Abstract
Objectives:
We sought to determine the safety and feasibility of esophagectomy after neoadjuvant immunotherapy and chemoradiotherapy in clinical trial patients with locally advanced esophageal cancer.
Methods:
We retrospectively identified patients who were treated with neoadjuvant immunotherapy and chemoradiotherapy (n = 25) or chemoradiotherapy alone (n = 143) at our institution between 2017 and 2020. The primary end point was risk of 30-day major complications (Clavien-Dindo classification system grade ≥ 3), which was assessed between groups using a multivariable log-binomial regression model to obtain adjusted relative risk ratios. Secondary end points were interval to surgery, 30-day readmission rate, and 30-day mortality.
Results:
All included patients successfully completed neoadjuvant therapy and underwent esophagectomy with negative margins. Age, sex, performance status, clinical stage, histologic subtype, procedure type, and operative approach were similar between groups. Neoadjuvant immunotherapy was not associated with a statistically significantly increased risk of developing a major pulmonary (relative risk, 1.43; 95% confidence interval, 0.53-3.84; P = .5), anastomotic (relative risk, 1.34; 95% confidence interval, 0.45-3.94; P = .6), or other complication (relative risk, 1.29; 95% confidence interval, 0.26-6.28; P = .8). Median (interquartile range) interval to surgery was 54 days (47-61 days) in the immune checkpoint inhibitor group versus 53 days (47-66 days) in the control group (P = .6). Minimally invasive approaches were successful in 72% of cases, with only 1 conversion. Thirty-day mortality and readmission rates were 0% and 17%, respectively, in the immune checkpoint inhibitor group and 1.4% and 13%, respectively, in the control group.
Conclusions:
On the basis of our preliminary experience, esophagectomy appears to be safe and feasible following combined neoadjuvant immunotherapy and standard chemoradiotherapy for locally advanced esophageal cancer.
Keywords: esophagectomy, immunotherapy, chemoradiotherapy, esophageal cancer
Graphical Abstract
The current standard of care for locally advanced, surgically resectable esophageal cancer is neoadjuvant chemoradiotherapy followed by esophagectomy. However, due to the high rate of distant recurrence, 5-year survival remains <50%.1,2 In fact, limited progress has been made in the treatment of locally advanced esophageal cancer since the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study was first reported in 2012.3 More recently, encouraging results from the Cancer and Leukemia Group B 80803 trial (www.ClinicalTrials.gov/NCT01333033) indicate that a positron emission tomography (PET)-directed strategy using 5–fluorouracil-based neoadjuvant chemotherapy results in a higher rate of pathologic complete response.4 However, long-term survival data are still pending.
Immune checkpoint inhibitors (ICIs) have rapidly become a pillar of cancer treatment, alongside chemotherapy, radiation, and surgery. These agents, which allow for the recognition of tumor antigens by the patient’s own T cells by blocking programmed cell death protein 1– or programmed death-ligand 1–mediated immune-evasion mechanisms on the tumor-cell surface, have been deemed safe and effective as part of multimodality therapy for several cancer types, including non–small cell lung cancer (NSCLC).5 The use of ICIs in the neoadjuvant setting, in particular, has gained traction over the past few years, but only a handful of studies have specifically examined surgical safety following treatment with ICIs.6,7 For example, Bott and colleagues8 demonstrated that major lung resections could be performed safely after 2 cycles of neoadjuvant nivolumab, without any unexpected perioperative morbidity or mortality.
To date, the experience with ICIs for the treatment of esophagogastric cancer has been largely limited to patients with advanced disease. Data from The Cancer Genome Atlas Research Network show that 5% to 20% of esophagogastric cancers exhibit microsatellite instability.9 Moreover, esophagogastric cancers tend to have a higher mutation burden than other solid tumors.10 Given that both microsatellite instability and hypermutation are known biomarkers of response to immunotherapy, early-phase clinical trials have investigated the efficacy of ICIs, either alone or in combination with other chemotherapeutic or targeted agents, for the treatment of metastatic esophagogastric cancer.11,12 Thus far, results have revealed durable responses, with acceptable toxicity, in a subset of patients, which has prompted progression to ongoing phase 3 trials.13 However, the safety and efficacy of immunotherapy for the treatment of locally advanced, surgically resectable esophageal cancer remain unknown.
