Abstract
Background:
Maternal depression is prevalent during and following pregnancy and is related to adverse outcomes in offspring. Perinatal depression is associated with risk for difficulties in offspring; however, the mechanisms underlying this association are not clear. We examined whether maternal prenatal and postnatal depressive symptoms are associated with infant white matter organization and with behavioral problems in toddlerhood.
Methods:
37 mother–infant dyads (20 male; ages 5.95-7.66 months) participated in this study. We conducted diffusion MRI with infants during natural sleep. Mothers reported on their prenatal and postnatal depressive symptoms at six months postpartum. We calculated fractional anisotropy (FA), radial, axial, and mean diffusivity, and assessed offspring behavioral problems at age 18 months.
Results:
Prenatal depressive symptoms were associated with FA of the corpus callosum; postnatal depressive symptoms were not associated with FA of limbic tracts or corpus callosum segmentations. Higher levels of prenatal depressive symptoms were associated with higher FA and lower radial diffusivity of the corpus callosum genu; FA of this region was positively associated with behavioral problems at age 18 months.
Limitations:
This study had a small sample size; therefore, findings should be replicated. Further, we used retrospective reports of maternal prenatal depression, but validated them in this study.
Conclusions:
Depressive symptoms during pregnancy may affect infant corpus callosum development and, in turn, offspring behaviors. These findings suggest that early maternal stress accelerates infant neurodevelopment in a manner that may increase risk for behavioral problems. Thus, efforts to reduce maternal prenatal depression should be a public health priority.
Introduction
As many as 20 percent of mothers experience clinically significant depression or depressive symptoms during pregnancy (Bennett et al., 2004; Gavin et al., 2005) and/or after childbirth (Sanger et al., 2015). These rates are even higher when including mothers with subclinical levels of depressive symptoms (Meaney, 2018). Thus, at least one in five infants is exposed to maternal depression in utero or in the year following birth (Gavin et al., 2005; O’Hara & Wisner, 2014). Maternal depressive symptoms have adverse consequences for both mothers (Gotlib, 1992;-Moehler et al., 2006) and offspring (Goodman & Gotlib, 1999), including increased risk for children’s attentional (Zou et al., 2019), emotional (Nolvi et al., 2020), and behavior problems (Cents et al., 2013; Hay et al., 2020; Sandman et al., 2015). The mechanisms underlying the intergenerational transmission of risk, however, are unclear (Gotlib et al., 2020). One possible mechanism involves alterations in brain structure in offspring in early life (Hay et al., 2020; Sandman et al., 2015; Zou et al., 2019). Indeed, investigators have reported findings consistent with the formulation that maternal depressive symptoms adversely affect brain structure in young children. Much of this research has been conducted with school-age and older children and adolescents. For example, investigators have reported greater cortical thinning in preschool-age (Lebel et al., 2016), adolescent (Foland-Ross et al., 2015; Peterson et al., 2009), and pre-adolescent (Sandman et al., 2015), children of depressed mothers. Further, Wen et al. (2017) reported that whereas prenatal depressive symptoms were associated with larger right amygdala volume, postnatal depressive symptoms were associated with higher fractional anisotropy (FA) of the right amygdala. Recently, Hay et al. (2020) found that prenatal depressive symptoms were associated with higher mean diffusivity (MD) of the cingulum and with higher MD and lower FA of the amygdala pathway in 4-year-old children and, further, that limbic-prefrontal connectivity mediated the association in boys between maternal prenatal depressive symptoms and externalizing problems.
Investigators have begun to examine neural characteristics of infants whose mothers experienced depressive symptoms in the perinatal period. Rifkin-Graboi et al. (2013) reported that neonates of mothers with higher levels of prenatal depressive symptoms had lower FA and AD of the amygdala. Similarly, Posner et al. (2016) reported that greater prenatal depressive symptoms were related in neonates to decreased structural connectivity between the right amygdala and the right ventral prefrontal cortex, and Dean et al. (2018) found that greater maternal prenatal depressive and anxious symptoms were associated with higher diffusivity in right frontal regions in one-month-old infants. Further, they report that infant sex moderated the association between maternal symptoms and diffusion properties of the splenium (Dean et al., 2018). Finally, Nolvi and colleagues (2020) reported that maternal postnatal depressive symptoms predicted offspring emotional reactivity at age 6 months, but only in newborns with higher mean FA across the corpus callosum. Thus, while a small number of studies indicate that maternal prenatal depressive symptoms are associated with infant white matter organization, there is variability in the specific tracts that are implicated and in the direction of these associations. Further, moderating variables, such as offspring sex, may explain significant variance in the effects of maternal depressive symptoms on infant white matter organization (Dean et al., 2018; Hay et al., 2020; Lebel et al., 2016; Wen et al., 2017). For example, given the rapid development of the infant brain during the perinatal period (Dubois et al., 2014), maternal depressive symptoms at different peripartum timepoints (i.e., prenatal vs. postnatal, broadly reflecting changes in the hormonal milieu of the intrauterine environment versus difficulties in postnatal caregiving behavior) may differentially affect neurodevelopment (Bock et al., 2015). In fact, researchers have found different associations between prenatal and postnatal maternal depressive symptoms and brain structure in youth (El Marroun et al., 2018; Lebel et al., 2016; Wen et al., 2017; Zou et al., 2019), suggesting that different mechanisms are involved in these relations. Identifying when in the perinatal period maternal depressive symptoms are most consequential for infant white matter organization may inform strategies to prevent the intergenerational transmission of risk for psychosocial difficulties in the earliest stages of life.
