Abstract
Cyanosporus is a cosmopolitan brown-rot fungal genus, recognizable by blue-tinted basidiocarps. Species in this genus were usually treated as belonging to the Postia caesia complex, however, recent phylogenetic analyses showed that this complex represents an independent genus. During further studies on Cyanosporus, five new species were discovered based on morphological features and molecular data. Phylogenetic analyses of Cyanosporus were conducted using the internal transcribed spacer (ITS) regions, the large subunit of nuclear ribosomal RNA gene (nLSU), the small subunit of nuclear ribosomal RNA gene (nSSU), the small subunit of mitochondrial rRNA gene (mtSSU), the largest subunit of RNA polymerase II (RPB1), the second largest subunit of RNA polymerase II (RPB2), and the translation elongation factor 1-α gene (TEF); illustrated descriptions of the new species are provided. In addition, fifteen species previously belonging to the Postia caesia complex are transferred to Cyanosporus and proposed as new combinations.
Keywords: brown-rot fungi, multi-loci phylogeny, new species, Postia caesia complex, species identification
Introduction
Boletus caesius Schrad. was described based on material from Germany (Schrader, 1794), and this name was subsequently sanctioned by Fries (1821), who considered B. coeruleus Schumach. a synonym of Polyporus caesius (Schrad.) Fr. In 1881 Karsten transferred Boletus caesius to Postia Fr. as Postia caesia (Schrad.) P. Karst. (Karsten, 1881). Murrill (1907) transferred this species to Tyromyces P. Karst. as T. caesius (Schrad.) Murrill and later McGinty (1909) proposed a new monotypic genus Cyanosporus McGinty for Polyporus caesius, based on its cyanophilous basidiospores, but Cyanosporus caesius was not widely used in subsequent studies (Donk, 1960; Jahn, 1963; Lowe, 1975), while Tyromyces caesius was commonly used. Later, Postia caesia was widely used (Papp, 2014). David (1974, 1980) described another two species: P. luteocaesia (A. David) Jülich and P. subcaesia (A. David) Jülich from Europe besides Postia caesia (Schrad.) P. Karst. Jahn (1979) noted that David’s P. subcaesia included many forms and introduced P. subcaesia “f. minor,” which was described as P. alni Niemelä & Vampola by Niemelä et al. (2001). Subsequently, Pieri and Rivoire (2005) introduced the fifth European species, P. mediterraneocaesia M. Pieri & B. Rivoire in the Postia caesia complex. Papp (2014) provided a detailed nomenclatural review on the Postia caesia complex and proposed the subgenus Postia subg. Cyanosporus (McGinty) V. Papp for this complex which included five species viz., P. alni, P. caesia, P. luteocaesia, P. mediterraneocaesia and P. subcaesia. Miettinen et al. (2018) focused on the taxonomy of the Postia caesia complex based on morphological features and molecular evidence and increased the species number of the Postia caesia complex from 10 to 24. In their study, they only focused on the species concept of the Postia caesia complex, the taxonomic status of this complex among Postia and related genera are not mentioned.
Shen et al. (2019) carried out a comprehensive study on Postia and related genera and confirmed that the genus Cyanosporus is an independent genus rather than subgenus which containing 12 accepted species including seven new species of the Postia caesia complex. Furthermore, phylogenetic analyses showed that Cyanosporus belongs to the antrodia clade (Shen et al., 2019). Morphologically, Cyanosporus differs from other related genera by its bluish basidiocarps, usually narrow allantoid and thin- to slightly thick-walled basidiospores (McGinty, 1909; Shen et al., 2019). In the current study, the phylogenetic analysis of Cyanosporus was carried out based on the combined sequence dataset of ITS + nLSU + nSSU + mtSSU + RPB1 + RPB2 + TEF rRNA gene regions. Combining morphological characters and molecular evidence, thirty-one species belonging to the Postia caesia complex are now recognized in Cyanosporus, including five new species and fifteen new combinations.
Materials and Methods
Taxa Sampling and Morphological Study
The examined specimens were deposited at the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC) with some duplicates at the Institute of Applied Ecology, Chinese Academy of Sciences (IFP). Morphological descriptions and abbreviations used in this study following Liu et al. (2019); Sun et al. (2020).
DNA Extraction and Molecular Analyses
The procedures for DNA extraction and polymerase chain reaction (PCR) used in this study were the same as described by Chen et al. (2017); Song and Cui (2017). The primer pairs ITS5 and ITS4 for ITS regions, LR0R and LR7 for nLSU regions, NS1 and NS4 for nSSU regions, MS1 and MS2 for mtSSU regions, RPB1-Af and RPB1-Cr for RPB1 gene, fRPB2-f5F and bRPB2-7.1R for RPB2 gene, EF1-983 F and EF1-1567R for TEF gene used in this study are the same as previous studies (White et al., 1990; Rehner, 2001; Matheny et al., 2002; Matheny, 2005).
The PCR cycling schedules for different DNA sequences of ITS, nLSU, nSSU, mtSSU, RPB1, RPB2, and TEF genes used in this study followed those used in Liu et al. (2019); Shen et al. (2019), Zhu et al. (2019); Sun et al. (2020) with some modifications. The PCR products were purified and sequenced at the Beijing Genomics Institute (BGI), China, with the same primers. All newly generated sequences were deposited in GenBank (Table 1). Additional sequences for phylogenetic analyses were downloaded from GenBank (Table 1). All sequences were aligned in MAFFT 7 (Katoh and Standley, 20131) and manually adjusted in BioEdit (Hall, 1999). Alignments were spliced in Mesquite (Maddison and Maddison, 2017). The missing sequences were coded as “N,” ambiguous nucleotides were coded as “N” following Chen et al. (2017). The final concatenated sequence alignment was deposited in TreeBase (2 submission ID: 27274).
TABLE 1.
A list of species, specimens, and GenBank accession number of sequences used for phylogenetic analyses in this study.
| Species | Sample no. | Locality | GenBank accessions |
||||||
| ITS | nLSU | nSSU | mtSSU | RPB1 | RPB2 | TEF | |||
| Amaropostia stiptica | Cui 10043 | China | KX900906 | KX900976 | KX901119 | KX901046 | KX901167 | KX901219 | / |
| A. stiptica | Cui 10981 | China | KX900907 | KX900977 | KX901120 | KX901047 | KX901168 | KX901220 | |
| Amylocystis lapponica | HHB-13400-Sp | United States | KC585237 | KC585059 | / | / | / | / | / |
| A. lapponica | OKM-4418-Sp | United States | KC585238 | KC585060 | / | / | / | / | / |
| Antrodia serpens | Dai 7465 | Luxemburg | KR605813 | KR605752 | KR605913 | KR606013 | / | KR610832 | KR610742 |
| A. serpens | Dai 14850 | Poland | MG787582 | MG787624 | MG787731 | MG787674 | / | MG787798 | MG787849 |
| Cyanosporus alni | Petr Vampola 12.10.1995 | Slovakia | MG137026 | / | / | / | / | / | / |
| C. alni | Cui 7185 | China | KX900879 | KX900949 | KX901092 | KX901017 | KX901155 | KX901202 | KX901254 |
| C. alni | Dai 14845 | Poland | KX900880 | KX900950 | KX901093 | KX901018 | KX901156 | KX901203 | KX901255 |
| C. arbuti | Viacheslav Spirin 8327 | United States | MG137039 | / | / | / | / | / | MG137132 |
| C. auricoma | Dai 20992 | Belarus | MW182169 | / | / | / | / | / | / |
| C. auricoma | Cui 13518 | China | KX900887 | KX900957 | KX901100 | KX901025 | / | KX901209 | / |
| C. auricoma | Cui 13519 | China | KX900888 | KX900958 | KX901101 | KX901026 | / | / | / |
| C. auricoma | Tuomo Niemelä 8310 | Finland | MG137040 | / | / | / | / | / | / |
| C. auricoma | Viacheslav Spirin 4586 | Russia | MG137042 | / | / | / | / | / | / |
| C. bifaria | Viacheslav Spirin 6402 | Russia | MG137043 | / | / | / | / | / | MG137133 |
| C. bifaria | Cui 17445 | China | MW182170 | MW182223 | MW182187 | MW182206 | / | MW191562 | MW191528 |
| C. bifaria | Cui 17806 | China | MW182171 | MW182224 | MW182188 | MW182207 | MW191546 | / | MW191529 |
| C. bubalinus | Cui 16976 | China | MW182172 | MW182225 | MW182189 | MW182208 | MW191547 | MW191563 | MW191530 |
| C. bubalinus | Cui 16985 | China | MW182173 | MW182226 | MW182190 | MW182209 | MW191548 | MW191564 | MW191531 |
| C. caesiosimulans | Viacheslav Spirin 4199 | Russia | MG137061 | / | / | / | / | / | MG137140 |
| C. caesiosimulans | Otto Miettinen 16976 | United States | MG137054 | / | / | / | / | / | MG137137 |
| C. caesius | Otto Miettinen 14156 | Finland | MG137048 | / | / | / | / | / | MG137134 |
| C. caesius | Gerhard Schuster 51 | Germany | MG137045 | / | / | / | / | / | / |
| C. caesius aff AR | CIEFAP 350 | Argentina | JX090110 | JX090130 | / | / | / | / | / |
| C. caesius aff AR | CIEFAP 174 | Argentina | JX090109 | JX090129 | / | / | / | / | / |
| C. caesius aff GB | K 32713 | United Kingdom | AY599576 | / | / | / | / | / | / |
| C. caesius aff GB | K 32425 | United Kingdom | AY599575 | / | / | / | / | / | / |
| C. coeruleivirens | Dai 11834 | China | KF699119 | / | / | / | / | / | / |
| C. coeruleivirens | Otto Miettinen 12214 | Indonesia | MG137063 | / | / | / | / | / | / |
| C. coeruleivirens | Dai 19220 | China | MW182174 | MW182227 | MW182191 | MW182210 | MW191549 | / | MW191532 |
| C. comata | Otto Miettinen 14755,1 | United States | MG137066 | / | / | / | / | / | / |
| C. comata | Cui 18388 | China | MW182175 | MW182228 | MW182192 | / | MW191550 | / | MW191533 |
| C. comata | Cui 18546 | China | / | MW182229 | MW182193 | / | / | / | MW191534 |
| C. cyanescens | Otto Miettinen 13602 | Finland | MG137067 | / | / | / | / | / | MG137142 |
| C. cyanescens | Otto Miettinen 15919,2 | Spain | MG137071 | / | / | / | / | / | MG137144 |