Therefore, our center has initiated phase 1 and 2 clinical trials to evaluate the safety and efficacy of neoadjuvant immunotherapy combined with standard chemoradiotherapy in patients with locally advanced esophageal adenocarcinoma. The objective of the present study was to assess the safety and feasibility of esophagectomy after this regimen by examining 30-day complications and perioperative outcomes. On the basis of previously reported outcomes of lung resection, we hypothesized that there would be no difference in perioperative outcomes of esophagectomy following the addition of neoadjuvant immunotherapy. However, esophagectomy is undoubtedly among the most technically challenging and morbid operations that thoracic surgeons routinely perform. Patients in the Society of Thoracic Surgeons General Thoracic Surgery Database had a 33% rate of major complications and mortality of 3% at 30 days, irrespective of a center’s surgical volume.14 As a result, surgeons are obligated to review and report surgical outcomes of esophagectomy after this novel treatment strategy.
METHODS
ICI Group
We queried our prospectively maintained multimodality surgical database to identify patients with esophageal adenocarcinoma who underwent neoadjuvant immunotherapy and standard chemoradiotherapy followed by esophagectomy between 2017 and 2020 at Memorial Sloan Kettering Cancer Center. All patients had provided written informed consent for enrollment in the following single-arm clinical trial: a phase 1/2 study of induction chemotherapy and durvalumab (MEDI4736) with chemoradiation for esophageal and gastroesophageal junction adenocarcinoma (www.ClinicalTrials.gov/NCT02962063; institutional review board-approved protocol No. 16-1405). Figure 1, A, depicts a Consolidated Standards of Reporting Trials diagram of our ICI group.
FIGURE 1.
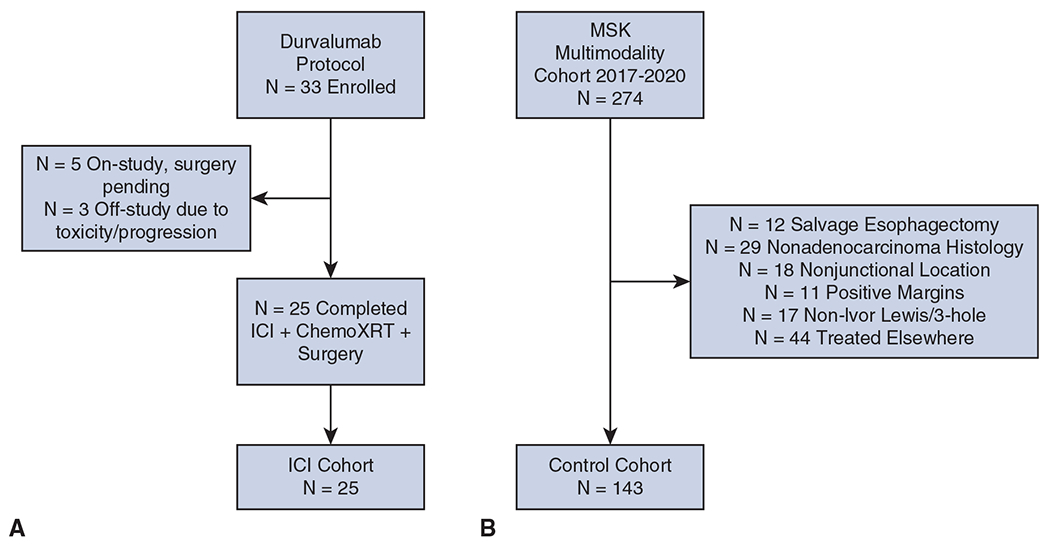
Consolidated Standards of Reporting Trials diagrams of the immune checkpoint inhibitor (ICI) group (A) and control group (B) in the context of ongoing clinical trial enrollment. Information is current as of May 2020. MSK, Memorial Sloan Kettering Cancer Center.
In phase 1 of the durvalumab study, patients were treated with 2 doses of induction durvalumab followed by concurrent chemoradiotherapy, underwent surgery 6 to 8 weeks later, and then received adjuvant durvalumab for 6 months (Figure E1, A). The durvalumab study evolved significantly from phase 1 to phase 2 to incorporate elements of the Cancer and Leukemia Group B 80803 trial—in particular, induction combination chemotherapy regimen of leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin (FOLFOX) using a PET-directed approach (Figure E1, B). Eligibility for the durvalumab study includes Siewert I through III junctional adenocarcinoma with either T (any), N+, or T3 or T4, N (any) disease, as determined by staging PET-computed tomography scan and endoscopic ultrasound. All patients were required to undergo upfront evaluation by a thoracic surgeon to confirm surgical resectability and candidacy. Patients were specifically excluded from enrollment if they were immunosuppressed or had an active infectious or autoimmune disease, significant underlying comorbidities, recent or concurrent malignancy, or prior immunotherapy treatment. Observed toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (https://ctep.cancer.gov/).
