Abstract
Purpose:
There is substantial variation in the radiologic terms used to characterize renal masses, leading to ambiguity and inconsistency in clinical radiology reports and research studies. The purpose of this study was to develop a standardized lexicon to describe renal masses at CT and MRI.
Materials and Methods:
This multi-institutional, prospective, quality improvement project was exempt from IRB oversight. Thirteen radiologists belonging to the Society of Abdominal Radiology (SAR) disease-focused panel on renal cell carcinoma representing nine academic institutions participated in a modified Delphi process to create a lexicon of terms used to describe imaging features of renal masses at CT and MRI. In the first round, members voted on terms to be included and proposed definitions; subsequent voting rounds and a teleconference established consensus. One non-voting member developed the questionnaire and consolidated responses. Consensus was defined as ≥80% agreement.
Results:
Of 37 proposed terms, 6 had consensus to be excluded. Consensus for inclusion was reached for 30 of 31 terms (13/14 basic imaging terms, 8/8 CT terms, 6/6 MRI terms and 3/3 miscellaneous terms). Despite substantial initial disagreement about definitions of ‘renal mass’, ‘necrosis’, ‘fat’, and ‘restricted diffusion’ in the first round, consensus for all was eventually reached. Disagreement remained for the definition of ‘solid mass’.
Conclusions:
A modified Delphi method produced a lexicon of preferred terms and definitions to be used in the description of renal masses at CT and MRI. This lexicon should improve clarity and consistency of radiology reports and research related to renal masses.
Keywords: Renal mass; renal cell carcinoma; CT, MRI, lexicon, Delphi method
Introduction
Diagnostic radiologists translate imaging data into words that influence patient care (1). These words must be precise and accurate to optimize communication between radiologists, referring providers, and patients (2-10). Emerging data indicate the need for structured reports (11-15) that use specific well-defined terms (16-21) to improve clarity and understandability. To address these goals, schemas such as the Breast Imaging Reporting and Data System (BI-RADS) (2,3) have created standardized lexicons that define terms to be used when radiologists are describing imaging findings, either in a clinical radiology report or in a research study. Such lexicons define terms and provide formal recommendations about which specific terms should be used or avoided (12,13).
No such lexicon exists for CT and MRI of renal masses. Terminology used in radiology reports and research studies remains highly variable (22-25). For example, numerous terms have been used to describe the presence of ‘fat’ in a renal mass, including terms that refer to fat cells (e.g., ‘macroscopic fat’, ‘macroscopic lipid’, ‘extracellular fat’, ‘bulk fat’) and terms that refer to both fat cells and other cells that contain fat (e.g., ‘microscopic fat’, ‘intracellular fat’, ‘intracellular lipid’, ‘intracytoplasmic fat’, ‘intra-voxel fat’) (26-29). Terms used inconsistently or inaccurately, or that are not based on explicit definitions, lead to confusing or ambiguous clinical reports (29), interobserver variability, and difficulty interpreting and reproducing research studies (23-25).
There is a need to develop standard terms for imaging features of renal masses that will promote accurate and consistent reporting in both clinical practice and research. The purpose of this study was to develop a standardized lexicon and image atlas to describe renal masses at CT and MRI.
Methods
This study was undertaken by the Society of Abdominal Radiology (SAR) Disease-Focused Panel (DFP) on renal cell carcinoma (RCC). Only terms relevant to CT and MRI were included because CT and MRI are the most common modalities used to evaluate indeterminate renal masses. Terms describing imaging protocols (e.g., the phases of enhancement after intravenous contrast material administration), anatomic structures (e.g., renal cortex), and specific disease entities (e.g., renal cell carcinoma) were not evaluated because they are outside the scope of this project and definitions for these terms and concepts exist throughout the literature. In addition, some terms that are specific to cystic masses (e.g., ‘nodule’, ‘septa’ and the adjectives used to describe them) were excluded from this project because they were being fully defined as part of a parallel effort to update the Bosniak classification (30). All 13 radiologist members of the RCC DFP from nine academic institutions in the US and Canada participated in the study. All participants considered renal masses a primary area of clinical and research interest.
Study design: Modified Delphi method
This multi-institutional, prospective, quality improvement project was exempt from IRB oversight, and utilized a modified Delphi method (31-34). One radiologist member of the panel (AS), with the help of a radiology clinical fellow (HP) served as the ‘coordinator’ who compiled the initial list of terms, prepared the questionnaires, and collected and analyzed the data. The remaining twelve radiologist members of the RCC DFP were invited to participate in the creation of the lexicon. All members are fellowship-trained abdominal radiologists with mean 13 years of experience as attending radiologist (range 5-30 years). Figure 1 outlines the process that was followed, and Appendix 1 provides the detailed methodology. Three rounds of questionnaires and one teleconference (after the second round) were conducted to build consensus (Appendix 1). Following the third round of questionnaires, the manuscript was created. Blinded edits to the manuscript were made and final consensus was reached. Based on prior literature, ≥80% agreement at the end of three rounds was considered sufficient ‘consensus’ regarding the inclusion or exclusion of each term and its definition (35-38). Individual panelist responses remained anonymous in all three rounds. After the lexicon was finalized, official endorsement was obtained from the SAR Board of Directors.
Figure 1.
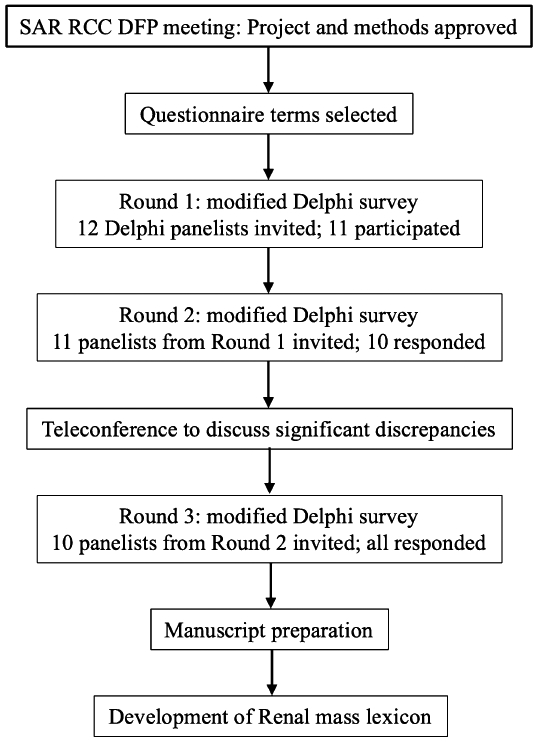
Flowchart demonstrating the modified Delphi process used to create a lexicon of terms for the description of renal masses at CT and MRI.
