) | 531 | 77 | 35 | 14 | 14 | 5 | 0 | 4.9 | CV-specific and all-cause mortality |
| Tonelli et al. [52] | 2011 | Canada | Prospective observational cohort | Secondary care (outpatient) | 920,985 | NR | 64.9 | 8.7 | 1.8 | 0.4 | 0 | 2.9 | All-cause mortality, acute MI, stroke or TIA |
| Clase et al. [53] | 2011 | Multinational | Prospective observational cohort | Secondary care (outpatient) | 27,620 | 73 | 37.7 | 19 | 5 | 0.3 | 0.3 | 4.6 | Composite of CV death, MI, stroke, or HF |
| Matsushita et al. [54] | 2010 | USA and the Netherlands | Prospective observational cohort | Population | 1,128,310 | NR | NR | NR | NR | NR | NR | 7.9 | CV-specific and all-cause mortality |
| Ninomiya et al. [55] | 2009 | Multinational | Pooled analysis from a randomised controlled trial | Secondary care (outpatient) | 10,640 | 76 | NR | NR | NR | NR | NR | 4.0 | Composite of CV disease and CV-specific death |
| Wang et al. [56] | 2018 | USA and China | Prospective observational cohort | Database | 25,269 | NR | NR | NR | NR | NR | NR | 6.9 | All-cause mortality |
CKD chronic kidney disease, CV cardiovascular, eGFR estimated glomerular filtration rate, HF heart failure, MI myocardial infarction, NR not reported, TIA transient ischaemic attack, UK United Kingdom, USA United States of America
*All studies listed in this table reported combined associations between CKD and albuminuria stage and outcome; however, many did not report the proportions of patients at each CKD stage alone
Of these 29 studies, 26 were prospective observational cohort studies. Seven studies were undertaken in US populations, and eight in European populations, and sample sizes ranged from 531 to 1,128,310 patients. A quality assessment of the 29 included studies is included in Table S6. Furthermore, funnel plot analysis indicated the potential presence of publication bias (Fig. S2); however, there was consistency in results between the random- and fixed-effects meta-analysis between the sensitivity analysis which excluded studies with fewer than 10,000 patients (Table S11).
Risk of Adverse Clinical Outcomes with Advancing CKD
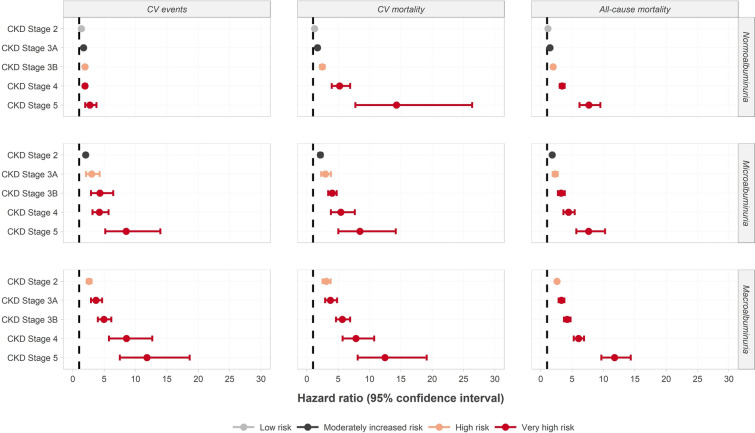
The risks of CV morbidity, CV mortality and all-cause mortality were strongly associated with more advanced CKD (Fig. 1; Table S7). The results of meta-analyses showed that across all outcomes, risk increased with CKD stage and increased further with the worsening of albuminuria. Compared to stage 1 (or without) CKD with normoalbuminuria the relative risks [mean HR (95% confidence interval)] of CV morbidity, CV mortality and all-cause mortality were 2.58 (2.22–2.99), 3.10 (2.52–3.81) and 2.59 (2.40–2.81) respectively at stage 2 CKD with macroalbuminuria. Stage 5 CKD with macroalbuminuria was associated with further increases in the relative risks of CV morbidity, CV mortality and all-cause mortality of 11.82 (7.50–18.64), 12.46 (8.12–19.12) and 11.77 (9.66–14.36) respectively [versus stage 1 (or without) CKD with normoalbuminuria].
