Abstract
Metabolism differs in women and men at homeostasis. Critically ill patients have profound dysregulation of homeostasis and metabolism. It is not clear if the metabolic response to critical illness differs in women compared to men. Such sex-specific differences in illness response would have consequences for personalized medicine. Our aim was to determine the sex-specific metabolomic response to early critical illness. We performed a post-hoc metabolomics study of the VITdAL-ICU trial where subjects received high dose vitamin D3 or placebo. Using mixed-effects modeling, we studied sex-specific changes in metabolites over time adjusted for age, Simplified Acute Physiology Score II, admission diagnosis, day 0 25-hydroxyvitamin D level, and 25-hydroxyvitamin D response to intervention. In women, multiple members of the sphingomyelin and lysophospholipid metabolite classes had significantly positive Bonferroni corrected associations over time compared to men. Further, multiple representatives of the acylcarnitine, androgenic steroid, bile acid, nucleotide and amino acid metabolite classes had significantly negative Bonferroni corrected associations over time compared to men. Gaussian graphical model analyses revealed sex-specific functional modules. Our findings show that robust and coordinated sex-specific metabolite differences exist early in critical illness.
Subject terms: Metabolomics, Randomized controlled trials
Introduction
Though inclusiveness of women subjects in clinical research was mandated by the National Institutes of Health (NIH) in 1993, most clinical research studies do not account for sex-specific differences1–3. The research that does exist shows that robust differences exist between women and men with respect to disease incidence, disease severity, metabolism and pharmacodynamics of interventions4,5. Although firm evidence exists for improved outcomes for female animals in experimental models of severe illness, such differences are not consistently observed in studies on critically ill patients6–9. Mechanistic understanding of sex-specific differences in the response to illness is essential if we are to progress to personalized medicine10.
Existing data show that metabolism differences are present in healthy women relative to men. At homeostasis, women incorporate free fatty acids into triglycerides whereas men oxidize circulating free fatty acids11. Circulating acylcarnitines which are reflective of energy metabolism, are generally lower in women12. Women also have less free fatty acid-induced insulin resistance13. Healthy women have increases in circulating lipid sphingomyelins which act in cell signaling and may reflect glucose metabolism14–17. Sex-specific differences in lipid and cholesterol metabolism are well established and likely due to sex chromosome and sex-specific hormone action18. The overall sex-specific metabolism differences at homeostasis are probably due to variation in metabolism related gene expression which contributes to sexual dimorphism12,19.
Metabolomics provides a window into the large number of circulating substrates and products of patient’s cellular metabolism20. A few large metabolomics studies on healthy individuals are notable for robust metabolite differences related to sex12,19,21–23. Data from healthy subjects has little relevance to critically ill patients where metabolic homeostasis is profoundly disturbed24. Heterogenous critical illness is not defined by a precise phenotypic framework and studies have provided limited mechanistic insights into pathophysiology25. Metabolomic studies performed early in critical illness can reflect illness severity and predict outcomes. But such work does not address sex-specific differences in the response to critical illness26–28. Therefore, to see whether sex-specific metabolism differences apply to the critically ill, we studied differences between women and men with regards to changes in metabolism during critical illness.
To test the hypothesis that significant sex-specific plasma metabolomic profile differences exist in the response to critical illness, we performed a metabolomics analysis of 1215 plasma samples from 428 subjects collected during the VITdAL-ICU trial29. The VITdAL-ICU trial randomized 492 critically ill adults (166 of whom were women) with 25-hydroxyvitamin D [25(OH)D] levels ≤ 20 ng/ml to high dose oral vitamin D3 or placebo. The VITdAL-ICU trial did not find significant differences in length of hospital stay or mortality outcomes. We assessed the effect of sex on changes in individual metabolites and plasma metabolite families over three time points early in the course of critical illness. Further, with the metabolite change data we determined if regulated metabolite modules exist that associate with sex.
Results
In the 428 subject analytic cohort, 35% of subjects were women. Baseline characteristics were balanced between subjects stratified by sex for C-reactive protein, Simplified Acute Physiology Score (SAPS) II, day 0 25(OH)D levels, intervention status and ICU type. Differences existed by sex with respect to age (see Table 1 and Supplementary Table S1). The overall 28-day mortality of the 428 subject analytic cohort was 22.2%. The 28-day mortality in women was 22.5% and in men was 22.0%.
Table 1.
Cohort characteristics.
| Characteristic | Female | Male | Total | P-value |
|---|---|---|---|---|
| No | 151 | 277 | 428 | |
| Age years Mean (SD) | 68.2 (13.3) | 62.0 (15.3) | 64.2 (14.9) | < 0.001* |
| Day 0 25(OH)D ng/ml Mean (SD) | 13.2 (5.7) | 14.4 (10.1) | 13.9 (8.8) | 0.17* |
| SAPS II Mean (SD) | 34.6 (14.7) | 32.7 (15.8) | 33.4 (15.4) | 0.24* |
| Day 0 C-reactive protein μg/mL Mean (SD) | 119.9 (96.4) | 127.6 (86.0) | 124.9 (89.8) | 0.40* |
| Day 0 Procalcitonin ng/ml Median [IQR] | 0.45 [0.14, 1.98] | 0.77 [0.20, 3.02] | 0.66 [0.17, 2.79] | < 0.001† |
| Vitamin D3 Intervention No. (%) | 78 (51.7) | 134 (48.4) | 212 (49.5) | 0.52 |
| Change in 25(OH)D ng/ml Mean (SD) | 11.3 (18.0) | 10.0 (15.5) | 10.4 (16.4) | 0.43* |
| ICU | 0.22 | |||
| Anesthesia ICU No. (%) | 24 (15.9) | 59 (21.3) | 83 (19.4) | |
| Cardiac Surgery ICU No. (%) | 42 (27.8) | 84 (30.3) | 126 (29.4) | |
| Surgical ICU No. (%) | 7 (4.6) | 16 (5.8) | 23 (5.4) | |
| Medicine ICU No. (%) | 31 (20.5) | 59 (21.3) | 90 (21.0) | |
| Neurological ICU No. (%) | 47 (31.1) | 59 (21.3) | 106 (24.8) |
Data presented as No. (%) unless otherwise indicated. P-values determined by chi-square unless designated by (*) then P-value determined by ANOVA or by (†) determined by Kruskal–Wallis test.
