Abstract
Mild traumatic brain injury (mTBI) is a major public health concern. Cerebrovascular alterations play a significant role in the evolution of injury sequelae and in the process of post-traumatic brain repair. Arterial spin labeling (ASL) is an advanced perfusion magnetic resonance imaging technique that permits noninvasive quantification of cerebral blood flow (CBF). This is the first systematic review of ASL research findings in patients with mTBI. Our approach followed the American Academy of Neurology (AAN) and PRISMA guidelines. We searched Ovid/MEDLINE, Web of Science, Scopus, and the Cochrane Index for relevant articles published as of February 20, 2020. Full-text results were combined into Rayyan software for further evaluation. Data extraction, including risk of bias ratings, was performed using American Academy of Neurology’s four-tiered classification scheme. Twenty-three articles met inclusion criteria comprising data on up to 566 mTBI patients and 654 control subjects. Of the 23 studies, 18 reported some type of regional CBF abnormality in mTBI patients at rest or during a cognitive task, with more findings of decreased than increased CBF. The evidence supports the conclusion that mTBI likely causes ASL-derived CBF anomalies. However, synthesis of findings was challenging due to substantial methodological variations across studies and few studies with low risk of bias. Thus, larger-scale prospective cohort studies are needed to more definitively chart the course of CBF changes in humans after mTBI and to understand how individual difference factors contribute to post-injury CBF changes.
Keywords: mild traumatic brain injury, concussion, cerebral blood flow, arterial spin labeling, magnetic resonance imaging
Introduction
Traumatic brain injury (TBI) is a significant public health concern and one of the leading causes of morbidity and mortality (Faul et al. 2010). In the United States, at least 3.5 million people experience a TBI and require medical evaluation each year (Coronado et al. 2012). Mild TBI (mTBI) represents the vast majority of all cases of head trauma and may cause significant, and sometimes persistent, neurocognitive and neurobehavioral dysfunction (McInnes et al. 2017). Despite an increase in knowledge of the acute and potential long-term effects of mTBI, its underlying mechanisms remain to be fully elucidated (Manley and Maas 2013; Slobounov et al. 2012; McInnes et al. 2017; Giza and Hovda 2014; Werner and Engelhard 2007a; McAllister 2011). It is largely recognized that after the primary mechanical insult to brain tissue, TBI leads to delayed secondary injury because of neurochemical, metabolic, and cellular changes (Werner and Engelhard 2007b; Loane and Faden 2010; McAllister 2011; Giza and Hovda 2014; Pearn et al. 2017; Toth et al. 2016; Jassam et al. 2017). Although the pathogenesis has yet to be entirely revealed, cerebrovascular alterations appear to contribute to the evolving secondary injury and slow brain repair (Dijkhuizen 2011; Len and Neary 2011; Pop and Badaut 2011; Tan et al. 2014; A. J. Gardner et al. 2015).
Several neurobiological mechanisms may contribute to cerebral blood flow (CBF) changes after mTBI (Len and Neary 2011; Pop and Badaut 2011; Giza and Hovda 2014; Toth et al. 2016). Generally, local CBF is coupled with neuronal metabolism through the neurovascular unit (NVU), a physiological entity structurally defined by interactions amongst endothelial cells, pericytes, smooth muscle cells, astrocytes, and neurons (Iadecola and Nedergaard 2007; Pop and Badaut 2011). The NVU contributes to the pathogenesis of TBI, either directly from physical trauma or as part of the cascade of secondary injury after TBI (Kenney et al. 2016; Pop and Badaut 2011). The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells and related cellular constituents of the NVU (such as astrocytes and pericytes). The BBB has been proposed to be central for the proper functioning of the NVU, as the BBB maintains brain homeostasis through nutrient regulation and directly contributes to CBF (Pop and Badaut 2011). Post-injury changes in the NVU, such as disruption of the BBB, are primarily observed in the first week post-injury. It is unknown how these changes in the NVU evolve over a long time period (Pop and Badaut 2011; Kenney et al. 2016).
Cerebral autoregulation, the intrinsic ability of the brain to maintain a constant CBF in response to variations in systemic blood pressure, is impaired following mTBI (Junger et al. 1997; Strebel et al. 1997; Rangel-Castilla et al. 2008; Len and Neary 2011). TBI appears to impair CBF autoregulation in response to both decreasing and increasing perfusion pressure in adults and children (Toth et al. 2016). Changes of CBF autoregulatory function could take place in cerebral circulation after TBI, as cortical spreading depolarization-related neurovascular dysfunction has been speculated to play an important role in molecular and cellular mechanisms of autoregulatory dysfunction (Toth et al. 2016). Links between autoregulatory dysfunction, impaired myogenic response, microvascular impairment, and the development of secondary brain damage also have been detected (Toth et al. 2016). In addition, the autonomic and cardiovascular systems may become uncoupled after acute brain injury (Goldstein et al. 1998), and deficits in neuroautonomic control following brain injury could be associated with abnormal cerebrovascular responses (Zhang et al. 2002).
Emerging evidence indicates inflammatory changes are also triggered by mTBI (Giza and Hovda 2014). Neuroinflammation is well-established as an important aspect of secondary injury in animal and human studies (Kumar and Loane 2012; Smith et al. 2013; McAllister 2011; Bigler 2013; Ramlackhansingh et al. 2011; W. Wang et al. 2017a). Microglia and astrocytes play a crucial role in the neuroinflammatory processes, presumably sensing neuronal damage and initiating the brain’s immune response to injury (Smith et al. 2013). Recent animal studies suggest CBF recovery in the perilesional areas after TBI could be attributed to increased glial response, and CBF might be a sensitive biomarker for assessment of neuroinflammation and drug efficacy in the TBI model (W. Wang et al. 2017b). A detailed description of these neuroinflammatory consequences of mTBI is beyond the scope of the present discussion.
Several nuclear imaging methods are available to assess CBF after mTBI, such as single-photon emission computed tomography (Audenaert et al. 2003; Gowda et al. 2006) and perfusion computed tomography (Metting et al. 2014). They have several disadvantages including financial cost, ionizing radiation, and limited repetition of acquisitions. In contrast, arterial spin labeling (ASL) is a class of advanced techniques for perfusion magnetic resonance imaging (MRI) that permits noninvasive quantification of CBF using magnetically labeled arterial blood water as an endogenous contrast tracer (Detre et al. 2009; J. Wang et al. 2003; Y. Wang et al. 2011; Wong 2014; Alsop et al. 2015). Through a number of methodological advances, high-quality whole brain perfusion images can be obtained in just a few minutes using an ASL scan (Alsop et al. 2015; Detre et al. 2012; Wong 2014). ASL perfusion MRI has been validated extensively against other methods (Alsop et al. 2015; Ewing et al. 2005). Various ASL MRI sequences using the main labeling techniques, particularly pulsed ASL (PASL) or pseudo-continuous ASL (pCASL), are now commercially available on all major MRI platforms, with demonstrated reproducibility (Gevers et al. 2009; Petersen et al. 2010). ASL perfusion brain MRI has been used to assess CBF as a surrogate marker of brain function and metabolism in numerous clinical populations, such as stroke, neurodegeneration, brain tumor, and neurovascular diseases (Haller et al. 2016).
An increasing body of preclinical research indicates ASL perfusion MRI is feasible for the serial, noninvasive measurement of CBF after experimental TBI (Forbes et al. 1997; Hayward et al. 2010; Hendrich et al. 1999; Kochanek et al. 2002). ASL was successfully used to evaluate CBF after cortical contusion in anesthetized and paralyzed rats and showed the clear heterogeneity of CBF at 24 hours post-injury (Forbes et al. 1997). In one study that used the controlled cortical impact (CCI) model, ASL MRI detected reduced global CBF at ~3 hours with 85% and 49% reductions in the injury side and contralateral cortex, respectively (Hendrich et al. 1999). At 8–9 months post-lateral fluid-percussion (LFP) TBI, ASL MRI showed reduced regional CBF that could not be attributed to changes in histologically assessed vascular density (Hayward et al. 2010). In another rat sample one year after CCI, ASL MRI found dramatically reduced CBF at or near the impact site, including injured cortex and hippocampus, translating into reduced hemispheric CBF (Kochanek et al. 2002). These results suggest changes in CBF can be detected using ASL MRI at different stages of TBI recovery in an animal model. The combination of altered CBF and other structural and functional changes can potentially be a source of secondary injury after TBI.
Objectives
This review aimed to systematically evaluate the available evidence regarding the question: In humans, does mTBI cause changes in CBF measurable through ASL imaging?
Method
General Approach
This review was designed to be consistent with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al. 2009), as well as the methodology published by the American Academy of Neurology (AAN) for conducting systematic reviews (Gronseth et al. 2015; Gronseth et al. 2011; Gronseth et al. 2017; Rae-Grant et al. 2019). The following methodology was adapted from the protocols set forth by Pertab et al. (Pertab et al. 2018) and Gardner et al. (A. Gardner et al. 2012). Studies reporting ASL perfusion MRI data in mTBI patients were considered in the current review. We expected the majority of the literature would evaluate the relationship between mTBI and ASL metrics at various stages of recovery (vs. non-mTBI groups) to inform this question. However, we also planned to include any literature on the relationship between ASL measures and clinical signs of injury severity (e.g., symptoms, neurocognitive performance, symptom duration) to inform the degree to which there may be a dose-response relationship between mTBI severity and ASL perfusion. Per AAN guidelines, this could contribute to our conclusion about the plausibility of the biological effect of mTBI on CBF via ASL imaging (Gronseth et al. 2017; Rae-Grant et al. 2019).
Search Strategy
The review was conducted in three stages:
Stage 1:
Articles were retrieved with the assistance of a medical librarian with a preliminary search through four electronic databases (Ovid/MEDLINE, Web of Science, Scopus, and Cochrane) to find articles of interest. These articles were combined with the private collection of the authors to identify key articles relevant to the research question. Eligible articles were published in English from 1998 to February 20, 2020. A start date of 1998 was selected because utilizing MRI with ASL is a relatively new technique mostly performed in animals before this time (Talagala and Noll 1998; Koretsky 2012; Detre et al. 2009). The following keywords and combinations were used to search the databases in a comprehensive manner: arterial spin labeling, arterial spin tagging, cerebral blood flow, perfusion magnetic resonance imaging, perfusion weighted MRI, perfusion MRI, magnetic resonance imaging, mild traumatic brain injury, mTBI, traumatic brain injury, concussion, sports-related concussion, brain concussion, brain injury, brain damage, brain trauma.
Stage 2:
The full-text results were combined into Rayyan software (Ouzzani et al. 2016) and reviewed by two independent raters who removed duplicates and excluded irrelevant articles based on the following prespecified inclusion and exclusion criteria:
Full text article published in a peer reviewed medical journal presenting original data. Excluded review or commentary articles summarizing the work of others. Excluded abstracts without full text and published poster abstracts.
Study conducted on humans.
Study included analysis of a group diagnosed with mTBI or concussion. When mixed brain injury samples were studied, the results must have been provided separately for a subgroup of participants with mTBI. Our author group considers concussion to be a mild form of a broader spectrum of mTBI. In particular, mTBI is often defined as admission Glasgow Coma Scale score 13–15 or through somewhat more restrictive criteria that place a cap on the duration of any unconsciousness or posttraumatic amnesia (e.g., per definition of the American Congress of Rehabilitation Medicine) (Kay et al. 1993). Concussion, on the other hand, is typically reserved to describe mTBIs for which there are no acute intracranial findings on neuroimaging; in the context of sport-related injuries, concussions tend to be characterized by acute signs/symptoms suggesting much milder injuries (e.g., low prevalence of unconsciousness/amnesia) than those in civilian/hospital-recruited populations. Due to the small number of studies eligible for inclusion, we planned to include any sample characterized as mTBI by the author, so long as admission Glasgow Coma Scale scores were at least 13.