Results
The Round 1 questionnaire was sent to 12 panelists by one coordinator; 11 completed it. Of the 11 panelists who participated in Round 1, one was not able to participate further; as a result, ten panelists completed the questionnaires in Rounds 2 and 3.
A total of 35 terms were included in the first round (Table 1). There were 17 basic imaging terms, eight CT terms, six MRI terms, and four miscellaneous terms. Two additional terms were proposed in the first round (‘magnetic susceptibility’ and ‘growth rate’). By the end of the third round, of the 37 terms, 31 had ≥80% agreement to remain in the lexicon (Table 2) and six had ≥80% agreement to be removed from the lexicon (Table 3). Of the 31 included terms, 29 had 100% agreement to include and two had 90% agreement to include. Of the six excluded terms, four had 100% agreement to exclude, one had 90% agreement to exclude, and one had 80% agreement to exclude (Table 3).
Table 1.
Initial list of imaging terms evaluated during the first round of a modified Delphi process to create a lexicon of standard terms to be used in the description of renal masses at CT and MRI
| Basic imaging terms (n=17) | CT terms (n=8) |
| Renal mass Composition Solid mass Cystic mass Size Laterality Margin Well-defined margin Ill-defined margin Outline Growth pattern Categories of growth pattern Anteroposterior location Craniocaudal location Necrosis Capsule Central scar |
Attenuation Internal reference for attenuation Fluid attenuation Macroscopic fat at CT Calcification at CT Morphologic categories of calcification Hemorrhage at CT Enhancement at CT |
| MRI terms (n=6) | |
| Signal intensity Fat at MRI Calcification at MRI Hemorrhage at MRI Enhancement at MRI Restricted (impeded) diffusion | |
| Miscellaneous terms (n=4) | |
| Heterogeneity Rate of enhancement Degree of enhancement Internal vascularity |
Table 2.
Final consensus (≥80% agreement) terms and definitions to be used in the description of renal masses at CT and MRI. Arrows, solid and dotted lines on figures refer to the relevant term, each of which are defined in the column labeled ‘definition’.
| Category | Term | Level of Agreement for Inclusion |
Level of Agreement for Definition |
Definition | Counterpoints and Other Comments |
|---|---|---|---|---|---|
| Basic imaging terms | Renal mass | 100% | 100% | Any space-occupying abnormality thought to originate from or contiguous with renal parenchyma. Abnormalities include space-occupying features which deviate from the normal appearance of renal parenchyma and surrounding tissues. | One panelist agreed with the proposed definition but also recommended that benign entities with pathognomonic appearance, such as hypertrophic column of Bertin, should not be considered a renal mass. |
| Composition | 100% | 100% | The internal make-up of a renal mass, which may be further categorized as solid or cystic. | ||
Solid mass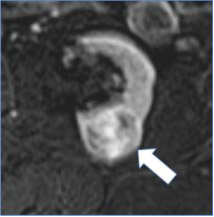
|
100% | 60% | Mass composed of greater than or equal to 25% solid enhancing components or fat. | Four (of 10) panelists commented that solid component does not always enhance and may appear as heterogeneous non-enhancing tissue. | |
Cystic mass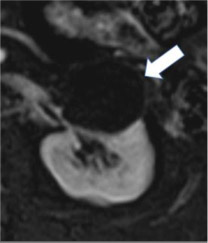
|
100% | 90% | Mass composed of less than 25% enhancing components or fat. | ||
Size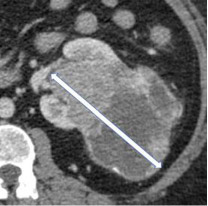
|
100% | 100% | The single longest dimension of a renal mass in any plane. | ||
| Laterality | 100% | 100% | Localization of renal mass(es) in the right, left or bilateral kidneys | ||
| Margin | 100% | 100% | The interface between the mass and adjacent uninvolved tissue. | ||
Well-defined margin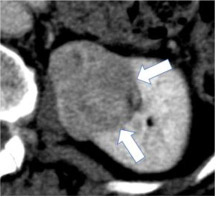
|
100% | 90% | A sharp pencil-thin demarcation between the mass and surrounding tissue over at least 90% circumference of the mass. | One panelist suggested using sharp demarcation over "most of the circumference of the mass" instead of using 90% threshold. | |
Ill-defined margin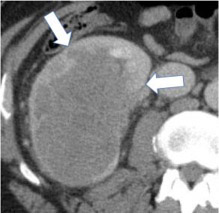
|
100% | 100% | An indistinct or blurred demarcation between the mass and surrounding tissue over greater than 10% circumference of the mass. | ||
| Growth pattern | 100% | 100% | The location of a renal mass relative to the renal parenchymal contour. | ||
| Categories of growth pattern -Endophytic 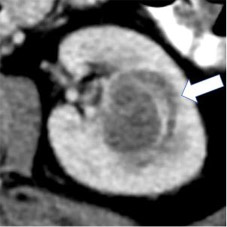 - <50% Exophytic 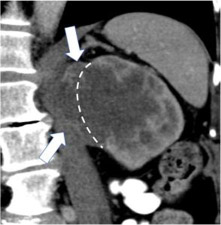 - ≥50% Exophytic 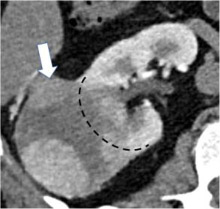
|
100% | 100% | Growth pattern may be categorized into: 1. Endophytic: Located within the renal parenchyma such that the outer renal contour is not altered. 2. <50% Exophytic: The renal contour is altered and <50% of the mass projects out from the renal contour. 3. ≥50% Exophytic: The renal contour is altered and ≥50% of the mass projects out from the renal contour. | ||
| Anteroposterior location - Anterior 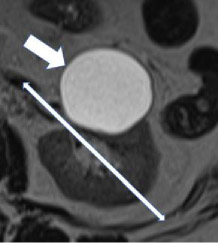 - Posterior 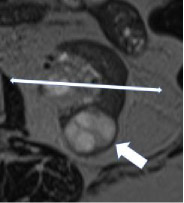