| C. fusiformis | Cui 10775 | China | KX900868 | KX900938 | KX901081 | KX901006 | / | KX901191 | KX901245 |
| C. fusiformis | Dai 15036 | China | KX900867 | KX900937 | KX901080 | KX901005 | / | KX901190 | KX901244 |
| C. glauca | Viacheslav Spirin 5317 | Russia | MG137078 | / | / | / | / | / | / |
| C. glauca | Viacheslav Spirin 6580 | Russia | MG137081 | / | / | / | / | / | MG137145 |
| C. gossypina | Bernard Rivoire 6658 | France | / | / | / | / | / | / | MG137146 |
| C. hirsutus | Cui 17050 | China | MW182176 | MW182230 | MW182194 | MW182211 | MW191551 | MW191565 | MW191535 |
| C. hirsutus | Cui 17053 | China | MW182177 | MW182231 | MW182195 | MW182212 | MW191552 | MW191566 | MW191536 |
| C. hirsutus | Cui 17055 | China | MW182178 | MW182232 | MW182196 | MW182213 | MW191553 | MW191567 | MW191537 |
| C. hirsutus | Cui 17083 | China | MW182179 | MW182233 | MW182197 | MW182214 | MW191554 | MW191568 | MW191538 |
| C. livens | Viacheslav Spirin 8728 | United States | MG137090 | / | / | / | / | / | MG137150 |
| C. livens | Otto Miettinen 17177 | United States | MG137082 | / | / | / | / | / | MG137147 |
| C. luteocaesia | Bernard Rivoire 2605 | France | MG137091 | / | / | / | / | / | / |
| C. magna | Cui 16983 | China | MW182180 | MW182234 | MW182198 | MW182215 | MW191555 | MW191569 | MW191539 |
| C. magna | Dai 10854 | China | KF699117 | / | / | / | / | / | / |
| C. magna | Otto Miettinen 10634 | China | KC595944 | KC595944 | / | / | / | / | MG137151 |
| C. mediterraneocaesius | LY BR 4274 | France | KX900886 | / | KX901099 | KX901024 | / | / | / |
| C. microporus | Cui 11014 | China | KX900878 | KX900948 | KX901091 | KX901016 | / | KX901201 | / |
| C. microporus | Dai 11717 | China | KX900877 | KX900947 | KX901090 | KX901015 | / | KX901200 | / |
| C. nothofagicola | Cui 16697 | Australia | MW182181a | MW182235a | MW182199a | MW182216a | MW191556a | MW191570a | MW191540a |
| C. nothofagicola | Dai 18765 | Australia | MW182182a | MW182236a | MW182200a | MW182217a | MW191557a | / | MW191541a |
| C. piceicola | Cui 10626 | China | KX900862 | KX900932 | KX901075 | KX901001 | / | KX901185 | / |
| C. piceicola | Cui 12158 | China | KX900866 | KX900936 | KX901079 | KX901004 | KX901153 | KX901189 | KX901243 |
| C. populi | Cui 17087a | China | MW182183 | MW182237 | MW182201 | MW182218 | MW191558 | MW191571 | MW191542 |
| C. populi | Cui 17549 | China | / | MW182238 | MW182202 | MW182219 | MW191559 | MW191572 | MW191543 |
| C. populi | Tuomo Niemelä 8379 | Finland | MG137097 | / | / | / | / | / | MG137154 |
| C. populi | Otto Miettinen 17043 | United States | MG137092 | / | / | / | / | / | MG137153 |
| C. simulans | Otto Miettinen 20422 | Finland | MG137110 | / | / | / | / | / | MG137160 |
| C. simulans | Tuomo Niemelä 8846 | Finland | MG137103 | / | / | / | / | / | / |
| C. subcaesius | Josef Vlasák 0110/24 | Czech Republic | MG137117 | / | / | / | / | / | MG137164 |
| C. subcaesius | Alix David 652 | France | MG137116 | / | / | / | / | / | / |
| C. subhirsutus | Cui 11330 | China | KX900873 | KX900943 | KX901086 | KX901011 | / | KX901196 | KX901250 |
| C. subhirsutus | Dai 14892 | China | KX900871 | KX900941 | KX901084 | KX901009 | / | KX901194 | KX901248 |
| C. submicroporus | Cui 16306 | China | MW182184 | MW182239 | MW182203 | MW182220 | MW191560 | MW191573 | MW191544 |
| C. submicroporus | Cui 17750 | China | MW182185 | MW182240 | MW182204 | MW182221 | MW191561 | / | MW191545 |
| C. submicroporus | Cui 18156 | China | MW182186 | MW182241 | MW182205 | MW182222 | / | MW191574 | / |
| C. subviridis | Viacheslav Spirin 8774a | United States | MG137120 | / | / | / | / | / | MG137166 |
| C. subviridis | Reijo Penttilä 14376 | Finland | / | / | / | / | / | / | MG137165 |
| C. tenuis | Cui 10788 | China | KX900885 | KX900955 | KX901098 | KX901023 | KX901161 | KX901208 | / |
| C. tenuis | Dai 12974 | China | KX900884 | KX900954 | KX901097 | KX901022 | KX901160 | KX901207 | KX901258 |
| C. tricolor | Cui 12233 | China | KX900876 | KX900946 | KX901089 | KX901014 | / | KX901199 | KX901253 |
| C. tricolor | Cui 10790 | China | KX900875 | KX900945 | KX901088 | KX901013 | / | KX901198 | KX901252 |
| C. ungulatus | Cui 10778 | China | KX900870 | KX900940 | KX901083 | KX901008 | / | KX901193 | KX901247 |
| C. ungulatus | Dai 12897 | China | KX900869 | KX900939 | KX901082 | KX901007 | KX901154 | KX901192 | KX901246 |
| C. yanae | Heikki Kotiranta 27606 | Russia | MG137122 | / | / | / | / | / | MG137168 |
| C. yanae | Heikki Kotiranta 27454 | Russia | MG137121 | / | / | / | / | / | MG137167 |
| Calcipostia guttulata | Cui 10028 | China | KF727433 | KJ684979 | KX901139 | KX901066 | KX901182 | KX901237 | KX901277 |
| C. guttulata | KHL 11739 (GB) | Finland | EU118650 | EU118650 | / | / | / | / | / |
| Cystidiopostia hibernica | Cui 2658 | China | KX900905 | KX900975 | KX901118 | KX901045 | / | KX901218 | / |
| C. hibernica | K(M)17352 | Austria | AJ006665 | / | / | / | / | / | / |
| Fuscopostia fragilis | Cui 10020 | China | KX900912 | KX900982 | KX901126 | KX901054 | / | KX901226 | KX901270 |
| F. fragilis | JV 0610-8 | Czech | JF950573 | / | / | / | / | / | / |
| Oligoporus rennyi | KEW 57 | Unknown | AY218416 | AF287876 | / | / | / | / | / |
| O. rennyi | MR 10497 | Argentina | JX090117 | / | / | / | / | / | / |
| Osteina obducta | Cui 9959 | China | KX900923 | KX900993 | KX901143 | KX901070 | / | KX901239 | / |
| O. obducta | Cui 10074 | China | KX900924 | KX900994 | KX901144 | KX901071 | / | KX901240 | / |
| Postia amurensis | Cui 1044 | China | KX900902 | KX900972 | / | KX901043 | / | / | / |
| P. amurensis | Dai 903 | China | KX900901 | KX900971 | / | KX901042 | / | / | / |
| P. hirsuta | Cui 11180 | China | KJ684971 | KJ684985 | / | KX901039 | / | / | / |
| P. hirsuta | Cui 11237 | China | KJ684970 | KJ684984 | KX901113 | KX901038 | / | / | KX901266 |
| P. lactea | Cui 9319 | China | KX900894 | KX900964 | KX901106 | KX901031 | KX901165 | KX901213 | KX901262 |
| P. lactea | Cui 11511 | China | KX900893 | KX900963 | KX901105 | KX901030 | KX901164 | KX901212 | KX901261 |
| Spongiporus leucospongia | JV 0709/123-J | United States | KX900918 | KX900988 | KX901137 | KX901064 | / | / | KX901275 |
| S. leucospongia | OKM 4335 | United States | KC585395 | KC585228 | / | / | / | / | / |
New sequences for this study are indicated in bold.
Phylogenetic analyses approaches used in this study followed Han et al. (2016); Cui et al. (2019). The congruences of the 7-gene (ITS, nLSU, nSSU, mtSSU, RPB1, RPB2, and TEF) were evaluated with the incongruence length difference (ILD) test (Farris et al., 1994) implemented in PAUP∗ 4.0b10 (Swofford, 2002), under heuristic search and 1000 homogeneity replicates. The sequences of Antrodia serpens (Fr.) Donk obtained from GenBank were used as outgroups for phylogenetic reconstruction. Maximum parsimony (MP) analysis was performed in PAUP∗ version 4.0b10 (Swofford, 2002). Maximum Likelihood (ML) analysis was performed in RAxmL v.7.2.8 with a GTR + G + I model (Stamatakis, 2006). Bayesian inference (BI) was calculated by MrBayes 3.1.2 (Ronquist and Huelsenbeck, 2003) with a general time reversible (GTR) model of DNA substitution and a gamma distribution rate variation across sites determined by MrModeltest 2.3 (Posada and Crandall, 1998; Nylander, 2004). Clade robustness was assessed using a bootstrap (BT) analysis with 1000 replicates (Felsenstein, 1985). Descriptive tree statistics tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each Most Parsimonious Tree (MPT) generated. The branch support was evaluated with a bootstrapping method of 1000 replicates (Hillis and Bull, 1993). Branches that received bootstrap supports for MP, ML greater than or equal to 75% and Bayesian posterior probabilities (BPP) greater than or equal to 0.95 were considered as significantly supported. The phylogenetic tree was visualized using FigTree v1.4.23.
Results
Molecular Phylogeny
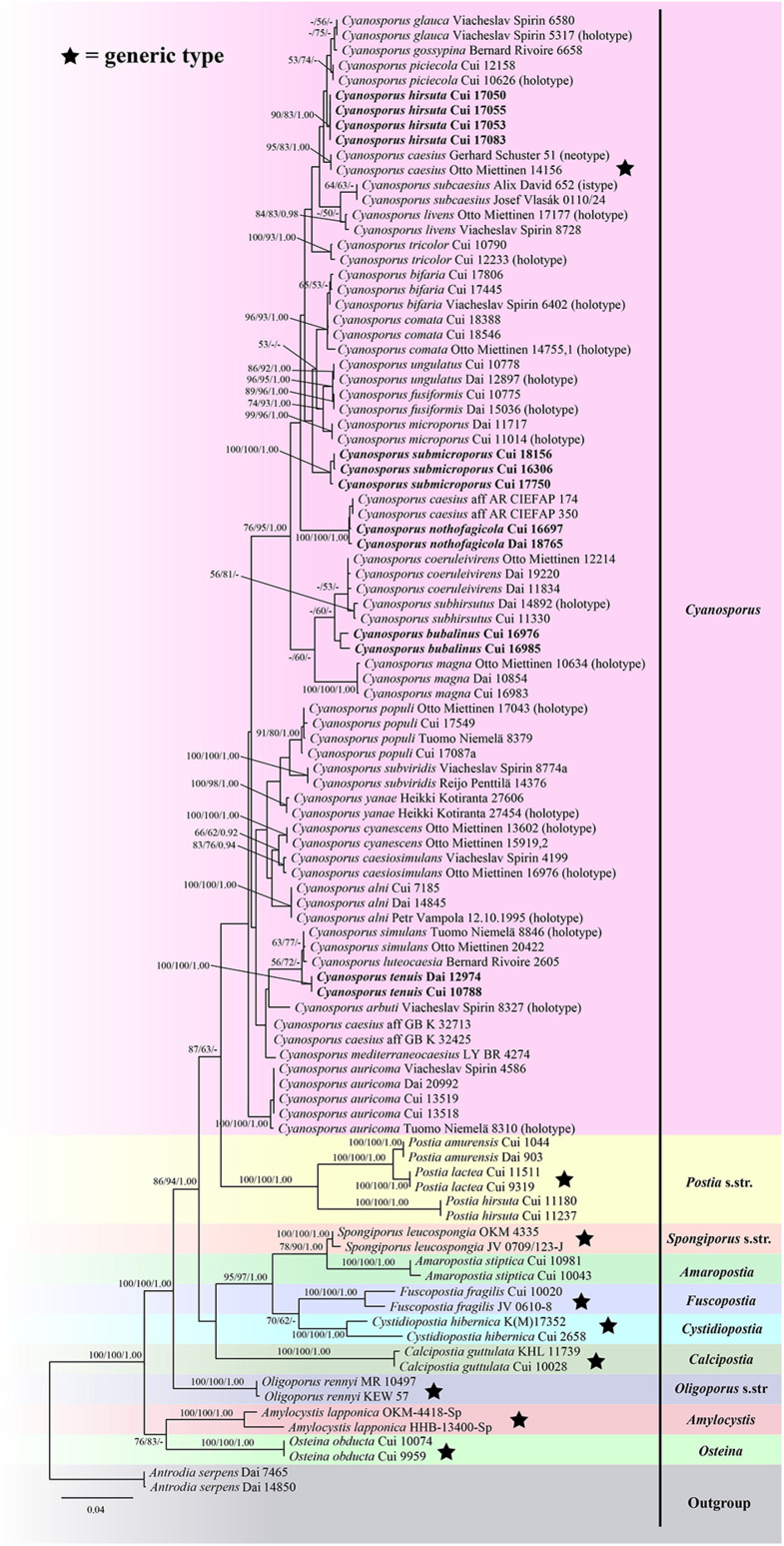
The ITS + TEF sequences dataset had an aligned length of 1166 characters, of which 646 characters were constant, 68 were variable and parsimony-uninformative, and 452 were parsimony-informative. MP analysis yielded 4 equally parsimonious trees (TL = 1983, CI = 0.417, RI = 0.726, RC = 0.303, HI = 0.583). The best model for the concatenate sequence dataset estimated and applied in the Bayesian inference was GTR + I + G with an equal frequency of nucleotides. ML analysis resulted in a similar topology as MP and Bayesian analyses, and only the ML topology is shown in Figure 1.
FIGURE 1.
Maximum likelihood tree illustrating the phylogeny of Cyanosporus and its related genera in the antrodia clade based on sequences dataset of ITS + TEF. Branches are labeled with maximum likelihood bootstrap higher than 50%, parsimony bootstrap proportions higher than 50% and Bayesian posterior probabilities more than 0.90 respectively. Bold names = New species.
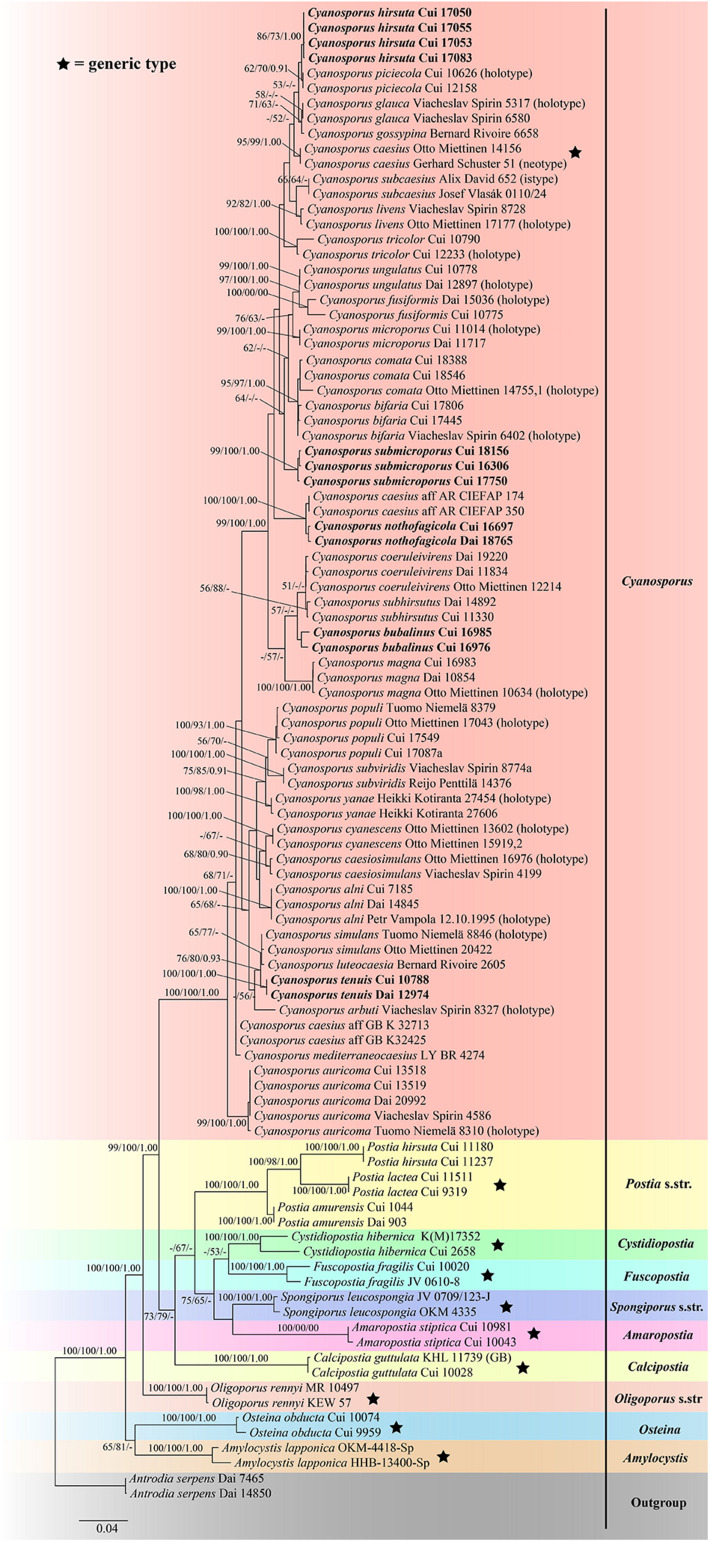
The combined three-gene (ITS + nLSU + TEF) sequences dataset had an aligned length of 2475 characters, of which 1752 characters were constant, 134 were variable and parsimony-uninformative, and 589 were parsimony-informative. MP analysis yielded 8 equally parsimonious trees (TL = 2222, CI = 0.473, RI = 0.770, RC = 0.365, HI = 0.527). The best model for the concatenate sequence dataset estimated and applied in the Bayesian inference was GTR + I + G with equal frequency of nucleotides. ML analysis resulted in a similar topology as MP and Bayesian analyses, and only the ML topology is shown in Figure 2.