Study presented data on at least one variable assessing CBF using ASL, including various different ASL MRI techniques, such as two or three dimensional (2D or 3D) pCASL, 2D or 3D PASL, VS-ASL (velocity selective ASL), SNS-ASL (spatially nonselective ASL), true FISP (true fast imaging with steady state precession) ASL, etc.
A third independent rater compared the results for final consensus of the included articles.
Stage 3:
Based on the final included articles, two independent raters were given the full text in order to extract study characteristics for quality. These characteristics were entered into independent evidence tables that summarized key components of each study including sample and control demographics and descriptions, time since injury, concussion definition, results, limitations, etc. The two evidence tables were analyzed by a third party for comparison, clarification, and discrepancy resolution.
The third party created a final master evidence table, which was used as the basis for the study results. The flow chart depicted in Figure 1 reports the number of articles identified during screening and excluded at each phase of review.
Figure 1.
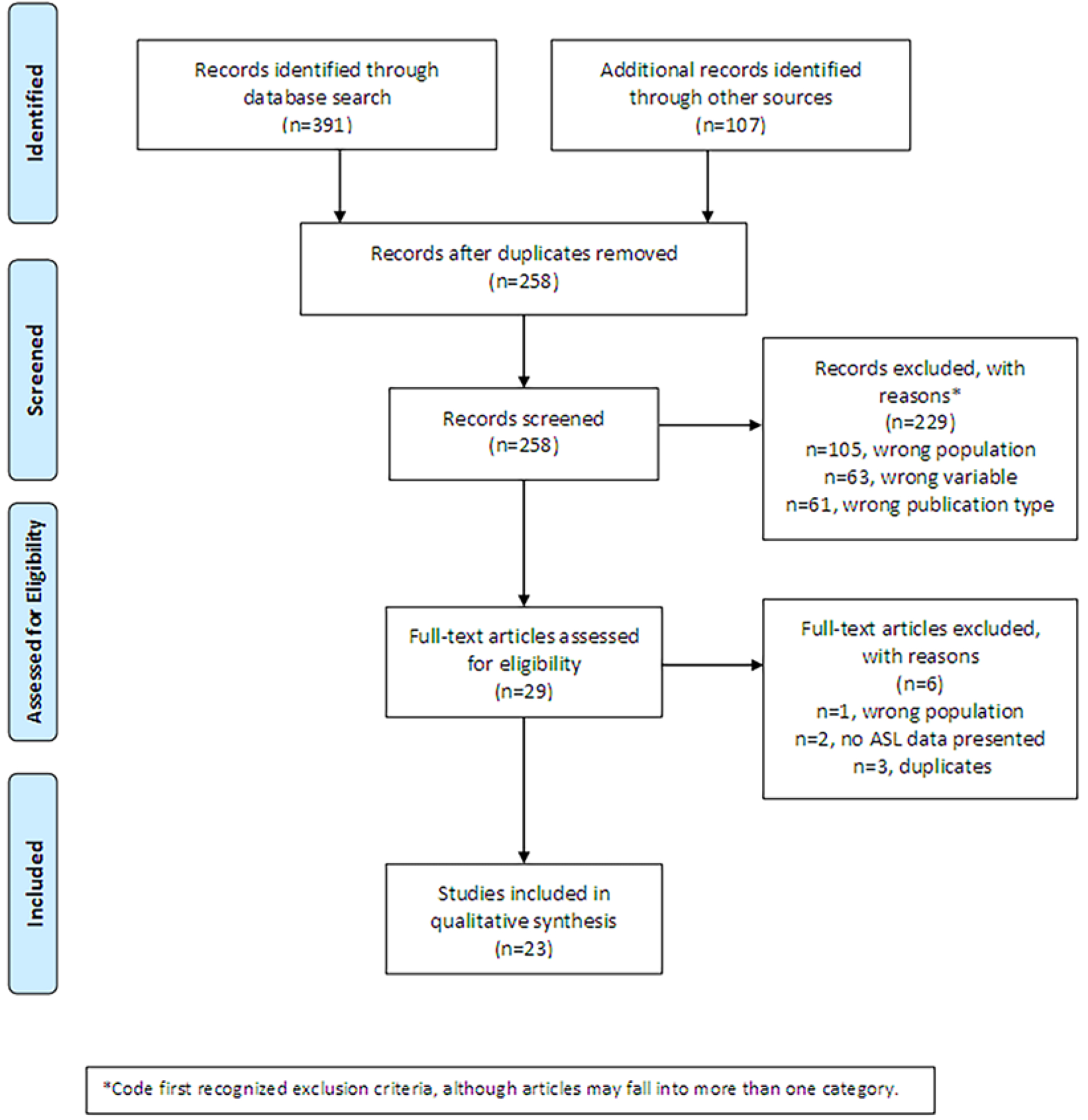
PRISMA Flow Diagram
Risk of Bias Ratings and Data Analysis
For those articles that met the inclusion criteria, the quality of the evidence was evaluated by employing the qualitative and quantitative tools described in the AAN guidelines (Gronseth et al. 2015; Gronseth et al. 2011; Gronseth et al. 2017; Rae-Grant et al. 2019). The guidelines include processes for evaluating both the risk of systematic bias in individual studies (grading criteria) and the risk at the outcome/conclusion level. The recommended approach for establishing studies’ risk of bias includes overlapping but different considerations for diagnostic, prognostic, and causation research questions. Given our objective, we primarily relied on the AAN guidelines for evaluating bias for questions of causation. In particular, to assign a Class I rating we required all of the following criteria to be met: (a) clearly defined inclusion/exclusion criteria, (b) primary outcome defined, (c) at least 80% complete data on key metrics at any time point, (d) objective outcome measurement, and (e) all relevant confounding characteristics presented and substantially equivalent between groups or appropriately accounted for statistically.
While there appears to be some variability in how the phrase “prospective study” is interpreted across investigators, we decided to declare a study as a prospective cohort study if subject enrollment and some data collection (e.g., clinical assessment) occurred prior to participants’ injuries occurring. However, pre-injury measurement of the outcome (CBF changes) was not conducted in any study identified and would further strengthen the rigor of a study. We felt this decision was most consistent with the AAN definition of prospective cohort study and perhaps leaned toward being more conservative relative to the many studies that recruit acutely post-injury and could meet the definition of a prospective cohort study. Formally, the AAN defines a prospective study as one that enrolled subjects and performed some data collection before the outcome is experienced, whereas a retrospective study enrolled patients and collected some data after at least some patients reached the outcome. Since the timepoint at which the outcome in this study (CBF changes due to injury) occurs is uncertain and the clarity of reporting around how subjects were identified in the studies that enrolled patients post-injury was highly variable, we felt it most straightforward and conservative to declare any study that enrolled subjects post-injury to be a retrospective cohort or case control study (i.e., one in which subjects who had already experienced the outcome were selected to clearly be either an mTBI or a non-mTBI patient and then that historical event used to predict CBF changes).
Second, to consider a study to have adequate inclusion/exclusion criteria, we primarily considered whether the definition of mTBI was clear. We declared studies that either cited a specific definition (e.g., ACRM [American Congress of Rehabilitation Medicine] or CISG [Concussion in Sport Group] consensus guidelines) or listed the elements of widely accepted definitions (e.g., head trauma with altered mental status or new-onset symptoms) to be adequate. We declared studies relying on clinician diagnoses without specific criteria to be inadequate, as well as definitions written so broadly they conflicted with widely accepted definitions (e.g., allowing for cases with bodily trauma and perfect mental status to be declared as mTBI) (Barlow et al. 2017).
Finally, after rating each study’s risk of bias, we synthesized the evidence to develop conclusions. AAN recommends specific language for certainty (“highly likely,” “likely,” “possible,” or “insufficient evidence”) based on the number of studies available in each class and consideration of other factors. Risk of bias is intended to primarily address sources of systematic bias, but factors that might affect the strength of findings (e.g., low power) should be considered in determining certainty for one’s conclusions. For studies that did not perform multiple comparison correction, we did so using the false discovery rate control method when possible from the reported data, and we described how the author’s conclusions were affected. Other factors such as the consistency of findings, statistical power of studies, effect sizes, biological plausibility, and evidence for a dose-response relationship were considered. Risk of bias decisions are documented in Table 1. Because all studies were determined to have sufficiently objective outcome measures, these variables are excluded from Table 1. Additional data on the study methods are presented in Tables 2–5 including (1) participant demographics, (2) participant characteristics (injury cause, concussive history), (3) time lapsed (acute, subacute, or chronic assessment), (4) ASL technique and protocol, (5) ASL data analysis approaches, (6) other imaging or clinical assessments, (7) main results of the study, and (8) study conclusion (see Tables 1–5 for data extraction results).
Table 1.
Risk of bias of studies assessing CBF using ASL in mTBI (AAN rating criteria)
| Class I Requirements1 | ||||||
|---|---|---|---|---|---|---|
| Prospective cohort | Relevant confounds equivalent/controlled for | Clear inclusion/exclusion criteria | ≥80% complete data | Downgrades | Risk of bias | |
| Maximum class if criterion not met: | II | III | IV | III | III | |
| Barlow, 2017 | N | Y | N5 | N7 | - | IV |
| Brooks, 2019 | N | Y | Y | Y | - | II |
| Churchill, 2017a | N | Y | Y | Y | - | II |
| Churchill, 2017b | N | Y | Y | Y | - | II |
| Churchill, 2019a | N | Y | Y | Y | - | II |
| Churchill, 2019b | N | Y | Y | Y | - | II |
| Coverdale, 2020 | N | Y | Y | Y | - | II |
| Doshi, 2018 | N | Y | Y | N8 | - | III |
| Ge, 2009 | N | Y | N5 | Y | IV | |
| Hamer, 2019 | N | Y (female group); N (male group)2 |
N | Y | IV | |
| Lin, 2016 | N | Y | Y | Y | - | II |
| Liu, 2016 | N | Y | Y | Y | - | II |
| Meier, 2018 | N | Y | N6 | N (Time 3) | - | IV |
| Militana, 2016 | N | Y | N6 | Y | - | IV |
| Moller, 2017 | N | Y | Y | Y | narrow patient spectrum10 | III |
| Mutch, 2016 | N | N3 | N6 | Y | narrow patient spectrum | IV |
| Mutch, 2018 | N | N3 | Y | N9 | historical controls | III |
| Peng, 2016 | N | Y | N5 | Retention NR | - | IV |
| Sours, 2015 | N | Y (primary group comparison); N (secondary analysis)4 |
N5 | Excluded cases NR | - | IV |
| Stephens, 2018 | N | Y | Y | Y | narrow patient spectrum | III |
| Wang, 2015 | N | Y | N5 | Y | narrow patient spectrum | IV |
| Wang, 2016 | Y | Y | Y | Y | - | I |
| Wang, 2019 | Y | Y | Y | Excluded cases NR | - | I |
Note. Studies are listed by the first author last name and publication year. Because of ambiguity around when patients met the outcome (cerebral blood flow changes), studies were conservatively only considered prospective if they enrolled subjects pre-injury. AAN = American Academic of Neurology; NR = not reported
Requirements met by all studies (objective outcome, clearly defined primary outcomes) are not listed
Analyses did not control for age despite significant age difference between male cases and controls
Age/gender matching not reported in the paper but was verified as not significantly different between groups based on the data presented
Second analysis of mTBI subgroups did not control for age despite significant age difference between groups
Definitions of concussion/mTBI that did not clearly require both trauma and either AMS or concussion symptoms were considered insufficient
Physician diagnoses made without clearly indicating a specific definition of mTBI/concussion were considered insufficient
79% of symptomatic mTBI group could complete the MRI
2/7 patients excluded for motion artifact
21% of controls excluded for motion artifact
Narrow patient spectrum declared for any sample recruited entirely from a concussion/mTBI clinic
Table 2.
