Abstract
Objective:
Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent and impairing neurodevelopmental disorder. While early childhood is a crucial time for early intervention, it is characterized by instability of ADHD diagnosis. Neural correlates of ADHD have potential to improve diagnostic accuracy; however, minimal research has focused on early childhood. Research indicates that disrupted neural connectivity is associated with ADHD in older children. Here, we explore network connectivity as a potential neural correlate of ADHD diagnosis in early childhood.
Method:
We collected EEG data in 52 medication-naïve children with ADHD and in 77 typically developing controls (3–7 years). Data was collected with the EGI 128 HydroCel Sensor Net System, but to optimize the ICA, the data was down sampled to the 10–10 system. Connectivity was measured as the synchronization of the time series of each pair of electrodes. Subsequent analyses utilized graph theoretical methods to further characterize network connectivity.
Results:
Increased global efficiency, which measures the efficiency of information transfer across the entire brain, was associated with increased inattentive symptom severity. Further, this association was robust to controls for age, IQ, SES, and internalizing psychopathology.
Conclusions:
Overall, our findings indicate that increased global efficiency, which suggests a hyper-connected neural network, is associated with elevated ADHD symptom severity. These findings extend previous work reporting disruption of neural network connectivity in older children with ADHD into early childhood.
Keywords: ADHD, Early Childhood, EEG, Externalizing Disorders, Connectivity
Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder, affecting 3–7% of the population (Danielson et al., 2017; Polanczyk et al., 2014; Visser et al., 2014). ADHD diagnosis confers an increased risk of social, academic, and substance use problems and is associated with continued impairment into adulthood (Loe & Feldman, 2007; Wehmeier et al., 2010; Wilens et al., 2011).
Despite agreement across numerous studies that the prevalence rate of ADHD is about 6% worldwide (Polanczyk et al., 2014), diagnostic rates (i.e., the rates at which children receive a diagnosis) are much more variable. Broadly, evidence suggests that diagnosis of ADHD is not standard across different populations defined by gender, age, race and ethnicity. Diagnosis of ADHD in early childhood (e.g. less than 8 years old) is highly unstable, with rates of remission or partial remission as high as 50% in this age range (Campbell & Ewing, 1990; Law et al., 2014; McGee et al., 1991; Tandon et al., 2011). It may be that in early childhood parental report of ADHD symptoms is less accurate, primarily because many of the diagnostic criteria are hard to separate from normative behaviors during this developmental period (Smidts & Oosterlaan, 2007). Developing biological indicators of ADHD to compliment current diagnostic criteria would be beneficial for improving diagnosis, particularly in early childhood.
The initial step towards this goal is to identify neural markers of ADHD symptomatology, e.g., increased inattention and hyperactivity. Broadly, network dysfunction has been observed in individuals with ADHD across multiple networks that are implicated in higher-level cognitive functions (Castellanos & Proal, 2012; Henry & Cohen, 2019; Konrad & Eickhoff, 2010; Liston et al., 2011; Qiu et al., 2011). Recent work has applied increasingly sophisticated analytic methods to enhance our understanding of which aspects of neural connectivity are disrupted in individuals with ADHD. In particular, graph theoretical methods, which utilize a mathematical framework for understanding network organization, have proven to be particularly useful for advancing our understanding of brain connectivity in typical and atypical populations (Fornito et al., 2015; Meunier et al., 2010; Petersen & Sporns, 2015; Sporns & Betzel, 2016; Vértes & Bullmore, 2015). Previous work using fMRI has employed graph theoretical techniques to characterize differences in functional connectivity between older children with and without ADHD (Henry & Cohen, 2019). In these prior studies, children with ADHD had increased modularity, decreased global efficiency, and increased local efficiency in comparison to typically developing (TD) controls of the same age (Beare et al., 2017; Cao et al., 2013; Henry & Cohen, 2019; Lin et al., 2014; Wang et al., 2009). Taken together, these studies suggest that in middle childhood and adolescence, children with ADHD have increased segregation and decreased integration compared to TD children (Beare et al., 2017; Cao et al., 2013; Henry & Cohen, 2019; Lin et al., 2014). Increased segregation indicates that local information is not being processed effectively due to hyper-connectivity within subnetworks. This may impact processing speed even for fairly low-level cognitive functions. Decreased integration indicates that a network is displaying inefficient long-range communication, which is crucial for higher-level cognitive functions. This includes executive function, a set of cognitive abilities which are commonly impaired in individuals with ADHD (Baum et al., 2017; Marek et al., 2015). Overall, previous work with children 8 years old and older has suggested that dysfunctional integration and segregation of brain connectivity, as characterized by modularity, local efficiency, and global efficiency, are neural correlates of ADHD. Importantly, this approach has not been explored in early childhood.
While work with older children has primarily utilized fMRI, particularly for connectivity analyses, there is a rich history of work applying EEG metrics in studies of ADHD. Furthermore, given the ease and expense of acquiring resting-state EEG (rsEEG), it could be used more easily in complement to other metrics (self-report, behavioral observations) when translating this area of research into clinical use. Previous rsEEG studies of older children with ADHD have found differences in coherence, a measure of the synchronicity of oscillating brain activity that underlies functional brain networks (Ahmadlou & Adeli, 2010; Alba et al., 2016; Barry et al., 2002, 2011; Bowyer, 2016; Clarke et al., 2007; Robbie et al., 2016). However, in these studies differences have been observed in alpha, beta, theta, and delta frequency bands (Ahmadlou & Adeli, 2010; Alba et al., 2016; Barry et al., 2002, 2011; Clarke et al., 2007; Robbie et al., 2016). Studies also report both increased and decreased coherence values in children with ADHD, depending on the specific method used to calculate coherence and which frequency band was examined (Ahmadlou & Adeli, 2010; Alba et al., 2016; Barry et al., 2002, 2011; Robbie et al., 2016). Overall, existing work examining coherence indicates that children with and without ADHD differ in regards to neural network connectivity, but does not provide a clear understanding of the network organization properties that differ. Ample evidence from studies utilizing traditional EEG methods indicates that differences in alpha power (approximately 8–13 Hz) distinguish between individuals with ADHD in studies of both children and adults with ADHD when compared to TD individuals (Barry et al., 2003; Hale et al., 2009, 2010; Koehler et al., 2009; Robbie et al., 2016). Furthermore, previous work in the sample included in the current paper also identified that alpha power distinguishes between ADHD and TD groups and is associated with increased symptom severity (Furlong et al., 2020; Robertson et al., 2019). The application of graph theoretical methods to this population early in childhood has potential to resolve inconsistent findings in studies of rsEEG coherence in this population and build upon previous work implicating the alpha band in ADHD symptomatology.
Importantly, ADHD is highly comorbid with both internalizing and externalizing disorders (Angold et al., 1999) and increased levels of comorbid externalizing and internalizing symptoms are associated with greater ADHD symptom severity (Connor et al., 2003). For example, individuals with ADHD have a greater likelihood of being diagnosed with or displaying behaviors and symptoms consistent with oppositional, conduct, and substance use disorders (Lee, Lahey, Owens, & Hinshaw, 2008; Murphy & Barkley, 1996) and ample evidence suggests that the etiology of externalizing disorders overlaps with the etiology of ADHD (Beauchaine et al., 2010; Cosgrove et al., 2011). This overlap is evident in regard to neural correlates: previous research suggests that dysfunctional network connectivity between prefrontal and subcortical regions underlies externalizing psychopathology in children and adolescents (Finger et al., 2012; Hwang et al., 2016; White et al., 2015). Conversely, although studies have placed the prevalence of anxiety disorders comorbid with ADHD at 15–35%, (Schatz & Rostain, 2006), previous research has identified distinct neural correlates that underlie internalizing and externalizing forms of psychopathology, such that internalizing disorders are less likely to share neural correlates with ADHD and other externalizing disorders (Hinton et al., 2019; Moadab et al., 2010). In follow up analyses, we control for internalizing and externalizing symptoms in significant models.
In the present study we use graph theoretical methods to characterize the properties of the neural networks, which are calculated from the measurement of synchronization of signal between pairs of electrodes during rsEEG. As with previous studies of children with ADHD, we expect to observe network dysfunction in children with ADHD in early childhood compared to age and gender matched controls. Further, we expect that increased dysfunction in measures of network structure will be associated with increased symptom severity. Dysfunction in network connectivity will be measured by (1) calculating the weighted phase lag index (WPLI), which measures the synchronization of the time series of each pair of electrodes, and (2) applying graph theoretical methods to the network connectivity matrices to characterize the extent to which the networks are globally communicating (integration) and locally communicating (segregation). If the neural development of ADHD in young children is consistent with that of older children with ADHD, we predict that in the alpha frequency band the children with ADHD in our study will show increased network segregation, characterized by increased modularity and local efficiency, and decreased network integration, characterized by decreased global efficiency, in comparison to TD children. Lastly, if local and global efficiency in the alpha band are related to ADHD specifically, we expect these findings to be robust to controls for internalizing or externalizing symptoms.
Methods
Participants
A sample of 52 medication naïve children with ADHD and 77 typically developing controls (3 years, 0 months −7 years, 4 months) from a larger sample of 150 children, which included children with ADHD that were not medication naïve, participated in the current study. Participants were recruited from community events, schools, a database of families who had expressed interest in research compiled by the Labs of Cognitive Neuroscience at Boston Children’s Hospital, and from a database of participants who were seen for ADHD at Boston Children’s Hospital. All study procedures were approved by the Institutional Review Board at Boston Children’s Hospital and complied with the Helsinki Declaration. The primary caregiver of each child participant provided informed consent and each child provided verbal assent. This data was collected as part of an ongoing longitudinal study of stability of ADHD diagnosis in young children.
