Abstract
Introduction
Patients with asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) present with chronic respiratory symptoms with features of both asthma and COPD. New ACO diagnostic criteria (2018) were suggested by the Japanese Respiratory Society (JRS). This prospective, multicenter, observational cohort study was conducted to investigate the proportion of patients who meet the JRS ACO diagnostic criteria among COPD patients in clinical practice.
Methods
This 2-year ongoing study enrolled consecutive outpatients aged ≥ 40 years with COPD who visited one of 27 Japanese centers at which the medical examinations/tests required for ACO diagnosis were routinely conducted. At registration, the proportion of ACO or non-ACO patients was determined using the JRS diagnostic criteria, and the characteristics of the two groups were compared using analysis of variance and chi-square test.
Results
Of 708 COPD patients analyzed at registration, 396 (55.9%) had the data necessary for ACO diagnosis to be conducted, and 312 (44.1%) were lacking these data. Of the 396 patients who had the data necessary for ACO diagnosis, 101 (25.5%) met the diagnostic criteria for ACO, and 295 (74.5%) did not (non-ACO patients). ACO patients were younger, had a greater rate of asthma based on a physician’s diagnosis, and used more medications, including inhaled corticosteroids (p < 0.05), compared with non-ACO patients.
Conclusions
We have determined the proportion of patients with the data necessary to diagnose ACO using the JRS criteria, and the proportion of these who met the ACO criteria among the COPD population at the time of registration. Patients, including those lacking necessary examination/test data at registration, will continue to undergo follow-up to explore changes in their testing and ACO diagnostic status over time. Analyses of study data over 2 years will provide relevant information on the ACO symptoms, clinical course, and real-world treatment patterns.
Trial registration
ClinicalTrials.gov identifier, NCT03577795.
Electronic supplementary material
The online version of this article (10.1007/s12325-020-01573-x) contains supplementary material, which is available to authorized users.
Keywords: ACO, Airflow limitation, Asthma, Clinical course, COPD, Diagnostic criteria, Japan, Observational study, Respiratory symptoms, Treatment outcome
Key Summary Points
| Why carry out this study? |
| New diagnostic criteria (2018) for asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) were suggested by the Japanese Respiratory Society (JRS). |
| This prospective, multicenter, observational cohort study investigated the proportion of COPD patients who meet the JRS ACO diagnostic criteria, and an ongoing follow-up analysis will explore how this changes over a 2-year period. |
| What was learned from the study? |
| At registration, of 708 COPD patients analyzed, 312 (44.1%) were lacking the necessary data for ACO diagnosis to be conducted, suggesting that some of the examinations/tests required for the diagnosis of ACO are not routine in clinical practice. |
| Of the 396 patients with necessary data for diagnosis, 101 (25.5%) met the JRS diagnostic criteria for ACO and 295 (74.5%) were non-ACO. |
| Analyses of study data over 2 years will provide relevant information on the ACO symptoms, clinical course, and real-world treatment patterns. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13221611.
Introduction
Among patients who present with persistent airflow limitation, a proportion display the clinical features of both asthma and chronic obstructive pulmonary disease (COPD) [1]. While both are common chronic respiratory conditions, asthma and COPD arise via different pathophysiologic mechanisms [2, 3]. Patients with asthma generally present with allergic airway inflammation and airway narrowing due to smooth muscle contraction, vascular congestion, and edema [4]. In contrast, the predominant causes of airway obstruction in COPD include inflammation, mucus hypersecretion, loss of elastic recoil through parenchymal tissue destruction, and fibrosis, which lead to narrowing of airways, particularly the small airways [4].
It is controversial whether asthma-COPD overlap (ACO) is a single disease entity or a collection of common traits [5]. Previously, guidelines from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recognized the existence of ACO [6], but the 2020 guidelines no longer refer to ACO, instead choosing to emphasize that patients with asthma and COPD have different diseases although they may share some clinical features [3].
Previous reports describing the prognosis for COPD patients with asthma characteristics have been inconsistent. Some prior studies have reported that the clinical outcomes of patients with ACO may be worse than those of patients with asthma or COPD alone [2, 7–9], and patients with ACO have been reported to have an increased risk of exacerbations and rapid lung function decline, worsened quality of life and mortality rates, and increased healthcare utilization, compared with patients who have only asthma or COPD [8–11]. However, some researchers have reported that ACO patients might have a better prognosis [12, 13]. These reported differences in clinical outcomes are partially due to variation in ACO diagnostic criteria used in previous studies, as clear definitions and diagnostic criteria for ACO had not been published [14]. For example, Diaz-Guzman et al. defined ACO based on self-reported physician diagnosis and reported poorer outcomes for ACO patients [8]. Alternatively, Suzuki et al. [12] and Cosio et al. [13] included objective criteria, such as airway reversibility and an elevated blood eosinophil count, for ACO diagnosis and reported better prognosis in ACO patients compared with that of COPD patients without ACO.
Owing to the lack of an established definition and diagnostic criteria for ACO, a great deal of uncertainty remains among physicians around the key diagnostic features and optimal therapeutic regimen for ACO [10, 14–16]. Historical estimates of the proportion of ACO patients within the COPD population have varied considerably, depending on the geographic region, patient characteristics, and diagnostic criteria used. Outside of Japan, the proportion of ACO among COPD patients ranges from 12.1 to 66.0% [17–20]; within Japan, proportions of 4.2–49.7% have been reported [12, 21–24]. The Japanese Respiratory Society (JRS) recently published their 2018 diagnostic criteria for ACO [25, 26]; these were based on more objective diagnostic criteria, which were considered to be better suited for the Japanese medical environment. Compared with previously proposed ACO diagnostic criteria [10, 27], which are considered by some researchers to be more subjective and insufficiently quantitative [26], the JRS diagnostic criteria for ACO are primarily founded on objective measurements (such as computed tomography imaging and fractional exhaled nitric oxide [FeNO]) in addition to medical history and patient reports of symptoms. Moreover, because the asthma component and the COPD component can be diagnosed separately, it is possible to show that ACO is an overlap between asthma and COPD. Using these new criteria, a single-center, cross-sectional study of patients with airflow limitations suggested that the prevalence of ACO in Japan was 30.6% [28].
