Abstract
Background:
Fanconi anemia (FA) is a genetic disorder that results in bone marrow failure, physical abnormalities, and solid organ malignancies. The diagnosis of FA is often delayed as the early disease characteristics have not been well established.
Objective:
To outline the spectrum of cutaneous findings seen in patients with FA.
Methods:
A cross-sectional study, where patients with FA received a full-body skin exam. Patient characteristics were summarized with mean (sd) for continuous and count (%) for categorical variables. Poisson regression and logistic regression models were used to examine the relationships between pigmentary changes and patient characteristics.
Results:
Nearly all of the patients (96.8%) had at least one cutaneous pigmentary alteration, most arising prior to the teenage years. The most common finding was café-au-lait macules (CALM). Other findings included hypopigmented macules, skin-fold freckle-like macules, extensive sun-exposed freckling, and both hypo- and hyperpigmented pigment macules.
Limitations:
Patients received a single assessment, so the number of pigmentary changes could not be assessed over time.
Conclusions:
Characteristic morphology of FA includes faint and ill-defined CALM, hypopigmented skin-fold freckle-like macules and the concurrence of hypo- and hyperpigmented macules. The recognition of these findings could aid clinicians in making earlier diagnoses.
Keywords: Fanconi anemia, café-au-lait macules, hypopigmented macules, hyperpigmented macules, hypopigmentation, hyperpigmentation, skin-fold freckling, skin-fold freckle-like macules, sun-exposed freckling, diagnosis, hematopoietic stem cell transplantation, radiation, graft-versus-host disease, voriconazole
Introduction:
Fanconi anemia (FA) is a rare, inherited disorder that can result in bone marrow failure, a heterogeneous array of physical features, and hematologic and solid organ malignancies. FA is caused by a genetic mutation in the proteins needed for activation of the FA pathway, which is involved in DNA damage repair. Defects in this pathway result in increased susceptibility to DNA damage and crosslinking (1–3), which can often be identified on chromosomal fragility tests (4). Specific physical features that aid in diagnosing FA include short stature, radial ray abnormalities, skin pigmentary changes, and microphthalmia. Even with characteristic exam findings, most patients are not diagnosed until they become symptomatic from bleeding, infection, and associated malignancy (3–6). Given the high risk of bone marrow failure and malignancy, patients with FA undergo rigorous cancer screening based on guidelines established by the Fanconi Anemia Research Fund. Cancer screening only begins after the diagnosis of FA, and unfortunately, in approximately 25% of patients a cancer diagnosis precedes that of FA (6).
Fanconi anemia-associated skin findings have not been specifically characterized and are not currently part of the diagnostic criteria. Case series have reported that altered pigmentation is the most common cutaneous clinical feature, found in up to 68% of FA patients. These pigment changes include café-au-lait macules, flexural hyperpigmentation, and hypopigmented macules (1, 2, 4, 7). The pattern, characteristics, and timing of these changes have not been specifically documented in the literature (1–3). Our study sought to provide details of the cutaneous findings in FA patients to better aid clinicians in earlier recognition and initiation of life-saving treatments, and to help discern FA from other genetic diseases with pigmentary alterations.
Methods:
We performed an institutional review board-approved cross-sectional study of skin changes in adults and children with FA. Patients with FA were recruited (1) during scheduled dermatology visits, (2) at a camp which hosts children with FA and their families for a week each summer, and (3) at the 2018 Meeting for Adults with Fanconi Anemia. Patients were enrolled from July 2015 to September 2018. Patients seen in clinic had genetically or molecularly confirmed diagnoses of FA, while patients recruited from camp and the 2018 Meeting for Adults with FA were self-identified, and medical records were not available to the investigators.
Patients received a full-body skin examination by one of four pediatric dermatologists. Demographic information and exam findings were documented. Consent and medical histories were obtained from adult patients and the parents of pediatric patients. The medical record was used to confirm elements of the past medical history when available.
Patient characteristics were summarized using mean (sd) for continuous and count (%) for categorical variables. Poisson regression with robust standard errors were used to evaluate the relationship between number of CALM and hypopigmented macule, where each of age, history of HSCT (hemopoietic stem cell transplantation), voriconazole use, graft-versus-host disease (GvHD), and radiation exposure were independent variables of separate models. Similarly, logistic regression models were used for outcomes of sun-exposed freckling and skin-fold freckle-like macules with the same independent variables. Incidence rate ratios (IRR) and odds ratios (OR) were calculated for Poisson and logistic regressions, respectively. All p-values are two sided and evaluate at the 0.05 level for statistical significance. Analysis was conducted in R, version 3.6.1.
