Abstract
Purpose
Menopause modifies women’s lipid profiles. However, the fact that it is still unclear whether high-density lipoprotein-cholesterol (HDL-C) levels decrease in postmenopausal women necessitated a systematic review and meta-analysis.
Methods
The PubMed, EMBASE, Cochrane Library, and Web of Science databases were searched and 498 articles published between 1987 and 2020 were retrieved. Studies reporting HDL-C, low-density lipoprotein-cholesterol (LDL-C), total cholesterol (TC), and triglyceride (TG) levels in both postmenopausal and premenopausal populations were included. The quality of the included studies was assessed using the Cross-Sectional/Prevalence Study Quality tool. The standard mean difference (SMD) and 95% confidence interval (CI) were estimated using random effects models. A meta-regression analysis and subgroup analysis were performed to identify potential modifiers. Egger’s test and funnel plots were constructed to evaluate publication biases.
Results
Lipid profiles from 18 cross-sectional studies and two cohort studies including 5652 postmenopausal women and 7825 premenopausal women were meta-analyzed. HDL-C levels were not significantly different between the postmenopausal and premenopausal women (SMD = − 0.053, 95% CI − 0.171 to 0.066, p = 0.383) and were not affected by country, publication year, study quality in the meta-regression analysis, or significant publication bias. Higher LDL-C, TC, and TG levels were detected in postmenopausal women than in premenopausal controls.
Conclusion
Unlike increased LDL-C, TC, and TG levels, HDL-C levels in pre- and postmenopausal women were not different in this first meta-analysis of lipid profiles in premenopausal and postmenopausal women. Prospective studies with large populations examining HDL-C levels and functions in women with different menopausal statuses are essential in the future.
Trial registration number
None.
Electronic supplementary material
The online version of this article (10.1007/s42000-020-00216-8) contains supplementary material, which is available to authorized users.
Keywords: High-density lipoprotein-cholesterol, Low-density lipoprotein-cholesterol, Triglycerides, Total cholesterol, Postmenopausal, Premenopausal, Cross-sectional
Introduction
The menopause transition is an inevitable physiological process that occurs in women and is characterized by biological changes such as reductions in estradiol levels and proatherogenic lipid profiles [1]. An increased risk of cardiovascular disease (CVD) has been observed during this phase of life. In particular, the risk doubles in women aged 45–54 years [2], indicating harmful changes in the metabolic pattern between the pre- and postmenopausal periods. High-density lipoprotein cholesterol (HDL-C), a robust, independent, and traditional CVD risk factor, has exhibited inconsistent changes in a quantitative analysis of menopause-related changes. In addition to the dichotomous findings of either lower or higher HDL-C levels in postmenopausal women [3–5], recent cross-sectional studies did not identify a significant difference in HDL-C levels between pre- and postmenopausal women [6, 7]. The long-established view acknowledges that HDL-C levels negatively correlate with the risk of CVD in the overall population [8]. However, accumulating evidence of a positive association between HDL-C levels and atherosclerosis-related diseases in postmenopausal women challenges the cardioprotective effect of high HDL-C levels [9, 10]. An open-ended question is whether a high HDL-C level is a CVD risk marker after menopause. Elevated levels of low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and total cholesterol (TC) are related to CVD, with substantial interrelationships with HDL-C levels. An improved understanding of the changes in the levels of HDL-C and other lipids in response to menopause status will ensure a more reliable investment in strategies aiming to reduce CVD risk.
To our knowledge, we have performed the first meta-analysis to clarify whether HDL-C, LDL-C, TG, and TC levels are altered after the menopausal transition. The results of the meta-regression and subgroup analyses may explain the heterogeneity of changes in HDL-C levels during the menopause transition.
Methods
Data sources and database searches
We searched four public electronic databases, namely PubMed, Web of Science, EMBASE, and the Cochrane Library, for related articles. The following search terms were used: “Postmenopause” or “Postmenopausal period,” and “Premenopause” or “Premenopausal period” in combination with “Lipoproteins, HDL” or “High-density Lipoproteins.” The search was restricted to articles published in the English language that included human participants. Subsequent to our search, the articles included studies published from the beginning of 1987 to 2020. All titles and abstracts yielded by the search were then screened for potential suitability. Those articles that appeared to be relevant were retrieved and examined in more detail. This search was supplemented by manual searches of the reference lists of all retrieved studies. We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines to conduct and report the results of our meta-analyses [11].
Inclusion and exclusion criteria
We included comparative studies of postmenopausal and premenopausal women published in the English language that met the following criteria: (1) original articles that included, (2) both healthy pre- and postmenopausal women, and (3) simultaneously reported HDL-C, LDL-C, TC, and TG levels. Postmenopausal status was defined as more than 12 months of amenorrhea and premenopausal state was defined as regular menstrual cycles. Studies were excluded when (1) the pre- or postmenopausal women had lipid-altering diseases, including diabetes, coronary artery disease, malignancy, and thyroid functional abnormalities; (2) the pre- or postmenopausal women received hormone replacement therapy (HRT) or other medications affecting lipid metabolism; (3) the premenopausal women were pregnant; or (4) the women underwent menopause due to surgery or other unnatural causes. Studies were also excluded if they used only menopause status as a covariate or if they reported overlapping data or consisted of studies of a single case or reviews.
Data extraction and methodological quality assessment
Two authors (HWL and QC) independently extracted and summarized the data from the included studies. The papers were required to have indicated the total numbers of postmenopausal subjects and premenopausal subjects as well as the mean HDL-C level ± standard deviation (SD) or the mean HDL-C level ± standard error of mean (SE). When multiple publications reported the same or overlapping data, we included the most recent study with the largest sample size. The following data were extracted from each study: first author, year, country, sample sizes of the case and control groups, mean age, and the levels of TC, TG, LDL-C, and HDL-C, as well as their SD or SE. We extracted the baseline data from cohort studies if multiple time points were provided. The level of quality of all cross-sectional studies was evaluated using the Cross-Sectional/Prevalence Study Quality tool recommended by the Agency for Healthcare Research and Quality. An item received a score of “1” if the answer was “Yes” and a score of “0” if the answer was “No” or “Unclear” [12]. Studies with scores of 0–3 points were classified as “low quality,” studies with a score of 4–7 points were classified as “moderate quality,” and studies with a score of 8–11 points were classified as “high quality” [13]. Two reports were cohort studies [5, 14], and the same checklist was also used to assess their quality. One of the selected papers was a high-quality study and the remaining 19 papers were moderate quality. The majority of the included studies were considered to display an acceptable level of quality [see Table S1 in Additional file 1].
