Abstract
Although beta 2 adrenergic receptors (β2ADR) are present in the keratinocytes, their role in cutaneous squamous cell tumorigenesis needs to be ascertained. For the first time, we report here that selective β2ADR antagonists by inhibiting β2ADR actions in the keratinocytes significantly retarded the progression of ultraviolet B (UVB) induced premalignant cutaneous squamous cell lesions. These antagonists acted by inhibiting vascular endothelial growth factor-A (VEGF) mediated angiogenesis to prevent UVB radiation-induced squamous cell carcinoma of the skin.
Keywords: Beta 2 adrenergic receptor blockers, UVB, Skin, Tumorigenesis
1. INTRODUCTION
Among the subtypes of adrenergic receptors (α1, α2, β1, β2 and β3), it is now well established that keratinocytes, the cells that make up the majority of the epidermis, express both α1 and β2 receptors1–4. Several studies indicate that β2 ADR play essential roles in wound healing and dermatological diseases such as atopic dermatitis, vitiligo and psoriasis2, 4. However, although there are reports demonstrating the effects of carvedilol, a non-specific βADR blocker on skin tumorigenesis, i.e., it acts on all the three β receptors, this drug also has α1 adrenergic activity5–7. Therefore, it will be interesting to identify the role of β2 ADR in this process.
Ultraviolet radiation is considered a major risk factor for squamous cell carcinoma of the skin8–10. UVB component of ultraviolet radiation is a complete carcinogen capable of cancer initiation, promotion, and progression9. It is therefore clinically relevant in the etiology of squamous cell carcinoma of the skin11–13. Accordingly, the role of β2ADR and their selective antagonists on preventing the progression of UVB induced squamous cell tumorigenesis of the skin in SKH-1 hairless mice, a standard animal model used for these studies, have been investigated.
2. MATERIALS AND METHODS
2.1. Cell Culture and Reagents
Mycoplasma free keratinocyte derived VEGF secreting A-431 cells14 (Cat#CRL-1555, ATCC, MD, USA) was authenticated using short tandem repeat profiling and cultured in Dulbecco’s Modified Eagle’s Medium (Cat#30–2002, ATCC) supplemented with 10% FBS (Cat#30–2020, ATCC). The cells were then serum and growth factor starved for 12 h and treated with selective β₂ ADR antagonists butoxamine (Cat#B1385, Sigma) or ICI-118,551 (ICI) (Cat#0821, Tocris, MN, USA). Forskolin was procured from Sigma, MO, USA.
2.2. UVB Irradiation and Treatment
The animal experiments were approved by the institutional animal care and use committee of the Ohio State University. 6–8 week-old male Skh-1 mice (Charles River Laboratories, CA, USA) were dorsally exposed to UVB radiation (290–320 nm) thrice a week for 16 weeks at a consistent dose of 2240 J/m2, using Phillips FS40UVB lamps (American Ultraviolet Company, IN, USA) fitted with Kodacel filters (Eastman Kodak, NY, USA). The UVB dose was determined by a UVX radiometer (UVB, Inc., CA, USA)12. Following UVB irradiation, the randomly grouped animals were treated with selective β₂ADR antagonists, butoxamine or ICI at a dose of 10 mg/kg/d i.p.15, 16 for 14 consecutive days. Skin tumors greater than 1 mm were counted and measured in each mouse using a caliper before the first day of treatment and thereafter, every alternate day throughout the treatment. Finally, at the end of the study, the animals were euthanized, and multiple dorsal skin lesions and tumors were harvested and fixed in 10% neutral buffered formalin12, 17.
2.3. Immunohistochemistry
Skin tissue sections were first stained with anti-CD31 antibody (Cat#ab28364, Abcam, MA, USA) or anti-VEGFA antibody (Cat#ab46154, Abcam) and kept overnight at 4°C and then incubated with secondary antibody (Vector, CA, USA). Images were obtained using Axio Scope upright light microscope (Carl Zeiss, Oberkochen, Germany). Microvessel density, i.e., CD31 staining, a marker of angiogenesis, was enumerated from 10 random fields per section using ImageJ software (National Institute of Health, MD, USA)18.
