Abstract
Introduction
Daratumumab is a CD38-targeting monoclonal antibody that has demonstrated clinical benefit for multiple myeloma. Daratumumab inhibition of CD38, which is expressed on immune cell populations and cardiomyocytes, could potentially affect cardiac function. This QTc substudy of the phase 2 CENTAURUS study investigated the potential effect of intravenous daratumumab monotherapy on QTc prolongation and other electrocardiogram (ECG) parameters, including concentration-QTc effect modeling.
Methods
Patients had intermediate- or high-risk smoldering multiple myeloma. Patients with QT interval corrected by Fridericia’s formula (QTcF) > 470 ms, QRS interval ≥ 110 ms, or PR interval ≥ 200 ms were excluded. Triplicate ECGs were collected at screening, Dose 1, and Dose 8. Analyses of on-treatment ECGs were conducted with a time-matched baseline (primary analysis). By time-point, pharmacokinetic-pharmacodynamic (PK/PD), and outlier analyses were conducted.
Results
Of 123 patients in CENTAURUS, 31 were enrolled in the QTc substudy. Daratumumab produced a small increase in heart rate (5–12 beats per minute) of unclear significance. There was a small but clinically insignificant effect on QTc, as measured by both time-matched time-point and PK/PD analyses. The primary analysis demonstrated a maximum mean increase in QTcF of 9.1 ms (90% 2-sided upper confidence interval [CI], 14.1 ms). The primary PK/PD analysis predicted a maximum QTcF increase of 8.5 ms (90% 2-sided upper CI, 13.5 ms). No patient had an abnormal U wave, a new QTcF > 500 ms, or > 60 ms change from baseline for QTcF.
Conclusion
Analysis of ECG intervals and concentration-QTc relationships showed a small but clinically insignificant effect of daratumumab.
Trial Registration
ClinicalTrials.gov Identifier: NCT02316106.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-020-01601-w.
Keywords: Daratumumab, Monoclonal antibody, Pharmacokinetic-pharmacodynamic analysis, QTc substudy, Smoldering multiple myeloma
Key Summary Points
| Why carry out this study? |
| There are a limited number of QT studies of intravenous drugs administered over 3 h in duration. |
| Daratumumab, a monoclonal antibody administered intravenously, is used to treat multiple myeloma; it targets CD38, a 45-kD transmembrane glycoprotein that is highly expressed on myeloma cells. |
| CD38 is also expressed on cardiomyocytes, and daratumumab therapy may have an effect on patient cardiac function; therefore, a QTc substudy was conducted using patients from the phase 2 CENTAURUS study, which evaluated daratumumab 16 mg/kg administered intravenously in patients with intermediate- or high-risk smoldering multiple myeloma. |
| What was learned from the study? |
| Primary time-matched time-point and pharmacokinetic-pharmacodynamic analyses showed a small but clinically insignificant effect of daratumumab monotherapy on QTc in patients with intermediate- or high-risk smoldering multiple myeloma, which was confirmed in pre-specified sensitivity analyses. |
| The small increase in QTcF observed in this substudy was of similar magnitude to that reported for many other approved oncologic therapies. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13342100.
Introduction
Daratumumab is a human IgG1κ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action [1–6]. Daratumumab monotherapy achieves deep and durable responses in patients with heavily treated relapsed or refractory multiple myeloma (MM) and has a favorable safety profile [7, 8]. In a pooled final analysis of the daratumumab monotherapy studies GEN501 and SIRIUS, overall response rate was 30.4%, and median overall survival was 20.5 months [9]. After a median follow-up of approximately 3 years, no new safety signals were identified, and deep and durable responses were maintained in these heavily pre-treated, highly refractory patients. Daratumumab has also demonstrated clinical benefit in combination with standard-of-care regimens, inducing rapid, deep, and durable responses and significantly reducing the risk of disease progression or death by ≥ 50% [10–12].
In contrast to its low expression on normal lymphoid and myeloid cells [13], CD38 is consistently expressed at high levels in myeloma cells [14, 15]. Daratumumab is ideal in this regard for targeting and killing malignant cells. However, CD38 is also expressed in some tissues of nonhematopoietic origin, including cardiomyocytes [16]. Thus, inhibition of CD38 with daratumumab therapy could theoretically affect cardiovascular function and safety. Daratumumab inhibits the cyclase activity of CD38 [6], which is involved in the modulation of endoplasmic reticulum calcium release [17]. The resulting lower intracellular calcium concentrations could potentially affect the function of cardiomyocytes.
Based on the lack of cardiac safety data for daratumumab in MM patients at the time, eligibility criteria for daratumumab clinical trials typically excluded patients with clinically significant cardiovascular conditions [7, 8, 18]. For example, in the SIRIUS monotherapy trial, patients with myocardial infarction within 1 year, uncontrolled or unstable angina, congestive heart failure (New York Heart Association [NYHA] Class III or IV), arrhythmia (grade 2 or higher), or QT interval corrected by Fridericia’s formula (QTcF) > 470 ms were excluded from participation [8]. Nevertheless, initial observations from Part 2 of the phase 1 GEN501 trial [7], which evaluated daratumumab 8 and 16 mg/kg, revealed no clinically relevant changes in the QT interval corrected for heart rate.