Statistical analysis
All statistical analyses were conducted using STATA software (version 15.0). A two-tailed p value of < 0.05 for any test or model was considered significant. All extracted data on TC, HDL-C, LDL-C, and TG levels were converted into mg/dL. The pooled standardized mean difference (SMD) and its 95% confidence interval (CI) were calculated for the meta-analysis of the lipid (HDL-C, LDL-C, TC, and TG) levels, which were included as continuous variables, in both cross-sectional studies and the baseline data from cohort studies, as mentioned in similar studies [15]. Heterogeneity was assessed using the I2 statistic, which describes the percentage of variability in the effect estimates due to heterogeneity rather than chance. An approximated normality test (z-test) was used to analyze the aggregated results. Heterogeneity was considered significant when the probability value of the chi-square test was < 0.05. As recommended by the Cochrane Handbook for the Systematic Reviews of Interventions, an I2 value < 40% suggests that “heterogeneity might not be important,” whereas an I2 value > 75% suggests “considerable heterogeneity.” We pooled the data across trials using the fixed effects model (Mantel-Haenszel) if heterogeneity was absent or the random effects model if heterogeneity was present. A meta-regression was performed to explore the effects of publication year, sample size, country, and study quality on the effect estimates and identified the potential sources of the heterogeneity. Subgroup analyses were performed after stratification according to the publication year, sample size, and country to evaluate the association between HDL-C levels and menopausal status. Additionally, a sensitivity analysis was performed by repeating the meta-analysis after one study or a few studies were sequentially removed to assess whether a particular study significantly affected the pooled estimates. Potential publication bias was tested by creating a funnel plot that represented the standard error as the measure of the sample size and SMD as the measure of the pooled effect and Egger’s test.
Results
Search results
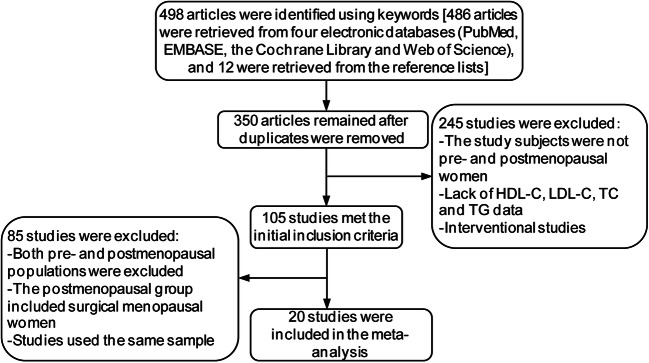
The literature search yielded 498 related papers. Three hundred fifty studies remained after removing duplicates. Two hundred forty-five articles were then excluded because they did not include pre- and postmenopausal women as subjects; lacked HDL-C, LDL-C, TC, and TG data; and were interventional studies. One hundred and five studies met the initial inclusion criteria, of which 85 studies were excluded because of exclusion of both pre- and postmenopausal populations, inclusion of surgical menopausal women, and studies using the same populations. The remaining 20 original articles were included in the meta-analysis. The literature selection criteria and the reasons for exclusion are presented in Fig. 1. Eighteen of the 20 original articles were cross-sectional studies, and the remaining two articles were cohort studies.
Fig. 1.
Flow chart of the literature search and study selection process
Study characteristics
The characteristics of all 20 selected studies, which included 5652 postmenopausal women and 7825 premenopausal women, are summarized in Table 1 [4, 5, 7, 14, 16–31]. Asian participants from China, Korea, Japan, and India included 4979 postmenopausal women (88.1%) and 7132 premenopausal controls (91.1%), while non-Asian participants consisted of 673 postmenopausal women (11.9%) and 693 premenopausal females (8.9%). These eligible studies were conducted in ten countries, four of which were from the USA [4, 14, 23, 27], three articles were from Argentina [18, 24, 25], three articles were from Korea [19, 20, 28], two articles were from Japan [22, 31], two articles were from China [5, 23], and two articles were from Slovakia [7, 17]. The other four articles were from Brazil [21], India [16], Turkey [30], and the Netherlands [29].
Table 1.
