Abstract
The Coronaviridae family includes the seven known human coronaviruses that cause mild to moderate respiratory infections (HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1) as well as severe illness and death (MERS-CoV, SARS-CoV, SARS-CoV-2). Severe infections induce hyperinflammatory responses that are often intensified by host adaptive immune pathways to profoundly advance disease severity. Proinflammatory responses are triggered by coronavirus entry mediated by host cell-surface receptors. Interestingly, five of the seven strains utilize three cell-surface metallopeptidases (CD13, CD26, and ACE2) as receptors, while the others employ O-acetylated-sialic acid (a key feature of metallopeptidases) for entry. Why coronaviruses evolved to use peptidases as their receptors is unknown, but the peptidase activities of the receptors are dispensable suggesting the virus utilizes/benefits from other functions of these molecules. Indeed, these receptors participate in the immune modulatory pathways that contribute to the pathological hyperinflammatory response. This review will focus on the role of coronavirus receptors in modulating immune responses.
Introduction
The recent pandemic of SARS-CoV-2 infection, its pleiotropic and enigmatic presentation in patients of varying age and race and the recurring waves of infection by less severe, yet often lethal, coronavirus strains clearly illustrates the limitations of our knowledge regarding these viruses. How they so efficiently exploit the immune system and what determines severe, mild, or even asymptomatic disease outcomes among infected patients remain important outstanding questions and would benefit from comprehensive investigation. The coronaviruses comprise a large family of enveloped RNA viruses that derive from a common ancestor and, due to the characteristically high mutation rate of RNA viruses, their progeny are quite diverse (1, 2). The resulting coronaviruses are classified into 4 sub-groups, namely alpha, beta, gamma, and delta. Seven of the combined alpha (HCoV-229E and HCoV-NL63) and beta (HCoV-OC43, HCoV-HKU1, MERS-CoV, SARS-CoV and SARS-CoV-2) sub-group coronaviruses infect humans. Human coronaviruses produce respiratory infections ranging from mild to moderate to critical illness and death. MERS-CoV, SARS-CoV and SARS-CoV-2 are the most virulent and can cause severe lower respiratory tract disease, while the other four remain in the nasopharyngeal tract and are responsible for 15% of common colds (3). According to WHO, MERS has a high fatality rate of ~34.4% as of November 2019 with 858 fatalities across 27 countries but its low human-to-human transmission limits widespread outbreaks (R0=0.45–0.98 in Saudi Arabia and R0=2.5–8.09 in early stages in South Korea but dropping to R0<1 in a later period or with intervention) (4). Similarly, SARS-CoV also has a high global fatality rate of ~9.5% with a low transmission rate (R0=2–4, dropping to <1 with control measures). Contrary to these two viruses, SARS-CoV-2 has a lower global mortality rate of ~3.5–4% but a high reproductive number (R0=2.4–5.7) (5–7) making it highly contagious and pervasive, resulting in devastating death tolls.
Coronavirus infections can eventually lead to an intense pathological inflammatory response that is accompanied by excessive activation of host innate immune mechanisms that further the damage. Viral entry is mediated by specific cell-surface receptors which are recognized by the C-terminal receptor-binding domains (RBD) of the distinctive coronavirus spike proteins (2, 8). The receptors for two of the viruses, HCoV-OC43 and HCoV-HKU1, have not been identified, but are known to employ the 9-O-acetylated-sialic acid modifications on glycoproteins as entry points (Table 1) (9, 10). By contrast, the remaining five coronaviruses utilize three cell-surface metallopeptidases as receptors: CD13/ANPEP/APN (aminopeptidase N), CD26/DPP4 (dipeptidyl peptidase 4), and ACE2 (angiotensin-converting enzyme 2) (11–16). Internalized viral RNA binds to cytosolic, extracellular, and endosomal pattern recognition receptors that activate standard downstream inflammatory signaling cascades (17). However, patients with severe coronavirus infections often have a massive overproduction of inflammatory cytokines, resulting in extensive neutrophil and macrophage infiltration and with dampened adaptive immunity (fewer CD4+ and CD8+ T cells), culminating in increased cell and tissue death and eventually, organ failure (18–29). Why coronaviruses have evolved to use peptidases as their receptors is unknown, but the possibility that this has occurred by chance is highly unlikely (30). Interestingly, viral entry is unaffected by abrogating the receptors’ peptidase activities (11, 12, 14, 31), suggesting that these molecules contribute additional functions that may facilitate viral persistence. Indeed, independent of viral infection, these peptidases as well as 9-O-acetylated sialic acid have been implicated as multifunctional modulators of immune cells such as inflammatory cytokine production, inflammatory cell adhesion, phagocytosis, angiogenesis and immune receptor trafficking (32–41). Viral activation of these pathways could clearly amplify the immune response, resulting in hyperinflammation leading to pathology in the context of viral infection. In this review, we will focus on the potential role of the human coronavirus receptors CD13/APN, CD26/DPP4 and ACE2 in mediating immune responses observed in coronavirus infections.