|
100% | 100% | The anterior or posterior location of a mass within the renal parenchyma on axial images. The mass is 'anterior' if the center of the mass lies anterior to the line bisecting the renal hilum on axial images and 'posterior' if the center of the mass lies posterior to this line. | ||
Craniocaudal location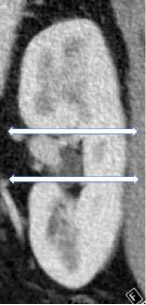
|
100% | 100% | The location of a mass with respect to the polar lines. A renal mass can be centered in the upper pole, interpolar region or lower pole. | ||
Necrosis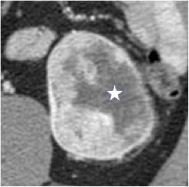
|
100% | 80% | Necrosis is a pathology term and cannot be diagnosed with certainty at imaging; necrosis, fibrosis, and cystic change can appear similar. Necrosis may be considered when non-enhancing ill-defined components of variable CT attenuation or MRI signal intensity are identified. | ||
| CT terms | Attenuation Homogenous mass 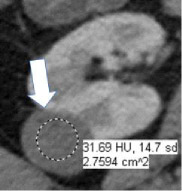 Heterogenous mass 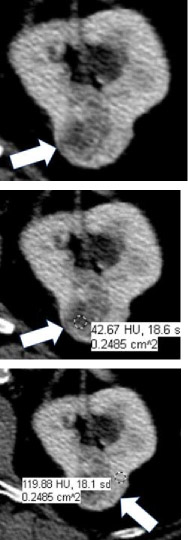
|
100% | 100% | CT attenuation can be determined in one or more regions of a renal mass. If the mass is homogeneous, the attenuation can be obtained with a single region of interest (ROI) that includes at least 2/3rds of the mass, being careful not to include tissues outside the mass. If the mass is heterogeneous, multiple smaller ROIs should be placed, taking care not to volume-average areas of different attenuation within the mass. Since attenuation may change depending on the phase of enhancement, reference to CT attenuation should be clear about when attenuation is being measured. | |
| Internal reference for attenuation | 100% | 100% | Renal mass attenuation (e.g., classified as hypo-, iso- or hyper-attenuating) is generally considered relative to renal cortex. However, reference standard anatomy should be specified in the report. When using the term 'attenuation' without referencing renal cortex or other reference standard, 'hypoattenuating' ‘isoattenuating’, and ‘hyperattenuating’ refer to a comparison to simple fluid (−9 to 20 HU). | ||
Fluid attenuation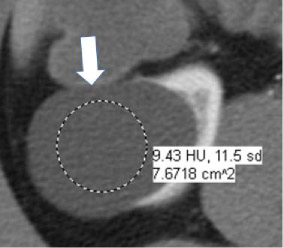
|
100% | 100% | Non-enhancing content within a mass measuring −9 HU to +20 HU. | ||
Macroscopic fat at CT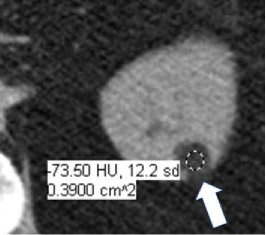
|
100% | 100% | Attenuation −10 HU or less (not reaching attenuation of air which is close to −1000). Pixel-based region of interest assessments will measure quantum mottle and are not reliable. Presence of fat cannot be confidently excluded on contrast-enhanced CT. | ||
Calcification at CT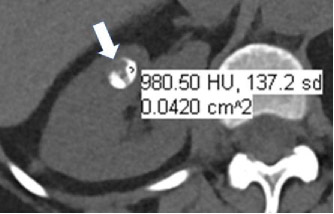
|
100% | 90% | Attenuation greater than 100 HU on unenhanced CT (excluding metal). Smaller calcifications may have lower attenuation because of volume averaging. Calcification cannot be confidently excluded on contrast-enhanced CT. | ||
| Morphologic categories of calcification - Thin 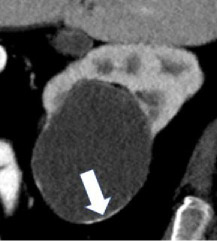 - Thick 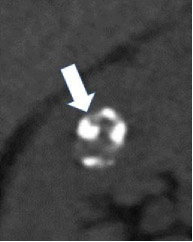 - Linear 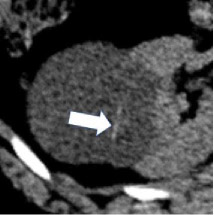 - Punctate 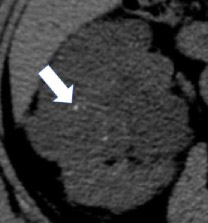 - Border-forming 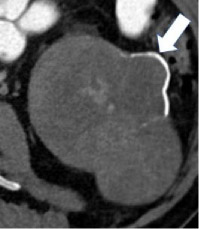 - Non-border-forming 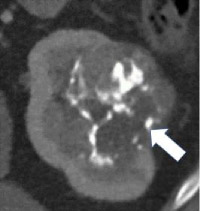
|
100% | 90% | Calcification may be further characterized as thin or thick, linear or punctate, border-forming or non-border-forming. | Clinical significance of morphologic appearance of calcification within a renal mass is controversial. | |
Hemorrhage at CT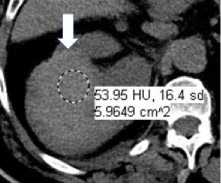
|
100% | 90% | Hyperattenuating nonenhancing area(s) within or around a renal mass. Since the CT attenuation of hemorrhage is variable and dependent on factors such as hemoglobin concentration and age of hemorrhage, there is no specific attenuation range that is diagnostic of hemorrhage; however, hemorrhage typically ranges from 40-100 HU. | One panelist suggested using attenuation range of 40-70 HU for hemorrhage at CT. | |
Enhancement at CT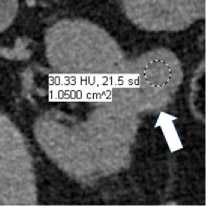 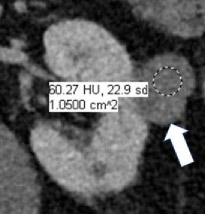
|
100% | 90% | Increase in attenuation ≥20HU using region(s) of interest on any contrast-enhanced phase relative to unenhanced imaging. Equivocal enhancement is an increase in attenuation ≥10 HU but < 20 HU, and no enhancement is an increase in attenuation <10 HU. The post-contrast phase should be acquired ≤2 minutes after contrast administration using the same parameters as the unenhanced imaging. When determining if a mass is enhancing at CT, the attenuation of the most enhancing portion of the mass is measured and compared to the same region on unenhanced imaging. | One panelist suggested removing the 2-minute time limit for assessment of enhancement. | |
