Abstract
Purpose
Oesophageal squamous cell carcinoma (ESCC) has a poor prognosis. Advanced tumours are treated with fluoropyrimidine/platinum chemotherapy followed by irinotecan or taxane monotherapy, but resistance is common and new treatments are needed. Approximately 20% of ESCCs carry copy number gain (CNG) of the epidermal growth factor receptor (EGFR) gene. Previous trials show that while anti-EGFR monotherapy benefits biomarker-selected patients with EGFR CNG and/or high EGFR expression, combining anti-EGFR therapies with platinum fluoropyrimidine chemotherapies is not effective, and uncertainty remains regarding the optimal cytotoxic chemotherapy partner for anti-EGFR therapies in ESCC.
Methods
The effects of EGFR CNG on fluoropyrimidine/platinum chemotherapy sensitivity in a cohort of gastroesophageal cancer patients (n = 302) was evaluated. Drug combination studies using the EGFR inhibitor gefitinib with cytotoxic chemotherapies, docetaxel, cisplatin, oxaliplatin and irinotecan, on cell proliferation and cell death of EGFR CNG ESCC cell lines were assessed.
Results
EGFR CNG in gastroesophageal cancer patients was associated with improved overall survival following fluoropyrimidine/platinum chemotherapy. However, co-administration of gefitinib and oxaliplatin or cisplatin was frequently antagonistic in cell-based assays in EGFR CNG ESCC, whereas the combination of gefitinib with docetaxel or irinotecan was more efficacious. Co-administration of gefitinib/docetaxel and sequential administration of docetaxel before gefitinib showed synergy, but docetaxel given after gefitinib was antagonistic.
Conclusion
Gefitinib/platinum co-administration demonstrated antagonism suggesting a possible explanation for the lack of benefit from addition of anti-EGFR therapies to fluoropyrimidine/platinum chemotherapy in trials. Gefitinib/docetaxel co-administration demonstrated synergy suggesting taxanes could be the most effective cytotoxic partner for anti-EGFR therapies in EGFR CNG-positive ESCC, but careful consideration of drug scheduling is required.
Keywords: Gastroesophageal cancer, Gefitinib, EGFR, Docetaxel, Platinum, ESCC, Chemotherapy combinations
Background
Oesophageal cancer is the sixth most common cause of death from cancer globally, and squamous cell carcinomas of the oesophagus (ESCC), is the dominant histological subtype of oesophageal cancer worldwide [1]. Patients frequently present with advanced disease and, as a result of late stage diagnosis and limited treatment options, 5-year survival rates remain low at around 15%. Current treatments depend on the tumour stage, co-morbidities and patient performance status; surgery is curative in fewer than half of patients and the majority of patients receive palliative treatment, including chemotherapy [2]. Currently, cytotoxic chemotherapy provides a systemic therapy option for palliative treatment of ESCC, but there are no licenced targeted therapies or predictive biomarkers and, therefore, an unmet need for more effective approaches [3].
First-line palliative chemotherapy usually involves a fluoropyrimidine/platinum combination but, eventually, all patients develop progressive disease with some receiving second-line treatment with a taxane or irinotecan monotherapy [4]. Recently, a study in patients progressing after fluoropyrimidine/platinum chemotherapy demonstrated that the PD-1 inhibitor nivolumab improved overall survival compared to taxane monotherapy (ATTRACTION-3 trial [5]). Although the progression free survival and the proportion of patients responding were similar in both groups, the responses to nivolumab were more durable but took longer to occur than responses to taxanes. These findings highlight the importance of identifying the minority subgroup of patients who would benefit long-term from nivolumab, but, in the short term, taxanes are superior. However, the low objective response rate (20%) and poor long-term survival with taxanes in this setting, indicates that treatment resistance is a major clinical challenge that needs to be addressed.
One approach to develop novel therapies is to identify and target oncogenic drivers and efforts to characterise genome alterations within tumour tissue is now enabling the selection of biomarkers for precision medicine targeted therapies. Potential drivers of oesophageal tumourigenesis include the epidermal growth factor receptor (EGFR). Copy number gain is detected in around 20% of tumours [6, 7], while EGFR is overexpressed in around 50% of ESCC tumours and correlates significantly with tumour invasion [6]. Targeting EGFR with EGFR tyrosine kinase inhibitors (TKi), such as gefitinib, erlotinib or afatinib, inhibits the proliferation of oesophageal cancer cell lines in vitro [8, 9], but, clinical trials of EGFR inhibitors in oesophageal cancer, including ESCC, have shown mixed results. Monotherapy trials in unselected patients with EGFR inhibitors indicate that there is an EGFR-driven minority ESCC subgroup who gain survival, symptomatic control and health-related quality of life benefits from EGFR inhibitors [10, 11]. In ESCC, EGFR CNG assessed by FISH, and/or EGFR protein over-expression have shown promise as predictive biomarkers to identify this benefiting subgroup, but needs prospective validation [3, 7, 12, 13]. Cell line models and patient-derived xenografts also demonstrate an EGFR-driven subgroup of ESCC sensitive to EGFR inhibitors and characterised by EGFR CNG and/or EGFR protein over-expression. However, even in these biomarker-selected groups, intrinsic and acquired resistance to EGFR inhibitors remains significant [8, 14, 15]. The considerable heterogeneity of EGFR CNG and protein over-expression observed in ESCC may be a key determinant of resistance [16], with rapid selection and outgrowth occurring of EGFR CNG and protein overexpression-negative tumour cell sub-clones that are unresponsive to EGFR inhibitors. This emphasises the importance of combining EGFR inhibitors with a therapy that is effective against EGFR ‘negative’ sub-clones, and ideally one that would also synergise with EGFR inhibitors towards the EGFR ‘positive’ driven sub-clones. To address this, a number of clinical trials have investigated the combination of EGFR inhibitors and cytotoxic chemotherapy. Clinical trials combining EGFR inhibitors and platinum/fluoropyrimidine chemotherapy in the advanced stage setting or with platinum fluoropyrimidine-based concurrent chemoradiotherapy in the curative treatment setting, have not shown an incremental benefit [17]. In the largest randomised trial in ESCC, in molecularly unselected patients with advanced stage disease, the addition of the anti-EGFR monoclonal antibody pantitumumab to cisplatin and 5FU chemotherapy did not improve overall survival [18]. Similarly, in unselected advanced stage gastroesophageal adenocarcinoma (GOA) patients, a negative impact on overall survival was observed with addition of panitumumab to epirubicin, oxaliplatin and capecitabine [19, 20]. Conflicting results have also been reported in trials of platinum-based chemotherapy in combination with EGFR TKi in non-small cell lung cancer patients (NSCLC) [21–23]. In contrast, the addition of the EGFR TKi erlotinib, to definitive chemoradiotherapy for ESCC, which included a taxane (paclitaxel and cisplatin) was beneficial [24].
Overall, there is evidence of an EGFR-driven and EGFR inhibitor-responsive subgroup of ESCC and, thus, the potential to combine current standard of care cytotoxic chemotherapies with EGFR inhibitors to improve outcomes. The development and clinical use of EGFR inhibitors for oesophageal cancer, however, has been hampered by conflicting trial results, which could be a consequence of biomarker-unselected patient cohorts, and also because questions regarding potential antagonistic effects of co-administration of EGFR inhibitors with cytotoxic chemotherapy have been raised [25–28]. Furthermore, the clinical relevance of this is increased by findings which suggest that, consistent with observation in other tumour types, EGFR signalling is a key determinant of resistance to immune checkpoint inhibitors in ESCC [29], and accordingly EGFR-driven ESCC are likely to be a subgroup that derive less, or no, benefit from immune checkpoint inhibitors.
To address this, and to identify the drug combinations most likely to benefit ESCC patients, in this study, we have investigated the outcomes from platinum/fluoropyrimidine chemotherapy in EGFR CNG positive and negative gastroesophageal cancer patients and then the combinatorial activity of EGFR inhibitors with cytotoxic drugs in ESCC cell lines with EGFR CNG and varying intrinsic sensitivity to gefitinib. Cytotoxic drugs included platinum-based chemotherapies (cisplatin and oxaliplatin), a taxane (docetaxel) and a topoisomerase inhibitor (irinotecan) which were tested in concurrent and sequential administration settings.