Study characteristics
| First author, year | mTBI | Control | Control/matching sample and criteria | mTBI recruitment site | Patient population | Injury cause | mTBI stage | Time lapse post-injury | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|
| Barlow, 2017 | n=51 (27 with PCS) Age: 14.1 (13–15) Male: 47% | n=21 Age: 14.4 (13–15) Male: 40% | Healthy controls without history of TBI, similar age and sex | ED or concussion clinic | ED patients | Heterogeneous, 60% SRC | Subacute (later) |
M: 40 days (SD: 7.6, 95% CI: 3644) | None |
| Brooks, 2019 | n=37 Age: 14.8 (13.9–15.7) Male: 54% |
n=16 Age: 14.3 (12.715.9) Male: 56% |
Orthopedic injury without HOC No difference on age, sex, handedness, ethnicity, parents’ education |
Existing research database | ED patients | Unknown | Chronic | M: 2.7 years (range: 2.33.0 yrs) | None |
| Churchill, 2017a | n=35 Age: 20.3 ± 2.2 Male: 46% |
n=35 Age: 20.3 ± 1.7 Male: 46% |
Healthy controls without concussion in past 6 months, matched on sex, age, concussion history | University sports medicine clinic | Athlete | SRC | Acute or subacute (early) | M: 4.2 days (SD:1.3, range: 1–7 days) | None |
| Churchill, 2017b | n=26 Age: 20 (17–23) Male: 46% |
n= Age: 20 (18–23) Male: 46% |
Healthy controls without concussion in past 6 months, matched on sex, age, concussion history | University sports medicine clinic | Athlete | SRC | Acute or subacute (early) | M: 4 days (range: 1–7 days) | None |
| Churchill, 2019a | n=21 Age: 20.2 ± 2.2 Male: 40% |
n=56 Age: 20.1 ± 2.2 Male: 51% |
Healthy controls without concussion in past 6 months, matched on sex, age, concussion history | University sports medicine clinic | Athlete | SRC | Acute or subacute (early) | Med: 4 days (range: 1–7 days) | None |
| Churchill, 2019b | n=24 Age: 20.0 ± 1.9 Male: 46% |
n=122 Age: 20.3 ± 2.0 Male: 51% |
Healthy controls without concussion in past 6 months, matched on sex, age, concussion history | University sports medicine clinic | Athlete | SRC | Acute or subacute (early) | M: 4 days (range: 1–6 days) | RTP and 1 year after RTP |
| Coverdale, 2020 | n=10 Age: 21± 2 Male: 60% |
n=10 Age: 21± 2 Male: 40% |
Healthy controls without HOC | Unstated | Athlete (8/10) | SRC (8/10) | Chronic | 126 ± 15 days | None |
| Doshi, 2015 | n=7/14 Age: 27.1 ± 5.5 (19–56) Male: 71% |
n=12 Age: 30.1 ± 10.2 (21–66) Male: 61% |
Community healthy controls, similar age | ED | ED patients | Mixed (MVC, pedestrian, fall, assault) | Acute or subacute (early) | M: 55 hrs (SD: 69, Med: 32 hrs, range: 3 hrs - 10 days) | None |
| Ge, 2009 | n=21 Age: 34.1 ±8.6 (22–54) Male: 71% |
n=18 Age: 36.1 ±10.6 Male: 78% |
Community healthy controls without history of head injury, similar age | Unstated | Unknown | Mixed (MVC, fall, sport) | Chronic | Med: 24.6 months (range: 6 mo - 7 years) | None |
| Hammer, 2019 | n=56 with HOC Age: 21.7 ± 2.5 (M), 20.4 ± 1.5 (F), Male: 50% | n=56 without HOC, age:19.8 ± 1.9 (M), 20.1 ± 1.7 (F), Male: 50% | Unstated | Varsity sport teams | Athlete with HOC | SRC | Chronic | M:3.5 years (range: 0.92 – 14.25 years) | None |
| Lin, 2016 | n=23 Age: 52.1 ± 9.7 Male: 30% |
n=21 Age: 51.6 ±8.4 Male: 32% |
Age- and gender-matched healthy volunteers from a staff of hospital coworkers or volunteers through advertisement | ED | ED patients | Mixed (MVC, fall, assault, sport, struck by object) | Subacute (early) |
< 1 mo (12.57 ± 4.13 days) | None |
| Liu, 2016 | n=46 (25 acute, 21 chronic) Age: acute: ± 9.8, chronic: ± 12.8 Male: 59% |
n=20 Age: 31.9 ± 7.9 Male: 60% |
Healthy hospital staff | Military hospital | Unknown | Unknown | Subacute and chronic | Acute group: <2 weeks; chronic group: > 12 months |
None |
| Meier, 2015 | n=17 Age: 20.6 ±1.6 Male: 100% |
n=27 Age: 20.7 ±1.4 Male: 100% |
Healthy football athletes 3+ months out from any prior concussion, matched on age, trend for HOC | NCAA Division I school | Athletes | SRC | Acute, subacute (early and later) | 1.4 ± 0.94 days (range: 0–3 days), 8.7 days (613 days), and 31.5 days (25–44 days) | 8.7 days (range: 6–13 days) and 31.5 days (25–4 days) |
| Militana, 2016 | n=7 Age: 19.7 ±1.2 Male: 57% |
n=11 Age: 20.0 ±1.6 Male: 45% |
Healthy college students without prior concussion, matching unreported | University sports teams | Athletes | SRC | Subacute (early) | <1 week (range 3–6 days) | None |
| Moller, 2017 | n=10 Age: 37.5 ± 11.2 Male: 50% |
n=10 Age: 36.9 ± 11.0 Male: 50% |
Healthy controls matched for age, gender, years of education | Neuropsychology clinic | Chronic mTBI with persistent cognitive impairments and fatigue symptoms | Mixed (fall, MVC, horse riding, bicycle) | Chronic | Med = 5 years (range 0.5–9 years) | None |
| Mutch, 2016 | n=15 adolescents with PCS Age: 17.3 (17–22) Male: 27% |
n=17 Age: 18.3 (13–25) Male: 47% |
Healthy controls recruited via word of mouth (including patient relatives), excluded if (1) symptomatic concussion or (2) history of prior concussion/TBI resulting in structural brain injury on neuroimaging | Multidisciplinary pediatric concussion clinic | Pediatric chronic concussion patient | SRC | Chronic | M: 327 days (range: 33993 days) | None |
| Mutch, 2018 | n=15 with PCS Age: 16.3 (14–20) Male: 60% |
n=27 Age: 17.6(13–21) Male: 48% |
Healthy controls (sample overlapped with Mutch, 2016), excluded if (1) symptomatic concussion, (2) diagnosis of prior moderate or severe TBI or neurologic condition resulting in structural brain injury on neuroimaging, or (3) diagnosis of a neurologic condition requiring prescription medication | Multidisciplinary pediatric concussion clinic | Pediatric chronic concussion patient | SRC | Subacute | M:16 days (range: 3–32 days) | None |
| Peng, 2016 | n=20 Age: 39.1 ± 5.6 Male: 65% |
n=21 Age: 38.7 ± 6.5 Male: 62% |
Healthy controls recruited by advertisement, matched on sex, age, and education | ED and hospital department of neurosurgery | ED patients or Neurosurgery patients | Mixed (MVC, fall, combat) | Acute, subacute And chronic |
Acute (<72 hours), subacute (3 days - 3 weeks), chronic (>3 months) | Subacute (3 days - 3 weeks), chronic (>3 months) |
| Sours, 2015 | n=28 (12 of them having PCS at 6 months follow-up) Age: 38.9 ± 15.9 Male: 64% |
n=28 Age: 39.25 ± 17.2 Male: 57% |
Neurologically intact participants, matched on age and education | Trauma center | ED patients | Mixed (MVC, fall, sport, insult, bicycle) | Acute, subacute and chronic | Initial stage: M: 6 ± 3 days (range: 1–11 days), subacute stage: M: 36 ± 13 days (range: 2588 days), chronic stage: M: 198 ± 26 days (range: 137–266 days) | Subacute stage: M: 36 +/−13 days (range: 2588 days), chronic stage: M: 198 ± 26 days (range: 137–266 days) |
| Stephens, 2018 | n=15 Age: 15.6 ± 1.2 (13–17) Male: 67% |
n=15 Age: 15.2 ± 1.7 (1317) Male: 67% |
Never-concussed adolescent athletes with no behavioral/ educational diagnoses; matched on age and sex | Clinics (e.g., concussion clinic) | Adolescent athletes | SRC | Subacute | 2 wks (8.8 ± 3.4 days) & 6 wks (43.2 ± 12.5 days) | Subacute |
| Wang, 2015 | n=14 Age: 15.1 ± 0.92 sex unknown |
n=15 Age:15.6 ± 0.99 sex unknown |
Healthy controls with no history of TBI; gender unstated; similar in age, gender, maternal education, and neuropsychological performance | Clinics (e.g., sports medicine clinic) | Pediatric chronic concussion patient | Sports and recreational activities | Chronic | M: 7.4 months (SD=2.4; range 3–12 months) | None |
| Wang, 2016 | n=18 Age: 17.8 ± 1.5 Male: 100% |
n=19 Age: 18.0 ± 1.8 Male: 100% |
Teammate healthy controls; matched at group-level on age, level of competition, race, height/weight, history of neurodevelopmental disorder, word reading ability; trend difference in concussion history | High school and Division III college football teams | Athletes | SRC (football, 78% college) | Acute and subactue (early) | <24 hrs (M: 20.3, range: 13–24 hrs) and 8 days (M: 8.4, range 7–11 days) | 8 days (M=8.4, range 7–11 days) |
| Wang, 2019 | n=24 Age: 19.0 ± 1.2 Male: 79% |
n=24 Age: 19.3 ± 1.5 Male: 79% |
Matched teammate (contact sport) controls; matched on age, gender, body mass index, concussion history, word reading ability | Two Division I colleges | Athletes | SRC (50% football) | Acute | 24–48 hrs | Not included in the report |
Note. M = mean; Med = median; ED=emergency department; mTBI=mild traumatic brain injury; MVC=motor-vehicle collision; SRC=sport-related concussion; HOC=history of concussion.
Table 5.