Table 1:
Receptors used by the seven different human coronaviruses
| Coronavirus | Receptor |
|---|---|
| Human coronavirus 229E (HCoV-229E) – alpha coronavirus | CD13 |
| Human coronavirus NL63 (HCoV-NL63) – alpha coronavirus | ACE2 (only group I to use ACE2) |
| Human coronavirus OC43 (HCoV-OC43) – beta coronavirus | 9-O-acetylated sialic acid (9-O-Ac-Sia) residues on glycoproteins |
| Human coronavirus HKU1 (HCoV-HKU1) – beta coronavirus | 9-O-acetylated sialic acid (9-O-Ac-Sia) residues on glycoproteins |
| Middle East respiratory syndrome coronavirus (MERS-CoV) – beta coronavirus | CD26 |
| Severe acute respiratory syndrome coronavirus (SARS-Cov) – beta coronavirus | ACE2 |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – beta coronavirus | ACE2 |
Immune response in coronavirus infections
To better understand the role of coronavirus receptors in modulating immune responses, a brief overview of immune responses observed in coronavirus infections is necessary, specifically among the more severe MERS-CoV, SARS-CoV and SARS-CoV-2 coronaviruses. The common characteristic immunological features of these infections are: elevated levels of proinflammatory chemokines and cytokines, increased neutrophil accumulation leading to toxic neutrophil extracellular traps (NETs) and lymphopenia (fewer CD4+ T cells, CD8+ T cells and B cells) (Figure 1) (18–28). Patients with severe SARS-CoV had extensive myeloid infiltrates, but lower levels of T cells (28) as did COVID-19 patients, (42) while patients who successfully recovered from COVID-19 had reestablished T cell and B cell numbers close to normal levels (18). COVID-19 patients also elicit a strong humoral response as IgM, IgA and IgG antibodies against SARS-CoV-2 spike protein become detectable by 14 days after the onset of symptoms and persist much longer (42). High titers of neutralizing IgG antibodies against SARS-CoV-2 were detected in 13/14 convalescent patients (43). Interestingly, a study comparing unexposed and SARS-CoV-2 infected patients revealed that ~40–60% of the unexposed individuals carried SARS-CoV-2-cross-reactive CD4+ T cells, presumably produced against common cold coronaviruses that could underlie the natural immunity against SARS-CoV-2 seen in a sub-population of individuals (44). Taken together, these findings emphasize the importance of the adaptive immune response in fighting SARS-CoV-2 infection in addition to suppressing the hyperactivated innate immune response. This may hold true for SARS-CoV and MERS-CoV infections as well.
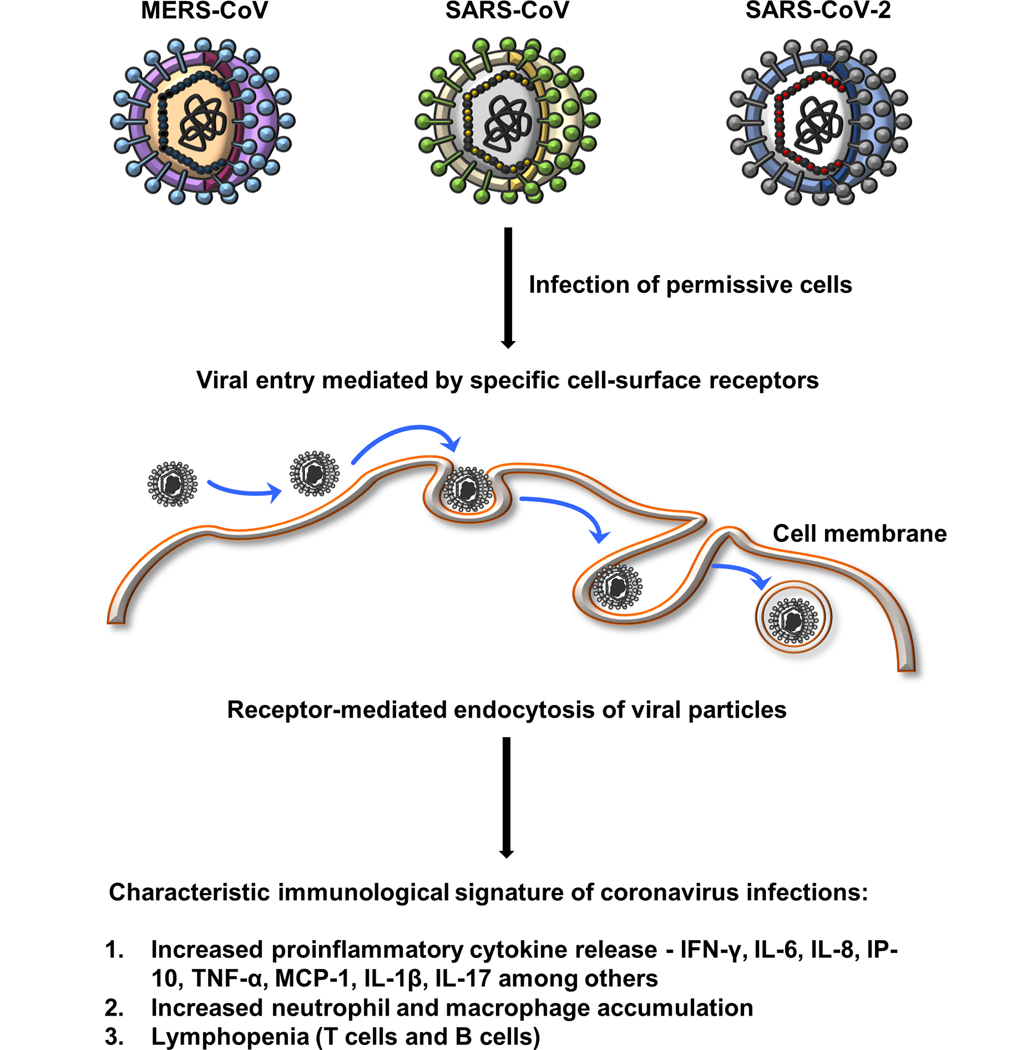
Figure 1: Common characteristic immunological features of coronavirus infections.
Induction of immune response begins with the binding and internalization of coronaviruses leading to elevated levels of proinflammatory chemokines and cytokines, specifically IFN-γ, IL-6, IL-8, IP-10, TNF-α, MCP-1, IL-1β and IL-17 among others. Increased chemokine and cytokine release causes neutrophil and macrophage accumulation as well as lymphopenia (CD4+ T cells, CD8+ T cells and B cells).