Single time point data
In day 0 plasma samples (N = 428), significant differences exist in 12 individual metabolites (all multiple test-corrected threshold of P-value < 8.65 × 10–5, − log10(P) > 4.06) and in metabolomic profiles (CV-ANOVA P-value < 0.001) in female subjects relative to males (see Supplementary Table S2). Regarding subject metabolomic profiles, though the multivariable OPLS-DA model had marginal predictability (Q2 = 0.42), the permutation test confirmed the stability and robustness of the model (Q2 intercept of − 0.387) with a negative permutation Q2 intercept indicating model validity (see Supplementary Table S2)30,31. Day 0 differences are present with increased individual sphingomyelin species and decreased androgenic steroids in women relative to men (see Supplementary Table S3).
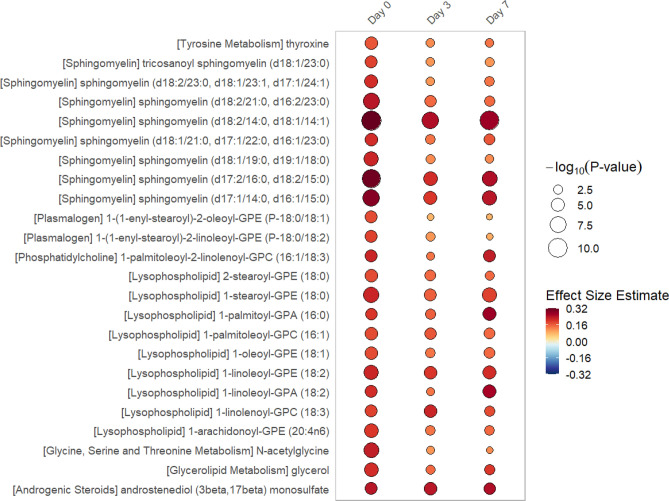
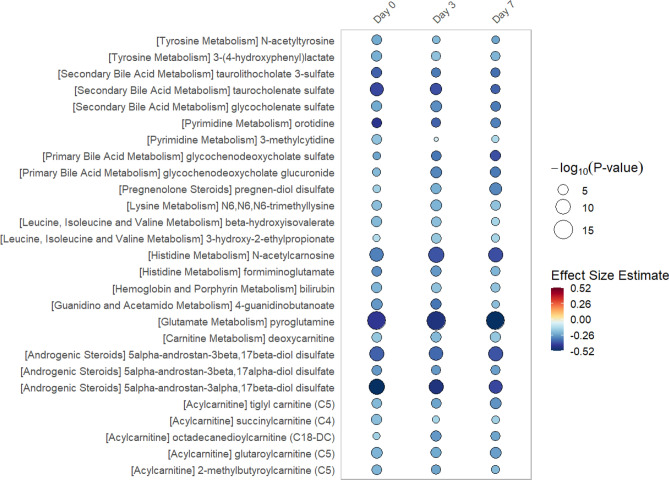
In linear regression models of metabolite data from single time points (day 0, 3 or 7), we find significant differences exist in 51 individual metabolites at 1 or more time point (all multiple test-corrected threshold of P-value < 8.65 × 10–5, − log10(P) > 4.06). The rain plots32 separately show the metabolites that increase (see Fig. 1) or decrease (see Fig. 2) in women relative to men, with greater significance shown by an increase in circle size. In the data from single time points, significant increases in individual sphingomyelin species and lysophopholipids are found in women when compared to men. Decreases in androgenic steroids as well as bile acid and amino acid metabolism are found in women relative to men.
Figure 1.
Rain Plot of single time point metabolites Increased in Women. Correlations between individual metabolites and sex at day 0, 3 or 7 were determined utilizing linear regression models correcting for age, SAPS II, admission diagnosis, 25(OH)D at day 0. Day 3 and 7 estimates were also corrected for absolute change in 25(OH)D level at day 3. The magnitude of beta coefficient estimates (effect size) is shown by a color fill scale and the corresponding significance level (− log10(P-value)) is represented by size of the circle. The intensity of the red fill color represents an increase in effect size for that metabolite in women compared to men. Statistical significance is the multiple test-corrected threshold of − log10(P-value) > 4.06 which is equivalent to P-value < 8.65 × 10−5.
Figure 2.
Rain Plot of single time point metabolites Decreased in Women. Correlations between individual metabolites and sex at day 0, 3 or 7 were determined utilizing linear regression models correcting for age, SAPS II, admission diagnosis, 25(OH)D at day 0. Day 3 and 7 estimates were also corrected for absolute change in 25(OH)D level at day 3. The magnitude of beta coefficient estimates (effect size) is shown by a color fill scale and the corresponding significance level (− log10(P-value)) is represented by size of the circle. The blue fill color represents an decrease in effect size for that metabolite in women compared to men. Statistical significance is the multiple test-corrected threshold of − log10(P-value) > 4.06 which is equivalent to P-value < 8.65 × 10–5.
Multiple time point data
In the repeated measures data, mixed-effects modeling of 1215 total day 0, 3 and 7 plasma samples from the analytic cohort (N = 432) shows 50 metabolites had significantly positive associations in women relative to men highlighted by increases in individual sphingomyelin species and lysophospholipids (see Summarized data in Table 2, Full data in Supplementary Table S4). One hundred five metabolites had significantly negative associations in women relative to men primarily by decreases in acylcarnitine, androgenic steroid, bile acid, nucleotide and amino acid metabolites (see Summarized data in Table 3, Full data in Supplementary Table S5). The mixed-effects modeling of only those subjects who received placebo (N = 216), though limited in power, showed similar patterns as the analytic cohort (N = 432) with Benjamini–Hochberg adjustment33 (Supplementary Data 1). data A bipartite graph34 highlights metabolites of the lysophospholipid, acylcarnitine, androgenic steroid, bile acid, nucleotide and amino acid metabolite sub-pathways and individual sphingomyelin species that significantly increase or decrease in women relative to men over days 0, 3 and 7 (see Fig. 3).