Inclusion/Exclusion Criteria
Exclusion criteria included history of known chromosomal abnormalities such as fragile X or down syndrome, prenatal substance exposure as reported in medical records or by caregivers, presence of autism spectrum disorder, lack of English language comprehension, sub-threshold symptoms (4 or 5 out of 9 symptoms) of ADHD on the Swanson Nolan and Pelham checklist (SNAP-4) (Swanson, 1992), or psychotropic medication use, including previous or current use of ADHD medications. Of the total sample (N=150), 6 participants were removed from the analyses because they did not meet our inclusion/exclusion criteria. Of those that were removed, 3 participants were excluded because their caregivers reported genetic abnormalities or prenatal exposure to substances, 1 participant was excluded because of a parent report of a diagnosis of autism spectrum disorder that was confirmed during initial assessments for the study, 1 participant was excluded because their parent did not speak English and could not comprehend the study procedures, and 1 participant was using psychotropic medication at the time of participation. An additional 13 participants had unusable EEG data due to movement artifacts, which were determined to be artifacts based on visual evaluation by a trained technician, and were not included in the final analyses (6 ADHD, 7 Control). One participant was excluded due to refusal to participate in the study after the time of consent. The final sample of 129 participants included 52 children who met criteria for ADHD and were medication-naïve and 77 TD controls who did not meet criteria for ADHD. All of these participants completed eyes-open (EO) and eyes-closed (EC) data collection, but N=2 controls and N=2 ADHD participants had unusable EC data. Thus, EC analyses included 50 medication-naïve children in the ADHD group and 75 TD controls. Age and gender did not differ between groups for EO (age: t(127) = 0.25, p = .80, gender: X2(1,N = 129) = 2.04, p = .15) or EC (age: t(125) = 0.20, p = .84, gender: X2(1,N = 125) = 2.02, p = .16). See Table 1 for demographic variables.
Table 1.
Group Demographics
| Eyes-open data | Eyes-closed data | |||
|---|---|---|---|---|
| TD | ADHD | TD | ADHD | |
| Female | 42.8 (33) | 28.8 (15) | 41.6 (32) | 28.0 (14) |
| Dominant Hand (Right) | 90.9 (70) | 90.4 (47) | 92.2 (71) | 88.0 (44) |
| Race | ||||
| White | 62.3 (48) | 65.4 (34) | 62.3 (48) | 62.0 (31) |
| Black/African American | 14.3 (11) | 21.2 (11) | 16.9 (13) | 22.0 (11) |
| Asian | 6.5 (5) | 0 (0) | 6.5 (5) | 0 (0) |
| Other/Multiracial | 16.9 (13) | 13.5 (7) | 14.3 (11) | 16.0 (8) |
| Hispanic/Latino | 5.2 (4) | 15.4 (8) | 5.2 (4) | 20.0 (10) |
| Age (months) | 68.14 ± 14.83 | 67.48 ± 14.80 | 68.23 ± 14.78 | 67.70 ± 14.62 |
Note. Values are percentage of total group, with the raw number in parentheses; age is expressed as mean ± SD.
ADHD Diagnosis
ADHD diagnosis was determined at the study visit. Caregivers reported symptoms of ADHD on the Diagnostic Structured Interview Schedule – young child version (DISC-IV) (Shaffer et al., 2000), the Achenbach child behavior checklist (CBCL) (Achenbach, 1991b) and the SNAP-4. If children met diagnostic criteria on the DISC-IV (N=42) they were included in the ADHD group. There were an additional N=6 children who received a sub-threshold diagnosis on the DISC-IV and met clinical thresholds on the CBCL (t-score on the attention problems subscale above 70) or the SNAP-4 (caregiver indicated they had 6/9 inattention or hyperactivity symptoms ‘quite a bit’ or ‘very much’) and N=4 children, who met criteria on both the SNAP and CBCL. These children (N=10) were also included in the ADHD group. To be included in the TD group, children had to have 3 or fewer symptoms of inattention and hyperactivity on the SNAP-4. There were two ADHD participants that did not have EC data, but were still included in the EO group. There was one TD participant with EO data only and one TD participant with EC data only.
To corroborate diagnostic status, teacher report of symptoms was assessed in 41% of all participants (N=53) in the EO condition and 42% of all participants (N=53) in the EC condition using either the Achenbach Teacher Report Form (TRF) or the Conners-3 Teacher Rating Scale (Achenbach, 1991a; Conners, 2001). Teacher report was available on a minority of participants because teachers found it difficult to make time to complete the checklist despite being remunerated for their participation. See Tables 2 and 3 for ADHD symptoms on the DISC, CBCL, SNAP-4, TRF, and Conners-3 by group membership in the EO and EC conditions.
Table 2.
Distribution of ADHD, ODD, CD, and Internalizing Symptoms in the Eyes-open Sample
| Group differences | ||||
|---|---|---|---|---|
| TD | ADHD | t-value | p-value | |
| ADHD measures | ||||
| DISC ADHD symptoms (0–23) | 3.87 ± 3.94 (69) | 16.26 ± 4.20 (50) | −16.19 | <0.001* |
| CBCL ADHD subscale t-score | 51.28 ± 2.52 (75) | 65.49 ± 7.09 (51) | −13.74 | <0.001* |
| SNAP-IV total (0–20) | 1.77 ± 2.4 (74) | 11.72 ± 4.27 (50) | −14.97 | <0.001* |
| Conners – Teacher Form | ||||
| Inattentive t-score | 49.39 ± 10.47 (18) | 61.25 ± 11.77 (16) | −3.09 | 0.004* |
| Hyperactivity t-score | 56.19 ± 16.72 (16) | 74.69 ± 12.97 (16) | −3.50 | 0.002* |
| TRF ADHD subscale t-score | 53.00 ± 4.33 (12) | 58.85 ± 11.91 (6) | −1.16 | 0.29 |
| Additional externalizing disorders | ||||
| DISC ODD symptoms | 5.32 ± 3.4 (68) | 5.94 ± 2.84 (48) | −1.06 | 0.29 |
| DISC CD symptoms | 0.64 ± 1.32 (67) | 1.17 ± 1.76 (50) | −1.78 | 0.08 |
| Internalizing disorders | ||||
| CBCL internalizing subscale t-score | 45.78 ± 10.06 (74) | 55.67 ± 9.8 (51) | −5.48 | <0.001* |
| DISC GAD symptoms | 1.57 ± 1.72 (68) | 1.34 ± 2.02 (50) | 0.66 | 0.51 |
| DISC depression symptoms | 2.75 ± 2.4 (68) | 3.22 ± 2.77 (50) | −0.96 | 0.34 |
Note. Values are means ± SD, with the number of participants with scores for each measure in parentheses. As expected, the ADHD group had significantly more ADHD symptoms compared with the typically developing control group, with the exception of the Teacher Report Form (TRF), which was completed in a small number of total cases. There was a significant difference in internalizing subscale t-scores on the CBCL, and this measure was included in the regression models as a covariate. DISC, Diagnostic Structured Interview Schedule – young child version; CBCL, Child Behavior Checklist; SNAP-IV, Swanson Nolan and Pelham checklist; ODD, Oppositional Defiant Disorder; CD, Conduct Disorder; GAD, Generalized Anxiety Disorder.
p<0.05
Table 3.
Distribution of ADHD, ODD, CD, and Internalizing Symptoms in the Eyes-closed Sample
| Group differences | ||||
|---|---|---|---|---|
| TD | ADHD | t-value | p-value | |
| ADHD measures | ||||
| DISC ADHD symptoms (0–23) | 3.87 ± 3.94 (69) | 16.16 ± 4.19 (49) | −16.10 | <0.001* |
| CBCL ADHD subscale t-score | 51.19 ± 2.44 (75) | 65.61 ± 6.99 (49) | −13.90 | <0.001* |
| SNAP-IV total (0–20) | 1.77 ± 2.4 (74) | 11.58 ± 4.26 (48) | −14.55 | <0.001* |
| Conners – Teacher Form | ||||
| Inattentive t-score | 49.24 ± 10.77 (17) | 61.25 ± 11.77 (16) | −3.05 | 0.005 |
| Hyperactivity t-score | 56.93 ± 17.03 (16) | 74.69 ± 12.97 (16) | −3.25 | 0.003 |
| TRF ADHD subscale t-score | 52.77 ± 4.23 (13) | 58.83 ± 11.91 (6) | −1.21 | 0.27 |
| Additional externalizing disorders | ||||
| DISC ODD symptoms | 5.00 ± 3.14 (66) | 6.49 ± 3.08 (49) | −2.54 | 0.01* |
| DISC CD symptoms | 0.47 ± 1.26 (68) | 1.32 ± 1.67 (48) | −2.99 | 0.004* |
| Internalizing disorders | ||||
| CBCL internalizing subscale t-score | 45.76 ± 10.08 (74) | 55.49 ± 9.79 (49) | −5.34 | <0.001* |
| DISC GAD symptoms | 0.93 ± 1.23 (68) | 2.20 ± 2.25 (49) | −3.60 | <0.001* |
| DISC depression symptoms | 1.94 ± 2.3 (68) | 4.29 ± 2.27 (49) | −5.47 | <0.001* |
Note. Values are means ± SD, with the number of participants with scores for each measure in parentheses. As expected, the ADHD group had significantly more ADHD symptoms compared with the typically developing control group, with the exception of the Teacher Report Form (TRF), which was completed in a small number of total cases. There was a significant difference in internalizing subscale t-scores on the CBCL, as well as on measures of ODD, CD, GAD, and depression on the DISC. DISC, Diagnostic Structured Interview Schedule – young child version; CBCL, Child Behavior Checklist; SNAP-IV, Swanson Nolan and Pelham checklist; ODD, Oppositional Defiant Disorder; CD, Conduct Disorder; GAD, Generalized Anxiety Disorder.
p<0.05
Covariates
To control for potential confounds, covariates included measures of socioeconomic status (SES), age, and IQ. SES was measured via each family’s income to needs ratio, which was calculated based upon their reported income on the MacArthur Subjective Social Status questionnaire (Adler, Epel, Castellazzo, & Ickovics, 2000) and the poverty income threshold for their household. Age was measured in months of age at the time of participation. The Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) performance IQ (PIQ) composite was used as a measure of IQ (Wechsler, 2002). There were significant group differences for SES and PIQ for participants in the EO condition (for SES, t(117) = 2.11, p = .04; for PIQ, t(126)=2.10, p = .04). In addition, bivariate correlations revealed associations between age, PIQ, and SES with either inattentive or hyperactive symptoms (see Table 4). Further, given previous evidence that these factors, and SES in particular, can impact brain structure and function in such a way that elevates the risk for increased ADHD symptoms (Machlin et al., 2020), they were included in the regression models to ensure robustness of the findings.