Mechanistically, induction of these immune responses begins with the binding and internalization of coronaviruses which leads to activation of the NF-kB and NLRP3 inflammasome and cleavage of pro-IL-1β to yield active proinflammatory IL-1β that can then induce expression of IL-6 (45, 46). Release of inflammatory mediators by the infected cells as well as the subsequently recruited innate immune cells, including neutrophils and inflammatory macrophages, promotes the proinflammatory response. Increased serum levels of proinflammatory cytokines, such as IL-6, is a common theme across coronavirus infections compared to healthy controls (26, 47, 48). The defensive antiviral IFN response against coronavirus infections is believed to be suppressed (lower levels of IFNβ), however an increase in the expression of interferon-stimulated genes has also been observed (47, 49). This suggests that the anti-viral interferon response could be dependent on the stage of the infection, while the proinflammatory status remains far more consistent (18–24). Plasma levels of IFN-α, IP-10, IL-6, and MCP-1 are highest in the acute phase (within 2 weeks of the onset of symptoms) of moderate and severe MERS-CoV cases and diminish in the convalescent phase (21). Similarly, moderately infected SARS-CoV patients have higher plasma levels of IFN-γ, IL-1β, IL-8, IL-6, MCP-1, and IP-10 for 19 consecutive days after onset of disease than healthy individuals (20). The initially high cytokine levels drop to normal levels in convalescent patients (19) and in patients treated with methylprednisolone (standard care of treatment) (20). Successful anti-viral therapy of COVID-19 patients also leads to reductions in the levels of proinflammatory cytokines and reestablishes CD4+ and CD8+ T cell numbers close to normal levels (18). Overall, increased levels of proinflammatory cytokines correlate with the viral load and track with the progression and regression of the disease, making them useful markers of pathology.
IL-6 – master regulator
As is evident from the various studies, the cytokine storm consists of pro-inflammatory cytokines such as IL-6, IL-8, MCP-1, IL-1β and TNF-α among others and is associated with the development of acute respiratory distress syndrome (50). However, in the fight against COVID-19, IL-6 has taken the center stage as it regulates a wide array of biological responses which can amplify respiratory distress (51–57). IL-6 is a unique cytokine in that it can switch between anti-inflammatory (classical signaling) and pro-inflammatory (trans-signaling) responses depending on upon the cleavage state of its receptor. IL-6 binding to membrane bound IL-6Rα mediates anti-inflammatory responses, while binding its soluble receptor (sIL-6Rα) mediates pro-inflammatory responses and acts as a positive feedback signal to further generate IL-6 that is more potent than classical signaling (58). IL-6 knockout or neutralization with anti-IL-6 antibody leads to reduced inflammation and fibrosis (59). Therefore, targeting IL-6 presents a viable approach to mitigate the multitude of proinflammatory events resulting from coronavirus infection. Antibodies against IL-6 such as tocilizumab have shown promising results in severe COVID-19 patients and are in clinical trials for various inflammatory disorders (60–62).
Role of coronavirus receptors in modulating the inflammatory response.
The peptidase receptor molecules modulate IL-6 levels both as bona fide coronavirus-binding receptor molecules as well as participating as accessory molecules in immune modulatory pathways to amplify or attenuate the immune response observed in coronavirus infections. Although direct binding of the individual coronaviruses to their primary receptor molecules has been validated, there is ample evidence suggesting that these, as well as other host cell accessory proteins, also facilitate viral entry. Prior to virus binding to receptors, cleavage of coronavirus spike proteins by host cell proteases, designated ‘S protein priming’, is often required (63, 64). For example, cleavage of the SARS-CoV-2 spike protein by the membrane bound serine protease TMPRSS2 is essential to enable virus binding to its ACE2 receptor (13). It has also been postulated that the high infection rate of SARS-CoV-2 may be due to its enhanced ability to exploit host cell factors such as glycan modifications to facilitate attachment, (65, 66). Similarly, MERS-CoV replication is impaired in human monocyte-derived macrophages and dendritic cells regardless of CD26 expression, presumably due to a lack of accessory proteins involved in its internalization (67). Additionally, potential cross-reactivity with other receptors could provide additional modes of entry, as suggested by results from analyses predicting the SARS-CoV-2 spike protein bound CD26 with high affinity, albeit lower than that to ACE2 (68). Therefore, SARS-CoV-2 could potentially use CD26 as a receptor and its binding to either the CD26 or ACE2 receptor molecules could depend on differences in the affinity and/or on the relative abundance of CD26 and ACE2 on host cells. Although in vitro assays have shown that CD13 is not the receptor for SARS-CoV-2 (13), CD13 and CD26 expression is co-regulated with ACE2 in various monkey and human tissues (69), suggesting that CD13 and/or CD26 could be potential accessory molecules contributing to viral entry in vivo. Therefore, we propose that although individual coronaviruses may not use CD13, CD26 and ACE2 as their primary receptors, these molecules could conceivably influence coronavirus uptake and overall infectivity, as well as amplify the ensuing immune response (Figure 2). This thereby emphasizes the necessity of understanding the contribution of these receptors to disease progression and severity.
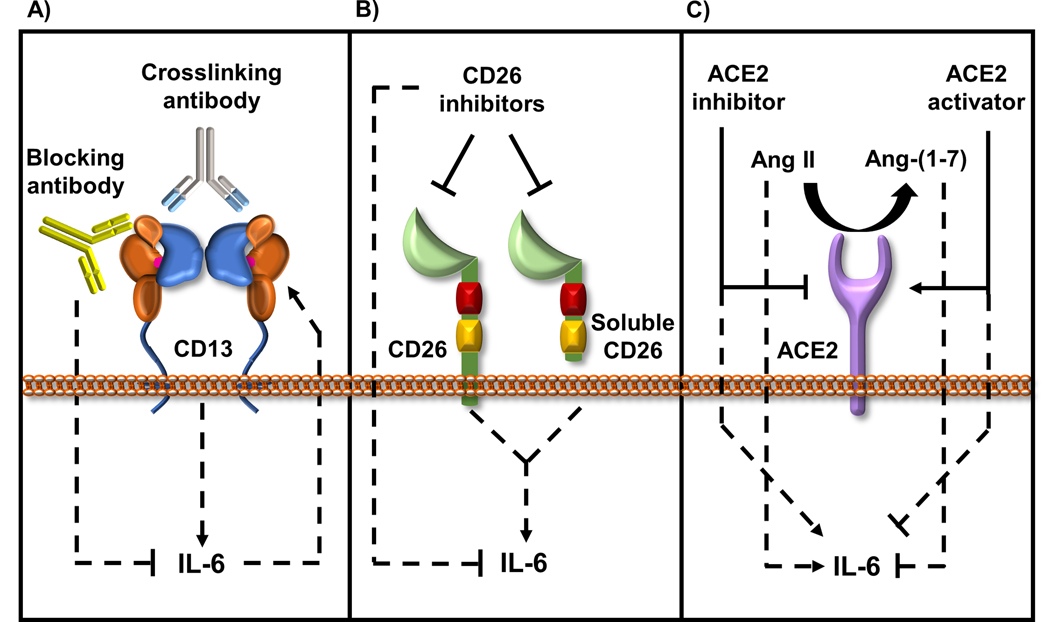
Figure 2: Role of coronavirus receptors in modulating IL-6 levels.