Main findings of each study
| First author, year | Main findings | Relationship between CBF and other measures | Study conclusion |
|---|---|---|---|
| Barlow, 2017 | 1. Both global and regional CBF were higher in the symptomatic group and lower in the asymptomatic group compared with controls. 2. Post-injury symptom score could be predicted by pre-injury PCSI and CBF in presence of mTBI. |
Pre-injury PCSI and mTBI × CBF were significant predictors of postinjury PCSI, but not CBF or TBI independently | Symptomatic children have higher CBF. Children who “recovered” quickly, have decreased CBF suggesting that clinical recovery precedes the cerebral recovery. |
| Brooks, 2019 | 1. Regional CBF analyses suggested that youth with a HOC had hypoperfusion in posterior and inferior regions and hyperperfusion in anterior/frontal/temporal regions as compared to those with orthopedic injury. 2. Global CBF did not differ between groups. |
Neither global nor regional CBF associated with demographics, number of concussions, time since injury, symptoms or cognitive abilities. | Youth with a HOC demonstrate differences in regional CBF (not global CBF), but without clear clinical expression |
| Churchill, 2017a | 1. For concussed athletes, greater total symptom severity was associated with elevated posterior cortical CBF. 2. Athletes reporting greater cognitive symptoms also had lower frontal and subcortical CBF, relative to athletes with greater somatic symptoms. 3. The “cognitive” and “somatic” subgroups also exhibited significant differences in CBF relative to controls. A subgroup of athletes with worse cognitive (vs. somatic) symptoms had significantly lower frontal and subcortical CBF, while athletes with worse somatic symptoms showed CBF effects in the opposite direction. |
CBF correlated with symptoms in recently concussed athletes | Specific symptom clusters (cognitive or somatic) may have distinct patterns of altered CBF. |
| Churchill, 2017b | Athletes scanned at the early acute injury stage (1–3 days) had elevated CBF and global functional connectivity and reduced FA, but those scanned at the late acute injury stage (5–7 days) had the opposite response (decrease CBF). In contrast, MD showed a more complex, spatially dependent relationship with days post-injury. | CBF reliably decreased as a function of days post-injury | The acute injury time interval has significant implications for studies relating to acute MRI data to concussion outcomes. |
| Churchill, 2019a | No significant differences on global resting CBF between concussed athletes and matched controls. | No significant associations between the global CBF. Inclusion of CBF as a regression covariate for the BOLD respiratory challenge showed no significant effects on acute BOLD response | Concussion was associated with greater reductions in BOLD activity during the early phase of the respiratory task, while no significant effects on resting global CBF were observed. It highlights the importance of examining neurovascular response to physiological stressors after a concussion. |
| Churchill, 2019b | CBF was elevated at SYM, restricted to the superior frontal gyri. At RTP, no significant effects of concussion were detected for CBF. At 1 year post- RTP, however, significant reductions in CBF were observed within middle frontal and temporal regions | Negative correlation of CBF with CS score at SYM. At RTP, no significant effects were observed, whereas at 1 year after RTP, positive correlations were seen between CBF and CS score in frontal areas | The study findings suggest the effects of concussion on CBF may be more subtle than for Gconn but persist over a longer time frame. |
| Coverdale, 2020 | CVR, not CBF, differed between concussed and control groups in regions where working memory fMRI showed difference between groups (ventral anterior cingulate cortex, superior frontal gyrus, the medial temporal gyrus and the lateral occipital cortex). | Unstated | BOLD results should be normalized to CVR in order achieve a clearer understanding of the neural and vascular contributions to the differences in the signal between groups. |
| Doshi, 2015 | Increases in regional CBF in the left striatum and in frontal and occipital lobes in patients as compared to controls. [all CBF group differences would be nonsignificant after FDR correction, applied for this review] | Neither susceptibility nor CBF measures were found to correlate with symptoms. | The increased CBF combined with increased venous oxygenation suggests an increase in CBF that exceeds the oxygen demand of the tissue. This may represent a neuroprotective response following mTBI, which warrants further investigation. |
| Ge, 2009 | The mean regional CBF was significantly lower in patients with mTBI as compared to normal controls in both sides of thalamus. | Decrease of thalamic CBF was significantly correlated with several neurocognitive measures including processing and response speed, memory/learning, verbal fluency, and executive function in patients. | Hemodynamic impairment can occur and persist in patients with mTBI in thalamic regions and correlate with neurocognitive dysfunction during the extended course of disease. |
| Hamer, 2019 | Males with HOC had lower CBF bilaterally than males without HOC (predominantly in the temporal lobes). Females with HOC showed no significant differences relative to females without HOC. Females with multiple concussions had lower CBF posteriorly compared with those with a single concussion, whereas males showed no significant effects. |
Unstated | Sex differences in CBF associated with HOC |
| Lin, 2016 | Reduction in CBF in the bilateral frontal and left occipital cortex in mTBI as compared with controls. [group differences would be nonsignificant after FDR correction, applied for this review] | Correlation between PCS and CBF in areas with hypoperfusion. | Changes in cerebral hemodynamics may play a role in pathophysiology underlying the symptoms. |
| Liu, 2016 | The first 5-min PVT increased CBF of patients in acute phase in attention network, and decreased CBF in DMN areas. | Regional CBF and PVT task performance RT showed significant difference between groups and between quarters of the 20-min PVT task | Mental fatigue of mTBI patients persists for more than 12 months and can be mitigated partly within the first year after injury. The bottom-up and top-down attention deficits result in mental fatigue of mTBI patients. |
| Meier, 2015 | 1. Both cognitive and neuropsychiatric symptoms at one day post-injury that resolved at either one week (cognitive) or one month (neuropsychiatric) postinjury. 2. Imaging data suggested both cross-sectional and longitudinal evidence of CBF recovery in the right insular and superior temporal cortex. 3. CBF in the dorsal mid-insular cortex was both decreased at one-month post-concussion in slower- to-recover athletes and was inversely related to the magnitude of initial psychiatric symptoms, suggesting a potential prognostic indication for CBF as a biomarker. |
Concussed athletes with poor outcomes had significantly lower CBF at one month relative to those with good outcome. Inverse relationship between dorsal mid- insular cortex CBF at one-month and initial concussion severity. | The resolution of CBF abnormalities show real-world validity for predicting outcomes following concussion. |
| Militana, 2016 | 1. No significant CBF changes between SRC and controls. 2. CVR was increased after concussion within some DMN regions, the anterior cingulate, and the right thalamus. [FDR correction applied for this review separately for patient vs. all control and patient vs. athlete control analyses found group differences only significant for patients vs. athlete controls] 3. The FC was increased in the concussed athletes within the DMN, with measures being linearly related to CVR in the hippocampus in the concussed athletes. |
None | This study provides evidence for increased CVR and FC in the medial regions of the DMN within days of a single SRC in college athletes. These findings emphasize the utility of complementary cerebrovascular measures in the interpretation of alterations in functional connectivity following concussion. |
| Moller, 2017 | Significant interaction effect between the subject group and performance time during PVT in a mainly frontal/thalamic network. In the mTBI patients, fatigability at the end of the PVT was related to increased rCBF in the right middle frontal gyrus, while self-rated fatigue was related to increased rCBF in left medial frontal and anterior cingulate gyri and decreases of rCBF in a frontal/thalamic network during this period. | Significant positive correlation for mTBI between RT and CBF; significant correlations for mTBI between CBF and the VAS-f ratings after MRI | Patients suffering from fatigue after mTBI used different brain networks compared with healthy controls during a vigilance task and in mTBI; there was a distinction between rCBF changes related to fatigability vs. perceived fatigue. |
| Mutch, 2016 | 1. Patient-specific differences in regional CBF (including diffuse areas) and CO2 BOLD responsiveness were observed in all PCS patients. 2. No group differences in global mean CBF between PCS patients and healthy controls. |
Unstated | Adolescent PCS is associated with patient- specific abnormalities in regional CBF and BOLD CVR that occur in the setting of normal global resting CBF. |
| Mutch, 2018 | 1. Significant group and patient-specific differences in CVR were observed with SRC patients demonstrating a predominant pattern of increased CVR. 2. No significant group differences in global mean resting CBF. |
No correlation between abnormal voxel counts of CVR and PCS among SRC patients | Acute and subacute SRCs are associated with alterations in CVR that can be reliably detected by brain MRI CO2 stress testing in individual patients |
| Peng, 2016 | 1. At the acute and subacute stages, CBF was reduced in the occipital lobe, parietal lobe, central region, subcutaneous region, and frontal lobe. CBF was restored at the chronic stage. 2. CBF in the temporal lobe and limbic lobe diminished at the acute and subacute stages but was restored at the chronic stage. |
Unstated | ASL can precisely measure CBF in various brain regions and may play a reference role in evaluating a patient’s condition and judging prognosis after traumatic brain injury. |
| Sours, 2015 | 1. No group differences in network CBF values for either the DMN CBF or TPN CBF at any of the three time points; no differences in the DMN/TPN CBF ratio at any time point. 2. Chronic mTBI patients demonstrate increased FC between the DMN and regions associated with the SN and TPN compared to the control populations, as well as reduced strength of FC within the DMN at the acute stage of injury. 3. Chronic mTBI patients demonstrate an imbalance in the ratio of CBF between nodes of the DMN and TPN. |
Compared with those without chronic PCS, patients with chronic PCS reveal an imbalance in the ratio of CBF between the DMN nodes and TPN nodes across multiple stages of recovery. | Findings suggest that the altered networkperfusion with the associated changes in FC may be a possible predictor of which mTBI patients will develop chronic PCS. |
| Stephens, 2018 | 1. At two weeks post-injury, the SRC group had significantly higher rCBF in the left dorsal ACC and left insula than controls. 2. At six weeks post-injury, elevated rCBF persisted in the SRC group in the left dorsal ACC. |
Perfusion in the left dorsal ACC was higher in athletes reporting physical symptoms six weeks postinjury compared with asymptomatic athletes and controls. | Findings are inconsistent with reports of reduced rCBF after mTBI but consistent with studies that report increased perfusion in persons with greater or persistent mTBI- related symptomology. |
| Wang, 2015 | Despite normal conventional MRI and neuropsychological performance, chronic pediatric patients showed significantly lower CBF than healthy controls in bilateral frontotemporal regions. | No | Pediatric concussion may produce a pathophysiologic process resulting in altered CBF, with a variable and possibly protracted time frame for resolution. |
| Wang, 2016 | While the control group did not show any changes in CBF between the two time-points, concussed athletes demonstrated a significant decrease in CBF at eight days relative to within 24 h after injury, diffusely across cortical gray matter, mainly in bilateral prefrontal regions, temporal lobes, some parietal regions, as well as the thalamus. Scores on the clinical symptoms and cognitive measures demonstrated significant impairment compared to pre-season baseline levels at 24 hrs but returned to baseline levels at eight days. | No | These data support the hypothesis that underlying neurophysiological recovery from injury beyond the point of clinical recovery after SRC. |
| Wang, 2019 | Significantly less CBF was detected in several brain regions (predominantly in left inferior parietal lobule, right supramarginal gyrus, right middle frontal gyrus, posterior cingulate cortex, left occipital gyrus, and thalamus) in acute concussed athletes, while clinical assessments also indicated clinical symptom and performance impairments in SRC patients. | Correlations between decreased CBF in acute CBF and clinical assessments including BESS and ImPACT scores, as well as days from injury to asymptomatic. | Although using different ASL MRI sequences, preliminary results from the two sites are consistent with previous reports of reduced CBF in acute SRC and suggest advanced ASL MRI methods might be useful for detecting acute neurobiological changes in acute SRC. |
Note: PCSI=Post-Concussion Symptom Inventory; PCS=post concussive symptoms; SRC=sports-related concussion; FA=fractional anisotropy; MD=mean diffusivity; DMN=default mode network; Gconn=global connectivity; PVT=psychomotor vigilance test; VAS-f=Self-rated current fatigue; FC=functional connectivity; CVR=cerebrovascular reactivity; SN=salience network; TPN=task positive network; rCBF=relative CBF; BESS=Balance Error Scoring System; BOLD=blood oxygen level dependent; SYM=early symptomatic injury (< 1 week after inlury); RTP = return to play; CS score=Clinical Severity score, i.e., symptom severity and time to RTP; HOC=history of concussion
Results
The initial search strategy was extremely liberal in order to capture all possible articles for inclusion in this review. The flow chart in Figure 1 depicts the number of articles identified and excluded at each stage as well as reasons for exclusion. Raters coded the first recognized reason for exclusion, which may underestimate the actual frequency of the reasons. In summary, 258 unique records were identified upon the initial search, 229 which were excluded due to studying the wrong population (n=105 non-humans or non-mTBI), not collecting or presenting ASL measures of CBF (n=63), being the wrong publication type (e.g., conference presentation, commentary, review; n=61). In the rest of 29 articles with full-text, 6 of them were further excluded due to reporting the wrong population (n=1 mixed mild and moderate TBI sample) or not reporting ASL main findings (n=2) or being a duplicate not identified as such until full-text review (n=3). The final 23 articles that met the inclusion/exclusion criteria are summarized below.
Quality indices and AAN grading for studies including a control group are summarized in Table 1. All studies were determined to have sufficiently objective outcome measures and clearly defined outcomes, so these factors are excluded from the table. Per the AAN guidelines, the 23 articles comprised two Class I, eight Class II, four Class III, and nine Class IV studies.
