Abstract
Objectives
The use of ultrabrief pulse (UBP) stimuli and individual seizure threshold determination have been proposed as ways of minimizing the cognitive side effects of electroconvulsive therapy, but large samples of patients receiving UBP ECT are lacking. This study reports a retrospective cohort of patients receiving an index course of right unilateral UBP ECT, and characterizes the amount of charge required to induce a seizure.
Methods
A single-center retrospective chart review was conducted of adult patients receiving a first lifetime course of ECT using an UBP titration from 2005–2017.
Results
2,328 patients received a dose titration during the study period, with a mean dose of 21.82 ± 21.61 mC applied to induce a seizure. During the early part of the study period a mean dose of 18.73 ± 8.99 mC was used, with 28% of patients seizing at a dose of less than 19.2 mC. In the later period the initial step of dose titration was standardized at 19.2 mC, yielding a mean initial dose to induce a seizure of 22.72 ± 24.00 mC.
Conclusions
Mean seizure thresholds may be lower than reported in previous studies, and a significant proportion of patients may have a seizure threshold lower than the first step of many existing dose titration protocols.
Keywords: electroconvulsive therapy, dose titration, seizure thresholds, cohort studies
Introduction
As the most effective treatment of depression, the single greatest cause of disability worldwide,1 electroconvulsive therapy (ECT) remains a critical tool in psychiatry.2,3 While modern anesthesia techniques have minimized the physical risks of the procedure,4 cognitive and memory side effects remain of concern.5,6 One strategy that has emerged to lessen cognitive effects is the use of ultrabrief pulse (UBP) treatments,7,8 in which the duration of each electrical pulse is reduced to <0.5 ms compared to 0.5–2 ms for brief pulse stimuli or 8.3–10 ms for the original sinusoidal waveform.9 The UBP stimulus is more efficient at inducing a seizure compared to other stimulus types, and UBP treatments have been found to be effective if given at a multiple (generally 6× or more) of the charge required to induce a seizure.10 Although predictive models have been proposed to estimate an individual’s seizure threshold (ST),11 clinical practice in many regions relies on an empirical dose titration at the time of first treatment to measure seizure threshold.12 Given multiple possible titration protocols and starting doses, clinical evidence of usual effective doses can guide future titrations. Here we present a large cohort of patients undergoing right unilateral UBP titration. For reasons that are not entirely clear, two titration paradigms emerged in the clinical practice. At the beginning of the study period a range of titrations were explored; later a switch occurred and 19.2 mC became the usual starting dose for the titration. This provides a natural experiment allowing for comparisons between the two time periods.
Methods
Population and Setting
This was a single center retrospective cohort study of patient receiving an index course of right unilateral UBP ECT (defined as a pulse width of 0.3 or 0.37 ms) at a freestanding academic psychiatric hospital during the study period of June 2005 to June 2017. Patients were excluded from the study population if they were known to have had prior ECT at any point in their life, if their ECT course used brief pulse or bilateral treatments, if they were younger than 18, or if they did not undergo dose titration on their first treatment. This chart review study was approved by the Partners Healthcare Institutional Review Board.
Treatment procedure
All patients received ECT using a Mecta Spectrum 5000Q (Tualatin, OR). All unilateral treatments utilized the D’Elia placement. As part of routine clinical practice for all twelve psychiatrists in the ECT group during the study period, seizure threshold was determined by dose titration for the first treatment. From 2005 through October 2010, a range of starting doses and titration steps were used at the discretion of the treating psychiatrist. Around this time an informal consensus emerged among treating psychiatrists setting the default starting dose for titrations at 19.2 mC (pulse width 0.3 ms, frequency 20 Hz, amplitude 800 mA, duration 2 s), with subsequent steps of the titration doubling duration according to the MECTA ultrabrief titration tables (step 2: 4s, 38.4 mC; step 3: 8s, 76.8 mC). Subsequent treatments were then targeted at 6× seizure threshold based on the MECTA ultrabrief tables. Generally methohexital was used as the anesthetic agent, but etomidate, propofol, or ketamine were used at the discretion of the treating psychiatrist or anesthesiologist. Succinylcholine was used as the muscle relaxant. Seizure presence and duration were determined based on two lead frontomastoid or bifrontal EEG and by observing motor response using the “cuff method” of inflating a BP cuff on one calf prior to muscle relaxant administration. If a seizure was ongoing at 120 seconds, propofol was given.