Fig. 1.
Associations between eGFR (left axis), albuminuria (right axis) and the risk of cardiovascular events (first column), cardiovascular mortality (second column) and all-cause mortality (third column). Data are mean (95% CI) hazard ratios for the risk of outcome at each eGFR and albuminuria stage from the subgroup of 29 studies that met the inclusion criteria and reported combined associations between eGFR, albuminuria and the risk of adverse clinical outcomes. Risk is for each CKD and albuminuria stage vs. stage 1 (or without) CKD and normoalbuminuria. Increase in risk is vs. stage 1 (or without) CKD with normoalbuminuria
The presence of CV comorbidity at baseline was associated with a higher risk of all-cause mortality (Table 2). Based on eGFR categorisation alone (i.e. independent of albuminuria stage), the relative risks of all-cause mortality in patients with diabetes and stage 4 or 5 CKD were 6.42 and 9.49 respectively compared with 3.01 and 4.26 respectively across the entire cohort. The relative risks of all-cause mortality in patients with prior MI were also elevated compared to the entire cohort, at 4.82 and 5.21 at stages 4 and 5 CKD respectively. There was insufficient data available to evaluate the effect of baseline CV morbidity on all-cause mortality at different stages of albuminuria.
Table 2.
Associations between baseline comorbidity, CKD stage and all-cause mortality
| Entire cohort | Baseline comorbidity | ||||
|---|---|---|---|---|---|
| Diabetes | Hypertension | MI | Stroke* | ||
| Stage 2 CKD | 1.11 (1.03–1.18) | 1.27 (0.92–1.29) | 1.14 (0.91–1.37) | 1.21 (1.13–1.36) | OR 1.02 (0.96–1.26) |
| Stage 3a CKD | 1.54 (1.43–1.67) | 1.23 (1.23–1.23) | 1.23 (1.23–1.23) | 1.56 (1.54–2.05) | OR 1.71 (1.03–2.40) |
| Stage 3b CKD | 2.22 (1.80–2.73) | 1.40 (1.40–1.40) | 1.40 (1.40–1.40) | 1.99 (1.98–2.00) | OR 1.17 (1.17–1.17) |
| Stage 4 CKD | 3.01 (2.40–3.76) | 6.42 (5.19–9.50) | 3.51 (2.62–3.54) | 4.82 (4.11–5.83) | OR 3.62 (2.84–3.79) |
| Stage 5 CKD | 4.26 (3.21–5.63) | 9.49 (9.49–9.49) | – | 5.21 (3.88–6.54) | – |
Data are median (interquartile range; IQR) hazard ratios from all 323 studies that met the inclusion criteria and reported associations between CKD stage and all-cause mortality. Risks are for each CKD stage vs. stage 1 (or without) CKD
CKD chronic kidney disease, MI myocardial infarction, OR odds ratio
*There were no studies identified that reported the risk of all-cause mortality with advancing CKD in patients with prior stroke as hazard ratios; therefore, data from one study that reported risk as odds ratios [OR: median (IQR)] is presented
Health Economic Analyses
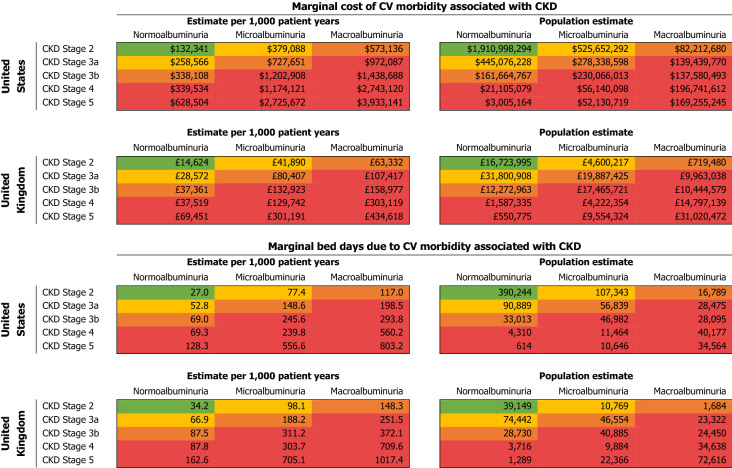
The increased risk of CV morbidity reported in patients with CKD translated into significant additional costs and bed days associated with its management in both US and UK settings (Table 3). Albuminuria was the largest driver of increased cost, with the additional cost of managing CV morbidity in patients with stage 2 CKD and macroalbuminuria (versus stage 1 (or without) CKD and normoalbuminuria) estimated to be $573,136 and £63,332 for the US and UK respectively per 1000 patient years. In patients with stage 5 CKD and macroalbuminuria, the estimated additional costs are $3,933,141 and £434,618 per 1000 patient years compared with stage 1 (or without) CKD and normoalbuminuria.