FIGURE 2.
Maximum likelihood tree illustrating the phylogeny of Cyanosporus and its related genera in the antrodia clade based on the combined sequences dataset of ITS + nLSU + TEF. Branches are labeled with maximum likelihood bootstrap higher than 50%, parsimony bootstrap proportions higher than 50% and Bayesian posterior probabilities more than 0.90 respectively. Bold names = New species.
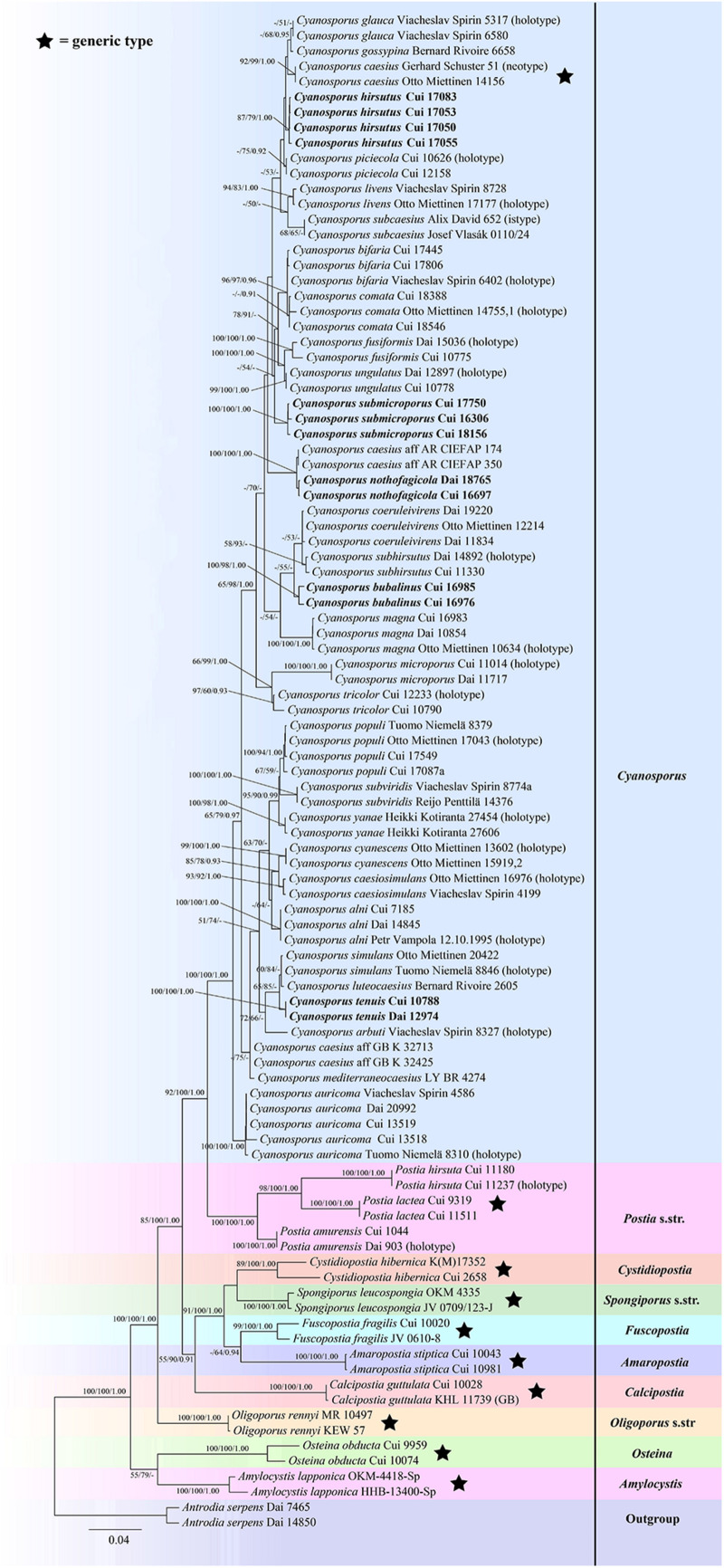
The combined seven-gene (ITS + nLSU + nSSU + mtSSU + RPB1 + RPB2 + TEF) sequences dataset had an aligned length of 5855 characters, of which 4194 characters were constant, 300 were variable and parsimony-uninformative, and 1361 were parsimony-informative. MP analysis yielded 10 equally parsimonious trees (TL = 3951, CI = 0.585, RI = 0.806, RC = 0.471, HI = 0.415). The best model for the concatenate sequence dataset estimated and applied in the Bayesian inference was GTR + I + G with equal frequency of nucleotides. ML analysis resulted in a similar topology as MP and Bayesian analyses, and only the ML topology is shown in Figure 3.
FIGURE 3.
Maximum likelihood tree illustrating the phylogeny of Cyanosporus and its related genera in the antrodia clade based on the combined sequences dataset of ITS + nLSU + nSSU + mtSSU + RPB1 + RPB2 + TEF. Branches are labeled with maximum likelihood bootstrap higher than 50%, parsimony bootstrap proportions higher than 50% and Bayesian posterior probabilities more than 0.90 respectively. Bold names = New species.
The phylogenetic trees (Figures 1–3) inferred from ITS + TEF, ITS + nLSU + TEF and ITS + nLSU + nSSU + mtSSU + RPB1 + RPB2 + TEF gene sequences were obtained from 99 fungal samples representing 45 taxa of Cyanosporus and its related genera in the antrodia clade. Seventy-five samples representing thirty-one taxa of Cyanosporus clustered together and separated from species of Postia and other related genera.
Taxonomy
Cyanosporus bubalinus B.K. Cui & Shun Liu, sp. nov. (Figures 4A–B, 5)
FIGURE 4.
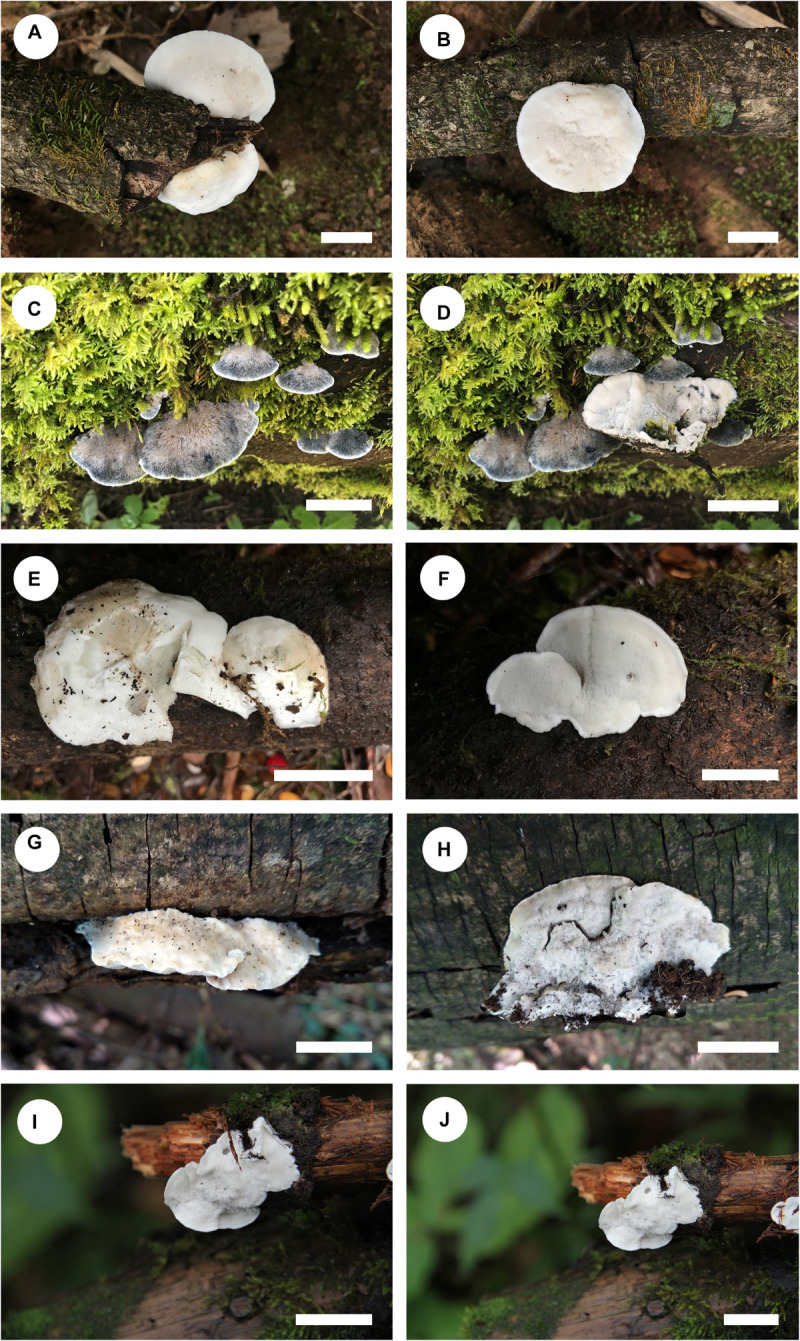
Basidiocarps of Cyanosporus species. (A,B) C. bubalinus; (C,D) C. hirsutus; (E,F) C. nothofagicola; (G,H). C. submicroporus; (I,J). C. tenuis (scale bars: A,B,I,J = 1 cm; C,D,E,F,G,H = 2 cm).
FIGURE 5.
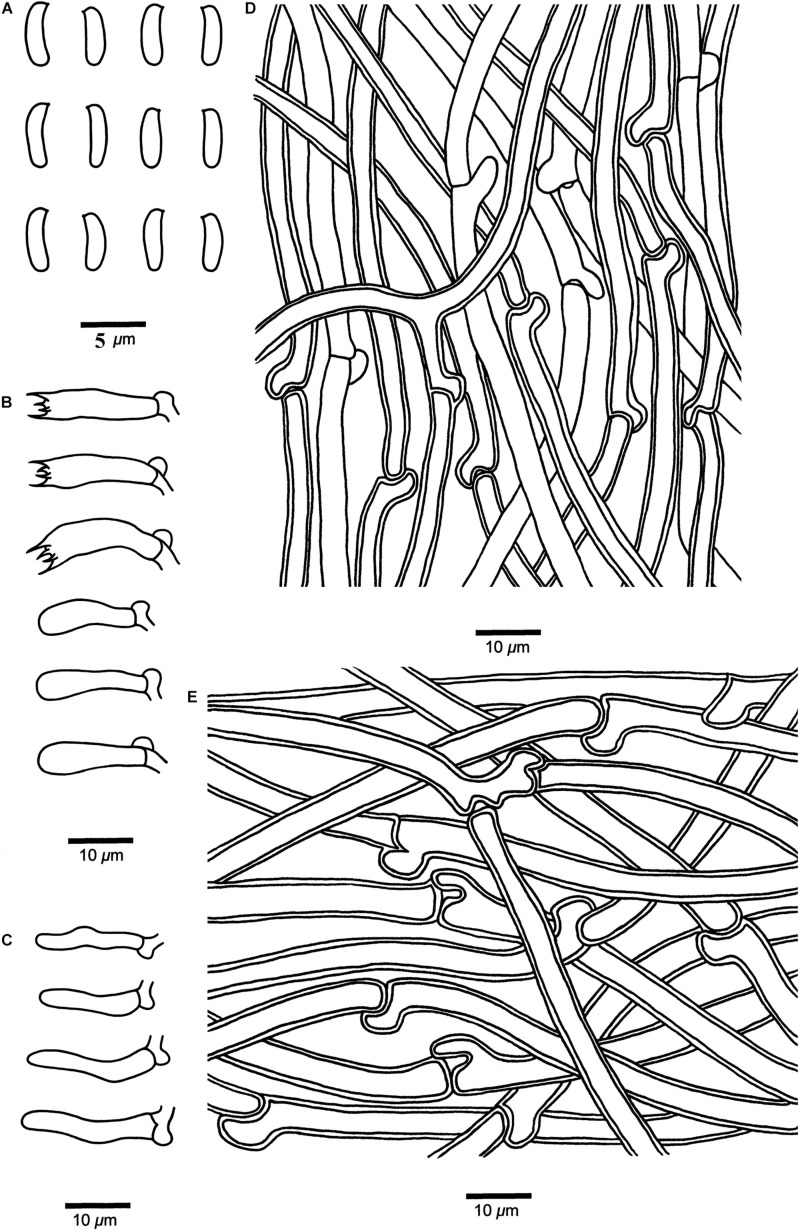
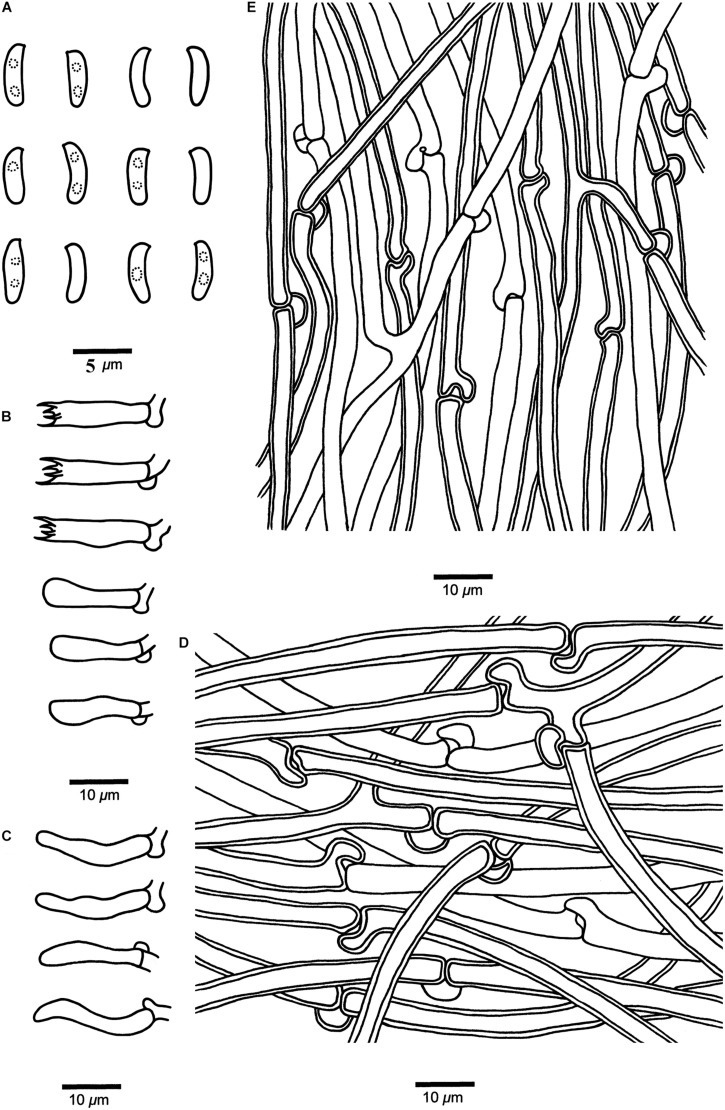
Microscopic structures of Cyanosporus bubalinus (drawn from the holotype). (A) Basidiospores; (B) Basidia and basidioles; (C) Cystidioles; (D) Hyphae from trama; (E) Hyphae from context. Bars: (A) = 5 μm; (B–E) = 10 μm.