| MRI terms | Signal intensity | 100% | 100% | MRI signal intensity (‘brightness’) can be determined in one or more regions of a renal mass. Since signal intensity may change depending on the sequence type and phase of enhancement, reference to signal intensity should be clear about what is being measured. The intensity is generally compared to normal renal cortex. If another reference standard is used it should be specified (such as "hyperintense compared to the muscle"). | |
| Fat at MRI - Microscopic 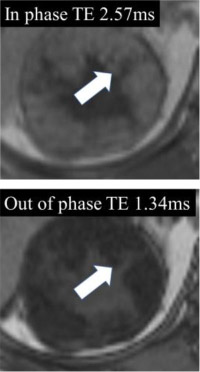 - Macroscopic 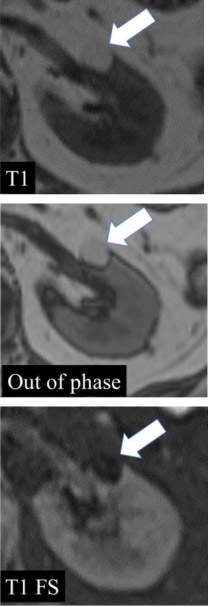
|
100% | 90% | When describing ‘fat’ at MRI, either ‘macroscopic fat’ or ‘microscopic fat’ should be used. Macroscopic fat is thought to be present when there is 1) loss of intratumoral signal intensity before and after application of fat-suppression (i.e with no changes in any of the other acquisition parameters), or 2) linear or curvilinear chemical shift artifact causing India-ink (etching) artifact within or at the periphery of the mass at macroscopic fat-water interfaces, while the central area (i.e. inside the etching artifact) remains hyperintense (i.e., following the signal intensity of subcutaneous and intraabdominal fat). Microscopic fat is thought to be present when there is non-linear non-curvilinear focal or diffuse signal intensity loss in any portion of a renal mass on the opposed-phase relative to in-phase T1-weighted images. |
One panelist suggested that 1-19% proton density fat fraction on multi-echo DIXON acquisitions in any portion of a renal mass may indicate microscopic fat. | |
Hemorrhage at MRI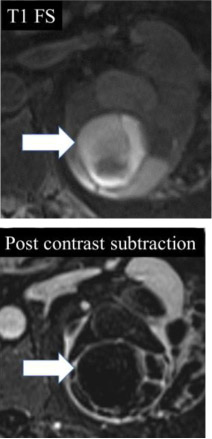
|
100% | 100% | Non-enhancing high signal intensity (relative to renal cortex) on pre-contrast fat-suppressed T1-weighted images. Proteinaceous content can appear similar. | ||
Enhancement at MRI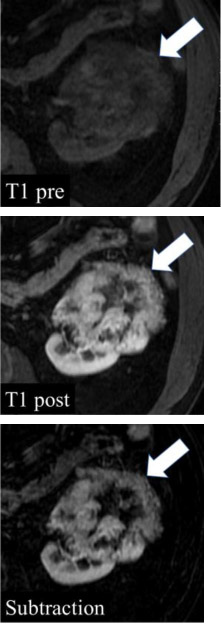
|
100% | 90% | Unequivocal visual enhancement or increase in signal intensity ≥15% using region(s) of interest on any contrast-enhanced phase relative to unenhanced imaging. No enhancement is an increase in signal intensity <15%. The post-contrast phase should be determined ≤2 minutes after contrast administration using the same parameters as the unenhanced imaging. Subtraction images (post-contrast minus pre-contrast) may be used to detect enhancement qualitatively by noting any signal in a portion of a mass; however, subtraction images need to be properly registered to avoid misregistration artifact. When determining if a mass is enhancing at MRI, the signal intensity of the most enhancing portion of the mass is measured and compared to the same region on unenhanced imaging. | One panelist suggested removing the 2-minute time limit for assessment of enhancement. | |
Restricted (impeded) diffusion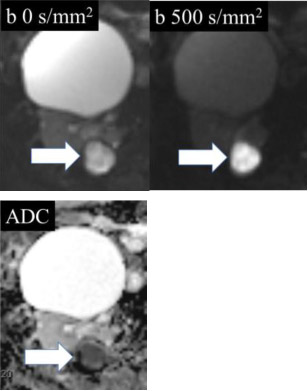
|
100% | 80% | Relative to the renal cortex, higher signal intensity in the mass on diffusion-weighted images with b-values >500 s/mm2 compared to a b-value of 0 s/mm2, or lower signal intensity on apparent diffusion coefficient map relative to renal cortex. | ||
Magnetic susceptibility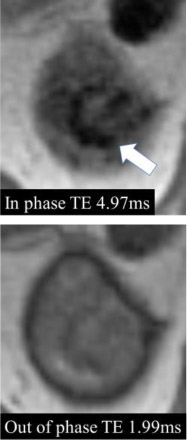
|
90% | 100% | Magnetic susceptibility is defined as a decrease in signal intensity on MRI images acquired with a longer echo time compared to images obtained with otherwise identical acquisition parameters except for shorter echo time. | One of the panelists pointed out that common causes of magnetic susceptibility artifact are hemosiderin, calcification, and gas. | |
| Miscellaneous terms | Heterogeneity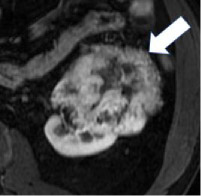
|
100% | 90% | Variable CT attenuation or MRI signal intensity. Assessment of heterogeneity may be done using visual inspection or by manually placing multiple regions of interest within a mass. There is no established quantitative definition. This definition does not include variable attenuation or signal as a result or artifacts or image noise. | Heterogeneity may be assessed quantitatively using texture analysis, but there are limited data on which features to use and methods of assessment. |
| Degree of enhancement - Hypovascular 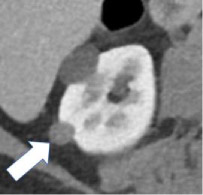 - Hypervascular 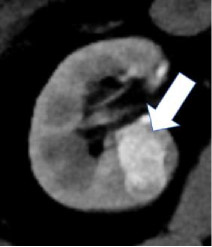
|
100% | 90% | Relative enhancement of a renal mass at a specific timepoint (e.g. corticomedullary phase), generally compared to the renal cortex. Degree of enhancement may be assessed qualitatively or quantitatively. Qualitatively, hypervascular masses typically enhance more than normal renal cortex and hypovascular masses enhance but less than normal renal cortex. Quantitatively, hyperenhancement refers to a mass with ≥70HU of absolute enhancement during the corticomedullary phase, and hypoenhancement refers to a mass with 20-40 HU of absolute enhancement during the corticomedullary phase. | No specific quantitative thresholds for degree of enhancement at MRI were established. | |
Growth rate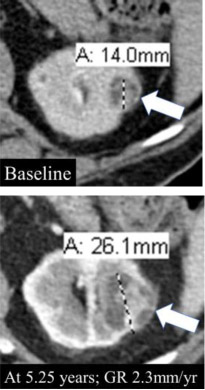
|
90% | 90% | Change in size divided by the length of time over which the size changed. *GR: Growth rate |
Table 3.