Methods
Patients and tumour samples
EGFR FISH was performed, to classify tumours as EGFR copy number gain (CNG) positive with either high polysomy (defined as having EGFR copy number ≥ 4 in ≥ 40% of cells) or gene amplification (defined by presence of tight gene clusters and a ratio of gene/chromosome per cell ≥ 2, or ≥ 15 copies of the genes per cell in ≥ 10% of analysed cells) or EGFR CNG negative (EGFR disomy, low trisomy, high trisomy and low polysomy) as described previously [16, 30], on formalin-fixed paraffin embedded tumour samples from the following patient cohorts (Table 1). First, a consecutive cohort of 52 patients with advanced stage (TNM version 7), gastroesophageal cancer treated with platinum/fluoropyrimidine-based palliative chemotherapy in 2015 at Tayside Cancer Centre (Table 1); all patients received up to 6 cycles of epirubicin 50 mg/m2 intravenously, day 1 cisplatin 60 mg/m2 or oxaliplatin 130 mg/m2 intravenously on day 1, plus capecitabine 1250 mg/m2 orally days 1–21 as 2 divided doses, or a continuous intravenous infusion of 5-fluorouracil 200 mg/m2/24 h, days 1–21 on a 21 day cycle. Secondly, a consecutive cohort of 250 patients with operable gastroesophageal cancer (TNM version 7) treated with surgical resection ± perioperative chemotherapy with 3 cycles before surgery and 3 cycles after surgery of: epirubicin 50 mg/m2 intravenously on day 1, cisplatin 60 mg/m2 intravenously on day 1, plus capecitabine 1250 mg/m2 orally days 1–21 as 2 divided doses, or a continuous intravenous infusion of 5-fluorouracil 200 mg/m2/24 h on days 1–21 on a 21-day cycle (Table 1) between 2004 and 2009 in Ninewells Hospital Dundee or Aberdeen Royal Infirmary. The use of all tumour specimens and clinical data was consistent with the patient consent provided and was approved by the appropriate UK regional research ethics committees prior to the work being undertaken.
Table 1.
Clinical features of patients tested for epidermal growth factor receptor (EGFR) copy number gain (CNG)
| (a) Neoadjuvant cohort | |||
|---|---|---|---|
| Clinical feature | EGFR copy number gain (N = 40) | EGFR no copy number gain (N = 109) | p |
| Age, mean (SD) | 64.3 (10.1) | 64.9 (9.4) | 0.733 |
| Sex, no. (%) | |||
|
Male Female |
25 (62.5%) 15 (37.5%) |
72 (66%) 37 (37%) |
0.687 |
| Histological diagnosis, no. (%) | |||
|
Squamous Adenocarcinoma Other |
8 (20%) 31 (77.5%) 1 (2.5%) |
18 (16.6%) 83 (76.1%) 8 (7.3%) |
0.51 |
| Disease site, no. (%) | |||
|
Oesophageal Junctional Gastric |
23 (57.5%) 1 (2.5%) 16 (40%) |
53 (48.6%) 16 (14.7%) 40 (36.7%) |
0.114 |
| Stage, no. (%) | |||
|
I II III IV |
7 (17.5%) 11 (27.5%) 20 (50%) 2 (5%) |
19 (17.4%) 34 (31.2%) 53 (48.6%) 3 (2.8%) |
0.90 |
| Neoadjuvant chemotherapy, no. (%) | |||
|
Yes No |
29 (72.5%) 11 (27.5%) |
71 (65.1%) 38 (34.9%) |
0.35 |
| (b) Advanced stage cohort | |||
|---|---|---|---|
| Clinical feature | EGFR copy number gain (N = 25) | EGFR no copy number gain (N = 27) | p |
| Age, mean (SD) | 63.1 (9.6) | 59.7 (8.8) | 0.191 |
| Sex, no. (%) | |||
|
Male Female |
22 (88%) 3 (12%) |
18 (67%) 9 (33%) |
0.068 |
| Histological diagnosis, no. (%) | |||
|
Squamous Adenocarcinoma Other |
5 (20%) 19 (76%) 1 (4%) |
6 (22.2%) 19 (70.3%) 2 (7.4%) |
0.84 |
| Disease site, no. (%) | |||
|
Oesophageal Junctional Gastric |
19 (76%) 6 (24%) 0 (0%) |
19 (70.3%) 8 (29.6%) 0 (0%) |
0.647 |
| Stage, no. (%) | |||
|
III IV |
3 (12%) 22 (88%) |
6 (22.2%) 21 (77.8%) |
0.33 |
Cell lines
Human KYSE520, OE21, and TE8 oesophageal squamous carcinoma cells (ESCC) with 14, 14, and 11 EGFR CNG, respectively, were obtained from the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University, Japan. The cell lines were passaged in Roswell Park Memorial Institute RPMI medium 1640 supplemented with l-glutamine (GIBCO) and 10% foetal bovine serum (FBS) (GIBCO). Cells were tested negative for mycoplasma by the in-house testing facility (Mycoalert) and were authenticated by STR profiling (NorthGene Ltd, Newcastle UK).
Reagents
Stock solutions were prepared as follows: Gefitinib (Iressa) (Tocris), 20 mM in DMSO; cisplatin [cis-Diamineplatinum (II) dichloride, (Sigma Adrich), 3 mM in sterile PBS; oxaliplatin (Selleckchem), 10 mM in sterile water; docetaxel (Selleckchem), 20 µM in DMSO and irinotecan (Tocris) (SN-38—active metabolite of CPT-11)], 20 mM in DMSO.
Cell proliferation assays
1000 (OE21, KYSE520, and TE8) viable cells/well were seeded overnight in 96-well plates. Cells were then treated with either solvent control or two or fourfold dilutions of gefitinib, docetaxel, cisplatin, oxaliplatin or SN-38 (active metabolite of irinotecan). Where possible, drug titrations used were selected to be within the range of reported peak plasma concentrations of each drug which are: gefitinib—1–1.4 µM [31], oxaliplatin—3.6 µM [32], cisplatin—165 µM [33], docetaxel—4 µM [34], SN-38—0.03–0.17 µM [35]. The relative insolubility of cisplatin in PBS restricted its maximum working concentration (40 µM) to below peak plasma levels. Proliferation assay endpoints (control wells 80% confluent during log-phase growth) were analysed by CellTitre-Glo® luminescent cell viability assay (Promega) according to the manufacturers’ instructions.
Spheroid assays
Microtitre plates (TPP, U-bottomed) were coated with 0.5% poly(2-hydroxyethyl methacrylate) (poly-HEMA) prepared in 95% ethanol (two applications of 50 µL dried at 37 °C) and were stored at 4 °C until required. TE8 cells (1000 cells/100µL in growth media) were seeded into the poly-HEMA coated plates and were centrifuged for 10 min, 2000 rpm. After overnight incubation, tumour cell aggregates/spheroids were treated with 100 µL of the appropriate drug concentrations for 5 days before analysis by CellTitre-glo® assay.
Colony formation assay
OE21 cells were seeded overnight at 1000 cells/well in the middle wells of 24-well plates to avoid edge effects, before being treated with appropriate drug concentrations. Docetaxel and gefitinib monotherapy doses were selected to induce approximately 50% reduction in colony number. After seven days media was removed, colonies washed in PBS, and then fixed in methanol for 30 min. Plates were washed, air dried and colonies stained with crystal violet staining solution (0.5%w/v in 20% methanol). Excess stain was washed off with water, and the plates air-dried before scanning. Stained colonies were quantified by solubilisation in 1% SDS, and the absorbance of the resulting solution measured at 570 nm.
Cell death assays
The proportion of dead cells was determined by CellTox™ Green cytotoxicity assay reagent (Promega) by imaging prior to, and following, addition of 50µL triton-x aqueous permeabilising solution (0.2%); non-viable cells were first labelled with CellTox™ Green cytotoxicity assay reagent (Promega) (4 µL/mL, 10 µL/well) and monitored during a drug treatment time course by IncuCyte® Zoom real-time imaging and software (Essen Biosciences, Sartorius) (first reading). The percentage of dead cells was then determined by permeabilisation/dye-uptake and imaging of total cell number (second reading) after triton addition and the equation (first reading/second reading)*100.
Drug co-administration
Studies were designed to conform to the requirements outlined for analysis by the Chou–Talalay mathematical model of drug combinations [36], namely, that combination drugs were used at equimolar dilution ratios at predetermined concentrations, where they had an effect on cell growth (around the IC50 values determined by prior CellTitre-Glo® cell proliferation assays). Cells were seeded at 1000 cells/well in 96-well plates, divided into four groups and treated for four days as follows: solvent control group; gefitinib alone; cytotoxic drug alone; concurrent group (gefitinib plus cytotoxic). Cell proliferation was assessed by CellTitre-Glo® assay.
Sequential drug administration study design
Cells were seeded overnight in 96-well microtitre plates and were divided into six groups for treatment for 96-h as follows: (1) solvent control group, (2) cytotoxic drug alone group—cells were treated continuously with docetaxel, cisplatin or oxaliplatin, (3) gefitinib alone group—cells were treated continuously with gefitinib, (4) cytotoxic drug followed by gefitinib group—cells were incubated with docetaxel or cisplatin or oxaliplatin or 48 h followed by addition of gefitinib for 48 h, (5) gefitinib followed by cytotoxic group—cells were treated with gefitinib for 48 h followed by addition of docetaxel or cisplatin or oxaliplatin for 48 h. (6) Concurrent group (both)—cells were incubated concurrently with cytotoxic chemotherapy and gefitinib for 96 h. All groups were retreated with the appropriate drug dilution on each treatment day and drug dilutions in media were balanced for solvent concentration. No drug washouts were carried out. Cell proliferation was assessed by CellTitre-Glo® assay.