Clinical studies of daratumumab have most commonly used the 16 mg/kg formulation that is administered intravenously (IV), with median duration of infusion being 7.0, 4.3, and 3.4 h for first, second, and subsequent infusions, respectively [19]. However, there are a limited number of QT studies focusing on IV drugs administered over such time periods. To investigate the potential effect of daratumumab monotherapy on QTc prolongation and other electrocardiogram (ECG) parameters, a QTc substudy was incorporated into CENTAURUS, a phase 2 study of daratumumab 16 mg/kg IV monotherapy in patients with intermediate- or high-risk smoldering MM (SMM). These patients represent an ideal study population, given the lack of confounding factors, such as known clinically significant cardiovascular sequelae (e.g., dehydration from hypercalcemia, anemia, or bone pain), and absence of prior therapies often observed in myeloma patients. Two different time-matched analyses were performed using different definitions of baseline. In addition, a pharmacokinetic-pharmacodynamic (PK/PD) analysis was performed using all patients with paired ECG and serum concentrations for daratumumab.
Methods
Study Design
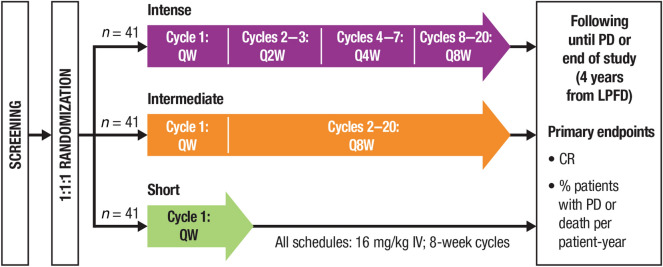
CENTAURUS (ClinicalTrials.gov Identifier: NCT02316106) is an ongoing randomized, open-label, multicenter, phase 2 study of daratumumab monotherapy in patients with intermediate- or high-risk SMM. The study design and primary results were recently published [20]. Briefly, patients with intermediate- or high-risk SMM were randomly assigned in a 1:1:1 ratio to daratumumab monotherapy 16 mg/kg IV in 8-week cycles with intense, intermediate, or short dosing schedules (Fig. 1). Stratification was based on the number of risk factors for progression to symptomatic MM (< 2 versus ≥ 2) per the criteria proposed by Dispenzieri [21]. Pre-infusion medications included methylprednisolone 60 to 100 mg, diphenhydramine 25 to 50 mg, acetaminophen 650 to 1000 mg, and montelukast 10 mg (optional).
Fig. 1.
CENTAURUS study design [20]. QW once weekly, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, IV intravenously, PD progressive disease, LPFD last patient first dose, CR complete response.
This figure was adapted from Fig. 1a from Landgren et al. (10.1038/s41375-020-0718-z) [20], which is licensed under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
The study protocol was approved by an independent ethics committee or institutional review board at each study site (see Supplementary Table 1 in the electronic supplementary material for details), and the study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Patients
Eligible patients had a confirmed diagnosis of intermediate- or high-risk SMM for < 5 years, defined as bone marrow plasma cells ≥ 10% to < 60% and ≥ 1 of the following: serum M-protein ≥ 3 g/dl (IgA ≥ 2 g/dl), urine M-protein > 500 mg/24 h, abnormal free light chain (FLC) ratio (< 0.126 or > 8) and serum M-protein < 3 g/dl but ≥ 1 g/dl, or absolute involved serum FLC ≥ 100 mg/l with an abnormal FLC ratio (< 0.126 or > 8, but not ≤ 0.01 or ≥ 100). The presence of clinically significant cardiac disease, including significant ischemic coronary disease, congestive heart failure (NYHA Class III or IV), unstable arrhythmias, myocardial infarction, or unstable angina within 6 months before randomization, a history of additional risk factors for torsades de pointes (e.g., electrolyte abnormalities, family history of Long QT Syndrome), a family history of sudden cardiac death before age 40, or screening QTcF > 470 ms also warranted patient exclusion from the overall study. Additional cardiac exclusion criteria for the QTc substudy included QRS interval ≥ 110 ms or PR interval ≥ 200 ms based on the mean of three tracings; pulse rate < 45 or > 90 beats per minute (bpm); skin condition likely to interfere with ECG electrode placement, breast implant, or thoracic surgery likely to cause abnormality in electrical conduction; and receipt of medications with known or possible risk of torsades de pointes within 4 weeks prior to ECG screening day. Additional exclusion criteria are presented in the Supplementary Methods section of the electronic supplementary material.