The goal of the current study was to examine the differential associations of maternal depressive symptoms during the prenatal period (reported retrospectively at six months postpartum) and during the postnatal period (reported contemporaneously) with white matter organization in six-month-old infants. Further, we examined the longer-term correlates of brain organization by assessing behavior problems at 18 months.
Given the equivocal findings reviewed above, we tested two competing hypotheses: i) higher maternal depressive symptoms delay the development of infant white matter organization, reflected in lower FA in white matter tracts; and ii) higher maternal depressive symptoms accelerate the development of infant white matter organization, reflected in higher FA in white matter tracts. We examined both prenatal and postnatal depressive symptoms to assess possible differential effects on infant white matter organization of symptoms occurring in these two periods. Given that previous studies have implicated limbic regions, frontal white matter, and diffusion properties of the corpus callosum in the association between maternal depressive symptoms and infant white matter organization, in addition to alterations in these brain regions in youth with a family history of Major Depressive Disorder (Huang et al., 2011; Hung et al., 2017), we focused on limbic tracts and corpus callosum segmentations as tracts of interest.
Methods
Study Design and Participants
Participants were 155 mother–infant dyads recruited from the San Francisco Bay Area for the Brain and Behavior Infant Experiences Study (BABIES) project, assessing the effects of the caregiving environment on infant brain and toddler behavioral development between May, 2016-June, 2020. Mothers were recruited during pregnancy (between 12-37 weeks’ gestation; n=59) or at six months postpartum (when infants were ages 5-8 months; n=96) using flyers and online advertisements. Participants recruited prenatally and postpartum completed identical assessments when infants were ages 5-8 months. A subset of the infants of these mothers (n=38; 18 recruited prenatally) provided usable diffusion-weighted imaging (DWI). Of these dyads, one mother reported an outlying score greater than 3 SDs above the mean on the Center for Epidemiologic Studies Depression Scale (see below) and, therefore, was excluded from analyses. Thus, the sample in the current analyses included 37 mothers (ages 21.26-44.44 years; M=32.53, SD=4.81; 18 recruited prenatally and 19 postnatally) and their infants (ages 5.95-7.66 months; M=6.73, SD=0.42). In an exploratory analysis, we also assessed whether infant FA was associated with behavioral functioning one year later (N=34 ages 17.52-21.15 months; M=18.27, SD=0.63; 1 lost to follow-up; 2 with missing data).
Mothers were screened for eligibility through a telephone interview, assessing whether they were currently pregnant or had an infant between five and eight months of age, were ≥18 years old, were fluent in English, and had no immediate plans to leave the geographic area. Exclusion criteria included maternal bipolar disorder, maternal psychosis, dyslexia, or visual processing issues, severe complications during birth, infant preterm birth (<36 weeks gestational age), infant head trauma or medical conditions (e.g., congenital, genetic, or neurological disorders), and contraindication for infant MRI. Eligible mothers reported on both their prenatal and postnatal depressive symptoms during the infants’ scanning session at six months postpartum. Infants were scanned during natural sleep. One year later, mothers were e-mailed a link for a survey in which they reported on their toddler’s behavioral functioning at 18 months postpartum. The Stanford University Institutional Review Board approved the research protocol; mothers provided informed written consent for themselves and their infant and were compensated for their time.
Measures
Maternal and infant characteristics.
Mothers reported on their education level (ranging from 1: no schooling completed to 9: graduate degree), their annual household income (binned from 1: $0-50,000 to 7: >$150,000), Latinx ethnicity (0: not Latinx; 1: Latinx), race (0: non-white; 1: white), and antidepressant use in pregnancy (0: no antidepressant use in pregnancy; 1: antidepressant use in pregnancy). Infant gestational age at birth was calculated using the infants’ due date and date of birth.
Maternal Depressive Symptoms.
Mothers provided retrospective reports of depressive symptoms experienced across their pregnancy using the Edinburgh Postnatal Depression Scale (EPDS; Cox et al., 1987). Specifically, we modified the prompt to: “please answer these questions in relation to how you felt DURING your pregnancy”. The EPDS consists of 10 items assessing symptoms of depression, measured on a 4-point scale, yielding total scores ranging from 0 to 30, with a clinical cutoff score of 15 (α=.86). A subsample of mothers who were recruited during pregnancy completed the EPDS during pregnancy and at six months postpartum (reporting retrospectively on their depressive symptoms during pregnancy) to assess the validity of these reports. Mothers also completed the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) during the infant’s scan session, rating their depressive symptoms over the past two weeks. The CES-D consists of 20 items that assess depressive symptoms, each measured on a 4-point scale, yielding total scores from 0 to 60, with a clinical cutoff score of 16 (α=.90).
Behavioral Problems in Toddlerhood.
One year after their infant’s scanning session, mothers completed the Early Childhood Screening Assessment (ECSA; Gleason et al., 2010) through an online survey. The ECSA consists of 36 items assessing infant behavioral problems, measured on a 3-point scale, yielding total scores ranging from 0 to 72, with a clinical cutoff score of 18 (α=.80).