2.4. Immunofluorescence
Mouse skin sections were probed with two unconjugated primary antibodies anti-pan keratin antibody (Cat#4545, Cell Signaling Technology, MA, USA) and anti-β₂ ADR antibody (Cat#ab182136, Abcam) overnight at 4°C and then incubated with fluorochrome-conjugated secondary antibodies (Invitrogen, CA, USA) for 1h at room temperature. 4, 6-diamidino-2-phenylindole (DAPI) from Electron Microscopy Sciences, PA, USA, was used as a nucleus counterstain. Slides were visualized using a confocal scanning microscope (FBV-1000; Olympus Corporation, Center Valley, PA, USA)19, 20.
2.5. Enzyme-Linked Immunosorbent Assay
A-431 cells were serum-starved for 12 h and then treated with 1 μM of Butoxamine or ICI for 48 h, and the supernatant was collected. Enzyme-linked immunosorbent assay (ELISA) was done using a Quantikine Enzyme-linked immunosorbent assay kit (Cat#DVE00, R&D Systems) following the protocol of the manufacturer to determine VEGF concentrations in the supernatant. The absorbance was measured at 450 nm, using a PowerWave XS Microplate reader (BioTek Instruments, USA)21, 22.
2.6. Tumor grading
Tumor tissue sections were graded in a blinded manner by board-certified pathologists. The area of the tumor was measured by multiplying the length and width. Tumor burden per mouse was counted from the total tumor area per mouse, which was the sum of all tumor areas from each mouse12, 17, 23, 24.
2.7. Western Blot
A-431 cell lysates were harvested and used for the western blot to assess phospho-cyclic AMP responsive element-binding protein (p-CREB) protein expression (Cat#9198, Cell Signaling Technology, USA). The immunoblot signals were developed using SuperSignal chemiluminescent substrates (Thermo Scientific, USA, Cat# 34580) and visualized using the C-DiGit® Blot Scanner (LI-COR Biosciences, USA, Model# 3600). Protein loading was normalized using an anti-GAPDH antibody (Cat#sc-25778, Santa Cruz Biotechnology). The antibody reactive bands were then quantified through densitometry25.
2.8. Statistics
Data are expressed as mean ± SEM values. Student’s t-test and ANOVA were performed to analyze results from independent groups. Repeated measures ANOVA was done to analyze data obtained on days 0–14. p<0.05 was considered statistically significant. Bonferroni correction was performed for multiple comparisons25.
3. RESULTS
3.1. VEGF and CD31 expressions in UVB-irradiated skin lesions
VEGF is a potent stimulator of angiogenesis26, 27. Therefore, we assessed VEGF and CD31 expressions in UVB irradiated murine skin lesions. The immunohistochemistry results revealed significant upregulation of VEGF and CD31expressions in these lesions compared to normal skin controls (FIGURES 1A–C) (*P < 0.05).
FIGURE 1.

(A) Immunohistochemistry staining of VEGF in normal mouse skin and UVB induced premalignant mouse skin lesions. The arrows show the VEGF expression. N=10 for both groups. (B and C) Immunohistochemistry staining of CD31, i.e., microvessel density in normal mouse skin and UVB induced premalignant mouse skin lesions. The arrows show microvessels. Data are expressed as mean ± SEM. N=10 for both groups. *p < 0.05 versus normal skin, Student’s t test. Scale bar = 100 μm. The figure is representative of ten separate experiments with similar results.
3.2. β2 ADR are present on keratinocytes in UVB-induced skin lesions
We next determined the status of β2 adrenergic receptors on the keratinocytes in UVB-induced cutaneous lesions in mice. Immunofluorescence staining revealed colocalization of β2 adrenergic receptors with pan-keratin, a marker of keratinocytes, thereby indicating expression of β2 adrenergic receptors in the keratinocytes of these lesions (FIGURE 2A).
FIGURE 2.