MycoBank: MB 838417
Differs from other Cyanosporus species by its tomentose, cream to buff pileal surface when fresh becoming cream to pinkish buff when dry and a white to cream pore surface when fresh becoming straw yellow to buff when dry, and round to angular small pores (5–8 per mm).
Type. — CHINA. Yunnan Province, Binchuan County, Jizu Mountain, on fallen branch of Pinus, 14 September 2018, Cui 16985 (holotype, BJFC).
Etymology. — Bubalinus (Lat.): refers to the cream to buff pileal surface.
Basidiocarps. — Annual, pileate, solitary, soft and watery when fresh, becoming soft corky to fragile upon drying. Pileus shell-shaped, projecting up to 2.5 cm, 3.5 cm wide and 1.5 cm thick at base. Pileal surface white to cream when fresh, finely tomentose, becoming cream to pinkish buff upon drying; margin acute. Pore surface white to cream when fresh, becoming straw yellow to buff when dry; sterile margin narrow to almost lacking; pores round to angular, 5–8 per mm; dissepiments thin, entire to lacerate. Context white, corky, up to 1.2 cm thick. Tubes cream, fragile, up to 5 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH.
Context. — Generative hyphae hyaline, slightly thick-walled with a wide lumen, occasionally branched, loosely interwoven, 2.5–7.3 μm in diam.
Tubes. — Generative hyphae hyaline, thin- to slightly thick-walled, occasionally branched, interwoven, 2–4.2 μm in diam. Cystidia absent; cystidioles present, fusoid, thin-walled, 13.3–23.4 × 2.9–4.2 μm. Basidia clavate, bearing four sterigmata and a basal clamp connection, 11.6–19.8 × 4.3–5.6 μm; basidioles dominant, in shape similar to basidia, but smaller.
Basidiospores. — Cylindrical, slightly curved, hyaline, thin- to slightly thick-walled, smooth, occasionally with small oily drops, IKI–, CB–, (4.2–)4.3–4.8 × 1.2–1.7(–1.8) μm, L = 4.65 μm, W = 1.55 μm, Q = 2.98–3.09 (n = 60/2).
Notes. — In the phylogenetic trees (Figures 1–3), two specimens of Cyanosporus bubalinus formed a highly supported lineage (Figures 1–3), closely related to C. coeruleivirens (Corner) B.K. Cui, Shun Liu & Y.C. Dai, C. magna (Miettinen) B.K. Cui & Shun Liu and C. subhirsutus B.K. Cui, L.L. Shen & Y.C. Dai. Morphologically, C. coeruleivirens differs by having a white, cream to bluish-grayish pore surface, smaller basidia (8.8–13.5 × 3.3–4.3 μm) and smaller basidiospores (3.8–4.8 × 1–1.3 μm) (Table 2); C. magna differs from C. bubalinus in its larger pores (4–5 per mm), smaller basidia (10–12.5 × 3.2–4 μm) and smaller basidiospores (3.6–4.4 × 1.0–1.2 μm) (Table 2); C. subhirsutus differs by having a hirsute pileal surface when dry, larger pores (2–3 per mm), smaller basidia (10–12 × 4–6 μm) and smaller basidiospores (4–4.5 × 0.9–1.3 μm) (Table 2). Cyanosporus microporus B.K. Cui, L.L. Shen & Y.C. Dai and C. piceicola B.K. Cui, L.L. Shen & Y.C. Dai were also discovered from Yunnan Province. Cyanosporus microporus differs from the new species by its narrower basidiospores (4.5–4.9 × 1–1.2 μm, Table 2); C. piceicola differs by having a velutinate, cream to clay-buff pileal surface, with bluish gray zonation when fresh, larger and round pores (3–5 per mm) and narrower basidiospores (4–4.5 × 0.9–1.3 μm). Cyanosporus bifaria (Spirin) B.K. Cui & Shun Liu, C. glauca (Spirin & Miettinen) B.K. Cui & Shun Liu and C. subviridis (Ryvarden & Guzmán) B.K. Cui & Shun Liu are similar to C. bubalinus as they all have pileate basidiocarps and similar pores, however, they differ by narrower tramal hyphae (2.5–3.8 μm in C. bifaria, 2.6–3.3 μm in C. glauca, 2.5–3.2 μm in C. subviridis) and smaller basidia (9.8–14.8 × 3.4–4.5 μm in C. bifaria, 9.8–14.8 × 3.1–4.3 μm in C. glauca, 10–13 × 3.2–4.4 μm in C. subviridis) (Table 2); in addition, they are distantly related from C. bubalinus in phylogeny.
TABLE 2.
Comparisons of the main morphological characters of species in Cyanosporus.
| Species | Distribution | Basidio-Carps | Pileal surface when dry | Pores per mm | Context hyphae (μm) | Tramal hyphae (μm) | Basidia (μm) | Basidiospores |
Ref. | |
| L × W | Q = L/W | |||||||||
| (μm) | ||||||||||
| C. alni | China (Guizhou, Hebei), Czech Republic, Denmark, Finland, Germany, Norway, Poland, Russia, Slovakia | Pileate/Effused-reflexed | Velutinate | 4–6 | 3.9–5.5 | 2.9–3.6 | 10–14.8 × 3.3–4.2 | 4.3–6.1 × 1.1–1.3 | 4.22 | Miettinen et al., 2018 |
| C. arbuti | United States | Pileate/Effused-reflexed | Glabrous | 6–8 | 3.2–4.6 | 2.4–3.1 | 11–17 × 3.3–4.2 | 4.1–5.1 × 1–1.2 | 4 | Miettinen et al., 2018 |
| C. auricoma | China (Inner Mongolia), Finland, Poland, Russia | Pileate/Effused-reflexed/Resupinate | Hirsute | 4–6 | 4.2–5.2 | 3.1–4.0 | 14–20 × 3.8–5.3 | 4.4–5.6 × 1.5–1.8 | 3.06 | Miettinen et al., 2018 |
| C. bifaria | China (Jilin, Sichuan), Japan, Russia | Pileate | Velutinate | 6–8 | 4–7 | 2.5–3.8 | 9.8–14.8 × 3.4–4.5 | 3.7–4.4 × 1–1.2 | 3.6 | Miettinen et al., 2018 |
| C. bubalinus | China (Yunnan) | Pileate | Tomentose | 5–8 | 2.5–7.3 | 2–4.2 | 11.6–19.8 × 4.3–5.6 | 4.3–4.8 × 1.5–2 | 2.6–2.83 | this study |
| C. caesius | Czech Republic, Denmark, Finland, France, Germany, Russia, Slovakia, Spain, United Kingdom | Pileate/Effused-reflexed | Hirsute | 4–5 | 3.7–5.2 | 2.8–3.6 | 10–15 × 3.7–4.5 | 4.1–5.3 × 1.3–1.7 | 3.13 | Miettinen et al., 2018 |
| C. caesiosimulans | Finland, Russia, United States | Pileate/Effused-reflexed | Glabrous | 5–7 | 3.4–5.2 | 2.9–3.8 | 10.5–15.5 × 3.2–5.2 | 4.2–5.5 × 1.1–1.4 | 3.93 | Miettinen et al., 2018 |
| C. coeruleivirens | China (Hunan, Jilin, Zhejiang), Indonesia, Russia | Pileate | Velutinate | 6–8 | 3.6–6 | 2.4–3.4 | 8.8–13.5 × 3.3–4.3 | 3.8–4.8 × 1–1.3 | 3.64 | Miettinen et al., 2018 |
| C. comata | China (Sichuan, Xizang), United States | Pileate/Effused-reflexed | Velutinate | 4–6 | 4.2–5.3 | 2.8–3.8 | 8.8–14.2 × 3.7–4.9 | 4.1–4.9 × 1.1–1.3 | 3.62 | Miettinen et al., 2018 |
| C. cyanescens | Estonia, Finland, France, Poland, Russia, Spain, Sweden | Pileate/Effused-reflexed | Glabrous | 5–6 | 4.1–5.2 | 2.9–3.7 | 11.4–19.8 × 3.7–5.4 | 4.7–6.1 × 1.1–1.6 | 3.92 | Miettinen et al., 2018 |
| C. fusiformis | China (Guizhou, Sichuan) | Pileate/Effused-reflexed | Tomentose | 4–5 | 3–5 | 2–4 | 12–15 × 4.5–6 | 4.5–5.2 × 0.8–1.1 | 5.21–5.45 | Shen et al., 2019 |
| C. glauca | China (Jilin), Russia | Pileate | Hirsute | 5–8 | 3.4–5.1 | 2.6–3.3 | 9.8–14.8 × 3.1–4.3 | 4.1–5.4 × 1.1–1.5 | 3.64 | Miettinen et al., 2018 |
| C. gossypina | France | Pileate | Glabrous | 4–6 | 3.6–4.8 | 2.3–3 | 8.7–16.8 × 3.8–5 | 4.1–5.1 × 1.2–1.7 | 3.11 | Miettinen et al., 2018 |
| C. hirsutus | China (Yunnan) | Pileate | Hirsute | 5–7 | 2.7–8.2 | 2–5 | 13.6–15.5 × 3.4–4.7 | 4–4.7 × 1.2–1.5 | 3.18–3.52 | this study |
| C. livens | Canada, United States | Pileate | Velutinate | 4–6 | 3.7–5.3 | 2.9–4 | 9.3–14.3 × 4–5.3 | 4.1–5.7 × 1.1–1.5 | 3.74 | Miettinen et al., 2018 |
| C. luteocaesius | France | Pileate/Effused-reflexed | Tomentose | 3–5 | 4.1–5.8 | 2.7–3.2 | 11.1–16.2 × 4–5.2 | 4.3–6.1 × 1.5–1.9 | 3.02 | Miettinen et al., 2018 |
| C. magna | China (Jilin, Hainan, Yunnan) | Pileate | Velutinate | 4–5 | 4.2–6 | 2.2–3.3 | 10–12.5 × 3.2–4 | 3.6–4.4 × 1–1.2 | 3.51 | Miettinen et al., 2018 |
| C. mediterraneocaesius | France, Spain | Pileate/Effused-reflexed | Velutinate | 5–6 | 3.1–4 | 2.3–3.2 | 12–18.5 × 3.5–4.6 | 4.2–5.8 × 1.3–1.7 | 3.26 | Miettinen et al., 2018 |
| C. microporus | China (Yunnan) | Pileate | Velutinate | 6–8 | 3.5–6 | 2–4 | 11–13.5 × 4–5 | 4.5–4.9 × 1–1.2 | 4.47–4.52 | Shen et al., 2019 |
| C. nothofagicola | Australia | Pileate/Effused-reflexed | Tomentose | 4–6 | 2.3–7.5 | 2–5 | 13.6–17.2 × 3.4–4.2 | 3.8–5 × 1–1.7 | 4.08–4.22 | this study |
| C. piceicola | China (Sichuan, Xizang, Yunnan) | Pileate | Velutinate | 3–5 | 5–7 | 2.5–4 | 13–16 × 4–5 | 4–4.5 × 0.9–1.3 | 3.75–3.97 | Shen et al., 2019 |
| C. populi | China (Jilin, Sichuan, Yunnan), Finland, Norway, Poland, Russia, United States | Pileate/Effused-reflexed | Glabrous | 5–7 | 3.2–4.8 | 2.7–3.3 | 10–16 × 3.5–4.2 | 4.2–5.6 × 1–1.3 | 4.14 | Miettinen et al., 2018 |
| C. simulans | Canada, China, Estonia, Finland, France, Germany, Norway, Russia, United States | Pileate/Effused-reflexed/Resupinate | Glabrous | 5–7 | 3.9–5 | 2.8–3.6 | 10–14.8 × 3.7–5.2 | 4.4–6.3 × 1.3–1.8 | 3.6 | Miettinen et al., 2018 |
| C. subcaesius | Czech Republic, Finland, France, Russia, United Kingdom | Pileate/Effused-reflexed | Glabrous | 4–6 | 4.2–6.6 | 3.1–4.1 | 10.3–17.8 × 3.3–4.6 | 4–5.3 × 1–1.4 | 3.8 | Miettinen et al., 2018 |
| C. subhirsutus | China (Guizhou, Fujian, Yunnan) | Pileate | Hirsute | 2–3 | 4–6 | 3–4.5 | 10–12 × 4–6 | 4–4.5 × 0.9–1.3 | 3.67–3.79 | Shen et al., 2019 |
| C. submicroporus | China (Sichuan, Yunnan) | Pileate | Velutinate | 6–9 | 2.3–6.2 | 2–4.8 | 12.2–20.5 × 3.4–5.6 | 3.6–4.7 × 1–1.3 | 3.45–3.52 | this study |
| C. subviridis | Finland, Mexico, United States | Pileate | Glabrous | 6–8 | 4.8–5.8 | 2.5–3.2 | 10–13 × 3.2–4.4 | 3.8–4.5 × 1–1.3 | 3.58 | Miettinen et al., 2018 |
| C. tricolor | China (Sichuan, Xizang) | Pileate | Velutinate | 4–5 | 3–5 | 2–3 | 12–15 × 4–5 | 4–4.8 × 0.8–1.2 | 4.55–4.87 | Shen et al., 2019 |
| C. tenuis | China (Sichuan) | Pileate/Effused-reflexed | Tomentose | 5–7 | 2.6–7 | 2.2–4.8 | 18.2–27.6 × 3.7–6 | 4.7–6 × 1.3–2 | 2.89–2.93 | This study |
| C. ungulatus | China (Sichuan) | Pileate | Glabrous | 4–6 | 2.5–4.5 | 2–3 | 12–15 × 4–5 | 4.5–5 × 0.9–1.2 | 4.79–4.83 | Shen et al., 2019 |
| C. yanae | Russia | Pileate/Effused-reflexed | Glabrous | 5–7 | 3–4 | 2.2–2.9 | 9–14 × 3.5–4.2 | 4.3–5.8 × 1.2–1.6 | 3.56 | Miettinen et al., 2018 |
Additional specimen (paratype) examined: CHINA. Yunnan Province, Binchuan County, Jizu Mountain, on wood of Pinus, 14 September 2018, Cui 16976 (BJFC).