Data Extraction
During the study period, the ECT service maintained two record sources: paper records, which included details of ECT consults, consents, and procedure notes, and a separate electronic medical record containing the ECT treatment parameters and anesthesia medications and doses used. Data for this analysis is extracted from the electronic medical record.
Statistical Analysis
Analysis were completed using R (v 3.6.2, Vienna, Austria) and Prism (v 8.2.1, San Diego CA). Differences in the means of continuous variables were assessed using two-sided t-tests. Differences in categorical variables were assessed using a chi-square test.
Results
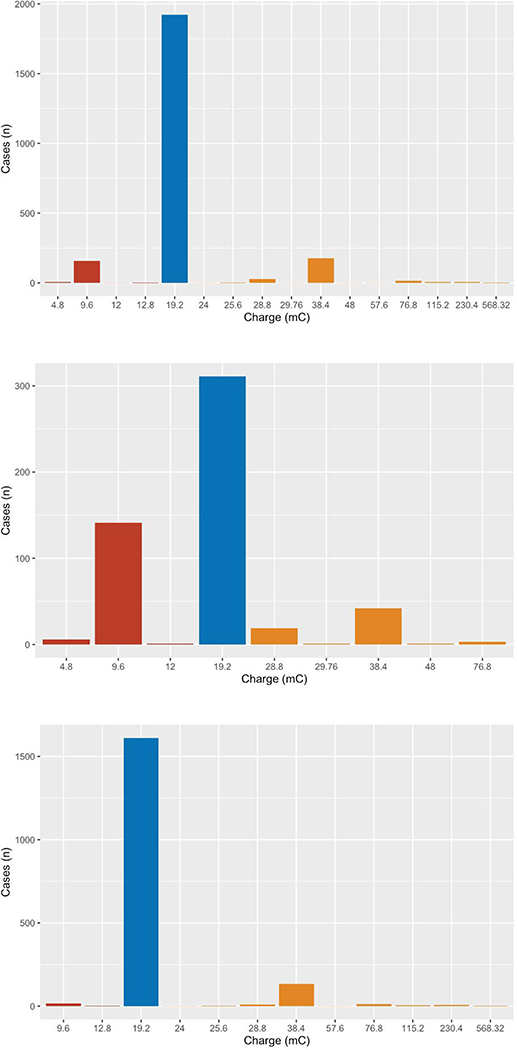
During the study period 3,416 patients had a first ECT treatment at the study site. Of these, 776 had right unilateral brief pulse and 146 had bilateral electrode placement for the initial treatment. Of those receiving right unilateral ultrabrief treatments, 134 had previously received ECT at a different hospital, 31 were under age 18, and 1 did not receive a dose titration due to medical complications, leaving a total of 2,328 patients who met the inclusion criteria. Of these, 983 (42.2%) were male, and the mean age at the time of titration was 45.4. Gender breakdown was not different in the pre-2010 and post-2010 periods, although the later sample was older (mean age 42.0 pre-2010 and 46.4 post-2010, P<0.0001). Demographics and cohort characteristics are shown in Table 1, with breakdowns of pre-Oct 2010 treatments and post-Oct 2010 treatments. Overall the median dose resulting in a seizure was 19.2 mC, with a mean of 21.82 mC. The amount of charge applied to reach a seizure was significantly less in the pre-2010 period than afterwards (mean of 18.73 mC vs. 22.72 mC, p<0.0001; median for both groups 19.2 mC). In total 166 patients (7.1%) seized at a dose of less than 19.2 mC, 1,922 (82.6%) seized at a dose of 19.2 mC, and 240 (10.3%) seized at a dose of greater than 19.2. A histogram of doses is shown in Figure 1, with a breakdown of pre-Oct 2010 treatments and post-Oct 2010 treatments. Prior to the protocol change 28.2% of patients seized at a dose of less than 19.2 mC, while afterwards only 1.0% did.