Table 4.
Bivariate Correlations of Variables of Interest
| DISC-IN | DISC-HI | Age | PIQ | SES | |
|---|---|---|---|---|---|
| DISC-IN | -- | 0.72* | 0.05 | −0.22* | −0.22* |
| DISC-HI | -- | −0.28* | −0.12 | −0.36* | |
| Age | -- | −0.07 | 0.21* | ||
| PIQ | -- | 0.17 | |||
| SES |
Note. Results of bivariate correlations of variables of interest (inattentive symptoms and hyperactivity symptoms on the DISC-IV) and potential confounds (age, IQ, and SES, measured as income to needs ratio). All three potential confounds were significantly correlated with the at least one of the variables of interest, thus, they were included in the subsequent regression analyses. DISC-IN, DISC Inattentive Symptoms; DISC-HI, DISC Hyperactivity/Impulsivity Symptoms; PIQ, WPPSI-III performance IQ; SES, socioeconomic status.
p<0.05
Procedure
EEG Acquisition
Children completed an rsEEG recording. EEG data were collected for a total of seven minutes, split into three and a half minutes each for EO and EC conditions. The recordings were collected over seven intervals, during which the participants alternated between 30 seconds of EO data collection, 15 second breaks, and 30 seconds of EC data collection (Vuga et al., 2008). This approach, previously utilized by Vuga et al., was chosen due to the young age of the children participating in the study and to maximize the amount of data collected without artifact. During the EO condition, the participants were instructed to sit as still as possible while directing their attention toward a cartoon image of open eyes. During the EC condition, participants were instructed to sit quietly and calmly with their eyes closed. A research assistant accompanied each child for the duration of EEG data collection to maintain motivation and cooperation during data acquisition. In this paper we present data from both the EO and EC conditions.
EEG recordings were collected using a 128 HydroCel Sensor Net System (EGI, Inc, Eugene, OR). The net was comprised of an elastic tension structure forming a geodesic tessellation of the head surface. At each vertex is a sensor pedestal housing an Ag/AgCl- coated, carbon-filled plastic electrode and sponge. Prior to fitting the cap on the participant, the net was soaked in an electrolyte solution (6cc KCl/liter distilled water) in order to facilitate electrical contact between the scalp and the electrode. The child’s head was measured and marked in the center using a wax pencil to ensure proper placement across participants. Once placed over the scalp, impedance for each electrode was checked using NetStation software. To decrease impedance, electrodes were re-wet with the electrolyte solution and firmly placed in close contact with the scalp (moving aside hair). The process of attempting to decrease impedance continued until less than 10% of the 128 electrodes had impedances less than 50 mV. EEG data was acquired using NetAmps 200 Amplifiers and the NetStation software. The data were amplified, filtered (bandpass 0.1 to 100.0 Hz), and sampled at an effective rate of 250 Hz. They were digitized with a 12-bit National Instruments Board (National Instruments Corp., Woburn, MA).
EEG Preprocessing
Using NetStation, EEG data were re-referenced using an average reference that was applied after excluding the most radial channels, including those in close proximity to the eye, as these channels were most prone to artifact and were not used in subsequent analyses (Umilta’ et al., 2012). Using an average reference has been shown to yield low re-referencing errors when using high-density EEG data (Liu et al., 2015). Additional electrodes were excluded if they were outside of a +/− 80 mV range, which is a typical threshold for detecting eye blinks and captures the typical eye blink deflection range of 50–100 mV (Luck, 2014). Data segments were included if there were no artifacts outside of +/− 80 mV. Any potential remaining artifacts would be less distinct than eye blink artifacts that exceed that threshold, and thus it is better practice to leave these for removal during an Independent Component Analysis (Luck, 2014). For each participant, data was exported to Matlab (http://www.mathworks.com/products/matlab/) for further analysis. All subjects had 60–150 seconds of data in the EO condition (except for one ADHD participant with 30 seconds) and 30–210 seconds in the EC condition. Groups did not have different lengths of data in EO, t(74.12) = 1.82, p = .07 (ADHD group: M = 110.56, SD = 21.26; TD group: M = 116.49, SD = 12.30) or EC, t(125) = −1.09, p = .28 (ADHD group: M = 112.57, SD = 27.07; TD group: M = 107.50, SD = 24.62). The average amount of data exported for all participants in the EO condition was 114.10 seconds and 109.49 seconds in the EC condition.
Independent Component Analysis.
In order to further remove noise contributions to the signal, we conducted an independent component analysis (ICA). In Matlab, prior to conducting an ICA, we ran a high pass filter at 1 Hz, as this has been found to consistently improve results in terms of signal-to-noise ratio and classification accuracy (Winkler, Debener, Müller, & Tangermann, 2015). The ICA was conducted using the infomax ICA algorithm implemented in EEGLAB (Delorme & Makeig, 2004; Onton & Makeig, 2006).
In order to reduce highly correlated signal from nearby electrodes, we down-sampled to the 10–10 international electrode system, resulting in 71 electrode channels (Onton & Makeig, 2006). In order to inform decisions about which components are artifacts and which are neural components, we utilized the Multiple Artifact Rejection Algorithm (MARA), an open-source EEGLAB plug-in that automatizes the process of labeling components for artifact rejection (Winkler et al., 2011, 2014). A trained researcher (SF) reviewed the decisions made by MARA for all components that accounted for more than 1% of the variance. Due to the previous findings in children with ADHD across studies and within traditional power analyses in the current sample, the remaining analytic steps were conducted within the alpha frequency band, defined as 8–12 Hz in accordance with previous studies (de Munck et al., 2009; Gasser et al., 2003; Shackman et al., 2010).
Statistical Methods
After completion of data cleaning, data was exported from EEGLAB into FieldTrip (Oostenveld et al., 2011) for further analysis.
Weighted Phase Lag Index.
Functional connectivity measures the temporal synchronization between brain regions or signals of brain activity (Bartolomei et al., 2006; Lee, Harrison, & Mechelli, 2003). In order to account for volume conduction in EEG data, it is necessary to utilize a synchronization measure that does not rely on correlations or partial correlations (Stam et al., 2007). Weighted Phase Lag Index (WPLI) is one such measure of the synchronization of neural signal that addresses these problems when assessing functional connectivity with EEG (see Ortiz et al., 2012 for an application of WPLI; see Vinck, Oostenveld, van Wingerden, Battaglia, & Pennartz, 2011 for a detailed review).
WPLI measures the asymmetry in the distribution of the phase differences obtained from the instantaneous phases of the two time series, and critically, in WPLI the contribution of the observed phase leads and lags is weighted by the magnitude of the imaginary component of the cross-spectrum. WPLI was calculated as defined by Vinck and colleagues (see Vinck et al., 2011). Using FieldTrip, WPLI was calculated for each pair of electrodes for every participant, resulting in WPLI matrices (71 electrodes × 71 electrodes, with WPLI values in each cell) for all participants. Next, graph theoretical methods were applied to the matrices in order to analyze characteristics of the networks.
Graph Theoretical Metrics.
Using graph theoretical methods, the network of interest is divided into nodes and edges. In the current study the nodes were the electrodes and the edges were defined as the WPLI values between two electrodes. There are a multitude of measures to examine within graph theory; based on previous work, we focused on the following 3 measures: modularity, global efficiency, and local efficiency. We performed these analyses using the Brain Connectivity Toolbox (BCT) (Rubinov & Sporns, 2010).
Modularity.
Modularity is the ratio of the number of within-module connections to the number of expected within-module connections in a random network (Cohen & D’Esposito, 2016; Rubinov & Sporns, 2010; Sporns, 2013). The extent of modularity (measured from 0 to 1) can be described as a spectrum ranging from more integrated to more segregated (Sporns, 2013). Segregation refers to strong interconnectivity within modules, which results in efficient local processing. Integration refers to increased connections across modules, which represents global processing. Modularity was calculated using the BCT algorithm (see Rubinov & Sporns, 2010). We used the Louvain Method for community detection, which maximizes network modularity (De Meo et al., 2011). The structure (and thus modularity) of the network is determined by subdividing the network into groups of nodes, with a maximally possible number of within-group edges, and a minimally possible number of between-group edges (Rubinov & Sporns, 2010).
Global Efficiency.