Table 2.
Metabolites significantly increased in women relative to men over time.
| Metabolite | P-value | Bonferroni corrected P-value | − log10p | β coefficient | Super pathway | Sub pathway |
|---|---|---|---|---|---|---|
| 1-linoleoyl-GPE (18:2) | 3.71 E−15 | 2.14 E−12 | 14.43 | 0.21 | Lipid | Lysophospholipid |
| 1-palmitoyl-GPA (16:0) | 1.77 E−11 | 1.02 E−08 | 10.75 | 0.20 | Lipid | Lysophospholipid |
| 1-linoleoyl-GPA (18:2) | 5.69 E−10 | 3.29 E−07 | 9.24 | 0.20 | Lipid | Lysophospholipid |
| 1-linolenoyl-GPC (18:3) | 1.57 E−11 | 9.08 E−09 | 10.80 | 0.20 | Lipid | Lysophospholipid |
| 1-stearoyl-GPE (18:0) | 6.74 E−16 | 3.90 E−13 | 15.17 | 0.19 | Lipid | Lysophospholipid |
| 1-palmitoleoyl-GPC (16:1) | 5.95 E−11 | 3.44 E−08 | 10.23 | 0.17 | Lipid | Lysophospholipid |
| 1-arachidonoyl-GPE (20:4n6) | 2.74 E−10 | 1.58 E−07 | 9.56 | 0.16 | Lipid | Lysophospholipid |
| 2-stearoyl-GPE (18:0) | 2.13 E−09 | 1.23 E−06 | 8.67 | 0.16 | Lipid | Lysophospholipid |
| 1-oleoyl-GPE (18:1) | 5.81 E−10 | 3.36 E−07 | 9.24 | 0.16 | Lipid | Lysophospholipid |
| 2-palmitoyl-GPC (16:0) | 7.59 E−09 | 4.39 E−06 | 8.12 | 0.15 | Lipid | Lysophospholipid |
| 1-lignoceroyl-GPC (24:0) | 3.89 E−06 | 2.25 E−03 | 5.41 | 0.15 | Lipid | Lysophospholipid |
| 1-linoleoyl-GPI (18:2) | 9.30 E−08 | 5.37 E−05 | 7.03 | 0.14 | Lipid | Lysophospholipid |
| 1-arachidonoyl-GPC (20:4) | 8.26 E−07 | 4.78 E−04 | 6.08 | 0.13 | Lipid | Lysophospholipid |
| Sphingomyelin (d18:2/14:0, d18:1/14:1) | 6.93 E-29 | 4.01 E−26 | 28.16 | 0.28 | Lipid | Sphingomyelin |
| Sphingomyelin (d17:2/16:0, d18:2/15:0) | 1.33 E−21 | 7.70 E−19 | 20.88 | 0.26 | Lipid | Sphingomyelin |
| Sphingomyelin (d17:1/14:0, d16:1/15:0) | 1.92 E−18 | 1.11 E−15 | 17.72 | 0.24 | Lipid | Sphingomyelin |
| Sphingomyelin (d18:2/21:0, d16:2/23:0) | 4.11 E−13 | 2.38 E−10 | 12.39 | 0.18 | Lipid | Sphingomyelin |
| Sphingomyelin (d18:1/21:0, d17:1/22:0, d16:1/23:0) | 3.76 E−10 | 2.17 E−07 | 9.43 | 0.17 | Lipid | Sphingomyelin |
| Sphingomyelin (d18:1/19:0, d19:1/18:0) | 1.28 E−09 | 7.38 E−07 | 8.89 | 0.15 | Lipid | Sphingomyelin |
| Sphingomyelin (d18:2/23:0, d18:1/23:1, d17:1/24:1) | 2.74 E−09 | 1.58 E−06 | 8.56 | 0.15 | Lipid | Sphingomyelin |
| Tricosanoyl sphingomyelin (d18:1/23:0) | 5.28 E−08 | 3.05 E−05 | 7.28 | 0.13 | Lipid | Sphingomyelin |
| Sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0, d19:1/24:0) | 4.60 E−05 | 2.66 E−02 | 4.34 | 0.11 | Lipid | Sphingomyelin |
Significant results presented following mixed-effects modeling of each of the 578 individual metabolites measured at day 0, 3 and 7. All estimates adjusted for age, SAPS II, admission diagnosis, 25(OH)D at day 0, absolute change in 25(OH)D level at day 3 and plasma day (as the random-intercept). A multiple test-corrected threshold of P-value < 8.65 × 10–5 was used to identify all significant associations. GPC is glycerophosphorylcholine; GPE is glycerophosphoethanolamine; GPI is glycosylphosphatidylinositol. Positive β coefficient values indicate higher abundance in females relative to males.
Table 3.
Metabolites significantly decreased in Women relative to Men over time.