Imaging Protocol
Infants were scanned between five and eight months of age during natural sleep using a 3T MRI scanner (Discovery MR750 scanner, General Electric Healthcare, Milwaukee, WI, USA) with the 32-channel NovaMedical head coil (Wilmington, MA) (see Camacho et al., 2019; Dennis et al., 2019; and King et al., 2020 for details regarding the scanning procedure).
Magnetic Resonance Imaging Data Acquisition
Diffusion data were acquired with a multi-shell diffusion scheme using b-values of 700 s/mm2 (30 volumes) and 2000 s/mm2 (64 volumes), including 11 volumes with no diffusion weighting (2.0mm x 2.0mm x 2.0mm voxel, 16 axial slices, TR=3400ms, TE=80ms, flip angle=77 degrees). Infants were swaddled and fed prior to scanning and were immobilized using sandbags and a MedVac bag (CFI Medical Solutions). Scan noise was reduced using earplugs and headphones. The multiband Diffusion-Weighted Imaging (DWI) protocol lasted approximately six minutes and took place at the end of the scanning protocol. 106 infants attempted one scanning sequence and 66 infants attempted the DWI scan. Of the 66 infants who attempted the scan, 12 had a different DWI protocol, 5 woke up prior to the conclusion of the sequence, 2 infants’ data did not save, 7 were excluded due to motion (more than 5 volumes with >2mm shifts or any volumes with >3mm shift), and 2 were removed due to image artifacts (e.g., striping or halo effects). Because infants were scanned at two different sites (31 scanned at the Stanford Center for Cognitive and Neurobiological Imaging and 6 scanned at the Richard M. Lucas Center for Imaging), we included scanner as a covariate in analyses; importantly, the MRI magnet and scanning parameters were the same at both locations. Further, each scanner regularly goes through preventative maintenance with GE Healthcare field service engineers to assess system performance and stability in a variety of tests, and each scanner passed all of these tests. Each system was running the same major release of the scanner software (DV26.0 in GE parlance) and was using the same model NovaMedical 32-channel whole-brain receive array for data acquisition.
Tractography
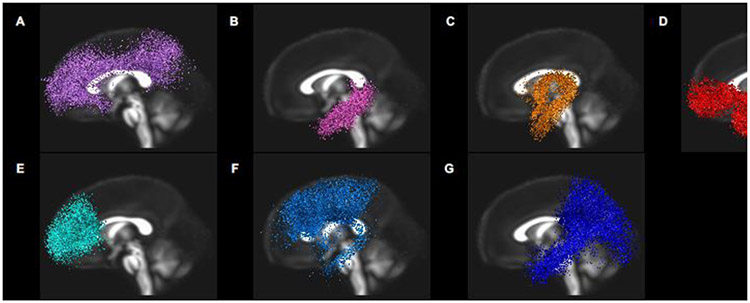
The preprocessing pipeline has been described by Dennis et al. (2019). In sum, we quality-checked scans and masked volumes using FSL BET and calculated tensor maps using FSL dtifit. Prior to conducting deterministic tractography, diffusion scans were unwarped and eddy-corrected using FSL Eddy/TopUp (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). DWI data were reconstructed using DSI Studio (http://dsi-studio.labsolver.org/). We implemented generalized q-sampling imaging (Yeh et al., 2010) with a diffusion sampling length ratio of 1.5. Further, we calculated restricted diffusion with restricted diffusion imaging (Yeh et al., 2017) and completed tractography using a deterministic fiber tracking algorithm (Yeh et al., 2013). We placed 200,000 seeds across the brain using an angular threshold of 75 degrees. We selected a large number of seeds and a high angular threshold to improve the tracking in smaller regions of interest (ROIs). The step size was randomly selected from 0.1 voxel to 3 voxels. The anisotropy threshold (quantitative index) was automatically determined using 0.6 * (Otsu’s threshold). Fiber trajectories were smoothed by averaging the propagation direction with a percentage of the previous direction, which was randomly selected from 0% to 95%. We discarded tracts from analyses that were shorter than 20 mm or longer than 250 mm (Dennis et al., 2019). We visually inspected fiber renderings for each tract for each infant prior to statistical analyses to ensure that each tract conformed to anatomical norms. With these steps, we were able to identify all of the tracts of interest – including limbic tracts (left and right cingulum, left and right cingulum hippocampus, left and right uncinate fasciculus, and fornix) and the corpus callosum segmentations (genu, body, and splenium) – in all infants (Figure 1).
Figure 1.
Panel 1=limbic tracts. A=cingulum; B=cingulum hippocampus; C=fornix; D=uncinate fasciculus. Panel 2=corpus callosum segmentations. E=genu; F=body; G=splenium.
We identified tracts of interest using TractStat (Corbin et al., 2017). We rigidly aligned the UNC Early Brain Development Study (https://www.nitrc.org/projects/uncebds_neodti/) template to the Type II JHU-Eve atlas (Zhang et al., 2010). Infants’ tracts were clustered using ROIs defined on the JHU atlas. We used ANTs to apply rigid, affine, and SyN non-linear registration to register each infant’s FA map to the infant template (Avants et al., 2011). We removed false-positive and spurious fibers using fiber orientation and neighborhood restraints, respectively. Using Dipy’s Minimum Average Direct Flip, fibers that had fewer than 20 neighbors within distance epsilon (3mm) were excluded. Streamlines were randomly reduced to 5,000 to decrease the computational load. We performed careful visual inspection on all tracts that were used to create the population bundle templates for white matter pathways. Diffusion metrics were calculated by averaging FA within the extracted tract bundles (Dennis et al., 2019).