Table 3.
Additional annual cost and bed days associated with CHD, ischaemic stroke and TIA in patients with advancing CKD and albuminuria based on KDIGO 2012 criteria in US and UK CKD populations
Colours are based on KDIGO 2102 categories: Green, low risk (if no other markers of kidney disease, no CKD); yellow, moderately increased risk; orange, high risk; red, very high risk. Increase in cost is vs. stage 1 (or without) CKD with normoalbuminuria
KDIGO kidney disease: improving global outcomes, CKD chronic kidney disease
The required bed days associated with CV morbidity also increased with CKD and albuminuria severity. Stage 2 CKD with macroalbuminuria was associated with an estimated additional 117 bed days per 1000 patient years in the USA and 148 in the UK versus stage 1 (or without) CKD and normoalbuminuria. Patients with stage 5 CKD and macroalbuminuria require an estimated additional 803 and 1017 bed days per 1000 patient years for the management of CV morbidity in the USA and UK respectively versus stage 1 (or without) CKD and normoalbuminuria.
At a population level, total estimated additional annual costs and bed days required for the management of CV morbidity varied according to the prevalence of each CKD stage and albuminuria level. In the USA, patients with stage 5 CKD with macroalbuminuria only make up 0.2% of the total CKD population but account for 4% of additional estimated costs and bed days associated with management (i.e. $169.3 million and 34,564 bed days annually). In the UK, patients with stage 5 CKD and macroalbuminuria make up 2% of the CKD population but account for 17% of additional estimated costs and bed days associated with its management (i.e. £31.0 million and 72,616 bed days annually). Furthermore, in the USA, although patients with stage 2 CKD with normoalbuminuria make up 75% of the CKD population (versus 33% in the UK), they only account for 43% of the additional annual estimated costs and bed days. There was insufficient data to explore the impact of comorbidities on additional costs and bed days due to CV morbidity in these patients.
Discussion
The objectives of this systematic literature review were to evaluate the risk of CV morbidity, CV mortality and all-cause mortality in patients with CKD at each eGFR and albuminuria stage (according to KDIGO categorisations [7]), and to quantify the cost and healthcare resource utilisation implications associated with the management of CV morbidity in CKD in both US and UK settings. In total, 29 studies reported on the risk of adverse clinical outcomes based on the combined relationship between eGFR and albuminuria stage as per KDIGO categorisation.
In 2012, KDIGO published guidelines on the evaluation and management of CKD [7]. Earlier KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines [21] focused on eGFR alone to categorise CKD and quantify patient risk and did not account for variations in albuminuria. KDIGO 2012 highlighted the importance of albuminuria to aid in the identification of higher-risk patients and recommended the measurement of urinary protein alongside eGFR to better evaluate CKD. Of the 29 studies included in this review, 10 were published from 2013 onwards. Of those, only six reported eGFR and albuminuria as per KDIGO categorisation, and indicates that further research is required to ensure that risk of adverse outcomes at each CKD and albuminuria stage can be accurately evaluated.