Characteristics of the 20 included studies
| First author | Year | Study design | Country | Number | Age, years | HDL-C (mg/dl) | LDL-C (mg/dl) | TG (md/dl) | TC (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|
| Cernanova et al. [7] | 2016 | Cross-sectional | Slovakia |
Postmenopausal women: 146 Premenopausal women: 146 |
53.51 ± 4.52 45.73 ± 3.77 |
61.16 ± 16.26 62.32 ± 16.26 |
126.76 ± 41.74 120.97 ± 32.85 |
127.43 ± 85.84 110.62 ± 60.18 |
211.97 ± 43.63 204.25 ± 35.91 |
| Mogarekar and Kulkarni [16] | 2015 | Cross-sectional | India |
Postmenopausal women: 40 Premenopausal women: 40 |
59.10 ± 10.04 30.13 ± 7.20 |
30.93 ± 7.47 36.68 ± 7.95 |
148.55 ± 40.54 135.13 ± 34.52 |
167.53 ± 68.79 118.95 ± 43.41 |
212.38 ± 48.43 195.35 ± 36.62 |
| Luptakova et al. [17] | 2012 | Cross-sectional | Slovakia |
Postmenopausal women: 129 Premenopausal women: 167 |
53.30 ± 0.32 45.59 ± 0.26 |
58.06 ± 17.41 61.16 ± 15.10 |
128.70 ± 39.42 123.29 ± 34.78 |
139.82 ± 93.81 115.93 ± 59.29 |
214.29 ± 40.93 206.56 ± 36.29 |
| Muzzio et al. [18] | 2011 | Cross-sectional | Argentina |
Postmenopausal women: 23 Premenopausal women: 13 |
53.00 ± 4.25 33.00 ± 5.25 |
52.26 ± 12.00 60.39 ± 13.16 |
168.89 ± 48.31 109.37 ± 31.30 |
138.05 ± 64.60 69.03 ± 36.28 |
241.70 ± 47.88 187.64 ± 31.27 |
| Jeon et al. [19] | 2011 | Cross-sectional | Korea |
Postmenopausal women: 805 Premenopausal women: 1166 |
51.20 ± 9.00 49.30 ± 8.50 |
61.60 ± 13.90 61.50 ± 13.20 |
130.60 ± 31.10 109.90 ± 27.40 |
95.00 ± 41.90 83.20 ± 37.40 |
209.40 ± 15.40 186.00 ± 30.80 |
| Zhou et al. [5] | 2010 | Cohort | China |
Postmenopausal women: 349 Premenopausal women: 380 |
53.80 ± 2.80 42.20 ± 3.80 |
60.20 ± 14.20 58.70 ± 12.30 |
117.40 ± 32.00 103.70 ± 25.70 |
118.00 ± 3.44 90.00 ± 2.37 |
204.50 ± 35.90 182.80 ± 28.60 |
| Jeong et al. [20] | 2010 | Cross-sectional | Korea |
Postmenopausal women: 2661 Premenopausal women: 4613 |
58.60 ± 6.40 43.50 ± 5.90 |
57.40 ± 13.60 60.00 ± 13.60 |
133.10 ± 31.00 112.80 ± 28.00 |
109.00 ± 66.20 82.40 ± 48.50 |
212.20 ± 33.50 189.30 ± 31.80 |
| Giribela et al. [21] | 2009 | Cross-sectional | Brazil |
Postmenopausal women: 18 Premenopausal women: 22 |
48.00 ± 3.00 45.00 ± 3.00 |
61.00 ± 14.00 61.00 ± 12.00 |
100.00 ± 24.00 103.00 ± 35.00 |
80.00 ± 44.00 103.00 ± 50.00 |
178.00 ± 24.00 191.00 ± 27.00 |
| Karita et al. [22] | 2008 | Cross-sectional | Japan |
Postmenopausal women: 59 Premenopausal women: 68 |
54.90 ± 3.40 42.00 ± 4.70 |
70.50 ± 12.50 71.40 ± 12.60 |
144.00 ± 37.00 127.00 ± 32.00 |
90.60 ± 48.40 65.80 ± 23.40 |
226.00 ± 38.00 200.00 ± 28.00 |
| Lin et al. [23] | 2006 | Cross-sectional | China |
Postmenopausal women: 234 Premenopausal women: 360 |
53.10 ± 4.40 46.00 ± 3.60 |
61.94 ± 17.03 56.52 ± 14.71 |
131.01 ± 35.94 125.60 ± 30.14 |
116.81 ± 71.68 91.15 ± 59.29 |
143.63 ± 44.02 144.40 ± 40.93 |
| Zern et al. [24] | 2005 | Cross-sectional | USA |