Results:
Ninety-three patients ranging in age from one to 51 years old were enrolled in the study. The median age was 18.4 years. Forty-three (46.2%) of the patients were adults (≥18 years). Forty-six (49.5%) were female and 74 (83.1%) were white. Fifty-eight patients (63%) had received HSCT. Of these, 47 (50.5%) had received radiation prior to transplantation, and 13 (14%) had a history of cutaneous graft-versus-host disease. Twenty-four (25.8%) had a known exposure to voriconazole (Table 1). Forty-one patients seen at the University of Minnesota Dermatology clinic had a genetic diagnosis of FA that could be confirmed with laboratory records. Of these, most (73.2%) had the FA-A (FANC-A) complementation group designation (Table 1). Given the low number of patients in other FANC complementation groups, the study was underpowered to determine if there was a statistically significant association between pigmentary findings and complementation group. In review of the patients with the most and least pigmentary lesions, there was not an easily apparent association. The other 52 patients were recruited from family camp or the 2018 Meeting For Adults with Fanconi Anemia and genetic testing information was not available
Table 1.
Patient Demographics and Clinical Characteristics, N = 93
| Overall |
|
|---|---|
| Age | |
| Mean (SD) | 18.37 (9.94) |
| Gender: Female, N (%) | 46 (49.5) |
| Race, N (%)4 | |
| African American | 2 (2.2) |
| Asian | 5 (5.6) |
| Hispanic | 8 (9.0) |
| White | 74 (83.1) |
| GvHD: Yes, N (%) | 14 (15.4)2 |
| Transplantation: Yes, N (%) | 58 (63.0)1 |
| Radiation: Yes, N (%) | 48 (52.2)1 |
| Voriconazole: Yes, N (%) | 24 (27.6)6 |
| Complementation Group*, N (%) | |
| FA-A | 30 (73.2) |
| FA-C | 3 (7.3) |
| FA-D1 | 1 (2.4) |
| FA-D2 | 2 (4.9) |
| FA-G | 2 (4.9) |
| FA-I | 2 (4.9) |
| Café-au-lait macules | 86 (92.5) |
| Mean number (SD) | 7.5 (6.0) |
| Hypopigmented macules | 43 (46.2) |
| Mean number (SD) | 2.5 (5.1)2 |
| Sun-exposed freckling: Yes, N (%) | 11 (11.8) |
| Skin-fold freckle-like macules+: Yes, N (%) | 23 (24.7) |
| Hyperpigmented | 1 |
| Hypopigmented | 13 |
| Hyper- and hypopigmented | 3 |
| Unknown | 8 |
| Flexural hyperpigmentation: Yes, N (%) | 6 (6.5) |
| Generalized hyperpigmentation: Yes, N (%) | 9 (9.7) |
Superscripts represent number missing for binary variables
Forty-one patients seen at the University of Minnesota Dermatology clinic had genetically confirmed diagnoses of Fanconi anemia; one patient’s genetic testing results were unknown.
Some patients had a combination of hyper-, hypopigmented freckling, or both and were included in multiple skin-fold freckle-like macule categories (double counted).