Statistical analysis
Survival analysis was performed using IBM SPSS statistics v22 (IBM Corporation, Armonk, NY, USA). Kaplan–Meier and Cox proportional hazards analysis were used for survival analysis and survival time was calculated in days from the date of histological diagnosis until the date of death. All reported p values are two sided. A p value of < 0.05 was considered statistically significant. ANOVA was performed using Graphpad prism software.
IC50 values were determined from cell proliferation assays using CalcuSyn (Biosoft Version 2.0) or Graphpad prism software. The antiproliferative effect of combination treatments was evaluated by determining the drug combination index (CI). Results were analysed according to the Chou–Talalay method [36] using CalcuSyn software (CalcuSyn, Inc. Paramus, USA) which generates Dm values (IC50), dose response curves and median effect plots. Recommended symbols for describing synergistic, additive or antagonistic effects in drug combination studies analysed with the CI method (CalcuSyn user manual) are given where appropriate.
Results
EGFR CNG status and outcomes from Platinum/fluoropyrimidine chemotherapy in gastroesophageal cancer patients.
First, we investigated the impact of EGFR signalling on clinical chemosensitivity by analysing outcomes in EGFR CNG positive and negative gastroesophageal cancer patients treated with platinum–fluoropyrimidine combination chemotherapy (PBC).
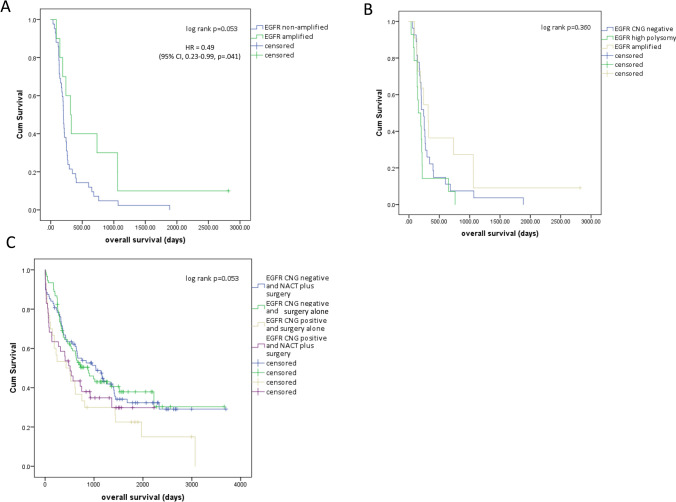
EGFR CNG status was not associated with patient clinical features (Table 1). Analysis of the cohort (n = 52) of advanced gastroesophageal cancer patients treated with palliative PBC revealed that patients with tumours containing amplified EGFR (n = 13) had longer median survival (315 days, 95% CI 183.3–446.7) than patients without EGFR CNG (201 days, 95% CI 184.1–217.9), HR 0.49, 95% CI 0.23–0.99, p = 0.041 (Fig. 1a). Shorter survival times compared to amplified EGFR cases, were also noted in patients with high polysomy (defined as having EGFR copy number ≥ 4 in ≥ 40% of cells) [16] (Fig. 1b).
Fig. 1.
EGFR copy number status and outcome following treatment with platinum-based chemotherapy in gastroesophageal cancers. a Advanced stage patients (n = 52) treated with palliative platinum-based combination chemotherapy categorised as EGFR amplified and EGFR non-amplified. b Advanced stage patients treated with palliative platinum-based combination chemotherapy categorised as EGFR amplified, EGFR high polysomy or EGFR copy number gain negative (CNG negative includes EGFR disomy, low trisomy, high trisomy and low polysomy). c Operable gastroesophageal cancers treated with surgical resection alone or platinum-based neo-adjuvant chemotherapy (NACT) followed by surgical resection categorised as EGFR copy number gain positive (CNG positive, includes EGFR amplification and high polysomy), or EGFR CNG negative
Operable patients with EGFR CNG positive tumours (high polysomy or amplification) had shorter median overall survival (284 days, 95% CI 284.5–737.5) than patients with EGFR CNG negative tumours (905 days 95% CI 566.9–1243.1), HR 1.51, 95% CI 1.09–2.12, p = 0.016). However, when analysed according to treatment received in this cohort, patients with EGFR CNG positive tumours who received neoadjuvant PBC had longer overall survival than patients with EGFR CNG positive tumours who did not receive neoadjuvant PBC (Fig. 1c). Patients without EGFR CNG positive tumours had similar overall survival regardless of whether they received pre-operative PBC or not (Fig. 1c). EGFR CNG positive patients who received neoadjuvant PBC had similar overall survival to those without EGFR CNG, but EGFR CNG positive patients who did not receive neoadjuvant PBC had shorter overall survival (Fig. 1c).
Overall, this suggests that gastroesophageal cancer patients with EGFR-driven tumours (as identified by EGFR CNG) benefit from, and are more sensitive to, PBC. This implies that therapeutic inhibition of EGFR-oncogenic pathways in EGFR CNG positive patients could negatively impact on the expected benefit derived from platinum-based chemotherapy and would be antagonistic. These observations could provide an explanation for negative clinical trials investigating PBC combined with EGFR inhibitors in gastroesophageal cancer.
Combinations of gefitinib and cytotoxic chemotherapy in EGFR CNG ESCC cell lines
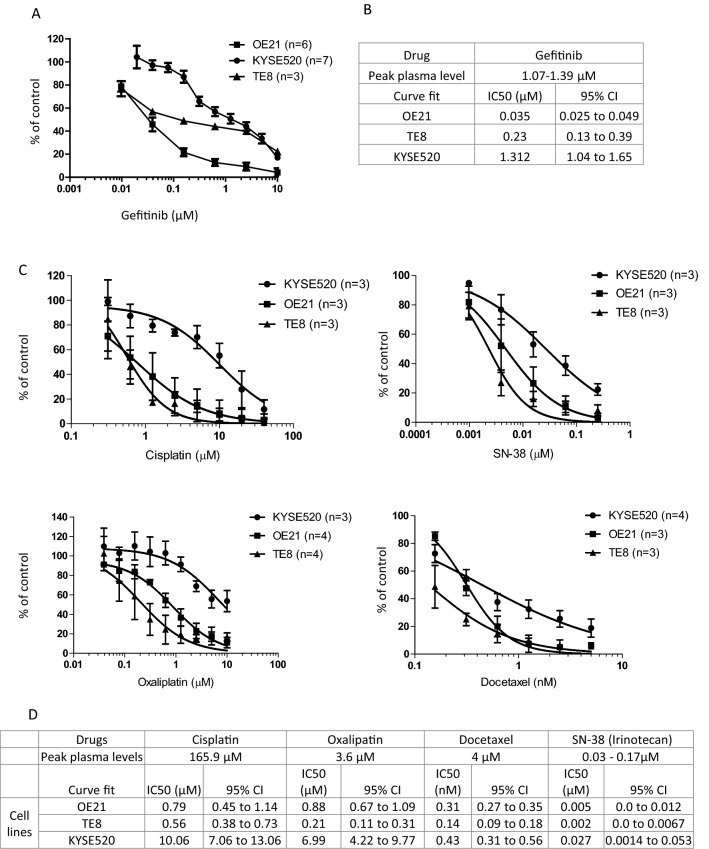
No gastroesophageal adenocarcinoma cell lines with EGFR CNG are available, so to investigate the potential antagonistic interaction between EGFR inhibitors and oxaliplatin and cisplatin, our subsequent experiments focused on ESCC. Three ESCC cell lines with EGFR copy number gain were selected. KYSE520 cells were considered resistant to gefitinib, having an IC50 at around the peak plasma levels (Fig. 2a), while inhibition of proliferation of OE21 and TE8 cells occurred at IC50s of 30-fold and fivefold lower than peak plasma levels, respectively (Fig. 2b). This range in sensitivity to gefitinib reflected the range in response seen in patients in the clinical setting. We also determined the sensitivity of these cell lines to oxaliplatin, cisplatin, docetaxel and irinotecan (administered as the active metabolite SN38) (Fig. 2c, d). KYSE520 cells were also least sensitive to cytotoxic agents in agreement with genomics of drug sensitivity in cancer data (https://www.cancerrxgene.org), while TE8 and OE21 cells were relatively sensitive (Fig. 2d).
Fig. 2.