Evidence for the role of coronavirus receptors in modulating IL-6 levels is strong and is observed in various normal and disease models.
A) IL-6 production is enhanced by crosslinking CD13, while CD13 blocking antibodies inhibit IL-6 production. Also, IL-6 and soluble IL-6R induce CD13 expression and activity suggesting a possible positive feedback loop between CD13 and IL-6 expression.
B) Both the membrane bound and soluble CD26 induce IL-6 expression that can be inhibited using CD26 inhibitors such as anagliptin and alogliptin.
C) Ang II, the substrate for ACE2 triggers the production of IL-6, while the product of ACE2 enzymatic activity, Ang-(1–7) opposes Ang II signaling and inhibits IL-6 production. Consequently, loss of ACE2 or treatment with an ACE2 inhibitor (MLN-4760) induces IL-6 production and alternatively, activation of endogenous ACE2 with XNT–(1-[(2-dimethylamino) ethylamino]-4-(hydroxymethyl)-7-[(4-methylphenyl) sulfonyl oxy]-9H-xanthene-9-one} reduces IL-6 production.
CD13/APN/ANPEP, inflammation and IL-6
Human CD13 is the prototypical member of the M1 family of zinc-binding metallopeptidases and is expressed on myeloid cells as well as in various tissues (70–72). CD13 is a 150-kD type II single-pass transmembrane protein consisting of a short, highly conserved, 7–9 amino acid cytoplasmic tail, a hydrophobic transmembrane region and 7 extracellular domains which contain both its zinc-coordinating and active sites. As a peptidase, CD13/APN participates in the metabolism of regulatory peptides by several cell types, including small intestinal and renal tubular epithelial cells, macrophages, granulocytes, and brain pericytes. However, the majority of CD13-mediated functions occur independently of its enzymatic activity, ranging from angiogenesis, monocyte/endothelial adhesion in inflammation, integrin recycling, receptor endocytosis and maintenance of the stem cell niche (34–36, 73–77). Pertinent to this review, monoclonal antibodies (mAbs) that blocked infection of newborn pigs with the porcine Transmissible Gastroenteritis Virus (TGEV) identified porcine CD13 (pCD13) as the receptor for this fatal intestinal pathogen (31). Subsequently, human CD13 (hCD13) was identified as the receptor for the human coronavirus HCoV-229E (11). Importantly, CD13’s enzymatic activity is not essential for receptor activity and neither HCoV-229E binding nor infection is affected by various peptidase inhibitors (11, 78, 79).
Evidence for the interconnection between CD13, inflammation and IL-6 in particular, is strong, and is observed in various normal and disease models (80–84). IL-6 and soluble IL-6R have been shown to induce CD13 expression and activity (81, 82), while anti-CD13 antibody inhibits IL-6 production (84), indicating a positive feedback loop between the expression of CD13 and IL-6. Indeed, antibody-mediated crosslinking of CD13 in mast cells leads to IL-6 production (83). Additionally, soluble CD13 is highly abundant in synovial fluid of rheumatoid arthritis (RA) patients where it increases expression of proinflammatory cytokines (84–91). With regard to HCoV-229E infection, treatment of primary human nasal and tracheal epithelial cells with GFB (glycopyrronium, formoterol, and budesonide), a cocktail of drugs used to treat chronic obstructive pulmonary disease caused by HCoV-229E infection, led to reduced CD13 expression, fewer acidic endosomes that are essential for the entry of HCoV-229E as well as decreased production of inflammatory cytokines (80). This suggests that in addition to preventing entry of HCoV-229E, this cocktail may also help mitigate proinflammatory signaling events observed in other coronavirus infections.
CD26/DPP4, inflammation and IL-6
CD26 is a type II cell membrane peptidase that is expressed in many tissues and hematopoietic cells (92). MERS-CoV utilizes CD26 as the receptor for viral entry and a neutralizing antibody (Mersmab1) against the receptor-binding domain of the MERS-CoV spike protein blocks viral entry (93). Independent of viral infection, CD26 activity promotes inflammation and inhibition of CD26 is beneficial in suppressing inflammation. CD26 is unique in the sense that its inhibition seems to have a dual effect on inflammation, discussed below.
Soluble CD26, generated by cleavage of membrane bound CD26, has been shown to upregulate the p-p65 NF-κB subunit, increase expression and secretion of IL-6, IL-8, and MCP-1 in human vascular smooth muscle cells, while CD26 inhibition and PAR2 silencing prevented this phenotype (94). Specific CD26 inhibition in different disease models has been shown to reduce expression of IL-6 and IL-1β and other proinflammatory cytokines, suggesting that CD26 intensifies inflammation (95). Therefore, it appears that inhibiting CD26 would suppress various inflammatory responses. However, contradictory evidence implicates CD26 as an inhibitor of lymphocyte chemotaxis. Young, diabetic Dpp4−/− mice on high fat diet have increased MCP-1 protein. Additionally, CD26 inhibition in older diabetic mice resulted in cardiac impairment and dysregulated expression of inflammatory and fibrosis genes (96). Mechanistically, CD26 cleaves and inactivates the proinflammatory chemokine CXCL10 to a nonfunctional truncated form, leading to decreased lymphocyte chemotaxis and NK cell infiltration, demonstrated primarily in tumor models (97). This highlights the role of CD26 in mediating certain aspects of anti-inflammatory responses by suppressing NK and T cell infiltration. Therefore, while inhibiting CD26 seems logical considering its role in IL-6 production, it could lead to increased immune cell infiltration, that can potentially exacerbate tissue damage in the context of severe coronavirus infections. Clearly, a careful consideration of the dual role of CD26 in inflammation is needed in the context of coronavirus treatment.
