Abstract
Dimethyl fumarate (DMF), a fumaric acid with antioxidant and immunomodulatory properties, is among the most commonly used oral therapies for relapsing multiple sclerosis. Progressive multifocal leukoencephalopathy (PML) has been associated with several disease-modifying therapies (DMT), including DMF in treating MS. We present detailed clinical characteristics of the 9 PML cases and show that the PML incidence in DMF treated patients is 0.02 per 1000 patients. In addition to persistent severe lymphopenia, older age appears to be a potential risk for PML. However, younger patients without lymphopenia were also observed to develop PML. DMF-associated PML has occurred in patients with absolute lymphocyte counts (ALC) above the guideline threshold, suggesting that changes in specific subsets might be more important than total ALC. Furthermore, since DMF has been found to decrease immune cell migration by decreasing the expression of adhesive molecules, the CSF immune profile may also be useful for assessing PML risk in DMF-treated patients. This review provides an up-to-date assessment of PML cases occurring in DMF treated patients and discusses other potential considerations in light of our current understanding of DMF’s mechanism of action on the immune system in the periphery and in the central nervous system (CNS).
Keywords: Multiple sclerosis, lymphopenia, immunosenescence, fumarate, PML
INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease caused by reactivation and viral replication of the John Cunningham Virus (JCV) in glial cells. The clinical presentation of PML varies widely based on the primary site of demyelination, and its diagnosis is based on radiographic MRI appearance, serum and CSF studies, as well as observed subacute neurologic decline.1 Despite the high rate of seroconversion in the general population2, PML is rare and typically occurs in the immunocompromised population. More specifically, PML occurs in patients with reduced cell-mediated immune systems, such as HIV infected individuals or patients who have undergone immune suppression in the setting of organ transplantation. In the past decade, PML has been described in patients who were treated with disease-modifying therapies (DMT) for multiple sclerosis (MS), including dimethyl fumarate (DMF). DMF is among the most commonly prescribed oral disease-modifying agents for MS. DMF became FDA approved for RRMS in 2013 based on two randomized placebo-controlled phase III clinical trials: DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS) and CONFIRM (Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis) studies,3, 4 which showed a decrease in annual relapse rate, progression of the disease, and MRI activities as compared to placebo.3, 4
This review describes PML pathophysiology, our current understanding of the mechanisms of action of DMF and how immune system modulation could be contributing to PM, potential risk factors in identifying DMF related PML, and the current known cases and the incidence of PML in DMF-treated MS patients.
REVIEW OF PML CASES IN DMF-TREATED PATIENTS
Based on a PubMed search and Biogen medical information, as of January 31, 2020, there are a total of 9 cases of PML (see Table 1) in >445,000 DMF-treated MS patients, representing >875,000 patient-years of exposure worldwide. It is estimated that the US real-world average age of patients on DMF is between 44–47 years old from various studies.5–8 We calculate that the estimated incidence is 0.02 per 1000 patients, and the incidence rate is 1.02 per 100,000 patient-years. This is about 3-fold less PML risk than fingolimod9 and 100-fold less than natalizumab.10 Previously, there was a report on 19 cases of PML associated with fumaric acid esters use in either psoriasis or MS.11 However, the study was poorly referenced, in a mostly psoriasis population, and it was unclear what other co-concomitant medications patients were taking. Fumaric acid esters are mixtures of 3 salts of monoethyl fumarate (MEF) and DMF; therefore, it is difficult to ascertain any of these PML cases to be linked directly to DMF. Therefore, they were not counted in our incident calculations.