Excluded terms and level of agreement for exclusion
| Category | Term | Level of Agreement for the Term to be Excluded |
Reason for Exclusion |
|---|---|---|---|
| Basic imaging terms | Capsule | 100% | Anatomic term (outside the scope of this lexicon) |
| Basic imaging terms | Central scar | 100% | Unclear clinical significance and not enough data for accurate definition |
| Basic imaging terms | Outline | 80% | Panel considered ‘margin’ to represent the same feature |
| MRI terms | Calcification at MR | 100% | Unclear clinical significance |
| Miscellaneous terms | Internal vascularity | 100% | Insufficient data for accurate definition |
| Miscellaneous terms | Rate of enhancement | 90% | insufficient data for accurate definition |
Of 31 included terms, consensus on definition was achieved for 30 (13/14 basic imaging terms, 8/8 CT terms, 6/6 MRI terms, and 3/3 miscellaneous terms). Of these, 17 terms (10 basic imaging terms, four CT terms, and three MRI terms) had 100% agreement, 11 terms (two basic imaging terms, four CT terms, two MRI terms, and three miscellaneous terms) had 90% agreement, and two terms (one basic imaging term and one MRI term) had 80% agreement. Consensus was not reached for the definition of one general term (solid mass, 60% agreement). Table 2 details the proposed definitions and points of disagreement. The lexicon was endorsed by the SAR Board of Directors.
Discussion
We developed a consensus-based lexicon to describe renal masses at CT and MRI that addresses a knowledge gap in clinical radiology reporting. As radiology practice becomes more value-based, there is an increasing emphasis on the quality and actionability of radiology reports. This renal mass lexicon may make radiology reports of renal masses more standardized, actionable, and easy to understand for referring providers, patients, and radiologists, and may improve the reproducibility and generalizability of related research. Further, a similar lexicon may be necessary in other clinical contexts; we also hope that this study provides a guide for similar such efforts in the future.
This lexicon addresses clinical reporting as well as the imaging-based research of renal masses. The terms and their definitions address a variety of imaging-based features which add clarity to radiology reports and renal mass imaging research. Some specific potential benefits include distinguishing between macroscopic and microscopic fat at MRI (26-29). These terms reflect challenging concepts and are critical to the diagnosis of masses which contain fat cells or other cells that contain fat in their cytoplasm (39). The lexicon also addresses imaging features used to differentiate solid and cystic masses, including the presence of intravenous contrast material enhancement (40-42); these are important considerations when applying the Bosniak classification (30). The lexicon does not include some of the terms used to describe cystic renal masses as the recent update proposal to the Bosniak classification provided a comprehensive description of features and definition of terms (30). Use of the lexicon may promote consistent terminology, decrease inter-observer variability, and improve reproducibility – issues that the Bosniak update proposal aimed to improve (30). This lexicon uses consistent terminology as in the Bosniak update, but expands it to encompass all terminology related to renal masses. The lexicon also adds clarity to the description of renal mass location and growth pattern; both have implications for prognosis and management, particularly surgical planning and calculating the R.E.N.A.L nephrometry score (43).
When conducting research, investigators from different institutions do not always analyze the same imaging features. When they do, the exact terms and their definitions are either incompletely expressed or inconsistent with prior studies (22-25). For example, in 2014 and 2015, Karlo et al and Shinagare et al each reported on the radiogenomics of renal cell carcinoma (23,24). While five of six mutations (VHL, BAP1, PBRM1, SETD2, KDM5C, MUC4) studied in these reports were the same, of the 10 imaging features evaluated, only four were evaluated in both studies. Furthermore, the conclusions reached for the four shared imaging features also differed despite that both studies utilized similar study cohorts; this was thought to be due to differences in the definitions of imaging features used in these studies (23,24). For some of the imaging features studied, the definitions were unclear. For example, Shinagare et al defined ‘necrosis’ as “hypodense, non-enhancing areas which were not sharply demarcated and lacked apparent walls”. Karlo et al defined ‘necrosis’ as “presence or absence of areas within the tumor that did not demonstrate contrast enhancement during the nephrographic and delayed phases” (23,24). These studies also differed in whether specific definitions were provided for well-defined margin and tumor architecture (23,24). This is not meant to be a criticism of either study; indeed, during our Delphi method, there was no initial consensus for the imaging-based definition of ‘necrosis’. Although consensus was eventually reached, the panel determined that necrosis cannot be diagnosed with certainty at imaging and therefore was difficult to define using imaging (Table 2). These difficulties highlight the need for a lexicon of standard terminology.
The modified Delphi process we used offered several advantages for arriving at expert consensus (31-34,38). Since each panelist completed the questionnaire independently and anonymously, their opinions were expressed freely and with sufficient time to research and formulate views, as opposed to the in-person roundtable method where a few members can dominate the discussion, and others may not feel as confident or comfortable expressing their opinions (31-34,38). We shared individual anonymous opinions and opposing views in Rounds 2 and 3, and geographically disparate members from different time zones discussed the sources of disagreement during the teleconference.
Given that the definitions were created using expert consensus, some of the features were by necessity arbitrarily defined (e.g., “well-defined”). We were forced to rely on expert experience in the absence of published data or published definitions. There was initial disagreement on the definition of some terms, including basic terminology that is used in daily practice. ‘Renal mass’ was one of them. The panel eventually agreed that a renal mass should include any space-occupying abnormality as opposed to including only cysts and neoplasms. The panel believed that using a broad term that encompasses any pathology would decrease the chance of a non-neoplastic condition such as focal bacterial pyelonephritis from being misdiagnosed as a neoplasm. Both ‘cystic’ and ‘solid’ were also difficult to define. Although discriminating cystic masses from solid masses is a fundamental tenant in renal mass evaluation, at the time this work began, there were no established imaging criteria for doing so. This likely explains the difficulty in reaching consensus for the definition of a ‘solid’ mass. Overall, there was general agreement that a renal mass is considered solid when it is composed of ≥25% enhancing components or fat (30); however, there was disagreement about what constitutes solid tissue. While all the panelists agreed that a solid mass may be comprised of enhancing tissue or fat, four of 10 panelists also commented that solid tissue does not always enhance and may appear as heterogeneous non-enhancing tissue at either CT or MRI.
‘Margin’ of a renal mass is often a source of confusion. The panel agreed that a ‘well-defined margin’ denotes a sharp, pencil-thin demarcation with an abrupt transition between the mass and the surrounding tissue encompassing at least 90% of the circumference of the mass. If a well-defined margin is not present, it is termed an ‘ill-defined margin’. There also may be confusion about the internal reference standard to assess ‘CT attenuation’, ‘MRI signal intensity’, and ‘enhancement’. There was consensus to use normal renal cortex as an internal reference standard. When a renal mass is heterogeneous, one or more regions of interest may need to be placed for accurate assessment. In prior literature, several terms are variably and interchangeably used to describe the presence of fat within a renal mass (26-29). The panel recommends use of ‘macroscopic fat’ and ‘microscopic fat’, and provides definitions for each (44).