ACE2, inflammation and IL-6
ACE2, the receptor for SARS-CoV and SARS-CoV-2 viruses is expressed in various tissues including small intestine, colon, breast, liver, testis, ovary, kidney, bladder, heart, thyroid, pancreas, lungs, adipose tissue, and adrenal gland (26, 98). This diverse expression profile may underlie the prevalence of widespread organ damage including kidney and cardiovascular tissue in severe SARS-CoV-2 infected patients (26, 99). ACE2 is a type I membrane metalloenzyme that cleaves angiotensin II (Ang II) to generate angiotensin 1–7 (Ang-(1–7) (13, 14). Full length Ang II binds to its receptor AT1 to trigger a strong pro-inflammatory response, including vasoconstriction, vascular permeability and proinflammatory signaling. By contrast, its cleavage product Ang-(1–7) produces antagonistic anti-inflammatory effects by acting as a competitive antagonist for AT1 (100) or by binding to and signaling through the Mas receptor (13, 14). AT1 receptor blockers, soluble ACE2, overexpressed ACE2, ACE2 inhibitors (MLN-4760) (101), Ace2 deletion and exogenous Ang-(1–7) have all been shown to induce anti-inflammatory immune responses in various models of inflammatory disease (101–110). ACE2 expression is elevated in injured lung epithelial cells in chronic obstructive pulmonary disease (COPD) patients and smokers (111, 112). Therefore, these individuals are potentially more susceptible to SARS-CoV-2 infection, although at this time there is no conclusive evidence in humans to substantially prove this hypothesis. Interestingly, Type 1 diabetic patients exhibit increased circulating (serum) ACE2 activity compared to healthy controls (113) and soluble human ACE2 can prevent viral entry by binding to SARS-CoV, HCoV-NL63 and SARS-CoV-2 (16, 114), suggesting that Type 1 diabetic patients may be protected. Therefore, ACE2 acts as a crucial modulator of the anti-inflammatory immune response and activation of ACE2 or use of exogenous Ang-(1–7) could potentially counter the pro-inflammatory response seen in severe COVID-19 patients. This is an important consideration since treatment strategies aimed at blocking ACE2 may also target its beneficial anti-inflammatory effects.
Conclusions
The massive inflammation characterized by the cytokine storm that develops upon infection by MERS-CoV, SARS-CoV and SARS-CoV-2 clearly underlies the high morbidity and death toll that accompanies these viral infections. While mitigating infection by blocking the initial entry of coronaviruses into host cells is logically the gold standard to prevent new infection, once infected, addressing the question of why these particular viruses trigger such extreme responses may be an effective means of controlling the damage resulting from these infections. To this end, it is essential that we extensively explore the physiological capabilities of these immune modulatory peptidases that also function as viral receptors. A clear understanding of their potential contributions to the exaggerated immune activation in advanced infections may lead to strategies to alleviate morbidity and mortality of these and the inevitable pandemic coronavirus infections of the future.
Acknowledgements
Figures were prepared using the Motifolio illustration toolkit.
Funding sources: This work was supported by grants R01-HL127449 and R01-HL125186
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of, V. 2020. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature microbiology 5: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters PS 2006. The molecular biology of coronaviruses. Advances in virus research 66: 193–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sariol A, and Perlman S. 2020. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 53: 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JE, Jung S, Kim A, and Park JE. 2018. MERS transmission and risk factors: a systematic review. BMC Public Health 18: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majumder MS, and Mandl KD. 2020. Early Transmissibility Assessment of a Novel Coronavirus in Wuhan, China. Ssrn: 3524675. [Google Scholar]
- 6.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, and Ke R. 2020. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerging infectious diseases 26: 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Arienzo M, and Coniglio A. 2020. Assessment of the SARS-CoV-2 basic reproduction number, R0, based on the early phase of COVID-19 outbreak in Italy. Biosafety and Health 2: 57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin MR,, Levantovsky, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salomé B, Selvan SME,MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N,, Brown BD, Greenbaum B, Gümüş ZH, Homann D, Horowitz A, Kamphorst AO, Curotto de, and Lafaille MA, Mehandru S, Merad M, Samstein RM, The Sinai Immunology Review Project,. 2020. Immunology of COVID-19: current state of the science. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulswit RJG, Lang Y, Bakkers MJG, Li W, Li Z, Schouten A, Ophorst B, van Kuppeveld FJM, Boons GJ, Bosch BJ, Huizinga EG, and de Groot RJ. 2019. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc Natl Acad Sci U S A 116: 2681–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Dong W, Milewska A, Golda A, Qi Y, Zhu QK, Marasco WA, Baric RS, Sims AC, Pyrc K, Li W, and Sui J. 2015. Human Coronavirus HKU1 Spike Protein Uses O-Acetylated Sialic Acid as an Attachment Receptor Determinant and Employs Hemagglutinin-Esterase Protein as a Receptor-Destroying Enzyme. J Virol 89: 7202–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, and Holmes KV. 1992. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, and Haagmans BL. 2013. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495: 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, and Pohlmann S. 2020. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181: 271–280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, and Farzan M. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letko M, Marzi A, and Munster V. 2020. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology 5: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, and Pohlmann S. 2005. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A 102: 7988–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, and Lanni C. 2020. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal transduction and targeted therapy 5: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni M, Tian FB, Xiang DD, and Yu B. 2020. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J Med Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, Ye L, Xu S, Sun R, Wang Y, and Lou J. 2004. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 72: 4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, and Sung JJ. 