Another complexity with accurate ACO diagnosis is the variability of the patients’ conditions. Indeed, data from the Spanish CHAIN study, which investigated changes in the prevalence of ACO over 1 year of follow-up, suggested that the proportion of patients who meet the ACO criteria can change over time in the same patient population [13]. To our knowledge, there have been no prospective longitudinal studies in Japan to examine the proportion and temporal changes of ACO patients using the JRS diagnostic criteria [25].
For ACO, as with other respiratory diseases, optimal management depends on accurate and timely diagnosis. ACO patients have asthma symptoms, and a lack of proper treatment, such as inhaled corticosteroid (ICS) therapy, may place them at a clinical disadvantage. Thus, it is critically important to accurately identify ACO patients and determine the prevalence of ACO in Japanese clinical settings to ensure that these patients receive the best possible care. Therefore, we are currently conducting a prospective, multicenter study, with the primary objective of investigating the proportion of patients within the COPD population who meet the JRS ACO diagnostic criteria in clinical practice and observing COPD patient transitions during a 2-year follow-up period. As secondary objectives, we aim to clarify the differences in demographics, clinical characteristics, symptoms, and exacerbation rate/severity between ACO and non-ACO patients and assess the implementation status of examinations/tests required for ACO diagnosis in Japanese clinical practice. Herein, we report the study design and data at registration of this ongoing study.
Methods
Study Design
This multicenter, observational, 2-year, prospective cohort study is being conducted at 27 study sites in Japan. The enrollment period was from June to December 2018. Patients were enrolled consecutively, and registration, including checking eligibility, was managed at a single registration office. Patients will be followed up over 2 years, which includes the visits at registration, 1 year later, and 2 years later. The selected study sites were the medical institutions with respiratory specialists that were able to perform the examinations/tests required for the diagnosis of ACO, as part of routine clinical practice for COPD patients. This capability was checked for each candidate site prior to their inclusion in the study. However, as this was an observational study that aimed to reveal the real-world management of ACO, having tests required for ACO diagnosis was not a prerequisite for patients at study entry, and study centers were not required to change or upgrade their routine clinical practices.
The examinations and tests required to confirm the JRS diagnostic criteria for ACO [25, 26] are listed in Table 1. These criteria were chosen because they are consistent with the diagnostic procedures used in clinical practice, whereby the asthma component and the COPD component are usually diagnosed separately and ACO is then diagnosed as the coexistence of both components [26]. Data were collected using an electronic case report form (completed by the investigator) and questionnaires (completed by the patient). Items to be collected included baseline patient demographics and clinical characteristics, medical history, symptoms (based on questionnaire scores compiled from the COPD assessment test [CAT] modified Medical Research Council questionnaire [mMRC], and the asthma control questionnaire [ACQ]), frequency and severity of exacerbations, results of respiratory function tests, and the outcomes of ACO diagnostic examinations/tests.
Table 1.
Japanese Respiratory Society diagnostic criteria for asthma and COPD overlap [25]
| Basic criteria | |
| Age ≥ 40 years and chronic airflow obstruction: post-bronchodilator FEV1/FVC < 70% | |
|
[Characteristics of COPD] One item from 1, 2, and 3 |
[Characteristics of asthma] Two items from 1, 2, and 3, or One item from 1, 2, and 3 and at least two items from 4 |
|
1. Smoking history (10 pack-years or more) or career involving significant air pollution or biomass exposure 2. Presence of low attenuation areas on chest CT demonstrating emphysematous changes 3. Impaired pulmonary diffusing capacity (%DLCO < 80% or %DLCO/VA < 80%) |
1. Variable (diurnally, daily, and seasonally) or paroxysmal respiratory symptoms (cough, sputum, and dyspnea) 2. History of asthma before age 40 years 3. FeNO > 35 ppb 4. (1) Concomitant perennial allergic rhinitis (2) Airway reversibility (change in FEV1 > 12% and > 200 mL) (3) Peripheral blood eosinophils > 5% or > 300/μL (4) High IgE level (total IgE, or IgE specific to perennial inhalant antigens) |
|
1. To be diagnosed as ACO, one item of the characteristics of COPD plus two items from 1, 2, and 3 or one item from 1, 2, and 3 and at least two items from criterion 4 of the characteristics of asthma are needed 2. If the characteristics of COPD alone are present, it is diagnosed as COPD, and if the characteristics of asthma alone are present, it is diagnosed as asthma (with remodeling) 3. If the characteristics of asthma cannot be confirmed when diagnosing ACO, it is important to monitor for the presence of the characteristics of asthma over time 4. Perennial inhalant antigens include house dust, mites, molds, scales from animals, and feathers, and seasonal inhalant antigens include pollen from trees, plants, and weeds Note 1. Diseases of differential diagnosis (diffuse panbronchiolitis, congenital sinobronchial syndrome, obstructive panbronchiolitis, bronchiectasis, pulmonary tuberculosis, pneumoconiosis, lymphangioleiomyomatosis, congestive heart failure, interstitial lung disease, and lung cancer) should be ruled out by standard chest x-rays, etc. Note 2. Respiratory symptoms such as cough, sputum, and dyspnea are variable (diurnally, daily, and seasonally) or paroxysmal in asthma and chronic and continuous in COPD | |
Adapted from [25] with permission
COPD chronic obstructive pulmonary disease, CT computed tomography, DLCO diffusion capacity for carbon monoxide, FeNO fractional exhaled nitric oxide, FEV1 forced expiratory volume in one second, FVC forced vital capacity, Ig immunoglobulin, VA alveolar volume
This study is being conducted in accordance with the Declaration of Helsinki and all applicable national and international ethical guidelines for medical and health research involving human participants. All study documentation was approved by the Ethics Committee of Tohoku University Hospital (approval reference: 2018-2-147-1). All participants gave informed consent prior to registration. Medical data were collected and stored in compliance with the relevant laws/regulations concerning data protection and the Personal Information Protection Act. This study was registered with ClinicalTrials.gov (identifier: NCT03577795).