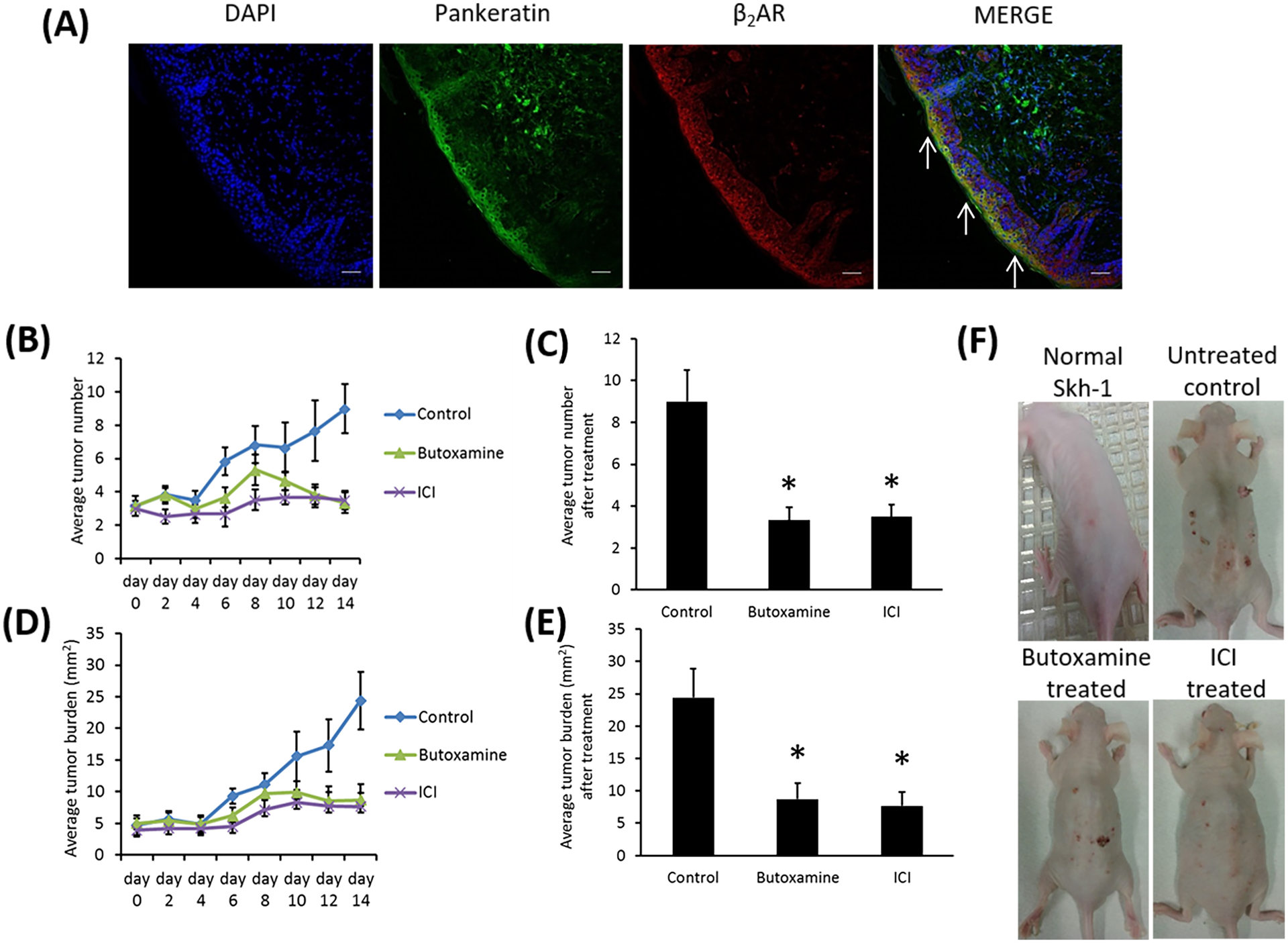
(A) Immunofluorescence staining and confocal microscopy were used to colocalize pan keratin (green) and β2AR (red) in mouse premalignant skin lesions. N= 10. Scale bar = 100 μm. The arrows show co-staining. (B and C) Average tumor numbers were recorded and compared between mice treated with selective Butoxamine or ICI and control mice treated with vehicle. Data are expressed as mean ± SEM. N=10 for all groups. *p< 0.05 versus vehicle-treated control, ANOVA with repeated measures. (D and E) Average tumor burdens were recorded and compared between mice treated with Butoxamine or ICI and control mice treated with vehicle. Data are expressed as mean ± SEM. N=10 for all groups. *p< 0.05 versus vehicle-treated control, ANOVA with repeated measures. (F) Gross pictures of the dorsal skin of ultraviolet B irradiated Skh-1 mice treated with Butoxamine or ICI or vehicle. N=10 for all groups. The figures are representative of ten separate experiments with similar results.
3.3. Treatment with β2 ADR antagonists decrease the number of tumors, the tumor burden, and the development of malignant skin tumors
The number of skin tumors and the tumor burden significantly decreased in UVB-irradiated mice following treatment with selective β2 ADR antagonists butoxamine or ICI (10 mg/kg once daily i.p. for 14 d) in comparison to vehicle-treated controls (FIGURES 2B–F) (*P < 0.05). On histopathological examination, the lesions were hyperplasia and papilloma (grades 1–3). Although 16.3% of skin lesions were squamous cell carcinomas in the vehicle-treated control group, malignant squamous cell carcinoma was not found in the groups treated with selective β2 ADR antagonists.