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin HS, Kim Y, Kim G, Lee JY, Jeong I, Joh JS, Kim H, Chang E, Sim SY, Park JS, and Lim DG. 2019. Immune Responses to Middle East Respiratory Syndrome Coronavirus During the Acute and Convalescent Phases of Human Infection. Clin Infect Dis 68: 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, and Lei HY. 2005. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 75: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alosaimi B, Hamed ME, Naeem A, Alsharef AA, AlQahtani SY, AlDosari KM, Alamri AA, Al-Eisa K, Khojah T, Assiri AM, and Enani MA. 2020. MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine 126: 154895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Liu L, Liu WJ, Zhou C, Chen L, Xu Z, Xing L, Cao M, Wu W, Peng L, Li J, Shen C, Li J, Wang Z, Tan S, Li S, Zhao D, Li X, Qin Y, Zhang Z, Yuan J, Wang F, Yang Y, Huang F, Zhang C, and Liu Y. 2020. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. National Science Review 7: 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, and Suliman BA. 2018. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 104: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, and Ning Q. 2020. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, and Zheng X. 2020. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55: 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, Chan PK, Ng MH, Yu LM, Hui DS, Tam JS, Cheng G, and Sung JJ. 2003. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Bmj 326: 1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Yale IT, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, and Iwasaki A. 2020. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F 2015. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol 89: 1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delmas B, Gelfi J, L’Haridon R, Vogel LK, Sjostrom H, Noren O, and Laude H. 1992. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 357: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schauer R, Srinivasan GV, Wipfler D, Kniep B, and Schwartz-Albiez R. 2011. O-Acetylated sialic acids and their role in immune defense. Adv Exp Med Biol 705: 525–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi WX, Chammas R, Varki NM, Powell L, and Varki A. 1996. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem 271: 31526–31532. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh M, Lo R, Ivic I, Aguilera B, Qendro V, Devarakonda C, and Shapiro LH. 2019. CD13 tethers the IQGAP1-ARF6-EFA6 complex to the plasma membrane to promote ARF6 activation, beta1 integrin recycling, and cell migration. Sci Signal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh M, Subramani J, Rahman MM, and Shapiro LH. 2015. CD13 Restricts TLR4 Endocytic Signal Transduction in Inflammation. J Immunol 194: 4466–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramani J, Ghosh M, Rahman MM, Caromile LA, Gerber C, Rezaul K, Han DK, and Shapiro LH. 2013. Tyrosine Phosphorylation of CD13 Regulates Inflammatory Cell-Cell Adhesion and Monocyte Trafficking. J Immunol 191: 3905–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikushima H, Munakata Y, Iwata S, Ohnuma K, Kobayashi S, Dang NH, and Morimoto C. 2002. Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cellular immunology 215: 106–110. [DOI] [PubMed] [Google Scholar]
- 38.Bank U, Heimburg A, Helmuth M, Stefin S, Lendeckel U, Reinhold D, Faust J, Fuchs P, Sens B, Neubert K, Tager M, and Ansorge S. 2006. Triggering endogenous immunosuppressive mechanisms by combined targeting of Dipeptidyl peptidase IV (DPIV/CD26) and Aminopeptidase N (APN/ CD13)--a novel approach for the treatment of inflammatory bowel disease. International immunopharmacology 6: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 39.Baumann AM, Bakkers MJ, Buettner FF, Hartmann M, Grove M, Langereis MA, de Groot RJ, and Muhlenhoff M. 2015. 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat Commun 6: 7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernstein KE, Khan Z, Giani JF, Cao DY, Bernstein EA, and Shen XZ. 2018. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol 14: 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao DY, Saito S, Veiras LC, Okwan-Duodu D, Bernstein EA, Giani JF, Bernstein KE, and Khan Z. 2020. Role of angiotensin-converting enzyme in myeloid cell immune responses. Cell Mol Biol Lett 25: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L, Ren L, Yang S, Xiao M, Chang, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang QW, Xu SY, Zhu HD, Xu YC, Jin Q, Sharma L, Wang L, and Wang J. 2020. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis 71: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, Wei P, Ge J, Gou M, Li X, Sun L, Cao T, Wang P, Zhou C, Zhang R, Liang P, Guo H, Wang X, Qin CF, Chen F, and Dong C. 2020. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 52: 971–977 e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, and Sette A. 2020. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181: 1489–1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siu KL, Yuen KS, Castano-Rodriguez C, Ye ZW, Yeung ML, Fung SY, Yuan S, Chan CP, Yuen KY, Enjuanes L, and Jin DY. 2019. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J 33: 8865–8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cahill CM, and Rogers JT. 2008. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem 283: 25900–25912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, and tenOever BR. 2020. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181: 1036–1045 e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Jiang M, Chen X, and Montaner LJ. 2020. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 108: 17–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, and Wang J. 2020. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell host & microbe 27: 883–890 e882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aisiku IP, Yamal JM, Doshi P, Benoit JS, Gopinath S, Goodman JC, and Robertson CS. 2016. Plasma cytokines IL-6, IL-8, and IL-10 are associated with the development of acute respiratory distress syndrome in patients with severe traumatic brain injury. Critical care 20: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wrighting DM, and Andrews NC. 2006. Interleukin-6 induces hepcidin expression through STAT3. Blood 108: 3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heinrich PC, Castell JV, and Andus T. 1990. Interleukin-6 and the acute phase response. Biochem J 265: 621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chomarat P, Banchereau J, Davoust J, and Palucka AK. 2000. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 1: 510–514. [DOI] [PubMed] [Google Scholar]