In order to further evaluate differences in the integration of the neural networks of children with ADHD and TD children, we examined global efficiency, which measures the efficiency of information transfer among all pairs of nodes (electrodes in this case) in the graph, and thus reflects the efficiency of interaction across the whole graph (de Pasquale et al., 2016; Rubinov & Sporns, 2010). Global efficiency was also calculated using the BCT algorithm (see de Pasquale et al., 2016; Rubinov & Sporns, 2010).
Local Efficiency.
To further assess for and characterize differences in the segregation of neural networks of children with ADHD compared to TD children, we calculated local efficiency, which measures the efficiency of information transfer limited to neighboring nodes (i.e., nodes with a direct connection to the node of interest) (Cohen & D’Esposito, 2016; Rubinov & Sporns, 2010; Sporns, 2013). Local efficiency was calculated using the BCT algorithm (see Latora & Marchiori, 2001; Rubinov & Sporns, 2010).
Network Costs.
In network analyses it is appropriate to threshold the network in order to analyze a sparse network that most closely represents the density of connection of human and non-human nervous systems, by focusing on the strongest functional connections in the network (Achard & Bullmore, 2007). All analyses were conducted over a range of costs (10–30%, in 5% increments) to ensure that any results were not due to a specific threshold. The range of thresholds was chosen based upon the range of values that have been shown to produce graphs with small world characteristics (Bullmore & Bassett, 2011). Results reported in this paper are for matrices created with weighted thresholds (e.g. the edge values were maintained after each threshold was performed).
Statistical Testing.
We conducted independent 2-sample t-tests to test for group differences (TD vs. ADHD) for modularity, global, and local efficiency in each frequency band. Additionally, multiple linear regressions were conducted in order to test if graph metrics were associated with symptom severity (total inattentive symptoms and total hyperactive/impulsive symptoms), while controlling for age, SES, and PIQ. To probe the impact of development on network organization in children with ADHD as compared to TD children, we tested for age × graph metric interactions in separate regression models in which age and graph metrics (modularity, global efficiency, and local efficiency) were predictors of symptom severity (inattentive symptoms and hyperactivity symptoms). In addition, for models with significant associations of ADHD symptoms with network metrics, we control for internalizing symptoms to test the robustness of the correlates as a unique measure of ADHD in early childhood. All tests were conducted for both EO and EC data. For each significant finding, we report whether it remained significant after controlling for multiple comparisons using False Discovery Rate (FDR).
Results
To confirm that the data in the current study consisted of typical EEG power spectra, we plotted the average spectra, across all participants (Figure 1). This confirmed that the data were what would be expected from an rsEEG task, and that across all participants the peak power was in the alpha band, as has been found across populations (Grandy et al., 2013). Effect sizes are reported with findings as well (cohen’s d for t-tests, standardized beta values for regressions).
Figure 1. Average Power Spectra Across All Participants.
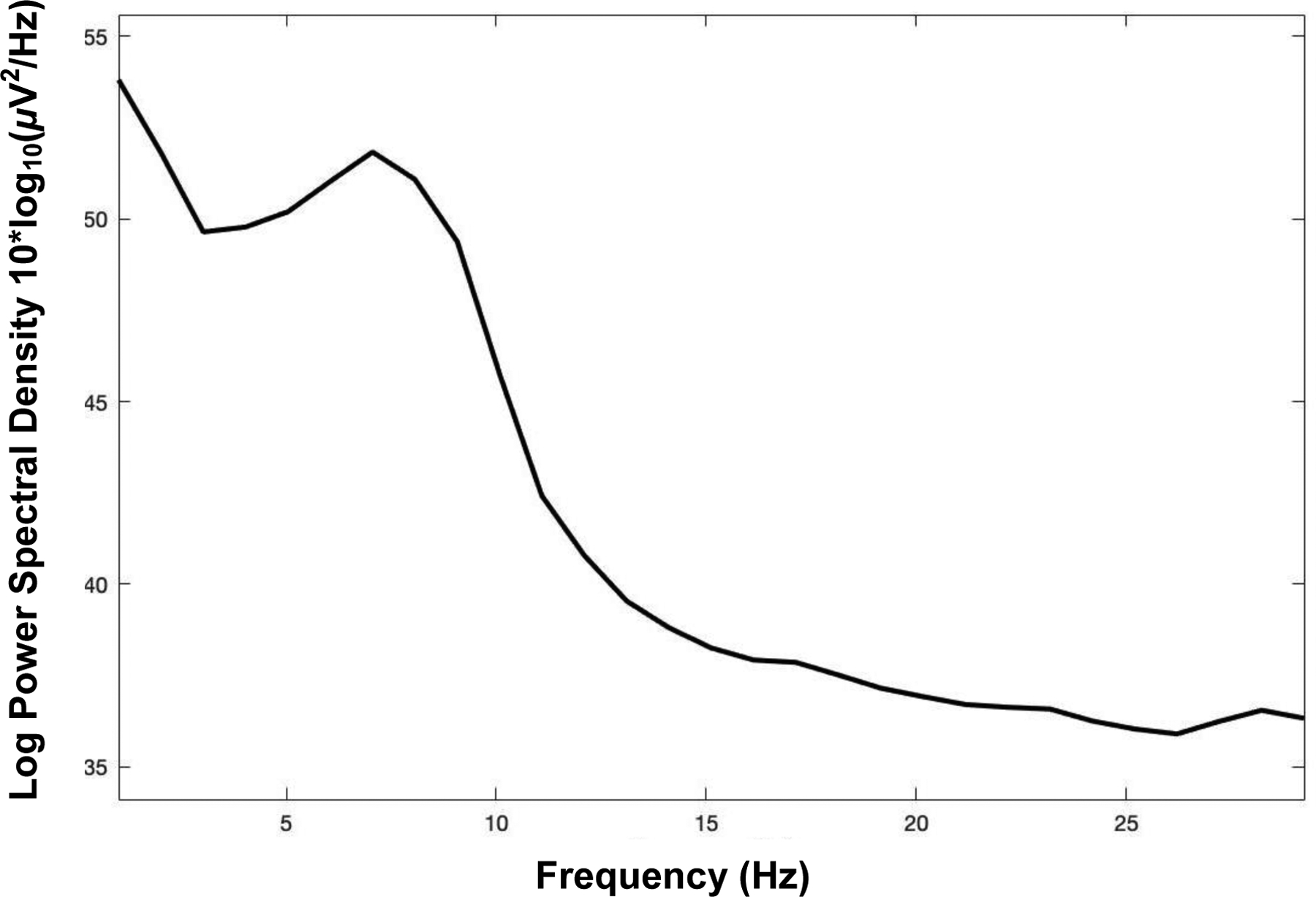
Note. The average power spectra, calculated across all participants to confirm that the data used in the current analysis include the expected peak in the alpha band.
Modularity
Group Differences
T-tests revealed that there were no significant differences in modularity between the ADHD group and the TD group when averaging across all network costs in alpha in EO, t(126)=0.74 , p=0.46, 95%CI [−0.01,0.03], d=0.13, or EC, t(125)=.46, p=0.65, 95%CI [−0.02,0.03], d=0.08.
ADHD Symptoms
Controlling for age, SES, and PIQ, modularity was not significantly associated with symptoms of inattention or hyperactivity when averaging across all network costs in alpha in EO (inattention: β=−0.06, p=0.52, hyperactivity: β=0.07, p=0.48) or EC (inattention: β=−0.02, p=0.85, hyperactivity: β=0.001, p=0.99).
Global Efficiency
Group Differences
There were no significant differences in global efficiency between the ADHD group and the TD group when averaging across all network costs in alpha in EO, t(90.53)=−1.77 , p=0.08, 95%CI [−0.02,0.001], d=0.33, or EC, t(122.33)=−1.57, p=0.12, 95%CI [−0.02,0.003], d=0.27.
ADHD Symptoms
Controlling for age, SES, and PIQ, multiple linear regression models revealed a significant association of increased global efficiency in alpha in EO with increased symptoms of inattention when averaging across all network costs (β=0.20, p=0.03) and at 3 out of 5 network costs (all p’s<0.05; see Figure 2). After FDR correction for multiple comparisons, this finding remained significant at the 3 network costs. There were no significant associations of global efficiency in EO with symptoms of hyperactivity and impulsivity when averaging across all network costs (β=0.13, p=0.14). Controlling for age, SES, and PIQ, multiple linear regression models revealed a significant association of increased global efficiency in alpha in EC with increased symptoms of inattention when averaging across all network costs (β=0.28, p=0.01) and at 4 out 5 network costs (all p’s<0.05, see Figure 3). After FDR correction, this finding remained significant at all 4 costs. There was also a significant association of global efficiency in EC with symptoms of hyperactivity and impulsivity when averaging across all network costs (β=0.22, p=0.04) and at 2 of 5 network costs (both p’s=0.03). However, this finding was no longer significant after FDR correction and findings at only 2 costs or less are often considered to be spurious.
Figure 2. Global Efficiency in Eyes-open Condition and Inattentive Symptoms at Individual Network Costs.