Table 1:
Cohort demographics, overall and broken down before and after the informal Oct 2010 dosing consensus. P values are for difference between the pre- and post-Oct 2010 groups.
| Overall | pre-Oct 2010 | post-Oct 2010 | P value (pre vs. post) | |
|---|---|---|---|---|
| n | 2,328 | 525 | 1,803 | |
| Sex = Male (%) | 983 ( 42.2) | 207 ( 39.4) | 776 ( 43.0) | 0.14 (Χ2) |
| Age (yrs; mean (SD)) | 45.38 (15.98) | 42.01 (13.40) | 46.37 (16.54) | <0.0001 (t-test) |
| Charge (mC; mean (SD)) | 21.82 (21.61) | 18.73 (8.99) | 22.72 (24.00) | 0.0002 (t-test) |
| Seizure Duration (sec; mean (SD)) | 76.19 (36.65) | 66.57 (30.49) | 79.00 (37.82) | <0.0001 (t-test) |
| Anesthesia Type (%) | ||||
| Ketamine | 1 ( 0.0) | 0 (0.0) | 1 ( 0.1) | |
| Etomidate | 3 ( 0.1) | 3 ( 0.6) | 0 (0.0) | |
| Methohexital | 2,250 ( 99.5) | 510 ( 98.8) | 1,740 ( 99.7) | |
| Propofol | 6 ( 0.3) | 3 ( 0.6) | 3 ( 0.2) | |
| Charge (%) | ||||
| <19.2 mC | 166 ( 7.1) | 148 ( 28.2) | 18 ( 1.0) | |
| 19.2 mC | 1,922 ( 82.6) | 311 ( 59.2) | 1,611 ( 89.4) | |
| >19.2 mC | 240 ( 10.3) | 66 ( 12.6) | 174 ( 9.7) | |
| Age (%) | ||||
| <35 | 694 ( 29.8) | 169 ( 32.2) | 525 ( 29.1) | |
| 35–50 | 685 ( 29.4) | 195 ( 37.1) | 490 ( 27.2) | |
| >50 | 949 ( 40.8) | 161 ( 30.7) | 788 ( 43.7) |
Figure 1:
histogram of energy applied to induce a seizure. Top: overall sample. Middle: sample prior to Oct 2010, when dose titration was determined by the treating psychiatrist. Bottom: sample after October 2010 with a consensus starting dose of 19.2 mC. Charges are binned into less than 19.2 mC (red), 19.2 mC (blue), and greater than 19.2 mC (orange)
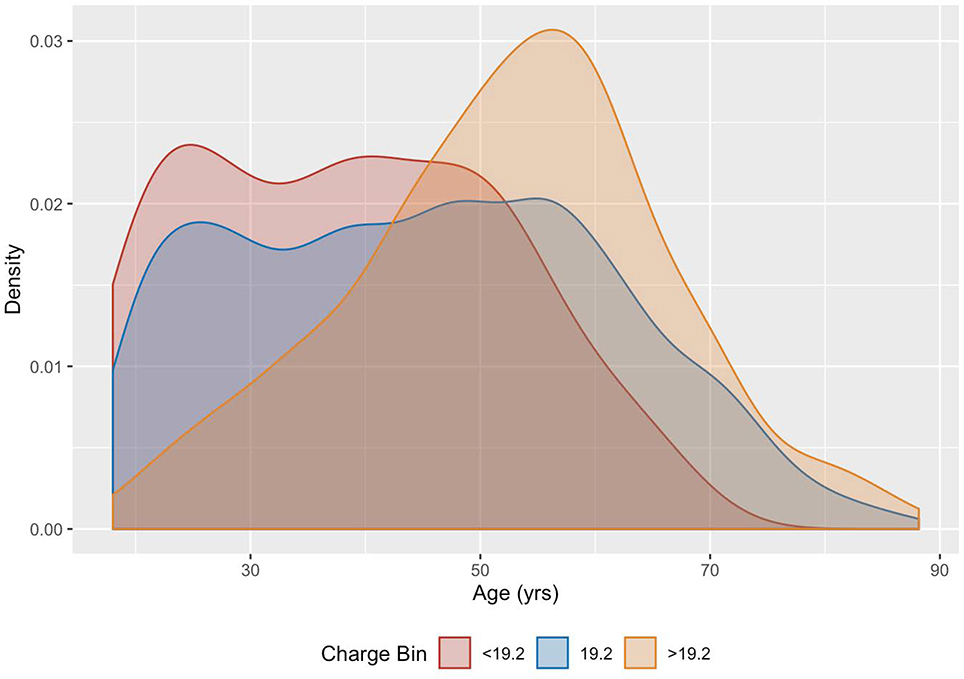
Looking at age vs. charge distributions, those patients who were treated at <19.2 mC and at 19.2 mC have similar age distributions, with patients treated at >19.2 mC skewing towards older patients (Figure 2). Mean ages for the three charge ranges are 38.9±13.4 yrs (<19.2 mC), 45.1±16.2 yrs (19.2 mC), and 52.3±13.6 yrs (>19.2 mC). In the post-2010 epoch, virtually all of the patients treated at doses less than the standard 19.2 mC were younger patients.