Postmenopausal women: 20 Premenopausal women: 24 |
58.50 ± 7.50 39.70 ± 8.50 |
69.68 ± 13.55 65.81 ± 8.90 |
112.08 ± 33.62 112.08 ± 30.92 |
132.74 ± 106.19 88.50 ± 48.67 |
216.22 ± 37.84 193.05 ± 39.77 |
| Zago et al. [25] | 2004 | Cross-sectional | Argentina |
Postmenopausal women: 30 Premenopausal women: 28 |
52.90 ± 9.90 32.70 ± 9.90 |
52.00 ± 17.52 60.10 ± 19.04 |
167.10 ± 66.26 108.50 ± 44.93 |
137.00 ± 89.10 69.00 ± 51.79 |
244.30 ± 67.02 187.10 ± 44.93 |
| Berg et al. [26] | 2004 | Cross-sectional | Argentina |
Postmenopausal women: 30 Premenopausal women: 20 |
57.00 ± 5.30 36.90 ± 4.10 |
58.45 ± 13.16 58.45 ± 10.06 |
154.59 ± 44.06 109.37 ± 23.96 |
124.78 ± 44.25 72.57 ± 22.12 |
237.84 ± 47.10 186.87 ± 22.01 |
| Kanaley et al. [27] | 2001 | Cross-sectional | USA |
Postmenopausal women: 27 Premenopausal women: 23 |
53.30 ± 4.24 49.00 ± 2.83 |
57.40 ± 30.03 53.60 ± 22.08 |
120.20 ± 60.06 115.60 ± 50.34 |
102.10 ± 91.85 95.00 ± 90.08 |
193.90 ± 68.89 194.90 ± 79.49 |
| Kim et al. [28] | 2000 | Cross-sectional | Korea |
Postmenopausal women: 821 Premenopausal women: 485 |
47.20 ± 2.90 47.10 ± 2.60 |
207.40 ± 34.70 190.00 ± 33.90 |
70.00 ± 17.30 66.50 ± 16.80 |
123.60 ± 27.20 112.50 ± 25.40 |
118.30 ± 69.10 102.00 ± 60.70 |
| Peters et al. [29] | 1999 | Cross-sectional | The Netherlands |
Postmenopausal women: 93 Premenopausal women: 93 |
51.10 ± 2.20 50.60 ± 2.40 |
250.19 ± 52.51 227.41 ± 52.51 |
63.48 ± 21.68 61.16 ± 21.68 |
166.96 ± 47.54 146.09 ± 47.54 |
102.65 ± 72.57 102.65 ± 72.57 |
| Oner et al. [30] | 1997 | Cross-sectional | Turkey |
Postmenopausal women: 18 Premenopausal women: 20 |
57.80 ± 5.10 28.90 ± 3.70 |
204.00 ± 38.80 153.00 ± 29.10 |
44.20 ± 7.80 51.10 ± 8.00 |
137.00 ± 37.40 87.20 ± 25.70 |
113.00 ± 37.70 71.10 ± 21.30 |
| Li et al. [4] | 1996 | Cross-sectional | USA |
Postmenopausal women: 74 Premenopausal women: 72 |
55.80 ± 7.40 41.20 ± 6.50 |
225.10 ± 34.30 193.40 ± 32.60 |
56.40 ± 14.10 61.00 ± 15.70 |
144.90 ± 29.70 117.60 ± 29.00 |
117.90 ± 69.10 72.80 ± 35.00 |
| Wakatsuki and Sagara [31] | 1994 | Cross-sectional | Japan |
Postmenopausal women: 10 Premenopausal women: 20 |
65.63 ± 5.58 42.75 ± 7.25 |
220.38 ± 43.48 170.68 ± 30.37 |
61.25 ± 12.74 62.70 ± 14.87 |
136.30 ± 39.00 89.85 ± 21.07 |
114.13 ± 39.42 96.45 ± 42.23 |
| Matthews et al. [14] | 1989 | Cohort | USA |
Postmenopausal women: 65 Premenopausal women: 65 |
47.80 ± 1.60 47.30 ± 1.50 |
192.66 ± 44.02 180.31 ± 44.02 |
60.39 ± 22.06 56.90 ± 17.81 |
114.40 ± 44.06 108.21 ± 44.06 |
89.38 ± 70.80 76.11 ± 60.18 |
TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride
Association between menopause and HDL-C
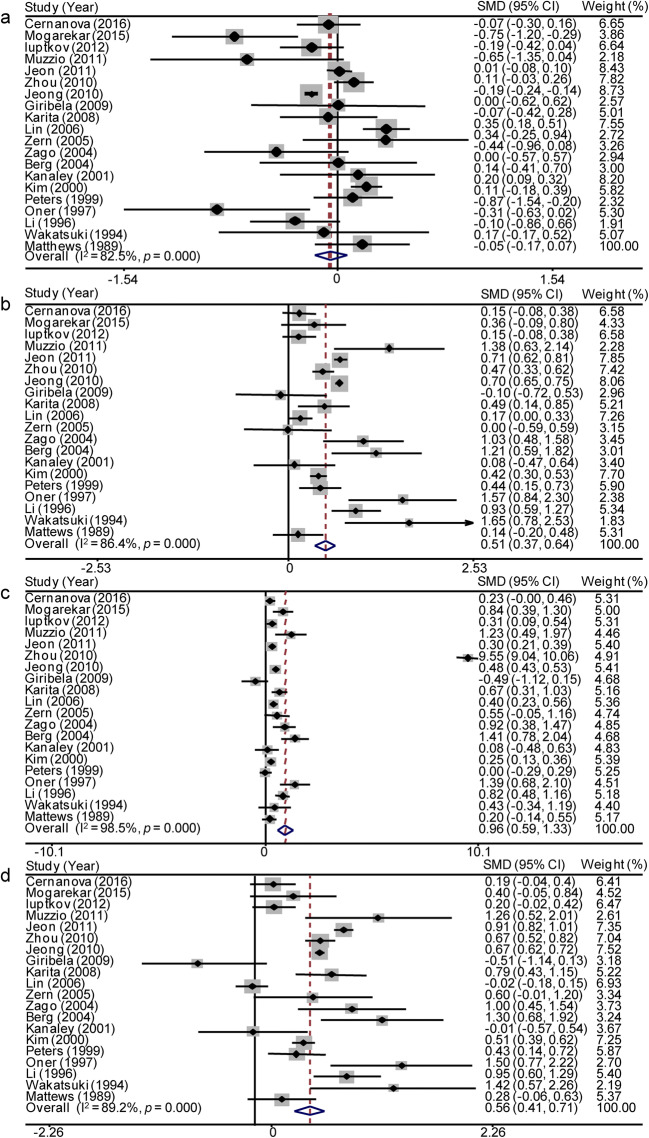
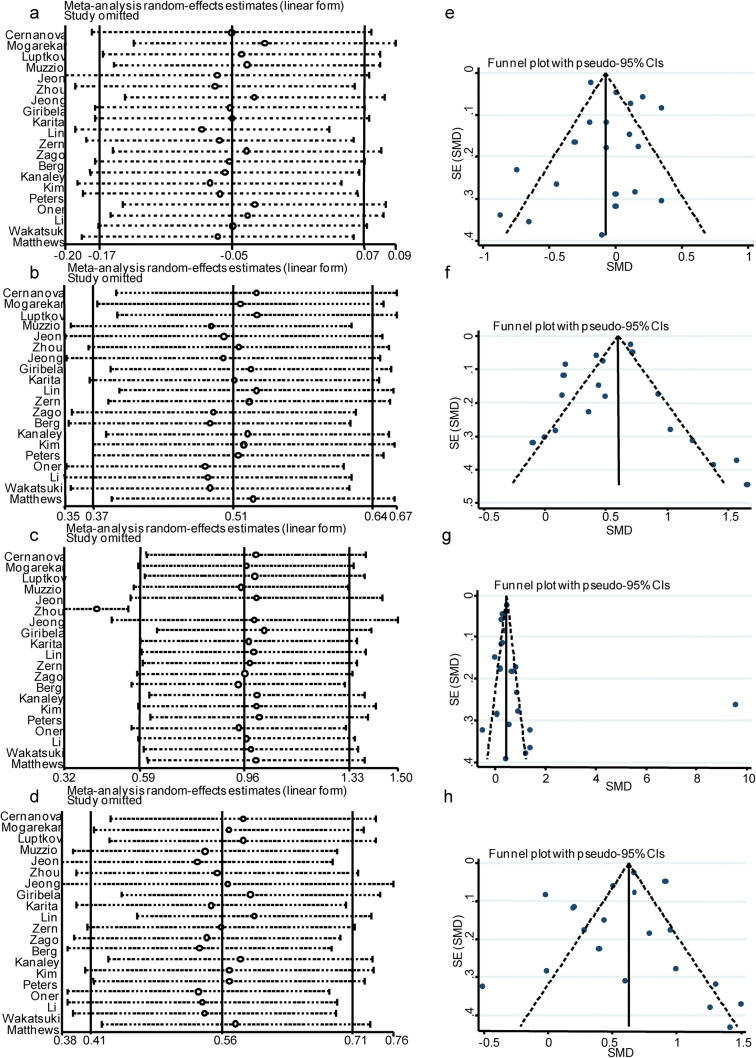