Control Group
To define our control group, we queried our multimodality surgical database for all patients who underwent neoadjuvant chemoradiotherapy followed by esophagectomy during the same period. Exclusion criteria were receipt of neoadjuvant therapy outside of our institution, histologic subtype other than adenocarcinoma, nonjunctional tumor location, salvage esophagectomy (ie, surgery >100 days from neoadjuvant treatment), transhiatal or thoracoabdominal procedure, or resection with positive margins. These exclusion criteria were designed to match the control group to the ICI group as much as possible, to eliminate heterogeneity between patient populations. Figure 1, B, depicts a Consolidated Standards of Reporting Trials diagram of our control group. Our aim was to delineate a group of patients who were treated with a PET-directed approach, as described in the Cancer and Leukemia Group B 80803 trial, to neoadjuvant chemoradiotherapy similar to that used to treat patients in the ICI group, for appropriate comparison.
Approval for retrospective review of patients in both groups was obtained from our institutional review board (No. 16-1631), which waived the requirement for informed consent.
Outcome Measures
We divided all complications into 4 main categories, which were coded as either present or absent, in conjunction with severity. Specifically, pulmonary complications included pneumonia, empyema, acute respiratory distress syndrome, respiratory failure requiring reintubation, pleural effusion, and pulmonary embolism. Cardiac complications included arrhythmia, demand ischemia, and myocardial infarction. Anastomotic complications included leak, dehiscence, and fistula. Other complications included chyle leak, deep venous thrombosis, wound infection, and recurrent laryngeal nerve injury. Interval to surgery was measured from the end of neoadjuvant treatment to the date of surgery, and operative time was measured from incision to wound closure. The primary end point was risk of 30-day major complications. A major complication was defined as grade ≥ 3, in accordance with the Clavien-Dindo classification system.15 Secondary end points were interval to surgery, 30-day readmission rate, and 30-day mortality.
Statistical Analyses
Characteristics were summarized as either median and interquartile range (IQR) or frequency and percent and were compared between the ICI and control groups using either a Wilcoxon rank sum test (continuous variables) or Fisher exact test (categorical variables). Relative risk ratios (RRs) of 30-day perioperative outcomes for the ICI versus control groups were estimated using a multivariable log-binomial regression model, with adjustment for a set of predefined clinical factors, including age, Zubrod performance status, pulmonary comorbidity, smoking status, and minimally invasive approach. Adjusted RRs were estimated only for outcomes with ≥10 events. Two-sided P < .05 was considered to indicate statistical significance. All statistical analyses were performed using Stata 15.0 (StataCorp, College Station, Tex) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
The ICI group included 25 patients. Of these, 5 were enrolled in the phase 1 durvalumab study (Figure E1, A) and 20 were enrolled in the phase 2 durvalumab study (Figure E1, B). The patients in this group were compared with the patients in the control group (n = 143). Patient characteristics are summarized in Table 1. All patients in the ICI group underwent surgical resection with negative margins. The distribution of age, sex, Zubrod performance status, clinical stage, procedure type, operative approach, and extent of lymphadenectomy were well balanced between the 2 groups. The majority of patients in both groups were men, aged between 61 and 64 years, and had symptomatic clinical T3 N1 or stage III junctional adenocarcinoma. However, patients in the ICI group had significantly less tobacco exposure (31% vs 64%; P = .003) and fewer pulmonary comorbidities (0% vs 15%; P = .048), compared with patients in the control group. Of note, 18 of 25 patients (72%) in the ICI group underwent a minimally invasive Ivor Lewis or 3-incision esophagectomy (robotic-assisted, n = 2; conventional laparoscopy and thoracoscopy, n = 16). There was 1 unplanned conversion to an open procedure, owing to the extent of disease along the lesser curvature of the stomach, necessitating a total gastrectomy with esophagojejeunostomy. In the control group, 90 of 143 esophagectomies (63%) were minimally invasive, and 5 of 143 (3%) unplanned conversions occurred.
TABLE 1.