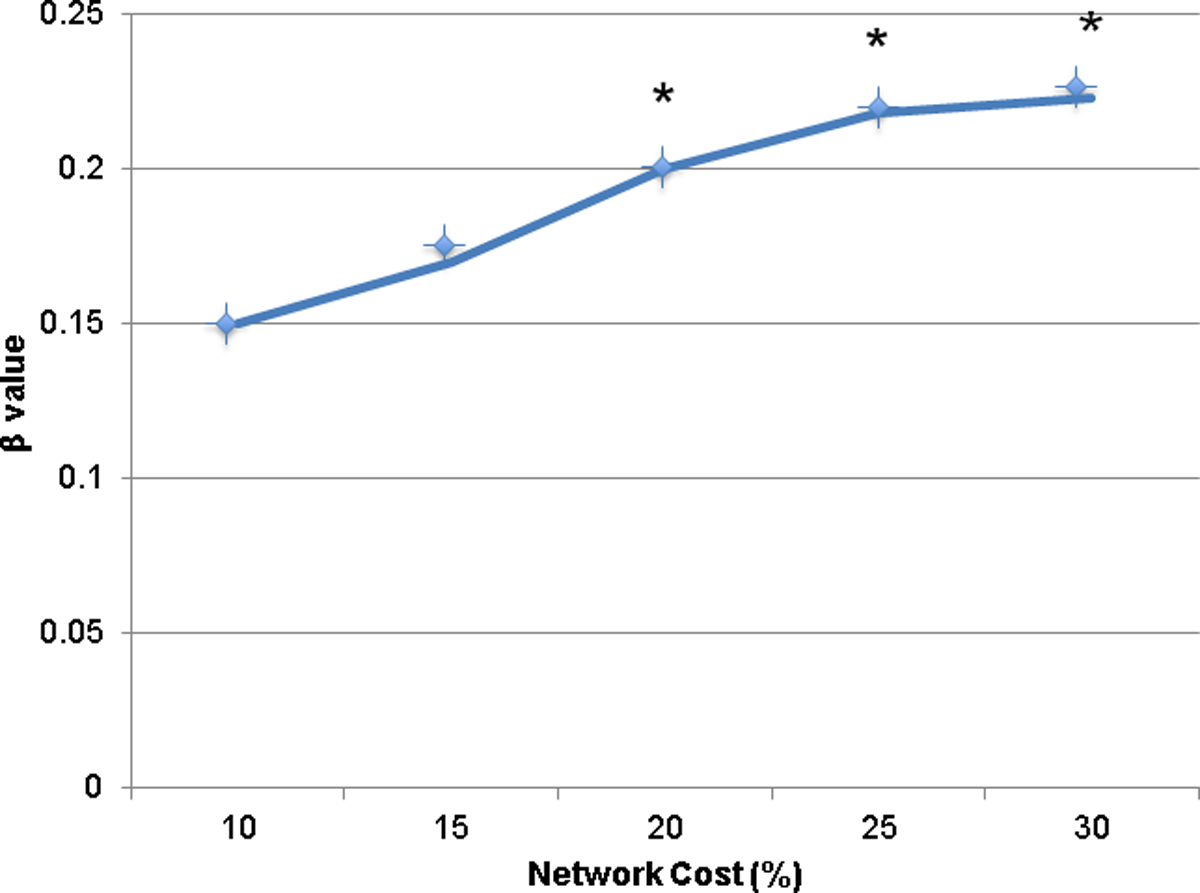
Note. A plot of the relationship of global efficiency in alpha in EO and inattention at each network cost. The x-axis has each network cost value, and the y-axis contains the β values of the relationship of global efficiency and inattentive symptoms. An * indicates a significant (p<0.05) association of global efficiency and inattentive symptoms.
Figure 3. Global Efficiency in Eyes-closed and Inattentive Symptoms at Individual Network Costs.

Note. A plot of the relationship of global efficiency in alpha in EC and inattentive symptoms at each network cost. The x-axis has each network cost value, and the y-axis contains β values of the relationship of global efficiency and inattentive symptoms. An * indicates a significant (p<0.05) association of global efficiency and inattentive symptoms.
Local Efficiency
Group Differences
T-tests revealed that there were no significant differences in average local efficiency between the ADHD group and the TD group when averaging across all network costs in alpha in EO, t(127)=0.16 , p=0.87, 95%CI [−0.01,0.01], d=0.03, or EC t(124.28)=−0.85, p=0.40, 95%CI [−0.03,0.01], d=0.14.
ADHD Symptoms
Controlling for age, SES, and PIQ, multiple linear regression modeling revealed that increased average local efficiency in alpha was not associated with increased symptoms of inattention in EO or EC when averaging across all network costs (EO: β=0.08, p=0.38; EC: β=0.18, p=0.11). In the EC condition, there was a significant association of increased local efficiency with increased inattentive symptoms at one network cost (β=0.23, p=0.04), but this was not significant after FDR correction. There were no significant associations of local efficiency with symptoms of hyperactivity and impulsivity when averaging across all network costs in EO (β=−0.01, p=0.93) or EC (β=0.12, p=0.29).
Interaction with Age
Further analyses were conducted to probe the impact of age on the results reported above. Since the significant results above were only present in the relationship of inattentive symptoms to global efficiency in EO and EC, we limited our further investigation of the impact of age to models that tested if an interaction of age with global efficiency in the alpha band in EO or EC predicted inattentive symptoms.
Multiple regression analysis was used to test if age interacted with average global efficiency to predict inattentive symptom severity. To test this, we used two models, both included age, SES, and PIQ as predictors of inattentive symptoms. One of these models additionally included global efficiency in EO and the interaction of global efficiency in EO and age, the other additionally included global efficiency in EC and the interaction of global efficiency and age. In these models, neither the interaction of age and global efficiency in EO, nor the interaction of age and global efficiency in EC significantly predicted inattentive symptoms (EO global efficiency × age: β=−0.69, p=0.28; EC global efficiency × age: β=−0.02, p=0.98).
Tests of Robustness
Externalizing Symptoms
Due to the high comorbidity of ADHD with oppositional defiant disorder (ODD) and conduct disorder (CD) (Lee et al., 2008; Murphy & Barkley, 1996), we tested the robustness of the findings as a neural correlate that is specific to ADHD symptoms. To do so, additional regression models included symptoms of ODD and CD (measured on the DISC-IV) as covariates. Importantly, these additional tests function as an initial step to test the specificity of the neural correlates identified in the current study. These additional models were tested with inattentive symptoms as an outcome since there was no relationship of global or local efficiency with hyperactivity symptoms. The association between increased inattentive symptoms and increased global efficiency in EC remained significant when averaging across network costs (β=0.20, p=0.03) and at all 5 network costs (all p’s<0.05). These findings remained significant after FDR correction. The results in EO were more equivocal, the association between increased inattentive symptoms and increased global efficiency in alpha in EO was significant at 1 out of 5 network costs (p<.05) but not when averaging across network costs (β=0.16, p=0.07). This finding did not remain significant after FDR correction.
Internalizing Symptoms
We also tested the robustness of the findings when internalizing symptoms (measured on the CBCL) were included in regression models as a covariate. Critically, these additional models provide further testing of the specificity of the neural correlates identified in the current study. These additional models were tested with inattentive symptoms as an outcome since there was no relationship of global or local efficiency with hyperactivity symptoms. The association between increased inattentive symptoms and increased global efficiency in alpha in EO was not significant when averaging across network costs (β=0.16, p=0.05), but remained significant at 2 out of 5 network costs (p’s=0.03, 0.04). This finding did not remain significant after FDR correction. However, the association between increased inattentive symptoms and increased global efficiency in EC remained significant when averaging across network costs (β=0.32, p=0.002) and at all 5 network costs (all p’s<0.01).
Motion Confounds
Given that a symptom of ADHD is hyperactivity, we sought to perform follow-up analyses to confirm that our results were unlikely to be caused by motion. As the main priority of the ICA analysis is to remove signal generated from motion (i.e., eye blinks, facial muscle movements) and retain neural signal, the number of components removed serves as a measure of the amount of motion artifact for each participant. Thus, to determine if the reported findings were related to motion, we compared the number of components removed during ICA between groups and in association with symptoms of ADHD.
There was no difference in the number of components removed between groups in the EO condition (TD group: M=42.84, SD=10.17; ADHD group: M=42.71, SD=9.85; t(127)=0.07, p=0.94) or the EC condition (TD group: M=40.45, SD=13.02; ADHD group: M=37.46, SD=10.53; t(125)=1.36, p = .18). The total number of ADHD symptoms was not associated with the number of components removed in EO (r=0.10, p=0.35) or EC (r=−0.14, p=0.17). Similarly, inattentive symptoms alone (EO: r=0.28, p=0.28; EC: r=−0.15, p=0.16) and symptoms of hyperactivity and impulsivity alone (EO: r=0.07, p=0.53; EC: r=−0.12, p=0.25) were not associated with the number of components removed during ICA analysis. The results of this analysis are consistent with the idea that motion artifact does not account for our observations.
Discussion
Here we demonstrate, using rsEEG and a graph theoretical framework, that in early childhood, increased ADHD symptoms are associated with increased global efficiency in the alpha band in eyes-open and eyes-closed resting-state EEG. Specifically, global network efficiency in alpha in EO and EC was associated with symptoms of inattention, but not hyperactivity, across all participants (although at two network costs prior to FDR correction there was a significant association of global efficiency in EC with hyperactivity symptoms, this was not significant after FDR and findings at only 2 network costs are often considered to be spurious). Activity in the alpha band has previously been associated with inhibitory control and information processing (Klimesch, 2012), both of which are functions that are impaired in individuals with ADHD. These findings are consistent with prior work that has found that children ages 8–13 years with ADHD had excess alpha power compared to TD controls (Barry et al., 2011). Additionally, across populations, the peak power is within the alpha band (Grandy et al., 2013), which thus might lend itself to supporting the identification of subtle and nuanced differences in brain activity. Given the importance of identifying neural correlates of ADHD in early childhood, and the paucity of research in this age range, this work represents an important first step in understanding the neural underpinnings and markers of ADHD symptomatology in this critical developmental time period.