Statistical Analyses
All analyses were conducted in R (Team, 2019) with statistical significance set atp<0.05 (two-tailed). All continuous variables were z-scored.
Preliminary analyses and identification of covariates.
We conducted group comparisons to determine whether mothers or infants in the current sample (completed DWI) differed from mothers or infants who were not included (no DWI) with respect to demographic and clinical variables. Next, we assessed the test-retest reliability of the EPDS using data from the larger sample of mothers who were recruited during pregnancy and reported on their prenatal depressive symptoms during pregnancy and six months postpartum (n=55).
We considered the following covariates: maternal education, annual income, infant gestational age at birth, infant sex, and infant age at scan. We tested each covariate separately to determine whether it explained variance in FA of the limbic tracts or corpus callosum segmentations. We included only those covariates that improved the model fit for either set of tracts; however, we covaried for the dummy-coded “scanner” variable in both multivariate regressions regardless of its statistical significance.
Primary analyses.
We conducted two multivariate linear regression analyses using the “Manova” function from the “car” package to test the associations of maternal prenatal and postnatal depression scores with infant FA of limbic tracts (left and right cingulum, left and right cingulum hippocampus, left and right uncinate fasciculus, and fornix) and corpus callosum segmentations (genu, body, and splenium), respectively. Using multivariate regression allowed us to reduce the number of tests conducted, thereby decreasing the chance of a false positive.
In the presence of a significant omnibus effect in the multivariate regression, we conducted univariate follow-up analyses to determine which tracts were related to prenatal or postnatal depression scores. If FA of any tracts was significantly associated with maternal depression scores, we conducted exploratory analyses to probe whether other diffusion metrics (RD, AD, MD) were also associated with depression scores and whether FA of the respective tract was associated with behavioral problems during toddlerhood. Finally, we repeated the main analyses excluding mother-infant dyads in which mothers took antidepressants during pregnancy (n=5).
Results
Participant Characteristics
Demographic and clinical characteristics of infants and their mothers are presented in Table 1. On average, mothers did not have high levels of depressive symptoms. Scores on the EPDS and CES-D were positively correlated (rs(35)=0.49, p=.002, 95% CI[0.21, 0.76]).
Table 1.
Demographic and clinical characteristics of mother-infant dyads.
| Variable | # and % or M (SD) | Range |
|---|---|---|
| Infant characteristics | ||
| Sex (male) | 20 (54%) | |
| Gestational age at birth (weeks) | 39.62 (1.29) | 36.00-41.71 |
| Age at scan session (months) | 6.73 (0.42) | 5.95-7.66 |
| Age at follow-up (months) | 18.27 (0.63) | 17.52-21.15 |
| ECSA scorea | 7.43 (5.08) | 0.00-24.00 |
| Maternal characteristics | ||
| Age (years) | 32.53 (4.81) | 21.26-44.44 |
| Annual income | ||
| $15,000-30,000 | 1 (3%) | |
| $30,001-60,000 | 4 (11%) | |
| $60,001-90,000 | 2 (5%) | |
| $90,001-150,000 | 13 (35%) | |
| >$150,000 | 17(46%) | |
| Education ≥ 4-year college degree | 32 (86%) | high school grad - grad degree |
| Race | ||
| White | 26 (70%) | |
| Asian/Asian American | 7 (19%) | |
| Black/African American | 1 (3%) | |
| Native Hawaiian/Pacific Islander | 1 (3%) | |
| Other race | 2 (5%) | |
| SSRIb use in pregnancy | 5 (14%) | |
| EPDS scorec | 5.62 (4.77) | 0.00-19.00 |
| CES-D scored | 8.81 (8.53) | 0.00–31.00 |
Notes. N=37.
ECSA=Early Childhood Screening Assessment.
SSRI=selective serotonin-reuptake inhibitors.
EPDS=Edinburgh Postnatal Depression Scale; prenatal depressive symptoms.
CES-D=Center for Epidemiologic Studies Depression Scale; postnatal depressive symptoms.
Mothers whose infants provided usable DWI scans did not differ from mothers whose infants did not in age (t(152)=1.49, p=.140), education (t(152)=0.11, p=.914), annual income (t(151)=−0.19,p=.851), Latinx (t(151)=−0.36, p=.717), race (t(152)=−1.50, p=.136), prenatal (t(142)=1.50, p=.136), or postnatal (t(152)=1.00, p=.321) depressive symptoms. Further, infants who completed the DWI protocol did not differ from infants who did not complete the DWI protocol in gestational age at birth (t(152)=−0.22, p=.829), sex (t(152)=−0.56, p=.574), Latinx (t(150)=−0.30, p=.764), race (t(152)=−1.56, p=.121), or behavioral problems at age 18 months (t(145)=1.78, p=.077). EPDS scores obtained during pregnancy were positively correlated with retrospectively reported EPDS scores about pregnancy depression obtained at 6 months postpartum (r(53)=0.68, p<.001, 95% CI[0.51, 0.80]) in the sample of women who were recruited during pregnancy.