Cyanosporus hirsutus B.K. Cui & Shun Liu, sp. nov. (Figures 4C–D, 6)
FIGURE 6.
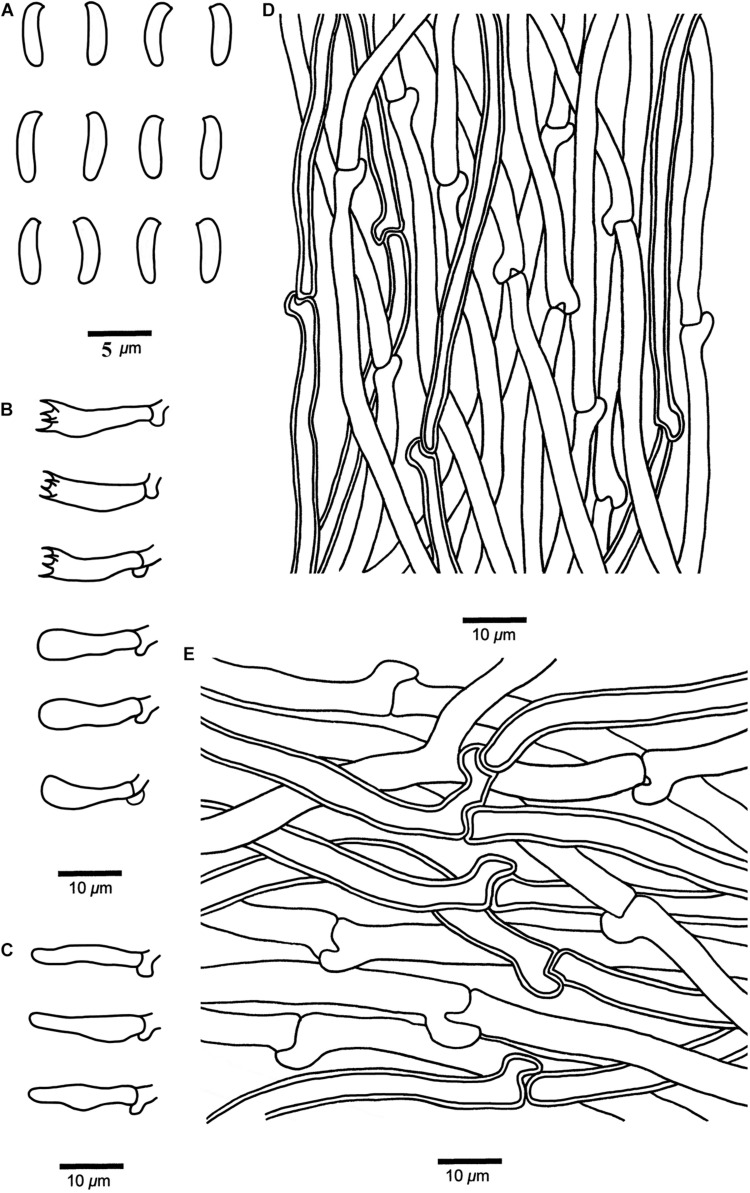
Microscopic structures of Cyanosporus hirsutus (drawn from the holotype). (A) Basidiospores; (B) Basidia and basidioles; (C) Cystidioles; (D) Hyphae from trama; (E) Hyphae from context. Bars: (A) = 5 μm; (B–E) = 10 μm.
MycoBank: MB 838418
Differs from other Cyanosporus species by its flabelliform to semicircular and distinctly hirsute pileus with ash gray to light grayish brown pileal surface, and cylindrical and slightly curved basidiospores (4.1–4.7 × 1.2–1.5 μm).
Type. — CHINA. Yunnan Province, Lijiang, Yulong Snow Mountain, on fallen trunk of Picea, 14 September 2018, Cui 17083 (holotype, BJFC).
Etymology. — Hirsutus (Lat.): refers to the distinctly hirsute pileal surface.
Basidiocarps. — Annual, pileate, soft corky, without odor or taste when fresh, becoming corky to fragile upon drying. Pileus flabelliform to semicircular, projecting up to 5.2 cm, 9.5 cm wide and 1.5 cm thick at base. Pileal surface ash gray to light grayish brown with bluish gray zone when fresh, becoming grayish to grayish brown when dry, distinctly hirsute; margin acute. Pore surface cream when fresh, becoming straw yellow to olivaceous buff when dry; sterile margin narrow to almost lacking; pores angular, 5–7 per mm; dissepiments thin, entire. Context white, soft corky, up to 9 mm thick. Tubes cream, fragile, up to 7 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH.
Context. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, loosely interwoven, 2.7–8.2 μm in diam.
Tubes. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, interwoven, 2–5 μm in diam. Cystidia absent; cystidioles present, fusoid, thin-walled, 13.2–22.5 × 2.7–4.3 μm. Basidia clavate, bearing four sterigmata and a basal clamp connection, 13.6–15.5 × 3.4–4.7 μm; basidioles dominant, in a shape similar to basidia, but smaller.
Basidiospores. — Cylindrical, slightly curved, hyaline, thin- to slightly thick-walled, smooth, occasionally with small oily drops, IKI–, CB–, 4–4.7(–4.9) × (1–)1.2–1.5(–1.8) μm, L = 4.42 μm, W = 1.33 μm, Q = 3.18–3.52 (n = 90/3).
Notes. — Phylogenetically, Cyanosporus hirsutus grouped together with C. caesius, C. glauca, C. gossypina (Moug. & Lév.) B.K. Cui & Shun Liu, C. mediterraneocaesius (M. Pieri & B. Rivoire) B.K. Cui, L.L. Shen & Y.C. Dai and C. piceicola. Morphologically, C. hirsutus is similar to C. caesius as both species share hairy and distinctly bluish basidiocarps, but C. caesius differs from C. hirsutus by its larger pores (4–5 per mm), and narrower hyphae of the context (3.7–5.2) (Table 2); C. hirsutus and C. glauca have similar-sized basidiospores, but C. glauca has a plumbeous to bluish gray or grayish brown pileal surface (Miettinen et al., 2018); C. hirsutus and C. gossypina have similar-sized pores and basidiospores, but C. gossypina has smaller basidiocarps with a cream to light gray pileal surface (Miettinen et al., 2018); C. hirsutus and C. mediterraneocaesius share similar-sized and similar-shaped pores, but C. mediterraneocaesius has larger basidiospores (4.2–5.8 × 1.3–1.7) (Table 2); C. hirsutus and C. piceicola both have a flabelliform pileus, with bluish gray zonate pileal surface, similar-sized pores and basidiospores, but C. piceicola has smaller basidiocarps (projecting up to 3 cm, 5.5 cm wide and 1.8 cm thick at base), thinner hyphae in the context (5–7 μm) and smaller basidia (13–16 × 4–5 μm) (Shen et al., 2019). Cyanosporus microporus and C. subhirsutus both have pileate basidiocarps with a blue tint to the pileal surface and slightly thick-walled basidiospores like C. hirsutus, but C. microporus differs in its smaller pores (6–8 per mm) and basidia (11–13.5 × 4–5 μm) (Table 2); C. subhirsutus differs in having larger pores (2–3 per mm) and smaller basidia (10–12 × 4–6 μm) (Table 2).
Additional specimens (paratypes) examined. CHINA. Yunnan Province, Lijiang, Yulong Snow Mountain, on fallen trunk of Picea, 14 September 2018, Cui 17050, 17053, 17055, 17070, 17082 (BJFC).
Cyanosporus nothofagicola B.K. Cui, Shun Liu & Y.C. Dai, sp. nov. (Figures 4E–F, 7)
FIGURE 7.
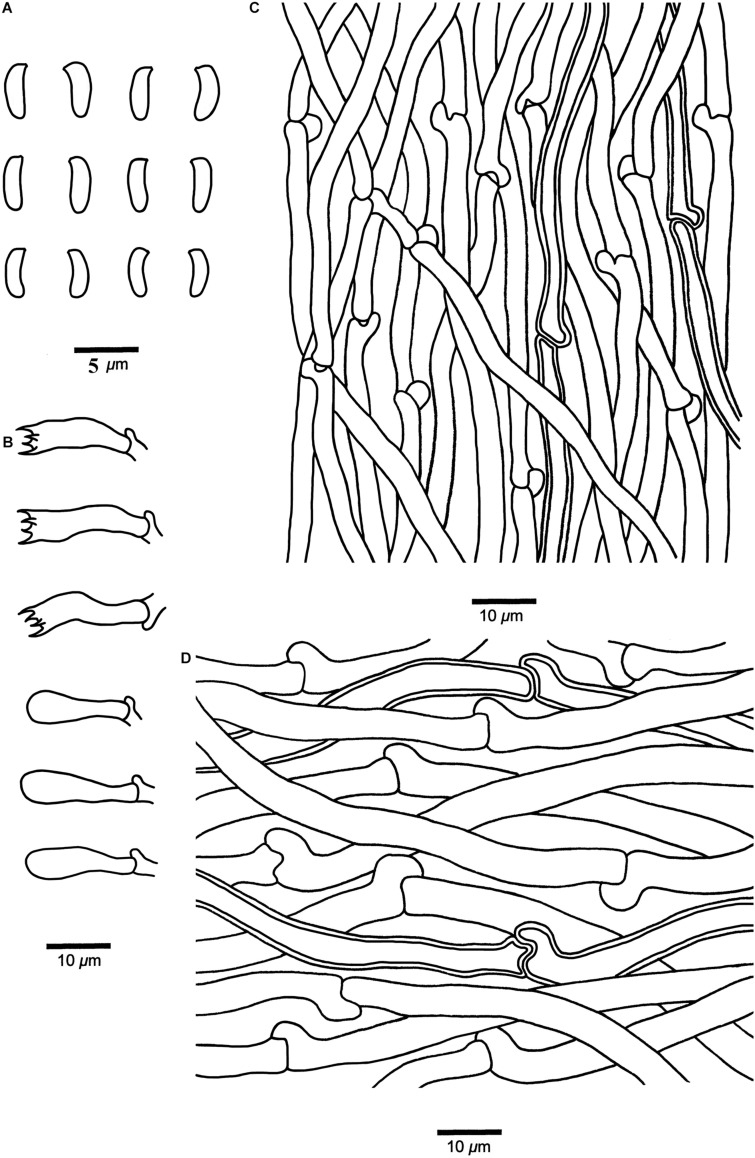
Microscopic structures of Cyanosporus nothofagicola (drawn from the holotype). (A) Basidiospores; (B) Basidia and basidioles; (C) Cystidioles; (D) Hyphae from trama; (E) Hyphae from context. Bars: (A) = 5 μm; (B–E) = 10 μm.
MycoBank: MB 838419
Differs from other Cyanosporus species by its flabelliform to semicircular pileus with pale mouse gray to buff yellow pileal surface and cream to buff yellow pore surface when dry, cylindrical to allantoid basidiospores (3.8–5 × 1–1.7 μm), and growth on Nothofagus.
Type. — AUSTRALIA. Tasmania, Arve River Streamside Reserve, on fallen branch of Nothofagus, 15 May 2018, Cui 16697 (holotype, BJFC).
Etymology. — (Lat.): refers to the frequent occurrence on Nothofagus.