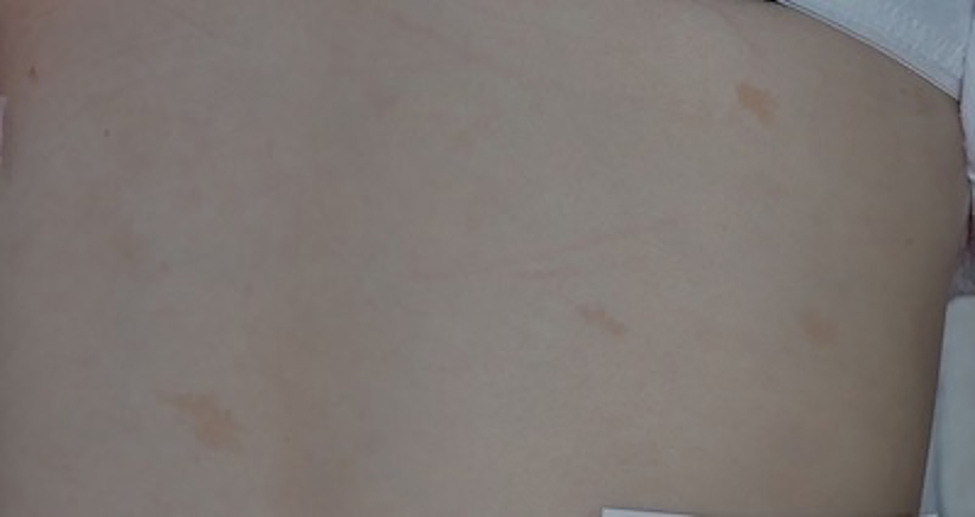
Ninety (96.8%) patients had at least one pigmentary change. The most common of these were café-au-lait macules (CALM). Café-au-lait macules are defined as evenly pigmented, light brown macules or patches of any size without pigment networks (10). The CALM were noted on all areas of the body and varied in size from several millimeters to >10 cm. Café-au-lait macules tended to be faint in pigmentation and were ill-defined (Figure 1); these findings, in addition to their isolated appearance, differentiated CALM from other hyperpigmented changes. Eighty-six (92.5%) patients had at least one CALM and 74.2% had three or more. The average number of CALM per patient was 7.5. Sixty-four patients (68.8%) had at least one pigmentary change in addition to CALM, most commonly hypopigmented macules or patches. This combination of CALM and hypopigmented macules was seen in 42 (45.2%) patients. Café-au-lait macules were the most common finding in children. All patients in the youngest cohort (≤ 5 years old) had at least one CALM (Table 2). Fifty-six percent of adults (18 years and older) had more than five CALM. Adult patients had an average of eight CALM per person, while those younger than 18 years old had an average of seven CALM per person (Table 2). No association was found between patients’ age and number of CALM (Table 3).
Figure 1.
Fanconi anemia: Faint, ill-defined café-au-lait macules or “shadow spots”.
Table 2.
Number of Café-au-lait Macules and Hypopigmented Macules in Patients of Different Age Groups
| 0–5 Years Old | 6–17 Years Old | 18+ Years Old | |
|---|---|---|---|
|
| |||
|
Café-au-lait macules
|
|||
| Mean number | 3.0 | 7.3 | 8.0 |
| None | 0 | 3 | 4 |
| 1–5 | 4 | 18 | 15 |
| 6–20 | 0 | 22 | 20 |
| >20 | 0 | 3 | 4 |
| Total |
4 | 46 | 43 |
|
Hypopigmented macules
|
|||
| Mean number | 1.0 | 2.5 | 2.7 |
| None | 2 | 20 | 26 |
| 1–5 | 2 | 17 | 11 |
| 6–20 | 0 | 7 | 1 |
| >20 | 0 | 1 | 4 |
| Total | 4 | 45 * | 42 * |
Available data varied slightly: hypopigmented macules = missing 2
Table 3.
Regression Results of Secondary Risk Factors on Cutaneous Changes
| Age | HSCT* | Voriconazole Use | GvHD* | Radiation | |
|---|---|---|---|---|---|
|
|
|||||
| Poisson Regression: Incidence Rate Ratio (95% CI) P-value | |||||
|
|
|||||
| Cafe-au-lait macules | 1.002 (0.601, 1.673) 0.759 | 1.093 (0.766, 1.560) 0.623 | 1.224 (0.867, 1.728) 0.250 | 0.986 (0.591, 1.646) 0.957 | 0.909 (0.655, 1.261) 0.988 |
| Hypopigmented macules | 1.002 (0.970, 1.035) 0.889 | 1.789 (0.695, 4.606) 0.228 | 1.009 (0.491, 2.071) 0.981 | 1.275 (0.443, 3.670) 0.653 | 1.039 (0.243, 1.835) 0.995 |
|
|
|||||
| Logistic Regression: Odds Ratio (95% CI) P-value | |||||
|
|
|||||
| Sun-exposed freckling | 0.924 (0.837, 1.000) 0.080 | 2.939 (0.701, 20.101) 0.185 | 15.250 (3.428, 107.947) 0.001 | 0.581 (0.030, 3.504) 0.621 | 1.707 (0.478, 6939) 0.421 |
| Skin-fold freckle-like macules | 1.067 (1.009, 1.111) 0.023 | 1.778 (0.644, 5.457) 0.284 | 0.420 (0.091, 1.431) 0.203 | 1.311 (0.329, 4.461) 0.677 | 1.853 (0.703, 5.164) 0.221 |
HSCT = Hematopoietic stem cell transplantation; GvHD = Graft-versus-host-disease
HSCT, Voriconazole use, GvHD, Radiation = secondary risk factors
Hypopigmented macules or patches were found in 43 (46.2%) patients. They were noted on all areas of the body and varied in size from millimeters to centimeters. The average number of hypopigmented macules or patches per patient was 2.5 (Table 1). Adult patients had an average of 2.7 hypopigmented macules per person, while those younger than 18 years old had an average of 2.4 hypopigmented macules per person (Table 2). No association was found between patients’ age and number of hypopigmented macules (Table 3).