Endpoints and Assessments
For patients included in the QTc substudy, triplicate 12-lead ECGs were collected according to the following schedule: screening (within 28 days of randomization), Cycle 1 Dose 1 (pre-dose, end of infusion [EOI]), and Cycle 1 Dose 8 (at the end of weekly dosing; pre-dose, EOI, 1 h after EOI). ECGs were performed with the patient in the same supine resting position for ≥ 10 min before the ECG tracings (see Supplementary Methods in the electronic supplementary material). Pre-dose ECGs were obtained in the time period following administration of pre-infusion medications but before the daratumumab infusion. The 12-lead ECGs were collected in triplicate (three 10-s digital ECGs within 5 min) to provide a more robust point estimate of the value of that interval parameter. All three ECGs (or whatever number was available) derived data that were averaged to provide a single set of ECG intervals for each time point. The actual test time was intended to be consistent with a time point for both the screening and on-study ECGs to minimize variability in the results obtained. Similarly, for patients participating in ECG data collection, daratumumab infusions were administered beginning at approximately the same time of day for Doses 1 and 8 in Cycle 1. ECGs were collected prior to PK collections. Medications with known or possible risk of torsades de pointes were prohibited during Cycle 1.
ECGs were transmitted from the clinical sites via modem to a third-party central ECG laboratory for measurement of intervals, diagnostics of abnormalities, and review of ECG waveform morphology. Measurements of the RR, PR, QRS, and QT interval durations were performed, and heart rate and QTcF were calculated from the interval measurements. Morphological analyses were performed with regard to the ECG waveform interpretation provided by the central ECG laboratory cardiologist. “New” findings were defined as findings that were not present on any ECG recorded prior to the start of infusion on Cycle 1 Dose 1 but that were present on at least one on-treatment ECG.
Blood samples for patients enrolled in the QTc substudy were collected for analysis of daratumumab serum concentration at Cycle 1 Dose 1 (pre-dose, EOI) and Cycle 1 Dose 8 (pre-dose, EOI, 1 h after EOI).
Statistical Analysis
The ECG analysis included ECG data for all patients enrolled in the QTc substudy who had at least one available centrally evaluated baseline ECG and at least one centrally evaluated on-treatment ECG recorded on Cycle 1 Dose 1 or Cycle 1 Dose 8 (ECG population). For the PK/PD analysis, a time-matched serum concentration was also necessary (PK/PD population).
The PK/PD analysis was a concentration response analysis of baseline-adjusted QTc (ΔQTcF) against the independent variables of serum concentration of daratumumab and time. The primary endpoint was change from baseline in QTcF (see Supplementary Methods in the electronic supplementary material). A linear mixed-effects modeling approach was used to examine the relationship between the change from baseline in QTc intervals (QTcF) and serum concentration of daratumumab. The model included serum concentration, time (categorical), and treatment with random patient effects on serum concentration and the intercept included in the model. This model was used to estimate the population slope and the standard error of the slope of the relationship between change from baseline in QTc intervals and other ECG continuous parameters (QTcF) as well as serum concentration of daratumumab.
Central tendency, outlier, and sensitivity analyses are described in the Supplementary Methods in the electronic supplementary material.
Results
Patients
At the first interim analysis (clinical cutoff date of February 8, 2017), 123 patients with SMM were randomized into the phase 2 study; of these, 31 patients enrolled in the QTc substudy. Baseline demographics of the 31 patients enrolled in the substudy were consistent with those of the CENTAURUS study population as a whole [20]. The median (range) age was 64.0 (39–77) years (Table 1). Twenty-eight patients (90.3%) had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0, and the remaining three patients (9.7%) had an ECOG performance status score of 1. One patient withdrew study consent prior to the first dose of daratumumab. Thus, 31 patients had screening ECGs, but only 30 patients were included in the ECG analysis population. Twenty-nine patients in the QTc substudy were included in the PK/PD analysis; two patients were excluded because of lack of matching ECG and PK data points.
Table 1.
Demographics and baseline characteristics of patients in the QTc substudy
| All patients pooled (N = 31) | |
|---|---|
| Median (range) age, years | 64.0 (39–77) |
| Female, n (%) | 19 (61.3) |
| Race, n (%) | |
| White | 29 (93.5) |
| Black or African American | 1 (3.2) |
| Asian | 1 (3.2) |
| Median (range) weight, kg | 75.00 (50.0–115.3) |
| ECOG performance status score, n (%) | |
| 0 | 28 (90.3) |
| 1 | 3 (9.7) |
ECOG Eastern Cooperative Oncology Group
Time-Point Analyses
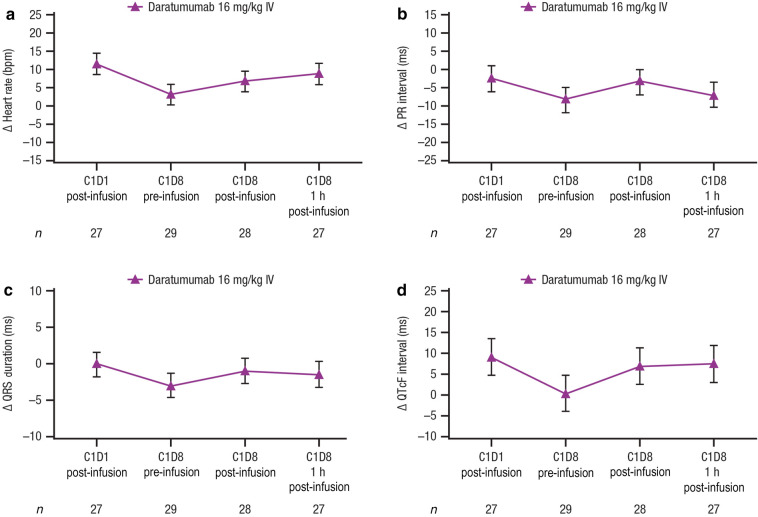
The time-point analysis showed a small increase in heart rate at all time points ranging from 3.3 to 11.5 bpm (Fig. 2a), but the clinical significance of this small change in this patient population is uncertain. There was no evidence of any effect of daratumumab on PR interval (Fig. 2b) or QRS duration (Fig. 2c). The time-matched time-point data analyses showed a small but clinically insignificant effect (upper confidence interval [CI] < 20 ms [22]) of daratumumab on cardiac repolarization (Fig. 2d; Supplementary Table 2 in electronic supplementary material). The peak magnitude for the QTcF change from baseline was 9.1 ms at Cycle 1 Dose 1 (90% 2-sided upper CI, 14.1 ms).