Table 1:
Details of PML reported cases associated with Dimethyl Fumarate (Tecfidera) as of January 31, 2020
| Case | Age (years) | Sex | Prior treatment for MS and duration of washout prior to DMF | DMF exposure (months) | ALC (cells/μl) | MRI suggestive/CSF JCV PCR/Biopsy Proven | Location of lesion on MRI | Year Case Diagnosed | Serum JCV Index | CSF JCV |
|---|---|---|---|---|---|---|---|---|---|---|
| 116 | 54 | F | Glatiramer, with 2 year washout | 54 | 290–580 | +/+/NA | Bilateral middle cerebellar peduncle and cerebellums and pons | 2014 | NA | NA |
| 217 | 64 | M | None | 22 | 300–530 | +/+/NA | Singular left frontal lesion | 2015 | 1.31 | 12 copies/ml |
| 3 | 59 | M | None | 16 | 400–524 | +/−/+ | NA | 2015 | NA | 172 copies/ml |
| 4 | 61 | F | 76 month Natalizumab exposure with 2 month washout | 20 | 600–1000 | +/+/NA | NA | 2015 | NA | NA |
| 5 | 66 | NK | None | 41 | Unavailable; lymphopenia reported | NA/NA/NA | NA | 2016 | NA | NA |
| 6 | 60 | F | None | 36 | Unavailable; lymphopenia reported | +/NA/NA | NA | 2016 | NA | NA |
| 718 | 66 | F | Interferon-beta-1a | 52 | 810–840 | +/+/NA | Multifocal (cerebellar hemisphere and peduncles, brainstem) | 2017 | NA | 2020 copies/ml |
| 814 | 39 | F | 24 month Natalizumab exposure with 9 month washout | 42 | 640–2180 | +/+/NA | Multifocal (right thalamus, right mesencephalon, pons, bilateral cerebellums | 2018 | 2.873 | 35–43GEq/ml |
| 915 | 76 | F | Mitroxantrone, 2 month washout Concurrent Intrathecal triamcinolone | 28 | 1240 | −/+/NA | No visible PML lesion | 2017 | 3.68–6.72 | 1,090,110 copies/ml |
NA = information not available
In the general population, lymphocyte number and function decline with age12 and older age is associated with increased risk of developing lymphopenia.13 Table 1 shows PML cases in DMF treated patients seem to be associated with two main risk factors: lymphopenia and age >54. We want to draw attention to two case reports of PML in patients with absolute lymphocyte counts >800 (cases 8 and 9), as well as young age (case 8).14, 15
PATHOPHYSIOLOGY OF PML
Primary JCV infection is subclinical and thought to occur primarily via respiratory transmission, with infection of the tonsillar lymphocytes, and further systemic spread from tonsillar lymphocytes to the kidney and bone marrow, which are the primary sites of reservoir for the virus, allowing the virus to replicate asymptomatically (add ref 23).19 Fecal/oral transmission is also plausible, as shedding in urine was found in 19–45% of healthy infected individuals, with an increase in shedding rate with increasing age.20–22 The virus’s noncoding region (NCCR) nucleotide sequence mutates from a non-pathological archetype to a pathogenic prototype thought to occur through a series of deletions and duplications.23 What drives these mutations is still under investigation, but EBV co-infection has been implicated as a possible catalyst.24 Since the JCV NCCR is present with putative recombination sites, it may be possible that the activation of class-switch recombinases in B cells gives rise to pathogenic mutations in its genome.25 Spread into the CNS has been proposed to be carried out through trafficking of the virus in infected B cells.26 Once inside the CNS, infection of oligodendrocytes cause cytolytic death, leading to subcortical white matter demyelination.27
While JCV antibodies are produced after initial infection, there is little clinical evidence proving these antibodies are useful in preventing PML, even though in vivo data suggests JCV antibodies block viral uptake into cells.23 CD4+ and CD8+ T cells seem to play an important role in the prevention of PML in a JCV positive immunocompromised person. CD4+ T cells against the four major viral proteins have been shown to be essential against reactivation of the virus in natalizumab-treated MS patients.28 It is likely these CD4+ T cells are integral in stimulating cytotoxic JCV CD8+ T cells that have been shown to play a role in disease containment and patient survival.29, 30
DIMETHYL FUMARATE’S EFFECTS ON PERIPHERAL LYMPHOCYTES
There are several hypotheses on how DMF may modulate its immune responses to promote a therapeutic effect.31 We and others have examined DMF effects on peripheral lymphocytes. Within six months of DMF treatment, there is a significant decrease in the CD4+ and CD8+ T cell populations, with a predilection for the CD8+ population. The CD8+ population decreases even more after 12 and 18 months, increasing the CD4+/CD8+ ratio.32–34 Additionally, DMF affects both central memory (Tcm) and effector memory (Tem) T cells, with the loss of activated and/or pro-inflammatory subsets being more reduced.33, 35 Throughout treatment with DMF, CD4+ Tcm cells remain decreased, but CD8+ Tcm cells rebound after an initial decrease.33 Both CD4+ and CD8+ Tem cells eventually stabilized after an initial decrease in the first 4–6 months after DMF treatment, but naïve T cells, CD3+CD4+ and CD3+CD4−, increased for at least 18 months.33, 35 There was no change in the absolute number of terminally differentiated effector memory (TERMA) T cells with DMF treatment, but the percentage of CD69+ TERMA cells and activated CD69+ T cells did decrease.33
DMF has been shown to inhibit GAPDH and downregulate aerobic glycolysis in lymphoid cells, resulting in a decrease of immune response and a shift from a pro-inflammatory to regulatory/anti-inflammatory state.36 Glycolysis is crucial for the survival and differentiation of many cells, such as classically activated macrophages and effector T cells, such as CD8+ T cells, and Th1 and Th17 cells,37–43 whereas the differentiation of regulatory T cells more often occurs under oxidative metabolism.44 DMF also affects dendritic cell maturation, producing immature cells incapable of driving CCXR3+ Th1 and CCR6+ Th17 cell production, leading to a subsequent decrease in pro-inflammatory cytokines IFN-γ and IL-17 production.45 With DMF treatment, these T helper subsets decrease in absolute number35 and proportion relative to CD4+ T cells.33, 34 Furthermore, DMF can reduce IL-17 producing CD161+ T cells46 and eventually increase the proportion of anti-inflammatory cells, such as CCR3+ Th2 and Tregs (CD4+CD25hiCD127lo) amongst a decrease in the total number of Tregs.33–35 DMF treatment increases Th2/Th1Th17 and Treg/Th1Th17 ratios, demonstrating a shift to a more anti-inflammatory state in the peripheral blood (Figure 1).