Across all outcomes, risk increased with eGFR decline and increased further with the worsening of albuminuria; even at the same level of renal function, worsening of albuminuria could increase the risk of CV morbidity, CV mortality or all-cause mortality by over fourfold. An increase in the risk of adverse outcomes was apparent even in patients with early-stage CKD. Patients with stage 2 CKD with normoalbuminuria had up to 36% increases in the risk of adverse clinical outcomes, increasing up to threefold for those with stage 2 CKD and macroalbuminuria; the risk increased 12-fold in patients with stage 5 CKD and macroalbuminuria.
The additional costs and bed day provision associated with the management of CV morbidity in patients with CKD are substantial in the UK, but are significantly higher in the USA. These differences are likely driven by the greater cost of healthcare in the USA compared with the UK and reflect differences in per capita costs of healthcare between the two countries [US$4018 (£2989) per person in the UK in 2017 vs. US$10,398 (£7736) per person in the USA] [22].
Whilst micro- and macroalbuminuria (at any CKD stage) are less prevalent than normoalbuminuria in the overall CKD population—13% and 24% of all patients with CKD in the USA and UK respectively—the cost and healthcare resource burden associated with the management of CKD-related CV morbidity in these patients is significant compared to those with normoalbuminuria. At a patient level, the additional costs and bed days associated with managing a patient with macroalbuminuria are four to eight times greater than for a patient with normoalbuminuria at the same CKD stage. At a population level, 42% and 66% of estimated additional costs and bed days in the USA and UK respectively that are required to manage CV morbidity in patients with CKD are consumed by those with micro- or macroalbuminuria.
Given this substantial increase in cost and healthcare resource utilisation associated with the management of CV morbidity in micro- and macroalbuminuria and in latter-stage CKD, it should be a priority of healthcare providers to avoid patients reaching macroalbuminuria and advanced stage CKD through early identification of the disease and proactive management—especially in patients with more rapidly advancing disease—to slow CKD and albuminuria progression. Any reduction in the number of patients reaching latter-stage CKD has the potential to alleviate the burden of CV morbidity and its impact on healthcare resources.
Studies included in this systematic review were conducted across a broad population of patients with CKD, with approximately one-third conducted in higher-risk patient cohorts, including patients with diabetes, hypertension, prior MI or stroke, amongst others. Risks of all-cause mortality based on worsening eGFR in the subgroups of patients with baseline comorbidity tended to be higher than across the entire cohort, with patients with diabetes at highest risk. Therefore, it would be prudent for healthcare providers to both prioritise CKD screening in patients with comorbidities to ensure early identification of CKD, and ensure optimal management of CKD in those already diagnosed to slow progression and thus reduce the already elevated risk of morbidity and early mortality in those with more advanced disease.
The major strength of this systematic literature review is the applicability of the findings across a wide CKD population. Approximately one third of the studies included were conducted in higher-risk patient cohorts and the remaining were based on general population cohorts, many of which included patients with significant comorbidity. However, this study is not without limitations. There was significant heterogeneity in the categorisation of eGFR and albuminuria across studies and many did not align with KDIGO categories, preventing their inclusion in the overall analysis. There is also potential for publication bias to impact the generalisability of the results; if fewer studies with non-significant outcomes are published, it could result in an overestimation of the level of risk associated with more advanced CKD. Whilst the funnel plot of included studies indicated the potential presence of publication bias, this is thought to be primarily as a result of the inclusion of large observational studies, and the clinical demographics of the CKD population rather than the presence of significant publication bias, as high-risk patients, such as those with stage 5 CKD, make up a comparatively small proportion of the overall CKD population. This conclusion was supported by consistency in results between the random- and fixed-effects meta-analysis and also the sensitivity analysis which excluded studies with fewer than 10,000 patients. There is also the potential that these findings are confounded as a result of significant differences in patient characteristics (including comorbidities such as type 2 diabetes, heart failure etc.) between different KDIGO categorisations; however, many of the included studies adjusted results for relevant patient characteristics and there is a well-established causal relationship between renal impairment and the incidence of adverse clinical outcomes in patients with CKD.