The lexicon does not dictate when each term should be used. The terms in the lexicon complement the structured reporting template previously derived by the SAR RCC DFP (45-47). These studies addressed what is preferred to be included in structured reports, such as mass type (cystic mass according to Bosniak classification vs. a solid mass), presence of fat, presence of enhancement, and radiologic stage – all considered ‘core’ features when reporting indeterminate renal masses (47). This lexicon complements the prior work and provides definitions for various imaging terms that would help standardize the use of terminology in clinical reports and research studies.
The modified Delphi method has some inherent limitations, including its reliance on expert opinion informed by evidence, possible bias in selecting experts, dependence on questionnaire design, and ability of the coordinator to effectively compile the data. We tried to minimize the dependence on questionnaire design by the coordinator by allowing the panelists to propose additional imaging terms. The reliance on the ability of the coordinator to compile data was offset by using three voting rounds and a teleconference, as well as by allowing all authors to propose any final edits to the definitions. The panel included diverse members of the SAR RCC DFP with specific expertise in renal mass imaging, representing a broad range of academic experience. We did not attempt to define terms of specific disease entities (e.g., renal cell carcinoma, angiomyolipoma); these are defined elsewhere in the literature. We only included terms used at CT and MRI as these are the most common modalities used to evaluate indeterminate renal masses. An updated lexicon that includes other modalities (e.g., ultrasound) is planned.
The Radiological Society of North American (RSNA) endorses RadLex as a lexicon designed to achieve similar goals of consistency and reproducibility for all radiology reporting (4,48). We did not specifically incorporate terms defined by RSNA RadLex. While there is some common ground between RadLex and this lexicon, RadLex does not contain all the terms relevant to renal mass evaluation (e.g., ‘renal mass’ or ‘growth pattern’). Additionally, some of the terms used in RadLex are not appropriate for evaluation of a renal mass (e.g., instead of ‘well-defined margin’ and ‘ill-defined margin’, which are germane to renal mass assessment, RadLex uses ‘smooth’, ‘lobulated’ and ‘irregular’, which are less relevant). The panel excluded from consideration several terms because they were either confusing, non-contributory, or lacking sufficient evidence. The term ‘renal capsule’ is an anatomic term, and hence outside the scope of this lexicon. The term ‘outline’ was excluded because the panel considered ‘margin’ to represent the same feature. Terms such as calcification at MRI, central scar, and rate of enhancement were excluded because either the clinical significance of these terms was unclear, or there was not enough data to define them. Finally, terms related to texture analysis were not included because the techniques for conducting this type of analysis are not standardized, data on accuracy and reproducibility are scant, and its clinical utility is uncertain. The members of the SAR RCC DFP acknowledge that some of these terms may need to be reevaluated for potential inclusion in the lexicon in the future as new data emerge about their importance in the management of renal masses. Finally, despite our efforts to standardize terminology, there may be persistent reader-level variability in the assessment of a renal mass (24). We hope the specific definitions included in the lexicon will reduce this variability and take us a step closer to standardizing both radiology reports and research. Whether these goals are accomplished is a topic of further research.
In summary, this SAR-endorsed lexicon for the description of renal masses at CT and MRI has been created that attempts to address the inconsistencies and ambiguities that currently exist in clinical radiology reports and research related to renal masses. The process we used to create a renal mass lexicon may serve as a guide for the creation of lexicons in other imaging settings.
Acknowledgements:
We thank the Society of Abdominal Radiology Board of Directors for officially endorsing this lexicon
Appendix 1. Detailed Study Design
Study design: Modified Delphi method
This multi-institutional, prospective, quality improvement project was exempt from IRB oversight. A modified Delphi method was used (31-34). The Delphi technique is a structured process that relies on expert opinion and uses a series of questionnaires or ‘rounds’ to gather the required information from a selected group of experts (panelists), in this case the SAR RCC DFP. Delphi technique involves iterative, sequential, one-on-one panelist interviews. Modified Delphi technique (used in this study) involves reaching consensus by simultaneously collecting information from all panelists (49). One radiologist member of the panel (AS) served as the ‘coordinator’ who compiled the initial list of terms, prepared the questionnaires, and collected and analyzed the data. A radiology clinical fellow (HP) helped create the questionnaires used in each round. To avoid bias, the coordinator and clinical fellow did not participate in voting.
The remaining twelve radiologist members of the RCC DFP were invited to participate in the creation of the lexicon. All members are fellowship-trained abdominal radiologists with mean 13 years of experience as attending radiologist (range 5-30 years). Figure 1 outlines the process that was followed. Three rounds of questionnaires and one teleconference (after the second round) were conducted. Following the third round of questionnaires, a manuscript was created. Blinded edits to the manuscript were made and final consensus was reached. Based on prior literature, ≥80% agreement at the end of three rounds was considered sufficient ‘consensus’ regarding the inclusion or exclusion of each term and its definition (35-38). Individual panelist responses remained anonymous in all three rounds.
Initial selection of terms
The coordinator created an initial list of renal mass imaging features based on prior clinical and research experience and a literature search. A PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) search through recent literature over a period of two years was performed from January 2016 to December 2017 using the following search string: “(renal or kidney) and (imaging or computed or CT or magnetic or MRI) and (features or findings)”. This yielded 60 publications. The coordinator screened the full text of these publications for imaging terms used to describe renal masses. This literature search was performed mainly to ensure that no commonly used imaging terms were missed; the actual selection of imaging terms occurred in Rounds 1 and 2 of the Delphi process during which the panelists voted on inclusion of each term and also suggested additional imaging terms if needed. The selected imaging terms were categorized into four categories: ‘basic imaging terms’, ‘CT terms’, ‘MRI terms’ and ‘miscellaneous terms’.
Round 1 questionnaire
The Round 1 questionnaire containing the list of the selected terms was administered using REDCap (https://redcap.partners.org/redcap/index.php), a secure web application for building and managing online questionnaires. Each panelist had a unique link to access their questionnaire. Each panelist was emailed up to 4 automated reminders, one week apart, to complete the questionnaire. All responses were submitted anonymously while blinded to the responses of the other participants.
For each term, the panelists were asked if the term should be included (Options: ‘include’ or ‘exclude’). If they selected ‘include’, they were asked to suggest a definition for that term in the form of free text without a word limit. During Round 1, panelists also were asked to suggest additional terms to include in the lexicon.