Patients
The target patient population was outpatients aged ≥ 40 years with post-bronchodilator forced expiratory volume in one second/forced vital capacity (FEV1/ FVC) < 70% and who had characteristics relevant to COPD according to the JRS ACO diagnostic criteria [25]. Patients were also required to confirm their anticipated ability to visit the study site at least once per year. Full details of the inclusion and exclusion criteria are provided in Table 2.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
1. Patients who provided written informed consent for study participation 2. Patients aged ≥ 40 years 3. Patients with lung function (FEV1/FVC) < 70% after inhalation of a bronchodilator within the past year or at the time of registration 4. Patients who fulfill one of the following criteria: smoking history (≥ 10 pack-years) or equivalent air pollution exposure (including passive smoking) at the time of registration; presence of a low attenuation area showing emphysematous change on chest computed tomography imaging as assessed by the investigator based on the most recent scan within the past year; lung diffusion impairment (defined as %DLCO < 80% or %DLCO/VA < 80%) assessed within the past year 5. Outpatients who were able to regularly visit the study sites in Japan at least once per year |
1. Patients participating in an interventional study within 8 weeks prior to registration 2. Patients who experienced an exacerbation of COPDa or asthmab within the last 8 weeks 3. Patients with a condition that could not be distinguished from COPD or asthma at the time of obtaining consent (diffuse panoramic bronchitis, congenital sinus bronchial syndrome, obstructive panbronchitis, bronchiectasis, pulmonary tuberculosis, pneumoconiosis, lymphangiomyomatosis, interstitial lung disease, lung cancer, vocal fold dysfunction, intratracheal tumor, tracheal softening, bronchial tuberculosis, pulmonary thromboembolism, congestive heart failure). However, patients with certain diseases could be registered if remission or stable disease was confirmed by the physician (laryngitis, epiglottitis, airway foreign body, cough induced by a drug such as an angiotensin converting enzyme inhibitor, spontaneous pneumothorax, hyperventilation syndrome, and psychogenic cough) 4. Any patient who could not comply with study procedures or was otherwise determined by the investigator to be ineligible |
COPD chronic obstructive pulmonary disease, DLCO diffusion capacity for carbon monoxide, FEV1 forced expiratory volume in one second, FVC forced vital capacity, VA alveolar volume
aCOPD exacerbation was defined as the need to change stable treatment due to the observation of increasing symptoms (shortness of breath, cough, and sputum and discomfort or strange sensation in the chest), with the exception of symptoms relevant to other preexisting diseases (e.g., cardiac failure, pneumothorax, pulmonary thromboembolism)
bAsthma exacerbation was defined as the development of paroxysmal/variable symptoms of cough, sputum, wheezing, and dyspnea induced by factors such as respiratory infection
Study Endpoints
The primary study endpoints are (1) the proportions of patients who met the ACO criteria at least once at the time of registration, 1 year later, and 2 years later, among all patients, and (2) the number and proportion of ACO and non-ACO patients among patients who had the data necessary for ACO diagnosis at registration.
Secondary endpoints include comparison of patient groups (those who met the ACO criteria, those who did not, and those who were lacking data necessary for the diagnosis) in terms of patient demographics, exacerbations and lung function decline, symptoms, and treatments as well as investigation of the implementation status of the examinations/tests required to identify ACO. Full details of the study endpoints are given in Table S1.
Statistical Analysis
This study planned to enroll 700 patients. This number was an assumption based on previous reports in which 16.3% of Japanese patients met the criteria of ACO using a cutoff value of FeNO > 35 ppb [24] and 15% of Caucasian patients met the ACO diagnostic criteria with a blood eosinophil count > 5%, with a further 8% meeting the criteria after 1 year [13]. Assuming that the proportion of patients meeting the ACO diagnostic criteria was 15–30% at each time point, and the dropout rate over 2 years was 20% (based on a 17% dropout rate over 3 years in a previous long-term COPD study [29]), a sample size of 700 patients would provide a 95% confidence interval (CI) that falls within 3.1–4.0% for the proportion of patients fulfilling the ACO diagnostic criteria in this study.
The full analysis set (FAS) included patients who had information on at least one item required for ACO diagnosis (clinical characteristics, symptoms, or examinations/tests) collected during at least one of the time points after registration. Categorical variables were summarized using numbers and proportions of patients; quantitative variables were summarized using frequency, mean and standard deviation (SD), median and range, and quartiles. For the primary outcome of this first analysis of patients meeting ACO criteria at registration, the number, proportion, and 95% CI were calculated. Tests performed for comparisons between patient groups included one-way analysis of variance for quantitative variables and chi-square test for categorical variables. All statistical tests were conducted in an exploratory manner, and there were no adjustments for multiplicity and no imputation for missing or incomplete data. All statistical analyses were performed using SAS® Enterprise Guide Analytics version 7.1 (SAS Institute, Cary, NC, USA).
Results
Participant Characteristics
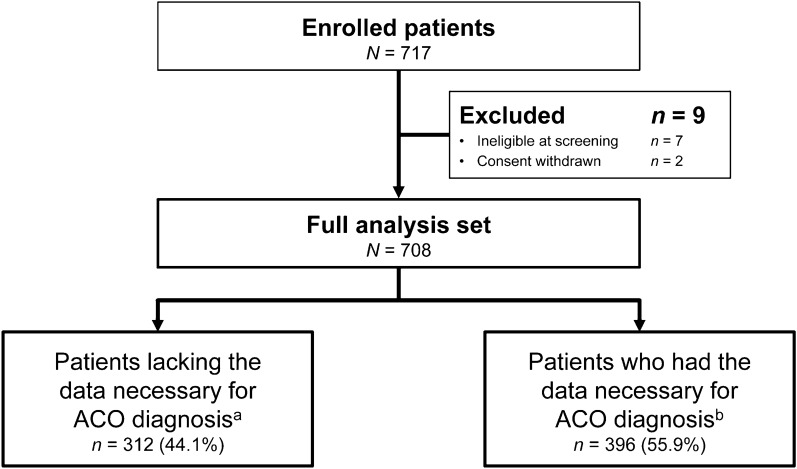
A total of 717 patients were enrolled at 27 centers, and after the exclusion of 9 patients owing to ineligibility or withdrawal of consent, 708 patients were included in the FAS (Fig. 1). Of the FAS, 312 patients lacked the data necessary for ACO diagnosis at registration, while 396 patients had the data necessary for the ACO diagnostic criteria to be applied. Regarding the breakdown by study center, 20 or more patients were registered from each of 19 centers, 15 or more patients from 4 centers, and 10 or fewer patients from the remaining 4 centers.