HDL-C levels in the postmenopausal and premenopausal women extracted from the 20 studies were synthesized. Due to heterogeneity, which was > 70%, we conducted a meta-analysis of the 20 included studies using the random effects model to identify differences in HDL-C levels between postmenopausal women and premenopausal women (Fig. 2a). The HDL-C levels were not significantly different between postmenopausal and premenopausal women (SMD = − 0.053, 95% CI − 0.171 to 0.066, p = 0.383). Significant heterogeneity was observed among the available studies (p < 0.001 and I2 = 82.5% for the heterogeneity test). A meta-regression analysis was conducted to identify potential moderators of this heterogeneity, but associations between HDL-C levels and covariates, including the publication year, sample size, and country, were not observed (Table 2). We performed a subgroup analysis after stratification by publication year, sample size, and country (Table 3). In the subgroups with a sample size ≤ 500 and non-Asian countries, the I2 value was significantly reduced by more than 20%. In the sensitivity analysis, no studies which were sequentially omitted affected the association between the menopause status and HDL-C levels (Fig. 3a). A funnel plot was generated and Egger’s test was performed to assess the publication bias of the studies included in this meta-analysis. The shapes of the funnel plots did not reveal any obvious asymmetry and Egger’s test revealed β = 0.390 and p = 0.616. Both the appearance of the funnel plot and Egger’s test results confirmed a lack of significant publication bias (Fig. 3e).
Fig. 2.
Forest plots of the meta-analysis of HDL-C (a), LDL-C (b), TG (c), and TC (d) levels using random effects models. SMD standardized mean difference, CI confidence interval
Table 2.
The meta-regression analysis of HDL-C, LDL-C, TG, and TC levels
| 95% CI | ||||
|---|---|---|---|---|
| Β | Lower CI limit | Upper CI limit | p | |
| HDL-C | ||||
| Publication year | 0.094 | − 0.123 | 0.312 | 0.360 |
| Sample size | 0.000 | − 0.000 | 0.000 | 0.823 |
| Country | 0.096 | − 0.118 | 0.311 | 0.344 |
| Study quality | 0.064 | − 0.045 | 0.173 | 0.235 |
| LDL-C | ||||
| Publication year | − 0.014 | − 0.045 | 0.017 | 0.350 |
| Sample size | 0.000 | − 0.000 | 0.000 | 0.734 |
| Country | − 0.042 | − 0.120 | 0.036 | 0.276 |
| Study quality | − 0.067 | − 0.244 | 0.109 | 0.433 |
| TG | ||||
| Publication year | 0.044 | − 0.094 | 0.183 | 0.508 |
| Sample size | 0.000 | − 0.001 | 0.001 | 0.951 |
| Country | − 0.187 | − 0.519 | 0.145 | 0.252 |
| Study quality | 1.090 | 0.454 | 1.726 | 0.002* |
| TC | ||||
| Publication year | − 0.013 | − 0.045 | 0.019 | 0.395 |
| Sample size | 0.000 | − 0.000 | 0.000 | 0.826 |
| Country | − 0.024 | − 0.106 | 0.059 | 0.558 |
| Study quality | − 0.036 | − 0.223 | 0.152 | 0.693 |
The correlation coefficients of publication year, sample size, country, and study quality with 95% confidence intervals (CI) for the effect size of HDL-C, LDL-C, TG, and TC of this meta-analysis are presented and considered significant to be heterogeneity sources with p < 0.05(*)
Table 3.