Modularity
We initially explored modularity, as previous findings provided evidence for increased modularity (increased neural network segregation) in older children and adults with ADHD when compared to controls (Henry & Cohen, 2019). However, in our sample of younger children, we observed no differences in modularity between groups, and no association of modularity with symptoms of ADHD. While this differs from previous findings in older children and adults with ADHD, no study to date has examined modularity in individuals with ADHD in this age range, a dynamic period of brain development. It is possible that the neurodevelopmental trajectory of children with ADHD begins with typical levels of modularity early in childhood, but later on in development children with ADHD display increased modularity. This possibility is consistent with patterns of network development in TD populations where modularity increases from early childhood through adulthood (Chen & Deem, 2014).
Global and Local Efficiency
Our findings show that increased global efficiency was significantly associated with increased inattentive symptoms. Interestingly, there was no significant association of global efficiency or local efficiency with hyperactivity symptoms. Moreover, additional models tested the robustness of these findings and indicated that the relationship between symptoms of inattention and global efficiency in alpha is particular to ADHD as opposed to being indicative of broader psychopathology, was not due to data loss in the ADHD group, or to increased movement for children with versus without ADHD. Increased global efficiency in atypical populations has been suggested to reflect overactive functional integration, such that it might be disruptive to information transfer across the brain, hindering complex cognitive functions (Ma et al., 2018; Zhang et al., 2015). In sum, previous literature has suggested that it is possible to have levels of global efficiency that are too elevated to be helpful, and are in fact harmful, for cognitive and behavioral functioning.
This interpretation could be consistent with our observation that global efficiency was associated with disruption of inattentive symptoms specifically. Alternately, the specificity of associations between global efficiency and inattentive symptoms could have to do with overall severity. Given the young age of our sample, and that inattentive symptoms are less noticeable and more difficult to identify early in childhood, it may be that reports of increased inattentive symptoms in early childhood are indicative of increased overall severity of ADHD symptoms and impairment. Therefore, increased global efficiency might be a marker of increased severity of ADHD, rather than of inattention in particular. Future studies should further probe this question in this early age range.
Previous studies reported that older children and adults with ADHD had decreased global efficiency, increased modularity, and increased local efficiency in comparison to TD controls of the same age (Beare et al., 2017; Wang et al., 2009). The interpretation of these findings was that the symptoms of ADHD, and the associated decreased levels of global efficiency, were related to a loss of long-range communication due to the presence of structural abnormalities in individuals with ADHD (Qiu et al., 2011; Wang et al., 2009). Increased local efficiency has been associated with ADHD diagnosis in older children, and has been interpreted as an indication of increased tolerance to network disruption, such that loss of a subset of nodes or connections within a subnetwork does not cause as much harm in a network with increased local efficiency (Latora & Marchiori, 2001; Wang et al., 2009). Taken with their finding of decreased global efficiency in children with ADHD, Wang et al. (2009) interpreted increased local efficiency in individuals with ADHD to be a response to the impairment caused by decreased global efficiency. Overall findings of increased segregation and decreased integration in previous research with older children, adolescents, and adults with ADHD have been suggested to be evidence of a delayed maturational trajectory of neurodevelopment in individuals with ADHD compared to controls (Beare et al., 2017; Henry & Cohen, 2019; Lin et al., 2014); however, these interpretations have been developed without empirical data about neural network organization in individuals with ADHD in early childhood.
Given the large quantity of neural and cognitive development and maturation that occurs between early childhood and adolescence, the difference between previous findings and the results of the current study might be attributable to maturational changes; albeit following a pattern of development that is not only delayed as was thought based upon the previous literature, but different from typical neurodevelopment. Viewing the current work in conjunction with previous literature suggests that children with ADHD follow a neurodevelopmental trajectory such that early in childhood the neural function of children with ADHD is characterized by increased global efficiency compared to TD controls, with the levels of global efficiency possibly spurring the development of increased local efficiency, and then dropping to less than the level of global efficiency of TD controls later in development. It may be that an over-proliferation of long-range connections in early childhood actually drives subsequent heightened pruning of these connections in response. This maturational pattern would be further reflected in only observing modularity differences in middle childhood and adolescence, described above. Future longitudinal studies of the development of neural connections in children with ADHD throughout developmental stages are necessary to probe this possibility further. It should be noted that previous work with older children with ADHD where differences in both modularity and efficiency were observed has been primarily conducted with fMRI, and thus the differences in modalities is an additional confounding factor in understanding the differences in the present findings with younger children compared to those with older children.
Additional analyses tested if the associations of global efficiency with inattentive symptoms were robust to controls for internalizing symptoms and externalizing symptoms (ODD and CD symptoms). The association of global efficiency and inattentive symptoms remained significant in the EC condition. It is possible that we observe more robust associations between global efficiency and inattentive symptoms for EC vs. EO because we were examining global efficiency in alpha and alpha power is a more prominent contributor to signal in EC (Kirschfeld, 2005). This increase in overall alpha power may function to increase signal to noise ratio, allowing observed differences in connectivity to be more clear in EC compared to EO. The association of global efficiency in alpha measured during EC rsEEG with increased inattentive symptoms appears to be robust to internalizing symptoms and symptoms of ODD and CD, suggesting that global efficiency in alpha during EC rsEEG might be particularly helpful in further studies of early neural markers of ADHD symptoms.
While we did not observe that any network measure predicted symptoms of hyperactivity, we observed, consistent with existing literature (Bresnahan et al., 1999; Brown & Borden, 1986), that increased age predicted decreased hyperactivity symptoms. Further, while previous findings have shown a relationship between head movement and ADHD symptoms, and with hyperactivity symptoms in particular (Couvy-Duchesne et al., 2016), there was no difference in motion between groups in the current study according to our available metrics. The finding that the network metrics of interest were unrelated to age and that there was no difference in movement between groups highlight the likelihood that our observations of network differences between groups and in association with symptoms of inattention are not due to motion confounds.
Limitations
There are several limitations to be considered in this study. First, due to the young age of the children, the amount of data collected was fairly short in comparison to other studies with older populations. Additionally, no metrics of cognitive function (working memory, task-switching, etc.) were measured in this study, thus we cannot relate our resting state network measures to indices of cognitive ability. Further, given that the network structure differences between children with and without ADHD were dissimilar to what has been observed in middle childhood and adolescence, future longitudinal studies are necessary to understand how this network structure changes from early childhood through adolescence. Finally, this work represents an initial step to identify neural correlates of ADHD in early childhood. In future research, these findings could be explored in terms of their utility for improving diagnostic accuracy of ADHD in early childhood. As a future direction, the children in this study are being followed longitudinally to assess the potential of these network organization differences to predict long-term diagnostic status.
Conclusions
Crucially, the present findings provide evidence for a neural correlate of ADHD symptom severity in early childhood. One neural network metric, global efficiency, was related to symptoms of inattention in early childhood. Given the relatively low cost of EEG equipment in comparison to other neuroimaging modalities, the relative ease of data collection with young children, and the potential for automated analyses, there is potential that network measures such as these could ultimately be used as complimentary metrics to aid existing diagnostic tools to improve diagnostic accuracy of ADHD.
In contrast to previous theoretical interpretations (Wang et al., 2009), the present study provides evidence that in early childhood, ADHD is characterized by increased global efficiency. More longitudinal studies are needed to explore the shift that occurs in development leading to older children with ADHD to present with decreased global efficiency and increased local efficiency. The differences between the present findings with younger children in comparison to studies of older children with ADHD suggest that the neural correlates of ADHD are not permanent during development, and thus highlight the potential of early intervention to shape these developmental trajectories.