Fig. 1.
Patient flow. ACO asthma-COPD overlap, COPD chronic obstructive pulmonary disease, JRS Japanese Respiratory Society. aPatients who were lacking data necessary for the JRS ACO criteria to be applied and were not given an ACO diagnosis at registration. bPatients who had the data necessary for the JRS ACO criteria to be applied and were able to be diagnosed as ACO or non-ACO at registration. The enrollment period was from June 2018 to December 2018. Patients were categorized based on examination/test data obtained within 1 year prior to registration
The characteristics of all patients at registration are reported in Table 3. The majority of patients were male (638/708, 90.1%) and had a history of smoking (ex-smokers 616/707, 87.1% or current smokers 79/707, 11.2%). The mean (SD) age was 73.5 (7.7) years, and the mean (SD) body mass index was 22.9 (3.5) kg/m2. The mean (SD) duration of COPD was 5.7 (5.2) years, and almost half of the patients (328/703, 46.7%) were classified as GOLD II. Asthma had previously been diagnosed in 36.0% of patients (255/708); other comorbidities included diabetes (12.6%), allergic rhinitis (10.3%), and heart failure (8.5%). Overall, 42.1% of patients used ICS either as monotherapy or in any combination.
Table 3.
Characteristics of all patients at registration
| Full analysis set, N = 708 | ||
|---|---|---|
| Sex, male, n (%) | 638 (90.1) | |
| Age, years, mean (SD) | 73.5 (7.7) | |
| BMI, kg/m2, mean (SD) | n = 707 | 22.9 (3.5) |
| Smoking status, n (%) | n = 707 | |
| Current smoker | 79 (11.2) | |
| Never smoker | 12 (1.7) | |
| Ex-smoker | 616 (87.1) | |
| Smoking history (pack-years), mean (SD) | n = 690 | 56.2 (34.3) |
| Duration of COPD, years, mean (SD) | n = 692 | 5.7 (5.2) |
| GOLD stage, n (%) | n = 703 | |
| I | 197 (28.0) | |
| II | 328 (46.7) | |
| III | 134 (19.1) | |
| IV | 44 (6.3) | |
| Post-bronchodilator FEV1/FVC%, mean (SD) | n = 704 | 52.0 (12.7) |
| Post-bronchodilator %FEV1 predicted, mean (SD) | n = 703 | 65.1 (22.2) |
| FEV1 reversibility (change in FEV1 > 12% and > 200 mL), n (%) | n = 331 | 20 (6.0) |
| Complication/comorbidity, n (%) | ||
| Asthma (based on physician's diagnosis) | 255 (36.0) | |
| Age at onset < 40 years | 24/240 [10.0] | |
| Age at onset ≥ 40 years | 216/240 [90.0] | |
| Allergic rhinitis | 73 (10.3) | |
| Heart failure | 60 (8.5) | |
| Diabetes | 89 (12.6) | |
| Respiratory medication at registration, n (%) | ||
| ICS (as monotherapy or any combinations) | 298 (42.1) | |
| ICS monotherapy | 12 (1.7) | |
| LAMA monotherapy | 81 (11.4) | |
| LABA monotherapy | 40 (5.6) | |
| LAMA and LABAa | 271 (38.3) | |
| ICS and LABAa | 99 (14.0) | |
| ICS and LAMA | 7 (1.0) | |
| ICS, LAMA, and LABAa | 180 (25.4) | |
BMI body mass index, COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in one second, FVC forced vital capacity, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroids, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, SD standard deviation
aIncluding any combinations of separate and combined inhalers
Primary Outcome: Proportion of ACO Patients at Registration
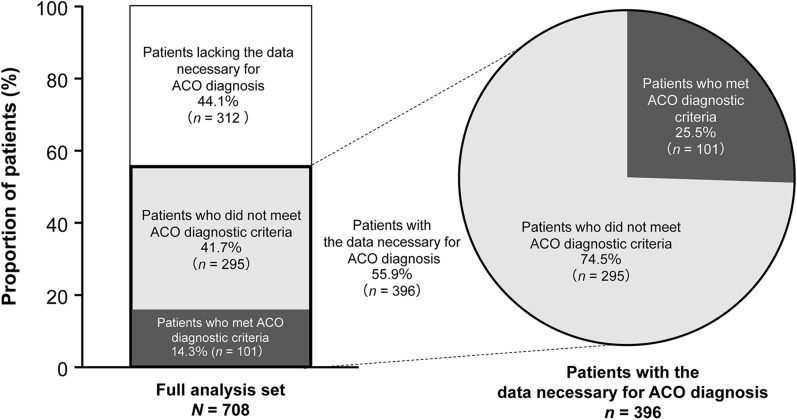
Of the 708 patients analyzed, 101 (14.3%) met the diagnostic criteria for ACO at registration (Fig. 2). Of the 396 (55.9%) patients who had the data necessary for diagnosis of ACO at registration, the proportion of patients who met the ACO criteria was 25.5% (101/396). The remaining 312 (44.1%) patients who lacked the examination/test data necessary for ACO diagnosis at registration will continue in the study and will be followed up over the next 2 years to explore changes in their testing and diagnostic status.
Fig. 2.