The subgroup analyses of HDL-C, LDL-C, TG, and TC levels
| N | SMD (95% CI) (mg/dl) | p | I2 (%) | Weight (%) | |
|---|---|---|---|---|---|
| HDL-C | |||||
| Total | 20 | − 0.053 (− 0.171, 0.066) | 0.383 | 82.5 | 100.00 |
| Sample size | |||||
| > 500 | 5 | 0.089 (− 0.109, 0.286) | 0.379 | 95.0 | 40.73 |
| ≤ 500 | 15 | − 0.147 (− 0.296, 0.001) | 0.052 | 47.1 | 59.27 |
| Year | |||||
| ≥ 2008 | 9 | − 0.119 (− 0.247, 0.010) | 0.070 | 77.8 | 51.89 |
| < 2008 | 11 | 0.036 (− 0.137, 0.210) | 0.681 | 65.4 | 48.11 |
| Country | |||||
| Asia | 10 | − 0.024 (− 0.190, 0.142) | 0.775 | 90.7 | 58.91 |
| Not Asia | 10 | − 0.105 (− 0.242, 0.032) | 0.134 | 20.9 | 41.09 |
| LDL-C | |||||
| Total | 20 | 0.507 (0.373, 0.642) | < 0.001 | 86.4 | 100.00 |
| Sample size | |||||
| > 500 | 5 | 0.506 (0.332, 0.680) | < 0.001 | 93.3 | 38.29 |
| ≤ 500 | 15 | 0.553 (0.323, 0.783) | < 0.001 | 77.5 | 61.71 |
| Year | |||||
| ≥ 2008 | 9 | 0.467 (0.304, 0.631) | < 0.001 | 86.6 | 51.27 |
| < 2008 | 11 | 0.584 (0.353, 0.815) | < 0.001 | 80.6 | 48.73 |
| Country | |||||
| Asia | 10 | 0.529 (0.373, 0.686) | < 0.001 | 88.9 | 57.36 |
| Non-Asian country | 10 | 0.490 (0.215, 0.766) | < 0.001 | 78.4 | 42.64 |
| TG | |||||
| Total | 20 | 0.958 (0.587, 1.330) | < 0.001 | 98.5 | 100.00 |
| Sample size | |||||
| > 500 | 5 | 2.094 (1.261, 2.926) | < 0.001 | 99.7 | 26.47 |
| ≤ 500 | 15 | 0.529 (0.308, 0.749) | < 0.001 | 75.7 | 73.53 |
| Year | |||||
| ≥ 2008 | 9 | 1.436 (0.727, 2.146) | < 0.001 | 99.4 | 45.64 |
| < 2008 | 11 | 0.500 (0.291, 0.710) | < 0.001 | 76.2 | 54.36 |
| Country | |||||
| Asia | 10 | 0.467 (0.190, 0.744) | < 0.001 | 99.3 | 49.29 |
| Non-Asian country | 10 | 1.416 (0.842, 1.991) | 0.001 | 79.0 | 50.71 |
| TC | |||||
| Total | 20 | 0.563 (0.415, 0.711) | < 0.001 | 89.2 | 100.00 |
| Sample size | |||||
| > 500 | 5 | 0.555 (0.331, 0.779) | < 0.001 | 96.0 | 36.09 |
| ≤ 500 | 15 | 0.592 (0.363, 0.822) | < 0.001 | 77.5 | 63.91 |
| Year | |||||
| ≥ 2008 | 9 | 0.535 (0.352, 0.718) | < 0.001 | 89.5 | 50.32 |
| < 2008 | 11 | 0.634 (0.374, 0.894) | < 0.001 | 85.2 | 49.68 |
| Country | |||||
| Asia | 10 | 0.610 (0.427, 0.793) | < 0.001 | 92.1 | 56.08 |
| Non-Asian country | 10 | 0.511 (0.229, 0.793) | < 0.001 | 79.6 | 43.92 |
SMD, standardized mean difference; CI, confidential interval
Fig. 3.
Sensitivity analysis and funnel plot of the meta-analysis of HDL-C, LDL-C, TG, and TC levels. Sensitivity analysis (a, b, c, d) and funnel plots (e, f, g, h) were performed to examine the heterogeneity of this meta-analysis. SMD standardized mean difference, CI confidence interval
Association between menopause and the levels of other lipid components
Higher LDL-C (SMD = 0.507, 95% CI = 0.373 to 0.642, p < 0.001) (Fig. 2b), TG (SMD = 0.958, 95% CI = 0.587 to 1.330, p < 0.001) (Fig. 2c), and TC levels (SMD = 0.563, 95% CI = 0.415 to 0.711, p < 0.001) (Fig. 2d) were detected in postmenopausal women than in premenopausal women. No associations between the modifiers publication year, sample size, country, and LDL-C, or TC levels were identified (Table 2) in meta-regression analyses. However, TG levels tended to be affected by study quality (Table 2). The subgroups of a sample size of ≤ 500, year < 2008, and non-Asian countries presented a greater than 20% decrease in the I2 value. For TC and LDL-C levels, no subgroup showed a significant reduction in the I2 value (Table 3). The sensitivity analysis revealed a decrease in the random effects estimates after omitting the study conducted by Zhou et al., [5] indicating that this study was a potential source of heterogeneity (Fig. 3c). The funnel plot of the meta-analysis of TG levels showed apparent asymmetry (Fig. 3g), although Egger’s test did not reveal significant publication bias (β = 2.779, p = 0.281). After removing this study, no association was observed between TG levels and study quality in the meta-regression analysis (β = − 0.084, p = 0.467), but the synthesized higher TG levels observed in postmenopausal women compared with premenopausal controls were not affected (SMD = 0.433, 95% CI 0.319 to − 0.548, p < 0.001). In the sensitivity analysis, no studies which were sequentially omitted affected the association between the menopause status and LDL-C and TC levels (LDL-C: Fig. 3b; TC: Fig. 3d). Both the appearance of the funnel plot (LDL-C: Fig. 3f; TC: Fig. 3h) and Egger’s test results (LDL-C: β = − 1.015, p = 0.242; TC: β = − 0.720, p = 0.465) showed no significant publication bias in the meta-analysis of LDL-C and TC levels in postmenopausal and premenopausal women.
Discussion
To the best of our knowledge, this is the first meta-analysis conducted to identify an association between the menopause status and blood lipid profiles. Based on the results of our study, HDL-C levels in postmenopausal women were not significantly different from those in premenopausal women. This finding is consistent with some large population studies [5, 19], which indicated that menopause did not affect HDL-C levels. In the subgroup analysis, sample size ≤ 500 and non-Asian countries affected the heterogeneity of this meta-analysis. Although the value was not significant, a small sample size potentially affected the association between HDL-C levels and menopause status (Table 3). The difference in HDL-C levels between pre- and postmenopausal women potentially originates from the analysis of cross-sectional data. Wang et al. [32] identified significantly lower HDL-C levels in postmenopausal women than in premenopausal women in longitudinal studies but not in cross-sectional studies. Due to insufficient evidence of HDL-C changes during the menopause transition, more large population-based prospective studies are crucial to determine the association between HDL-C levels and the menopause transition.
Unlike HDL-C levels, LDL-C, TG, and TC levels were significantly increased in postmenopausal women compared with premenopausal controls. A recent large population-based study also observed significantly higher LDL-C and TC levels in postmenopausal women than in premenopausal women, while HDL-C levels were not different between postmenopausal and premenopausal women [33]. Menopause-induced proatherogenic lipid profile changes may mainly affect LDL-C, TG, and TC levels.