Gestational age at birth significantly improved model fit for both the limbic tracts [F(7,29)=3.35, p=.010] and corpus callosum segmentations [F(3,33)=3.51, p=.026] and, therefore, were controlled for in subsequent analyses. However, infant sex [F(7,29)=0.76, p=.623], infant age at scan [F(7,29)=1.18, p=.342], maternal education [F(7,29)=0.70, p=.671], and annual income [F(7,29)=0.34, p=931] did not improve fit for the model in which FA of limbic tracts were entered jointly as dependent variables. Similarly, infant sex [F(3,33)=0.29, p=.831], infant age at scan [F(3,33)=0.71, p=.554], maternal education [F(3,33)=0.33, p=.803], and annual income [F(3,33)=0.59, p=.624] did not improve the model in which FA of the corpus callosum segmentations was the dependent variable and, therefore, were not included as covariates.
Primary analyses.
Neither prenatal nor postnatal depressive symptoms were associated with FA of limbic tracts (prenatal: F(7,26)=0.54, p=.797, postnatal: F(7,26)=0.23, p=.974). Further, postnatal depressive symptoms were not associated with FA of corpus callosum segmentations (F(3,30)=0.99, p=.413). In contrast, prenatal depressive symptoms were significantly positively associated with FA of the corpus callosum segmentations (F(3,30)=4.13, p=.015). Follow-up analyses indicated that infants whose mothers reported higher prenatal depressive symptoms had higher FA in the corpus callosum genu (β=0.47, SE=0.19, t(32)=2.47, p=.019, 95% CI[0.08, 0.85], ΔR212.10% (Figure 2A).
Figure 2.

EPDS=Edinburgh Postnatal Depression Scale. Data points with similar x- and y-axis values are indicated by overlapping circles that appear darker and more opaque.
Given the significant association between prenatal maternal depressive symptoms and FA of the corpus callosum genu, we computed bivariate Spearman correlations assessing the association between EPDS scores and MD, AD, and RD of this tract. These analyses yielded a significant negative correlation between EPDS scores and RD of the corpus callosum genu (rs(35)=−0.36, p=.028, 95% CI[−0.67, −0.05]) (Figure 2B), but not between EPDS scores and MD (rs(35)=−0.30, p=.068, 95% CI[−0.61, 0.02]) or AD (rs(35)=−0.08, p=.646, 95% CI[−0.44, 0.28]).
Finally, we examined whether FA of the corpus callosum genu was associated with mothers’ reports of their toddlers’ behavioral problems. We found that 6-month-old infants with higher FA of the corpus callosum genu had higher ECSA scores in toddlerhood (rs(32)=0.36, p=.039, 95% CI[<0.01 0.72]) (Figure 3). These results held when we excluded mothers who took antidepressants in pregnancy (see Supplement Table S1)
Figure 3.

ECSA=Early Childhood Screening Assessment. Data points with similar x- and y-axis values are indicated by overlapping circles that appear darker and more opaque.
Discussion
The present study was conducted to examine the relation between maternal perinatal depressive symptoms and 6-month-old infant white matter organization. In addition, we explored associations of white matter organization with behavioral problems assessed one year later when offspring were age 18 months. Maternal prenatal depressive symptoms were associated with higher FA of infant corpus callosum segmentations, even after adjusting for postnatal depressive symptoms. Specifically, higher maternal prenatal depressive symptoms were associated with higher FA and lower RD of the corpus callosum genu segment, an interhemispheric white matter tract that connects the frontal lobes, but not with MD or AD. We also found that higher FA of the corpus callosum genu at age six months was associated with more behavioral problems in these offspring at age 18 months.
FA typically increases with age (Dubois et al., 2014), and importantly, the corpus callosum genu is one of the fastest developing white matter pathways (Uda et al., 2015). Lebel et al. (Lebel et al., 2008) reported that FA of this region reaches peak development at earlier ages compared to association fibers. In addition, a recent study of a cross-sectional cohort of participants ranging from 0.6-18.8 years of age found that the corpus callosum genu matured earlier than did all other tracts (Lynch et al., 2020). Researchers have documented that the majority of myelination occurs in the first year of life (Dubois et al., 2014). In fact, researchers have shown that myelination in the corpus callosum genu is “mature” by six months of age and that the myelin water fraction increases in this region between six and eight months of age (Dubois et al., 2014). Our findings suggest that maternal prenatal depressive symptoms are associated with lower diffusivity of the corpus callosum genu in infants during this critical period. While we cannot make strong claims given our diffusion model that the underlying neurobiological substrate is myelin, investigators have demonstrated that RD is sensitive to myelin loss (Song et al., 2002).
The observed positive association between prenatal depressive symptoms and FA, coupled with the negative association with RD, suggests that infants of mothers who have higher depressive symptoms have lower diffusivity. These findings support previous results reported by Lebel et al. (2016) and Wen et al. (2017), suggesting that maternal perinatal depressive symptoms are associated with lower diffusivity in offspring. Lower diffusivity may be indexed by accelerated myelination, increased fiber density/axonal packing, or more uniform fiber orientation. Taken together, these findings support the stress acceleration hypothesis (Callaghan & Tottenham, 2016), in which maternal prenatal depressive symptoms serve as an early stressor that promotes accelerated fetal brain development.
While several investigators have reported an association between maternal depressive symptoms and frontal white matter organization, many of these findings have reported higher diffusivity in this region (Dean et al., 2018; Posner et al., 2016; Zou et al., 2019). Inconsistencies across studies are likely due to differences in the age of the participants and when maternal depressive symptoms were measured. Longitudinal work is needed to assess whether the effects of maternal depressive symptoms on brain structure persists across infancy and childhood in the same sample, especially given the dynamic nature of white matter properties.