Round 2 questionnaire
The responses from Round 1 were analyzed by the coordinator using simple descriptive statistics. The percentage of responses for inclusion or exclusion of each term were summarized. The proposed definitions of each term were compiled to create either a single unified definition, or 2-4 alternative definitions if the content of the proposed definitions varied substantially and the coordinator was unable to coalesce them into a single definition. The summary statistics regarding inclusion and exclusion and proposed summary definition(s) were incorporated into the Round 2 questionnaire. Any rationale provided by the panelists in Round 1 to support their conflicting views was included for consideration by the other members in Round 2. In Round 2, the panelists were asked again to vote to ‘include’ or ‘exclude’ each term. For each term, if a single unified definition was suggested, the panelists were asked if they agreed with the proposed definition (Options: ‘agree’ or ‘disagree’). If they disagreed, they were required to provide an alternative definition. When more than one definition was provided, they were asked to select one of the options or to provide a new definition.
Two new terms were proposed to be added during Round 1 (‘magnetic susceptibility’ and ‘growth rate’). These were included in Round 2. Panelists were asked if these terms should be included and, if so, they were asked to suggest a definition, similar to Round 1.
The same anonymous blinded method was used to administer the Round 2 questionnaire.
Teleconference
A teleconference was conducted after the completion of Round 2 data analysis to address issues that prevented reaching consensus for terms with persistent disagreement. A summary of the discussion at the teleconference was provided to all the panelists as part of the Round 3 questionnaire.
Round 3 questionnaire
Data extracted from the Round 2 questionnaire were analyzed by the coordinator in the same fashion as the data from Round 1. A consensus definition was provided for each term. Whenever new definitions were provided, an attempt was made to reconcile these with the original proposed definition to create either a single proposed definition or a set of alternatives from which to select.
If there was 100% consensus regarding inclusion or exclusion of a particular term or definition of a term, these terms and definitions were considered ‘finalized’. This information was provided in the Round 3 questionnaire with no further questions regarding these terms. Terms and definitions that had not met 100% consensus were included in the Round 3 questionnaire. The Round 3 questionnaire was administered to all panelists from Round 2 in the same blinded and anonymous manner as the first two questionnaire rounds. The results were summarized at the end of Round 3.
Footnotes
Disclosures:
Relevant to this work: None
Not relevant:
Atul Shinagare: Consultant, Arog Pharmaceuticals, Virtualscopics
Matthew Davenport: Royalties from Wolters Kluwer
Hyesun Park: No disclosures listed
Hersh Chandarana: Research support in form of hardware and software from Siemens Healthcare, Patent and provisional patents: MR technique GRASP and automated assessment of Image Quality with Deep Learning.
Ankur M. Doshi: No disclosures listed
Ivan Pedrosa: Honorarium for a Bayer Scientific Advisory Board, co-inventor of patents with Philips Healthcare
Erick M. Remer: No disclosures listed
Nicola Schieda: No disclosures listed
Andrew Smith: President of eMASS LLC, patents pending, and President of Radiostics LLC
Raghunandan Vikram: No disclosures listed
Zhen J. Wang: Consultant, GE Healthcare; Shareholder, Nextrast, Inc.
Stuart Silverman: Grant support NIH 1R21CA216796-01A1
References
- 1.Baron RL. The radiologist as interpreter and translator. Radiology. 2014. July;272(1):4–8. [DOI] [PubMed] [Google Scholar]
- 2.Breast Imaging Reporting & Data System [Internet]. [cited 2018 Jul 2]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads
- 3.Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiol Clin North Am. 2002. May;40(3):409–30, v. [DOI] [PubMed] [Google Scholar]
- 4.Langlotz CP. RadLex: a new method for indexing online educational materials. Radiogr Rev Publ Radiol Soc N Am Inc. 2006. December;26(6):1595–7. [DOI] [PubMed] [Google Scholar]
- 5.Bertaud V, Lasbleiz J, Mougin F, Burgun A, Duvauferrier R. A unified representation of findings in clinical radiology using the UMLS and DICOM. Int J Med Inf. 2008. September;77(9):621–9. [DOI] [PubMed] [Google Scholar]
- 6.Filice RW, Kahn CE. Integrating an Ontology of Radiology Differential Diagnosis with ICD-10-CM, RadLex, and SNOMED CT. J Digit Imaging. 2019. January 31; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Radiology (ACR) Illustrated Breast Imaging Reporting and Data System (BIRADS TM) American College of Radiology (ACR) Reston, VA: (1998). [Google Scholar]
- 8.LI-RADS [Internet]. [cited 2018 Jul 2]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS
- 9.Lung Rads [Internet]. [cited 2018 Jul 2]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
- 10.PI-RADS [Internet]. [cited 2018 Jul 2]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/PI-RADS
- 11.Boland GW, Duszak R. Structured Reporting and Communication. J Am Coll Radiol JACR. 2015. December;12(12 Pt A):1286–8. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz LH, Panicek DM, Berk AR, Li Y, Hricak H. Improving communication of diagnostic radiology findings through structured reporting. Radiology. 2011. July;260(1):174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn CE, Heilbrun ME, Applegate KE. From guidelines to practice: how reporting templates promote the use of radiology practice guidelines. J Am Coll Radiol JACR. 2013. April;10(4):268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herts BR, Gandhi NS, Schneider E, Coppa CP, Mody RN, Baker ME, et al. How We Do It: Creating Consistent Structure and Content in Abdominal Radiology Report Templates. AJR Am J Roentgenol. 2019. January 8;1–7. [DOI] [PubMed] [Google Scholar]
- 15.Brook OR, Brook A, Vollmer CM, Kent TS, Sanchez N, Pedrosa I. Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning. Radiology. 2015. February;274(2):464–72. [DOI] [PubMed] [Google Scholar]
- 16.Khorasani R, Bates DW, Teeger S, Rothschild JM, Adams DF, Seltzer SE. Is terminology used effectively to convey diagnostic certainty in radiology reports? Acad Radiol. 2003. June;10(6):685–8. [DOI] [PubMed] [Google Scholar]
- 17.Mityul MI, Gilcrease-Garcia B, Searleman A, Demertzis JL, Gunn AJ. Interpretive Differences Between Patients and Radiologists Regarding the Diagnostic Confidence Associated With Commonly Used Phrases in the Radiology Report. AJR Am J Roentgenol. 2017. October 12;1–4. [DOI] [PubMed] [Google Scholar]
- 18.Bosmans JML, Weyler JJ, De Schepper AM, Parizel PM. The radiology report as seen by radiologists and referring clinicians: results of the COVER and ROVER surveys. Radiology. 2011. April;259(1):184–95. [DOI] [PubMed] [Google Scholar]