The study quality of the included articles was not associated with HDL-C, LDL-C, TG, and TC in meta-regression analyses (Table 2). We strictly followed the inclusion and exclusion criteria to include relevant studies in our meta-analysis. No significant source of heterogeneity was identified and no low-quality studies were included. The sensitivity analysis identified the study conducted by Zhou et al. [5] as a source of heterogeneity in the pooled analysis of TG levels (Fig. 3c), but its omission did not affect the conclusion of increased TG levels in postmenopausal women compared with premenopausal women.
Besides quantitative HDL-C levels, novel metrics for evaluating HDL quality strongly pointed to these levels being independent cardiovascular risk factors. Compared with HDL levels of premenopausal women, less oxidative resistance of HDL among postmenopausal women was demonstrated, together with an impaired ability to inhibit LDL oxidation [25]. On the other hand, large HDL particle concentrations and HDL-mediated cholesterol efflux capacity has been reported to be increased in the early menopausal transition [34]. These novel HDL metrics may explain the pathophysiological changes in late perimenopausal and postmenopausal women, which needs to be confirmed by acquiring additional evidence and performing further studies.
Estrogen, which is the most important female sex hormone, displays decreased levels in postmenopausal women. Hormone replacement therapy and estrogen receptor modulators have been reported to improve lipoprotein levels in postmenopausal populations [35, 36]. Additional studies are needed to explore the effects of sex hormones on postmenopausal lipid functions.
Strengths and limitations
Our study is, as far as we know, the first meta-analysis to compare the association of HDL-C levels between healthy postmenopausal and premenopausal women, which increases the knowledge base regarding the potential association between HDL-C levels and the menopause status. One of the limitations of this study is that most of the participants came from Asia because of large sample size–based Korean and Japanese studies; thus, more clinical data derived from further studies from Western countries are required to better represent global populations. The definition of menopause status is based on the time from the last bleeding pattern, regardless of sex hormone levels. Additionally, although some studies have used FSH levels as a criterion to confirm menopause status, consensus on the cut-off values for postmenopausal FSH levels has not as yet been established [22, 28]. Another limitation is the small number of included cohort studies and sample sizes; therefore, further prospective studies would help to improve the inferences of causality.
Conclusions
Based on the evidence of this meta-analysis, HDL-C levels are not associated with menopause status. Higher LDL-C, TG, and TC levels are observed in postmenopausal women than in premenopausal women. Prospective studies with large sample sizes examining HDL-C levels and HDL functions in women with different menopause statuses are needed.
Electronic supplementary material
(DOCX 44 kb).
Authors’ contributions
HWL mainly performed the search for the relevant articles, extracted the data, performed the statistical analyses, and wrote the paper. RLS participated in drafting the paper and settling any conflicts regarding article inclusion. QC helped search for articles, extract the data from the included articles, and evaluate the quality of the articles by assessing the methodological quality using the Cross-Sectional/Prevalence Study Quality tool. QG and JJW generated the figures and tables. LML provided guidance for the statistical analyses. YLZ was the chief investigator who directed this study. All authors read and approved the final manuscript.
Funding information
The authors disclose the receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the Natural Science Foundation of China (No. 81970388), the Key S&T Special Project of Guangzhou (201704020044), and the Yat-sen Scientific Research Launching Project (YXQH201804).
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El Khoudary SR. Gaps, limitations and new insights on endogenous estrogen and follicle stimulating hormone as related to risk of cardiovascular disease in women traversing the menopause: a narrative review. Maturitas. 2017;104:44–53. doi: 10.1016/j.maturitas.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 3.Matthan NR, Jalbert SM, Lamon-Fava S, Dolnikowski GG, Welty FK, Barrett HR, Schaefer EJ, Lichtenstein AH. TRL, IDL, and LDL apolipoprotein B-100 and HDL apolipoprotein A-I kinetics as a function of age and menopausal status. Arterioscler Thromb Vasc Biol. 2005;25(8):1691–1696. doi: 10.1161/01.ATV.0000172629.12846.b8. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, McNamara JR, Fruchart JC, Luc G, Bard JM, Ordovas JM, Wilson PW, Schaefer EJ. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res. 1996;37(9):1886–1896. doi: 10.1016/S0022-2275(20)37553-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhou JL, Lin SQ, Shen Y, Chen Y, Zhang Y, Chen FL. Serum lipid profile changes during the menopausal transition in Chinese women: a community-based cohort study. Menopause. 2010;17(5):997–1003. doi: 10.1097/gme.0b013e3181dbdc30. [DOI] [PubMed] [Google Scholar]
- 6.Cho EJ, Min YJ, Oh MS, Kwon JE, Kim JE, Lee WS, Lee KJ, Kim SW, Kim TH, Kim MA, Kim CJ, Ryu WS. Effects of the transition from premenopause to postmenopause on lipids and lipoproteins: quantification and related parameters. Korean J Intern Med. 2011;26(1):47–53. doi: 10.3904/kjim.2011.26.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cernanova V, Dankova Z, Luptakova L, Cvicelova M, Sivakova D. The association of Asn453Ser polymorphism in CYP1B1 gene with selected somatic and biochemical variables in Slovak women of different menopause status. Menopause. 2016;23(5):577–583. doi: 10.1097/GME.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 8.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. Jama. 1986;256(20):2835–2838. doi: 10.1001/jama.1986.03380200073024. [DOI] [PubMed] [Google Scholar]