Figure 1: Mechanism of action for dimethyl fumarate and postulated mechanism for acquiring PML.
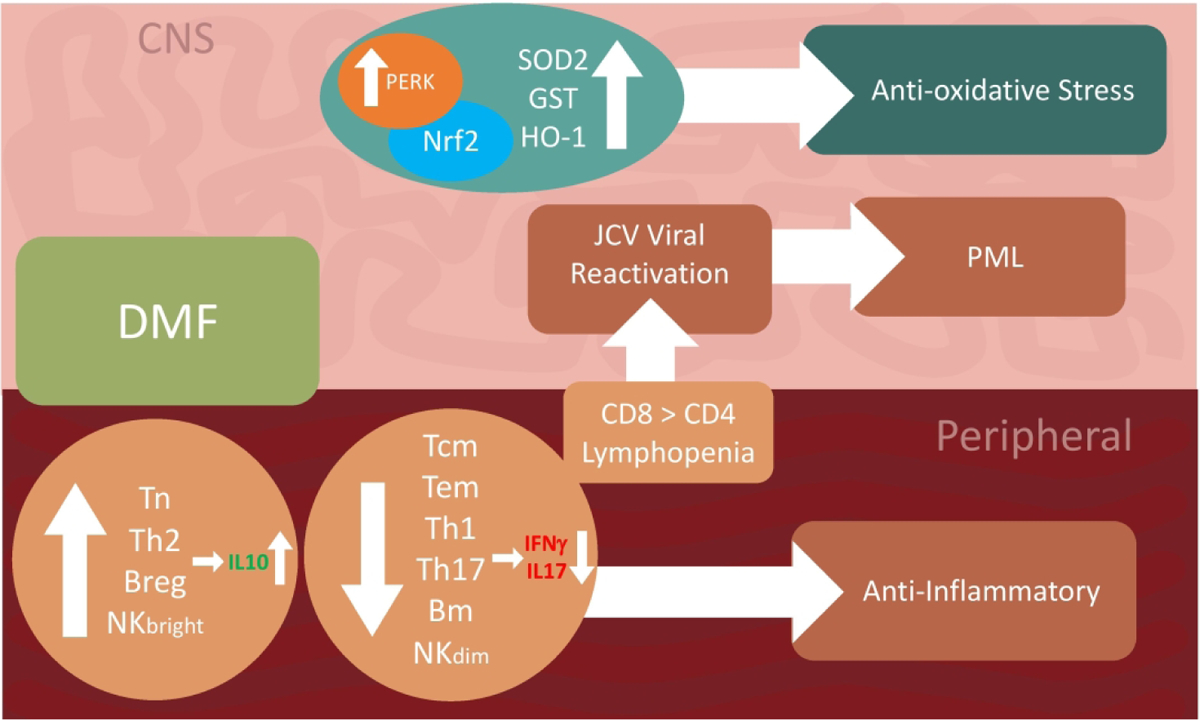
The treatment of dimethyl fumarate leads to an anti-inflammatory state in the peripheral. It upregulates of Tn, Th2, Breg, and NKbright cells, which produce anti-inflammatory cytokines, such as IL-10, and downregulates Tcm, Tem, Th1, Th17, Bmem, and NKdim cells, which produce pro-inflammatory cytokines, such as IFN-γ and IL17. In addition, lymphopenia affects CD8 more than CD4. Possibly some of these changes could contribute to JCV reactivation, and ultimately PML. In the CNS, another benefit of DMF is the increased activities of PERK and NrF2, leading to a state of anti-oxidative stress that resulted from an increase in SOD2, GST, and HO-1.