Sensitivity of ESCC cell lines to gefitinib and cytotoxic monotherapy. Dose–response curves (a, c) and IC50s (b, d) for gefitinib (a, b) and cytotoxic agents, cisplatin, oxaliplatin, docetaxel, and irinotecan (c, d) in three EGFR dysregulated ESCC cell lines. a, c Cells were seeded overnight in 96-well plates and treated with concentrations of gefitinib, docetaxel, cisplatin, oxaliplatin or SN38 (irinotecan) as indicated. Cells were harvested with CellTitre-Glo® assay reagent (Promega) and graphs depict cell proliferation relative to solvent control treated cells (set at 100%). The non-linear curve fit was generated using Graphpad from at least three independent assays (n) as indicated in the graph legend. IC50 values and 95% confidence intervals (b, d) were determined using graphpad prism. Peak plasma levels are also given for each agent
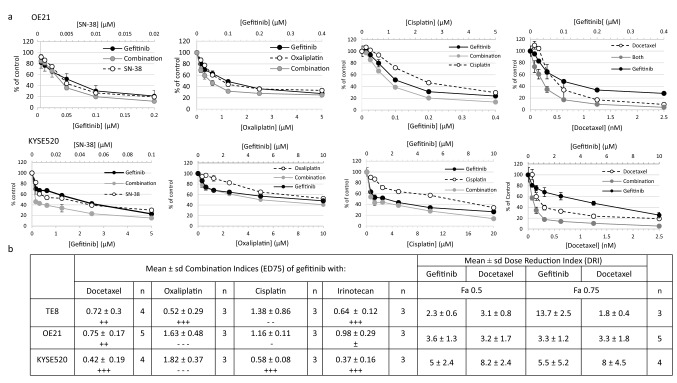
Having determined the dose response of the agents, we conducted combination experiments in gefitinib sensitive (OE21, TE8) and resistant (KYSE520) cells using drug titrations at equimolar ratios (representative dose responses in OE21 and KYSE520 cells are shown in Fig. 3a) and then calculated combination indices using CalcuSyn software based on Chou–Talalay methodology. Mean ED75 combination indices ± SD from independent experiments are summarised in Fig. 3b. Consistent with our hypothesis, platinum-based cytotoxic drugs, cisplatin and oxaliplatin, frequently had antagonistic activity when used in combination with gefitinib (CI > 1). The level of antagonism varied the with agent and cell line. Both cisplatin and oxaliplatin were antagonistic in combination with gefitinib in OE21 cells, the line most sensitive to gefitinib as a monotherapy. SN38 combined with gefitinib induced responses ranging from nearly additive to synergistic. This observation is consistent with reports of synergistic interactions between irinotecan and gefitinib in colorectal cancer cell lines [37]. Docetaxel plus gefitinib, however, consistently showed synergistic activity across the cell line panel, and was highly effective at inhibiting the proliferation of the previously gefitinib refractory cell line KYSE520. Importantly, the combination of gefitinib with docetaxel led to dose reduction indices (DRI) for gefitinib (ED50, range 2.3–5 fold; ED75 range 3.3–13.7 fold) and docetaxel (ED50, range 3.1–8.2 fold; ED75 range 1.8–8 fold) across the cell line panel (Fig. 3b). These data suggest that similar efficacy can be achieved using reduced doses of gefitinib and docetaxel when used in combination.
Fig. 3.
Dose response curves of gefitinib co-administration with cytotoxic chemotherapies. a Gefitinib sensitive (OE21) and gefitinib resistant (KYSE520) cells were treated with gefitinib alone, cytotoxic chemotherapy alone or equimolar titrations of both drugs (combination). Cell proliferation was determined by CellTitre-Glo® assay, and representative graphs depict proliferation relative to solvent control treated cells (set at 100%). b Table of combination indices (CI) and dose reduction indices (DRI) of gefitinib and cytotoxic chemotherapies in ESCC cell lines. Average (± SD) CI values at ED75 determined by CalcuSyn software from at least three (n) independent assays [representative dose response curves are shown in a]. CI values < 1 indicate synergistic interactions, CI = 1 additive, CI values > 1 indicate antagonistic interactions. CalcuSyn recommended symbols are also provided to indicate the degree of the effect: antagonistic (– – –); moderate antagonism (– –); slight antagonism (−); nearly additive (±); moderate synergism (++); synergism (+++). Mean (± SD) DRI determined from independent 96-h assays (n) of gefitinib in combination with docetaxel evaluated by endpoint CellTitre-Glo®. DRI were calculated by CalcuSyn software. Fa = fraction affected equivalent to ED50 and ED75 at 0.5 and 0.75, respectively
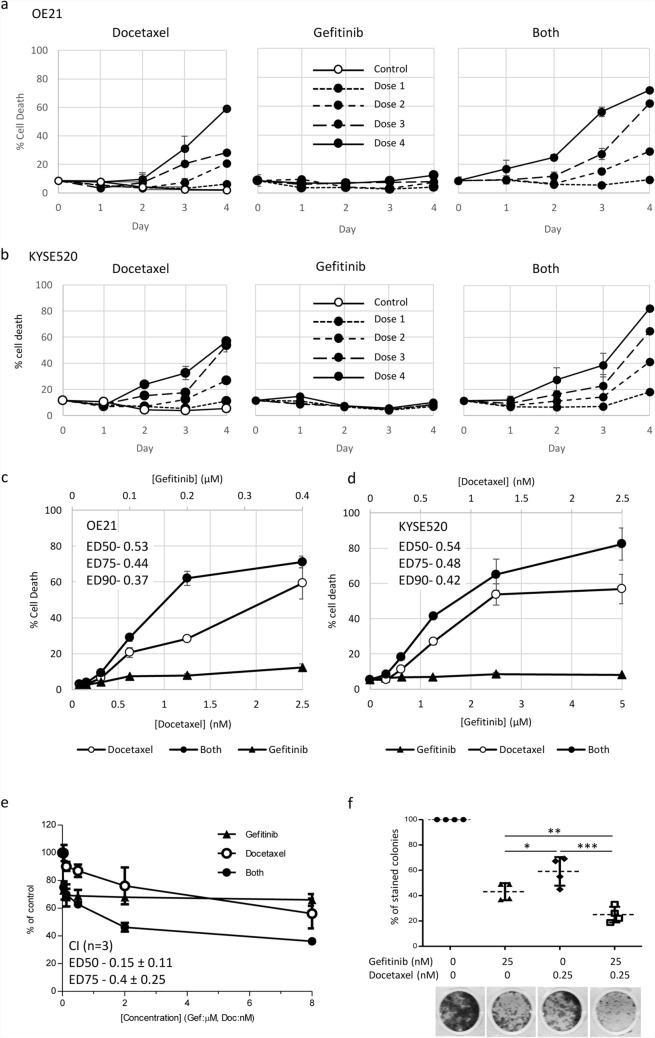
We next tested docetaxel and gefitinib as monotherapy and combination therapy in kinetic cell death assays in OE21 and KYSE520 cells over increasing doses (dose 1–4) (Fig. 4). As expected, gefitinib induced little cell death over the 4-day time course in line with its primary mode of action being induction of G1 cell cycle arrest. Docetaxel induced dose-dependent increases in cell death in both cell lines. When docetaxel and gefitinib were used in combination, synergistic levels of cell death were induced (Fig. 4c, d) and with more rapid kinetics (Fig. 4a). In addition, we tested responses to gefitinib and docetaxel in 3-D cell aggregates/spheroids and 2D colony formation, which can more closely model aspects of in vivo tumour biology such as reduced proliferation rates with increased survival, cell: cell adhesions, hypoxic cores and long term replicative capacity (colony formation). Gefitinib and docetaxel displayed synergistic activity against TE8 cell spheroids at ED50 and ED75 (Fig. 4e) (CI ED50, 0.15 ± 0.11; ED75, 0.4 ± 0.25. n = 3). OE21 and KYSE520 cells did not survive for the duration of the 5-day spheroid assay so we were unable to use these cells; however, OE21 cells were able to form discrete 2-D colonies in colony formation assays and OE21 colonies were more significantly inhibited by the combination of gefitinib and docetaxel than either drug as a monotherapy (n = 4). These results indicated that gefitinib in combination with docetaxel had the most consistent activity in ESCC inducing synergistic effects on proliferation and cell death in 2-D and 3-D model systems.
Fig. 4.