Basidiocarps. — Annual, effused-reflexed to pileate, solitary, soft and watery, without odor or taste when fresh, becoming soft corky to fragile upon drying. Pileus flabelliform to semicircular, projecting up to 2.4 cm, 4.2 cm wide and 0.7 cm thick at base. Pileal surface buff to olivaceous buff when fresh, finely tomentose, becoming smooth, pale mouse gray to buff yellow when dry; margin acute. Pore surface white to cream when fresh, becoming cream to buff yellow when dry; sterile margin narrow to almost lacking; pores angular, 4–6 per mm; dissepiments thin, entire to lacerate. Context white, soft corky, up to 4 mm thick. Tubes cream, fragile, up to 3 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH.
Context. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, loosely interwoven, 2.3–7.5 μm in diam.
Tubes. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, interwoven, 2–5 μm in diam. Cystidia absent; cystidioles present, fusoid, thin-walled, 14.2–16.7 × 2.6–5.2 μm. Basidia clavate, bearing four sterigmata and a basal clamp connection, 13.6–17.2 × 3.4–4.2 μm; basidioles dominant, in shape similar to basidia, but smaller.
Basidiospores. — Cylindrical to allantoid, slightly curved, hyaline, thin- to slightly thick-walled, smooth, IKI–, CB–, 3.8–5 × (0.8–)1–1.7 μm, L = 4.62 μm, W = 1.15 μm, Q = 4.08–4.22 (n = 60/2).
Notes. — Cyanosporus caesius is also found in Tasmania, Australia. It has pileate or effused reflexed basidiocarps with a tomentose pileal surface and similar pores to C. nothofagicola, but it differs from the later by its plumbeous to bluish gray or grayish brown pileal surface (Miettinen et al., 2018).
Additional specimen (paratype) examined. AUSTRALIA. Tasmania, Arve River Streamside Reserve, on fallen trunk of Nothofagus, 15 May 2018, Dai 18765 (BJFC).
Cyanosporus submicroporus B.K. Cui & Shun Liu, sp. nov. (Figures 4G–H, 8)
FIGURE 8.
Microscopic structures of Cyanosporus submicroporus (drawn from the holotype). (A) Basidiospores; (B) Basidia and basidioles; (C). Hyphae from trama; (D) Hyphae from context. Bars: (A) = 5 μm; (B–D) = 10 μm.
MycoBank: MB 838420
Differs from other Cyanosporus species by its cream to pinkish buff pileal surface and white to smoke gray pore surface when fresh, buff to buff yellow pileal surface and buff to olivaceous buff pore surface when dry.
Type. — CHINA. Yunnan Province, Baoshan, Gaoligongshan Nature Reserve, on angiosperm wood, 8 November 2019, Cui 18156 (holotype, BJFC).
Etymology. — Submicroporus (Lat.): refers to the new species resembling Cyanosporus microporus in morphology.
Basidiocarps. — Annual, pileate, soft and watery, without odor or taste when fresh, becoming corky to woody hard upon drying. Pileus flabelliform to semicircular, projecting up to 3.2 cm, 6.5 cm wide and 1.3 cm thick at base. Pileal surface cream to pinkish buff when fresh, velutinate, becoming rugose, buff to buff yellow when dry; margin acute. Pore surface white to smoke gray when fresh, becoming buff to olivaceous buff when dry; sterile margin narrow to almost lacking; pores round, 6–9 per mm; dissepiments thin, entire. Context cream to buff, corky, up to 5 mm thick. Tubes pale mouse gray to cream, fragile, up to 7 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH.
Context. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, rarely branched, loosely interwoven, 2.3–6.2 μm in diam.
Tubes. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, interwoven, 2–4.8 μm in diam. Cystidia and cystidioles absent. Basidia clavate, bearing four sterigmata and a basal clamp connection, 12.2–20.5 × 3.4–5.6 μm; basidioles dominant, in shape similar to basidia, but smaller.
Basidiospores. — Allantoid, slightly curved, hyaline, thin- to slightly thick-walled, smooth, occasionally with small oil drops, IKI–, weakly CB+, 3.6–4.7 × (0.9–)1–1.3 μm, L = 4.18 μm, W = 1.19 μm, Q = 3.45–3.52 (n = 90/3).
Notes. — In the phylogenetic tree the three specimens of Cyanosporus submicroporus formed a highly supported lineage (Figures 1-3), and are usually grouped together with C. bifaria, C. comata (Miettinen) B.K. Cui & Shun Liu, C. fusiformis B.K. Cui, L.L. Shen & Y.C. Dai and C. ungulatus B.K. Cui, L.L. Shen & Y.C. Dai. Morphologically, both C. submicroporus and C. bifaria have similar pores and basidiospores, but C. bifaria differs in having ochraceous hues, a strigose pileal surface, and smaller basidia (9.8–14.8 × 3.4–4.5 μm) (Table 2); C. comata has similar basidiospores to C. submicroporus, but differs in having conchate or effused-reflexed basidiocarps, and smaller basidia (8.8–14.2 × 3.7–4.9 μm) (Table 2); C. fusiformis and C. ungulatus differ in having larger pores (Table 2). Cyanosporus submicroporus is similar to C. microporus by having pileate basidiocarps with a velutinate pileal surface, and small and angular pores, but C. microporus differs in having thick-walled tramal generative hyphae, smaller basidia (11–13.5 × 4–5 μm) and longer basidiospores (4.5–4.9 × 1–1.2 μm) (Table 2).
Additional specimens (paratypes) examined. CHINA. Sichuan Province, Shimian County, on fallen angiosperm trunk, 14 September 2019, Cui 17750 (BJFC); Yunnan Province, Chuxiong, Zixishan Nature Reserve, on fallen angiosperm trunk, 20 September 2017, Cui 16306 (BJFC).
Cyanosporus tenuis B.K. Cui, Shun Liu & Y.C. Dai, sp. nov. (Figures 4I–J, 9)
FIGURE 9.
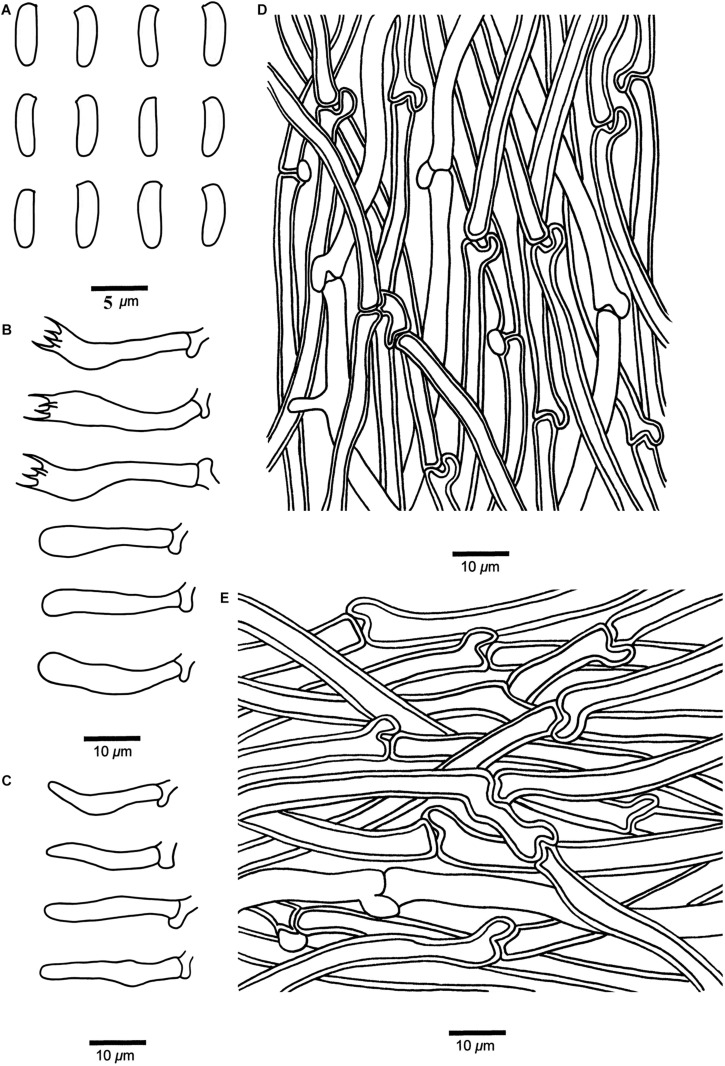
Microscopic structures of Cyanosporus tenuis (drawn from the holotype). (A) Basidiospores; (B) Basidia and basidioles; (C) Cystidioles; (D) Hyphae from trama; (E) Hyphae from context. Bars: (A) = 5 μm; (B–E) = 10 μm.
MycoBank: MB 838421
Differs from other Cyanosporus species by the thin basidiocarps, larger cystidioles (16.4–25.4 × 2.8–4.2 μm), and bigger basidiospores (4.7–6 × 1.3–2 μm).
Type. — CHINA. Sichuan Province, Luding County, Hailuogou Forest Park, on fallen trunk of Picea, 20 October 2012, Cui 10788 (BJFC).
Etymology. — Tenuis (Lat.): refers to the thin basidiocarps.
Basidiocarps. — Annual, effused-reflexed to pileate, soft corky, without odor or taste when fresh, becoming corky to fragile upon drying. Pileus flabelliform, projecting up to 1.5 cm, 1.8 cm wide and 0.4 cm thick at base. Pileal surface buff to olivaceous buff when fresh, finely tomentose, becoming cream to olivaceous buff when dry; margin acute. Pore surface white to cream when fresh, becoming buff yellow to pinkish buff when dry; sterile margin narrow to almost lacking; pores angular, 5–7 per mm; dissepiments thin, entire to lacerate. Context cream, soft corky, up to 2 mm thick. Tubes pinkish buff, fragile, up to 2 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae with clamp connections, IKI–, CB–; tissues unchanged in KOH.
Context. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, loosely interwoven, 2.6–7 μm in diam.
Tubes. — Generative hyphae hyaline, thin- to slightly thick-walled with a wide lumen, occasionally branched, interwoven, 2.2–4.8 μm in diam. Cystidia absent; cystidioles present, fusoid, thin-walled, 16.4–25.4 × 2.8–4.2 μm. Basidia clavate, bearing four sterigmata and a basal clamp connection, 18.2–27.6 × 3.7–6 μm; basidioles dominant, in a shape similar to basidia, but smaller.
Basidiospores. — Cylindrical, slightly curved, hyaline, thin- to slightly thick-walled, smooth, occasionally with small oil drops, IKI–, weakly CB+, (4.5–)4.7–6 × 1.3–2 μm, L = 5.44 μm, W = 1.76 μm, Q = 2.89–2.93 (n = 60/2).
Notes. — Phylogenetically, Cyanosporus tenuis grouped together with C. arbuti (Spirin) B.K. Cui & Shun Liu, C. luteocaesius (A. David) B.K. Cui, L.L. Shen & Y.C. Dai and C. simulans (P. Karst.) B.K. Cui & Shun Liu. Morphologically, C. arbuti differs in having narrower hyphae in the context and smaller basidiospores (4.1–5.1 × 1–1.2 μm) (Table 2); C. luteocaesius differs in larger pores (3–5 per mm; Table 2); C. simulans differs in smaller basidia (10–14.8 × 3.7–5.2 μm; Table 2). Cyanosporus fusiformis, C. piceicola, C. tricolor B.K. Cui, L.L. Shen & Y.C. Dai and C. ungulatus are also distributed in Sichuan Province, but C. fusiformis has a semicircular pileus, fusiform cystidioles presenting in the hymenium and narrow allantoid basidiospores (4.5–5.2 × 0.8–1.1 μm) (Table 2); C. piceicola has sub-rotund pileus, small angular pores, and slightly thick-walled and allantoid basidiospores (4–4.5 × 0.9–1.3 μm) (Table 2); C. tricolor has a white, blue, and pale mouse gray pileal surface when fresh (Shen et al., 2019); C. ungulatus has ungulate basidiocarps (Shen et al., 2019).
Additional specimen (paratype) examined. CHINA. Sichuan Province, Puge County, Luoji Mountain, on fallen trunk of Picea, 19 September 2012, Dai 12974 (BJFC).
Cyanosporus arbuti (Spirin) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838422
Basionym: Postia arbuti Spirin, Fungal Systematics and Evolution 1: 113, 2018.
Notes. — Postia arbuti was described from the United States (Miettinen et al., 2018). It is characterized by conchate, pendant to effused-reflexed basidiocarps with almost glabrous to matt pileal surfaces. It usually grows on Arbutus menziesii and is widely distributed in temperate areas of North America (North-West). In our study, the phylogenetic analysis (Figures 1–3) strongly supports its placement in Cyanosporus. For a detailed description of Postia arbuti, see Miettinen et al. (2018).
Cyanosporus auricoma (Spirin & Niemelä) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838423
Basionym: Postia auricoma Spirin & Niemelä, Fungal Systematics and Evolution 1: 115, 2018.
= Cyanosporus mongolicus B.K. Cui, L.L. Shen & Y.C. Dai, Persoonia 42: 115, 2019.
Notes. — Postia auricoma was described by Miettinen et al. (2018) and Cyanosporus mongolicus was described by Shen et al. (2019). Phylogenetically, the two species formed a highly supported lineage (Figures 1–3) and as the morphological characters of C. mongolicus fit well with Postia auricoma, we treat C. mongolicus as a synonym of P. auricoma, and P. auricoma is transferred to Cyanosporus as a new combination.
Specimens examined: CHINA. Inner Mongolia, Ewenk, Honghuaerji Nature Reserve, on fallen trunk of Pinus, 19 October 2015, Cui 13518, 13519 (BJFC).
Cyanosporus bifaria (Spirin) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838424
Basionym: Postia bifaria Spirin, Fungal Systematics and Evolution 1: 115, 2018.
Notes. — Postia bifaria was described by Miettinen et al. (2018). The morphological characters of the two specimens from China fit well with Postia bifaria and phylogenetically they clustered together within the genus Cyanosporus. Based on morphological characters and phylogenetic analysis, we transferred Postia bifaria to Cyanosporus as a new combination. For a detailed description of Postia bifaria, see Miettinen et al. (2018).