Figure 2:
Distribution of patient age by charge level visualized using kernel density estimation. Those who had a seizure at < 19.2 mC (red); at 19.2 mC (blue); at > 19.2 mC (orange).
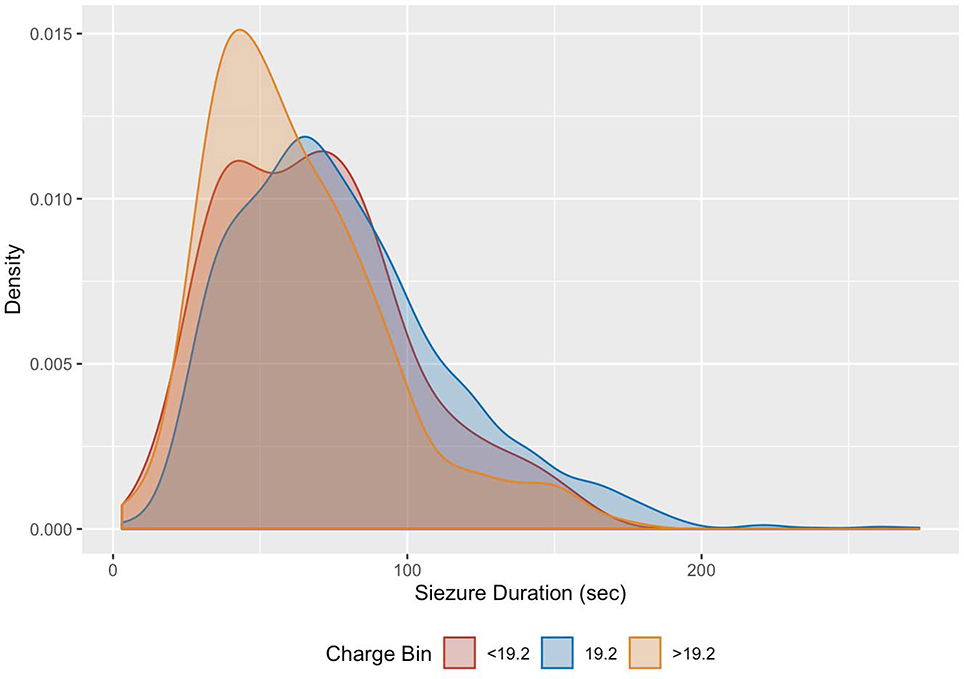
EEG seizure duration differed for the three charge bins (Figure 3), with mean seizure durations of 69.2±32.4 sec (<19.2 mC), 78.5±37.3 sec (19.2 mC), and 62.9±30.9 sec (>19.2 mC).
Figure 3:
Distribution of EEG seizure duration by charge level visualized using kernel density estimation. Stimulus < 19.2 mC (red), 19.2 mC (blue), > 19.2 mC (orange).
Discussion
Our study of ECT dose titration in 2,328 patients receiving an index course of right unilateral ultrabrief pulse treatments is the largest cohort ever reported for this modality. An informal change in our clinical protocol in the year 2010 without change in ECT machine, anesthesia protocol, or clinical staff provides a valuable natural experiment, allowing for analysis of patient treatment before and after the policy change.
The patients before and after the 2010 protocol change are similar in gender breakdown, although the later patients are on average 4.4 years older, a difference made up by an increase in the proportion of patients older than 50 rather than by a decrease in patients younger than 35. The key effect was to eliminate the lowest dose step in the titration protocol for most patients, with an increase the average charge applied to reach a seizure from a mean of 18.73 mC to 22.72 mC. Consistent with this, the proportion of patients who seized at a dose of less than 19.2 mC dropped from 28.2% to 1.0%. This was partially offset by a decrease in the number of patients who seized at a dose greater than 19.2 mC from 12.6% to 9.7%.