Patient characteristics
| Characteristic | Immune checkpoint inhibitor (n = 25) | Control (n = 143) | P value |
|---|---|---|---|
| Age (y) | 61.5 (53.0-67.0) | 64.0 (56.0-70.0) | .2 |
| Male | 22 (88) | 123 (86) | 1 |
| Zubrod performance status | .8 | ||
| 0 | 8 (32) | 53 (37) | |
| 1 | 17 (68) | 88 (62) | |
| 2 | 0 (0) | 2 (1.4) | |
| Pulmonary comorbidity | 0 (0) | 22 (15) | .048 |
| Cardiac comorbidity | 12 (48) | 81 (57) | .5 |
| Renal insufficiency | 1 (4.0) | 5 (3.5) | 1 |
| Diabetes | 5 (20) | 32 (22) | 1 |
| Smoking history | 9 (36) | 98 (69) | .003 |
| cT | .4 | ||
| 1 | 1 (4.0) | 2 (1.4) | |
| 2 | 2 (8.0) | 12 (8.4) | |
| 3 | 21 (84) | 127 (89) | |
| 4 | 1 (4.0) | 2 (1.4) | |
| cN | .2 | ||
| 0 | 10 (40) | 35 (24) | |
| 1 | 14 (56) | 80 (56) | |
| 2 | 1 (4.0) | 27 (19) | |
| 3 | 0 (0) | 1 (0.7) | |
| cStage | .14 | ||
| 1 | 0 (0) | 1 (0.7) | |
| 2 | 2 (8.0) | 6 (4.2) | |
| 3 | 22 (88) | 108 (76) | |
| 4 | 1 (4.0) | 28 (20) | |
| Procedure type | .4 | ||
| 3-incision | 1 (4.0) | 3 (2.1) | |
| Ivor Lewis | 24 (96) | 140 (98) | |
| Minimally invasive approach | 18 (72) | 90 (63) | .4 |
| Lymph nodes removed | 23.0 (17.0-28.0) | 23.0 (18.0-28.0) | .8 |
Values are presented as n (%) or median (interquartile range). cT, Clinical tumor stage; cN, clinical nodal stage; cStage, clinical overall stage.
Immune-Related Adverse Events
Of the patients enrolled in durvalumab study, 1 was taken off study midprotocol because of a hypersensitivity reaction to paclitaxel. This patient received a single dose of neoadjuvant durvalumab and ultimately completed concurrent chemoradiotherapy with cisplatin and irinotecan and underwent surgery on schedule. Another patient was taken off study postoperatively because of nephritis; this patient did not receive adjuvant durvalumab. Finally, 1 patient presented with a fundus perforation1 month after completion of immunotherapy and chemoradiation, while awaiting surgery. This patient required urgent laparotomy with Graham patch repair. Biopsy of the area of perforation did not reveal any evidence of cancer and was consistent with an ulcer, and the area of perforation was considered to be outside of the radiation field. The patient recovered without incident and was able to undergo esophagectomy 2 months later.
Overall, the incidence of immune toxicity in the preoperative setting was low: 2 patients each with grade 2 and grade 3 colitis and 1 patient each with grade 3 hepatitis, grade 2 dermatitis, and grade 1 nephritis. Thus, 7 patients in total (28%) experienced immune-related toxicity. None of these events delayed surgery by more than 1 to 2 weeks, and no episodes of pneumonitis have been documented thus far.
Surgical Outcomes
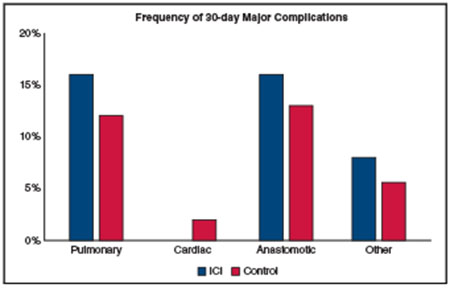
In the ICI group, 6 patients (24%) experienced a pulmonary complication, 3 (12%) experienced a cardiac complication, 4 (16%) experienced an anastomotic complication, and 9 (36%) experienced other complications (Table 2). The frequency of these complications was not statistically significantly different from that in the control group. All cardiac complications and 7 of 9 other complications were coded as minor, whereas 4 of 6 pulmonary complications and all 4 anastomotic complications occurred in overlapping patients and were coded as major, requiring either reintervention, critical care management, or both. Treatment with neoadjuvant immunotherapy was not associated with a statistically significantly increased risk of developing a major pulmonary (RR, 1.43; 95% confidence interval [CI], 0.53 to 3.84; P = .5), anastomotic (RR, 1.34; 95% CI, 0.45-3.94; P = .6), or other complication (RR, 1.29; 95% CI, 0.26-6.28; P = .8), after adjustment for age, sex, Zubrod performance status, pulmonary comorbidity, smoking status, and minimally invasive approach. Because there were no major cardiac complications in the ICI group (all 3 minor cardiac complications were atrial arrhythmias) and only 2 in the control group, RR could not be calculated.
TABLE 2.