Proportion of patients who met Japanese Respiratory Society ACO diagnostic criteria. ACO asthma-COPD overlap, COPD chronic obstructive pulmonary disease
Analysis According to Each ACO Diagnostic Criterion at Registration
The number of patients who underwent evaluation for each JRS ACO diagnostic criterion and the proportion of those patients who met the criterion at registration are shown in Table 4. Of the patients who met the ACO criteria, 85.7% met asthma criterion #1 (variable or paroxysmal respiratory symptoms), 27.3% met criterion #2 (history of asthma before age 40 years), 68.6% met criterion #3 (FeNO > 35 ppb), and 76.6% met at least two of the items in criterion #4 (perennial allergic rhinitis, airway reversibility, elevated blood eosinophil count, or high IgE level). Although 295 patients did not meet the diagnostic criteria for ACO at registration, 14.4% met asthma criterion #1 (variable or paroxysmal respiratory symptoms). In addition, 14.5% presented with at least two of the items in criterion #4; notably, of the patients who underwent evaluation, 58.6% met criterion 4–4(4) (high IgE level).
Table 4.
Proportion of patients who underwent evaluation for each ACO diagnostic criterion and the proportion who met each criterion
| Characteristic, n (%) | Met ACO criteria (n = 101) |
Did not meet ACO criteria (n = 295) |
|||
|---|---|---|---|---|---|
| Patients who underwent evaluationa | Patients who met the criterionb | Patients who underwent evaluationa | Patients who met the criterionb | ||
| Characteristics of COPD | 1. Smoking history (10 pack-years or more) or career involving significant air pollution or biomass exposure | 101 (100.0) | 98 (97.0) | 295 (100.0) | 292 (99.0) |
| 2. Presence of low attenuation areas on chest CT demonstrating emphysematous changes | 80 (79.2) | 64 (80.0) | 231 (78.3) | 201 (87.0) | |
| 3. Impaired pulmonary diffusing capacity (%DLCO < 80% or %DLCO/VA < 80%) | 53 (52.5) | 31 (58.5) | 130 (44.1) | 99 (76.2) | |
| Characteristics of asthma | 1. Variable (diurnally, daily, and seasonally) or paroxysmal respiratory symptoms (cough, sputum, and dyspnea) | 98 (97.0) | 84 (85.7) | 292 (99.0) | 42 (14.4) |
| 2. History of asthma before age 40 years | 99 (98.0) | 27 (27.3) | 294 (99.7) | 1 (0.3) | |
| 3. FeNO > 35 ppb | 86 (85.1) | 59 (68.6) | 269 (91.2) | 16 (5.9) | |
| 4. Applies to the following two or more items | 77 (76.2) | 59 (76.6) | 200 (67.8) | 29 (14.5) | |
| (1) Concomitant perennial allergic rhinitis | 101 (100.0) | 37 (36.6) | 295 (100.0) | 20 (6.8) | |
| (2) Airway reversibility (change in FEV1 > 12% and > 200 mL) | 61 (60.4) | 8 (13.1) | 178 (60.3) | 6 (3.4) | |
| (3) Peripheral blood eosinophils > 5% or > 300/μL | 92 (91.1) | 49 (53.3) | 278 (94.2) | 47 (16.9) | |
| (4) High IgE level (total IgE, or IgE specific to perennial inhalant antigens) | 64 (63.4) | 56 (87.5) | 128 (43.4) | 75 (58.6) | |
ACO asthma-COPD overlap, COPD chronic obstructive pulmonary disease, CT computed tomography, DLCO diffusion capacity for carbon monoxide, FeNO fractional exhaled nitric oxide, FEV1 forced expiratory volume in one second, Ig immunoglobulin, VA alveolar volume
aBased on the total number of patients in the group and data collected within the year prior to registration
bBased on the number of patients evaluated
Comparison of Patient Characteristics, Biomarkers, and Lung Function Between ACO and Non-ACO Patients at Registration
In total, there were 396 patients with the data necessary for ACO diagnosis at registration; this number included 101 patients who were diagnosed as ACO (patients who met the JRS criteria) and 295 who were diagnosed as non-ACO (patients who did not meet the criteria). Patient characteristics (Table 5) and biomarkers and lung function (Table 6) were compared between these two groups of patients.
Table 5.
Characteristics of patients with or without ACO at registration among those who had the data necessary for ACO diagnosis
| Patients with the data necessary for ACO diagnosis | p value | |||
|---|---|---|---|---|
| All (n = 396) | ACO (n = 101) | Non-ACO (n = 295) | (ACO vs non-ACO) | |
| Sex, male, n (%) | 353 (89.1) | 90 (89.1) | 263 (89.2) | 0.990 |
| Age, years, mean (SD) | 73.2 (8.0) | 71.5 (9.6) | 73.8 (7.3) | 0.025 |
| BMI, kg/m2, mean (SD) | 23.0 (3.4) | 23.0 (3.1) | 23.0 (3.5) | 0.869 |
| Smoking status, n (%) | ||||
| Current smoker | 42 (10.6) | 6 (5.9) | 36 (12.2) | 0.108 |
| Never smoker | 4 (1.0) | 2 (2.0) | 2 (0.7) | |
| Ex-smoker | 350 (88.4) | 93 (92.1) | 257 (87.1) | |
| Smoking history (pack-years) | n = 392 | n = 99 | n = 293 | |
| Mean (SD) | 55.1 (35.8) | 57.7 (40.7) | 54.2 (34.1) | 0.441 |
| Duration of COPD, years | n = 390 | n = 99 | n = 291 | |
| Mean (SD) | 5.3 (5.4) | 5.3 (4.6) | 5.3 (5.7) | 0.997 |
| GOLD stage, n (%) | ||||
| I | 125 (31.6) | 30 (29.7) | 95 (32.2) | 0.207 |
| II | 194 (49.0) | 54 (53.5) | 140 (47.5) | |
| III | 59 (14.9) | 16 (15.8) | 43 (14.6) | |