Acknowledgments
This research was supported by the National Institutes of Health (K01-MH092555 and R03-DA037405 to Sheridan). These funders and the author’s employers (UNC-CH and BCH) provided support for all data collection and analysis. We thank the caregivers and children who participated in this project.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Achard S, & Bullmore E (2007). Efficiency and Cost of Economical Brain Functional Networks. PLoS Computational Biology, 3(2), e17. 10.1371/journal.pcbi.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM (1991a). Teacher report form. Burlington, VT: Department of Psychiatry, University of Vermont. [Google Scholar]
- Achenbach Thomas M. (1991b). Manual for the Child Behavior Checklist/4–18 and 1991 profile. Department of Psychiatry, University of Vermont Burlington, VT. [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, & Ickovics JR (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women. Health Psychology, 19(6), 586–592. [DOI] [PubMed] [Google Scholar]
- Ahmadlou M, & Adeli H (2010). Wavelet-synchronization methodology: A new approach for EEG-based diagnosis of ADHD. Clinical EEG and Neuroscience, 41(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Alba G, Pereda E, Mañas S, Méndez LD, Duque MR, González A, & González JJ (2016). The variability of EEG functional connectivity of young ADHD subjects in different resting states. Clinical Neurophysiology, 127(2), 1321–1330. 10.1016/j.clinph.2015.09.134 [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, & Erkanli A (1999). Comorbidity. Journal of Child Psychology and Psychiatry, 40(1), 57–87. 10.1111/1469-7610.00424 [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, Dupuy FE, McCarthy R, & Selikowitz M (2011). EEG coherence and symptom profiles of children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology, 122(7), 1327–1332. 10.1016/j.clinph.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, & Johnstone SJ (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology, 114(2), 171–183. 10.1016/S1388-2457(02)00362-0 [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, & Selikowitz M (2002). EEG coherence in attention-deficit/hyperactivity disorder: A comparative study of two DSM-IV types. Clinical Neurophysiology, 113(4), 579–585. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Bosma I, Klein M, Baayen JC, Reijneveld JC, Postma TJ, Heimans JJ, van Dijk BW, de Munck JC, de Jongh A, Cover KS, & Stam CJ (2006). Disturbed functional connectivity in brain tumour patients: Evaluation by graph analysis of synchronization matrices. Clinical Neurophysiology, 117(9), 2039–2049. 10.1016/j.clinph.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, Kahn AE, Vandekar SN, Rupert PE, Quarmley M, Cook PA, Elliott MA, Ruparel K, Gur RE, Gur RC, Bassett DS, & Satterthwaite TD (2017). Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Current Biology: CB, 27(11), 1561–1572.e8. 10.1016/j.cub.2017.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare R, Adamson C, Bellgrove MA, Vilgis V, Vance A, Seal ML, & Silk TJ (2017). Altered structural connectivity in ADHD: A network based analysis. Brain Imaging and Behavior, 11(3), 846–858. 10.1007/s11682-016-9559-9 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Hinshaw SP, & Pang KL (2010). Comorbidity of Attention-Deficit/Hyperactivity Disorder and Early-Onset Conduct Disorder: Biological, Environmental, and Developmental Mechanisms. Clinical Psychology: Science and Practice, 17(4), 327–336. 10.1111/j.1468-2850.2010.01224.x [DOI] [Google Scholar]
- Bowyer SM (2016). Coherence a measure of the brain networks: Past and present. Neuropsychiatric Electrophysiology, 2, 1. 10.1186/s40810-015-0015-7 [DOI] [Google Scholar]
- Bresnahan SM, Anderson JW, & Barry RJ (1999). Age-related changes in quantitative EEG in attention- deficit/hyperactivity disorder. Biological Psychiatry, 46(12), 1690–1697. 10.1016/S0006-3223(99)00042-6 [DOI] [PubMed] [Google Scholar]
- Brown RT, & Borden KA (1986). Hyperactivity at Adolescence: Some Misconceptions and New Directions. Journal of Clinical Child Psychology, 15(3), 194–209. 10.1207/s15374424jccp1503_1 [DOI] [Google Scholar]
- Bullmore ET, & Bassett DS (2011). Brain Graphs: Graphical Models of the Human Brain Connectome. Annual Review of Clinical Psychology, 7(1), 113–140. 10.1146/annurev-clinpsy-040510-143934 [DOI] [PubMed] [Google Scholar]
- Campbell SB, & Ewing LJ (1990). Follow-up of hard-to-manage preschoolers: Adjustment at age 9 and predictors of continuing symptoms. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 31(6), 871–889. [DOI] [PubMed] [Google Scholar]
- Cao Q, Shu N, An L, Wang P, Sun L, Xia M-R, Wang J-H, Gong G-L, Zang Y-F, Wang Y-F, & He Y (2013). Probabilistic Diffusion Tractography and Graph Theory Analysis Reveal Abnormal White Matter Structural Connectivity Networks in Drug-Naive Boys with Attention Deficit/Hyperactivity Disorder. Journal of Neuroscience, 33(26), 10676–10687. 10.1523/JNEUROSCI.4793-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, & Proal E (2012). Large-Scale Brain Systems in ADHD: Beyond the Prefrontal-Striatal Model. Trends in Cognitive Sciences, 16(1), 17–26. 10.1016/j.tics.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, & Deem MW (2014). Development of Modularity in the Neural Activity of Children’s Brains. Biophysical Journal, 106(2), 795a. 10.1016/j.bpj.2013.11.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Johnstone SJ, Hsu C-I, Magee CA, Lawrence CA, & Croft RJ (2007). Coherence in children with Attention-Deficit/Hyperactivity Disorder and excess beta activity in their EEG. Clinical Neurophysiology, 118(7), 1472–1479. 10.1016/j.clinph.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Cohen JR, & D’Esposito M (2016). The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. Journal of Neuroscience, 36(48), 12083–12094. 10.1523/JNEUROSCI.2965-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C (2001). Conners Rating Scales-Revised. Multihealth Systems. [Google Scholar]
- Connor DF, Edwards G, Fletcher KE, Baird J, Barkley RA, & Steingard RJ (2003). Correlates of Comorbid Psychopathology in Children With ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 42(2), 193–200. 10.1097/00004583-200302000-00013 [DOI] [PubMed] [Google Scholar]
- Cosgrove VE, Rhee SH, Gelhorn HL, Boeldt D, Corley RC, Ehringer MA, Young SE, & Hewitt JK (2011). Structure and Etiology of Co-occurring Internalizing and Externalizing Disorders in Adolescents. Journal of Abnormal Child Psychology, 39(1), 109–123. 10.1007/s10802-010-9444-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvy-Duchesne B, Ebejer JL, Gillespie NA, Duffy DL, Hickie IB, Thompson PM, Martin NG, de Zubicaray GI, McMahon KL, Medland SE, & Wright MJ (2016). Head Motion and Inattention/Hyperactivity Share Common Genetic Influences: Implications for fMRI Studies of ADHD. PLoS ONE, 11(1). 10.1371/journal.pone.0146271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson ML, Visser SN, Gleason MM, Peacock G, Claussen AH, & Blumberg SJ (2017). A national profile of attention-deficit hyperactivity disorder diagnosis and treatment among US children aged 2 to 5 years. Journal of Developmental and Behavioral Pediatrics: JDBP, 38(7), 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meo P, Ferrara E, Fiumara G, & Provetti A (2011). Generalized Louvain Method for Community Detection in Large Networks. ArXiv:1108.1502 [Physics], 88–93. 10.1109/ISDA.2011.6121636 [DOI] [Google Scholar]
- de Munck JC, Gonçalves SI, Mammoliti R, Heethaar RM, & Lopes da Silva FH (2009). Interactions between different EEG frequency bands and their effect on alpha–fMRI correlations. NeuroImage, 47(1), 69–76. 10.1016/j.neuroimage.2009.04.029 [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Sporns O, Romani GL, & Corbetta M (2016). A Dynamic Core Network and Global Efficiency in the Resting Human Brain. Cerebral Cortex, 26(10), 4015–4033. 10.1093/cercor/bhv185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, Schneider MR, Sims C, Pope K, Fowler K, Sinclair S, Tovar-Moll F, Pine D, & Blair RJ (2012). Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research: Neuroimaging, 202(3), 239–244. 10.1016/j.pscychresns.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, & Breakspear M (2015). The connectomics of brain disorders. Nature Reviews Neuroscience, 16(3), 159–172. 10.1038/nrn3901 [DOI] [PubMed] [Google Scholar]
- Furlong S, Robertson MM, Snyder J, Kehm K, Way M, Goines K, Machlin L, & Sheridan MA (2020). Neural correlates of ADHD and exposure to adversity: A resting state EEG study [Manuscript submitted for publication]. Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill. [Google Scholar]
- Gasser T, Rousson V, & Gasser US (2003). EEG power and coherence in children with educational problems. Journal of Clinical Neurophysiology, 20(4), 273–282. [DOI] [PubMed] [Google Scholar]
- Grandy TH, Werkle-Bergner M, Chicherio C, Schmiedek F, Lövdén M, & Lindenberger U (2013). Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults: Alpha stability. Psychophysiology, 50(6), 570–582. 10.1111/psyp.12043 [DOI] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Dang J, Hanada G, Macion J, McCracken JT, McGough JJ, & Loo SK (2010). ADHD familial loading and abnormal EEG alpha asymmetry in children with ADHD. Journal of Psychiatric Research, 44(9), 605–615. 10.1016/j.jpsychires.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Hanada G, Macion J, McCracken JT, McGough JJ, & Loo SK (2009). Atypical alpha asymmetry in adults with ADHD. Neuropsychologia, 47(10), 2082–2088. 10.1016/j.neuropsychologia.2009.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T,R, & Cohen J,R (2019). Dysfunctional brain network organization in neurodevelopmental disorders. In Connectomics: Applications to Neuroimaging (pp. 83–100). Academic Press. 10.1016/B978-0-12-813838-0.00005-4 [DOI] [Google Scholar]
- Hinton KE, Lahey BB, Villalta-Gil V, Meyer FAC, Burgess LL, Chodes LK, Applegate B, Van Hulle CA, Landman BA, & Zald DH (2019). White matter microstructure correlates of general and specific second-order factors of psychopathology. NeuroImage: Clinical, 22, 101705. 10.1016/j.nicl.2019.101705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Nolan ZT, White SF, Williams WC, Sinclair S, & Blair RJR (2016). Dual neurocircuitry dysfunctions in disruptive behavior disorders: Emotional responding and response inhibition. Psychological Medicine, 46(07), 1485–1496. 10.1017/S0033291716000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschfeld K (2005). The physical basis of alpha waves in the electroencephalogram and the origin of the “Berger effect.” Biological Cybernetics, 92(3), 177–185. 10.1007/s00422-005-0547-1 [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S, Lauer P, Schreppel T, Jacob C, Heine M, Boreatti-Hümmer A, Fallgatter AJ, & Herrmann MJ (2009). Increased EEG power density in alpha and theta bands in adult ADHD patients. Journal of Neural Transmission, 116(1), 97–104. 10.1007/s00702-008-0157-x [DOI] [PubMed] [Google Scholar]