3.4. β2 ADR antagonists inhibit angiogenesis
Since β2 ADR are expressed in the keratinocytes in UVB-irradiated skin lesions (FIGURE 2A), we examined the effects of inhibition of β2ADR actions in these cells by β2 ADR antagonists on VEGF induced angiogenesis. Mice irradiated with UVB thrice a week with consistent dosing of 2240 J/m2 for 16 weeks were treated intraperitoneally (i.p.) on week 17 with selective β2 ADR antagonists/blockers butoxamine or ICI at a dose of 10 mg/kg once daily for 14 consecutive days. After the end of treatment, i.e., on day 14, our results showed significant inhibition of CD31 and VEGF expressions in the skin lesions of UVB irradiated animals in comparison to vehicle-treated controls (FIGURES 3A–C) (*P < 0.05).
FIGURE 3.

(A, B) Immunohistochemistry staining of CD31, i.e., microvessel density (MVD) in premalignant skin lesions from vehicle-treated control and β2AR antagonists treated mice. The arrows show the microvessels. Data are expressed as mean ± SEM. N= 10 for both groups. *p < 0.05 versus vehicle-treated control, Student’s t test. Scale bar = 100 μm. The figures are representative of ten separate experiments with similar results. (C) Immunohistochemistry staining of VEGF in premalignant skin lesions from β2AR antagonists Butoxamine or ICI treated mice compared to vehicle-treated control. The arrows show VEGF expression. N= 10 for all groups. Scale bar = 100 μm. The figures are representative of ten separate experiments with similar results. (D) VEGF concentration in the A-431 cell culture supernatant detected by Enzyme-linked immunosorbent assay. Data are expressed as mean ± SEM. *p < 0.05 versus untreated control, Student’s t test. The figures is representative of six separate experiments under similar conditions. (E) Western blot of A-431 cells treated with Butoxamine or ICI. Forskolin is the positive protein kinase A control. The blots were first probed with phospho-CREB antibody and re-probed with GAPDH antibody to compare equal protein load. The pCREB signals were normalized using the GAPDH antibody. *p < 0.05 versus untreated control, +p < 0.05 versus untreated control (one-way ANOVA). The molecular weight of pCREB is 43 kDa. The figures are representative of six separate experiments under similar conditions.
3.5. Inhibition of β2 ADR actions in keratinocytes suppress VEGF synthesis
Suppression of VEGF synthesis through inhibition of β2 ADR actions in the keratinocytes was further confirmed by enzyme-linked immunosorbent assay, which showed significant inhibition of VEGF concentration in the cell culture supernatant of β2 ADR expressing A-431 cells28, 29 upon treatment with 1 μM of selective β2 ADR blockers butoxamine or ICI for 48 h (FIGURE 3D) (*P < 0.05).
3.6. Inhibition of β2 ADR function in keratinocytes suppresses VEGF production through the β2ADR-CREB Pathway
Inhibition of β2ADR actions in the keratinocytes downregulates VEGF expressions in these cells (FIGURE 3C, D) and subsequent angiogenesis in UVB-irradiated premalignant skin lesions (FIGURES 3A, B). As β2ADR classically act through the protein kinase A signaling pathway30, and stimulation of the PKA pathway upregulates VEGF synthesis through the phosphorylation or activation of the cAMP response element-binding protein (CREB) transcription factor31, therefore, we investigated the effects of treatment with 1 μM of selective β2ADR butoxamine or ICI on CREB phosphorylation (phospho-CREB) in A-431 cells. Western blot analysis revealed significant inhibition of phospho-CREB in butoxamine- or ICI-treated A-431 cells compared to the untreated controls (FIGURE 3E) (*P < 0.05). Protein kinase A stimulator forskolin (10 μM)22 was a positive control (FIGURE 3E) (+P < 0.05) (See Supplemental FIGURE 1 for full blots).
4. DISCUSSION
This study, for the first time, indicated that suppression of actions of β2 adrenergic receptors by selective β2ADR antagonists significantly reduced the number of tumors, tumor burden and incidence of malignant squamous cell tumors in UVB-induced premalignant skin lesions through suppression of angiogenesis. These antagonists acted by downregulating VEGF expression in keratinocytes by inhibiting CREB phosphorylation via the protein kinase A/CREB pathway. It is to be noted here that stimulation of β2ADR by agonists induces cyclic adenosine monophosphate production, which activates protein kinase A32.