Thirty-day complications
| Complication | Immune checkpoint inhibitor (n = 25) | Control (n = 143) | P value | Adjusted risk ratio (95% confidence interval)* | Adjusted P value |
|---|---|---|---|---|---|
| n (%) | |||||
| Pulmonary | 6 (24) | 43 (30) | .6 | 0.96 (0.48-1.90) | .9 |
| Major pulmonary | 4 (16) | 17 (12) | .5 | 1.43 (0.53-3.84) | .5 |
| Cardiac | 3 (12) | 25 (17) | .8 | 1.07 (0.36-3.17) | .9 |
| Major cardiac | 0 (0) | 2 (1.4) | 1 | – | |
| Anastomotic | 4 (16) | 21 (15) | .8 | 1.16 (0.41-3.25) | .8 |
| Major anastomotic | 4 (16) | 18 (13) | 1 | 1.34 (0.45-3.94) | .6 |
| Other | 9 (36) | 46 (32) | 1 | 1.06 (0.57-1.99) | .8 |
| Major other | 2 (8.0) | 8 (5.6) | .6 | 1.29 (0.26-6.28) | .8 |
Adjusted risk ratios were estimated only for outcomes with ≥10 events.
Interval to surgery, 30-day readmissions, and 30-day mortality did not differ significantly between the ICI and control groups (Table 3). The median interval to surgery was 54 days (IQR, 47-61 days) in the ICI group and 53 days (IQR, 47-66 days) in the control group (P = .6). There were no 30-day or 90-day deaths in the ICI group, compared with 2 (1.4%) and 3 (2.1%), respectively, in the control group. Readmission rates at 30-days were also comparable: 4 of 25 (17%) in the ICI group versus 19 of 143 (13%) in the control group (P = .7). Median operative time was 502 minutes (IQR, 419-560 minutes) in the ICI group and 467 minutes (IQR, 419-533 minutes) in the control group, although this difference was not statistically significant (P = .3). Intraoperative blood loss and perioperative transfusion rates were also similar between groups. Median length of hospital stay was 1 day shorter in the ICI group (8.0 days; IQR, 7.0-9.0 days vs 9.0 days; IQR, 7.0-11.0 days; P = .12).
TABLE 3.
Thirty-day perioperative outcomes
| Outcome | Immune checkpoint inhibitor (n = 25) | Control (n = 143) | P value |
|---|---|---|---|
| Interval to surgery* (d) | 54.0 (47.0-61.0) | 53.0 (47.0-66.0) | .6 |
| Operative time (min) | 502.0 (419.0-560.0) | 467.0 (419.0-533.0) | .3 |
| Length of hospital stay (d) | 8.0 (7.0-9.0) | 9.0 (7.0-11.0) | .12 |
| Intraoperative blood loss (mL) | 200.0 (150.0-300.0) | 200.0 (100.0-350.0) | .6 |
| Perioperative transfusion | 2 (8.0) | 21 (15) | .5 |
| 30-d readmissions | 4 (17) | 19 (13) | .7 |
| 30-d deaths | 0 (0) | 2 (1.4) | 1 |
Values are presented as median (interquartile range) or n (%).
Between end of neoadjuvant treatment and surgery.
DISCUSSION
In this study, 30-day perioperative outcomes were not statistically significantly different between patients treated with combined neoadjuvant immunotherapy and chemoradiotherapy or chemoradiotherapy alone followed by esophagectomy (Figure 2). The addition of neoadjuvant immunotherapy did not result in a statistically significantly increased risk of 30-day major complications, death, or readmission. Immune-related adverse events were relatively infrequent and did not cause any significant delays to surgery after completion of neoadjuvant therapy.
FIGURE 2.
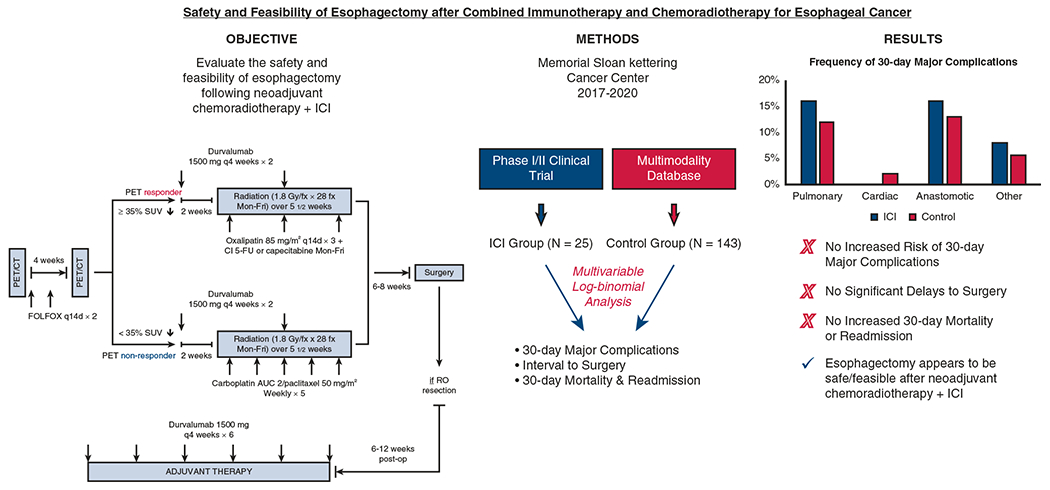
On the basis of this preliminary experience, esophagectomy appears to be safe and feasible after combined immunotherapy and chemoradiotherapy for esophageal cancer. ICI, Immune checkpoint inhibitor; PET, positron-emission tomography; CT, computed tomography; SUV, standardized uptake value; fx, fraction; AUC, area under the curve.