| IV | 18 (4.5) | 1 (1.0) | 17 (5.8) | |
| Complication/comorbidity, n (%) | ||||
| Asthma (based on physician’s diagnosis) | 177 (44.7) | 68 (67.3) | 109 (36.9) | < 0.001 |
| Age at onset < 40 years | n = 175 | n = 67 | n = 108 | |
| 24 (13.7) | 23 (34.3) | 1 (0.9) | < 0.001 | |
| Age at onset ≥ 40 years | n = 175 | n = 67 | n = 108 | |
| 151 (86.3) | 44 (65.7) | 107 (99.1) | < 0.001 | |
| Allergic rhinitis | 57 (14.4) | 37 (36.6) | 20 (6.8) | < 0.001 |
| Heart failure | 45 (11.4) | 3 (3.0) | 42 (14.2) | 0.002 |
| Diabetes | 58 (14.6) | 15 (14.9) | 43 (14.6) | 0.946 |
| Respiratory medication at baseline, n (%) | n = 396 | n = 98 | n = 289 | |
| ICS (monotherapy or any combinations) | 180 (45.5) | 75 (76.5) | 105 (36.3) | < 0.001 |
| ICS monotherapy | 4 (1.0) | 3 (3.1) | 1 (0.3) | 0.052 |
| LAMA monotherapy | 44 (11.1) | 10 (10.2) | 34 (11.8) | 0.674 |
| LABA monotherapy | 20 (5.1) | 1 (1.0) | 19 (6.6) | 0.032 |
| LAMA and LABAa | 138 (34.8) | 11 (11.2) | 127 (43.9) | < 0.001 |
| ICS and LABAa | 65 (16.4) | 22 (22.4) | 43 (14.9) | 0.083 |
| ICS and LAMA | 4 (1.0) | 2 (2.0) | 2 (0.7) | 0.267 |
| ICS, LAMA, and LABAa | 107 (27.0) | 48 (49.0) | 59 (20.4) | < 0.001 |
| Symptoms based on interview | ||||
| Presence of variable symptoms, n (%) | 96 (24.2) | 67 (66.3) | 29 (9.8) | < 0.001 |
| Presence of paroxysmal symptoms, n (%) | n = 387 | n = 95 | n = 292 | < 0.001 |
| 90 (23.3) | 57 (60.0) | 33 (11.3) | ||
| Questionnaire | ||||
| CAT | n = 395 | n = 101 | n = 294 | |
| Mean (SD) | 10.3 (7.0) | 10.7 (7.8) | 10.1 (6.7) | 0.492 |
| mMRC | n = 395 | n = 101 | n = 294 | |
| Mean (SD) | 1.0 (1.0) | 1.0 (1.0) | 1.1 (1.0) | 0.523 |
| ACQ | n = 394 | n = 101 | n = 293 | |
| Mean (SD) | 0.5 (0.8) | 0.9 (1.0) | 0.4 (0.6) | < 0.001 |
ACO asthma-COPD overlap, ACQ asthma control questionnaire, BMI body mass index, CAT COPD assessment test, COPD chronic obstructive pulmonary disease, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroids, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, mMRC modified Medical Research Council questionnaire, SD standard deviation
aIncluding any combinations of separate and combined inhalers
Table 6.
Biomarker and lung function measurements of patients with or without ACO at registration among those who had the data necessary for ACO diagnosis
| Patients with the data necessary for ACO diagnosis | p value | |||
|---|---|---|---|---|
| All (n = 396) | ACO (n = 101) | Non-ACO (n = 295) | (ACO vs non-ACO) | |
| Biomarkers | ||||
| FeNO, ppb | n = 355 | n = 86 | n = 269 | |
| Mean (SD) | 27.4 (23.5) | 47.8 (35.5) | 20.9 (12.5) | < 0.001 |
| Median | 22.0 | 41.5 | 20.0 | |
| FeNO > 35 ppb, n (%) | 75 (21.1) | 59 (68.6) | 16 (5.9) | < 0.001 |
| Peripheral eosinophils absolute count, cells/μL | n = 370 | n = 92 | n = 278 | |
| Mean (SD) | 241.1 (277.9) | 392.8 (472.2) | 190.9 (139.4) | < 0.001 |
| Median | 176.0 | 289.5 | 160.5 | |
| Eosinophil ratio, % | n = 370 | n = 92 | n = 278 | |
| Mean (SD) | 3.8 (3.8) | 5.9 (6.0) | 3.1 (2.3) | < 0.001 |
| Total IgE, IU/mL | n = 278 | n = 75 | n = 203 | |
| Mean (SD) | 415.1 (837.9) | 833.2 (1330.2) | 260.6 (475.1) | < 0.001 |
| Median | 133.5 | 398.0 | 92.0 | |
| Presence of ≥ 1 positive allergen detected in the specific IgE exam, n (%) | n = 147 | n = 39 | n = 108 | |
| 64 (43.5) | 24 (61.5) | 40 (37.0) | 0.008 | |
| Lung function | ||||
| Post-bronchodilator FEV1/FVC%, mean (SD) | 53.1 (12.0) | 53.3 (9.9) | 53.0 (12.7) | 0.545 |
| Post-bronchodilator %FEV1 predicted, mean (SD) | 68.1 (21.7) | 69.2 (20.6) | 67.7 (22.1) | 0.792 |
| FEV1 reversibility (change in FEV1 > 12% and > 200 mL) | n = 239 | n = 61 | n = 178 | |
| Yes, n (%) | 14 (5.9) | 8 (13.1) | 6 (3.4) | 0.010 |
| Presence of emphysema on CT, n (%) | n = 311 | n = 80 | n = 231 | |
| 265 (85.2) | 64 (80.0) | 201 (87.0) | 0.128 | |
| %DLCO < 80% or %DLCO/VA < 80%, n (%) | n = 183 | n = 53 | n = 130 | |
| 130 (71.0) | 31 (58.5) | 99 (76.2) | 0.017 | |
ACO asthma-COPD overlap, CT computed tomography, DLCO diffusion capacity for carbon monoxide, FeNO fractional exhaled nitric oxide, FEV1 forced expiratory volume in one second, FVC forced vital capacity, Ig immunoglobulin, SD standard deviation, VA alveolar volume
The ACO patients were younger (p = 0.025), and a greater proportion had asthma (based on the physician’s diagnosis; p < 0.001) or allergic rhinitis (p < 0.001) as a complication or comorbidity compared with non-ACO patients. In addition, more ACO patients had used ICS as monotherapy or in any combinations including triple therapy (p < 0.001) and had higher rates of variable and paroxysmal symptoms (p < 0.001 for both). All biomarkers investigated were also elevated in ACO patients compared with non-ACO patients; ACQ score was also higher in ACO patients compared with non-ACO patients (p < 0.001). Conversely, body mass index, smoking status, lung function (with the exception of FEV1 reversibility), and CAT and mMRC scores did not differ between the two groups.