Fig. 2.
Time-point analyses. Mean change from baseline in a heart rate, b PR interval, c QRS duration, and d QTcF interval. Values are means ± 90% CIs; estimates and CIs are model based. PR interval time from onset of P wave to the start of the QRS complex, QRS duration interval of time between Q wave and S wave, QTcF interval QT interval corrected for heart rate using Fridericia’s formula, CI confidence interval, IV intravenously, bpm beats per minute, C Cycle, D Dose
Outlier Analyses
The outlier analysis used categorical cut points to determine whether any patient showed a signal of a potential effect on cardiac repolarization not manifested in the central tendency data. No patients had an abnormal U wave, an absolute QTcF > 500 ms, or a QTcF increase > 60 ms (Table 2). Two (6%) patients had the nonspecific finding of QTcF > 30 to 60 ms change from baseline. One patient had QTcF increase from a baseline value of 454 ms to 493 ms at Cycle 1 Dose 8 (1 h post-dosing), but no adverse events were reported. The second patient had a mean baseline QTcF of 416 ms that increased to 450 ms at Cycle 1 Dose 1 post-infusion. The second patient also had an adverse event of tachycardia that was reported at Cycle 1 Dose 1, which was considered an infusion-related reaction. Mean heart rate increased from 66 bpm pre-dose to 87 bpm post-dose, and the patient reported nausea and received metoclopramide, a medication known to prolong QTc. No patient experienced ventricular arrhythmias following QTc prolongation (> 30–60 ms increase in QTcF).
Table 2.
Outlier and morphology analyses
| All patients pooled (N = 31) | |
|---|---|
| Heart rate outliers, n (%) | |
| Tachycardic | 1 (3) |
| Bradycardic | 0 |
| PR interval outliers, n (%) | 0 |
| QRS interval outliers, n (%) | 0 |
| QT interval outliers, n (%) | |
| New > 500 ms | 0 |
| QTcF outliers, n (%) | |
| New > 500 ms | 0 |
| New > 480 ms | 0 |
| > 30–60 ms increase | 2 (6) |
| > 60 ms increase | 0 |
| Morphology, n (%) | |
| New atrial fibrillation | 0 |
| New atrial flutter | 0 |
| New abnormal U waves | 0 |
| New ST segment depression changes | 1 (3) |
| New ST segment elevation changes | 0 |
| New T wave inverted | 0 |
| New second-degree heart block | 0 |
| New third-degree heart block | 0 |
| New complete right bundle branch block | 0 |
| New complete left bundle branch block | 0 |
| New myocardial infarction | 0 |
QTcF QT interval corrected by Fridericia’s formula
“New” means not present at baseline and only seen after baseline
Morphological Analyses
One patient developed a new ECG morphologic event during the study: a 0.5-mm ST segment depression post-dose at Cycle 1 Dose 1 (Table 2). This nonspecific ECG finding was only seen in one of the three ECGs collected, was not suggestive of myocardial ischemia, and was not observed on subsequent ECGs.
Pharmacokinetic-Pharmacodynamic Analyses
In the PK/PD analysis, the slope of the relationship between QTcF change from baseline and daratumumab serum concentration was flat to slightly positive (not statistically significant with P value = 0.9359). The overall predicted QTcF change from baseline at the geometric mean Cmax (935.88 μg/ml) was 8.5 ms, with a 90% two-sided upper CI of 13.5 ms. Figure 3 illustrates the relationship between the exposure (serum concentration of daratumumab) and the effect (change in QTcF from baseline) for daratumumab for QTcF. These data also demonstrate a small but not clinically significant effect of daratumumab on cardiac repolarization. Analysis of the model data showed good model fit.
Fig. 3.