In addition, this analysis has only addressed risk at absolute eGFR/albuminuria stages, whereas risk is also linked to the rate of eGFR decline/albuminuria rise. Future research could explore the risk of adverse clinical outcomes based on the rate of CKD progression and delve deeper into the role that comorbidities such as diabetes or heart failure play in the risk of adverse clinical outcomes. Furthermore, the economic analysis in this manuscript focused only on the additional costs and bed days associated with the management of CV morbidity associated with CKD; there are further costs (e.g. monitoring and treatment costs) that also need to be considered when evaluating the full economic burden of CKD. Additionally, costs associated with CV morbidity in the USA setting were derived in a population of patients with type 2 diabetes rather than CKD; however, costs represent the per-event cost associated with the incidence of CV events and as such costs are likely to be representative of other comorbid populations.
Lastly, the quality of our analysis is limited by the availability of source data to inform our cost and bed day estimations. During this analysis, we noted significant differences in the prevalence of stage 2 CKD with normoalbuminuria in US and UK (75% vs. 33% respectively) CKD populations, likely due to the availability of data rather than significant differences in clinical practice and the diagnosis of early-stage CKD. Indeed, epidemiological data to inform US estimates was sourced from the US Renal Data System [4] which includes both diagnosed and potentially undiagnosed patients with CKD. Given our outcome of interest was CV morbidity and CV events will happen in both diagnosed and undiagnosed patients, the US Renal Data System data are relevant; however, these differences between US and UK data indicate that the true number of patients with earlier-stage CKD in the UK may be underestimated.
Safe and effective therapies to slow the rate to CKD progression are essential to reduce the cost and healthcare resource burden of CV morbidity in CKD. Treatment options to delay CKD progression have previously been limited to renin–angiotensin–aldosterone system inhibitors, despite considerable residual risk of disease progression. However, following the observation that sodium–glucose co-transporter 2 (SGLT2) inhibitors demonstrated a reduced risk of CV and renal events in patients with type 2 diabetes [23–25], this class of drug is now being studied in CKD populations. Dapagliflozin has recently been evaluated in patients with and without type 2 diabetes in the DAPA-CKD study [26]. Dapagliflozin was associated with reductions in the risk of kidney failure, CV mortality, or hospitalisation for heart failure, and prolonged survival in patients with CKD with or without type 2 diabetes [27], potentially signalling a new class of therapy for patients with CKD, regardless of diabetes status.
In conclusion, the results of this systematic review and meta-analysis demonstrate that CKD (with or without albuminuria) is associated with poor patient outcomes even from an early stage. The risks increased with more advanced disease and the highest risk of CV morbidity, CV mortality and all-cause mortality was observed consistently in patients with stage 5 CKD with macroalbuminuria. The additional costs and bed days associated with management of the associated CV morbidity in patients with CKD are substantial and increase with disease severity. Early diagnosis and proactive management of CKD is important to be able to slow CKD progression, and reduce the risk of adverse clinical outcomes in patients with CKD. More effective management of the disease may result in a reduced healthcare, societal and patient burden of CKD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The systematic literature review, health economic analyses and medical writing related to this manuscript were conducted by Health Economics and Outcomes Research Ltd. The authors wish to thank Dr Sukhvir Rai of Health Economics and Outcomes Research Ltd for her assistance with conducting the systematic literature review.