Researchers have shown that exposure to maternal prenatal symptoms is associated with increased risk for depression in offspring (Pearson et al., 2013; Plant et al., 2015); however, the mechanisms underlying this association are not clear (Gotlib et al., 2020). Interestingly, researchers have provided evidence that brain structure in children mediates associations between maternal prenatal depression and subsequent behavioral problems in offspring (Hay et al., 2020; Sandman et al., 2015; Zou et al., 2019). In the present study we provide evidence that diffusivity of the corpus callosum genu in infancy, the tract associated with maternal prenatal depressive symptoms, predicted behavioral problems in toddlerhood. Thus, white matter of the corpus callosum genu may be one mechanism by which prenatal maternal depressive symptoms are associated with heightened risk for psychopathology in offspring.
Limitations
This study was the first to assess the relation between maternal perinatal depressive symptoms and white matter organization in six-month-old infants. We should note four limitations of this study. First, we had a relatively small sample size, and it is possible that with a larger sample we would have seen associations of maternal depressive symptoms in other tracts. As a related point, given our sample size, we did not have sufficient statistical power to conduct a mediation analysis assessing whether variation in FA of the corpus callosum genu at age 6 months explained variance in the association between maternal prenatal depressive symptoms and toddler behavioral problems (Fritz & MacKinnon, 2007). Second, we assessed prenatal depressive symptoms retrospectively, which can be subject to bias, particularly underreporting (Newport et al., 2008). We should note, however, that we documented a strong positive correlation between these retrospective reports and levels of depressive symptoms that were reported in pregnancy, indicating that retrospective reports were reliable with earlier contemporaneous reports. Third, we assessed prenatal and postnatal depressive symptoms and infant white matter organization concurrently and, therefore, cannot make strong claims regarding causal effects. Finally, the majority of mothers in our sample had relatively high levels of education and socioeconomic status. Therefore, our findings should be replicated in lower-income and higher-risk samples.
Conclusion
Early identification of depressive symptoms during pregnancy is important for the well-being of the mother and the neuroanatomical development of the child. Researchers have shown that changes in brain structure mediate the relation between maternal depressive symptoms and subsequent behavioral and attention problems in older offspring (Hay et al., 2020; Sandman et al., 2015; Zou et al., 2019); our findings support the formulation that white matter organization in infancy is also related to behavioral problems in toddlerhood. Future work should assess whether treating depressive symptoms during pregnancy improves infants’ outcomes, including white matter organization and subsequent behavioral outcomes.
Supplementary Material
Table 2.
Relation between maternal depressive symptoms and fractional anisotropy of limbic tracts and corpus callosum segmentations.
| Overall Model | |||
|---|---|---|---|
| Pillai | F | p | |
| Limbic Tractsa FAb | |||
| Scanner | 0.26 | 1.30 | .289 |
| Gestational age at birth | 0.39 | 2.35 | .054 |
| Prenatal depression (EPDS) | 0.13 | 0.54 | .797 |
| Postnatal depression (CES-D) | 0.06 | 0.23 | .974 |
| Corpus Callosum Segmentation FAc | |||
| Scanner | 0.15 | 1.83 | .163 |
| Gestational age at birth | 0.16 | 1.86 | .159 |
| Prenatal depression (EPDS)d | 0.29 | 4.13 | .015 |
| Postnatal depression (CES-D)e | 0.09 | 0.99 | .413 |
Notes. N=37.
limbic tracts=bilateral cingulum, bilateral cingulum hippocampus, bilateral uncinate fasciculus, and fornix.
FA=fractional anisotropy.
corpus callosum segmentations=genu, body, splenium.
EPDS=Edinburgh Postnatal Depression Scale.
CES-D=Center for Epidemiologic Studies Depression Scale.
Highlights.
Prenatal depression was associated with diffusivity of the corpus callosum in infants
Postnatal depression was not related to infant limbic or corpus callosum white matter
Diffusivity of the corpus callosum genu predicted behavioral problems in toddlerhood
Treating prenatal depression may improve infants’ brain and behavioral outcomes
Acknowledgments:
We thank Anna Cichocki, Daisy Feddoes, Cheyenne Garcia, Amar Ojha, Fran Querdasi, Marissa Roth, Jill Segarra, and Lucinda Sisk for their assistance in data collection and management. We also thank the mothers and infants who participated in this study.
Funding: The funders did not participate in the work. This work was supported by the National Institutes of Health [IHG, R21 MH111978; R21 HD090493]; the National Science Foundation [LSK, Graduate Student Research Fellowship]; and the Jacobs Foundation [KLH, Early Career Research Fellowship 2017-1261-05].