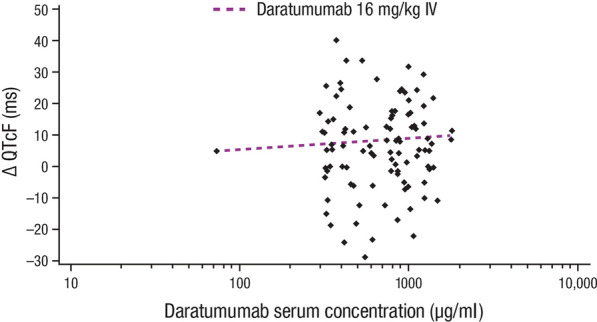
Relationship between QTcF change from baseline and serum concentration of daratumumab. Shown is a scatterplot of all QTcF change from baseline and daratumumab serum concentration pairs at each time point. Each of the 31 patients could have up to 4 pairs for each of the time points: Cycle 1 Dose 1 post-infusion, Cycle 1 Dose 8 pre-infusion, Cycle 1 Dose 8 post-infusion, and Cycle 1 Dose 8 1 h post-infusion. The prediction line was based on a mixed-effects regression model using concentration, treatment dose, and least square mean estimates of the time values. QTcF interval QT interval corrected for heart rate using Fridericia’s formula, IV intravenously
Sensitivity Analyses
Results from the sensitivity analyses are summarized in Table 3. The maximum QTcF change from baseline was 4.5 ms (90% 2-sided upper CI, 6.5 ms) at Cycle 1 Dose 1 post-dose in the time-point analysis. The PK/PD sensitivity analysis showed a flat to positive slope for the relationship between QTcF and plasma concentration (P = 0.3667). The overall predicted QTcF change from baseline at the geometric mean Cmax (935.88 μg/ml) was 2.7 ms, with a 90% two-sided upper CI of 6.5 ms.
Table 3.
Time-averaged mean change from baseline: sensitivity analyses
| Mean change from baseline | All patients pooled (N = 31) |
|---|---|
| Heart rate, bpm | 10.0 |
| PR interval, ms | −6.6 |
| QRS interval, ms | −1.7 |
| QTcF, ms | 1.9 |
bpm beats per minute, QTcF QT interval corrected by Fridericia’s formula.
Discussion
This QTc substudy was conducted to investigate whether daratumumab has an impact on QTc in patients with SMM. The study population was considered to be suitable for evaluation of QTc, as daratumumab was given as a single agent in a relatively healthy population of patients with SMM, compared with the GEN501 and SIRIUS studies, which enrolled patients with relapsed/refractory MM who were heavily pre-treated with immunomodulatory agents [7, 8]. The QTc substudy was designed to include centrally processed ECGs collected in triplicate, using a time-matched baseline, with stringent ECG inclusion criteria and prohibition of concomitant QT-prolonging medications. Additional measures undertaken to minimize variability in QTc assessments included using a centralized laboratory to interpret ECGs and employing a high-resolution on-screen caliper method with annotations to minimize inter-reader variability.
Results from the QTc substudy showed a small increase in heart rate (ranging from 3.3–11.5 bpm) of unclear significance. The contribution of discomfort caused by infusion-related reactions to increases in heart rate cannot be excluded; however, a strong association between infusion-related reactions and increased heart rate was not observed. There was no signal of any effect on atrioventricular conduction or cardiac depolarization, as measured by the PR and QRS interval durations. There were also no new clinically relevant morphological changes observed in the study. Overall, there was no evidence of acute cardiac toxicity at the end of weekly dosing with daratumumab monotherapy in patients with intermediate- or high-risk SMM. The long-term effects of daratumumab monotherapy on QTc prolongation and other ECG parameters in patients with intermediate- or high-risk SMM were not studied. However, to date, no evidence of daratumumab-associated QTc prolongation has emerged from daratumumab monotherapy or combination therapy studies with longer follow-up or from post-marketing data [19].
There was a small but not clinically significant effect of daratumumab on cardiac repolarization, as measured by the time-point and PK/PD analyses. In the primary analysis using the time-matched baseline ECGs collected primarily at screening, maximum mean increase in QTcF was 9.1 ms at Cycle 1 Dose 1, with a 90% two-sided upper CI of 14.1 ms. The primary PK/PD analysis predicted a maximum QTcF increase of 8.5 ms, with a 90% two-sided upper CI of 13.5 ms. These two analyses are in excellent agreement. Two patients met the outlier criterion of > 30 to 60 ms change in QTcF from baseline (mean change from baseline of 39 and 34 ms, respectively). However, daily variability in QTc in healthy patients is as high as 76 ms [23]; thus, the clinical significance of these QTc changes is unclear, so it is difficult to determine for an individual patient whether such nonspecific QTc changes represent normal variability or a drug-related effect.