DMF can decrease immune responses through the activation of the Hydrocarboxylic acid receptor 2 (HCA2) receptor47, 48 and inhibition of the GAPDH enzyme,36 which may reduce absolute lymphocyte counts and result in lymphopenia. The binding of monomethyl fumarate (MMF), a metabolite of DMF, on hydrocarboxylic acid receptor 2 (HCA2) receptors have shown to lead to a signaling cascade that results in the inhibition of NF-kB signaling, which leads to a suppression of pro-inflammatory cytokines and reduction of immune cells, such as neutrophils, from infiltrating the CNS.49 Additionally, DMF is hypothesized to promote an anti-inflammatory state through the activation of the HCA2 receptor in the small intestine,47 leading to prostaglandin E2 (PGE2) production,50 which results in a shift from pro- to anti-inflammatory cytokine production.51 Although downregulation of pro-inflammatory effector T cells may result from an inhibition of GAPDH, another potential hypothesis is T cell apoptosis is induced by DMF’s inactivation of NF-kB, which is independent of the HCA2 pathway.47 NF-kB inhibition also reduces the activation of antigen-presenting cells.52, 53
DMF treatment in MS patients increases anti-inflammatory cytokines, such as IL-10, and shifts the B lymphocytes’ profile in the peripheral blood towards a more anti-inflammatory state. Although DMF treatment results in a decrease in the total level of circulating CD19+ B cells, the most notable change is a decrease in mature CD27+ B cells.54–56 Additionally, after 12 months of DMF treatment, the proportion of CD24hiCD38hi and CD43+CD27+ regulatory B cells are increased (Figure 1).54
JCV-Specific Response
Koralnik’s group studied the JCV-specific CD8+ T cells in PML patients.29, 30 Although there is currently no information about the JCV-specific cytotoxic T lymphocyte response in DMF patients, the CD8+ T-cell lymphopenia associated with DMF suggests that the loss of JCV-specific CD8+ T-cells may be a contributing factor in the development of PML. Information regarding the activation and responsiveness of JCV-specific CD8+ T-cells from DMF-treated patients will be needed to determine how this population varies over the course of treatment and whether it can be used to assay risk for PML.
DIMETHYL FUMARATE’S EFFECTS ON THE CENTRAL NERVOUS SYTEM
Previous studies on experimental autoimmune encephalomyelitis (EAE) models have shown that DMF treatment decreases CD49d expression on peripheral lymphocytes,57 which may lead to difficulties in removing and presenting the JCV antigen. If this finding applies to human MS patients, then the monitoring of CD49d expression may be prognostically useful in DMF-treated patients as well. In vitro studies have shown that DMF can decrease the expression of adhesion molecules in endothelial cells, such as E-selectin, VCAM-1, and ICAM-1.58–60 These cytokine-induced adhesion molecules are essential for lymphocyte trafficking from the periphery to the CNS, which means their downregulation will reduce trans-endothelial migration and allow differences to arise in immune cell composition between the CNS and peripheral blood. This downregulation of adhesion molecules has also been described DMF-treated MS patients, with a decrease in frequency in T cell expressing CCR5, CCR6 in both CD4+ and CD8+ cells, as well as a reduction in MCAM-1+, CXCR3+, and CCR2+ CD4+ T cells in DMF-treated patients compared to untreated controls.61
In a very small study (N=3), CSF samples from DMF treated MS patients showed a decrease in CD4, but not CD8 cells.61 This finding is opposite peripheral data showing a reduction in CD8 cell, and it should be noted these patients were not compared to themselves but to other untreated MS patients.61 More studies are needed before we can say the CD4+/CD8+ ratio differs between peripheral and central compartments.
DISCUSSION
To date, the most commonly used metric to identify DMF-treated MS patients at risk for developing PML is absolute lymphocyte count. This is based on case reports showing the majority of DMF-treated patients who develop PML have ALC <800. The FDA DMF label includes a recommendation to consider treatment interruption in patients with ALCs <0.5 × 109/L persisting ≥6 months to minimize the risk of subsequently developing severe prolonged lymphopenia. The minimum exposure to DMF was 16 months in case 3. Longer exposure could lead to prolonged lymphopenia. Based on combined trial data analysis, the overall incidence of severe lymphopenia (ALC <0.5 × 109/L persisting for ≥6 months) is very small; about 2 – 2.5% of all DMF-treated patients.62 Our review mirrors previous reports showing ALC as a major risk factor for PML development.
Another risk factor highlighted in our review is age >54 years old. This suggests that age-associated decreased immune capacity due to immunosenescence plays an essential role.63, 64 Severe lymphopenia is more prevalent in older patients and has been demonstrated to increase the risk for DMF-associated PML.65 When breaking into different age range, severe lymphopenia (ALC <0.5 × 109/L persisting for ≥6 months) <40 year-old: 0.9%, 40–49: 3.3%, >50 4.2%. Therefore, it appears that older age is associated with a slightly greater decline in mean ALC from baseline and a higher incidence of severe lymphopenia.62, 66
Given DMF’s predilection for CD8+>CD4+ population and the important role that these CD8+ and CD4+ T cells play in controlling the JCV virus, it is not surprising that lymphopenia increases the risk for PML. Interestingly, when the relationship between ALC and lymphocyte subsets counts after DMF treatment in patients <50 and ≥50 years old was assessed, recent data showed at baseline there was less CD8+ naïve T cells in patients ≥50 years old; upon DMF treatment, both CD4 and CD8 memory populations were selectively reduced in all age groups, while the naïve compartment increased regardless of age.67 More information still needs to be gathered on JCV-specific CD8+ T cells to better understand its changes throughout the course of DMF treatment to assess whether this cell population can be used to monitor PML risk.