Specimens examined: CHINA. Sichuan Province, Xiangcheng County, on fallen trunk of Pinus, 12 August 2019, Cui 17445 (BJFC); Zhaojue County, on stump of Pinus, 16 September 2019, Cui 17806 (BJFC).
Cyanosporus caesiosimulans (G.F. Atk.) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838425
Basionym: Tyromyces caesiosimulans G.F. Atk., Annales Mycologici 6: 61, 1908.
= Postia caesiosimulans (G.F. Atk.) Spirin & Miettinen, Fungal Systematics and Evolution 1: 117, 2018.
Notes. — Tyromyces caesiosimulans was introduced primarily based on its globose basidiospores (Atkinson, 1908). Miettinen et al. (2018) transferred it to the Postia caesia complex based on morphological and molecular evidence. In our study, the phylogenetic analysis strongly supports its placement in Cyanosporus (Figures 1–3). For a detailed description of Postia caesiosimulans, see Miettinen et al. (2018).
Cyanosporus coeruleivirens (Corner) B.K. Cui, Shun Liu & Y.C. Dai, comb. nov.
MycoBank: MB 838426
Basionym: Tyromyces coeruleivirens Corner, Beihefte zur Nova Hedwigia 96: 163, 1989.
= Postia coeruleivirens (Corner) V. Papp, Mycotaxon 129 (2): 411, 2015.
Notes. — Tyromyces coeruleivirens was described from Borneo (Corner, 1989). It has a greenish pileus, a monomitic hyphal system, and allantoid inamyloid basidiospores (Corner, 1989; Hattori, 2002), and was considered as a member of the Postia caesia group (Hattori, 2002). Papp (2014) transferred Tyromyces coeruleivirens to the Postia caesia complex as a new combination. In our study, Postia coeruleivirens is transferred to Cyanosporus as a new combination; phylogenetically it is closely related to C. subhirsutus (Figures 1–3). Morphologically, both C. coeruleivirens and C. subhirsutus have annual basidiocarps, a monomitic hyphal system and allantoid basidiospores, but C. subhirsutus differs in having a disc-shaped pileus, hirsute and zonate pileal surface and larger pores (2–3 per mm; Shen et al., 2019). For a detailed description of Postia coeruleivirens, see Miettinen et al. (2018).
Specimens examined: CHINA. Hunan Province, Changde, Hefu Forest Park, on fallen angiosperm trunk, 17 October 2018, Dai 19220 (BJFC); Zhejiang Province, Hangzhou, Jiuxi Forest Park, on fallen angiosperm trunk, 17 October 2010, Dai 11834 (BJFC).
Cyanosporus comata (Miettinen) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838427
Basionym: Postia comata Miettinen, Fungal Systematics and Evolution 1: 118, 2018.
Notes. — Postia comata was described from the United States (Miettinen et al., 2018). It is morphologically similar to P. livens Miettinen & Vlasák but differs in having mostly thick-walled tramal hyphae and slightly smaller basidiospores (Miettinen et al., 2018). Its closest relative is the East Asian P. bifaria, which has smaller pores (6–8 per mm) and basidiospores (3.7–4.4 × 1.0–1.2 μm), and thin-walled tramal hyphae that collapse (Miettinen et al., 2018). Postia comata is transferred to Cyanosporus as a new combination based on morphological characters and molecular phylogeny. For a detailed description of Postia comata, see Miettinen et al. (2018).
Specimens examined: CHINA. Sichuan Province, Jiuzhaigou County, Zhangzha, on stump of Picea, 19 September 2020, Cui 18546 (BJFC); Xizang (Tibet), Mangkang County, on stump of Abies, 8 September 2020, Cui 18388 (BJFC).
Cyanosporus cyanescens (Miettinen) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838428
Basionym: Postia cyanescens Miettinen, Fungal Systematics and Evolution 1: 119, 2018.
Notes. — Postia cyanescens was described by Miettinen et al. (2018). This species is characterized by thin, conchate to flabelliform basidiocarps with light bluish grayish tinted pore surfaces, and long and narrow basidiospores (4.7–6.1 × 1.1–1.6 μm). In our study, the phylogenetic analysis (Figures 1–3) strongly supports its placement in Cyanosporus, so we transferred P. cyanescens to Cyanosporus as a new combination. For a detailed description of Postia cyanescens, see Miettinen et al. (2018).
Cyanosporus glauca (Spirin & Miettinen) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838429
Basionym: Postia glauca Spirin & Miettinen, Fungal Systematics and Evolution 1: 120, 2018.
Notes. — Postia glauca was described by Miettinen et al. (2018), it is characterized by thin, small and conchate basidiocarps with plumbeous to bluish gray or grayish brown pileal surfaces, and cream to light bluish grayish pore surfaces. In our study, the phylogenetic analysis (Figures 1–3) supports its placement in Cyanosporus, so we transferred it to Cyanosporus as a new combination. For a detailed description of Postia glauca, see Miettinen et al. (2018).
Cyanosporus gossypina (Moug. & Lév.) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838430
Basionym: Polyporus gossypinus Moug. & Lév., Annales des Sciences Naturelles Botanique 9: 124, 1848.
= Postia gossypina (Moug. & Lév.) Spirin & B. Rivoire, Fungal Systematics and Evolution 1: 120, 2018.
Notes. — Postia gossypina was proposed by Miettinen et al. (2018). Based on morphological characters and phylogenetic analysis, we transferred P. gossypina to Cyanosporus as a new combination. For a detailed description of Postia gossypina, see Miettinen et al. (2018).
Cyanosporus livens (Miettinen & Vlasák) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838431
Basionym: Postia livens Miettinen & Vlasák, Fungal Systematics and Evolution 1: 120, 2018.
Notes. — Postia livens was described from North America. It is the most common representative of the P. caesia complex in North America, and it can be easily identified by its plumbeous to bluish gray to ochraceous pileal surface often with bluish tints (Miettinen et al., 2018). In our study, it grouped with other species of the P. caesia complex in Cyanosporus (Figures 1–3), so, we transferred it into Cyanosporus as a new combination. For a detailed description of Postia livens, see Miettinen et al. (2018).
Cyanosporus magna (Miettinen) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838432
Basionym: Postia magna Miettinen, Fungal Systematics and Evolution 1: 121, 2018.
Notes. — Postia magna was described from China (Miettinen et al., 2018). Although we did not find the type specimen, we have examined two specimens from China, and its morphological characters fit well with this species. The phylogenetic analysis (Figures 1–3) also strongly supports its placement in Cyanosporus. So, we transferred Postia magna to Cyanosporus as a new combination. For a detailed description of Postia magna, see Miettinen et al. (2018).
Specimens examined: CHINA. Hainan Province, Ledong County, Jianfengling Nature Reserve, on fallen angiosperm trunk, 12 May 2009, Cui 10854 (BJFC); Jilin Province, Fusong County, Lushuihe Forest Park, on fallen angiosperm branch, 11 August 2011, Cui 10094 (BJFC); Yunnan Province, Binchuan County, Jizu Mountain, on fallen angiosperm branch, 14 September 2018, Cui 16983 (BJFC).
Cyanosporus populi (Miettinen) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838433
Basionym: Postia populi Miettinen, Fungal Systematics and Evolution 1: 122, 2018.
Notes. — Postia populi was described by Miettinen et al. (2018). Based on morphological characters and phylogenetic analyses, we transferred P. populi to Cyanosporus as a new combination. For a detailed description of Postia populi, see Miettinen et al. (2018).
Specimens examined: CHINA. Sichuan Province, Muli County, on fallen trunk of Pinus, 16 August 2019, Cui 17549 (BJFC); Yunnan Province, Lijiang, Yulong Snow Mountain, on fallen trunk of Picea, 16 September 2018, Cui 17087a (BJFC).
Cyanosporus simulans (P. Karst.) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838434
Basionym: Bjerkandera simulans P. Karst., Revue Mycologique Toulouse 10: 73, 1888.
= Postia simulans (P. Karst.) Spirin & B. Rivoire, Fungal Systematics and Evolution 1: 123, 2018.
Notes. — This species was described by Karsten (1888) as Bjerkandera simulans. Recently, Bjerkandera simulans was treated as an independent species in the Postia caesia complex by Miettinen et al. (2018). In our study, the phylogenetic analysis (Figures 1–3) supports its placement in Cyanosporus. For a detailed description of Postia simulans, see Miettinen et al. (2018).
Cyanosporus subviridis (Ryvarden & Guzmán) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838435
Basionym: Tyromyces subviridis Ryvarden & Guzmán, Mycotaxon 78: 252, 2001.
= Postia subviridis (Ryvarden & Guzmán) Spirin, Fungal Systematics and Evolution 1: 125, 2018.
Notes. — Tyromyces subviridis was described from a highland conifer forest in Mexico (Guzmán and Ryvarden, 2001). Miettinen et al. (2018) studied the type specimen, and transferred it to the Postia caesia complex. In our study, the phylogenetic analysis (Figures 1–3) supports its placement in Cyanosporus. For a detailed description of Postia subviridis, see Miettinen et al. (2018).
Cyanosporus yanae (Miettinen & Kotiranta) B.K. Cui & Shun Liu, comb. nov.
MycoBank: MB 838436
Basionym: Postia yanae Miettinen & Kotir., Fungal Systematics and Evolution 1: 125, 2018.
Notes. — Postia yanae was described by Miettinen et al. (2018). It is characterized by effused-reflexed or resupinate basidiocarps with detached or adnate margins, and white, with light to strong bluish grayish tinted pore surfaces. In our study, the phylogenetic analysis (Figures 1–3) supports its placement in Cyanosporus. For a detailed description of Postia yanae, see Miettinen et al. (2018).
Other Specimens of Cyanosporus Examined
Cyanosporus alni (Niemelä & Vampola) B.K. Cui, L.L. Shen & Y.C. Dai. CHINA. Guizhou Province, Suiyang County, Kuankuoshui Nature Reserve, on fallen angiosperm trunk, 26 June 2014, Dai 15060 (BJFC); Hebei Province, Xinglong County, Wulingshan Nature Reserve, on fallen angiosperm trunk, 29 August 2009, Cui 7185 (BJFC). CZECH REPUBLIC. Ceske Budejovice, on fallen trunk of Fagus, 22 November 2011, Dai 12709 (BJFC). FINLAND. Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Populus, 4 November 2011, Dai 12641 (BJFC). POLAND. Brynica, Mcrow, on fallen trunk of Fagus, 3 October 2014, Dai 14845 (BJFC).
Cyanosporus caesius. FINLAND. Uusimaa, Vantaa, Tamisto Nature Reserve, on fallen trunk of Picea, 3 November 2011, Dai 12605 (BJFC). SPAIN. Cadiz Province, Sierra Grazalema Natural Park, on fallen trunk of Abies, 22 November 2005, Dai 7438 (BJFC).
Cyanosporus fusiformis. CHINA. Guizhou Province, Suiyang County, Kuankuoshui Nature Reserve, on dead angiosperm tree, 26 November 2014, Dai 15036 (BJFC, holotype); Sichuan Province, Luding County, Hailuogou Forest Park, on dead tree of Rhododendron, 20 October 2012, Cui 10775 (BJFC, paratype).
Cyanosporus microporus. CHINA. Yunnan Province, Pu’er, Taiyanghe National Forest Park, on fallen angiosperm trunk, 8 July 2013, Cui 11014 (BJFC, holotype); Chuxiong, Zixishan Nature Reserve, on dead angiosperm tree, 28 August 2010, Dai 11717 (BJFC, paratype).
Cyanosporus piceicola. CHINA. Sichuan Province, Jiuzhaigou County, Jiuzhaigou Nature Reserve, on stump of Picea, 11 October 2012, Cui 10626 (BJFC, holotype), on fallen trunk of Picea, 11 October 2012, Cui 10617 (BJFC, paratype); Xizang (Tibet), Linzhi, Sejila Mountain, on fallen trunk of Picea, 18 September 2014, Cui 12158 (BJFC, paratype); Milin County, Nanyigou Forest Park, on fallen trunk of Picea, 16 September 2014, Cui 12088 (BJFC, paratype); Yunnan Province, Weixi County, Laojunshan Nature Reserve, on fallen trunk of Picea, 21 September 2011, Cui 10446 (BJFC, paratype).
Cyanosporus subhirsutus. CHINA. Fujian Province, Yongjing County, Huboliao Nature Reserve, on fallen angiosperm branch, 26 October 2013, Cui 11330 (BJFC, paratype); Guizhou Province, Jiangkou County, Fanjingshan Nature Reserve, on fallen trunk of Pterocarya, 21 November 2014, Dai 14892 (BJFC, holotype); Yunnan Province, Pu’er, Taiyanghe National Forest Park, on fallen angiosperm trunk, 8 July 2013, Cui 11019 (BJFC, paratype).
Cyanosporus subcaesius. FINLAND. Helsinki, Arabia, on angiosperm stump, 23 November 1996, Dai 2345 (IFP); Vantaa, on fallen trunk of Prunus, 4 October 1997, Dai 2725 (IFP).
Cyanosporus tricolor. CHINA. Sichuan Province, Luding County, Hailuogou Forest Park, on fallen trunk of Abies, 20 October 2012, Cui 10790 (BJFC, paratype), on fallen trunk of Picea, 20 October 2012, Cui 10780 (BJFC, paratype); Xizang (Tibet), Motuo County, on fallen branch of Abies, 20 September 2014, Cui 12233 (BJFC, holotype).
Cyanosporus ungulatus. CHINA. Sichuan Province, Mianning County, Lingshansi Park, on fallen branch of Castanopsis, 17 September 2012, Dai 12897 (BJFC, holotype); Luding County, Hailuogou Forest Park, on fallen trunk of Abies, 20 October 2012, Cui 10778 (BJFC, paratype).
Discussion
The genus Cyanosporus, usually with blue-tinted basidiocarps, is easy to recognize, but identification to species level is difficult as morphological features are quite similar among the species. The main morphological characters of the species in Cyanosporus are provided in Table 2.