Table 2 summarizes the basic design of each study including sample size, age and gender, mTBI definitions/diagnosis, time post-injury of assessment, injury cause, and prospective studies with follow-up. The term “mTBI” is used to describe a variety of mild brain injuries, with variability across different patient populations (e.g., sport, hospital) and diagnostic criteria. Up to 566 mTBI patients and 654 control subjects were evaluated across the 23 studies, although this is an overestimate given overlap in three study samples.1 The sample sizes of mTBI patients with ASL data ranged from seven (Coverdale et al. 2020) to 66 (Hamer et al. 2019). Four studies focused only on a pediatric sample (younger than 18 years old) (Barlow et al. 2017; Stephens et al. 2018; Y. Wang et al. 2015; Brooks et al. 2019), three studies enrolled both adolescents and young adults (14–22 years old) (Y. Wang et al. 2016; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018), ten studies were conducted on college athletes of young adult age (18–22 years old) (Churchill et al. 2017a; Churchill et al. 2017b; Meier et al. 2015; Militana et al. 2016; Y. Wang et al. 2019; Churchill et al. 2019a, 2019b; Hamer et al. 2019), and other reports studied adults with a wider range of ages (Doshi et al. 2015; Ge et al. 2009; Lin et al. 2016; Liu et al. 2016; Moller et al. 2017; Peng et al. 2016; Sours et al. 2015). Twenty of 23 studies recruited control subjects matched on age (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Doshi et al. 2015; Ge et al. 2009; Lin et al. 2016; Meier et al. 2015; Moller et al. 2017; Peng et al. 2016; Sours et al. 2015; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015; Brooks et al. 2019; Churchill et al. 2019a, 2019b; Coverdale et al. 2020; Hamer et al. 2019). Two studies on college football involved male athletes only (Meier et al. 2015; Y. Wang et al. 2016), while the other 21 studies included mixed-gender samples ranging from 27% to 71% males. Thirteen of these studies recruited control subjects matched on gender (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Lin et al. 2016; Moller et al. 2017; Peng et al. 2016; Stephens et al. 2018; Y. Wang et al. 2019; Brooks et al. 2019; Churchill et al. 2019a, 2019b; Hamer et al. 2019), and the majority of groups were also matched on age. Fifteen of 23 studies reported findings related to sport-related concussion (SRC) (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Meier et al. 2015; Militana et al. 2016; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015; Churchill et al. 2019a, 2019b; Coverdale et al. 2020; Hamer et al. 2019); seven studies reported mTBI from mixed injury causes including motor vehicle collision (MVC), fall, assault, bicycle, sports, etc. (Doshi et al. 2015; Ge et al. 2009; Lin et al. 2016; Moller et al. 2017; Peng et al. 2016; Sours et al. 2015; Brooks et al. 2019); and one study recruited patients from a military hospital and did not specify causes of injury (Liu et al. 2016). Fourteen studies reported clear definitions of mTBI: four used the ACRM definition (Doshi et al. 2015; Lin et al. 2016; Liu et al. 2016; Moller et al. 2017), one used the U.S. Department of Defense’s definition (Y. Wang et al. 2016), nine (Churchill et al. 2017a; Churchill et al. 2017b; Militana et al. 2016; W. A. C. Mutch et al. 2018; Churchill et al. 2019a, 2019b; Coverdale et al. 2020; Hamer et al. 2019; Y. Wang et al. 2019) used the definition of the Concussion in Sport Group (McCrory et al. 2017), and the remaining eight studies defined mTBI by the Glasgow Coma Scale (i.e., 13–15) or other criteria (Barlow et al. 2017; Ge et al. 2009; Meier et al. 2015; W. A. Mutch et al. 2016; Peng et al. 2016; Sours et al. 2015; Stephens et al. 2018; Brooks et al. 2019). The reasons why studies did not meet our criteria for an acceptable definition of mTBI are listed in the Table 1 note.
The studies sampled mTBI participants at varying intervals post-injury with inconsistent terminology of acute, subacute, and chronic stages used across the different reports. For uniformity in this review, we adopted the injury stage definition from the SRC Common Data Elements (CDE) Working Groups of the National Institute of Neurological Disorders and Stroke, as follows: acute, injury <72 hours;subacute, >72 hours and <3 months; and chronic, ≥3 months. Of the 14 cross-sectional studies, one research study focused on acute concussion only (24–48 hours) (Y. Wang et al. 2019), four studies included findings from mixed acute or early subacute stages (1–7 days post-injury) (Churchill et al. 2017a; Churchill et al. 2017b; Doshi et al. 2015; Churchill et al. 2019a), four studies reported findings from subacute mTBI with different time windows after injury (Barlow et al. 2017; Lin et al. 2016; Militana et al. 2016; W. A. C. Mutch et al. 2018), seven studies focused on chronic mTBI (Ge et al. 2009; Moller et al. 2017; W. A. Mutch et al. 2016; Y. Wang et al. 2015; Brooks et al. 2019; Coverdale et al. 2020; Hamer et al. 2019), and one study compared both subacute and chronic mTBI subjects with control subjects (Liu et al. 2016). Of the six studies with follow-up ASL exams, only one assessed patients at acute, subacute, and chronic stages (Peng et al. 2016). One study did not separate findings from the acute and early subacute stages but followed patients at later subacute and chronic stages (Sours et al. 2015); one followed concussed football players at acute and subacute phases (Y. Wang et al. 2016); one studied two time points during the subacute stage (two and six weeks after injury) (Stephens et al. 2018); one studied concussed athletes at acute, early, and later subacute stages (Meier et al. 2015); and one studied concussed athletes at acute or early subacute stages, and then followed them at return-to-play (RTP) and one year after RTP (Churchill et al. 2019b).
Table 3 presents the imaging parameters and clinical assessments performed in each study. While all ASL studies were conducted on a 3 Tesla magnetic resonance scanner, manufacturer and head coil channels varied across studies. Regarding the ASL acquisition method, ten studies applied the 2D PASL sequence (Churchill et al. 2017a; Churchill et al. 2017b; Doshi et al. 2015; Peng et al. 2016; Sours et al. 2015; Stephens et al. 2018; Y. Wang et al. 2015; Churchill et al. 2019a, 2019b; Hamer et al. 2019), whereas ten studies used the pCASL sequence, six of which used the 3D pCASL sequence (Barlow et al. 2017; Lin et al. 2016; Liu et al. 2016; Meier et al. 2015; Y. Wang et al. 2016; Brooks et al. 2019) and the other four used 2D pCASL sequence (Militana et al. 2016; Moller et al. 2017; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018). Notably, one study reported findings from two sites, one using 3D pCASL and another one using 2D PASL (Y. Wang et al. 2019). Another two studies applied very unique ASL sequences (Coverdale et al. 2020; Ge et al. 2009). Except for five studies from the same group (Churchill et al. 2017a; Churchill et al. 2017b; Churchill et al. 2019a, 2019b; Hamer et al. 2019), the ASL acquisition parameters (e.g., repetition time, echo time, labeling duration, post-labeling delay, spatial resolution, total labeling, control images) varied markedly across studies. Most studies (21 of 23) acquired ASL data during resting state to assess the resting CBF. Two studies conducted a task ASL study where subjects were requested to complete a 20-min psychomotor vigilance test (PVT) inside the scanner during simultaneous measurements of ASL and reaction time (Liu et al. 2016; Moller et al. 2017), and one of them also acquired additional resting ASL data (Moller et al. 2017). In addition to ASL results, findings from other imaging modalities were reported in ten studies, including functional connectivity (FC) using resting-state functional MRI (fMRI) (Churchill et al. 2017b; Militana et al. 2016; Sours et al. 2015; Churchill et al. 2019b) and cerebrovascular reactivity (CVR) measured using blood oxygenation level dependent (BOLD) fMRI during hypercapnia of controlled carbon dioxide (CO2) (Militana et al. 2016; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018; Churchill et al. 2019a; Coverdale et al. 2020); one of these reported findings for both FC and CVR in addition to resting CBF (Militana et al. 2016). Another study combined CBF with susceptibility-weighted imaging for venous blood oxygenation quantification (Doshi et al. 2015). In addition to anatomical imaging (T1 and FLAIR), several studies acquired resting-state fMRI, diffusion tensor imaging, or susceptibility-weighted imaging, but not all of them included related findings in their ASL reports (Churchill et al. 2017a; Churchill et al. 2017b; Doshi et al. 2015; Liu et al. 2016; Moller et al. 2017; Churchill et al. 2019b). Given the aims of this review, we only report on the studies’ CBF findings.
Table 3.
Study imaging and clinical assessments
| First author, year | MR specification | ASL sequence | ASL protocol | Other imaging modality | Clinical assessments | Preinjury / baseline assessment |
|---|---|---|---|---|---|---|
| Barlow, 2017 | 3T GE MR750w, 32- channel head coil | 3D pCASL | PLD: 2.0 sec. In-plane resolution: 3.2·mm2. 34 3.5-mm thick slices | T1 | PCSI; CNS Vital Signs computerized cognitive battery: NCI | Preinjury PCSI |
| Brooks, 2019 | 3T GE MR750w, 32- channel head coil | 3D pCASL | PLD: 2.0 sec. In-plane resolution: 3.2·mm2. Thirty-four 3.5mm thick slices | T1 | PCSI, BASC-2; CNS Vital Signs | No |
| Churchill, 2017a | 3T Siemens Skyra, 20- channel head receiver coil | 2D PASL using the PICORE QUIPSS II | TI1: 700ms, TI1s: 1600ms, TI2: 1800ms; TE/TR: 12/2500ms, flip angle: 90, voxel size: 4×4×8mm; 45 tag-control image pairs | T1, FLAIR, SWI* | SCAT3, SAC, BESS | Preseason baseline SCAT3 |
| Churchill, 2017b | 3T Siemens Skyra, 20- channel head receiver coil | 2D PASL using the PICORE QUIPSS II | TI1: 700ms, TI1s: 1600ms, TI2:1800ms; TE/TR: 12/2500ms, flip angle:90, voxel size:4×4×8mm; 45 tag-control image pairs | T1, FLAIR, SWI, rs- fMRI, DTI | SCAT3, SAC, BESS | Preseason baseline SCAT3 |
| Churchill, 2019a | 3T Siemens Skyra, 20- channel head receiver coil | 2D PASL using the PICORE QUIPSS II | TI1:700ms, TI1s:1600ms, TI2:1800ms; TE/TR: 12/2500ms, flip angle:90, voxel size:4×4×8mm; 45 tag-control image pairs | T1, BOLD fMRI for CVR, T1, FLAIR, SWI | SCAT3 | Preseason baseline SCAT3 |