Funding
This work was supported by AstraZeneca who provided support for the systematic literature review, health economic analysis and medical writing for this study, and funded the publication of this open access manuscript using the journal’s rapid publication service.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
JJGS, DS, PM and OD conceptualised and designed the study. CD was involved in the systematic review and responsible for the medical writing. OD was responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Disclosures
Juan Jose Garcia Sanchez, Elisabeth Sörstadius and Heleen van Haalen are employees of AstraZeneca. Marc Evans has received honoraria from AstraZeneca, NovoNordisk, Takeda and NAPP, and research support from NovoNordisk. Oliver Darlington, Carissa Dickerson, Phil McEwan and Daniel Sugrue have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35(2):329–344. doi: 10.1016/j.pop.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System. Annual data report. Epidemiology of kidney disease in the United States. 2018.
- 5.Kerr M, Bray B, Medcalf J, O'Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(Suppl 3):73–80. doi: 10.1093/ndt/gfs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Society of Nephrology. Global Kidney Disease Health Atlas. 2019. Available from: https://www.theisn.org/focus/ckd#health-atlas. Accessed 5 Dec 2019.
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 8.Tonelli M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System. Available from: http://www.cdc.gov/ckd. Accessed 5 Dec 2019.
- 10.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478–83. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Health and National Institute of Diabetes and Digestive and Kidney Disease. United States Renal Data System (USRDS). 2018. Available from: https://www.usrds.org/. Accessed 5 Dec 2019.
- 13.NHS Digital. Health Survey for England, 2016: Kidney and liver disease. 2017. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2016. Accessed 5 Dec 2019.
- 14.UK Renal Registry. UK Renal Registry 21st Annual Report. 2019. Available from: https://renal.org/sites/renal.org/files/publication/file-attachments/21st_UKRR_Annual_Report.pdf.
- 15.United States Census Bureau. United States Census. Available from: https://www.census.gov/. Accessed 5 Dec 2019.
- 16.Office for National Statistics. UK National Statistics. Available from: https://www.ons.gov.uk/. Accessed 5 Dec 2019.
- 17.Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012) J Med Econ. 2014;17(3):176–83. doi: 10.3111/13696998.2014.882843. [DOI] [PubMed] [Google Scholar]
- 18.National Health Service (NHS). 2018/19 National cost collection data. https://www.england.nhs.uk/national-cost-collection/. Accessed 07 Dec 2020
- 19.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lash JP, et al. Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 22.Office for National Statistics. How does UK healthcare spending compare with other countries? https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthcaresystem/articles/howdoesukhealthcarespendingcomparewithothercountries/2019-08-29. Accessed 03 Dec 2017
- 23.Neal B, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 24.Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 25.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 26.https://clinicaltrials.gov/ct2/show/NCT03036150. Accessed 05 Dec 2019.
- 27.McMurray JJV, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2020 [Online ahead of print]. [DOI] [PubMed]
- 28.Alonso A, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the atherosclerosis risk in communities (ARIC) study. Circulation. 2011;123(25):2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin AP, et al. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2013;61(4 Suppl 2):S12–23. doi: 10.1053/j.ajkd.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6(10):2444–51. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blecker S, et al. High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis. 2011;58(1):47–55. doi: 10.1053/j.ajkd.2011.02.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deo R, et al. Albuminuria, kidney function, and sudden cardiac death: findings from the reasons for geographic and racial differences in stroke (REGARDS) study. Heart Rhythm. 2017;14(1):65–71. doi: 10.1016/j.hrthm.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovesdy CP, et al. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. J Am Coll Cardiol. 2013;61(15):1626–1633. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muntner P, et al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011;6(9):2200–2207. doi: 10.2215/CJN.02030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuyun MF, et al. Microalbuminuria independently predicts all-cause and cardiovascular mortality in a British population: The European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) population study. Int J Epidemiol. 2004;33(1):189–198. doi: 10.1093/ije/dyh008. [DOI] [PubMed] [Google Scholar]