Due to the charting method in use during the study period, where only the final dose used to achieve a seizure was recorded in the electronic record, we are unable to assess systematically the number of subthreshold stimuli each patient received prior to the stimulus that did produce an adequate seizure. As a result, the recorded doses listed here do not represent the seizure thresholds of the patients, as the true threshold could be lower than the stimulus applied. This is particularly true in the pre-Oct 2010 period, when there was less consistency in titration schedules: it is unclear if patients who received particularly high stimulus doses did so because lower doses were unsuccessful or because the treating psychiatrist chose a higher than normal dose as a result of a clinical factor when the patient could potentially have been successfully treated at a lower dose. It is likely that some of the patients with seizure thresholds >19.2 in the pre-2010 era could have been treated at a lower dose, as fewer patients in the post-2010 era required doses higher than the default 19.2 mC. Likewise, a significant fraction of the patients treated at 19.2 mC in the post-2010 era potentially could have seized at a lower dose, as happened prior to the policy change. Put together, this evidence suggests that the true mean seizure threshold of this group may be even lower than the 18.73 mC observed for the earlier treatment period.
Multiple prior prospective studies have reported seizure thresholds for patients using a variety of dosing protocols (Table 2). Most of these are small, single-center trials. This study includes nearly three times as many patients as all prior trials put together, and the median applied charge to induce a seizure of 21.82 mC is lower than all but one previous trial. The next largest study is the PRIDE trial of Kellner et al.,13 in which 240 geriatric patients received right unilateral ultrabrief pluse ECT with titrations starting at a MECTA dose of 24 mC or the Thymatron 5% dose (24.8 mC). This study found a seizure threshold of 30.5 mC, with 84% of patients seized at the lowest step of the titration, implying that some may have had true seizure thresholds less than this. Galvez et al. report combined analyses of multiple trials totaling 179 patients, where seizure threshold was titrated beginning at lower doses (9.6 mC for MECTA, 2% or 10 mC for Thymatron).11 The authors conduct a multiple regression analysis of factors predicting seizure threshold in their sample, and recommend that initial titration steps be at 10 mC for patients younger than age 40, and 20 mC for patients older than this. Their sample and the one reported here are similar in mean age and gender breakdown, and it is unclear why fewer patients in their sample had seizures at the lowest doses although factors such as anesthetic (thiopentone and propofol in the Galvez et al. study vs methohexital here) and concomitant medications may play a role.
Table 2:
sample size, gender ratio, mean age, mean seizure threshold, and titration protocol for previous studies utilizing right unilateral UBP ECT. In total the studies include 810 patients.
| Paper | N | % female | Age (yr) | SD | ST (mC) | SD | Titration protocol |
|---|---|---|---|---|---|---|---|
| Sackeim et al 200810 | 22 | 55.0% | 54.0 | 16.0 | 22.0 | 8.0 | not stated; 1 pt at 4.8 mC, rest at 19.2 or 38.4 mC |
| Sienaert et al 200919 | 32 | 71.9% | 54.4 | 13.1 | 38.4 | 24.9 | starting at 24 mC |
| McCormick et al 200920 | 26 | 73.0% | 53.2 | 14.5 | 51.4 | 108.7 | not stated |
| Roepke et al 201121 | 17 | 55.0% | 54.3 | 15.5 | 37.5 | 12.1 | not stated; group 1 of trial |
| Roepke et al 201121 | 17 | 65.0% | 57.9 | 14.3 | 40.6 | 26.2 | not stated; group 2 of trial |
| Quante et al 201122 | 41 | 76.8% | 56.4 | 13.9 | 13.9 | 15.1 | MECTA starting at 9.2 mC |
| van Waarde et al 201223 | 63 | 62.6% | 59.1 | 15.0 | 45.6 | 16.8 | Thymatron starting at 25.2 mC for pt < 50; 50.4 mC for patients >50 |
| Rosa et al 201324 | 30 | 60.0% | 52.0 | 15.2 | 36.1 | 15.1 | MECTA table starting at 9.6 mC |
| Spaans et al 201325 | 58 | 70.7% | 60.4 | 16.3 | 23.9 | 10.2 | MECTA table starting at 9.6 mC |
| Mayur et al 201326 | 17 | 52.9% | 43.4 | 11.7 | 22.1 | 8.9 | MECTA table starting at 9.6 mC |
| Galvez et al 201511 | 179 | 60.9% | 45.7 | 14.7 | 31.7 | 22.2 | MECTA table starting at 9.6 mC or Thymatron 10 mC |
| Kellner et al 201613 | 240 | 57.5% | 69.9 | 7.6 | 30.5 | 14.3 | MECTA starting at 24 mC or Thymatron starting at 5% (25 mC) |
| Rasmussen at al 201627 | 20 | 80.0% | 47.7 | 12.9 | 32.7 | 17.8 | Thymatron starting at 5% (25 mC) |
| Galvez et al 201728 | 35 | 51.4% | 50.2 | 15.6 | 27.7 | 11.5 | MECTA table starting at 9.6 mC or Thymatron 10 mC |
| Wong et al 201929 | 13 | 23.1% | 20.3 | 12.6 | 39.2 | 28.8 | Thymatron, titration not stated |
Our results suggest that average seizure thresholds may be lower than previously believed, and that a significant fraction of patients (at least 28%) may have seizure thresholds lower than the 19.2 mC second step of the MECTA UBP titration tables. Although none of the patients in our sample were treated with the 900 mA current of the Thymatron device, which one study indicates may be more efficient for producing seizures,11 our results likewise suggest that the often-used 5% charge threshold (corresponding to a dose of 24.8 mC) is suprathreshold for many patients.