- 9.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles atherosclerosis study. Atherosclerosis. 2007;195(1):e191–e196. doi: 10.1016/j.atherosclerosis.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 10.El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol. 2016;10(4):962–969. doi: 10.1016/j.jacl.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.AHRQ . Comparative effectiveness reviews. Rockville: AHRQ; 2008. [Google Scholar]
- 13.Guan XL, Wang H, Huang HS, Meng L. Prevalence of dysphagia in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2015;36(5):671–681. doi: 10.1007/s10072-015-2067-7. [DOI] [PubMed] [Google Scholar]
- 14.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 15.Solmi M, Veronese N, Favaro A, Santonastaso P, Manzato E, Sergi G, Correll CU. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237–252. doi: 10.1016/j.psyneuen.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Mogarekar MR, Kulkarni SK. Small dense low density lipoprotein cholesterol, paraoxonase 1 and lipid profile in postmenopausal women: quality or quantity? Arch Med Res. 2015;46(7):534–538. doi: 10.1016/j.arcmed.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Luptakova L, Sivakova D, Sramekova D, Cvicelova M. The association of cytochrome P450 1B1 Leu432Val polymorphism with biological markers of health and menopausal symptoms in Slovak midlife women. Menopause. 2012;19(2):216–224. doi: 10.1097/gme.0b013e3182281b54. [DOI] [PubMed] [Google Scholar]
- 18.Muzzio ML, Miksztowicz V, Repetto EM, Brites F, Berg G, Schreier L. Increased MMP-2 in healthy postmenopausal women. Ann Clin Biochem. 2012;49(Pt 1):75–79. doi: 10.1258/acb.2011.011041. [DOI] [PubMed] [Google Scholar]
- 19.Jeon YK, Lee JG, Kim SS, Kim BH, Kim SJ, Kim YK, Kim IJ. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr J. 2011;58(2):87–93. doi: 10.1507/endocrj.K10E-297. [DOI] [PubMed] [Google Scholar]
- 20.Jeong IK, Cho SW, Kim SW, Choi HJ, Park KS, Kim SY, Lee HK, Cho SH, Oh BH, Shin CS. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif Tissue Int. 2010;87(6):507–512. doi: 10.1007/s00223-010-9427-3. [DOI] [PubMed] [Google Scholar]
- 21.Giribela AH, Melo NR, Latrilha MC, Baracat EC, Maranhao RC. HDL concentration, lipid transfer to HDL, and HDL size in normolipidemic nonobese menopausal women. Int J Gynaecol Obstet. 2009;104(2):117–120. doi: 10.1016/j.ijgo.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Karita K, Yamanouchi Y, Takano T, Oku J, Kisaki T, Yano E. Associations of blood selenium and serum lipid levels in Japanese premenopausal and postmenopausal women. Menopause. 2008;15(1):119–124. doi: 10.1097/gme.0b013e31806bf32c. [DOI] [PubMed] [Google Scholar]
- 23.Lin WY, Yang WS, Lee LT, Chen CY, Liu CS, Lin CC, Huang KC. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes. 2006;30(6):912–917. doi: 10.1038/sj.ijo.0803240. [DOI] [PubMed] [Google Scholar]
- 24.Zern TL, Wood RJ, Greene C, West KL, Liu Y, Aggarwal D, Shachter NS, Fernandez ML. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135(8):1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 25.Zago V, Sanguinetti S, Brites F, Berg G, Verona J, Basilio F, Wikinski R, Schreier L. Impaired high density lipoprotein antioxidant activity in healthy postmenopausal women. Atherosclerosis. 2004;177(1):203–210. doi: 10.1016/j.atherosclerosis.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Berg G, Mesch V, Boero L, Sayegh F, Prada M, Royer M, Muzzio ML, Schreier L, Siseles N, Benencia H. Lipid and lipoprotein profile in menopausal transition. Effects of hormones, age and fat distribution. Horm Metab Res. 2004;36(4):215–220. doi: 10.1055/s-2004-814450. [DOI] [PubMed] [Google Scholar]
- 27.Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, Sagendorf KS, Feiglin D, Jaynes EB, Meyer RA, Weinstock RS. Abdominal fat distribution in pre- and postmenopausal women: the impact of physical activity, age, and menopausal status. Metabolism. 2001;50(8):976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- 28.Kim CJ, Kim TH, Ryu WS, Ryoo UH. Influence of menopause on high density lipoprotein-cholesterol and lipids. J Korean Med Sci. 2000;15(4):380–386. doi: 10.3346/jkms.2000.15.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters HW, Westendorp IC, Hak AE, Grobbee DE, Stehouwer CD, Hofman A, Witteman JC. Menopausal status and risk factors for cardiovascular disease. J Intern Med. 1999;246(6):521–528. doi: 10.1046/j.1365-2796.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 30.Oner P, Mutlu-Turkoglu U, Omer B. Evaluation of the changes in serum lipid profile and ferritin concentrations in relation to body ascorbic acid status in healthy pre- and postmenopausal women. J Nutr Sci Vitaminol (Tokyo) 1997;43(1):1–9. doi: 10.3177/jnsv.43.1. [DOI] [PubMed] [Google Scholar]
- 31.Wakatsuki A, Sagara Y. Lipoprotein metabolism in postmenopausal and oophorectomized women. Obstet Gynecol. 1995;85(4):523–528. doi: 10.1016/0029-7844(94)00452-J. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Ferreira DLS, Nelson SM, Sattar N, Ala-Korpela M, Lawlor DA. Metabolic characterization of menopause: cross-sectional and longitudinal evidence. BMC Med. 2018;16(1):17. doi: 10.1186/s12916-018-1008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Kat AC, Dam V, Onland-Moret NC, Eijkemans MJ, Broekmans FJ, van der Schouw YT. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med. 2017;15:2. doi: 10.1186/s12916-017-0841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Khoudary SR, Hutchins PM, Matthews KA, Brooks MM, Orchard TJ, Ronsein GE, Heinecke JW. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. J Clin Endocrinol Metab. 2016;101(9):3419–3428. doi: 10.1210/jc.2016-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller VT. Effects of estrogen or estrogen/ progestin regimens on heart disease risk factors in postmenopausal women. Jama. 1995;273(3):199. doi: 10.1001/jama.1995.03520270033028. [DOI] [PubMed] [Google Scholar]
- 36.Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, Shah AS, Anderson PW. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. Jama. 1998;279(18):1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 44 kb).
Data Availability Statement
Not applicable.