| Churchill, 2019b | 3T Siemens Skyra, multichannel head coil | 2D PASL using the PICORE QUIPSS II | TI1:700ms, TI1s:1600ms, TI2:1800ms; TE/TR: 12/2500ms, flip angle:90, voxel size:4×4×8mm; 45 tag-control image pairs | T1, DTI, resting state fMRI, FLAIR, SWI, | SCAT3, BESS | Preseason baseline SCAT3; baseline imaging (control group) |
| Coverdale, 2020 | 3T Siemens Trio, 32- channel head coil | 2D dualecho pCASL | 25 slices, TR :4000ms, TE1/TE2: 10/30ms, voxel size:3.9×3.9×4.67mm, PLD:1000ms, tagging duration: 1665ms | T1, working memory fMRI, hypercapnic breathing task | Unstated | No |
| Doshi, 2015 | 3T Siemens Verio, 32- channel head coil | 2D PASL | TR/TE:2830/11ms, flip angle:90, voxel size: 4×4×4mm | SWI, FLAIR, DTI, rs- fMRI | SAC, patients were asked to grade each symptom from No, mild, moderate, to severe | No |
| Ge, 2009 | 3T Siemens Trio, 8- channel head coil | FISP ASL included a FAIR preparation | Selective IR: 15mm, TI: 1200ms, flip angle:50, TR/TE:5000/1.64ms, matrix size:256×256, FOV:220×220mm, four repetitions of both unlabeled and labelled images, one slice with 6mm thickness acquired per measurement. | T1 | A traditional consensus battery including assessments of executive functions, verbal ability, psychomotor ability, memory/learning and attention/concentration. | No |
| Hamer, 2019 | 3T Siemens Skyra, 20- channel head coil | 2D PASL using the PICORE QUIPSS II | TI1:700ms, TI1s:1600ms, TI2:1800ms; TE/TR: 12/2500ms, flip angle:90, voxel size:4×4×8mm; 45 tag-control image pairs | T1 | SCAT3, BESS, SAC, ANAM | No |
| Lin, 2016 | 3T GE Discovery MR750, 8 channel receive-only head coil | 3D pCASL | TR/TE/TI:5327/10.5/2525ms, labeling duration:1500ms, PLD:2525ms, FOV:240×240, matrix: 128×128, excitation:4, slice thickness:4mm, an echo train length of 36 to obtain 36 consecutive axial slices | T1 | DS, CPT, PCS score, DHI, SSQ, BAI, and WCST | No |
| Liu, 2016 | 3T GE Discovery MR750, 8 channel receive-only head coil | 3D pCASL | TR/TE:4632/10.5ms, slice thickness:4mm, PLD: 1525ms, each spiral arm included 512 sampling points and a total of eight arms acquired. Repeated four times during the PVT task. | T1, SWI, Task ASL study: subjects performed 20-min PVT | FAI, PSQI, ESS, RT during 20-min PVT task | No |
| Meier, 2015 | 3T GE Discovery MR750, 32- channel receive-only head coil | 3D pCASL | TR/TE:5161/12.08ms, FOV:240, spiral readout of eight arms and 512 samples, excitations:3, PLD:1.525s, label duration: 1.45s, voxel size: 1.875×1.875×2.0mm | T1 | HAM-D, HAM-A, ANAM Sports Medicine Battery | No |
| Militana, 2016 | 3T Philips, 32-channel receive-only head coil | 2D pCASL | Matrix:80×80, FOV:240×240, 17 axialslices, 7mm slice thickness with no gap, TE:13.78ms, SENSE:2.5, 35 pairs of labeled and control images. | T1, T2, CVR during a hypercapnia challenge, rs- fMRI | RPQ | No |
| Moller, 2017 | 3T Siemens Trio, 32- channel head coil | 2D pCASL | TR/TE:3330/18ms, labeling duration: 1600ms, PLD: 1200ms, FOV:230×230, matrix size:64×64, 18 slices of 6mm thickness, interslice gap of 0.6mm. Three pCASL measurements before, during, and after the PVT. | T1, T2, FLAIR, SWI, DTI, BOLD fMRI,. | FSS, VAS-f, PSQI, RT during the 20-min PVT task | No |
| Mutch, 2016 | 3T Siemens Verio, 12- channel head coil | 2D pCASL | TR/TE:4000/12ms, 20 slices; PLD:1200ms, voxel size 3.8×3.8×5 mm, 22 imaging pairs, plus M0 scan | T1, FLAIR, CVR using controlled CO2 challenge and BOLD fMRI | PCSS | No |
| Mutch, 2018 | 3T Siemens Verio, 12- channel head coil | 2D pCASL | TR/TE:4000/12ms, 20 slices; PLD:1200ms, voxel size 3.8×3.8×5 mm, 22 imaging pairs, plus M0 scan | T1, FLAIR, CVR using controlled CO2 challenge and BOLD fMRI | PCSS | No |
| Peng, 2016 | 3T Siemens, 16-channel head coil | 2D PASL | TR/TE:2500/11ms, TI1:700ms, TI2:1800ms, slice thickness 8mm, 11 slices, matrix: 64×64, FOV: 256 ×256mm, total 91 frame images | T1, T2, FLAIR | Unstated | No |
| Sours, 2015 | 3T Siemens Trio, 12- channel head coil | 2D PASL | TR/TE:2500/11ms, FOV:230mm, Matrix:64×64, 16 slices (thick:5mm with 1mm gap), 45 pairs of labeled and control | T1, rs-fMRI | ANAM, modified RPQ,mmSE, MACE at each visit. SWLS, GOSE and DRS at 6 months | No |
| Stephens, 2018 | 3T Philips, 8- channel receive-only head coil | 2D PASL | TR/TE:4000/12ms, labeling duration: 1650ms, PLD: 1525msec, matrix size:80×80, voxel size: 3×3×7mm3, 17 slices, no gap, 35 control and label pairs | T1 | ImPACT | No |
| Wang, 2015 | 3T Siemens Trio, 12- channel head coil | 2D PASL using Q2TIPS | TR/TE:3000/13ms, 24 axial slices with voxel size of 3.75×3.75×5mm, TI1/TI2:700/1800ms, 50 control and label image pairs plus one M0 map. Online 3D motion correction | T1 | A neuropsychological and parent-report battery focused on attention-related and executive functions and episodic memory. | No |
| Wang, 2016 | 3T GE Discovery MR750, 32- channel head coil | 3D pCAS] | L TR/TE:4632/10.54ms, spiral readout of eight arms and 512 samples; PLD:1.52s; label duration:1.45s; voxel size:1.875 × 1.875 × 4mm, 36 slices; excitations:3, background suppression | T1 | SCAT3, SAC, ANAM, ImPACT | Yes |
| Wang, 2019 | site 1: 3T Siemens Trio, 32-channel head coil site 2: 3T GE Discovery MR750, 32 channel head coil | site 1: 2D PASL siet 2: 3D pCASL | Site 1: 2D PASL, TR/TE:3204/13ms, FOV:224mm, matrix:64×64, TI1/TI 1s/TI2:700ms/1600ms/1800ms, 36 slices of 4.5mm thickness, 54 control and label image pairs plus one M0 | T1 | SCAT3, SAC, BESS, ImPACT, CNT battery, and other self-report and computerized measures | Yes |
| Site 2: 3D pCASL, TR/TE:4632/10.5ms, FOV:240mm, matrix:128×128, PLD:1525ms, labeling duration: 1450ms, spiral readout of eight arms and 512 samples, excitations:3, slice thickness:4mm, 36 slices, background suppression. |
Note: PASL=pulsed ASL; pCASL=pseudocontinuous ASL; SWI=susceptibility weighted imaging; FLAIR=fluid-attenuated inversion recovery; IR=Inversion recovery, TI=inversion time, AC-PC=anterior-posterior commissure; FOV=field of view; PLD=post labeling delay; FC=functional connectivity; CVR=cerebrovascular reactivity; BOLD=blood oxygen level dependent; rs-fMRI=resting-state functional MRI; SCAT3=Sport Concussion Assessment Tool 3; SAC=Standardized Assessment of Concussion; BESS=Balance Error Scoring System; ANAM=Automated Neuropsychological Assessment Metrics; ImPACT=Immediate Post-Concussion Assessment and Cognitive Testing, PCSI=Post-Concussion Symptom Inventory; RPQ=Rivermead Post Concussion Symptoms Questionnaire; PCSS=Post-Concussion Symptom Scale; NCI=Neurocognition Index of the CNS Vital Signs; CNT=Computerized neurocognitive test battery; BASC-2=Behavior Assessment System for Children, Second Edition; GOSE=Glasgow Outcome Scale Extended; DRS=Disability Rating Scale; DS=digit span; CPT=Continuous performance test; DHI=dizziness handicap index; SSQ=simulator sickness questionnaire; BAI=Beck anxiety inventory; WCST=Wisconsin card sorting test; FAI=Fatigue Assessment Index; PSQI=Pittsburgh Sleep Quality Index; ESS=Epworth Sleepiness Scale; HAM-D=Hamilton Depression rating scales; HAM-A=Hamilton Anxiety rating scales; FSS=Fatigue Severity Scale; VAS-f=Visual Analog Scale of Fatigue; PSQI=Pittsburgh Sleep Quality Index; RT=Reaction time; MMSE=Min- Mental State Exam; MACE=Military Acute Concussion Evaluation; SWLS=Satisfaction with Life Scale; FISP=fast imaging with steady state precession
Various clinical measures appropriate for the stage of mTBI and age of the subjects were utilized to determine the relationship between CBF and clinical assessments, most commonly a TBI/concussion symptom checklist such as the Sport Concussion Assessment Tool 3, Post-Concussion Symptom Inventory, Rivermead Post Concussion Symptoms Questionnaire, or Post-Concussion Symptom Scale. Assessments of cognitive function, postural stability, psychiatric symptoms, sleep, quality of life, and return to daily function were also employed by some studies. (See the Table 3 note for a detailed list of clinical assessments, which varied substantially across studies.) Of note, although four studies enrolled participants post-injury and were able to obtain pre-injury clinical data routinely collected in participants’ athletic programs (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Churchill et al. 2019a, 2019b), only two studies enrolled participants and conducted clinical assessments pre-injury (Y. Wang et al. 2016; Y. Wang et al. 2019).
Table 4 presents data analysis procedures across studies. To estimate individual CBF maps using the acquired ASL data, various imaging tools were utilized across studies including manufacturer-provided online ASL processing software or offline processing using different publicly available packages or in-house software, all of which were based on the established one-compartment model (Buxton 2005). For post-processing of CBF maps, different imaging analysis packages such as SPM (Statistical Parametric Mapping, www.fil.ion.ucl.ac.uk/spm), AFNI (Analysis of Functional Neuroimaging, afni.nimh.nih.gov), FSL (FMRIB Software Library, fsl.fmrib.ox.ac.uk/fsl/fslwiki), ASLtbx (cfn.upenn.edu/~zewang/ASLtbx.php), and additional in-house software were utilized. Care was taken during post-processing steps in 16 studies to control for the significant difference between perfusion in the grey matter (GM) and white matter (WM) (Parkes et al. 2004) and the limited ability of the standard ASL protocol to reliably measure WM perfusion (Alsop et al. 2015). Ten studies applied a GM mask generated from a segmentation of anatomical images to restrict analysis on GM only (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Meier et al. 2015; Militana et al. 2016; Sours et al. 2015; Brooks et al. 2019; Churchill et al. 2019a, 2019b; Coverdale et al. 2020). Two studies conducted the correction of partial volume effects on GM ASL data; one of them (Y. Wang et al. 2019) used a regression algorithm (Asllani et al. 2008) and another (Peng et al. 2016) used the GM density as covariate in data analysis. In order to reduce data noise caused by inter-subject variations in global CBF, three studies calculated rCBF (relative CBF) as normalized by the whole-brain average or mean GM CBF (Meier et al. 2015; Stephens et al. 2018; Y. Wang et al. 2019).
Table 4.