Several studies have demonstrated that esophageal resection is associated with relatively high perioperative morbidity and mortality and outcomes are associated with center surgical volume or individual surgeon experience.16,17 Because we are among the highest-volume programs in the region, we perform more than 100 esophagectomies for esophageal cancer annually. Overall, the outcomes reported here are consistent with those from other large series, especially with respect to rates of major pulmonary, cardiac, and anastomotic complications in both our control group and our ICI group.14 In particular, the anastomotic leak rate in the ICI group (16%) is similar to that reported by the Society of Thoracic Surgeons General Thoracic Surgery Database. All 4 anastomotic complications in the ICI group were classified as major, necessitating endoscopy, and 3 of the 4 anastomotic complications also required monitoring in the intensive care unit. However, no patients underwent reoperation in the form of repeat thoracoscopy or thoracotomy.
Patients in the ICI group had a lower frequency of pulmonary complications overall but a higher frequency of major pulmonary complications, compared with the control group. However, preoperative smoking history and pulmonary comorbidities were also significantly lower in the ICI group, secondary to the exclusion of patients with moderate or severe pulmonary disease from the clinical trials, given the potential risk of pneumonitis. After adjusting for these preoperative factors, the RR of developing any pulmonary complication was only 4% lower and the RR of developing a major pulmonary complication was actually 43% higher in the ICI group. Although neither difference was statistically significant, it will be important to assess this in future studies with larger numbers of patients. Similarly, the RR of developing a major anastomotic or other complication was 34% and 29% higher in the ICI group, respectively, although these differences were also not statistically significant.
Initial studies that examined the safety and feasibility of surgical resection of NSCLC after neoadjuvant immunotherapy have described increased difficulty and technical challenges. Chaft and colleagues18 were the first to report a dense fibrosis encountered during pulmonary resection of NSCLC. In a phase 2 clinical trial of neoadjuvant nivolumab, Bott and colleagues8 subsequently reported that 62% of patients with locally advanced NSCLC required conversion to thoracotomy because of hilar or mediastinal inflammation and fibrosis that appeared unrelated to pathologic response. Of note, no patients in either study received neoadjuvant chemoradiotherapy in addition to immunotherapy, and all underwent resection within a month of completing treatment. By all objective metrics, there were no findings in our study to suggest increased difficulty or technical challenges associated with esophageal resection that may be attributable to neoadjuvant immunotherapy. In fact, 18 of 25 esophagectomies were completed via a minimally invasive approach, with only 1 conversion, owing to extent of disease. Furthermore, operative time, intraoperative blood loss, and perioperative transfusion rates were fairly similar between ICI and control groups. In reviewing operative reports, subjective findings of increased inflammation, fibrosis, or difficulty of dissection, as well as the addition of increased procedural work modifier codes, were also rare. Therefore, we did not identify any greater technical difficulty secondary to inflammation or fibrosis in this initial experience.
Our study has several limitations. First, it is a retrospective analysis with a relatively small sample size of patients treated with immunotherapy, and thus statistical comparisons may be underpowered. Second, essentially all patients in the ICI group were treated with durvalumab and underwent Ivor Lewis esophagectomy for junctional adenocarcinoma. Therefore, our results may not be generalizable to populations treated with other immune checkpoint inhibitors or operative approaches or with other tumor histologic subtypes or locations.
CONCLUSIONS
Our results from this preliminary experience suggest that esophagectomy may be both safe and feasible following neoadjuvant immunotherapy combined with standard chemoradiotherapy for locally advanced esophageal cancer. Overall rates of perioperative morbidity and mortality were not significantly higher in patients treated with neoadjuvant immunotherapy and were generally consistent with nationally reported outcomes. However, randomized studies of larger groups of patients will be needed to confirm these results. Full reporting of the results of the clinical trials discussed here, in terms of pathologic response rates, survival, and genomic correlatives, will be forthcoming in future work, once target accruals are met.