In addition to the risk that stems from the increase in the CD4+/CD8+ ratio, we also postulate that DMF’s shift away from Treg/Th1Th17 phenotypes and toward the less inflammatory Th2/Th1Th17 cell phenotypes may leave the host with an inability to mount a sufficient attack against JCV (Figure 1). For example, the ability of IFN-γ to inhibit T-antigen in a Jak/Stat-dependent manner,68 suggests that its presence could help prevent JCV reactivation. Additionally, the shift from the production of pro-inflammatory IFN-γ to anti-inflammatory IL-10 as a result of DMF treatment may also contribute to the inability to adequately clear JCV from the CNS.69 Therefore, like double-edged swords, decreases in pro-inflammatory signaling is desirable in MS but could contribute to JCV reactivation in glial cells.
Given that lymphopenia has been remarked as the biggest risk for factor to PML in DMF-treated patients, it, therefore, becomes important to review in more detail case reports of patients with ALC >600 who developed PML, for other possible risk factors. Case 8 describes a young 39-year-old woman who developed PML with ALC >600 and, similarly, in case 4 with a 61-year-old woman. In these cases, our group argues that natalizumab may have played a prominent role in the risk of the development of PML. Natalizumab treatment does not reduce peripheral blood lymphocyte count but actually increases peripheral blood lymphocytes by about 50%. However, it does significantly decrease CSF levels of CD4+, CD8+, and CD19+ cells.70 These cell types remain depleted for several months after discontinuation of the drug, with normalization in the CSF occurring at 14 months after the cessation of therapy.70, 71 As stated above, DMF also has been shown to affect ALC in the CNS, and we hypothesize that perhaps this patient continued to have a persistent low ALC in the CNS, increasing the risk for PML development. This suggests that the risk for development of PML, in this case, was related to the lack of immunosurveillance in the CNS rather than in the periphery, a risk that was discussed previously in our comprehensive review.69 Case 9 is another potential example showing cell suppression in the CSF (in this case due to concurrent therapy with intrathecal triamcinolone) as a potential risk factor for the development of PML, even with normal peripheral ALC.64
The development of JCV-induced CNS pathology involves genetic mutations within the viral genome. NCCR region mutation was reported in case 9. No other case reports performed mutation analysis. The NCCR region mutations are commonly seen in supratentorial lobal type of PML as this mutation facilitates infection in glial cells. While MRI information is limited, both supratentorial and infratentorial lesion locations were seen in our review of DMF-treated PML cases. VP1 region mutations are commonly associated with the infratentorial granule cell neuronopathy variant, which could co-exist with NCRR region mutation.72 Case 1, 7, and 8 involves cerebellums and are likely due to VP1 mutations. Case 2 is likely due to NCCR mutations. Though case 9 did not have MRI visible lesions at the time with viremia and high CSF viral load, there could be diffuse pathological tissue-level changes as we showed in our previous case report.73 JCV was also found on autopsy in immunocompetent individuals74 without clinical evidence of PML.
CONCLUSION
In summary, DMF is an oral disease-modifying therapy that exerts its clinical effect by changing the lymphocyte profile and providing neuroprotection against oxidative injury in RRMS patients. The risk for the development of PML in a DMF-treated MS patient is rare, and estimated to be 0.02 per 1000 patients, and the incidence rate is 1.02 per 100,000 patient-years. This estimate could be limited by reporting bias. DMF-related PML appears to be linked to suppression of peripheral lymphocyte count with increased risk in patients with prolonged ALC counts <800 and increased susceptibility in the elderly. In cases with reported DMF-related PML and normal ALC counts, suggest that there is a probable associated CSF associated lymphopenia, advising the need for more CNS focused mechanism of action studies to be undertaken. Future real-world data is needed to further identify the risk factors of lymphopenia in various ethnic groups of various genetic backgrounds.
Acknowledgements
Conflict of Interest and Financial Disclosures
AJ, JY, and CF have nothing to disclose. MKR has served as a consultant for Teva Neuroscience and Genzyme-Sanofi. YMD has served as a consultant and/or received grant support from: Acorda, Bayer Pharmaceutical, Biogen Idec, Chugai Pharmaceutical, EMD Serono, Genzyme-Sanofi, Novartis, Questor, Genentech, and Teva Neuroscience. YMD is currently supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557, UM1-AI144298-01, CMSC, Chugai, PCORI, Genzyme-Sanofi, Novartis and Genentech.