Data analysis
| First author, year | CBF estimation | CBF maps analysis | Statistical approach | Confounding variables accounted for | Correction for multiple comparison |
|---|---|---|---|---|---|
| Barlow, 2017 | The 3D ASL scan was automatically processed into quantitative CBF maps using the scanner-integrated pipeline with default settings (partition coefficient of 0.9; blood T1 of 1.6 sec). | SPM 12 used to process CBF maps. GM mask (GM probability >40%) was used. |
1. Correlations between global CBF and age, group, family income, and the NCI 2. Voxelwise one-way ANOVA to compare the symptomatic, asymptomatic, and HCs. Post hoc independent samples t tests to detect differences between groups. 3. GLM with main effects for sex, group, PCSI score, NCI, and global CBF |
Sex, age, preinjury PSCI | No |
| Brooks 2019 | The 3D ASL scan was automatically processed into quantitative CBF maps using the scanner-integrated pipeline with default settings (partition coefficient of 0.9; blood T1 of 1.6 sec) | SPM 12 used to process CBF maps. GM mask (GM probability >40%) was used |
Voxelwise analysis of CBF between groups was conducted using a whole-brain F-contrast in SPM12. | Age | FDR correction |
| Churchill, 2017a | Voxelwise CBF was calculated based on the mean difference in magnetization ΔM averaged over all tag- control pairs using the kinetic model (partition coefficient of 0.98, blood T1 of 1.6 sec, labelling efficiency of 0.95, venous outflow of 0.85) | Analyzed using a combination of the ASLtbx package and in-house software. GM mask with GM likelihood > 0.33. | 1. Correlations using non-parametric Spearman correlations and bootstrapped 95% CIs based on 1000 resampling iterations. 2. Voxel-based ordinary least squares linear regression with CBF values regressed against symptom severity. Significance was evaluated by bootstrap resampling of 1000 iterations. 3. Using significant brain regions identified on voxel-based analysis as ROI, the mean CBF value was computed for participants in both groups. A bootstrap analysis was then performed (1000 iterations) to estimate the difference in mean CBF between concussed athletes and controls within these brain regions 4. Concussed subgroups with (cognitive > somatic) and (somatic > cognitive) symptoms were separately compared to the control cohort using bootstrap analyses. |
Sex, age, HOC | Clinical data using FDR of 0.05, imaging used FWE correction at p < 0.05 |
| Churchill, 2017b | Voxelwise CBF was calculated based on the mean difference in magnetization AM averaged over all tag- control pairs using the kinetic model (partition coefficient of 0.98, blood T1 of 1.6 sec, labelling efficiency of 0.95, venous outflow of 0.85) | Analyzed using a combination of the ASLtbx package and in-house software. GM mask with GM likelihood > 0.33. | Difference in voxelwise CBF of concussed athletes relative to their matched controls was estimated in a bootstrapped resampling framework. Voxelwise analysis was done using ordinary least squares linear regression, where MRI values were regressed against days post-injury. | Sex, age, HOC | Clinical data using FDR of 0.05, imaging used FWE correction at p < 0.05) |
| Churchill, 2019a | Voxelwise CBF estimates were calculated using the method as in Churchill 2017b | ASL data processed and analyzed by a combination of AFNI and in-house software. Mean CBF was calculated over all GM voxels. | Global CBF compared between groups. Spearman correlations along with bootstrapped 95% confidence bounds to test association between global CBF and symptom severity and supplementary demographic covariates. |
Sex, HOC, participation in collision sports, and days postinjury | Clinical data using FDR of 0.05, imaging used FWE correction at p < 0.05 |
| Churchill, 2019b | Voxelwise CBF estimates were calculated using the method as in Churchill 2017b | ASL data processed and analyzed via a combination of AFNI, FSL, and customized algorithms. GM mask and additional mask retaining only voxels with mean control CBF > 20 | Voxelwise nonparametric analyses. For each concussed athlete, CBF values converted into difference scores relative to the normative mean of a subgroup matched controls. | None | FWE correction at p < 0.05 |
| Coverdale, 2020 | Perfusion data converted into physiological units using the model proposed by (Wang, 2003) | ASL processed using FSL, AFNI and Matlab scripts. GM mask (GM probability >40%). | CBF and CVR extracted from regions where detected working memory task activation difference between groups. T- test to assess whether differences in BOLD activation were co-localized with changes in vascular physiology. | None | No |
| Doshi, 2015 | Perfusion data were processed automatically by the Siemens online software | Using predefined ROIs of the WFU pickatlas, regional rCBF values from the striatum, caudate nucleus, thalamus, globus pallidus, putamen, and frontal, occipital, parietal and temporal lobes of each subject were recorded. | Student’s t-test was performed to evaluate ROI-wise difference between groups. | None | No |
| Ge, 2009 | Absolute perfusion in each of these localized areas of deep GM was quantified with in-house MATLAB scripts using a general kinetic model | ROIs were drawn manually for the bilateral thalamus, putamen and head of caudate nuclei, as well as the frontal GM and WM. Mean CBF of ROI was extracted for each subject. | Student’s t-test to evaluate ROI-wise difference between groups. Cross correlation between neuropsychological testing Z-score and ROI CBF was calculated. | Age | Bonferroni correction |
| Hamer, 2019 | Voxelwise CBF estimates were calculated using the method as in Churchil 2017b | Unstated | Voxelwise two-sample t-tests | Sex | FWE correction at p < 0.05 |
| Lin, 2016 | Quantification of CBF obtained using GE Functool software | Based on the AAL template, predefined ROIs selected in the bilateral frontal, parietal, temporal, and occipital lobes, as well as the bilateral ACA, MCA, and PCA territories. Average CBF values were extracted for each subject. | Student’s t-test was performed to evaluate ROI-wise difference between groups. Regression analysis was performed between average CBF and neuropsychological tests in mTBI patients by Spearman’s rank correlation analysis |
Age, sex | No |
| Liu, 2016 | Quantification of CBF obtained automatically using GE online software | Voxelwise, whole-brain GLM analysis using the PET model in SPM. Contrasts were defined between Time of PVT within same group and between groups. GM density included as covariate. |
A two-way within subject ANOVA for repeated measures (time vs. group) to assess the main effects of the group and time (4 quarters) with regards to changes in the behavioral data of PVT. Univariate post hoc tests with Tukey corrections for multiple comparisons to investigate the interaction between Time and Group. | Age, sex, years of education | FWE correction at p < 0.05 |
| Meier, 2015 | Quantification of CBF obtained automatically using GE online software | AFNI used for all voxelwise calculations and analyses. A relative CBF image was then calculated by dividing the smoothed quantified CBF image by the average CBF value. Binary GM mask created from the segmentation. |
Voxelwise linear mixed-effects model to assess changes in CBF as a function of recovery in the concussed athletes. To avoid thresholding effects, post hoc analyses were performed on the average CBF from spherical (radius=5 mm) regions of interest created at peak voxels exhibiting significant main effects of time. | Age, HOC | Bonferroni correction at p < 0.05 |
| Militana, 2016 | Voxelwise CBF was quantified using the single blood compartment model (partition coefficient of 0.98, blood T1 of 1.7 sec, labelling efficiency of 0.85, venous outflow of 0.85, label duration 1.65 s, PLD 1.65 s). | Using SPM8 to analyze CBF maps. | Group comparisons were made within and between 18 ROIs: 16 chosen from three networks (DMN, DAN, and FPC), and left and right thalamus. A two-sample t-test was used to compare the CVR and the CBF values in each ROI between groups. | None | Uncorrected p value |
| Moller, 2017 | Voxelwise CBF computation used ASL data processing toolbox | PCASL data were processed using AFNI. The CBF data during the PVT were normalized voxelwise to each’s mean CBF at rest before the onset of the task. | Voxelwise three-way ANOVA, fixed factors are the time of PVT (five quantiles of the PVT paradigm) and group. Regression analysis to study the possible correlation between fatigue and CBF. | Age, gender, years of education | FWE correction at p < 0.05 |
| Mutch, 2016 | Voxelwise CBF computation used ASLtbx package | Standard preprocessing of ASL using SPM8, including batch processing by an SPM toolbox and in-house MatLab scripts. | Using SPM to determine voxelwise regional resting CBF and CVR to the CO2 stimulus. ROC curves were generated to compare voxel counts categorized by control or PCS. | None | No |
| Mutch, 2018 | Voxelwise CBF computation used ASLtbx package | Standard preprocessing of ASL and BOLD sequences with SPM8, including batch processing by an SPM toolbox and in-house MatLab scripts. | Using SPM to determine voxelwise regional CVR to the CO2 stimulus. Voxel- by-voxel comparisons on a group and individual basis for the SRC patients was conducted at the p = 0.005 level to identify voxels that responded less or greater than the mean control group responses to the CO2 stimulus from the atlas. ROC curves were generated to compare voxel counts categorized by control or PCS. | None | No |
| Peng, 2016 | Details unstated | CBF maps processed using SPM8, including standard preprocessing steps and correction for partial- volume effects | ROI based analysis: mean CBF extracted from 11 brain regions including whole brain, GM, and WM, based on AAL template. Between-group difference assessed by t-test. | None | No |
| Sours, 2015 | CBF maps were generated using in-house MATLAB program | The DMN and TPN ROIs generated from the resting state fMRI analysis were transformed from MNI space to native ASL space using SPM. A GM mask from segmentation of the T1 image was used. Average CBF values for the DMN and TPN as well as a CBF ratio (TPN CBF/DMN CBF) were calculated. | ROI-wise group differences in measures of CBF and rest FC were tested using ANCOVAs. Longitudinal changes in imaging measures within the first 6 months following mTBI were determined using repeated measures ANCOVAs. Within group differences between DMN CBF and TPN CBF were calculated using paired t-tests. | Age | Uncorrected results |
| Stephens, 2018 | The PCASL data were processed to generate CBF maps using the fully automated ASL-MRI Cloud tool (MRICloud.org). | T1 image was used to normalize the CBF maps into MNI space. Anatomic ROIs were applied to yield structure-based CBF values. Considering the baseline differences across imaging sessions and across subjects, rCBF images and ROI values, normalized by the whole- brain average. | SPM12 second-level t-test used to evaluate voxelwise between-group differences in rCBF. rCBF values from clusters showing significant difference between groups were entered into SPSS to evaluate differences between groups and differences between time points within the SRC group. Control analyses, Pearson correlations, and ANCOVAs, were completed in SPSS to ensure that neither age nor sex was influencing group differences. | Age, sex | FWE correction at p < 0.05 |
| Wang, 2015 | Quantitative CBF calculated based on a single compartment model incorporating in vivo measurement of blood T1 and labeling efficiency. | Individual CBF maps processed using SPM8, including standard preprocessing steps. | The GLM including age and sex as covariates was utilized in SPM8 for voxelwise group analysis. | Age, sex | FWE correction at p < 0.05 |
| Wang, 2016 | Quantification of CBF was obtained automatically using GE online software | CBF maps processed using SPM8, including standard preprocessing steps | For voxelwise group comparison, the AFNI mixed-effects multilevel analysis tool was applied to perform a two-way ANOVA with repeated measures that incorporates both the variability across subjects and the precision estimate of each effect of interest from individual subject analyses. | Age | FWE correction at p < 0.05 |
| Wang, 2019 | All ASL image processing was performed using previously published methods | CBF maps were preprocessed using SPM12. Partial volume effects was corrected using the regression algorithm (Asllani et al. 2008, 2009). A GM mask was applied. Relative CBF (rCBF) was calculated by normalizing CBF with mean GM CBF. | For voxelwise group comparison, the AFNI mixed-effects multilevel analysis tool was applied to perform an ANCOVA that incorporates both the variability across subjects and the precision estimate of each effect of interest from individual subject analyses. | Age, sex, site, HOC | FWE correction at p < 0.05 and p < 0.01 |
Note: MNI=Montreal Neurological Institute; SPM=Statistical Parametric Mapping (www.fil.ion.ucl.ac.uk/spm); GM=gray matter; WM=white matter; CSF=cerebral spinal fluid; HC=healthy control; PCSI=Post-Concussion Symptom Inventory. NC =Neurocognition Index; 95% CIs=95% Confidence intervals; FSL=FMRIB Software Library; AFNI=Analysis of Functional Neuroimaging (www.afni.nimh.nih.gov); FDR=False Discovery Rate; ASLtbx package=https://cfn.upenn.edu/~zewang/ASLtbx.php; ROI=regions of interest; ICBM=International Consortium for Brain Mapping; WFU pickatlas=the Wake Forest University PickAtlas; AAL=automated anatomical labeling; ACA=bilateral anterior cerebral artery; MCA=middle cerebral artery; PCA=posterior cerebral artery; PCSI=Post Concussion Symptom Inventory; GLM=general linear model; FWHM=full width at half maximum; ANOVA=analysis of variance; ANTS=Advanced Normalization Tools; DAN=dorsal attention network; FPC=frontal parietal control network; ROC=receiver operating characteristic; SN=salience network; TPN=Task Positive Network; SPSS=Statistical Package for the Social Sciences; FWE=family wise error; HOC=history of concussion; GLM=General Linear model
For statistical analysis on group-level comparisons, seven studies (Doshi et al. 2015; Ge et al. 2009; Lin et al. 2016; Militana et al. 2016; Peng et al. 2016; Sours et al. 2015; Coverdale et al. 2020) used region-of-interest (ROI) approaches, where ROIs were defined using the template atlas (Doshi et al. 2015; Lin et al. 2016; Peng et al. 2016), were manually drawn on anatomical images (Ge et al. 2009), used a network generated from an FC analysis using resting-state functional MRI (Militana et al. 2016; Sours et al. 2015), or were derived from a working memory task fMRI (Coverdale et al. 2020). Student’s t-test or analysis of covariance was applied on ROI-averaged CBF to assess group differences or longitudinal changes within group. The majority of the studies (16 of 23) conducted whole brain voxelwise analysis for group comparisons. Seven of the studies used SPM second-level analysis within a general linear model framework for group comparison (Barlow et al. 2017; Liu et al. 2016; Moller et al. 2017; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018; Y. Wang et al. 2015; Brooks et al. 2019), and five studies applied a bootstrapped resampling framework (Churchill et al. 2017a; Churchill et al. 2017b; Churchill et al. 2019a, 2019b; Hamer et al. 2019). The latter nonparametric approach avoids any distributional assumptions about the ASL data being analyze and is robust to the influence of outliers (Churchill et al. 2017b). Another three studies used the AFNI mixed-effects multilevel analysis tool to perform an analysis of covariance that incorporates both the variability across subjects and the precision estimate of each effect of interest from individual subject analyses, including appropriate covariates (Meier et al. 2015; Y. Wang et al. 2016; Y. Wang et al. 2019). Five of 13 studies using the whole brain voxelwise analysis also used the significant brain regions identified in a voxel-based analysis as the ROIs for further group analysis (Meier et al. 2015; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Brooks et al. 2019). Twelve studies took steps to account for the effects of potential confounding variables, such as age (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Ge et al. 2009; Liu et al. 2016; Meier et al. 2015; Moller et al. 2017; Sours et al. 2015; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015; Brooks et al. 2019), gender (Barlow et al. 2017; Churchill et al. 2017a; Churchill et al. 2017b; Liu et al. 2016; Moller et al. 2017; Stephens et al. 2018; Y. Wang et al. 2019; Y. Wang et al. 2015; Churchill et al. 2019a; Hamer et al. 2019), previous concussion (Churchill et al. 2017a; Churchill et al. 2017b; Meier et al. 2015; Y. Wang et al. 2019; Churchill et al. 2019a), years of education (Liu et al. 2016; Moller et al. 2017), participation in collision sports and days post injury (Churchill et al. 2019a), and study site (Y. Wang et al. 2019). One study specifically investigated gender differences (Hamer et al. 2019). Only thirteen studies applied a multiple comparison correction to control for type I error using different approaches including familywise error correction (Liu et al. 2016; Meier et al. 2015; Moller et al. 2017; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015; Churchill et al. 2019a, 2019b; Hamer et al. 2019), false discovery rate correction (Brooks et al. 2019), or Bonferroni correction (Ge et al. 2009; Meier et al. 2015).