Supplementary Material
FIGURE E1. Clinical trial design schema of the phase 1 durvalumab study (A) and phase 2 durvalumab study (B). fx, Fraction; AUC, area under the curve; PET, positron-emission tomography; CT, computed tomography; SUV, standardized uptake value; cCR, clinical complete response.
CENTRAL MESSAGE.
On the basis of preliminary experience, esophagectomy appears to be safe and feasible following combined neoadjuvant immunotherapy plus standard chemoradiotherapy for locally advanced esophageal cancer.
PERSPECTIVE.
Neoadjuvant immunotherapy has become more popular; however, only a handful of studies have examined surgical safety following this approach. In this retrospective analysis of clinical trial patients with locally advanced esophageal cancer treated with neoadjuvant chemoradiation plus immunotherapy, esophagectomy was not associated with increased risk of perioperative morbidity or mortality.
Discussion.
Presenter: Dr Smita Sihag

Dr Wayne L. Hofstetter (Houston, Tex). Congratulations, Smita, on a really nice paper; very well presented. First of all, I’d like to congratulate the group for recognizing that we are not making headway in the treatment of locally advanced esophageal cancer by debating different types of chemotherapy or chemoradiation. The need to move the needle forward is apparent, and bringing therapeutic options like immunotherapy to the clinical setting is critical. So congratulations on engaging in these protocols in the first place.
My first question for you is: Based on data not presented—based on just your eyes and your hands—were there any differences in operating on patients who have had immunotherapy compared with those who did not? Were these cases harder at all?

Dr Smita Sihag (New York, NY). Thank you very much, Dr Hofstetter; great question. We have not studied this formally and perhaps we should, but in an informal polling amongst my colleagues that have done these cases, we have really not appreciated any greater difficulty associated with the dissection during these cases following immunotherapy.
Obviously, these cases are hard to begin with, following chemoradiotherapy, but I would say that we have not appreciated any major differences with the addition of immunotherapy. We do think that there is probably a difference in the quality of fibrosis that we see, but as such, no increased difficulty.
Dr Hofstetter. I think that’s relatively interesting because with the lung, we’ve recognized that there’s sometimes often a significant difference in difficulty. Do you think that as we start doing tumors that are maybe a little bit higher up, say in the midesophagus rather than an easier area like the esophagogastric junction where your protocols focused, that we may start running into more fibrosis around the airway specifically? And what about larger tumors—were you able to look at smaller versus larger tumors?
Dr Sihag. That’s a terrific point. Obviously, the patients selected for these initial clinical trials are somewhat cherry-picked and so far we have not encountered very bulky tumors—T4A tumors in particular and so forth—that might be difficult to dissect off the airway or the pericardium. Therefore, as our experience evolves, we may actually notice more of a difference. But at this time, I would say that most surgeons in our group agree that there is no increased difficulty.
Dr Hofstetter. Smita, how did your team define response? Obviously, we can define it in terms of the tumor regression grade response just in the primary tumor. Did you also take a look at response within the lymph nodes? And, in the particular episode where you said there was 90% downstaging—did that result in actual pathologic downstaging of the tumor?
Dr Sihag. Yes, thank you for this question. So we did see nodal downstaging in 5 patients, and all of these patients had >90% treatment response in the primary tumor bed. I should mention that treatment response as defined in my slide refers to treatment response in the primary tumor bed only. So it does not account for nodal status or residual nodal disease. But 5 patients in this particular group actually did have evidence of treatment effect in the lymph nodes. The median number of lymph nodes that were harvested in our immunotherapy cohort in comparison to the control cohort were similar at 22, and overall downstaging was seen in 19 or 73% of patients in the immunotherapy cohort, as opposed to only 58% of patients in the control cohort.
Dr Hofstetter. That’s great. You’ve really answered the question of whether we can do it; there’s some nervousness about moving forward with surgery in a setting of chemoradiation and immunotherapy. I guess the next real question is: should we be doing this, and do these treatment responses just reflect heterogeneity in your patient population, or are these really related to the addition of immune therapy? So I’ll be really looking forward to the outcomes of these studies.
Dr Sihag. Yes, I agree. Thank you very much.
Dr Hofstetter. That concludes my questions. Great job, Smita; thank you. And thank you for having me discuss.

Dr Christine L. Lau (Baltimore, Md). Smita, where do you plan to take this from here on out? And if you had any patients that are further than eight weeks out that have had any problems with fibrosis, I’m assuming you continue the trial. Have there been any patients that have gone further out that have not been operated on within eight weeks?