- 54.Rincon M, Anguita J, Nakamura T, Fikrig E, and Flavell RA. 1997. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med 185: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jourdan M, Cren M, Robert N, Bollore K, Fest T, Duperray C, Guilloton F, Hose D, Tarte K, and Klein B. 2014. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia 28: 1647–1656. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, and Drobyski WR. 2009. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 114: 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alsaffar H, Martino N, Garrett JP, and Adam AP. 2018. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am J Physiol Cell Physiol 314: C589–C602. [DOI] [PubMed] [Google Scholar]
- 58.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, Mansell A, and Jenkins BJ. 2011. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol 186: 1199–1208. [DOI] [PubMed] [Google Scholar]
- 59.Pedroza M, Schneider DJ, Karmouty-Quintana H, Coote J, Shaw S, Corrigan R, Molina JG, Alcorn JL, Galas D, Gelinas R, and Blackburn MR. 2011. Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS One 6: e22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, and Wei H. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 117: 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbi L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, and Mussini C. 2020. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology 2: e474–e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka T, Narazaki M, and Kishimoto T. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menachery VD, Dinnon KH 3rd, Yount BL Jr., McAnarney ET, Gralinski LE, Hale A, Graham RL, Scobey T, Anthony SJ, Wang L, Graham B, Randell SH, Lipkin WI, and Baric RS. 2020. Trypsin Treatment Unlocks Barrier for Zoonotic Bat Coronavirus Infection. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Du L, Liu C, Wang L, Ma C, Tang J, Baric RS, Jiang S, and Li F. 2014. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci U S A 111: 12516–12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Hulswit RJG, Widjaja I, Raj VS, McBride R, Peng W, Widagdo W, Tortorici MA, van Dieren B, Lang Y, van Lent JWM, Paulson JC, de Haan CAM, de Groot RJ, van Kuppeveld FJM, Haagmans BL, and Bosch BJ. 2017. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci U S A 114: E8508-E8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park SS 2019. Post-Glycosylation Modification of Sialic Acid and Its Role in Virus Pathogenesis. Vaccines (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tynell J, Westenius V, Ronkko E, Munster VJ, Melen K, Osterlund P, and Julkunen I. 2016. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. The Journal of general virology 97: 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P, and Lu J. 2020. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. iScience 23: 101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi F, Qian S, Zhang S, and Zhang Z. 2020. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 526: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Look AT, Ashmun RA, Shapiro LH, and Peiper SC. 1989. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest 83: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winnicka B, O’Conor C, Schacke W, Vernier K, Grant CL, Fenteany FH, Pereira FE, Liang B, Kaur A, Zhao R, Montrose DC, Rosenberg DW, Aguila HL, and Shapiro LH. 2010. CD13 is dispensable for normal hematopoiesis and myeloid cell functions in the mouse. J Leukoc Biol 88: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Matteo P, Arrigoni GL, Alberici L, Corti A, Gallo-Stampino C, Traversari C, Doglioni C, and Rizzardi GP. 2011. Enhanced expression of CD13 in vessels of inflammatory and neoplastic tissues. J Histochem Cytochem 59: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhagwat SV, Lahdenranta J, Giordano R, Arap W, Pasqualini R, and Shapiro LH. 2001. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 97: 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Devarakonda CV, Pereira FE, Smith JD, Shapiro LH, and Ghosh M. 2019. CD13 deficiency leads to increased oxidative stress and larger atherosclerotic lesions. Atherosclerosis 287: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghosh M, Gerber C, Rahman MM, Vernier KM, Pereira FE, Subramani J, Caromile LA, and Shapiro LH. 2014. Molecular mechanisms regulating CD13-mediated adhesion. Immunology 142: 636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosh M, McAuliffe B, Subramani J, Basu S, and Shapiro LH. 2012. CD13 Regulates Dendritic Cell Cross-Presentation and T Cell Responses by Inhibiting Receptor-Mediated Antigen Uptake. J Immunol 188: 5489–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahman MM, Ghosh M, Subramani J, Fong GH, Carlson ME, and Shapiro LH. 2014. CD13 regulates anchorage and differentiation of the skeletal muscle satellite stem cell population in ischemic injury. Stem Cells 32: 1564–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wentworth DE, Tresnan DB, Turner BC, Lerman IR, Bullis B, Hemmila EM, Levis R, Shapiro LH, and Holmes KV. 2005. Cells of human aminopeptidase N (CD13) transgenic mice are infected by human coronavirus-229E in vitro, but not in vivo. Virology 335: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lassnig C, Sanchez CM, Egerbacher M, Walter I, Majer S, Kolbe T, Pallares P, Enjuanes L, and Muller M. 2005. Development of a transgenic mouse model susceptible to human coronavirus 229E. Proc Natl Acad Sci U S A 102: 8275–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, Shimotai Y, Momma H, Ichinose M, and Kawase T. 2020. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respiratory investigation 58: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang W, Gao B, Xu G, Weng D, Xie M, and Qian Y. 2014. Possible contribution of aminopeptidase N (APN/CD13) to migration and invasion of human osteosarcoma cell lines. International journal of oncology 45: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 82.Kido A, Krueger S, Haeckel C, and Roessner A. 1999. Possible contribution of aminopeptidase N (APN/CD13) to invasive potential enhanced by interleukin-6 and soluble interleukin-6 receptor in human osteosarcoma cell lines. Clin Exp Metastasis 17: 857–863. [DOI] [PubMed] [Google Scholar]
- 83.Zotz JS, Wolbing F, Lassnig C, Kauffmann M, Schulte U, Kolb A, Whitelaw B, Muller M, Biedermann T, and Huber M. 2016. CD13/aminopeptidase N is a negative regulator of mast cell activation. FASEB J 30: 2225–2235. [DOI] [PubMed] [Google Scholar]
- 84.Chomarat P, Rissoan M, Pin J, Banchereau J, and Miossec P. 1995. Contribution of IL-1, CD14, and CD13 in the increased IL-6 production induced by in vitro monocyte-synoviocyte interactions. J Immunol 155: 3645–3652. [PubMed] [Google Scholar]