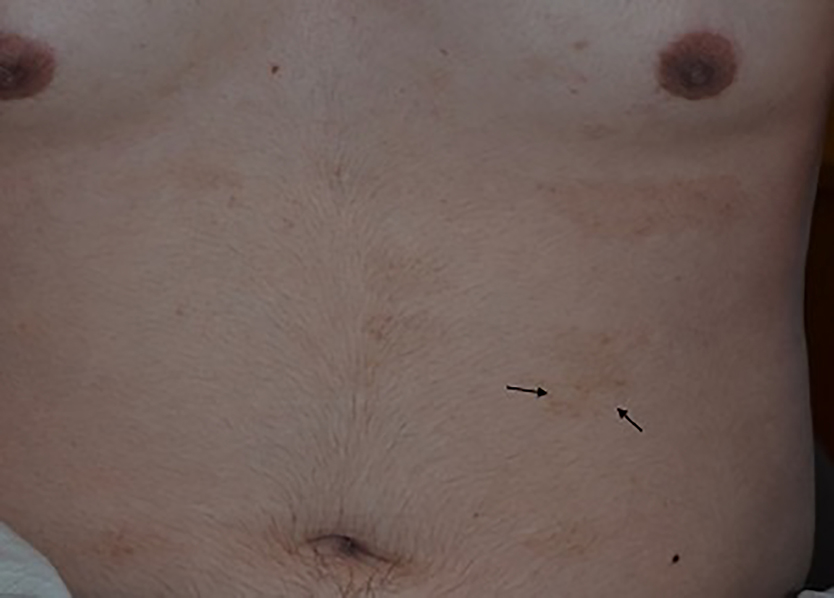
Skin-fold freckle-like macules were noted in 23 (24.7%) patients and found on the lateral neck, popliteal fossa, and axillary, inguinal, and suprapubic creases. The freckle-like macules were often hypopigmented (13 patients) or both hypo and hyperpigmented (three patients) (Table 1); these macules were all consistent in size, shape, and localized in clusters within the skin folds, making them easily differentiated from CALM, diffuse hyper- and hypopigmentation, and hypopigmented macules. Confluent flexural hyperpigmentation was noted in six (6.5%) patients (Table 1), one of which had superimposed hypopigmented freckle-like macules (Figure 2). Concurrent diffuse hyper- and hypopigmented macules were seen in four (4.3%) of the FA patients (Figure 3); the hyper- and hypopigmentation occurred together (often overlapping) with small, innumerable macules covering all or most body areas, and could be differentiated from the more isolated CALM and/or hypopigmented macules. Three of the patients with concurrent hyper- and hypopigmented macules had a history of HSCT, but no history of GvHD. Eleven (11.8%) patients had sun-exposed freckling. Three patients had no pigmentary changes, all of which were less than 15 years old; one of these patients was mosaic for FA.
Figure 2.
Fanconi anemia: Flexural hyperpigmentation with superimposed hypopigmented freckle-like macules within the skin fold (axilla).
Figure 3.
Fanconi anemia: Concurrent diffuse hyper- and hypopigmented macules. Black arrows show hypopigmented freckle-like macules.
No statistically significant associations were found between the outcomes and radiation exposure, cutaneous GvHD, or HSCT. There was not an increase or decrease in skin lesion number in those status-post HSCT. Increasing age was associated with an increase in the odds of skin-fold freckle-like macules (OR 1.067, 95% CI (1.009, 1.111) p value = 0.023) (Table 3). Eight patients with a history of voriconazole use had sun-exposed freckling; these patients had a higher odds of sun-exposed freckling than those without voriconazole use (OR 15.25, 95% CI (3.428, 107.947) p = 0.0012) (Table 3).