| Metabolite | P-value | Bonferroni corrected P-value | − log10p | β coefficient | Super pathway | Sub pathway |
|---|---|---|---|---|---|---|
| N-acetylvaline | 2.55 E−05 | 1.47 E−02 | 4.59 | − 0.10 | Amino acid | Leucine, isoleucine and valine metabolism |
| N-acetylleucine | 7.65 E−06 | 4.42 E−03 | 5.12 | − 0.12 | Amino acid | Leucine, isoleucine and valine metabolism |
| Alpha-hydroxyisocaproate | 9.27 E−07 | 5.36 E−04 | 6.03 | − 0.13 | Amino acid | Leucine, isoleucine and valine metabolism |
| 3-hydroxyisobutyrate | 1.37 E−06 | 7.90 E−04 | 5.86 | − 0.13 | amino acid | Leucine, isoleucine and valine metabolism |
| 3-hydroxy-2-ethylpropionate | 2.06 E−09 | 1.19 E−06 | 8.69 | − 0.15 | Amino acid | Leucine, isoleucine and valine metabolism |
| 2-hydroxy-3-methylvalerate | 5.00 E−08 | 2.89 E−05 | 7.30 | − 0.16 | Amino acid | Leucine, isoleucine and valine metabolism |
| 2,3-dihydroxy-2-methylbutyrate | 4.77 E−07 | 2.76 E−04 | 6.32 | − 0.17 | Amino acid | Leucine, isoleucine and valine metabolism |
| Beta-hydroxyisovalerate | 4.04 E−12 | 2.33 E−09 | 11.39 | -0.19 | Amino acid | Leucine, isoleucine and valine metabolism |
| Glycochenodeoxycholate | 1.59 E−06 | 9.18 E−04 | 5.80 | − 0.20 | Lipid | Primary bile acid metabolism |
| Taurocholate | 4.19 E−05 | 2.42 E−02 | 4.38 | − 0.21 | Lipid | Primary bile acid metabolism |
| Glycochenodeoxycholate glucuronide | 2.94 E−12 | 1.70 E−09 | 11.53 | − 0.28 | Lipid | Primary bile acid metabolism |
| Taurochenodeoxycholate | 2.20 E−08 | 1.27 E−05 | 7.66 | − 0.28 | Lipid | Primary bile acid metabolism |
| Glycochenodeoxycholate sulfate | 4.26 E−11 | 2.46 E−08 | 10.37 | − 0.32 | Lipid | Primary bile acid metabolism |
| Glycodeoxycholate sulfate | 4.01 E−06 | 2.32 E−03 | 5.40 | − 0.23 | Lipid | Secondary bile acid metabolism |
| Glycolithocholate sulfate | 5.82 E−09 | 3.36 E−06 | 8.24 | − 0.27 | Lipid | Secondary bile acid metabolism |
| Glycocholenate sulfate | 6.22 E−14 | 3.59 E−11 | 13.21 | − 0.28 | Lipid | Secondary bile acid metabolism |
| Taurolithocholate 3-sulfate | 3.11 E−12 | 1.80 E−09 | 11.51 | − 0.34 | Lipid | Secondary bile acid metabolism |
| Taurocholenate sulfate | 1.03 E−16 | 5.94 E−14 | 15.99 | − 0.38 | Lipid | Secondary bile acid metabolism |
Significant results presented following mixed-effects modeling of each of the 578 individual metabolites measured at day 0, 3 and 7. All estimates adjusted for age, SAPS II, admission diagnosis, 25(OH)D at day 0, absolute change in 25(OH)D level at day 3 and plasma day (as the random-intercept). A multiple test-corrected threshold of P-value < 8.65 × 10–5 was used to identify all significant associations. Negative β coefficient values indicate lower abundance in females relative to males.
Figure 3.
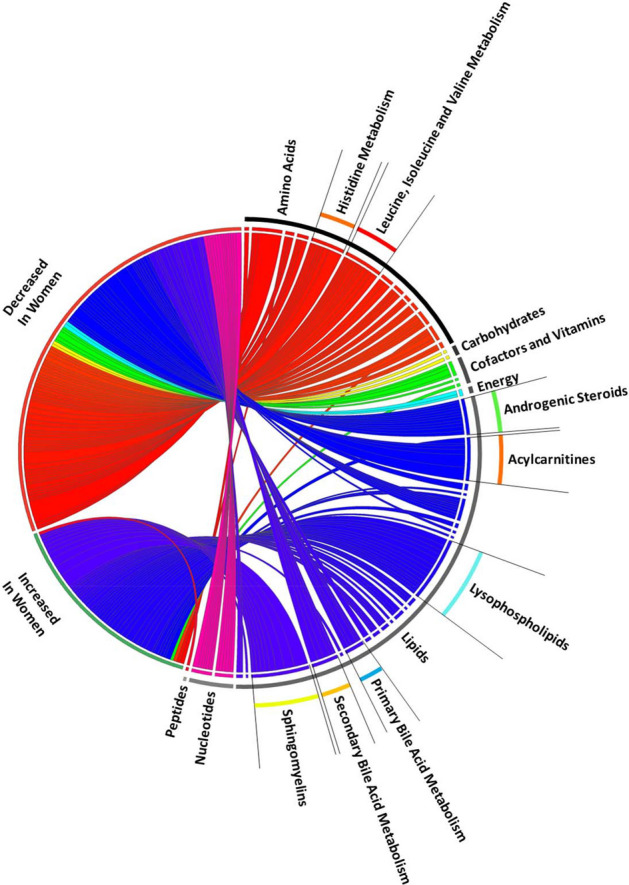
Circos Plot of metabolites over multiple time points. Bipartite graph of metabolites measured in 1215 plasma samples from 428 subjects. Metabolites shown are determined by mixed-effects linear regression to be significantly increased or decreased in women relative to men over the first seven days following trial enrollment. The graph connects the increase or decrease in metabolite on the left side with individual metabolites on the right side. Width of curves indicates strength of the significance (− log10(P-value)) as determined by mixed-effects regression. Colors differ for each sub-pathway (i.e. all amino acid metabolites are red, all lipids are blue). All curves shown have P-value < 8.65 × 10–5 in mixed-effects linear regression analysis.
Next, we explored the sex-specific associations of individual metabolites and 28-day mortality. We compared mixed-effects modeling of a total of 441 day 0, 3 and 7 plasma samples from 151 women in the analytic cohort to mixed-effects modeling of a total of 814 day 0, 3 and 7 plasma samples from 277 men in the analytic cohort. The data show that an increase in short chain acylcarnitines C4–C8 and branched-chain amino acids significantly associate with three fold higher 28-day mortality in women but not men (see Supplementary Table S6, Supplementary Fig. S1).
Metabolic networks and mediation
We investigated sex-specific metabolic networks by measuring pairwise correlations in metabolites which have similar effects via Gaussian graphical models (GGMs). The GGMs analysis revealed seven sex-specific functional modules at day 3 and seven at day 7 (see Supplementary Tables S7 & S8). Similar to the mixed-effects analyses, metabolism of branched chain amino acids, bile acids, androgenic steroids and lysophospholipids are prominently featured in the sex-specific GGM modules. Metabolites within in each functional module were either increased or decreased in women in unison and had biological or functional similarity. Of note, the sex-specific modules do include some individual metabolites that were not significantly associated with sex in our mixed-effects analysis (see Supplementary Tables S7 & S8: Modules B and E, H, I, K, M).