Docetaxel in combination with gefitinib synergistically enhances ESCC cell death. Cytotoxicity of drug treatments (docetaxel alone, gefitinib alone or gefitinib plus docetaxel combination) on the ESCC cell lines OE21 (a, c) and KYSE520 (b, d) was assessed by CellTox™ green assay and imaging (IncuCyte® Zoom). a, b Time course of treatment: cells were treated for 4 days with solvent (control) increasing doses of docetaxel (OE21 and KYSE520: dose 1 = 0.3215 nM; dose 2 = 0.625 nM; dose 3 = 1.25 nM; dose 4 = 2.5 nM), gefitinib (sensitive line OE21: dose 1 = 0.05 µM; dose 2 = 0.1 µM; dose 3 = 0.2 µM;, dose 4 = 0.4 µM and resistant line KYSE520: dose 1 = 1.25 µM; dose 2 = 2.5 µM; dose 3 = 5 µM;, dose 4 = 10 µM) or both drugs combined (both). c, d Endpoint IncuCyte® data from day 4 are presented as dose response curves in OE21 (c) and KYSE520 (d) (mean ± SEM of at least three replicate wells, 4 fields per well of a representative assay). Synergistic combination indices at effective dose 50%, 75% and 90% (ED50, ED75 and ED90) are indicated. e Five-day TE8 3-D anchorage independent spheroid assays treated with equimolar fourfold dilution series of gefitinib (from 8 µM) and docetaxel (from 8 nM) as single agents or in combination. Synergy ED50 and ED75 CI values were calculated using CalcuSyn and are presented as the mean CI ± SD from three independent assays. (ED90s were not reached and calculated CI ED90 were variable across independent assays). f OE21 colony formation assay using docetaxel and gefitinib doses selected to induce approximately 50% reduction in colony number as a monotherapy, and in combination. Statistical comparisons were performed using Graphpad prism software (ANOVA plus Bonferroni correction for multiple testing from n = 4 independent assays; *p = 0.01–0.05, **p = 0.001–0.01, ***p = < 0.001)
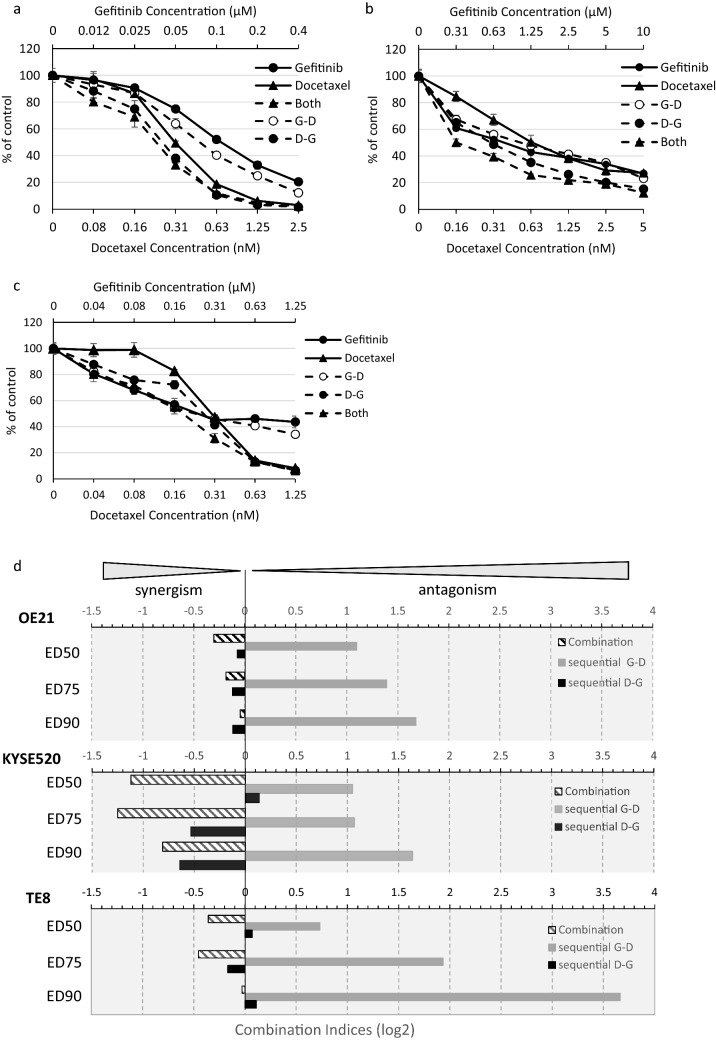
To determine whether synergistic effects could be affected by dosing schedules we tested gefitinib and docetaxel in sequential treatments over 96 h, D—G = docetaxel followed by gefitinib; G—D = gefitinib followed by docetaxel and compared the effects on cell proliferation of concurrent (combination) gefitinib and docetaxel treatment (Fig. 5). As confirmation of our previous results, concurrent administration of gefitinib and docetaxel induced synergistic inhibition of proliferation of all three cell lines. Similar results were noted when docetaxel was given prior to gefitinib (schedule D—G). However, there was a striking shift in response when gefitinib was given prior to docetaxel (G—D). Administered sequentially, gefitinib followed by docetaxel was antagonistic in all three cell lines, suggesting that careful dosing schedules should be devised to avoid deleterious drug interactions.
Fig. 5.
Efficacy of docetaxel/gefitinib combination treatment is sequence-dependent. a–c Representative dose response curves of gefitinib sensitive OE21 (a), gefitinib resistant KYSE520 (b) and gefitinib sensitive TE8 (c) ESCC cells were treated for 4 days with gefitinib or docetaxel and both drugs either in combination or sequentially: 4 days both drugs (both), 48-h gefitinib treatment followed by addition of docetaxel for the remaining 48-h (G—D) or 48-h docetaxel treatment followed by addition of gefitinib for the remaining 48 h (D—G). Cells were analysed by CellTitre-Glo® assay (d) Log2 combination indices were determined from assays described in a–c using CalcuSyn software, where 0 = additive effects, < 0 = synergistic effects, > 0 = antagonistic. log2 CI values at 50%, 75% and 90% effective dose ED50, ED75 and ED90, respectively, are displayed
Discussion
Oesophageal squamous cell carcinoma (ESCC) patients whose tumours have EGFR CNG and/or EGFR protein overexpression may represent a subgroup that benefits from EGFR inhibitor monotherapy [7, 12, 13]. However, even in this biomarker-selected subgroup of ESCC, significant proportions of patients do not respond to EGFR inhibitors and durable responses are uncommon, indicating that primary and acquired clinical resistance is a major clinical challenge. Heterogeneity is a predominant feature of ESCC, including for EGFR CNG and protein over-expression. Tumours with a higher number of genomic clonal subpopulations that are not EGFR-driven are less likely to respond significantly to monotherapy with an EGFR inhibitor. Therefore, EGFR combination treatments are likely to be important to optimise treatment effectiveness in EGFR CNG positive ESCC.
Several clinical trials have investigated the combination of EGFR inhibitors with cytotoxic chemotherapy or concurrent chemoradiotherapy [17–20, 24]. In the palliative setting, in both ESCC and GOA, the addition of EGFR inhibitors to platinum plus fluoropyrimidine chemotherapy has not improved overall survival [18–20]. Similarly, in the radical treatment setting, the addition of EGFR inhibitors to concurrent chemoradiotherapy, with a platinum and fluoropyrimidine chemotherapy backbone has not improved overall survival [17]. However, addition of EGFR inhibition to chemoradiotherapy in ESCC with a chemotherapy backbone incorporating paclitaxel did improve overall survival, even in biomarker-unselected patients [24]. Consistent with these clinical trial results we observed that EGFR CNG positive gastroesophageal cancers in both the palliative and neoadjuvant setting appear to be more sensitive to platinum fluoropyrimidine-based cytotoxic chemotherapy. This suggests that the use of an EGFR inhibitor could reduce, or negate, the benefit of platinum-based cytotoxic chemotherapy in patients with EGFR-driven tumours and would thus be antagonistic. We confirmed drug antagonism in EGFR CNG cell lines. Because no GOA cell lines with EGFR CNG are available, our cell line experiments were restricted to ESCC. This is a limitation of our work, and deriving EGFR CNG GOA cell lines to confirm our findings would be advantageous. The finding of synergy between docetaxel and gefitinib observed in 2-D cell culture was supported by our findings in longer-term 2-D colony formation assays and in 3-D spheroid culture models. However, not all cell lines used in this study were able to grow in 3-D culture and likewise, to overcome this limitation of the current study, testing of additional EGFR CNG positive cell lines that are able to survive anchorage-independent seeding would be advantageous. Investigation of drug synergy in an in vivo xenograft model, evaluation of the impact of EGFR CNG on survival in a cohort of ESCC patients treated with docetaxel monotherapy, as well as extension of investigation to other EGFR inhibitors would also be beneficial. In addition, elucidation of the mechanism of synergy between docetaxel and gefitinib, or other EGFR inhibitors, in EGFR CNG positive ESCC would also provide additional understanding and may suggest predictive biomarkers to identify those patients that might benefit most from this combination.