Discussion:
Cutaneous pigmentary changes are the most commonly associated physical finding in FA, seen in approximately 97% of the patients enrolled. Café -au-lait macules were present in 92.5% of patients, which is significantly higher than the incidence in the standard population (10–20%) (9). The number of lesions per patient ranged broadly from none to greater than 50 lesions, but most patients had less than 10. We suspect that the number of CALM may increase over time, as no child ≤ 5 years of age had more than five CALM, yet the majority of adult patients did (Table 2). Also, >20 CALM per person was noted most often in adult patients (Table 2). However, increased lesion count with age was not statistically significant. Further research with a larger sample size would give us a better idea of this association before generalizations can be made.
The CALM were distributed throughout the entire body and varied greatly in size. Other syndromes that share multiple CALM as a characteristic finding include neurofibromatosis 1 (NF1), McCune-Albright syndrome, Legius syndrome, tuberous sclerosis, and Noonan syndrome with multiple lentigines. In these syndromes, however, the CALM tend to be well-circumscribed (10). In this study, the CALM documented in FA patients were characteristic in that they were only subtly darker than the surrounding skin and had ill-defined edges. We termed them “shadow spots”, as they were often difficult to discern from shadows on the skin.
Hypopigmented macules and patches were the second most common pigmentary finding, noted in approximately half of the patients. This incidence is also much higher than in the general population, where 1 in 20 people (5%) have a hypopigmented macule (11). Most FA patients had between one and three hypopigmented macules, but some had over 50 macules.
Skin-fold freckle-like macules was present in a minority of the patients and developed during or after puberty. A subset of patients had both hyper and hypopigmented freckle-like macules in the skin folds. Hypopigmented skin-fold freckle-like macules has not previously been described in the literature in relation to other disease processes. In NF1, patients can have freckling that is both hyperpigmented and hypopigmented, however the macules are mainly solitary and located on the limbs or chest wall, not the flexural areas (8,12). Therefore, the presence of hypopigmented skin-fold freckle-like macules helps distinguish FA from other syndromes with pigmentary changes.
Concurrent diffuse hyper- and hypopigmented macules and a combination of CALM and hypopigmented macules were other notable findings that may help differentiate FA from other genetic skin conditions with similar presentations. Children with NF1 may present with hypopigmented macules in conjunction with CALM, but the hypopigmented macules tend to be few. The confetti-like macules noted in tuberous sclerosis complex are diffuse, but only hypopigmented and not limited to the skin folds. Piebaldism and familial progressive hyper- and hypopigmentation (FPHH) can be distinguished based on lesion morphology and history (13,14). Multiple other genetic disorders, such as dyskeratosis congenita and Naegeli-Franceschetti-Jadassohn syndrome, manifest with reticulate pigmentation, but not discrete hypo- and hyperpigmented macules (15).
Voriconazole, commonly used for antifungal prophylaxis in bone marrow transplant patients, has been known to cause phototoxic skin reactions, such as sun-exposed erythema, freckling, and hyperpigmentation (16). We therefore anticipated increased sun-exposed freckling in voriconazole-treated FA patients, however, the extent of this risk factor (OR 15.25, 95% CI (3.428, 107.947)) was surprising. We acknowledge that the confidence interval for this association is very wide and another study with a larger sample size would help us better understand this association. As this association is not well quantified in the published literature and the underlying mechanism of voriconazole-induced photosensitization is poorly understood, we do not know if these values would translate to all voriconazole-treated patients or are specific to our patient population with inherent DNA-repair defects (16).
CALM and hypopigmented macules were not more prevalent in FA patients with a history of radiation exposure. Additionally, the CALM and hypopigmented macules noted in FA patients were in both sun-exposed and non-exposed skin areas. This suggests that radiation and/or UV exposure are not the primary pathogenic factors for these skin changes in FA.
The diagnosis of FA is often dependent upon recognition of its associated physical findings. Only half of affected individuals are diagnosed prior to age 10 (17). As shown in this study, cutaneous pigmentary changes are nearly ubiquitous in individuals with FA and are seen as early as one year of age. Children with hypopigmented skin-fold freckle-like macules or hypo- and hyperpigmented macules should be assessed for other associated physical findings, such as radial ray anomalies, small body size, microcephaly, and microphthalmia. If present, FA-specific screening is suggested.
Limitations:
All patients had a known diagnosis of FA, and therefore may have had a higher burden of disease-specific findings compared to those with a delayed diagnosis. As patients were only assessed at one point in time, the study was not designed to track the longitudinal development of skin findings. Some of the analyses of the risk factors versus pigmentary changes were not statistically significant yet had extremely wide confidence intervals. Therefore, an additional study with a larger patient population is worth pursuing in the future.