As much of the trial evidence for dosing of ultrabrief unilateral ECT comes from studies that used dose titration procedures that may result in overestimation of seizure threshold, it is reasonable to consider the concept of dose in ECT and how it may affect efficacy. In particular, a trial of brief pulse ECT suggest that fixed high-dose treatments may have greater efficacy than titrated treatments, albeit with greater cognitive costs.14 As standard titration steps are spaced in multiples of two, a reduction in titrated seizure threshold by one step represents a 50% reduction in charge. If lower starting dose titrations for UBP ECT were to become the norm, it is likely that a significant fraction of patients will be treated with lower final doses which more accurately represent e.g. 6× seizure threshold. Subsequent studies will need to determine whether this changes efficacy and cognitive outcomes. Likewise some existing studies attempting consistent treatment at multiples of seizure threshold may have masked variability in dosing, with subpopulations actually dosed at twice the multiple of the rest of the sample due to erroneous seizure threshold determination.15 It may be that some patients require greater dosing than 6× seizure threshold, and some patients may actually have received 12× seizure threshold or more in existing trials. Furthermore, if most seizure thresholds are similar, the process of titration may expose patients to unnecessary subtherapeutic doses without gaining meaningful new information for most patients.16,17 While one study suggests that even a low intensity first stimulus may be clinically effective,18 this requires further study.
Notable limitations of this study include its retrospective observational nature. In particular, the lack of information about subthreshold stimuli or the number of titration steps applied to reach a seizure makes it impossible to accurately assess the true seizure thresholds of patients. In addition, our electronic ECT treatment records were not linked systematically to the patient’s general medical record, so we are unable to assess concomitant medications or medical conditions which may influence seizure threshold. This limitation has been corrected in our new electronic medical record that came into use at the end of the study period, which integrates ECT record into the patient’s overall chart and in which all subthreshold stimuli are routinely recorded. Additionally, while the use of consistent anesthesia methods and ECT machines allows for comparisons across time, it does not allow assessment of the effects of these variables on seizure threshold. Furthermore, treatment outcomes and cognitive side effect profile were not systematically collected for members of the cohort, so we are unable to assess whether the higher average dose applied after October 2010 affected the efficacy or tolerability of treatment.
Conclusions
In conclusion, this study reports a cohort of 2,328 patients receiving right unilateral UBP ECT dose titration over a 12-year period. The median stimulus dose resulting in a seizure was 21.82 ± 21.61 mC, lower than reported in previous smaller-scale studies. These results suggest that studies using dose titrations which start at doses above ~10 mC may overestimate the seizure thresholds. Further research is needed to prospectively characterize potential benefits of dose titration, and whether these benefits exceed the risks of the dose finding treatment itself.
Funding
This work was supported by the National Institute of Mental Health (R25MH094612, JL; R56MH115187 and R01MH120991, THM; 5R01MH112737–03, MEH) and the Brain and Behavior Research Foundation (26489; THM). The sponsors had no role in study design, writing of the report, or data collection, analysis, or interpretation.
Footnotes
Declaration of Interest
THM receives research funding from the Stanley Center at the Broad Institute, the Brain and Behavior Research Foundation, National Institute of Mental Health, and Telefonica Alfa. JL, SJS, and MEH have no disclosures to report.