The majority of earlier reports have suggested that EGFR protein expression and/or EGFR gene copy number gain are associated with shorter survival of gastroesophageal cancers including adenocarcinomas and ESCCs [38–40]. However, longer survival, or no impact, has been observed in other studies [38–40]. In contrast to our investigation, the influence of treatment modality in general and cytotoxic chemotherapy in particular on how EGFR impacts the clinical outcomes has not been investigated in the majority of these studies. Our data shows an interaction between neo-adjuvant PBC and the impact of EGFR and, consistent with the findings of previous studies, indicates that the treatment patients receive should be taken into account when the impact of EGFR is being considered. Furthermore, Smyth et al., reported that higher tumour EGFR RNA expression is associated with shorter overall survival in gastroesophageal cancer patients treated with peri-operative PBC [41]. These findings contrast with ours, and suggest that EGFR copy number measured by FISH and EGFR RNA expression measured by nanostring may be measuring different aspects of gastroesophageal cancer biology. For example EGFR FISH-positive tumours almost invariably overexpress EGFR RNA (and EGFR protein), but up to 50% of EGFR FISH-negative tumours also have high EGFR RNA and protein [42, 43]. The lack of benefit from gefitinib in EGFR CNG-negative patients (which might have still high expression EGFR RNA and or protein) suggests that EGFR protein or RNA expression may be a less reliable marker of EGFR-driven gastroesophageal cancers and a less reliable marker of the impact of EGFR signalling and EGFR inhibitors on PBC sensitivity, resistance or clinical outcomes. Nevertheless, these findings also indicate the importance of considering the type of EGFR assay used in gastroesophageal predictive and prognostic studies. To our knowledge the impact of EGFR CNG on overall survival in advanced stage patients treated with palliative platinum fluoropyrimidine chemotherapy (PBC) has not been previously reported.
Our data indicating that the combination of gefitinib with cisplatin is antagonistic (in TE8 and OE21 cells) are at odds with reports that treatment of TE8 xenograft tumours with cisplatin in combination with EGFR inhibition by cetuximab significantly reduces their size [44]. Such discrepancies may arise due to the nature of the mechanism of inhibition of EGFR (small molecule EGFR TKi versus blocking antibody with potential antibody-dependent cellular cytotoxicity effects). However, clinical results of EGFR monoclonal antibodies both as monotherapy and in combination with PBC in ESCC have been similar to those demonstrated with EGFR TKis [10, 18, 20–23, 27, 28, 35]. In addition, in line with our conclusions, the POWER phase III RCT in advanced ESCC did not demonstrate any benefit of the addition of the humanised monoclonal anti-EGFR antibody panitumumab to cisplatin plus fluoropyrimidine chemotherapy [18]. POWER enrolled molecularly unselected ESCC patients, but, a retrospective analysis demonstrated that EGFR IHC did not correlate significantly with overall survival, and EGFR copy number was not investigated. The antagonism between cisplatin and oxaliplatin and EGFR inhibition provides a key explanation for this, and other, negative clinical trials and suggests that, even if these trials had been undertaken in biomarker-selected patients, benefit from the addition of EGFR inhibitors may not have been observed.
Previous studies in KYSE30 cells have suggested that the sequence of administration of gefitinib in combination with cytotoxic agents determines efficacy. Synergy was noted with cisplatin, carboplatin, oxaliplatin, docetaxel and paclitaxel followed by gefitinib [45]. However, our studies in a wider cell panel suggest that the effect of concurrent gefitinib with platinum-based cytotoxic drugs is cell line-dependent and thus the effects of this combination may be unpredictable in the clinical setting. In contrast, we demonstrated that gefitinib and docetaxel was consistently synergistic. This observation is consistent with the demonstration that addition of erlotinib to chemoradiotherapy including paclitaxel was beneficial in ESCC [24]. In this study there was no biomarker selection, and we hypothesise that in this trial the greatest benefit from addition of erlotinib will be seen in those patients who are EGFR CNG and/or have EGFR protein overexpression. Our dose reduction analysis indicates that equivalent reductions in tumour cell growth could be achieved with lower doses of gefitinib and docetaxel if used in combination, which could have significant advantages in terms of reduced financial costs of the treatment and, more critically, reduced toxicity to the patient. The cell line studies also suggest that the sequence of administration of taxane and EGFR inhibitor is critical. The administration of gefitinib prior to docetaxel invariably resulted in antagonism which is consistent with studies in both an NSCLC cell line [46] and in KYSE30 ESCC cells [45]. This data suggests that careful scheduling, or drug holidays may be required to avoid possible antagonistic drug interactions. In support of this, when used in combination with paclitaxel, pulsatile administration of gefitinib has proved more effective than continuous dosing in murine models of breast cancer, [47].
The molecular mechanism of drug combination antagonism or synergy in this setting is unclear and is under investigation. In vitro studies analysing potential combination therapies on both head and neck SCC and NSCLC cell lines suggest that there is also antagonism between gefitinib and cisplatin in other tumour types [25–27]; the effects have been variously attributed to cisplatin cytotoxicity being dependent on EGFR phosphorylation and degradation [28], induction of epithelial to mesenchymal transition (EMT) which is associated with increased resistance to gefitinib [26], reduced cisplatin entry into the cell and increased DNA repair or cell cycle arrest in G1. However, antagonism can be overcome by concurrent use of autophagy inhibitors (without any apparent effect on the cell cycle) [27] suggesting that factors other than the phase of the cell cycle may be involved. Previous reports in colorectal cancer cells have suggested that treatment of cells with cytotoxic agents increases phosphorylation of EGFR rendering cells more sensitive to the effects of EGFR TKis, whereas antagonistic interactions result from a cytotoxic drug induced a decrease in EGFR phosphorylation [37, 48]. In NSCLC, the mechanism of synergistic interaction was also suggested to be due to increased docetaxel-induced phosphorylation of EGFR and its subsequent inhibition following gefitinib addition [6]; however, similar analysis of our ESCC cell lines over several repeat experiments was inconclusive. The antagonistic effect of sequential administration of gefitinib prior to docetaxel could be due to cell cycle effects, where gefitinib induces a G1 cell cycle arrest thus rendering taxanes (which are primarily mitotic spindle inhibitors acting in G2/M) ineffective [45, 46]. Since concurrent administration of gefitinib or administration after docetaxel is synergistic, in the clinic, this would suggest that an interrupted schedule of an oral TKi like gefitinib, might be needed in combination with a taxane. Alternatively, and in contrast to common clinical practice at present, an anti-EGFR monoclonal antibody should potentially be administered after docetaxel and not before, when combined with a taxane.
Recently, the ATTRACTION-3 study demonstrated that the PD-1 inhibitor nivolumab provided improved overall survival compared to taxane monotherapy suggesting that nivolumab is a new standard of care for ESCC after progression with fluoropyrimidine/platinum chemotherapy [5]. However, given that only a minority subgroup of patients respond to nivolumab and responses took a median of 2.6 months to occur, its use presents clinical challenges in this setting, where patients often have high tumour burdens and are very symptomatic. In addition, in squamous cell carcinomas including ESCC, EGFR activation is associated with depleted tumour infiltrating lymphocytes and resistance to immune checkpoint inhibition (ICI) [29]. EGFR activation leads to increased anaerobic glycolysis in tumour cells, glucose depletion and accumulation of lactate in squamous cell carcinomas, meaning that tumour-infiltrating T cells, may have to compete for metabolic fuels. ICI appear to be less effective in EGFR mutant positive NSCLC [29, 49], and EGFR activation has been associated with hyper-progression following ICI therapy [50]. Early phase trials in NSCLC have revealed problematic toxicity combining EGFR inhibitors and immune checkpoint inhibitors [51]. Together these data suggest that EGFR-driven ESCC, identified by EGFR CNG and /or EGFR protein overexpression are likely to be less sensitive to nivolumab which is unlikely as a monotherapy to provide an effective treatment for this group of patients. As such, taxanes will likely remain one standard of care for ESCC after progression with fluoropyrimidine/platinum chemotherapy, either before or after nivolumab. Overall, our results contribute additional evidence to support investigation of EGFR inhibitors in EGFR CNG positive ESCC and suggest that a combination strategy with taxanes has the potential for synergism thereby optimising clinical impact and effectiveness. Since taxanes are a standard of care for ESCC after progression with fluoropyrimidine/platinum chemotherapy evaluating the benefit of the addition of an EGFR inhibitor to docetaxel or paclitaxel in tumours that are EGFR CNG and/or EGFR protein overexpressed by IHC would be the most appropriate initial area of clinical investigation.
In summary, drug combination studies indicate that targeting EGFR in ESCC cells carrying EGFR copy number gain may negate or reduce anticancer effects of platinum-based chemotherapy; however, EGFR inhibitors are efficacious and synergistic in combination with docetaxel when scheduled correctly. We recommend further preclinical studies and consideration of clinical investigation of scheduled anti-EGFR therapies combined with taxanes for ESCC patients with tumours expressing high EGFR by IHC and/or EGFR CNG.