- 36.Bruno G, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia. 2007;50(5):941–948. doi: 10.1007/s00125-007-0616-1. [DOI] [PubMed] [Google Scholar]
- 37.Garofolo M, et al. Albuminuric and non-albuminuric chronic kidney disease in type 1 diabetes: association with major vascular outcomes risk and all-cause mortality. J Diabetes Complicat. 2018;32(6):550–557. doi: 10.1016/j.jdiacomp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Nerpin E, et al. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant. 2011;26(9):2820–2827. doi: 10.1093/ndt/gfq848. [DOI] [PubMed] [Google Scholar]
- 39.Sasso FC, et al. High cardiovascular risk in patients with type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate: the NID-2 prospective cohort study. Nephrol Dial Transplant. 2012;27(6):2269–2274. doi: 10.1093/ndt/gfr644. [DOI] [PubMed] [Google Scholar]
- 40.Solini A, et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2012;35(1):143–149. doi: 10.2337/dc11-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Velde M, Bakker SJ, de Jong PE, Gansevoort RT. Influence of age and measure of eGFR on the association between renal function and cardiovascular events. Clin J Am Soc Nephrol. 2010;5(11):2053–9. doi: 10.2215/CJN.08851209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlek AL, van der Graaf Y, Spiering W, Algra A, Visseren FL, S.s. group Cardiovascular events and all-cause mortality by albuminuria and decreased glomerular filtration rate in patients with vascular disease. J Intern Med. 2008;264(4):351–60. doi: 10.1111/j.1365-2796.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 43.Lim CC, et al. Chronic kidney disease, cardiovascular disease and mortality: a prospective cohort study in a multi-ethnic Asian population. Eur J Prev Cardiol. 2015;22(8):1018–1026. doi: 10.1177/2047487314536873. [DOI] [PubMed] [Google Scholar]
- 44.So WY, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29(9):2046–2052. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- 45.Wada T, et al. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol. 2014;18(4):613–620. doi: 10.1007/s10157-013-0879-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Wang F, Liu S, Zhou M, Zhang L, Zhao M. Reduced kidney function, albuminuria, and risks for all-cause and cardiovascular mortality in China: a population-based cohort study. BMC Nephrol. 2017;18(1):188. doi: 10.1186/s12882-017-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen CP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462,293 adults in Taiwan. Lancet. 2008;371(9631):2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 48.Yokoyama H, Oishi M, Kawai K, Sone H, G. Japan Diabetes Clinical Data Management Study Japan Diabetes Clinical Data Management Study, Reduced GFR and microalbuminuria are independently associated with prevalent cardiovascular disease in type 2 diabetes: JDDM study 16. Diabet Med. 2008;25(12):1426–32. doi: 10.1111/j.1464-5491.2008.02592.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, Wang X, He M, et al. Proteinuria is an independent risk factor for first incident stroke in adults under treatment for hypertension in China. J Am Heart Assoc. 2015;4(12):e002639. doi: 10.1161/JAHA.115.002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molnar AO, Eddeen AB, Ducharme R, et al. Association of proteinuria and incident atrial fibrillation in patients with intact and reduced kidney function. J Am Heart Assoc. 2017;6(7):e005685. doi: 10.1161/JAHA.117.005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salles GF, Cardoso CR, Pereira VS, Fiszman R, Muxfeldt ES. Prognostic significance of a reduced glomerular filtration rate and interaction with microalbuminuria in resistant hypertension: a cohort study. J Hypertens. 2011;29(10):2014–23. doi: 10.1097/HJH.0b013e32834adb09. [DOI] [PubMed] [Google Scholar]
- 52.Tonelli M, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80(12):1306–1314. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
- 53.Clase CM, et al. Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med. 2011;154(5):310–318. doi: 10.7326/0003-4819-154-5-201103010-00005. [DOI] [PubMed] [Google Scholar]
- 54.Chronic Kidney Disease Prognosis C, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ninomiya T, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, et al. Mortality risk of chronic kidney disease: a comparison between the adult populations in urban China and the United States. PLoS One. 2018;13(3):e0193734. doi: 10.1371/journal.pone.0193734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.