Squamous cell carcinoma of the skin is commonly seen globally, and UVB radiation increases the risk of this malignant tumor8–10. The presently available treatment does not result in a complete cure as the recurrence rate of this cancer is high, impacting the health budget10. Therefore, there is a need to develop newer and effective therapies to prevent this disease10. Our results demonstrate a novel role of β2ADR in regulating the development and progression of UVB-induced premalignant cutaneous lesions and the efficacy of selective β2ADR antagonists for their treatment. Interestingly, epidemiological studies have reported no increase in squamous cell carcinoma incidence in patients taking beta adrenergic blockers for other disorders33. These drugs also have a protective effect on melanoma34. Furthermore, as these classes of drugs with manageable side effects are currently being used in the clinics in other diseases, therefore these molecules may be used in the future to inhibit the progression of UVB-induced precancerous lesions for preventing squamous cell carcinoma of the skin.
Supplementary Material
Funding Information
DOD USA, W81XWH-12-1-0443, NIH/NHLBI, R01HL131405 and DOD USA, W81XWH1910233 to S.B.
Abbreviations
- β2ADR
Beta 2 adrenergic receptor
- UVB
Ultraviolet B
- VEGF
Vascular Endothelial Growth Factor-A
- ICI
ICI-118,551
- i.p.
intraperitoneally
- CREB
cAMP response element-binding protein
- ELISA
Enzyme-Linked Immunosorbent Assay
- cAMP
cyclic adenosine monophosphate
Footnotes
Conflict of Interest
The authors state no conflict of interest.
Data availability statement
The authors are committed to sharing their data, publishing the data, and making available the resources described in this publication to the scientific community.
REFERENCES
- 1.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 2012; 212: 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol Clin 2007; 25:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinkraus V, Steinfath M, Korner C, Mensing H. Binding of beta-adrenergic receptors in human skin. J Invest Dermatol 1992; 98: 475–480. [DOI] [PubMed] [Google Scholar]
- 4.Chakroborty D, Goswami S, Basu S, Sarkar C. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. FASEB J 2020; 34:14093–14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang KM, Liang S, Yeung S, Oiyemhonlan E, Cleveland KH, Parsa C, Orlando R, Meyskens FL Jr, Andresen BT, Huang Y. Topically Applied Carvedilol Attenuates Solar Ultraviolet Radiation Induced Skin Carcinogenesis. Cancer Prev Res (Phila) 2017; 10:598–606. [DOI] [PubMed] [Google Scholar]
- 6.Keating GM, Jarvis B. Carvedilol: a review of its use in chronic heart failure. Drugs 2003; 63:1697–1741. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Kang X, Wang Y, Wang F, Yu P, Shen J, Fu L. Effects of carvedilol on ventricular remodeling and the expression of β3-adrenergic receptor in a diabetic rat model subjected myocardial infarction. Int J Cardiol 2016; 222:178–184. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi N, Srivastava SK, Arora S, Omar Y, Ijaz ZM, Al-Ghadhban A, Deshmukh SK, Carter JE, Singh AP, Singh S. Comparative analysis of the relative potential of silver, Zinc-oxide and titanium-dioxide nanoparticles against UVB-induced DNA damage for the prevention of skin carcinogenesis. Cancer Lett 2016; 383:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigby CM, Roy S, Deep G, Guillermo-Lagae R, Jain AK, Dhar D, Orlicky DJ, Agarwal C, Agarwal R. Role of p53 in silibinin-mediated inhibition of ultraviolet B radiation-induced DNA damage, inflammation and skin carcinogenesis. Carcinogenesis 2017; 38:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mintie CA, Musarra AK, Singh CK, Ndiaye MA, Sullivan R, Eickhoff JC, Ahmad N. Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice. Cancers (Basel) 2020; 12:1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gloster HM Jr, Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996; 22:217–226. [DOI] [PubMed] [Google Scholar]
- 12.Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res 2007; 67:3468–3474. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Deep G, Agarwal R. An Overview of Ultraviolet B Radiation-Induced Skin Cancer Chemoprevention by Silibinin. Curr Pharmacol Rep 2015; 1:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mildner M, Weninger W, Trautinger F, Ban J, Tschachler E. UVA and UVB radiation differentially regulate vascular endothelial growth factor expression in keratinocyte-derived cell lines and in human keratinocytes. Photochem Photobiol 1999; 70:674–679. [PubMed] [Google Scholar]