Acknowledgements
We thank Alice Savage for technical laboratory assistance.
Abbreviations
- ESCC
Oesophageal squamous cell carcinoma
- EGFR
Epidermal growth factor receptor
- CNG
Copy number gain
- EAC
Oesophageal adenocarcinoma
- NSCLC
Non-small cell lung cancer
- IHC
Immunohistochemistry
- SD
Standard deviation
- ICI
Immune checkpoint inhibition
- TKi
Tyrosine kinase inhibitor
- EMT
Epithelial to mesenchymal transition
- GOA
Gastroesophageal adenocarcinoma
- CI
Combination index
- PBC
Platinum–fluoropyrimidine combination chemotherapy
Author contributions
LCS designed and conducted experiments, analysed data and prepared the manuscript. MM, PC, and JI designed, conducted and analysed experiments. MB analysed clinical data. JT and CC conducted experiments. GM and SW prepared and analysed clinical samples. DC conducted experiments and analysed data. ZM designed experiments. RP designed experiments, analysed data and prepared the manuscript.
Funding
The work undertaken was funded by Ninewells Cancer Campaign (Dundee) and Scottish Government Chief Scientist Office (Grant reference TCS/19/18).
Compliance with ethical standards
Conflict of interest
RDP has undertaken speaking, consulting and advisory roles for Eli Lilly, BMS, Pfizer, Sanofi, Servier; and received research funding (not related to the work in this manuscript) from Astra Zeneca, Roche, MSD, Merck serrano, Eli Lilly, Five Prime Therapeutics, Clovis, Boston Biomedical, and Janssen. LCS, MM, JI, PC, DC, JT, CC, GM, SW, ZM and MB declare no conflict of interest.
Ethics approval
Ethical approval for use of human tissue in this study was obtained from the Scientific Access Committees of the Grampian Tissue Biorepository and the Tayside Biorepository. The Biorepositories have delegated research ethics authority from The North of Scotland research ethics committee (Grampian) and the East of Scotland research ethics committee (Tayside) to approve research projects involving human tissue and data. All tissue and data were anonymised. The study was performed in accordance with the Declaration of Helsinki.
Consent to participate
Project specific written consent was not required for the retrospective use of archival tissue.
Availability of data and material
All data reported in this manuscript are included in the figures and tables.
Consent to publish
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Madusha Meemanage and Lindsay C. Spender contributed equally to this work.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomark Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.(2019) Healthcare Quality Improvement Partnership (HQIP): an audit of the care received by people with oesophago-gastric cancer and oesophageal high grade dysplasia in England and Wales. Version 2. https://www.nogca.org.uk. Accessed 2020
- 3.Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, Kato K, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Nakajima TE, Shitara K, Kawakami H, Narita Y, Yoshino T, Van Cutsem E, Martinelli E, Smyth EC, Arnold D, Minami H, Tabernero J, Douillard JY. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):34–43. doi: 10.1093/annonc/mdy498. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Chang J, Yu H, Wu X, Wang H, Li W, Ji D, Peng W. A phase II study of oxaliplatin in combination with leucovorin and fluorouracil as first-line chemotherapy in patients with metastatic squamous cell carcinoma of esophagus. Cancer Chemother Pharmacol. 2013;71(4):905–911. doi: 10.1007/s00280-013-2081-5. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 6.Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N, Ooi A. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer. 2006;118(5):1173–1180. doi: 10.1002/ijc.21454. [DOI] [PubMed] [Google Scholar]
- 7.Petty RD, Dahle-Smith A, Stevenson DAJ, Osborne A, Massie D, Clark C, Murray GI, Dutton SJ, Roberts C, Chong IY, Mansoor W, Thompson J, Harrison M, Chatterjee A, Falk SJ, Elyan S, Garcia-Alonso A, Fyfe DW, Wadsley J, Chau I, Ferry DR, Miedzybrodzka Z. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35(20):2279–2287. doi: 10.1200/JCO.2016.70.3934. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Wu Z, Wong G, Pectasides E, Nagaraja A, Stachler M, Zhang H, Chen T, Zhang H, Liu JB, Xu X, Sicinska E, Sanchez-Vega F, Rustgi AK, Diehl JA, Wong KK, Bass AJ. CDK4/6 or MAPK blockade enhances efficacy of EGFR inhibition in oesophageal squamous cell carcinoma. Nat Commun. 2017;8:13897. doi: 10.1038/ncomms13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CH, Ma BB, Hui CW, Tao Q, Chan AT. Preclinical evaluation of afatinib (BIBW2992) in esophageal squamous cell carcinoma (ESCC) Am J Cancer Res. 2015;5(12):3588–3599. [PMC free article] [PubMed] [Google Scholar]
- 10.Dutton SJ, Ferry DR, Blazeby JM, Abbas H, Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A, Falk S, Garcia-Alonso A, Fyfe DW, Hubner RA, Gamble T, Peachey L, Davoudianfar M, Pearson SR, Julier P, Jankowski J, Kerr R, Petty RD. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894–904. doi: 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 11.Adelstein DJ, Rodriguez CP, Rybicki LA, Ives DI, Rice TW. A phase II trial of gefitinib for recurrent or metastatic cancer of the esophagus or gastroesophageal junction. Investig New Drugs. 2012;30(4):1684–1689. doi: 10.1007/s10637-011-9736-z. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Niu H, Fan Q, Lu P, Ma C, Liu W, Liu Y, Li W, Hu S, Ling Y, Guo L, Ying J, Huang J. Predictive value of EGFR overexpression and gene amplification on icotinib efficacy in patients with advanced esophageal squamous cell carcinoma. Oncotarget. 2016;7(17):24744–24751. doi: 10.18632/oncotarget.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Fan Q, Lu P, Ying J, Ma C, Liu W, Liu Y, Tan F, Sun Y. Icotinib in patients with pretreated advanced esophageal squamous cell carcinoma with EGFR overexpression or EGFR gene amplification: a single-arm, multicenter phase 2 study. J Thorac Oncol. 2016;11(6):910–917. doi: 10.1016/j.jtho.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Wang C, Wang J, Chen D, Deng J, Deng J, Fan J, Badakhshi H, Huang X, Zhang L, Cai J, Guo S, Qian W, Nie Y, Li Q, Zhao K. A subset of esophageal squamous cell carcinoma patient-derived xenografts respond to cetuximab, which is predicted by high EGFR expression and amplification. J Thorac Dis. 2018;10(9):5328–5338. doi: 10.21037/jtd.2018.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Chen Z, Wang J, Zhang M, Li Z, Wang S, Dong B, Zhang C, Gao J, Shen L. Mouse avatar models of esophageal squamous cell carcinoma proved the potential for EGFR-TKI afatinib and uncovered Src family kinases involved in acquired resistance. J Hematol Oncol. 2018;11(1):109. doi: 10.1186/s13045-018-0651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahle-Smith A, Stevenson D, Massie D, Murray GI, Dutton SJ, Roberts C, Ferry D, Osborne A, Clark C, Petty RD, Miedzybrodzka Z. Epidermal growth factor (EGFR) copy number aberrations in esophageal and gastro-esophageal junctional carcinoma. Mol Cytogenet. 2015;8:78. doi: 10.1186/s13039-015-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14(7):627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 18.Moehler M, Maderer A, Thuss-Patience PC, Brenner B, Meiler J, Ettrich TJ, Hofheinz RD, Al-Batran SE, Vogel A, Mueller L, Lutz MP, Lordick F, Alsina M, Borchert K, Greil R, Eisterer W, Schad A, Slotta-Huspenina J, Van Cutsem E, Lorenzen S. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER) Ann Oncol. 2020;31(2):228–235. doi: 10.1016/j.annonc.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, Ferry D, Mansoor W, Crosby T, Coxon F, Smith D, Waters J, Iveson T, Falk S, Slater S, Peckitt C, Barbachano Y. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Gotte H, Melezinkova H, Moehler M, Arbeitsgemeinschaft Internistische O, Investigators E. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 21.Mok T, Ladrera G, Srimuninnimit V, Sriuranpong V, Yu CJ, Thongprasert S, Sandoval-Tan J, Lee JS, Fuerte F, Shames DS, Klughammer B, Truman M, Perez-Moreno P, Wu YL. Tumor marker analyses from the phase III, placebo-controlled, FASTACT-2 study of intercalated erlotinib with gemcitabine/platinum in the first-line treatment of advanced non-small-cell lung cancer. Lung Cancer. 2016;98:1–8. doi: 10.1016/j.lungcan.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA, Group TI. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Hida T, Sato T, Ando M, Seto T, Satouchi M, Ichinose Y, Katakami N, Yamamoto N, Kudoh S, Sasaki J, Matsui K, Takayama K, Kashii T, Iwamoto Y, Sawa T, Okamoto I, Kurata T, Nakagawa K, Fukuoka M. Randomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a west Japan thoracic oncology group trial (WJTOG0203) J Clin Oncol. 2010;28(5):753–760. doi: 10.1200/JCO.2009.23.3445. [DOI] [PubMed] [Google Scholar]