References
- 1.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013; 80: 1430–1438. 2013/04/10. DOI: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markowitz RB, Thompson HC, Mueller JF, et al. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. The Journal of infectious diseases 1993; 167: 13–20. 1993/01/01. DOI: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. DOI: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 4.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. DOI: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 5.Kresa-Reahl K, Repovic P, Robertson D, et al. Effectiveness of Delayed-release Dimethyl Fumarate on Clinical and Patient-reported Outcomes in Patients With Relapsing Multiple Sclerosis Switching From Glatiramer Acetate: RESPOND, a Prospective Observational Study. Clin Ther 2018; 40: 2077–2087. 2018/11/22. DOI: 10.1016/j.clinthera.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: A multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord 2018; 22: 27–34. 2018/02/26. DOI: 10.1016/j.msard.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Williams MJ, Amezcua L, Okai A, et al. Real-World Safety and Effectiveness of Dimethyl Fumarate in Black or African American Patients with Multiple Sclerosis: 3-Year Results from ESTEEM. Neurol Ther 2020. 2020/05/31. DOI: 10.1007/s40120-020-00193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinea A, Amezcua L, Vargas W, et al. Real-World Safety and Effectiveness of Dimethyl Fumarate in Hispanic or Latino Patients with Multiple Sclerosis: 3-Year Results from ESTEEM. Neurol Ther 2020. 2020/05/31. DOI: 10.1007/s40120-020-00192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger JR, Cree BA, Greenberg B, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 2018; 90: e1815–e1821. 2018/04/18. DOI: 10.1212/WNL.0000000000005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger JR and Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol 2016; 22: 533–535. 2016/02/03. DOI: 10.1007/s13365-016-0427-6. [DOI] [PubMed] [Google Scholar]
- 11.Gieselbach RJ, Muller-Hansma AH, Wijburg MT, et al. Progressive multifocal leukoencephalopathy in patients treated with fumaric acid esters: a review of 19 cases. J Neurol 2017; 264: 1155–1164. 2017/05/23. DOI: 10.1007/s00415-017-8509-9. [DOI] [PubMed] [Google Scholar]
- 12.Ponnappan S and Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal 2011; 14: 1551–1585. 2011/01/08. DOI: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity 2006; 24: 495–499. DOI: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diebold M, Altersberger V, Décard BF, et al. A case of progressive multifocal leukoencephalopathy under dimethyl fumarate treatment without severe lymphopenia or immunosenescence. Mult Scler 2019; 25: 1682–1685. 2019/06/18. DOI: 10.1177/1352458519852100. [DOI] [PubMed] [Google Scholar]
- 15.Motte J, Kneiphof J, Straßburger-Krogias K, et al. Detection of JC virus archetype in cerebrospinal fluid in a MS patient with dimethylfumarate treatment without lymphopenia or signs of PML. J Neurol 2018; 265: 1880–1882. 2018/06/15. DOI: 10.1007/s00415-018-8931-7. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz T, Novas M and Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. The New England journal of medicine 2015; 372: 1476–1478. 2015/04/09. DOI: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 17.Baharnoori M, Lyons J, Dastagir A, et al. Nonfatal PML in a patient with multiple sclerosis treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2016; 3: e274. 2016/08/23. DOI: 10.1212/NXI.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia J, Chavez Baroni H, Dubessy AL, et al. Progressive multifocal leukoencephalopathy in a patient treated by dimethyl fumarate for multiple sclerosis with no lymphopenia but exhausted T lymphocyte subpopulations. Abstract presented at the 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis and 24th Annual Conference of Rehabilitation in MS, Stockholm, Sweden, 12 Sept 2019. [Google Scholar]
- 19.Brew BJ, Davies NW, Cinque P, et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nature reviews Neurology 2010; 6: 667–679. 2010/12/07. DOI: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- 20.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. The Journal of infectious diseases 2009; 199: 837–846. 2009/05/13. DOI: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura T, Aso Y, Kuniyoshi N, et al. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. The Journal of infectious diseases 1990; 161: 1128–1133. 1990/06/01. DOI: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- 22.Agostini HT, Ryschkewitsch CF and Stoner GL. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. Journal of clinical microbiology 1996; 34: 159–164. 1996/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Major EO, Yousry TA and Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. The Lancet Neurology 2018; 17: 467–480. 2018/04/17. DOI: 10.1016/s1474-4422(18)30040-1. [DOI] [PubMed] [Google Scholar]