- 15.Arai M, Sato T, Takeuchi S, Goto S, Togari A. Dose effects of butoxamine, a selective β2-adrenoceptor antagonist, on bone metabolism in spontaneously hypertensive rat. Eur J Pharmacol 2013; 701:7–13. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, Peng H, Nguyen LP, Dudekula NB, Shardonofsky F, Knoll BJ, Parra S, Bond RA. Changes in beta 2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulm Pharmacol Ther 2008; 21:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns EM, Tober KL, Riggenbach JA, Kusewitt DF, Young GS, Oberyszyn TM. Differential effects of topical vitamin E and C E Ferulic® treatments on ultraviolet light B-induced cutaneous tumor development in Skh-1 mice. PLoS One 2013; 8:e63809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res 2004; 10:4349–4356. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 2001; 7:569–574. [DOI] [PubMed] [Google Scholar]
- 20.Chakroborty D, Sarkar C, Yu H, Wang J, Liu Z, Dasgupta PS, Basu S. Dopamine stabilizes tumor blood vessels by upregulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci U S A 2011; 108:20730–20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junker V, Becker A, Hühne R, Zembatov M, Ravati A, Culmsee C, Krieglstein J. Stimulation of beta-adrenoceptors activates astrocytes and provides neuroprotection. Eur J Pharmacol 2002; 446:25–36. [DOI] [PubMed] [Google Scholar]
- 22.Chakroborty D, Sarkar C, Lu K, Bhat M, Dasgupta PS, Basu S. Activation of Dopamine D1 Receptors in Dermal Fibroblasts Restores Vascular Endothelial Growth Factor-A Production by These Cells and Subsequent Angiogenesis in Diabetic Cutaneous Wound Tissues. Am J Pathol 2016; 186: 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gensler HL, Aickin M, Peng YM. Cumulative reduction of primary skin tumor growth in UV-irradiated mice by the combination of retinyl palmitate and canthaxanthin. Cancer Lett 1990; 53:27–31. [DOI] [PubMed] [Google Scholar]
- 24.Duncan FJ, Wulff BC, Tober KL, Ferketich AK, Martin J, Thomas-Ahner JM, Allen SD, Kusewitt DF, Oberyszyn TM, Vanbuskirk AM. Clinically relevant immunosuppressants influence UVB-induced tumor size through effects on inflammation and angiogenesis. Am J Transplant 2007; 7: 2693–2703. [DOI] [PubMed] [Google Scholar]
- 25.Lu K, Iwenofu OH, Mitra R, Mo X, Dasgupta PS, Basu S. Chebulinic acid is a safe and effective antiangiogenic agent in collagen-induced arthritis in mice. Arthritis Res Ther 2020; 22:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and disease: beyond discovery and development. Cell 2019; 176:1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvorak HF. Tumors: wounds that do not heal-a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin Thromb Hemost 2019; 45:576–592. [DOI] [PubMed] [Google Scholar]
- 28.Kashles O, Levitzki A. Characterization of the beta 2-adrenoceptor-dependent adenylate cyclase of A431 epidermoid carcinoma cells. Biochem Pharmacol 1987; 36:1531–1538. [DOI] [PubMed] [Google Scholar]
- 29.Wang HY, Berrios M, Malbon CC. Localization of beta-adrenergic receptors in A431 cells in situ. Effect of chronic exposure to agonist. Biochem J 1989; 263:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barisione G, Baroffio M, Crimi E, Brusasco V. Beta-Adrenergic Agonists. Pharmaceuticals (Basel). 2010; 3:1016–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Zhau HE, Huang WC, Iqbal S, Habib FK, Sartor O, Cvitanovic L, Marshall FF, Xu Z, Chung LW. cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: implication in human prostate cancer bone metastasis. Oncogene 2007; 26:5070–5077. [DOI] [PubMed] [Google Scholar]
- 32.Najafi A, Sequeira V, Kuster DW, van der Velden J. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest 2016; 46:362–374. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt SA, Schmidt M, Mehnert F, Lemeshow S, Sørensen HT. Use of antihypertensive drugs and risk of skin cancer. J Eur Acad Dermatol Venereol 2015; 29:1545–1554. [DOI] [PubMed] [Google Scholar]
- 34.Yap A, Lopez-Olivo MA, Dubowitz J, Pratt G, Hiller J, Gottumukkala V, Sloan E, Riedel B, Schier R. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth 2018;121:45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