Finally, we focused on the potential mediation of the relationship between individual metabolite abundance and sex by inflammation status. Mediation analyses in day 3 data revealed no influence of Procalcitonin or of C-reactive protein on the associations between sex and each of the individual 578 metabolites (all P-values were > 0.01 using 2000 bootstrap samples).
Discussion
Although previous work suggests that some sex-specific differences exist in the healthy at homeostasis35, our data argue that distinct nuanced alterations in metabolism are present in women and men during critical illness. In our single time point data and our mixed-effects methods analysis, we consistently find robust increases and decreases in groups of metabolites along similar sub-pathways that have conserved function (see Fig. 3, Supplementary Tables S4 & S5). Further, we illustrate how groups of metabolites with similar sex-specific effects form GGM modules which highlight the same sub-pathways as our single time point data and our mixed-effects methods analysis12. These modules serve to focus potential biological interpretation of our sex-specific metabolomics observations36. All three analyses highlight the importance of sex-specific metabolism in critical illness related to branched chain amino acids, bile acids, androgenic steroids and lysophospholipids.
Critically ill patients preferentially catabolize fatty acids and amino acids for mitochondrial energy production. Sex-specific differences exist in the utilization of carbohydrates and lipids as energy. With increased energy needs during cell stress, women preferentially oxidize lipids over carbohydrates while men utilize carbohydrates37. Firm evidence exists that female mitochondria have higher oxidative capacity, produce less reactive oxygen species, and preferentially utilize lipids for bioenergetics38–40. Elevated circulating even-chain C4–C22 acylcarnitines are shown to be due to incomplete mitochondrial β‐oxidation of fatty acids41,42. Studies find that increases in circulating acylcarnitines are common in severe critical illness and are associated with adverse outcomes27. To see whether these findings apply to critically ill women, we analyzed a total of 36 acylcarnitine species. We demonstrate that 15 circulating acylcarnitine species are significantly lower in women early in critical illness (see Supplementary Table S5). Our data are consistent with a more efficient fatty acid β-oxidation in critically ill women reflective of a sex-specific difference in mitochondrial response to critical illness.
The circulating amino acid pool is supplied by dietary amino acids, endogenous amino acid synthesis and cellular protein turnover43. Increases in circulating amino acids during critical illness are due to protein catabolism44. Skeletal muscle protein is rapidly metabolized in response to severity of illness to provide substrate for liver gluconeogenesis, immune function support and immunoglobulin synthesis45. Further, amino acid catabolism is a source for circulating C3, C4 and C5 acylcarnitines42. Our findings of decreases in C3, C4 and C5 acylcarnitines as well as in multiple amino acid metabolite sub-pathways suggest sex-specific protein catabolism and energy substrate utilization during critical illness. Of particular interest, are the GGM modules B and H (Supplementary Tables S7 & S8) which highlight the importance of decrease in branched chain amino acid metabolites in women. In women, we observe a combination of decreases in branch chain amino acid metabolites and in dicarboxylate fatty acids generated from fatty acid omega oxidation as well as in short-chain acylcarnitines C3 and C5 derived from branch chain amino acids (Supplementary Table S5). Such decreases strongly suggest improved mitochondrial function and more complete fatty acid β-oxidation in women relative to men46,47.
Our novel observations suggest that critically ill women have greater abundance of individual sphingomyelin species, plasmalogens and lysophospholipids compared to men. Studies show that during cell stress, specific sphingomyelin species regulate the initiation of apoptosis and autophagy48. Plasmalogens are known to be endogenous antioxidants that protect the endothelium from oxidative stress injury by controlling toxic oxidation products49. Lysophospholipids are signaling molecules that have chemoattractant effects and act to modulate the innate immune response50,51. Our data are consistent with the hypothesis that the response to cell stress differs in women and men.
The liver is the essential organ for glucose, protein, amino acid, lipid and cholesterol metabolism. Sex-specific differences in liver metabolism at homeostasis are postulated to be an evolutionary consequence of the metabolic flexibility required for reproduction10. Our data argue that bile acid metabolites are decreased in critically ill women. Though sex-specific differences in bile acid synthesis are reported52, such differences in bile acid homeostasis are not well characterized53. It is shown that cytochrome P450 enzymes are important for bile synthesis 52 and regulated in a sex-specific manner54,55. Bile acids activate the nuclear receptors farnesoid X receptor, pregnane X receptor and vitamin D receptor as well as the G-protein-coupled receptor TGR5. Such bile acid receptor activation results in gene expression which alters metabolism of bile acids, glucose, lipids, energy and inflammation56. As elevation in blood bile acids are common in critical illness57, and the synthesis and pool composition of bile acids are sex-specific, such differences have widespread downstream metabolism pathway effects.
Our novel study approach has several strengths. The use of a large number of plasma samples at multiple time points early in critical illness allows for a dynamic overview into sex-specific metabolomics (see Fig. 3). Mixed models are extremely useful for metabolomic data measured at multiple time points as they remove confounding variables with a fixed-effect (age, SAPS II, etc.) and also those with a random-effect (plasma sampling day)58,59. Importantly, by adjusting for the absolute change in 25(OH)D level at day 3, we mitigate the effect of the trial intervention on the observed sex-specific metabolomic changes which allow for study of the entire trial cohort increasing sample size and study power60,61. Further, our use of clinical trial data allows for modelling and normalization of metabolite abundance via adjustment for subject characteristics62. To account for multiple comparisons we utilized a conservative Bonferroni corrected P-value < 8.65 × 10–5 63. Finally, some of the metabolism differences we observe are known to be sex-specific thus increasing the biological plausibility and relevance of our work.
We do acknowledge potential limitations to our approach. Our VITdAL-ICU trial subject population is heterogenous with sex-specific imbalance in some admission diagnosis categories. Despite multivariable adjustment, our approach is subject to bias and confounding. Though our samples are derived from a randomized controlled trial, our study design is observational thus causal inference may be limited. Our subjects were all white with serum levels of 25(OH)D < 20 ng/ml, thus may not be representative of all critically ill. Our use of CRP as an indicator of inflammation is limited in the in the nine reproductive-aged women under study as CRP is associated with Progesterone and Estradiol levels64. The single-center setting may limit generalizability of our findings. It is important to recognize that although the function and biological relevance of a metabolite may be characterized, the clinical significance may not be known. Finally, our study is a hypothesis generating exploratory analysis requiring subsequent confirmation and careful interpretation.