Footnotes
Conflict of Interest Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Data linking: the data and code will be made available upon request following acceptance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, & Einarson TR (2004). Prevalence of Depression During Pregnancy: Systematic Review. Obstetrics & Gynecology, 103(4), 698–709. 10.1097/01.AOG.0000116689.75396.5f [DOI] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, & Segal M (2015). Stress In Utero: Prenatal Programming of Brain Plasticity and Cognition In Biological Psychiatry (Vol. 78, Issue 5, pp. 315–326). Elsevier; USA: 10.1016/j.biopsych.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior In Current Opinion in Behavioral Sciences (Vol. 7, pp. 76–81). Elsevier Ltd; 10.1016/j.cobeha.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina Camacho M, King LS, Ojha A, Garcia CM, Sisk LM, Cichocki AC, Humphreys KL, & Gotlib IH (2019). Cerebral blood flow in 5- to 8-month-olds: Regional tissue maturity is associated with infant affect. Developmental Science. 10.1111/desc.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cents RAM, Diamantopoulou S, Hudziak JJ, Jaddoe VWV, Hofman A, Verhulst FC, Lambregtse-Van Den Berg MP, & Tiemeier H (2013). Trajectories of maternal depressive symptoms predict child problem behaviour: The Generation R Study. Psychological Medicine, 43(1), 13–25. 10.1017/S0033291712000657 [DOI] [PubMed] [Google Scholar]
- Corbin CK, Gupta V, Villalon-Reina JE, Nir TM, Rashid FM, Thomopoulos SI, Jahanshad N, & Thompson PM (2017). White Matter Differences in Parkinson’s Disease Mapped Using Tractometry. bioRxiv. 10.1101/209858 [DOI] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry, 150(6), 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Dean DC, Planalp EM, Wooten W, Kecskemeti SR, Adluru N, Schmidt CK, Frye C, Birn RM, Burghy CA, Schmidt NL, Styner MA, Short SJ, Kalin NH, Goldsmith HH, Alexander AL, & Davidson RJ (2018). Association of Prenatal Maternal Depression and Anxiety Symptoms With Infant White Matter Microstructure. JAMA Pediatrics, 172(10), 973 10.1001/jamapediatrics.2018.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Singh A, Corbin CK, Jahanshad N, Ho TC, King LS, … & Gotlib IH (2019, April). Associations between maternal depression and infant fronto-limbic connectivity. In 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019) (pp. 126–130). IEEE; 10.1109/isbi.2019.8759513 [DOI] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, & Hertz-Pannier L (2014). The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. 10.1016/j.neuroscience.2013.12.044 [DOI] [PubMed] [Google Scholar]
- El Marroun H, Zou R, Muetzel RL, Jaddoe VW, Verhulst FC, White T, & Tiemeier H (2018). Prenatal exposure to maternal and paternal depressive symptoms and white matter microstructure in children. Depression and Anxiety, 35(4), 321–329. 10.1002/da.22722 [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Gilbert BL, Joormann J, & Gotlib IH (2015). Neural markers of familial risk for depression: An investigation of cortical thickness abnormalities in healthy adolescent daughters of mothers with recurrent depression. Journal of Abnormal Psychology, 124(3), 476–485. 10.1037/abn0000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, & MacKinnon DP (2007). Required sample size to detect the mediated effect. Psychological Science, 18(3), 233–239. 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, & Swinson T (2005). Perinatal Depression. Obstetrics & Gynecology, 106(5, Part 1), 1071–1083. 10.1097/01.AOG.0000183597.31630.db [DOI] [PubMed] [Google Scholar]
- Gleason MM, Zeanah CH, & Dickstein S (2010). Recognizing young children in need of mental health assessment: Development and preliminary validity of the early childhood screening assessment. Infant Mental Health Journal, 31(3), 335–357. 10.1002/imhj.20259 [DOI] [PubMed] [Google Scholar]
- Goodman SH, & Gotlib IH (1999). Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. In Psychological Review (Vol. 106, Issue 3). https://pdfs.semanticscholar.org/a233/bc7c9bb7ab3a4967792c6884db0c12656ba6.pdf [DOI] [PubMed] [Google Scholar]
- Gotlib IH (1992). Interpersonal and Cognitive Aspects of Depression. Current Directions in Psychological Science, 1(5), 149–154. 10.1111/1467-8721.ep11510319 [DOI] [Google Scholar]
- Gotlib IH, Goodman SH, & Humphreys KL (2020). Studying the Intergenerational Transmission of Risk for Depression: Current Status and Future Directions. Current Directions in Psychological Science, 29(2), 174–179. 10.1177/0963721420901590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RE, Reynolds JE, Grohs MN, Paniukov D, Giesbrecht GF, Letourneau N, Dewey D, & Lebel C (2020). Amygdala-prefrontal structural connectivity mediates the relationship between prenatal depression and behaviour in preschool boys. The Journal of Neuroscience, JN-RM-0481-20. 10.1523/JNEUROSCI.0481-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, & Rao U (2011). White matter changes in healthy adolescents at familial risk for unipolar depression: A diffusion tensor imaging study. Neuropsychopharmacology, 36(3), 684–691. 10.1038/npp.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y, Saygin ZM, Biederman J, Hirshfeld-Becker D, Uchida M, Doehrmann O, Han M, Chai XJ, Kenworthy T, Yarmak P, Gaillard SL, Whitfield-Gabrieli S, & Gabrieli JDE (2017). Impaired frontal-limbic white matter maturation in children at risk for major depression. Cerebral Cortex, 27(9), 4478–4491. 10.1093/cercor/bhw250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Camacho MC, Montez DF, Humphreys KL, & Gotlib IH (2020). Naturalistic Language Input is Associated with Resting-State Functional Connectivity in Infancy. The Journal of Neuroscience, JN-RM-0779-20. 