Contributor Information
James Luccarelli, Department of Psychiatry, Massachusetts General Hospital, Boston; Department of Psychiatry, McLean Hospital.
Thomas H. McCoy, Jr, Department of Psychiatry, Massachusetts General Hospital, Boston.
Stephen J. Seiner, Department of Psychiatry, McLean Hospital.
Michael E. Henry, Department of Psychiatry, Massachusetts General Hospital, Boston.
Works Cited
- 1.Friedrich MJ. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017;317(15):1517–1517. doi: 10.1001/jama.2017.3826 [DOI] [PubMed] [Google Scholar]
- 2.Bahji A, Hawken ER, Sepehry AA, Cabrera CA, Vazquez G. ECT beyond unipolar major depression: systematic review and meta-analysis of electroconvulsive therapy in bipolar depression. Acta Psychiatr Scand. 2019;139(3):214–226. doi: 10.1111/acps.12994 [DOI] [PubMed] [Google Scholar]
- 3.Ross EL, Zivin K, Maixner DF. Cost-effectiveness of Electroconvulsive Therapy vs Pharmacotherapy/Psychotherapy for Treatment-Resistant Depression in the United States. JAMA Psychiatry. 2018;75(7):713–722. doi: 10.1001/jamapsychiatry.2018.0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luccarelli J, Henry ME, McCoy TH. Quantification of fracture rate during electroconvulsive therapy (ECT) using state-mandated reporting data. Brain Stimul Basic Transl Clin Res Neuromodulation. 2020;13(3):523–524. doi: 10.1016/j.brs.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568–577. doi: 10.1016/j.biopsych.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 6.Kirov GG, Owen L, Ballard H, et al. Evaluation of cumulative cognitive deficits from electroconvulsive therapy. Br J Psychiatry. 2016;208(3):266–270. doi: 10.1192/bjp.bp.114.158261 [DOI] [PubMed] [Google Scholar]
- 7.Spaans H-P, H. Kho K, Verwijk E, Kok RM, Stek ML. Efficacy of ultrabrief pulse electroconvulsive therapy for depression: A systematic review. J Affect Disord. 2013;150(3):720–726. doi: 10.1016/j.jad.2013.05.072 [DOI] [PubMed] [Google Scholar]
- 8.Tor P-C, Bautovich A, Wang M-J, Martin D, Harvey SB, Loo C. A Systematic Review and Meta-Analysis of Brief Versus Ultrabrief Right Unilateral Electroconvulsive Therapy for Depression. J Clin Psychiatry. 2015;76(9):e1092–1098. doi: 10.4088/JCP.14r09145 [DOI] [PubMed] [Google Scholar]
- 9.Peterchev AV, Rosa MA, Deng Z-D, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159–174. doi: 10.1097/YCT.0b013e3181e48165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat. 2008;1(2):71–83. doi: 10.1016/j.brs.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gálvez V, Hadzi-Pavlovic D, Smith D, Loo CK. Predictors of Seizure Threshold in Right Unilateral Ultrabrief Electroconvulsive Therapy: Role of Concomitant Medications and Anaesthesia Used. Brain Stimulat. 2015;8(3):486–492. doi: 10.1016/j.brs.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Sackeim H Seizure Threshold in Electroconvulsive Therapy: Effects of Sex, Age, Electrode Placement, and Number of Treatments. Arch Gen Psychiatry. 1987;44(4):355. doi: 10.1001/archpsyc.1987.01800160067009 [DOI] [PubMed] [Google Scholar]
- 13.Kellner CH, Husain MM, Knapp RG, et al. Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Psychiatry. 2016;173(11):1101–1109. doi: 10.1176/appi.ajp.2016.15081101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated Moderately Suprathreshold vs Fixed High-Dose Right Unilateral Electroconvulsive Therapy: Acute Antidepressant and Cognitive Effects. Arch Gen Psychiatry. 2000;57(5):438–444. doi: 10.1001/archpsyc.57.5.438 [DOI] [PubMed] [Google Scholar]
- 15.McCall WV. Is Low-Dose Right Unilateral Electroconvulsive Therapy Sometimes a Strategy for Depression?: J ECT. 2013;29(2):81–82. doi: 10.1097/YCT.0b013e318284370f [DOI] [PubMed] [Google Scholar]