The importance of our study is that it offers a nuanced window into the differential metabolic response to critical illness between women and men. Beyond the known sex-dependent metabolism differences at homeostasis, we find that women respond to critical illness stressors in a dramatically different fashion than men. Our findings on sex-specific differences in metabolism pathways is an essential first step toward understanding how to provide patient-centered personalized medicine in the critically ill.
Methods
The details of the VITdAL-ICU trial29 as well as metabolomic processing and analysis are provided in Supplementary Methods. Briefly, the VITdAL-ICU trial randomized 475 critically ill adult patients to vitamin D3 or placebo once at a dose of 540,000 IU followed by 90,000 IU monthly29. At VITdAL-ICU trial enrollment, written informed consent was obtained and included permission for plasma specimens to be saved for future research studies29. The metabolomics study is considered post-hoc as it was designed following initiation completion of the of the VITdAL-ICU trial. The post-hoc study research protocol was approved by the Partners Human Research Committee Institutional Review Board at the Brigham and Women’s Hospital. All research was performed in accordance with the Declaration of Helsinki.
To generate metabolomic data, a total of 1215 plasma samples from 428 VITdAL-ICU trial subjects at day 0, 413 subjects at day 3 and 374 subjects at day 7 were analyzed using four ultra high-performance liquid chromatography/tandem accurate mass spectrometry methods by Metabolon, Inc65. Metabolomic profiling identified 769 metabolites (Supplementary Data 2). We reduced baseline noise by removing metabolites with the lowest interquartile range of variability, leaving 578 metabolites66. Metabolomic data underwent cube root transformation and Pareto scaling to normalize the distribution67.
For univariate analysis of day 0 data, Student’s t-test was performed to determine if significant sex-specific differences exist using MetaboAnalyst68. A Bonferroni multiple testing correction threshold of P-value < 8.65 × 10–5 was used to identify all significant differences63. Day 0 data were also analyzed using orthogonal partial least square-discriminant analysis (OPLS-DA), a supervised method to assess the significance of classification discrimination (SIMCA 15.0 Umetrics, Umea, Sweden). Permutation testing was performed for OPLS-DA model validation30,31. Sevenfold cross-validation analysis of variance (CV-ANOVA) was utilized to determine OPLS-DA model significance31.
For single time point data, correlations between individual metabolites and sex at day 0, 3 or 7 were separately determined utilizing linear regression models correcting for age, SAPS II, admission diagnosis, 25(OH)D at day 0 and absolute change in 25(OH)D level at day 3. A multiple test-corrected threshold of P-value < 8.65 × 10–5 was used to identify all significant associations in the single time point data63. All linear regression models were analyzed using STATA 14.1MP69. Rain plots were produced based on hierarchical clustering in R-3.6.2 adapted from source code published by Henglin et al.32.
For repeated measures data, correlations between individual metabolites and sex over time (day 0, 3 and 7) were determined utilizing linear mixed-effects models correcting for age, SAPS II, admission diagnosis, 25(OH)D at day 0, absolute change in 25(OH)D level at day 3 and plasma day (as the random-intercept). This analysis was performed in the analytic cohort (N = 428) with multiple test-corrected threshold of P-value < 8.65 × 10–5 was used to identify all significant associations. We repeated the analysis in only those subjects who received placebo (N = 216) with Benjamini–Hochberg adjustment of P-values33. All mixed-effects models were analyzed using STATA 14.1MP69. For data visualization purposes, a bipartite graph34 utilizing the Circos application (http://circos.ca/) in Perl was generated of metabolites which were significantly changed (increased or decreased) in females relative to males.
Mixed effects logistic regression was used separately in 151 women and in 277 men to estimate the odds of 28-day mortality of individual metabolites adjusted for age, SAPS II, admission diagnosis, 25(OH)D at day 0, absolute change in 25(OH)D level at day 3 and plasma day (as the random-intercept). A multiple test-corrected threshold of P-value < 8.65 × 10–5 was used to identify all significant associations in the repeated measures data63. All mixed-effects models were analyzed using STATA 14.1MP69. We used rain plots32 to separately visualize the mortality-dependent effect size and significance of individual metabolites in women and men.
As inflammation is important in response to critical illness, we evaluated a potential mediating effect of Procalcitonin or C-reactive protein on the association between sex and individual metabolite abundance adjusted for age, SAPS II, admission diagnosis, 25(OH)D at day 0, absolute change in 25(OH)D level at day 3. Analyses were performed on each of the 578 metabolites at day 3 using the R package mediation70 to obtain bootstrap P-values (N = 2000 samples)71,72. Significant mediation was present if the P-value was < 0.01 and 10% or more of the association was mediated through Procalcitonin or C-reactive protein levels71,72.
To identify sex-specific modules from metabolomics data, we estimated Gaussian graphical models (GGMs) for day 3 and 7. Modules serve to reconstruct pathway reactions from metabolomics data. GGMs are determined utilizing partial pairwise Pearson correlation coefficients following the removal of the effects of all other metabolites and covariates73. We inferred a sex-specific network for relative metabolite abundance. We included age, SAPS II, admission diagnosis, 25(OH)D at day 0, absolute change in 25(OH)D level at day 3 and plasma day as covariates into the model74. Edges between metabolites were allotted if both their Pearson correlations and partial correlations remained statistically significant at P-value < 0.05 following Bonferroni correction for 578 metabolites74. GGMs were produced using the GeneNet R package, version 1.2.13 in R-3.6.2 adapted from published source code74.
Supplementary Information
Acknowledgements
This article is dedicated to the memory of our dear friend and colleague Nathan Edward Hellman, MD, PhD.