- 85.Du Y, Lu C, Morgan RL, Stinson WA, Campbell PL, Cealey E, Fu W, Lepore NJ, Hervoso JL, Cui H, Urquhart AG, Lawton JN, Chung KC, Fox DA, and Amin MA. 2019. Angiogenic and Arthritogenic Properties of the Soluble Form of CD13. J Immunol 203: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morgan R, Endres J, Behbahani-Nejad N, Phillips K, Ruth JH, Friday SC, Edhayan G, Lanigan T, Urquhart A, Chung KC, and Fox DA. 2015. Expression and function of aminopeptidase N/CD13 produced by fibroblast-like synoviocytes in rheumatoid arthritis: role of CD13 in chemotaxis of cytokine-activated T cells independent of enzymatic activity. Arthritis Rheumatol 67: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan RL, Behbahani-Nejad N, Endres J, Amin MA, Lepore NJ, Du Y, Urquhart A, Chung KC, and Fox DA. 2016. Localization, Shedding, Regulation and Function of Aminopeptidase N/CD13 on Fibroblast like Synoviocytes. PLoS One 11: e0162008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamasaki SC, Murari-do-Nascimento S, and Silveira PF. 2012. Neutral aminopeptidase and dipeptidyl peptidase IV in the development of collagen II-induced arthritis. Regul Pept 173: 47–54. [DOI] [PubMed] [Google Scholar]
- 89.Riemann D, Kehlen A, Thiele K, Lohn M, and Langner J. 1997. Co-incubation of lymphocytes with fibroblast-like synoviocytes and other cell types can induce lymphocytic surface expression of aminopeptidase N/CD13. Adv Exp Med Biol 421: 75–79. [DOI] [PubMed] [Google Scholar]
- 90.Riemann D, Kehlen A, Thiele K, Lohn M, and Langner J. 1997. Induction of aminopeptidase N/CD13 on human lymphocytes after adhesion to fibroblast-like synoviocytes, endothelial cells, epithelial cells, and monocytes/macrophages. J Immunol 158: 3425–3432. [PubMed] [Google Scholar]
- 91.Riemann D, Rontsch J, Hause B, Langner J, and Kehlen A. 2000. Cell-cell contact between lymphocytes and fibroblast-like synoviocytes induces lymphocytic expression of aminopeptidase N/CD13 and results in lymphocytic activation. Adv Exp Med Biol 477: 57–66. [DOI] [PubMed] [Google Scholar]
- 92.Lambeir AM, Durinx C, Scharpe S, and De Meester I. 2003. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Critical reviews in clinical laboratory sciences 40: 209–294. [DOI] [PubMed] [Google Scholar]
- 93.Du L, Zhao G, Yang Y, Qiu H, Wang L, Kou Z, Tao X, Yu H, Sun S, Tseng CT, Jiang S, Li F, and Zhou Y. 2014. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol 88: 7045–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wronkowitz N, Gorgens SW, Romacho T, Villalobos LA, Sanchez-Ferrer CF, Peiro C, Sell H, and Eckel J. 2014. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim Biophys Acta 1842: 1613–1621. [DOI] [PubMed] [Google Scholar]
- 95.Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, and Huang Y. 2011. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. Journal of cardiovascular pharmacology 58: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulvihill EE, Varin EM, Ussher JR, Campbell JE, Bang KW, Abdullah T, Baggio LL, and Drucker DJ. 2016. Inhibition of Dipeptidyl Peptidase-4 Impairs Ventricular Function and Promotes Cardiac Fibrosis in High Fat-Fed Diabetic Mice. Diabetes 65: 742–754. [DOI] [PubMed] [Google Scholar]
- 97.Nishina S, Yamauchi A, Kawaguchi T, Kaku K, Goto M, Sasaki K, Hara Y, Tomiyama Y, Kuribayashi F, Torimura T, and Hino K. 2019. Dipeptidyl Peptidase 4 Inhibitors Reduce Hepatocellular Carcinoma by Activating Lymphocyte Chemotaxis in Mice. Cellular and molecular gastroenterology and hepatology 7: 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P, and Lu J. 2020. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. iScience 23: 101400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, and Zhang C. 2020. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galandrin S, Denis C, Boularan C, Marie J, M’Kadmi C, Pilette C, Dubroca C, Nicaise Y, Seguelas MH, N’Guyen D, Baneres JL, Pathak A, Senard JM, and Gales C. 2016. Cardioprotective Angiotensin-(1–7) Peptide Acts as a Natural-Biased Ligand at the Angiotensin II Type 1 Receptor. Hypertension 68: 1365–1374. [DOI] [PubMed] [Google Scholar]
- 101.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, and Tikellis C. 2010. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 107: 888–897. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Zeng Z, Cao Y, Liu Y, Ping F, Liang M, Xue Y, Xi C, Zhou M, and Jiang W. 2016. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-kappaB signaling pathways. Sci Rep 6: 27911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He H, Liu L, Chen Q, Liu A, Cai S, Yang Y, Lu X, and Qiu H. 2015. Mesenchymal Stem Cells Overexpressing Angiotensin-Converting Enzyme 2 Rescue Lipopolysaccharide-Induced Lung Injury. Cell Transplant 24: 1699–1715. [DOI] [PubMed] [Google Scholar]
- 104.He HL, Liu L, Chen QH, Cai SX, Han JB, Hu SL, Chun P, Yang Y, Guo FM, Huang YZ, and Qiu HB. 2015. MSCs modified with ACE2 restore endothelial function following LPS challenge by inhibiting the activation of RAS. J Cell Physiol 230: 691–701. [DOI] [PubMed] [Google Scholar]
- 105.Jin HY, Song B, Oudit GY, Davidge ST, Yu HM, Jiang YY, Gao PJ, Zhu DL, Ning G, Kassiri Z, Penninger JM, and Zhong JC. 2012. ACE2 deficiency enhances angiotensin II-mediated aortic profilin-1 expression, inflammation and peroxynitrite production. PLoS One 7: e38502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skurk T, van Harmelen V, and Hauner H. 2004. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-kappaB. Arterioscler Thromb Vasc Biol 24: 1199–1203. [DOI] [PubMed] [Google Scholar]
- 107.Funakoshi Y, Ichiki T, Ito K, and Takeshita A. 1999. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 34: 118–125. [DOI] [PubMed] [Google Scholar]
- 108.Wang L, Liang J, and Leung PS. 2015. The ACE2/Ang-(1–7)/Mas Axis Regulates the Development of Pancreatic Endocrine Cells in Mouse Embryos. PLoS One 10: e0128216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shoemaker R, Tannock LR, Su W, Gong M, Gurley SB, Thatcher SE, Yiannikouris F, Ensor CM, and Cassis LA. 2019. Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biology of sex differences 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh G, Imai J, Clemons KV, and Stevens DA. 2005. Efficacy of caspofungin against central nervous system Aspergillus fumigatus infection in mice determined by TaqMan PCR and CFU methods. Antimicrob Agents Chemother 49: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Milne S, and Sin DD. 2020. Biomarkers in Chronic Obstructive Pulmonary Disease: The Gateway to Precision Medicine. Clin Chest Med 41: 383–394. [DOI] [PubMed] [Google Scholar]
- 112.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, Dorscheid DR, and Sin DD. 2020. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH, and FinnDiane Study G. 2012. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. Journal of hypertension 30: 375–383. [DOI] [PubMed] [Google Scholar]
- 114.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, and Penninger JM. 2020. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 181: 905–913 e907. [DOI] [PMC free article] [PubMed] [Google Scholar]