- 24.Wortman MJ, Lundberg PS, Dagdanova AV, et al. Opportunistic DNA Recombination With Epstein-Barr Virus at Sites of Control Region Rearrangements Mediating JC Virus Neurovirulence. The Journal of infectious diseases 2016; 213: 1436–1443. 2015/12/23. DOI: 10.1093/infdis/jiv755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson EM, Wortman MJ, Dagdanova AV, et al. Polyomavirus JC in the context of immunosuppression: a series of adaptive, DNA replication-driven recombination events in the development of progressive multifocal leukoencephalopathy. Clin Dev Immunol 2013; 2013: 197807. 2013/04/15. DOI: 10.1155/2013/197807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durali D, de Goër de Herve MG, Gasnault J, et al. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Frontiers in immunology 2015; 6: 241. 2015/06/05. DOI: 10.3389/fimmu.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Major EO, Amemiya K, Tornatore CS, et al. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clinical microbiology reviews 1992; 5: 49–73. 1992/01/01. DOI: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkins MR, Ryschkewitsch C, Liebner JC, et al. Changes in JC virus-specific T cell responses during natalizumab treatment and in natalizumab-associated progressive multifocal leukoencephalopathy. PLoS pathogens 2012; 8: e1003014. 2012/11/13. DOI: 10.1371/journal.ppat.1003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. Journal of virology 2011; 85: 7256–7263. 2011/05/06. DOI: 10.1128/jvi.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzocchetti A, Lima M, Tompkins T, et al. Efficient in vitro expansion of JC virus-specific CD8(+) T-cell responses by JCV peptide-stimulated dendritic cells from patients with progressive multifocal leukoencephalopathy. Virology 2009; 383: 173–177. 2008/12/09. DOI: 10.1016/j.virol.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angiari S and O’Neill LA. Dimethyl fumarate: targeting glycolysis to treat MS. Cell Res 2018; 28: 613–615. DOI: 10.1038/s41422-018-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurology(R) neuroimmunology & neuroinflammation 2015; 2: e76. 2015/03/05. DOI: 10.1212/nxi.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Wang Q, Mao G, et al. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. Journal of immunology (Baltimore, Md : 1950) 2017; 198: 3069–3080. 2017/03/05. DOI: 10.4049/jimmunol.1601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross CC, Schulte-Mecklenbeck A, Klinsing S, et al. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurology(R) neuroimmunology & neuroinflammation 2016; 3: e183. 2016/01/15. DOI: 10.1212/nxi.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longbrake EE, Ramsbottom MJ, Cantoni C, et al. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Multiple sclerosis (Houndmills, Basingstoke, England) 2016; 22: 1061–1070. 2015/10/16. DOI: 10.1177/1352458515608961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornberg MD, Bhargava P, Kim PM, et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018; 360: 449–453. 2018/03/29. DOI: 10.1126/science.aan4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cham CM and Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol 2005; 174: 4670–4677. DOI: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 38.Cham CM, Driessens G, O’Keefe JP, et al. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol 2008; 38: 2438–2450. DOI: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macintyre AN, Gerriets VA, Nichols AG, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab 2014; 20: 61–72. 2014/06/12. DOI: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CH, Curtis JD, Maggi LB, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013; 153: 1239–1251. DOI: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerriets VA, Kishton RJ, Nichols AG, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015; 125: 194–207. 2014/12/01. DOI: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011; 35: 871–882. DOI: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013; 496: 238–242. 2013/03/24. DOI: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011; 208: 1367–1376. 2011/06/27. DOI: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghoreschi K, Brück J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 2011; 208: 2291–2303. 2011/10/10. DOI: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longbrake EE, Cantoni C, Chahin S, et al. Dimethyl fumarate induces changes in B- and T-lymphocyte function independent of the effects on absolute lymphocyte count. Multiple sclerosis (Houndmills, Basingstoke, England) 2018; 24: 728–738. 2017/05/10. DOI: 10.1177/1352458517707069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Glehn F, Dias-Carneiro RPC, Moraes AS, et al. Dimethyl fumarate downregulates the immune response through the HCA(2)/GPR109A pathway: Implications for the treatment of multiple sclerosis. Mult Scler Relat Disord 2018; 23: 46–50. 2018/05/16. DOI: 10.1016/j.msard.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Chen H, Assmann JC, Krenz A, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest 2014; 124: 2188–2192. 2014/04/01. DOI: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 2015; 130: 279–295. 2015/04/29. DOI: 10.1007/s00401-015-1422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanson J, Gille A, Zwykiel S, et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest 2010; 120: 2910–2919. 2010/07/26. DOI: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalinski P Regulation of immune responses by prostaglandin E2. J Immunol 2012; 188: 21–28. DOI: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diebold M, Sievers C, Bantug G, et al. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J Autoimmun 2018; 86: 39–50. 2017/09/27. DOI: 10.1016/j.jaut.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Peng H, Guerau-de-Arellano M, Mehta VB, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem 2012; 287: 28017–28026. 2012/06/25. DOI: 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lundy SK, Wu Q, Wang Q, et al. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurol Neuroimmunol Neuroinflamm 2016; 3: e211. 2016/03/03. DOI: 10.1212/NXI.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li R, Rezk A, Ghadiri M, et al. Dimethyl Fumarate Treatment Mediates an Anti-Inflammatory Shift in B Cell Subsets of Patients with Multiple Sclerosis. J Immunol 2017; 198: 691–698. 2016/12/14. DOI: 10.4049/jimmunol.1601649. [DOI] [PubMed] [Google Scholar]