Author contributions
S.C.: Conceptualization, Methodology, Formal analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization. K.A.: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Funding acquisition. J.L-S.: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Supervision, Project administration. H.D.: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Review & Editing, Supervision, Project administration, Funding acquisition. K.C.: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Funding
This work was supported by the National Institutes of Health [R01 GM115774]. The VITdAL-ICU trial was supported by the European Society for Clinical Nutrition and Metabolism (ESPEN), a research grant including provision of study medication from Fresenius Kabi (Germany), and the Austrian National Bank (Jubiläumsfonds, Project Nr. 14143).
Competing interests
Dr. Chary was a Masters student at Harvard Medical School during the implementation of the study. During her Masters, Dr. Chary was employed at Takeda Pharmaceutical Company and is currently employed by Biogen, Inc. Dr. Chary reports receiving salary and stock options from Takeda and from Biogen. Neither Takeda nor Biogen had any involvement in the VITdAL-ICU trial, the identification of metabolites, the post hoc study design, analysis of metabolomics, interpretation of the data, access to the data or writing of the manuscript. Dr. Lasky-Su served as a consultant and received personal fees from Metabolon, Inc., outside the submitted work. Dr. Amrein reports receiving lecture fees from Fresenius Kabi. Dr. Dobnig reports receiving lecture fees from Fresenius Kabi. Dr. Christopher reports no financial or other relationships that might lead to a conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83602-5.
References
- 1.Geller SE, et al. The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med. 2018;93:630–635. doi: 10.1097/ACM.0000000000002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woitowich NC, Woodruff TK. Opinion: research community needs to better appreciate the value of sex-based research. Proc. Natl. Acad. Sci. U. S. A. 2019;116:7154–7156. doi: 10.1073/pnas.1903586116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465:688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- 4.Regitz-Zagrosek V. Sex and gender differences in health. Sci. Soc. Ser. Sex Sci. EMBO Rep. 2012;13:596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 6.van Eijk LT, et al. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit. Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 7.Sheth SU, et al. Testosterone depletion or blockade in male rats protects against trauma hemorrhagic shock-induced distant organ injury by limiting gut injury and subsequent production of biologically active mesenteric lymph. J. Trauma. 2011;71:1652–1658. doi: 10.1097/TA.0b013e31823a06ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuelsson C, Sjoberg F, Karlstrom G, Nolin T, Walther SM. Gender differences in outcome and use of resources do exist in Swedish intensive care, but to no advantage for women of premenopausal age. Crit. Care. 2015;19:129. doi: 10.1186/s13054-015-0873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood K, Eldeirawi K, Wahidi MM. Association of gender with outcomes in critically ill patients. Crit. Care. 2012;16:R92. doi: 10.1186/CC11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Torre S, Maggi A. Sex differences: a resultant of an evolutionary pressure. Cell Metab. 2017;25:499–505. doi: 10.1016/j.cmet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am. J. Physiol. Endocrinol. Metab. 2005;288:E547–E555. doi: 10.1152/ajpendo.00340.2004. [DOI] [PubMed] [Google Scholar]
- 12.Mittelstrass K, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frias JP, et al. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50:1344–1350. doi: 10.2337/diabetes.50.6.1344. [DOI] [PubMed] [Google Scholar]
- 14.Mielke MM, et al. Factors affecting longitudinal trajectories of plasma sphingomyelins: the Baltimore Longitudinal Study of Aging. Aging Cell. 2015;14:112–121. doi: 10.1111/acel.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen PN, et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the Strong Heart Family Study. EBioMedicine. 2019;41:44–49. doi: 10.1016/j.ebiom.2018.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weir JM, et al. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trabado S, et al. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS ONE. 2017;12:e0173615. doi: 10.1371/journal.pone.0173615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varlamov O, Bethea CL, Roberts CT., Jr Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne) 2014;5:241. doi: 10.3389/fendo.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolz M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn WB, et al. Molecular phenotyping of a UK population: defining the human serum metabolome. Metabolomics. 2015;11:9–26. doi: 10.1007/s11306-014-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy MO, Loria AS. Sex-specific effects of stress on metabolic and cardiovascular disease: are women at higher risk? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;313:R1–R9. doi: 10.1152/ajpregu.00185.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiyama MG, Agellon LB. Sex differences in lipid metabolism and metabolic disease risk. Biochem. Cell Biol. 2012;90:124–141. doi: 10.1139/o11-067. [DOI] [PubMed] [Google Scholar]
- 24.Kiehntopf M, Nin N, Bauer M. Metabolism, metabolome, and metabolomics in intensive care: is it time to move beyond monitoring of glucose and lactate? Am. J. Respir. Crit. Care Med. 2013;187:906–907. doi: 10.1164/rccm.201303-0414ED. [DOI] [PubMed] [Google Scholar]
- 25.Podgoreanu, M. V. Metabolomics and other “-omic” approaches to characterize perioperative trajectories. In Surgical Metabolism (eds. Davis, K. & Rosenbaum, S.) 67–91 (Springer, Cham, 2020).