The small increase in QTcF noted in this substudy may be related to the observed small increase in heart rate, as drug-induced changes in heart rate are known to overcorrect QTc [24]. Direct effects of daratumumab on cardiac repolarization seem unlikely. Most drugs that have been associated with QT prolongation block the rapid component of the hERG-encoded delayed rectifier potassium channel (IKR) in cardiac ventricular myocytes [24, 25], and large monoclonal antibodies such as daratumumab are thought to have a low likelihood of direct ion channel interactions [26]. In addition, the small increase in QTcF observed in this substudy was first detected at the end of the first daratumumab infusion. This relatively acute effect observed at Cycle 1 Dose 1 suggests that an indirect effect on cardiac repolarization due to effects on hERG trafficking may not be a responsible underlying mechanism, as such mechanisms do not produce QTc prolongation acutely [27]. Nevertheless, the potential effect of daratumumab on cardiomyocyte intracellular calcium concentrations, and thereby on cardiomyocyte function, via direction inhibition of CD38 cyclase activity [6, 17] cannot be excluded.
Even if the observed small increase in QTcF does represent a true, drug-induced effect on cardiac repolarization, the small increase in QTcF observed in this substudy is similar to that observed for moxifloxacin, a fluoroquinolone antibacterial agent [28, 29], and is well within the range of QTc increases observed for many oncologic agents [30–32]. For oncologic agents in general, a QTcF increase with an upper CI below 20 ms is generally not considered to be clinically significant [22]. Of note, none of the patients in this study met any of the pre-specified categorical outlier criteria (i.e., QTcF > 480 ms, QTcF increase from baseline > 60 ms, or new abnormal U waves).
A series of sensitivity analyses were performed using a time-averaged baseline instead of a time-matched baseline, because the time-matched baseline values were primarily collected at the screening visit, which may have taken place up to 28 days prior to randomization and the initiation of treatment. The prolonged interval between the collection of the baseline ECGs and the post-treatment ECGs could potentially confound analyses. Therefore, for the sensitivity analyses, the baseline was defined as the mean of the values of the triplicate ECGs recorded at Cycle 1 Dose 1 pre-dose, thus ensuring that, at least for the Cycle 1 Dose 1 post-dose ECGs, the baseline ECGs had been collected in close temporal proximity to the on-treatment ECGs. Results of the sensitivity analyses were generally consistent with the primary analyses and showed a slightly larger effect of daratumumab on heart rate and confirmed that daratumumab had no clinically significant effect on QTcF (maximum time-point QTcF change from baseline of 4.5 ms [90% 2-sided upper CI, 6.5 ms]; predicted maximum QTcF change from baseline of 2.7 ms [90% 2-sided upper CI, 6.5 ms] in the PK/PD sensitivity analysis). The possibility of a change in patients’ baseline ECG interval duration measurements in the weeks between the collection of the screening and on-therapy ECGs suggests that the results of the sensitivity analyses, with baseline data collected on the day of dosing, may be more reliable than the results of the primary analysis.
The results of this study have clearly demonstrated minimal effects of daratumumab therapy on heart rate and QTcF in patients with intermediate- or high-risk SMM. However, our findings should be interpreted with some caution because of several limitations of the experimental design. The changes in heart rate and QTcF, or lack thereof, observed with daratumumab therapy may have been influenced by other confounding variables, including the use of pre- and post-infusion medications, such as steroids, psychological factors, and the underlying SMM itself. Nevertheless, it could be argued that all patients with SMM in the study were exposed to similar pre- and post-infusion medications in comparable experimental settings. In addition, certain cardiac risk groups were excluded from this study; therefore, the data generated may not necessarily be representative of all patients with SMM in a “real-world” setting. Specifically, the impact of daratumumab on heart rate and QTcF in patients with baseline QTc > 470 ms and with clinically significant cardiac disease, including concurrent cardiac amyloidosis, is not addressed by this study. Daratumumab was administered via IV infusion in this study, and the effect of the recently developed subcutaneous co-formulation of daratumumab and recombinant human hyaluronidase PH20 on heart rate and QTcF in patients with SMM remains to be determined [33].
Conclusion
In this QTc substudy of the phase 2 CENTAURUS study in patients with intermediate- or high-risk SMM, a patient population without known disease or prior treatment sequelae, a review of ECG data revealed that daratumumab produced a small increase in heart rate (5–12 bpm). Both the primary analysis and the sensitivity analysis of ECG data and the PK/PD relationships for daratumumab also demonstrated a small but not clinically significant effect on QTcF. The increase in QTcF seen in the primary analyses is on the order of magnitude of the QTcF prolongation reported for many other approved oncologic therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors and Janssen gratefully acknowledge the valued scientific contribution from Prof. Dr. Ihsan Karadogan, who has passed away. He will be dearly missed by the research teams that he contributed to or led. The authors also thank the patients who participated in this study, the staff members at the study sites, the data and safety monitoring committee, and others who were involved in data collection and analyses (Kristen Lantz, PhD; Huabin Sun, MD; and ERT).
Funding
This study (ClinicalTrials.gov Identifier: NCT02316106) was funded by Janssen Research & Development, LLC. Advances in Therapy’s Rapid Service and Open Access fee were funded by Janssen Global Services, LLC.