- 16.Bennett DM, Perrin JS, Currie J, et al. A comparison of ECT dosing methods using a clinical sample. J Affect Disord. 2012;141(2–3):222–226. doi: 10.1016/j.jad.2012.02.033 [DOI] [PubMed] [Google Scholar]
- 17.Rosenman SJ. Electroconvulsive therapy stimulus titration: Not all it seems. Aust N Z J Psychiatry. 2018;52(5):410–414. doi: 10.1177/0004867417743793 [DOI] [PubMed] [Google Scholar]
- 18.Lapidus KAB, Shin JSW, Pasculli RM, Briggs MC, Popeo DM, Kellner CH. Low-Dose Right Unilateral Electroconvulsive Therapy (ECT): Effectiveness of the First Treatment. J ECT. 2013;29(2):83–85. doi: 10.1097/YCT.0b013e31827e0b51 [DOI] [PubMed] [Google Scholar]
- 19.Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: Clinical efficacy. J Affect Disord. 2009;116(1–2):106–112. doi: 10.1016/j.jad.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 20.McCormick LM, Brumm MC, Benede AK, Lewis JL. Relative ineffectiveness of ultrabrief right unilateral versus bilateral electroconvulsive therapy in depression. J ECT. 2009;25(4):238–242. doi: 10.1097/YCT.0b013e31819fdff7 [DOI] [PubMed] [Google Scholar]
- 21.Roepke S, Luborzewski A, Schindler F, et al. Stimulus Pulse-Frequency-Dependent Efficacy and Cognitive Adverse Effects of Ultrabrief-Pulse Electroconvulsive Therapy in Patients With Major Depression: J ECT. 2011;27(2):109–113. doi: 10.1097/YCT.0b013e3181e63302 [DOI] [PubMed] [Google Scholar]
- 22.Quante A, Luborzewski A, Brakemeier E-L, Merkl A, Danker-Hopfe H, Bajbouj M. Effects of 3 different stimulus intensities of ultrabrief stimuli in right unilateral electroconvulsive therapy in major depression: A randomized, double-blind pilot study. J Psychiatr Res. 2011;45(2):174–178. doi: 10.1016/j.jpsychires.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 23.van Waarde JA, van Oudheusden LJB, Verwey B, Giltay EJ, van der Mast RC. Clinical predictors of seizure threshold in electroconvulsive therapy: a prospective study. Eur Arch Psychiatry Clin Neurosci. 2013;263(2):167–175. doi: 10.1007/s00406-012-0342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosa MA, Bueno CR, Andrade MA, Abdo GL, Rosa MO. Ultrabrief (0.3 ms) or brief (0.5 ms) pulses for right unilateral electroconvulsive therapy: is there a difference in seizure thresholds? J ECT. 2013;29(1):15–17. doi: 10.1097/YCT.0b013e31827134ba [DOI] [PubMed] [Google Scholar]
- 25.Spaans H-P, Verwijk E, Comijs HC, et al. Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: a randomized, double-blind, controlled study. J Clin Psychiatry. 2013;74(11):e1029–1036. doi: 10.4088/JCP.13m08538 [DOI] [PubMed] [Google Scholar]
- 26.Mayur P, Byth K, Harris A. Acute antidepressant effects of right unilateral ultra-brief ECT: A double-blind randomised controlled trial. J Affect Disord. 2013;149(1–3):426–429. doi: 10.1016/j.jad.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen KG, Johnson EK, Kung S, et al. An Open-Label, Pilot Study of Daily Right Unilateral Ultrabrief Pulse Electroconvulsive Therapy: J ECT. 2016;32(1):33–37. doi: 10.1097/YCT.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 28.Gálvez V, Hadzi-Pavlovic D, Waite S, Loo CK. Seizure threshold increases can be predicted by EEG quality in right unilateral ultrabrief ECT. Eur Arch Psychiatry Clin Neurosci. 2017;267(8):795–801. doi: 10.1007/s00406-017-0777-y [DOI] [PubMed] [Google Scholar]
- 29.Wong VKH, Tor PC, Martin DM, Mok YM, Loo C. Effectiveness and Cognitive Changes With Ultrabrief Right Unilateral and Other Forms of Electroconvulsive Therapy in the Treatment of Mania. J ECT. 2019;35(1):40–43. doi: 10.1097/YCT.0000000000000519 [DOI] [PubMed] [Google Scholar]