- 26.Langley RJ, et al. Integrative "omic" analysis of experimental bacteremia identifies a metabolic signature that distinguishes human sepsis from systemic inflammatory response syndromes. Am. J. Respir. Crit. Care Med. 2014;190:445–455. doi: 10.1164/rccm.201404-0624OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langley RJ, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013;5:195ra95. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson PI, et al. Plasma mitochondrial DNA and metabolomic alterations in severe critical illness. Crit. Care. 2018;22:360. doi: 10.1186/s13054-018-2275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amrein K, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 30.Westerhuis JA, et al. Assessment of PLSDA cross validation. Metabolomics. 2008;4:81–89. doi: 10.1007/s11306-007-0099-6. [DOI] [Google Scholar]
- 31.Eriksson L, Trygg J, Wold S. CV-ANOVA for significance testing of PLS and OPLS models. J. Chemom. 2008;22:594–600. doi: 10.1002/cem.1187. [DOI] [Google Scholar]
- 32.Henglin M. A single visualization technique for displaying multiple metabolite-phenotype associations. Metabolites. 2019;9:128. doi: 10.3390/metabo9070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling for false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodological. 1995;57:289–300. [Google Scholar]
- 34.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krumsiek J, et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitra K, Carvunis AR, Ramesh SK, Ideker T. Integrative approaches for finding modular structure in biological networks. Nat. Rev. Genet. 2013;14:719–732. doi: 10.1038/nrg3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson GC. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front. Endocrinol. (Lausanne) 2014;5:162. doi: 10.3389/fendo.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ventura-Clapier R, et al. Mitochondria: a central target for sex differences in pathologies. Clin. Sci. (Lond.) 2017;131:803–822. doi: 10.1042/CS20160485. [DOI] [PubMed] [Google Scholar]
- 39.Vina J, Borras C. Women live longer than men: understanding molecular mechanisms offers opportunities to intervene by using estrogenic compounds. Antioxid. Redox Signal. 2010;13:269–278. doi: 10.1089/ars.2009.2952. [DOI] [PubMed] [Google Scholar]
- 40.Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Hove JL, et al. Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: diagnosis by acylcarnitine analysis in blood. Am. J. Hum. Genet. 1993;52:958–966. [PMC free article] [PubMed] [Google Scholar]
- 42.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Ochoa Gautier JB, et al. How much and what type of protein should a critically Ill patient receive? Nutr. Clin. Pract. 2017;32:6S–14S. doi: 10.1177/0884533617693609. [DOI] [PubMed] [Google Scholar]
- 44.Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014;113:945–954. doi: 10.1093/bja/aeu187. [DOI] [PubMed] [Google Scholar]
- 45.Martindale RG, et al. Protein kinetics and metabolic effects related to disease states in the intensive care unit. Nutr. Clin. Pract. 2017;32:21S–29S. doi: 10.1177/0884533617694612. [DOI] [PubMed] [Google Scholar]
- 46.Mortensen, P. B. Mechanisms of dicarboxylic aciduria and dicarboxylic acid metabolism. In Fatty Acid Oxidation: Clinical, Biochemical and Molecular Aspects 249–64 (Alan R. Liss, Inc., New York, 1990). [PubMed]
- 47.Wanders RJ, Komen J, Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 2011;278:182–194. doi: 10.1111/j.1742-4658.2010.07947.x. [DOI] [PubMed] [Google Scholar]
- 48.Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013;54:5–19. doi: 10.1194/jlr.R031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallner S, Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids. 2011;164:573–589. doi: 10.1016/j.chemphyslip.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Lauber K, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/S0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 51.Peter C, et al. Migration to apoptotic "find-me" signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 52.Phelps T, Snyder E, Rodriguez E, Child H, Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol. Sex Differ. 2019;10:52. doi: 10.1186/s13293-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baars A, et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018;8:13426. doi: 10.1038/s41598-018-31695-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penaloza CG, et al. Sex-dependent regulation of cytochrome P450 family members Cyp1a1, Cyp2e1, and Cyp7b1 by methylation of DNA. FASEB J. 2014;28:966–977. doi: 10.1096/fj.13-233320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanwijngaerden YM, et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology. 2011;54:1741–1752. doi: 10.1002/hep.24582. [DOI] [PubMed] [Google Scholar]
- 58.Mei Y, Kim BS, Tsui K. Linear mixed effects models for feature selection in high dimensional NMR spectra. Exp. Syst. Appl. 2009;36:4703–4708. doi: 10.1016/j.eswa.2008.06.032. [DOI] [Google Scholar]
- 59.Ernest B, Gooding JR, Campagna SR, Saxton AM, Voy BH. MetabR: an R script for linear model analysis of quantitative metabolomic data. BMC Res. Notes. 2012;5:596. doi: 10.1186/1756-0500-5-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly RS. Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics. 2017;13:7. doi: 10.1007/s11306-016-1149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee-Sarwar K, et al. Dietary and plasma polyunsaturated fatty acids are inversely associated with asthma and atopy in early childhood. J. Allergy Clin. Immunol. Pract. 2019;7:529–538 e8. doi: 10.1016/j.jaip.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wanichthanarak K, Jeamsripong S, Pornputtapong N, Khoomrung S. Accounting for biological variation with linear mixed-effects modelling improves the quality of clinical metabolomics data. Comput. Struct. Biotechnol. J. 2019;17:611–618. doi: 10.1016/j.csbj.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaskins AJ, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am. J. Epidemiol. 2012;175:423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amrein K, Lasky-Su JA, Dobnig H, Christopher KB, et al. Metabolomic basis for response to high dose vitamin D in critical illness. Clin. Nutr. 2012 doi: 10.1016/j.clnu.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katajamaa M, Oresic M. Data processing for mass spectrometry-based metabolomics. J. Chromatogr. A. 2007;1158:318–328. doi: 10.1016/j.chroma.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 67.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong J, Xia J. Using MetaboAnalyst 4.0 for metabolomics data analysis, interpretation, and integration with other omics data. Methods Mol. Biol. 2020;2104:337–360. doi: 10.1007/978-1-0716-0239-3_17. [DOI] [PubMed] [Google Scholar]
- 69.StataCorp. Stata Statistical Software: Release 14. (StataCorp LP., College Station, TX, 2015).
- 70.Dustin T, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. J. Stat. Softw. 2014;59:1–38. [Google Scholar]
- 71.Masuch A, et al. Metabolomic profiling implicates adiponectin as mediator of a favorable lipoprotein profile associated with NT-proBNP. Cardiovasc. Diabetol. 2018;17:120. doi: 10.1186/s12933-018-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pietzner M, et al. Hepatic steatosis is associated with adverse molecular signatures in subjects without diabetes. J. Clin. Endocrinol. Metab. 2018;103:3856–3868. doi: 10.1210/jc.2018-00999. [DOI] [PubMed] [Google Scholar]
- 73.Krumsiek J, Suhre K, Illig T, Adamski J, Theis FJ. Gaussian graphical modeling reconstructs pathway reactions from high-throughput metabolomics data. BMC Syst. Biol. 2011;5:21. doi: 10.1186/1752-0509-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Do KT, et al. Phenotype-driven identification of modules in a hierarchical map of multifluid metabolic correlations. NPJ Syst. Biol. Appl. 2017;3:28. doi: 10.1038/s41540-017-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.