Conclusions:
This cross-sectional study demonstrated that nearly all examined children and adults with FA had skin pigmentary changes. Café-au-lait macules and hypopigmented macules were the most common pigmentary changes. Characteristic cutaneous findings included hypopigmented skin-fold freckle-like macules, ill-defined hypo- and hyperpigmented macules present simultaneously, and very faint ill-defined CALM leading to the new descriptive term “shadow spots”. Presence of these findings warrants additional screening for FA.
Capsule summary:
The cutaneous changes associated with Fanconi anemia are poorly understood. We found that nearly all Fanconi anemia patients had pigmentary changes and characterized these changes.
Clinicians should screen patients presenting with faint, ill-defined café-au-lait macules, hypopigmented skin-fold freckle-like macules, or concurrent hypo- and hyperpigmented macules for Fanconi anemia.
Acknowledgments
Funding sources: this research was supported by a grant from the Society for Pediatric Dermatology and the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494.
Footnotes
Conflict of Interest: None declared.
IRB approval status: Reviewed and approved by the University of Minnesota IRB; study #1504M68081
Reprint requests: Jenna Ruggiero
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Glanz A, Fraser FC. Spectrum of anomalies in Fanconi anaemia. J Med Genet 1982;19: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter BP. Inherited bone marrow failure syndromes. In: Nathan DG, Orkin SH, Look AT, Ginsburg D, editors. Nathan and Oski’s hematology of infancy and childhood. Philadelphia, PA: W.B. Saunders, 2003;280–365. [Google Scholar]
- 3.Dokal I, Vulliamy T. Inherited aplastic anaemias/bone marrow failure syndromes. Blood Rev 2008; 2(3):141–153. [DOI] [PubMed] [Google Scholar]
- 4.Green AM, Kupfer GM. Fanconi anemia. Hematol Oncol Clin North Am 2009;23:193–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 2010;24(3):101–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alter BP. Cancer in Fanconi anemia, 1927–2001. Cancer 2003;97:425–440. [DOI] [PubMed] [Google Scholar]
- 7.Tischkowitz M, Dokal I. Fanconi anaemia and leukaemia- clinical and molecular aspects. Br J Haematol 2004;126:176–191. [DOI] [PubMed] [Google Scholar]
- 8.Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Schepper S, Boucneau J, Vander Haeghen Y, Messiaen L, Naeyaert J, Lambert J. Café-au-lait spots in neurofibromatosis type 1 and in healthy control individuals: hyperpigmentation of a different kind? Arch Dermatol Res 2006;297(10):439–449. [DOI] [PubMed] [Google Scholar]
- 10.Shah KN. The diagnostic and clinical significance of café-au-lait macules. Pediatric Clinic of North Am. 2010;57(5):1131–53. [DOI] [PubMed] [Google Scholar]
- 11.Hill JP, Batchelor JM. An approach to hypopigmentation. BMJ 2017;356:i6534. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Martinez FJ, Duat A, Hernandez-Martin A. Hypopigmented macules: A common finding in neurofibromatosis type 1. J Am Acad Dermatol 2017;76(6):AB161. [DOI] [PubMed] [Google Scholar]
- 13.Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges 2010;8(3):187–202. [DOI] [PubMed] [Google Scholar]
- 14.Fang Xiao-Kai, He Yue-Xi, Li Yan-Jia, Chen Li-Rong, Wang He-Peng, Sun Qing. Familial progressive hyper- and hypopigmentation: a report on a Chinese family and evidence for genetic heterogeneity. An Bras Dermatol. 2017;92(3):329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnur RE, Heymann WR. Reticulate hyperpigmentation. Semin Cutan Med Surg 1997;16(1):72–80. [DOI] [PubMed] [Google Scholar]
- 16.Goyal RK, Gehris RP, Howrie D, Cogley KM, Windreich RM, Venkataramanan R. Phototoxic dermatoses in pediatric BMT patients receiving voriconazole. Pediatr Blood Cancer. 2014;61(7):1325–8. [DOI] [PubMed] [Google Scholar]
- 17.Fanconi Anemia. National Organization for Rare Disorders. Available at: https://rarediseases.org/rare-diseases/fanconi-anemia/. Accessed November, 29, 2019.