Medical Writing and Editorial Assistance
Medical writing and editorial support were provided by J. Matthew Kuczmarski, PhD (MedErgy), and were funded by Janssen Global Services, LLC.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
AC, MM, IK, EL, RK, TN, and CH participated in acquisition/collection of data and analysis or interpretation of data. KW and CB participated in conception and design of the work being described in the publication. MJ, MTP, NWCJvdD, MT, and FD participated in acquisition/collection of data. MB, MC, SK, and HG participated in analysis or interpretation of data. RB participated in conception and design of the work being described in the publication and analysis or interpretation of data. MQ and PLC participated in conception and design of the work being described in the publication, acquisition/collection of data, and analysis or interpretation of data. All authors participated in drafting the publication and/or revising it critically for important intellectual content and approved the final version for submission.
Disclosures
Ajai Chari received grants or has grants pending from Janssen, Celgene, Novartis, Amgen, Pharmacyclics, Seattle Genetics, and Takeda; and received consulting fees or honoraria from Janssen, Celgene, Novartis, Amgen, Bristol Myers Squibb, Karyopharm, Sanofi Genzyme, Seattle Genetics, Oncopeptides, Takeda, Antengene, GlaxoSmithKline, and Secura Bio. Markus Munder received grants or has grants pending from Bristol Myers Squibb and Incyte; received consulting fees or honoraria from Janssen, Takeda, Bristol Myers Squibb, GlaxoSmithKline, Sanofi, and Celgene; and received payment for lectures including service on speakers bureaus from Janssen, Amgen, Takeda, Bristol Myers Squibb, and Celgene. Katja Weisel received grants or has grants pending from Amgen, Celgene, Janssen, and Sanofi; received consulting fees or honoraria from Amgen, Adaptive Biotechnologies, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Sanofi, and Takeda; and received fees for participating in review activities from Amgen and Karyopharm. Matthew Jenner received honoraria from and served on advisory boards for Janssen, Takeda, Celgene, Amgen, Sanofi, and AbbVie; and received consulting fees and speaker fees from Janssen. Ceri Bygrave received grants or has grants pending from Celgene; received consulting fees or honoraria, support for travel to meetings for the study, manuscript preparation, or other purposes, and payment for lectures including service on speakers bureaus from Janssen, Celgene, Amgen, and Takeda; and received fees for participation in review activities from Sanofi. Maria Teresa Petrucci served as a consultant or on an advisory board for and received honoraria from Janssen-Cilag, Celgene, Bristol Myers Squibb, Amgen, Takeda, and Sanofi. Mario Boccadoro received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and AbbVie; and received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and Mundipharma. Michele Cavo received honoraria from Amgen, Celgene, Janssen, and Bristol Myers Squibb. Niels W.C.J. van de Donk received research support from Janssen, Amgen, Celgene, Novartis, and Bristol Myers Squibb; and served on advisory boards for Janssen, Amgen, Celgene, Bristol Myers Squibb, Roche, Novartis, Bayer, Takeda, and Servier. Mehmet Turgut received an honorarium from Johnson & Johnson. Fatih Demirkan received research support from AbbVie, Janssen, and Amgen; served on advisory boards for Roche, Amgen, and AbbVie; and received travel and accommodation support from Janssen, AbbVie, and Amgen. Edward Libby served as a consultant for Pharmacyclics and received payment for expenses related to conducting this study. Robert Kleiman is the chief medical officer and an employee of eResearch Technology, Inc., which provided consulting and core lab services to Janssen. Steven Kuppens, Christoph Heuck, Ming Qi, and Pamela L. Clemens are employees of Janssen and hold equity in Johnson & Johnson. Rajesh Bandekar is an employee of Janssen, and Tobias Neff is a former employee of Janssen and current employee of GlaxoSmithKline. Hartmut Goldschmidt received grants and/or provision of Investigational Medicinal Product (IMP) from Amgen, Bristol Myers Squibb, Celgene, Chugai, Dietmar-Hopp-Stiftung, Janssen, Johns Hopkins University, and Sanofi; received research support (institutions) from Amgen, Bristol Myers Squibb, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp & Dohme, Sanofi, Mundipharma, Takeda, and Novartis; served on advisory boards (institutions) for Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, Janssen, Sanofi, and Takeda; and received honoraria for speakers bureaus from Academy2 GmbH & Co. KG, Agentur Hogg Robinson Germany, Amgen, ArtTempi, Beupdated Helbig Consulting and Research AG Schweiz, Bristol Myers Squibb, Celgene, Chop GmbH, Chugai, Congress Culture Concept Dr. S. Stocker München, Connectmedia Warschau/Polen, Dr. Hubmann Tumorzentrum München, FomF GmbH, GlaxoSmithKline, GWT Forschung und Innovation Dresden, Institut für Versorgungsforschung in der Onkologie GbR, Janssen, Kompetenznetz Maligne Lymphome (KML) e.V., MedConcept GmbH, Medical Communication GmbH, Münchner Leukämie Labor Prof. Haferlach, New Concept Oncology, Novartis, OmniaMed Deutschland, Onko Internetportal dkg-web GmbH, Sanofi, STIL Forschungs GmbH, and Veranstaltungskonzept Gesundheit Mechernich. Ihsan Karadogan has no conflicts of interest to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by local ethics committees (see Supplementary Table 1 in the electronic supplementary material for details) and conducted per the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. Informed consent was obtained from all individual participants included in the study.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Footnotes
Ihsan Karadogan is deceased.