- 24.Wu SX, Wang LH, Luo HL, Xie CY, Zhang XB, Hu W, Zheng AP, Li DJ, Zhang HY, Xie CH, Lian XL, Du DX, Chen M, Bian XH, Tan BX, Jiang H, Zhang HB, Wang JH, Jing Z, Xia B, Zhang N, Zhang P, Li WF, Zhao FJ, Tian ZF, Liu H, Huang KW, Hu J, Xie RF, Du L, Li G. Randomised phase III trial of concurrent chemoradiotherapy with extended nodal irradiation and erlotinib in patients with inoperable oesophageal squamous cell cancer. Eur J Cancer. 2018;93:99–107. doi: 10.1016/j.ejca.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CM, Chen JT, Stewart DJ, Chiu CH, Lai CL, Hsiao SY, Chen YM, Chang KT. Antagonism between gefitinib and cisplatin in non-small cell lung cancer cells: why randomized trials failed? J Thorac Oncol. 2011;6(3):559–568. doi: 10.1097/JTO.0b013e3182021ff5. [DOI] [PubMed] [Google Scholar]
- 26.Kurokawa M, Ise N, Omi K, Goishi K, Higashiyama S. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci. 2013;104(7):904–911. doi: 10.1111/cas.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JT, Li WC, Gao S, Wang F, Li XQ, Yu HQ, Fan LL, Wei W, Wang H, Sun GP. Autophagy inhibition overcomes the antagonistic effect between gefitinib and cisplatin in epidermal growth factor receptor mutant non–small-cell lung cancer cells. Clin Lung Cancer. 2015;16(5):e55–66. doi: 10.1016/j.cllc.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Ahsan A, Hiniker SM, Ramanand SG, Nyati S, Hegde A, Helman A, Menawat R, Bhojani MS, Lawrence TS, Nyati MK. Role of epidermal growth factor receptor degradation in cisplatin-induced cytotoxicity in head and neck cancer. Cancer Res. 2010;70(7):2862–2869. doi: 10.1158/0008-5472.CAN-09-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottensmeier CH, Perry KL, Harden EL, Stasakova J, Jenei V, Fleming J, Wood O, Woo J, Woelk CH, Thomas GJ, Thirdborough SM. Upregulated glucose metabolism correlates inversely with CD8+ T-cell infiltration and survival in squamous cell carcinoma. Cancer Res. 2016;76(14):4136–4148. doi: 10.1158/0008-5472.CAN-15-3121. [DOI] [PubMed] [Google Scholar]
- 30.Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Gregorc V, Toschi L, Franklin WA, Crino L, Gazdar AF, Bunn PA, Jr, Hirsch FR. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol. 2005;23(22):5007–5018. doi: 10.1200/JCO.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, Kaye SB, Gianni L, Harris A, Bjork T, Averbuch SD, Feyereislova A, Swaisland H, Rojo F, Albanell J. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20(21):4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 32.Ehrsson H, Wallin I, Yachnin J. Pharmacokinetics of oxaliplatin in humans. Med Oncol. 2002;19(4):261–265. doi: 10.1385/MO:19:4:261. [DOI] [PubMed] [Google Scholar]
- 33.van Hennik MB, van der Vijgh WJ, Klein I, Elferink F, Vermorken JB, Winograd B, Pinedo HM. Comparative pharmacokinetics of cisplatin and three analogues in mice and humans. Cancer Res. 1987;47(23):6297–6301. [PubMed] [Google Scholar]
- 34.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16(1):187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 35.Rivory LP, Haaz MC, Canal P, Lokiec F, Armand JP, Robert J. Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin Cancer Res. 1997;3(8):1261–1266. [PubMed] [Google Scholar]
- 36.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 37.Koizumi F, Kanzawa F, Ueda Y, Koh Y, Tsukiyama S, Taguchi F, Tamura T, Saijo N, Nishio K. Synergistic interaction between the EGFR tyrosine kinase inhibitor gefitinib (“Iressa”) and the DNA topoisomerase I inhibitor CPT-11 (irinotecan) in human colorectal cancer cells. Int J Cancer. 2004;108(3):464–472. doi: 10.1002/ijc.11539. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Yu JM, Jing SW, Guo Y, Wu YJ, Li N, Jiao WP, Wang L, Zhang YJ. Relationship between EGFR over-expression and clinicopathologic characteristics in squamous cell carcinoma of the esophagus: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(14):5889–5893. doi: 10.7314/apjcp.2014.15.14.5889. [DOI] [PubMed] [Google Scholar]
- 39.Hong L, Han Y, Yang J, Zhang H, Jin Y, Brain L, Li M, Zhao Q. Prognostic value of epidermal growth factor receptor in patients with gastric cancer: a meta-analysis. Gene. 2013;529(1):69–72. doi: 10.1016/j.gene.2013.07.106. [DOI] [PubMed] [Google Scholar]
- 40.Guo YM, Yu WW, Zhu M, Guo CY. Clinicopathological and prognostic significance of epidermal growth factor receptor overexpression in patients with esophageal adenocarcinoma: a meta-analysis. Dis Esophagus. 2015;28(8):750–756. doi: 10.1111/dote.12248. [DOI] [PubMed] [Google Scholar]
- 41.Smyth EC, Nyamundanda G, Cunningham D, Fontana E, Ragulan C, Tan IB, Lin SJ, Wotherspoon A, Nankivell M, Fassan M, Lampis A, Hahne JC, Davies AR, Lagergren J, Gossage JA, Maisey N, Green M, Zylstra JL, Allum WH, Langley RE, Tan P, Valeri N, Sadanandam A. A seven-gene signature assay improves prognostic risk stratification of perioperative chemotherapy treated gastroesophageal cancer patients from the MAGIC trial. Ann Oncol. 2018;29(12):2356–2362. doi: 10.1093/annonc/mdy407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YL, Xu KL, Zhou Y, Gao X, Chen LR. Correlation of epidermal growth factor receptor overexpression with increased epidermal growth factor receptor gene copy number in esophageal squamous cell carcinomas. Chin Med J (Engl) 2012;125(3):450–454. [PubMed] [Google Scholar]
- 43.Jiang D, Li X, Wang H, Shi Y, Xu C, Lu S, Huang J, Xu Y, Zeng H, Su J, Hou Y, Tan L. The prognostic value of EGFR overexpression and amplification in esophageal squamous cell carcinoma. BMC Cancer. 2015;15:377. doi: 10.1186/s12885-015-1393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon J, Yoon HJ, Kim JH, Lee TS, Song IH, Lee HW, Kang MC, Park JH. Cetuximab inhibits cisplatin-induced activation of EGFR signaling in esophageal squamous cell carcinoma. Oncol Rep. 2014;32(3):1188–1192. doi: 10.3892/or.2014.3302. [DOI] [PubMed] [Google Scholar]
- 45.Morelli MP, Cascone T, Troiani T, De Vita F, Orditura M, Laus G, Eckhardt SG, Pepe S, Tortora G, Ciardiello F. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(Suppl 4):iv61–iv68. doi: 10.1093/annonc/mdi910. [DOI] [PubMed] [Google Scholar]
- 46.Chen B, Zheng J, Zeng Y, Li B, Xie B, Zheng J, Zhou J, Zhang W. Sequence-dependent antiproliferative effects of gefitinib and docetaxel on non-small cell lung cancer (NSCLC) cells and the possible mechanism. PLoS ONE. 2014;9(12):e114074. doi: 10.1371/journal.pone.0114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solit DB, She Y, Lobo J, Kris MG, Scher HI, Rosen N, Sirotnak FM. Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continuous dosing for sensitizing tumors to paclitaxel. Clin Cancer Res. 2005;11(5):1983–1989. doi: 10.1158/1078-0432.CCR-04-1347. [DOI] [PubMed] [Google Scholar]
- 48.Van Schaeybroeck S, Karaiskou-McCaul A, Kelly D, Longley D, Galligan L, Van Cutsem E, Johnston P. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin Cancer Res. 2005;11(20):7480–7489. doi: 10.1158/1078-0432.CCR-05-0328. [DOI] [PubMed] [Google Scholar]
- 49.Tsakonas G, Ekman S. Oncogene-addicted non-small cell lung cancer and immunotherapy. J Thorac Dis. 2018;10(Suppl 13):S1547–S1555. doi: 10.21037/jtd.2018.01.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):465–469. doi: 10.1080/14740338.2017.1300656. [DOI] [PubMed] [Google Scholar]