- Konrad K, & Eickhoff SB (2010). Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping, 31(6), 904–916. 10.1002/hbm.21058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, & Marchiori M (2001). Efficient Behavior of Small-World Networks. Physical Review Letters, 87(19). 10.1103/PhysRevLett.87.198701 [DOI] [PubMed] [Google Scholar]
- Law EC, Sideridis GD, Prock LA, & Sheridan MA (2014). Attention-deficit/hyperactivity disorder in young children: Predictors of diagnostic stability. Pediatrics, 133(4), 659–667. 10.1542/peds.2013-3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Harrison LM, & Mechelli A (2003). A report of the functional connectivity workshop, Dusseldorf 2002. NeuroImage, 19(2 Pt 1), 457–465. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Owens EB, & Hinshaw SP (2008). Few preschool boys and girls with ADHD are well-adjusted during adolescence. Journal of Abnormal Child Psychology, 36(3), 373–383. 10.1007/s10802-007-9184-6 [DOI] [PubMed] [Google Scholar]
- Lin P, Sun J, Yu G, Wu Y, Yang Y, Liang M, & Liu X (2014). Global and local brain network reorganization in attention-deficit/hyperactivity disorder. Brain Imaging and Behavior, 8(4), 558–569. 10.1007/s11682-013-9279-3 [DOI] [PubMed] [Google Scholar]
- Liston C, Cohen MM, Teslovich T, Levenson D, & Casey BJ (2011). Atypical Prefrontal Connectivity in Attention-Deficit/Hyperactivity Disorder: Pathway to Disease or Pathological End Point? Biological Psychiatry, 69(12), 1168–1177. 10.1016/j.biopsych.2011.03.022 [DOI] [PubMed] [Google Scholar]
- Liu Q, Balsters JH, Baechinger M, van der Groen O, Wenderoth N, & Mantini D (2015). Estimating a neutral reference for electroencephalographic recordings: The importance of using a high-density montage and a realistic head model. Journal of Neural Engineering, 12(5). 10.1088/1741-2560/12/5/056012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe IM, & Feldman HM (2007). Academic and Educational Outcomes of Children With ADHD. Journal of Pediatric Psychology, 32(6), 643–654. 10.1093/jpepsy/jsl054 [DOI] [PubMed] [Google Scholar]
- Luck SJ (2014). Chapter 6: Artifact Rejection and Correction. In Introduction to the Event-Related Potential Technique (2nd ed., p. 417). MIT Press. [Google Scholar]
- Ma X, Jiang G, Fu S, Fang J, Wu Y, Liu M, Xu G, & Wang T (2018). Enhanced Network Efficiency of Functional Brain Networks in Primary Insomnia Patients. Frontiers in Psychiatry, 9. 10.3389/fpsyt.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin L, McLaughlin KA, & Sheridan MA (2020). Brain structure mediates the association between socioeconomic status and ADHD status. Developmental Science, e12844. 10.1111/desc.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, & Luna B (2015). The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLOS Biology, 13(12), e1002328. 10.1371/journal.pbio.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee R, Partridge F, Williams S, & Silva PA (1991). A twelve-year follow-up of preschool hyperactive children. Journal of the American Academy of Child and Adolescent Psychiatry, 30(2), 224–232. 10.1097/00004583-199103000-00010 [DOI] [PubMed] [Google Scholar]
- Meunier D, Lambiotte R, & Bullmore ET (2010). Modular and Hierarchically Modular Organization of Brain Networks. Frontiers in Neuroscience, 4. 10.3389/fnins.2010.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moadab I, Gilbert T, Dishion TJ, & Tucker DM (2010). Frontolimbic activity in a frustrating task: Covariation between patterns of coping and individual differences in externalizing and internalizing symptoms. Development and Psychopathology, 22(2), 391–404. 10.1017/S0954579410000131 [DOI] [PubMed] [Google Scholar]
- Murphy K, & Barkley RA (1996). Attention deficit hyperactivity disorder adults: Comorbidities and adaptive impairments. Comprehensive Psychiatry, 37(6), 393–401. [DOI] [PubMed] [Google Scholar]
- Onton J, & Makeig S (2006). Information-based modeling of event-related brain dynamics. Progress in Brain Research, 159, 99–120. 10.1016/S0079-6123(06)59007-7 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, & Schoffelen J-M (2011). FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data [Research article]. Computational Intelligence and Neuroscience. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz E, Stingl K, Münßinger J, Braun C, Preissl H, & Belardinelli P (2012). Weighted Phase Lag Index and Graph Analysis: Preliminary Investigation of Functional Connectivity during Resting State in Children. Computational and Mathematical Methods in Medicine, 2012. 10.1155/2012/186353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Sporns O (2015). Brain Networks and Cognitive Architectures. Neuron, 88(1), 207–219. 10.1016/j.neuron.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, & Rohde LA (2014). ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. 10.1093/ije/dyt261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Ye Z, Li Q, Liu G, Xie B, & Wang J (2011). Changes of Brain Structure and Function in ADHD Children. Brain Topography, 24(3–4), 243–252. 10.1007/s10548-010-0168-4 [DOI] [PubMed] [Google Scholar]
- Robbie JC, Clarke AR, Barry RJ, Dupuy FE, McCarthy R, & Selikowitz M (2016). Coherence in children with AD/HD and excess alpha power in their EEG. Clinical Neurophysiology, 127(5), 2161–2166. 10.1016/j.clinph.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Robertson MM, Furlong S, Voytek B, Donoghue T, Boettiger CA, & Sheridan MA (2019). EEG power spectral slope differs by ADHD status and stimulant medication exposure in early childhood. Journal of Neurophysiology, 122(6), 2427–2437. 10.1152/jn.00388.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Schatz DB, & Rostain AL (2006). ADHD With Comorbid Anxiety: A Review of the Current Literature. Journal of Attention Disorders, 10(2), 141–149. 10.1177/1087054706286698 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, & Davidson RJ (2010). Identifying robust and sensitive frequency bands for interrogating neural oscillations. NeuroImage, 51(4), 1319–1333. 10.1016/j.neuroimage.2010.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 28–38. 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Smidts DP, & Oosterlaan J (2007). How Common are Symptoms of ADHD in Typically Developing Preschoolers? A Study on Prevalence Rates and Prenatal/Demographic Risk Factors. Cortex, 43(6), 710–717. 10.1016/S0010-9452(08)70500-8 [DOI] [PubMed] [Google Scholar]
- Sporns O (2013). Network attributes for segregation and integration in the human brain. Current Opinion in Neurobiology, 23(2), 162–171. 10.1016/j.conb.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Sporns O, & Betzel RF (2016). Modular Brain Networks. Annual Review of Psychology, 67(1), 613–640. 10.1146/annurev-psych-122414-033634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Nolte G, & Daffertshofer A (2007). Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Human Brain Mapping, 28(11), 1178–1193. 10.1002/hbm.20346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM (1992) School-based assessments and interventions for ADD students. K. C. Publishing. [Google Scholar]
- Tandon M, Si X, & Luby J (2011). Preschool Onset Attention-Deficit/Hyperactivity Disorder: Course and Predictors of Stability over 24 Months. Journal of Child and Adolescent Psychopharmacology; New Rochelle, 21(4), 321–330. http://dx.doi.org.libproxy.lib.unc.edu/10.1089/cap.2010.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta’ MA, Berchio C, Sestito M, Freedberg D, & Gallese V (2012). Abstract art and cortical motor activation: An EEG study. Frontiers in Human Neuroscience, 6. 10.3389/fnhum.2012.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértes PE, & Bullmore ET (2015). Annual Research Review: Growth connectomics - the organization and reorganization of brain networks during normal and abnormal development. Journal of Child Psychology and Psychiatry, 56(3), 299–320. 10.1111/jcpp.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, van Wingerden M, Battaglia F, & Pennartz CMA (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage, 55(4), 1548–1565. 10.1016/j.neuroimage.2011.01.055 [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, & Blumberg SJ (2014). Trends in the Parent-Report of Health Care Provider-Diagnosed and Medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 34–46.e2. 10.1016/j.jaac.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, & George CJ (2008). Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology, 67(1), 70–77. 10.1016/j.ijpsycho.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, & Wang Y (2009). Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Human Brain Mapping, 30(2), 638–649. 10.1002/hbm.20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler David. (2002). Wechsler Preschool and Primary Scale of Intelligence— Third Edition. The Psychological Corporation. [Google Scholar]
- Wehmeier PM, Schacht A, & Barkley RA (2010). Social and Emotional Impairment in Children and Adolescents with ADHD and the Impact on Quality of Life. Journal of Adolescent Health, 46(3), 209–217. 10.1016/j.jadohealth.2009.09.009 [DOI] [PubMed] [Google Scholar]
- White SF, VanTieghem M, Brislin SJ, Sypher I, Sinclair S, Pine DS, Hwang S, & Blair RJR (2015). Neural Correlates of the Propensity for Retaliatory Behavior in Youths With Disruptive Behavior Disorders. American Journal of Psychiatry, 173(3), 282–290. 10.1176/appi.ajp.2015.15020250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, & Biederman J (2011). Does ADHD Predict Substance-Use Disorders? A 10-Year Follow-up Study of Young Adults With ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 50(6), 543–553. 10.1016/j.jaac.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Brandl S, Horn F, Waldburger E, Allefeld C, & Tangermann M (2014). Robust artifactual independent component classification for BCI practitioners. Journal of Neural Engineering, 11(3), 035013. 10.1088/1741-2560/11/3/035013 [DOI] [PubMed] [Google Scholar]
- Winkler I, Debener S, Müller K-R, & Tangermann M (2015). On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. Engineering in Medicine and Biology Society (EMBC), 2015 37th Annual International Conference of the IEEE, 4101–4105. 10.1109/EMBC.2015.7319296 [DOI] [PubMed] [Google Scholar]
- Winkler I, Haufe S, & Tangermann M (2011). Automatic Classification of Artifactual ICA-Components for Artifact Removal in EEG Signals. Behavioral and Brain Functions, 7, 30. 10.1186/1744-9081-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang J, Liu X, Chen J, & Liu B (2015). Aberrant Brain Network Efficiency in Parkinson’s Disease Patients with Tremor: A Multi-Modality Study. Frontiers in Aging Neuroscience, 7. 10.3389/fnagi.2015.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]