In our current phylogenetic analyses, the genus Cyanosporus is supported as an independent genus; 31 species grouped together and formed a highly supported lineage (100% BS, 100% MP, 1.00 BPP; Figures 1–3), and were distant from other genera of Postia sensu lato. The current study also confirmed that Cyanosporus belongs to the antrodia clade phylogenetically and clusters with other brown-rot fungal genera, such as Amaropostia B.K. Cui, L.L. Shen & Y.C. Dai, Amylocystis Bondartsev & Singer ex Singer, Calcipostia B.K. Cui, L.L. Shen & Y.C. Dai, Cystidiopostia B.K. Cui, L.L. Shen & Y.C. Dai, Fuscopostia B.K. Cui, L.L. Shen & Y.C. Dai, Oligoporus Bref., Osteina Donk, Postia Fr. and Spongiporus Murrill, which is similar to previous studies (Ortiz-Santana et al., 2013; Ryvarden and Melo, 2014; Han et al., 2016; Shen et al., 2019).
The nomenclatural and taxonomic history of the Postia caesia complex was critically reviewed by Papp (2014), the generic name of the P. caesia complex have been changed many times, Papp proposed Cyanosporus as a subgenus of Postia to contain the P. caesia complex. Miettinen et al. (2018) revised the species concept of the Postia caesia complex based on morphology and two gene markers (ITS and TEF) and discussed that TEF sequences are more reliable for molecular identification of the P. caesia complex than ITS sequences. They also indicated that host tree is important for species identification of the P. caesia complex; their study raised the species number of the Postia caesia complex from 10 to 24, but their study did not focus on the taxonomic status for the P. caesia complex of Postia sensu lato.
Previously, species identification of the Postia caesia complex was only based on morphological characters and host trees in China. Samples grown on angiosperm woods were usually identified as Postia alni Niemelä & Vampola and those on gymnosperm woods as Postia caesia; only two species were recorded from China before Dai (2012). Shen et al. (2019) carried out a comprehensive phylogenetic and taxonomic study of Postia and related genera based on morphological characters and the combined seven-gene (ITS + nLSU + nSSU + mtSSU + RPB1 + RPB2 + TEF) sequences, Cyanosporus was confirmed as an independent genus of Postia sensu lato and 12 species belonging to the P. caesia complex were recognized in Cyanosporus, which was distant from Postia s. s.
In the current study, the novel species were supported by phylogenetic analyses based on ITS + TEF sequences, ITS + nLSU + TEF sequences and ITS + nLSU + nSSU + mtSSU + RPB1 + RPB2 + TEF sequences, respectively, but for the ITS sequences, several species have very similar base pairs and could not be separated by ITS sequences. The suitable DNA barcoding gene is the TEF gene for species identification of Cyanosporus, this result is consistent with a previous study by Miettinen et al. (2018). Our study expanded the number of Cyanosporus species to 31 around the world including 19 species from China. Our study indicated that more cryptic species could be discovered by combined evidence of morphological characters, molecular data, host trees and distribution areas in species complexes. However, several species of the Postia caesia complex, such as, P. africana (Ryvarden) V. Papp, P. amyloidea (Corner) V. Papp, P. atrostrigosa (Cooke) Rajchenb. and P. caesioflava (Pat.) V. Papp, are not included in the current phylogenetic analysis due to the lack of DNA sequences. Although the morphological characters of these species fit well with Cyanosporus, we did not transfer them to Cyanosporus in the current study. A fully resolved phylogeny for Cyanosporus and its related genera requires evolutionary information from more samples and more conserved gene markers.
In addition, some species with yellow basidiocarps, such as C. auricoma, C. caesioflava, and C. luteocaesius, and some species with white to cream colored basidiocarps, such as C. bubalinus and C. nothofagicola, are grouped together within Cyanosporus; this expanded the concept of the genus to include taxa without blue-tinted basidiocarps. More novel species might be discovered from different regions in future studies.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://purl.org/phylo/treebase, 27274.
Author Contributions
B-KC and SL designed the experiments. SL, L-LS, GG, and B-KC prepared the samples. SL, YW, and T-MX conducted the molecular experiments and analyzed the data. SL, GG, and B-KC drafted the manuscript. All the authors approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our gratitude to Drs. Tom May (Royal Botanical Gardens Victoria, Australia), Xiao-Lan He (Sichuan Academy of Agricultural Sciences, China), and Yu-Cheng Dai (Beijing Forestry University, China) for help during field collections.
Funding. This research was supported by the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (No. 2019HJ2096001006), the National Natural Science Foundation of China (Nos. U2003211 and 31750001), and the Beijing Forestry University Outstanding Young Talent Cultivation Project (No. 2019JQ03016).
Nomenclature
AR: Argentina; BI: Bayesian inference; BJFC: Herbarium of the Institute of Microbiology, Beijing Forestry University; BGI: Beijing Genomics Institute; BPP: Bayesian posterior probabilities; BT: Bootstrap; CB: Cotton Blue; CB +: cyanophilous; CB–: acyanophilous; GB: Great Britain; GTR + I + G: General time reversible + proportion invariant + gamma; CTAB: Cetyl trimethylammonium bromide; IFP: Herbarium of the Institute of Applied Ecology, Chinese Academy of Sciences; IKI: Melzer’s reagent, IKI–: neither amyloid nor dextrinoid; ILD: Incongruence length difference test; ITS: Internal transcribed spacer; KOH: 5% potassium hydroxide; L: mean spore length (arithmetic average of all spores); ML: Maximum likelihood; MP: Maximum parsimony; MPT: Most Parsimonious Tree; mtSSU: Mitochondrial small subunit rRNA; n (a/b): number of spores (a) measured from given number (b) of specimens; nLSU: Nuclear large subunit rDNA; nSSU: Nuclear small subunit rRNA; Q: variation in the L/W ratios between the specimens studied; RPB1: DNA-directed RNA polymerase II subunit 1; RPB2: DNA-directed RNA polymerase II subunit 2; TL: Tree length; W: mean spore width (arithmetic average of all spores); CI: Consistency Index; RI: Retention Index; RC: Rescaled Consistency Index; HI: Homoplasy Index; TEF: Translation elongation factor 1-α.
References
- Atkinson G. F. (1908). Notes on some new species of fungi from the United States. Ann. Mycol. 6 54–62. [Google Scholar]
- Chen Y. Y., Wu F., Wang M., Cui B. K. (2017). Species diversity and molecular systematics of Fibroporia (Polyporales, Basidiomycota) and its related genera. Mycol. Prog. 16 521–533. 10.1007/s11557-017-1285-1 [DOI] [Google Scholar]
- Corner E. J. H. (1989). Ad polyporaceas V. Beih. Nova Hedwigia 96 1–218. [Google Scholar]
- Cui B. K., Li H. J., Ji X., Zhou J. L., Song J., Si J., et al. (2019). Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 97 137–392. 10.1007/s13225-019-00427-4 [DOI] [Google Scholar]
- Dai Y. C. (2012). Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53 49–80. 10.1007/s10267-011-0134-3 [DOI] [Google Scholar]
- David A. (1974). Une nouvelle espèce de Polyporaceae: Tyromyces subcaesius. B. Mens. Soc. Linn. Lyon 43 119–126. [Google Scholar]
- David A. (1980). Étude du genre Tyromyces sensu lato: répartition dans les genres Leptoporus, Spongiporus et Tyromyces sensu stricto. B. Mens. Soc. Linn. Lyon 49 6–56. 10.3406/linly.1980.10404 [DOI] [Google Scholar]
- Donk M. A. (1960). The generic names proposed for Polyporaceae. Persoonia 1 173–302. [Google Scholar]
- Farris J. S., Källersjö M., Kluge A. G., Kluge A. G., Bult C. (1994). Testing significance of incongruence. Cladistics 10 315–319. 10.1006/clad.1994.1021 [DOI] [Google Scholar]
- Felsenstein J. (1985). Confidence intervals on phylogenies: an approach using the bootstrap. Evolution 39 783–791. 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- Fries E. M. (1821). Systema Mycologicum, Vol. 1 Lund: Officina Berlingiana. [Google Scholar]
- Guzmán G., Ryvarden L. (2001). Studies in neotropical polypores 12. New and noteworthy polypores from Mexico. Mycotaxon 78 245–256. [Google Scholar]
- Hall T. A. (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41 95–98. 10.1021/bk-1999-0734.ch008 [DOI] [Google Scholar]
- Han M. L., Chen Y. Y., Shen L. L., Song J., Vlasák J., Dai Y. C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: fomitopsis and its related genera. Fungal Divers. 80 343–373. 10.1007/s13225-016-0364-y [DOI] [Google Scholar]
- Hattori T. (2002). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. IV. Species described in Tyromyces (1). Mycoscience 43 307–315. 10.1007/s102670200045 [DOI] [Google Scholar]
- Hillis D. M., Bull J. J. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biodivers. 42 182–192. 10.1093/sysbio/42.2.182 [DOI] [Google Scholar]
- Jahn H. (1963). Mitteleuropäische Porlinge (Polyporaceae s. lato) und ihr Vorkommen in Westfalen. Westfälische Pilzbriefe 4 1–143. 10.1007/978-3-642-90915-3_1 [DOI] [Google Scholar]
- Jahn H. (1979). Pilze die an Holz Wachsen. Herford: Busse. [Google Scholar]
- Karsten P. A. (1881). Enumeratio boletinearum et polyporearum fennicarum, systemate novo dispositarum. Rev. Mycol. Toulouse 3 16–19. [Google Scholar]
- Karsten P. A. (1888). Diagnoses fungorum nonnullorum novorum, in Fennia detectorum. Rev. Mycol. Toulouse 10 73–75. [Google Scholar]
- Katoh K., Standley D. M. (2013). MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Song C. G., Cui B. K. (2019). Morphological characters and molecular data reveal three new species of Fomitopsis (Basidiomycota). Mycol. Prog. 18 1317–1327. 10.1007/s11557-019-01527-w [DOI] [Google Scholar]
- Lowe J. L. (1975). Polyporaceae of North America. The genus Tyromyces. Mycotaxon 2 1–82. 10.1007/978-3-319-23534-9_1 [DOI] [Google Scholar]
- Maddison W. P., Maddison D. R. (2017). Mesquite: A Modular System for Evolutionary Analysis, Version 3.2. Avaliable at: http://mesquiteproject.org. (accessed October 2020). [Google Scholar]
- Matheny P. B. (2005). Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Mol. Phylogenet. Evol. 35 1–20. 10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Matheny P. B., Liu Y. J., Ammirati J. F., Hall B. D. (2002). Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe. Agaricales). Am. J. Bot. 89 688–698. 10.3732/ajb.89.4.688 [DOI] [PubMed] [Google Scholar]
- McGinty N. J. (1909). A new genus, Cyanosporus. Mycol. Notes 33:436. [Google Scholar]
- Miettinen O., Vlasák J., Rivoire B., Spirin V. (2018). Postia caesia complex (Polyporales, Basidiomycota) in temperate Northern Hemisphere. Fungal Syst. Evol. 1 101–129. 10.3114/fuse.2018.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä T., Penttil R., Kinnunen J., Miettinen O., Turunen O. (2001). Novelties and records of poroid Basidiomycetes in Finland and adjacent Russia. Karstenia 41 1–21. 10.29203/ka.2001.373 [DOI] [Google Scholar]
- Nylander J. A. A. (2004). MrModeltest v2. Program Distributed by the Author. Uppsala: Uppsala University. [Google Scholar]
- Ortiz-Santana B., Lindner D. L., Miettinen O., Justo A., Hibbett D. S. (2013). A phylogenetic overview of the antrodia clade (Basidiomycota. Polyporales). Mycologia 105 1391–1411. 10.3852/13-051 [DOI] [PubMed] [Google Scholar]
- Papp V. (2014). Nomenclatural novelties in the Postia caesia complex. Mycotaxon 129 407–413. 10.5248/129.407 30528588 [DOI] [Google Scholar]
- Pieri M., Rivoire B. (2005). Postia mediterraneocaesia, une nouvelle espèce de polypore découverte dans le sud de l’Europe. Bull. Semestriel Féd. Assoc. Mycol. Méditerranéennes 28 33–38. [Google Scholar]
- Posada D., Crandall K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14 817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Rehner S. (2001). Primers for Elongation Factor 1-a (EF1-a). Avaliable at: https://www.docin.com/p-1613748809.html (accessed October 2020). [Google Scholar]
- Ronquist F., Huelsenbeck J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Ryvarden L., Melo I. (2014). Poroid fungi of Europe. Synop. Fungorum. 31 1–455. [Google Scholar]
- Schrader H. A. (1794). Spicilegium Florae Germanicae. Hannover: C. Ritscher. [Google Scholar]
- Shen L. L., Wang M., Zhou J. L., Xing J. H., Cui B. K., Dai Y. C. (2019). Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: postia (Polyporales, Basidiomycota) and related genera. Persoonia 42 101–126. 10.3767/persoonia.2019.42.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Cui B. K. (2017). Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evol. Biol. 17:102. 10.1186/s12862-017-0948-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analysis with thousands of taxa and mixed models. Bioinformatics 22 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Sun Y. F., Costa-Rezende D. H., Xing J. H., Zhou J. L., Zhang B., Gibertoni T. B., et al. (2020). Multi-gene phylogeny and taxonomy of Amauroderma s.lat. (Ganodermataceae). Persoonia 44 206–239. 10.3767/persoonia.2020.44.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D. L. (2002). PAUP∗. Phylogenetic Analysis Using Parsimony (∗ and other methods). Version 4.0b10. Sunderland, MA: Sinauer Associates, 10.1111/j.0014-3820.2002.tb00191.x [DOI] [Google Scholar]
- White T. J., Bruns T. D., Lee S., Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI] [Google Scholar]
- Zhu L., Song J., Zhou J. L., Si J., Cui B. K. (2019). Species diversity, phylogeny, divergence time and biogeography of the genus Sanghuangporus (Basidiomycota). Front. Microbiol. 10:812. 10.3389/fmicb.2019.00812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://purl.org/phylo/treebase, 27274.