Discussion
This prospective, observational study, which used the objective ACO diagnostic criteria proposed by the JRS, revealed that of the 396 patients who had the data necessary to diagnose ACO at registration, 25.5% were found to have ACO, and 74.5% did not (non-ACO patients). This analysis also revealed the actual status of examinations/tests required for the diagnosis of ACO that were conducted by respiratory specialists within the year prior to registration. Of the 708 patients enrolled in the study, 44.1% were found to be lacking the data necessary to make an ACO diagnosis at the time of registration. It is possible that some examinations/tests were omitted because the specialist had previously made a clinical judgement as to whether or not some patients had asthma overlap based on their past medical history and had subsequently not considered the possibility of new onset of ACO. Overall, our results showed that among patients who had available data for ACO diagnosis at registration, 101/396 (25.5%) patients were diagnosed with ACO. The remaining 312/708 (44.1%) patients lacked necessary data to determine whether or not they had ACO. This discrepancy suggests widespread undertesting, resulting in underdiagnosis of ACO among patients with COPD. Once routine diagnostic testing for ACO is implemented in clinical practice, it will be possible to identify more patients with ACO. Therefore, more widespread testing is crucial to facilitate the diagnosis of ACO among patients with COPD and to provide more appropriate treatments for these patients. It is recommended that appropriate objective tests, such as FeNO and peripheral eosinophil count, which have traditionally been less frequently performed, be conducted regularly to improve the precision of ACO diagnosis. All 708 patients will be followed up over the 2-year study duration to understand how routine testing may change over time and to identify how many of the patients who were lacking diagnostic data at registration are subsequently diagnosed with ACO.
The data obtained at registration showed that ICS use was relatively common in Japanese ACO patients (76.5%) compared with 36.3% of non-ACO patients. This is in line with the current treatment guidelines, which suggest that ICS provide clinical benefit for asthma symptoms [2]. Based on these data, it appears that some ACO patients could benefit from treatment with ICS. However, it should be noted that approximately 25% of ACO patients had not been prescribed ICS. We anticipate that our future analysis will yield additional information on the changes in treatment patterns during the study period.
Some non-ACO patients, who did not meet the diagnostic criteria for ACO at registration, nonetheless presented with one or more of the specified asthma characteristics. It is known that FeNO and peripheral blood eosinophil counts fluctuate because of treatment with ICS [30]. Therefore, as stated in the JRS ACO guidelines [25], if asthma characteristics cannot be accurately determined at the time of diagnosis, it is important to follow-up on the presence or absence of these characteristics over time. We also anticipate that some of the patients who were lacking the data necessary to diagnose ACO at registration will subsequently undergo the tests required in routine clinical practice and will be diagnosed as ACO or non-ACO during the study period. Overall, once our study is concluded, we expect to clarify how the ACO prevalence within a COPD population changes over a 2-year duration.
Our study has been designed to allow us to assess the clinical characteristics of several subgroups within our COPD population. The data from this initial analysis combined with the planned 2-year analysis will provide much-needed longitudinal information on the prevalence and clinical course of ACO. The need for this type of information has already been noted by other researchers [31]. Thus, we aim to follow (1) the population of patients who have been diagnosed with ACO at registration, (2) patients who were lacking the data necessary for ACO diagnosis at registration but who subsequently undergo the required examinations/tests, (3) patients who meet the ACO criteria but who are not diagnosed as having comorbid asthma by their attending physicians or did not use ICS, and (4) non-ACO patients who do not meet ACO criteria but yet are diagnosed as having comorbid asthma by their attending physicians or use ICS.
We acknowledge the limitations of our study. It should be noted that participation in the study is restricted to those outpatients who can visit the study sites regularly. Additionally, the study sites were chosen based on the availability of the examinations/tests required for the ACO diagnosis, and these were being carried out in routine clinical practice. Thus, the results obtained from our analyses cannot be directly extrapolated to centers and institutions in which the required examinations/tests are not conducted as part of routine practice. However, we hope that the implementation of a consecutive registration method will minimize some of the selection bias, which is inevitable in this type of study. We also note that some of the tests included in the ACO diagnostic criteria may be affected by the administration of therapeutic agents, particularly ICS. This effect will be taken into account when interpreting the results. Finally, although there have been previous epidemiologic investigations of ACO incidence and prevalence in Japan [12, 21–24], different diagnostic criteria were used, making it difficult to make valid cross-study comparisons. However, the proportion of patients diagnosed with ACO in our study (25.5%) was comparable with that reported in the recent single-center Japanese study [28], which also utilized the JRS criteria (30.6%), suggesting that consistent application of these criteria could provide a robust evidential basis to underpin future clinical analyses.
Conclusions
In this first analysis of an ongoing study of ACO in Japan, which will follow 708 COPD patients over 2 years, we have determined the proportion of patients with the data necessary to diagnose ACO using the JRS criteria and the proportion of these who met the ACO criteria at the time of registration. The patients in the FAS, including those lacking necessary examination/test data at registration, will continue to undergo follow-up to explore changes in their testing and ACO diagnostic status over time. Analyses of study data over 2 years will provide relevant information on the ACO symptoms, clinical course, and real-world treatment patterns.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants in the ACO Japan study.
Funding
This study was sponsored by AstraZeneca K.K., Osaka, Japan. The sponsor also funded the journal’s Rapid Service and Open Access Fees.
Medical Writing, Editorial, and Other Assistance
The authors thank Sally-Anne Mitchell, PhD, and Keyra Martinez Dunn, MD, of Edanz Evidence Generation for providing medical writing support in drafting earlier versions of this manuscript, which was funded by AstraZeneca K.K., Osaka, Japan, through EMC K.K. in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ryoko Sorimachi and Tatsunori Jinnai are employees of AstraZeneca K.K. Shu Hashimoto and Masakazu Ichinose have nothing to disclose.