- 56.Smith MD, Martin KA, Calabresi PA, et al. Dimethyl fumarate alters B-cell memory and cytokine production in MS patients. Ann Clin Transl Neurol 2017; 4: 351–355. 2017/04/17. DOI: 10.1002/acn3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kihara Y, Groves A, Rivera RR, et al. Dimethyl fumarate inhibits integrin α4 expression in multiple sclerosis models. Ann Clin Transl Neurol 2015; 2: 978–983. 2015/09/11. DOI: 10.1002/acn3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandermeeren M, Janssens S, Borgers M, et al. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun 1997; 234: 19–23. DOI: 10.1006/bbrc.1997.6570. [DOI] [PubMed] [Google Scholar]
- 59.Rubant SA, Ludwig RJ, Diehl S, et al. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J Invest Dermatol 2008; 128: 326–331. 2007/08/02. DOI: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- 60.Wallbrecht K, Drick N, Hund A-C, et al. Downregulation of endothelial adhesion molecules by dimethylfumarate, but not monomethylfumarate, and impairment of dynamic lymphocyte-endothelial cell interactions. [DOI] [PubMed]
- 61.Holm Hansen R, Højsgaard Chow H, Christensen JR, et al. Dimethyl fumarate therapy reduces memory T cells and the CNS migration potential in patients with multiple sclerosis. Mult Scler Relat Disord 2020; 37: 101451. 2019/10/16. DOI: 10.1016/j.msard.2019.101451. [DOI] [PubMed] [Google Scholar]
- 62.Fox RJ, Chan A, Gold R, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate-treated patients with MS: Patient management considerations. Neurol Clin Pract 2016; 6: 220–229. DOI: 10.1212/CPJ.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehmann-Horn K, Penkert H, Grein P, et al. PML during dimethyl fumarate treatment of multiple sclerosis: How does lymphopenia matter? Neurology 2016; 87: 440–441. 2016/06/28. DOI: 10.1212/wnl.0000000000002900. [DOI] [PubMed] [Google Scholar]
- 64.Mills EA and Mao-Draayer Y. Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult Scler 2018; 24: 1014–1022. 2018/05/19. DOI: 10.1177/1352458518775550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Longbrake EE, Naismith RT, Parks BJ, et al. Dimethyl fumarate-associated lymphopenia: Risk factors and clinical significance. Mult Scler J Exp Transl Clin 2015; 1 2015/11/10. DOI: 10.1177/2055217315596994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox R, Chan A, Gold R, et al. Characterization of Absolute Lymphocyte Count Profiles in MS Patients Treated with Delayed-Release Dimethyl Fumarate: Considerations for Patient Management. Poster presented at the 68th annual meeting of the American Academy of Neurology, Vancouver, Canada, 15–21 April 2016. [Google Scholar]
- 67.Giuliani F, Longbrake E, Mao-Draayer Y, et al. Immunomodulatory Effects of DMF Treatment on the Peripheral Lymphocyte Compartment: A Focus on Older MS Patients (478). Neurology 2020; 94: 478. [Google Scholar]
- 68.De-Simone FI, Sariyer R, Otalora YL, et al. IFN-Gamma Inhibits JC Virus Replication in Glial Cells by Suppressing T-Antigen Expression. PLoS One 2015; 10: e0129694. 2015/06/10. DOI: 10.1371/journal.pone.0129694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mills EA and Mao-Draayer Y. Understanding Progressive Multifocal Leukoencephalopathy Risk in Multiple Sclerosis Patients Treated with Immunomodulatory Therapies: A Bird’s Eye View. Front Immunol 2018; 9: 138. 2018/02/02. DOI: 10.3389/fimmu.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stüve O, Cravens PD, Frohman EM, et al. Immunologic, clinical, and radiologic status 14 months after cessation of natalizumab therapy. Neurology 2009; 72: 396–401. 2008/11/05. DOI: 10.1212/01.wnl.0000327341.89587.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stüve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006; 59: 743–747. DOI: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 72.Rempe T, Wang Q, Wu Q, et al. Progressive multifocal leukoencephalopathy and granule cell neuronopathy with novel mutation flanking VP1 C-terminus in natalizumab-extended interval dosing. Neurol Neuroimmunol Neuroinflamm 2020; 7 2020/03/20. DOI: 10.1212/NXI.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Himedan M, Camelo-Piragua S, Mills EA, et al. Pathologic Findings of Chronic PML-IRIS in a Patient with Prolonged PML Survival Following Natalizumab Treatment. J Investig Med High Impact Case Rep 2017; 5: 2324709617734248. 2017/09/27. DOI: 10.1177/2324709617734248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White FA 3rd, Ishaq M, Stoner GL, et al. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. Journal of virology 1992; 66: 5726–5734. 1992/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]