10.1523/jneurosci.0779-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, & Beaulieu C (2008). Microstructural maturation of the human brain from childhood to adulthood. Neuroimage, 40(3), 1044–1055. 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Lebel Catherine, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, & Dewey D (2016). Prepartum and Postpartum Maternal Depressive Symptoms Are Related to Children’s Brain Structure in Preschool. Biological Psychiatry, 80(11), 859–868. 10.1016/J.BIOPSYCH.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Lynch KM, Cabeen RP, Toga AW, & Clark KA (2020). Magnitude and timing of major white matter tract maturation from infancy through adolescence with NODDI. Neuroimage, 212, 116672 10.1016/j.neuroimage.2020.116672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ (2018). Perinatal Maternal Depressive Symptoms as an Issue for Population Health. American Journal of Psychiatry, 175(11), 1084–1093. 10.1176/appi.ajp.2018.17091031 [DOI] [PubMed] [Google Scholar]
- Moehler E, Brunner R, Wiebel A, Reck C, & Resch F (2006). Original contribution Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding A. Arch Womens Ment Health, 9, 273–278. 10.1007/s00737-006-0149-5 [DOI] [PubMed] [Google Scholar]
- Newport D, Brennan P, Green P, Ilardi D, Whitfield T, Morris N, Knight B, & Stowe Z (2008). Maternal depression and medication exposure during pregnancy: comparison of maternal retrospective recall to prospective documentation. BJOG: An International Journal of Obstetrics and Gynaecology, 115(6), 681–688. 10.1111/j.1471-0528.2008.01701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolvi S, Tuulari JJ, Lavonius T, Scheinin NM, Lehtola SJ, Lavonius M, Merisaari H, Saunavaara J, Korja R, Kataja EL, Pelto J, Parkkola R, Karlsson L, & Karlsson H (2020). Newborn white matter microstructure moderates the association between maternal postpartum depressive symptoms and infant negative reactivity. Social Cognitive and Affective Neuroscience, 15(6), 649–660. 10.1093/scan/nsaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MW, & Wisner KL (2014). Perinatal mental illness: Definition, description and aetiology. Best Practice & Research Clinical Obstetrics & Gynaecology, 28(1), 3–12. 10.1016/j.bpobgyn.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, O’Connor TG, & Stein A (2013). Maternal depression during pregnancy and the postnatal period risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry, 70(12), 1312–1319. 10.1001/jamapsychiatry.2013.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, & Weissman MM (2009). Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6273–6278. 10.1073/pnas.0805311106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant DT, Pariante CM, Sharp D, & Pawlby S (2015). Maternal depression during pregnancy and offspring depression in adulthood: Role of child maltreatment. British Journal of Psychiatry, 207(3), 213–220. 10.1192/bjp.bp.114.156620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, Raffanello E, Gingrich J, & Monk C (2016). Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry, 6, 935 10.1038/tp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT, Leutscher-Broekman B, Chong Y-S, Gluckman PD, Fortier MV, Meaney MJ, & Qiu A (2013). Prenatal Maternal Depression Associates with Microstructure of Right Amygdala in Neonates at Birth. Biological Psychiatry, 74(11), 837–844. 10.1016/J.BIOPSYCH.2013.06.019 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, & Davis EP (2015). Archival Report Fetal Exposure to Maternal Depressive Symptoms Is Associated With Cortical Thickness in Late Childhood. 10.1016/j.biopsych.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger C, Iles JE, Andrew CS, & Ramchandani PG (2015). Associations between postnatal maternal depression and psychological outcomes in adolescent offspring: a systematic review. Archives of Women ‘s Mental Health, 18(2), 147–162. 10.1007/s00737-014-0463-2 [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, & Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage, 17(3), 1429–1436. 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- Team RC (2019). A language and environment for statistical computing. [Google Scholar]
- Uda S, Matsui M, Tanaka C, Uematsu A, Miura K, Kawana I, & Noguchi K (2015). Normal Development of Human Brain White Matter from Infancy to Early Adulthood: A Diffusion Tensor Imaging Study. Developmental Neuroscience, 37(2), 182–194. 10.1159/000373885 [DOI] [PubMed] [Google Scholar]
- Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, Shek LP, Gluckman PD, Fortier MV, Meaney MJ, & Qiu A (2017). Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Translational Psychiatry, 7(4), e1103–e1103. 10.1038/tp.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Liu L, Hitchens TK, & Wu YL (2017). Mapping immune cell infiltration using restricted diffusion MRI. Magnetic Resonance in Medicine, 77(2), 603–612. 10.1002/mrm.26143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, & Tseng W-YI (2013). Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE, 8(11), e80713 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Wedeen VJ, & Tseng WYI (2010). Generalized q-sampling imaging. IEEE Transactions on Medical Imaging, 29(9), 1626–1635. 10.1109/TMI.2010.2045126 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, Akhter K, Rosa-Neto P, Pike GB, Evans A, Toga AW, Woods R, Mazziotta JC, Miller MI, van Zijl PCM, & Mori S (2010). Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage, 52(4), 1289–1301. 10.1016/j.neuroimage.2010.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R, Tiemeier H, Van Der Ende J, Verhulst FC, Muetzel RL, White T, Hillegers M, & El Marroun H (2019). Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: A population-based imaging study. American Journal of Psychiatry, 176(9), 702–710. 10.1176/appi.ajp.2019.18080970 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.