Compliance with Ethics Guidelines
This study is being conducted in accordance with the Declaration of Helsinki and all applicable national and international ethical guidelines for medical and health research involving human participants. All study documentation was approved by the Ethics Committee of Tohoku University Hospital (approval reference: 2018-2-147-1). All participants gave informed consent prior to registration. Medical data were collected and stored in compliance with the relevant laws/regulations concerning data protection and the Personal Information Protection Act. This study was registered with ClinicalTrials.gov (identifier: NCT03577795).
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Contributor Information
Shu Hashimoto, Email: hashimoto.shu@nihon-u.ac.jp.
Masakazu Ichinose, Email: ichinose@h-osaki.jp.
References
- 1.Knight A. Managing the overlap of asthma and chronic obstructive pulmonary disease. Aust Prescr. 2020;43:7–11. doi: 10.18773/austprescr.2020.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention; 2020. https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. Accessed Sep 2020.
- 3.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed Sep 2020.
- 4.Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond) 2017;131:1541–1558. doi: 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar Marques J, Lobato M, Leiria Pinto P, Neuparth N, Carreiro MP. Asthma and COPD "overlap": a treatable trait or common several treatable-traits? Eur Ann Allergy Clin Immunol. 2020;52:148–159. doi: 10.23822/EurAnnACI.1764-1489.138. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed Sep 2020.
- 7.Woodruff PG, van den Berge M, Boucher RC, et al. American Thoracic Society/National Heart, Lung, and Blood Institute asthma-chronic obstructive pulmonary disease overlap workshop report. Am J Respir Crit Care Med. 2017;196:375–381. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Guzman E, Khosravi M, Mannino DM. Asthma, chronic obstructive pulmonary disease, and mortality in the U.S. population. COPD. 2011;8:400–407. doi: 10.3109/15412555.2011.611200. [DOI] [PubMed] [Google Scholar]
- 9.Lange P, Çolak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med. 2016;4:454–462. doi: 10.1016/S2213-2600(16)00098-9. [DOI] [PubMed] [Google Scholar]
- 10.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ. 2017;358:j3772. doi: 10.1136/bmj.j3772. [DOI] [PubMed] [Google Scholar]
- 11.Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS ONE. 2015;10:e0136065. doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M, Makita H, Konno S, et al. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the Hokkaido COPD cohort study. Am J Respir Crit Care Med. 2016;194:1358–1365. doi: 10.1164/rccm.201602-0353OC. [DOI] [PubMed] [Google Scholar]
- 13.Cosio BG, Soriano JB, López-Campos JL, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149:45–52. doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 14.Uchida A, Sakaue K, Inoue H. Epidemiology of asthma-chronic obstructive pulmonary disease overlap (ACO) Allergol Int. 2018;67:165–171. doi: 10.1016/j.alit.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins C, FitzGerald JM, Martinez FJ, et al. Diagnosis and management of asthma, COPD and asthma-COPD overlap among primary care physicians and respiratory/allergy specialists: a global survey. Clin Respir J. 2019;13:355–367. doi: 10.1111/crj.13016. [DOI] [PubMed] [Google Scholar]
- 16.Rogliani P, Ora J, Puxeddu E, Cazzola M. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007–3013. doi: 10.2147/COPD.S54927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu JJ, Gibson PG, Simpson JL, McDonald VM. Longitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration. 2014;87:63–74. doi: 10.1159/000352053. [DOI] [PubMed] [Google Scholar]
- 18.Hardin M, Cho M, McDonald ML, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44:341–350. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 20.Izquierdo-Alonso JL, Rodriguez-Gonzálezmoro JM, de Lucas-Ramos P, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD) Respir Med. 2013;107:724–731. doi: 10.1016/j.rmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Inoue H, Nagase T, Morita S, Yoshida A, Jinnai T, Ichinose M. Prevalence and characteristics of asthma-COPD overlap syndrome identified by a stepwise approach. Int J Chron Obstruct Pulmon Dis. 2017;12:1803–1810. doi: 10.2147/COPD.S133859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitaguchi Y, Yasuo M, Hanaoka M. Comparison of pulmonary function in patients with COPD, asthma-COPD overlap syndrome, and asthma with airflow limitation. Int J Chron Obstruct Pulmon Dis. 2016;11:991–997. doi: 10.2147/COPD.S105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi Y, Yasunaga H, Matsui H, et al. Comparison of in-hospital mortality in patients with COPD, asthma and asthma-COPD overlap exacerbations. Respirology. 2015;20:940–946. doi: 10.1111/resp.12556. [DOI] [PubMed] [Google Scholar]
- 24.Tamada T, Sugiura H, Takahashi T, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176. doi: 10.2147/COPD.S88274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Japanese Respiratory Society . The JRS guidelines for the management of ACO 2018. Tokyo: Medical Review; 2017. [DOI] [PubMed] [Google Scholar]
- 26.Yanagisawa S, Ichinose M. Definition and diagnosis of asthma-COPD overlap (ACO) Allergol Int. 2018;67:172–178. doi: 10.1016/j.alit.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Global Initiative for Asthma (GINA). Asthma, COPD and asthma-COPD overlap syndrome (ACOS); 2015. https://ginasthma.org/asthma-copd-and-asthma-copd-overlap-syndrome-acos/. Accessed Sept 2020.
- 28.Yamamura K, Hara J, Kobayashi T, et al. The prevalence and clinical features of asthma-COPD overlap (ACO) definitively diagnosed according to the Japanese Respiratory Society Guidelines for the Management of ACO 2018. J Med Invest. 2019;66:157–164. doi: 10.2152/jmi.66.157. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura M, Makita H, Nagai K, et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 30.Tiotiu A. Biomarkers in asthma: state of the art. Asthma Res Pract. 2018;4:10. doi: 10.1186/s40733-018-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SY, Jung H, Kim JH, et al. Longitudinal analysis to better characterize Asthma-COPD overlap syndrome: Findings from an adult asthma cohort in Korea (COREA) Clin Exp Allergy. 2019;49:603–614. doi: 10.1111/cea.13339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.