- 19.Gunn AJ, Tuttle MC, Flores EJ, Mangano MD, Bennett SE, Sahani DV, et al. Differing Interpretations of Report Terminology Between Primary Care Physicians and Radiologists. J Am Coll Radiol JACR. 2016. December;13(12 Pt A):1525–1529.e1. [DOI] [PubMed] [Google Scholar]
- 20.Rosenkrantz AB, Kiritsy M, Kim S. How “consistent” is “consistent”? A clinician-based assessment of the reliability of expressions used by radiologists to communicate diagnostic confidence. Clin Radiol. 2014. July;69(7):745–9. [DOI] [PubMed] [Google Scholar]
- 21.Shinagare AB, Lacson R, Boland GW, Wang A, Silverman SG, Mayo-Smith WW, et al. Radiologist Preferences, Agreement, and Variability in Phrases Used to Convey Diagnostic Certainty in Radiology Reports. J Am Coll Radiol JACR. 2018. December 22; [DOI] [PubMed] [Google Scholar]
- 22.Egbert ND, Caoili EM, Cohan RH, Davenport MS, Francis IR, Kunju LP, et al. Differentiation of papillary renal cell carcinoma subtypes on CT and MRI. AJR Am J Roentgenol. 2013. August;201(2):347–55. [DOI] [PubMed] [Google Scholar]
- 23.Karlo CA, Di Paolo PL, Chaim J, Hakimi AA, Ostrovnaya I, Russo P, et al. Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology. 2014. February;270(2):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinagare AB, Vikram R, Jaffe C, Akin O, Kirby J, Huang E, et al. Radiogenomics of clear cell renal cell carcinoma: preliminary findings of The Cancer Genome Atlas-Renal Cell Carcinoma (TCGA-RCC) Imaging Research Group. Abdom Imaging. 2015. August;40(6):1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamshidi N, Jonasch E, Zapala M, Korn RL, Aganovic L, Zhao H, et al. The Radiogenomic Risk Score: Construction of a Prognostic Quantitative, Noninvasive Image-based Molecular Assay for Renal Cell Carcinoma. Radiology. 2015. October;277(1):114–23. [DOI] [PubMed] [Google Scholar]
- 26.Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014. June;39(3):588–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hindman N, Ngo L, Genega EM, Melamed J, Wei J, Braza JM, et al. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology. 2012. November;265(2):468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim RS, Flood TA, McInnes MDF, Lavallee LT, Schieda N. Renal angiomyolipoma without visible fat: Can we make the diagnosis using CT and MRI? Eur Radiol. 2018. February;28(2):542–53. [DOI] [PubMed] [Google Scholar]
- 29.Pokharel SS, Macura KJ, Kamel IR, Zaheer A. Current MR imaging lipid detection techniques for diagnosis of lesions in the abdomen and pelvis. Radiogr Rev Publ Radiol Soc N Am Inc. 2013. May;33(3):681–702. [DOI] [PubMed] [Google Scholar]
- 30.Silverman SG, Pedrosa I, Ellis JH, Hindman NM, Schieda N, Smith AD, et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology. 2019;292(2):475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000. October;32(4):1008–15. [PubMed] [Google Scholar]
- 32.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003. February;41(4):376–82. [DOI] [PubMed] [Google Scholar]
- 33.Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PloS One. 2011;6(6):e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphrey-Murto S, Varpio L, Wood TJ, Gonsalves C, Ufholz L-A, Mascioli K, et al. The Use of the Delphi and Other Consensus Group Methods in Medical Education Research: A Review. Acad Med J Assoc Am Med Coll. 2017;92(10):1491–8. [DOI] [PubMed] [Google Scholar]
- 35.Sun BC, Thiruganasambandamoorthy V, Cruz JD, Consortium to Standardize ED Syncope Risk Stratification Reporting. Standardized reporting guidelines for emergency department syncope risk-stratification research. Acad Emerg Med Off J Soc Acad Emerg Med. 2012. June;19(6):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berk L, Jorm AF, Kelly CM, Dodd S, Berk M. Development of guidelines for caregivers of people with bipolar disorder: a Delphi expert consensus study. Bipolar Disord. 2011. September;13(5–6):556–70. [DOI] [PubMed] [Google Scholar]
- 37.Eubank BH, Mohtadi NG, Lafave MR, Wiley JP, Bois AJ, Boorman RS, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016. 20;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum. 2011. October;41(2):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schieda N, Davenport MS, Pedrosa I, Shinagare A, Chandarana H, Curci N, et al. Renal and adrenal masses containing fat at MRI: Proposed nomenclature by the society of abdominal radiology disease-focused panel on renal cell carcinoma. J Magn Reson Imaging JMRI. 2019. April;49(4):917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Harbi F, Tabatabaeefar L, Jewett MA, Finelli A, O’Malley M, Atri M. Enhancement Threshold of Small (< 4 cm) Solid Renal Masses on CT. AJR Am J Roentgenol. 2016. March;206(3):554–8. [DOI] [PubMed] [Google Scholar]
- 41.Pallwein-Prettner L, Flöry D, Rotter CR, Pogner K, Syré G, Fellner C, et al. Assessment and characterisation of common renal masses with CT and MRI. Insights Imaging. 2011. October;2(5):543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kay FU, Canvasser NE, Xi Y, Pinho DF, Costa DN, Diaz de Leon A, et al. Diagnostic Performance and Interreader Agreement of a Standardized MR Imaging Approach in the Prediction of Small Renal Mass Histology. Radiology. 2018;287(2):543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009. September;182(3):844–53. [DOI] [PubMed] [Google Scholar]
- 44.Schieda N, Davenport MS, Pedrosa I, Shinagare A, Chandarana H, Doshi A, Curci N, Israel GM, Remer E, Wang J, Silverman SG. Renal and adrenal masses containing fat at MRI: proposed nomenclature by the Society of Abdominal Radiology Disease-Focused Panel on renal cell carcinoma. J Magn Reson Imaging (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davenport MS, Hu EM, Smith AD, Chandarana H, Hafez K, Palapattu GS, et al. Reporting standards for the imaging-based diagnosis of renal masses on CT and MRI: a national survey of academic abdominal radiologists and urologists. Abdom Radiol N Y. 2017;42(4):1229–40. [DOI] [PubMed] [Google Scholar]
- 46.Hu EM, Zhang A, Silverman SG, Pedrosa I, Wang ZJ, Smith AD, et al. Multi-institutional analysis of CT and MRI reports evaluating indeterminate renal masses: comparison to a national survey investigating desired report elements. Abdom Radiol N Y. 2018. December;43(12):3493–502. [DOI] [PubMed] [Google Scholar]
- 47.Davenport MS, Hu EM, Zhang A, Shinagare AB, Smith AD, Pedrosa I, et al. Standardized report template for indeterminate renal masses at CT and MRI: a collaborative product of the SAR Disease-Focused Panel on Renal Cell Carcinoma. Abdom Radiol N Y. 2018. December 3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.RadLex [Internet]. [cited 2017 Mar 9]. Available from: https://www.rsna.org/RadLex.aspx
- 49.Ozier W. The Delphi/Modified Delphi Technique: A Consensus Approach to Information Valuation. :16. [Google Scholar]