References
- 1.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 2.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474.
- 3.Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–321. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor–mediated cross-linking. J Immunol. 2016;197:807–813. doi: 10.4049/jimmunol.1501351. [DOI] [PubMed] [Google Scholar]
- 5.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Donk NW, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 8.Lonial S, Weiss BM, Usmani S, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–1560. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 9.Usmani S, Nahi H, Weiss BM, et al. Safety and efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed and refractory multiple myeloma: final results from GEN501 and SIRIUS. Poster presented at: The 59th American Society for Hematology (ASH) Annual Meeting & Exposition; December 9–12, 2017; Atlanta, GA.
- 10.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 12.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk Res. 2001;25:1–12. doi: 10.1016/S0145-2126(00)00093-X. [DOI] [PubMed] [Google Scholar]
- 14.Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–488. doi: 10.1309/74R4TB90BUWH27JX. [DOI] [PubMed] [Google Scholar]
- 15.Santonocito AM, Consoli U, Bagnato S, et al. Flow cytometric detection of aneuploid CD38++ plasmacells and CD19+ B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk Res. 2004;28:469–477. doi: 10.1016/j.leukres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez JE, Deaglio S, Donati D, et al. Analysis of the distribution of human CD38 and of its ligand CD31 in normal tissues. J Biol Regul Homeost Agents. 1998;12:81–91. [PubMed] [Google Scholar]
- 17.Wei W, Graeff R, Yue J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca2+ signaling pathway. World J Biol Chem. 2014;5:58–67. doi: 10.4331/wjbc.v5.i1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–981. doi: 10.1182/blood-2017-05-785246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DARZALEX® (daratumumab) injection, for intravenous use [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2020.
- 20.Landgren CO, Chari A, Cohen YC, et al. Daratumumab monotherapy for patients with intermediate-risk or high-risk smoldering multiple myeloma: a randomized, open-label, multicenter, phase 2 study (CENTAURUS) Leukemia. 2020;34:1840–1852. doi: 10.1038/s41375-020-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dispenzieri A, Stewart AK, Chanan-Khan A, et al. Smoldering multiple myeloma requiring treatment: time for a new definition? Blood. 2013;122:4172–4181. doi: 10.1182/blood-2013-08-520890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarapa N, Britto MR. Challenges of characterizing proarrhythmic risk due to QTc prolongation induced by nonadjuvant anticancer agents. Expert Opin Drug Saf. 2008;7:305–318. doi: 10.1517/14740338.7.3.305. [DOI] [PubMed] [Google Scholar]
- 23.Morganroth J, Brown AM, Critz S, et al. Variability of the QTc interval: impact on defining drug effect and low-frequency cardiac event. Am J Cardiol. 1993;72:26B–31B. doi: 10.1016/0002-9149(93)90037-D. [DOI] [PubMed] [Google Scholar]
- 24.Isbister GK, Page CB. Drug induced QT prolongation: the measurement and assessment of the QT interval in clinical practice. Br J Clin Pharmacol. 2013;76:48–57. doi: 10.1111/bcp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redfern W, Carlsson L, Davis A, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/S0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 26.Shah RR, Morganroth J, Kleiman RB. ICH E14 Q&A(R2) document: commentary on the further updated recommendations on thorough QT studies. Br J Clin Pharmacol. 2015;79:456–464. doi: 10.1111/bcp.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Heyden MAG, Smits ME, Vos MA. Drugs and trafficking of ion channels: a new pro-arrhythmic threat on the horizon? Br J Pharmacol. 2008;153:406–409. doi: 10.1038/sj.bjp.0707618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florian JA, Tornoe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration—QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152–1162. doi: 10.1177/0091270010381498. [DOI] [PubMed] [Google Scholar]
- 29.Yan LK, Zhang J, Ng MJ, Dang Q. Statistical characteristics of moxifloxacin-induced QTc effect. J Biopharm Stat. 2010;20:497–507. doi: 10.1080/10543400903581945. [DOI] [PubMed] [Google Scholar]
- 30.Fradley MG, Moslehi J. QT prolongation and oncology drug development. Card Electrophysiol Clin. 2015;7:341–355. doi: 10.1016/j.ccep.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Locatelli M, Criscitiello C, Esposito A, et al. QTc prolongation induced by targeted biotherapies used in clinical practice and under investigation: a comprehensive review. Target Oncol. 2015;10:27–43. doi: 10.1007/s11523-014-0325-x. [DOI] [PubMed] [Google Scholar]
- 32.Porta-Sanchez A, Gilbert C, Spears D, et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc. 2017;6:e007724. doi: 10.1161/JAHA.117.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usmani SZ, Nahi H, Mateos MV, et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood. 2019;134:668–677. doi: 10.1182/blood.2019000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.