Dr Sihag. Sure, that’s a great question. Thank you, Dr Lau. At this point, we do have some longer-term outcomes on some of these patients and we’ll actually be able to report 90-day outcomes in our manuscript. In terms of cases where we’ve had long delays to surgery, we actually have not had any significant delays at this point. The protocol dictates going to surgery within 6 to 8 weeks in our usual, standard fashion, after completing neoadjuvant therapy (including immunotherapy) and our goal has been to try to get patients done in that interval, on this protocol especially.
Dr Lau. Thank you.
Acknowledgments
Supported, in part, by the National Institutes of Health/National Cancer Institute Cancer Center Support grant No. P30 CA008748. Dr Sihag is supported by a grant from the Fiona and Stanley Druckenmiller Center for Lung Cancer Research at Memorial Sloan Kettering Cancer Center and the David J. Sugarbaker Research Scholarship from the American Association for Thoracic Surgery. Dr Jones is supported by the National Institutes of Health/National Cancer Institute (grant Nos. R01CA217169 and R01CA240472).
Conflict of Interest Statement
Dr Ku has received research funding from Arog, research funding/consulting fees from AstraZeneca, research funding/consulting fees from Bristol Myers Squibb, research funding from Daiichi, consulting fees from Eli Lilly, research funding/consulting fees from Merck, research funding/consulting fees from Pieris, and research funding from Zymeworks. Dr Wu has received research grants (institutional) from CivaTech Oncology, personal fees from AstraZeneca, and a travel grant from AlphaTau Medical. Dr Janjigian has financial relationships with Eli Lilly, ASCO, Michael J. Hennessy Associates, Paradigm Medical Communications, Zymeworks, AstraZeneca, Daiichi Sankyo, ONO Pharma, Merck, and Bristol Myers Squibb. Dr Jones serves as a senior medical advisor for Diffusion Pharmaceuticals and a consultant for AstraZeneca and Merck. Dr Molena serves as a consultant for Johnson & Johnson, Urogen, and Boston Scientific. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Abbreviations and Acronyms
- ICI
immune checkpoint inhibitor
- NSCLC
non–small cell lung cancer
- PET
positron emission tomography
Footnotes
Read at the 100th Annual Meeting of The American Association for Thoracic Surgery: A Virtual Learning Experience, May 22-23, 2020.
Scanning this QR code will take you to the article title page to access supplementary information. To view the AATS Annual Meeting Webcast, see the URL next to the webcast thumbnail.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/20AM/Presentations/Safety%20and%20Feasibility%20of%20Esophagect.mp4.

References
- 1.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in Oncology. J Natl Compr Cancer Netw. 2019;17:855–83. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 4.Goodman KA, Hall N, Bekaii-Saab TS, Ou F-S, Twohy E, Meyers MO, et al. Survival outcomes from CALGB 80803 (Alliance): a randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol. 2018;36:4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick SR. Adjuvant and neoadjuvant immunotherapy in non-small cell lung cancer. Thorac Surg Clin. 2020;30:215–20. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin Cancer Res. 2019;25:5743–51. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2019;158:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network, Analysis Working Group, Asan University, BC Cancer Agency, Brigham and Women’s Hospital, Broad Institute, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuza K, Nagahashi M, Watanabe S, Takabe K, Wakai T. Hypermutation and microsatellite instability in gastrointestinal cancers. Oncotarget. 2017;8:112103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku GY. The current status of immunotherapies in esophagogastric cancer. Hematol Oncol Clin North Am. 2019;33:323–38. [DOI] [PubMed] [Google Scholar]
- 13.Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. Check-Mate-032 Study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36:2836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Society of Thoracic Surgeons General Thoracic Surgery Database Task Force. The Society of Thoracic Surgeons composite score for evaluating esophagectomy for esophageal cancer. Ann Thorac Surg. 2017;103:1661–7. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CD, Kucharczuk JC, O’Brien SM, Grab JD, Allen MS, Society of Thoracic Surgeons General Thoracic Surgery Database. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137:587–95. [DOI] [PubMed] [Google Scholar]
- 17.Swisher SG, Deford L, Merriman KW, Walsh GL, Smythe R, Vaporicyan A, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126–32. [DOI] [PubMed] [Google Scholar]
- 18.Chaft JE, Hellmann MD, Velez MJ, Travis WD, Rusch VW. Initial experience with lung cancer resection after treatment with T-cell checkpoint inhibitors. Ann Thorac Surg. 2017;104:e217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE E1. Clinical trial design schema of the phase 1 durvalumab study (A) and phase 2 durvalumab study (B). fx, Fraction; AUC, area under the curve; PET, positron-emission tomography; CT, computed tomography; SUV, standardized uptake value; cCR, clinical complete response.