The main findings of each study are summarized in Table 5. Findings varied markedly across studies in terms of increases, decreases, or no changes in resting CBF measures. Though at different stages after injury, ten of the 23 studies reported decreased CBF in mTBI patients relative to controls (Churchill et al. 2017a; Churchill et al. 2017b; Ge et al. 2009; Lin et al. 2016; Meier et al. 2015; Peng et al. 2016; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015; Hamer et al. 2019), two studies showed increased CBF associated with mTBI (Doshi et al. 2015; Stephens et al. 2018), and one study found both higher and lower regional CBF in chronic mTBI (Brooks et al. 2019). Six studies found no significant group differences in resting CBF (Moller et al. 2017; W. A. C. Mutch et al. 2018; Militana et al. 2016; Churchill et al. 2019a; Coverdale et al. 2020; Liu et al. 2016). Markedly, four of those six studies only reported global mean CBF with no difference between groups (Churchill et al. 2019a; Militana et al. 2016; Moller et al. 2017; W. A. C. Mutch et al. 2018); however, another one of them found changes in CBF associated with the PVT cognitive task (Moller et al. 2017). In contrast, all eight reports focusing on voxelwise analyses found significant changes in resting CBF (Churchill et al. 2017a, 2019b; Hamer et al. 2019; Meier et al. 2015; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015). More interestingly, three additional studies showed significant findings not in global but only in regional resting CBF (Brooks et al. 2019; Churchill et al. 2017b; W. A. Mutch et al. 2016), while another report found changes in both global and regional CBF (Barlow et al. 2017).
Four of six longitudinal studies demonstrated decreased CBF over the post-mTBI recovery period. Meier et al. (Meier et al. 2015) reported reduced regional CBF from one day to one week and one month post-injury, a time frame in which neuropsychological symptoms resolved. Importantly, CBF was still decreased at one month post-concussion in slower-to-recover athletes and was inversely related to the magnitude of initial psychiatric symptoms (Meier et al. 2015). Similarly, Peng and colleagues found regional CBF was reduced at the acute stage and restored at the chronic stage (Peng et al. 2016), though no clinical correlation with dynamic CBF changes was stated. Furthermore, concussed athletes in another SRC study (Y. Wang et al. 2016) demonstrated a significant decrease in CBF at eight days relative to within 24 hours after injury, while clinical symptoms and cognitive measures demonstrated significant impairment compared with preseason baseline levels at 24 hours but returned to baseline levels at eight days. These findings support the hypothesis that physiological changes persist beyond the point of clinical recovery after SRC (Y. Wang et al. 2016). In one additional longitudinal study, mTBI patients with post-concussive syndrome (PCS) were compared with patients without PCS; these mTBI patients demonstrated an imbalance in the ratio of CBF between the default mode network (DMN) and task positive network nodes across multiple stages of recovery, without significant group differences in network average CBF. Only one longitudinal report showed higher regional CBF in SRC patients relative to controls at two and six weeks after injury, without significant changes between visit 1 and visit 2 within the SRC group (Stephens et al. 2018). Another recent longitudinal study reported elevated CBF at the symptomatic stage after SRC and no significant effects of concussion for CBF at RTP, but significant regional reductions in CBF at one year post-RTP, which may suggest persisting effects of concussion on CBF over a longer time scale (Churchill et al. 2019b).
Among reports cross-sectionally comparing mTBI patients with controls, one study found decreased CBF in acute SRC (Y. Wang et al. 2019), where correlations were also found between decreased CBF and clinical assessments as well as days from injury to asymptomatic (Y. Wang et al. 2019). Three studies from the same cohort showed decreased CBF in acute or subacute SRC (Churchill et al. 2017a; Churchill et al. 2017b) and demonstrated that CBF decreased as a reliable function of days post-injury (Churchill et al. 2017a; Churchill et al. 2017b). In addition, reduced CBF correlated with symptoms in recently concussed athletes (Churchill et al. 2017a; Churchill et al. 2017b). Another study showed decreased CBF in subacute mTBI and correlation between post-concussive symptoms and CBF in the hypoperfused areas (Lin et al. 2016). Two studies showed decreased CBF in chronic mTBI (Ge et al. 2009; Y. Wang et al. 2015; Hamer et al. 2019). One of them showed history of concussion is associated with decreased CBF in male athletes (Hamer et al. 2019). Another study also found that decreased thalamic CBF was significantly correlated with several neurocognitive measures including processing and response speed, memory/learning, verbal fluency, and executive function in patients (Ge et al. 2009). Only one cross-sectional study reported increased regional CBF in acute or subacute mTBI, in addition to decreases in local venous susceptibility, indicating increases in venous oxygenation (Doshi et al. 2015). However, neither CBF nor susceptibility measures were found to correlate with symptoms and neuropsychological testing (Doshi et al. 2015). Interestingly, Barlow and her group found in a pediatric study that global CBF at the later subacute stage was higher in symptomatic mTBI patients and lower in asymptomatic mTBI patients compared with control subjects (Barlow et al. 2017), where altered patterns were associated with the recovery trajectory. Another pediatric study also found both regional hypoperfusion and hyperperfusion in chronic mTBI but without clear clinical expression (Brooks et al. 2019).
Instead of voxelwise or ROI-wise group difference in CBF values, one study illustrated patients with chronic PCS with an imbalance in the ratio of CBF between the DMN and task positive network nodes across multiple stages of recovery after mTBI (Sours et al. 2015). Two reports from Mutch et al. (W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018) found no group difference in global mean resting CBF, but patient-specific abnormalities in regional CBF and CVR related to the control atlas were observed in patients with PCS at the subacute or chronic stage after injury (W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018). Another subacute SRC study (Militana et al. 2016) detected no significant resting CBF difference between groups but found increased CVR and FC within the DMN in the concussed athletes (Militana et al. 2016). Although no significant group differences were found in the CBF at rest, one PVT study using ASL showed that chronic mTBI patients with fatigue after injury used different brain networks compared with healthy control subjects during a vigilance task, and in mTBI, there was a distinction between CBF changes related to fatigability versus perceived fatigue (Moller et al. 2017). Likewise, another study observed CBF during PVT increased in the attention network but decreased in the DMN areas, which suggests that bottom-up and top-down attention deficits may result in mental fatigue in mTBI patients (Liu et al. 2016). Two recent studies reported no global CBF difference between mTBI and control groups, one at the acute/early subacute stage after SRC (Churchill et al. 2019a) and another at the chronic stage after injury (Coverdale et al. 2020), but found mTBI associated with reduced CVR as measured using the BOLD fMRI.
Discussion
A total of 23 studies published through February 20, 2020, met inclusion criteria for this review of ASL imaging in mTBI patients. Applying AAN guidelines for risk of bias assessments, we identified two Class I, eight Class II, four Class III, and nine Class IV studies. Overall findings, particularly combined from Class I and II studies included in the review, reinforce the notion that it is “likely” that aberrant CBF after mTBI can be detected using ASL perfusion MRI. In particular, most Class I and II studies of the acute to early subacute (e.g., within one week post-injury) found that concussed individuals demonstrated similar or lower CBF versus controls (Y. Wang et al. 2016; Y. Wang et al. 2019; Churchill et al. 2017b), which decreased further over the first week post-injury (e.g., Churchill et al., 2017b (Churchill et al. 2017b); Wang et al., 2016 (Y. Wang et al. 2016)). Findings of early reductions in CBF align with the preclinical literature but conflicted with some studies (e.g., Churchill et al., 2019 (Churchill et al. 2019b)). Thus, we settled on a conclusion of a likely, but not definitive, relationship between mTBI and ASL-measured CBF. Among the stronger (i.e., Class II) studies that investigated later stages of mTBI recovery, findings also tended to favor lower CBF or statistically equivalent CBF in mTBI patients versus controls between one month and one year post-injury (Churchill et al. 2019b). However, findings from studies with more risk of bias were variable and sometimes in the opposite direction. Obviously, there is considerable variability with respect to study design, time period of exam post-injury, MRI settings, ASL acquisition sequences, degree of control matching, and data analysis procedures across studies. It would be quite complicated to determine the extent to which these methodological variations across studies contribute to the variation reflected in the results, especially given that mTBI is highly heterogeneous (Kenzie et al. 2017). Taking this into account, it is understandable that the results from existing ASL studies in mTBI are diverse.
To illustrate variable study methodologies, four of six papers with null findings reported the global mean CBF only at rest (Churchill et al. 2019a; Militana et al. 2016; Moller et al. 2017; W. A. C. Mutch et al. 2018); another one of them, however, found changes in CBF during a cognitive task (Moller et al. 2017). In contrast, all eight reports focusing on voxelwise analyses showed significant relationships between mTBI and resting CBF (Churchill et al. 2017a, 2019b; Hamer et al. 2019; Meier et al. 2015; Stephens et al. 2018; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015). More interestingly, three additional studies showed significant findings only in regional resting CBF (Brooks et al. 2019; Churchill et al. 2017b; W. A. Mutch et al. 2016), while another report found changes in both global and regional CBF (Barlow et al. 2017). Apparently, these findings suggest that changes in ASL-derived CBF caused by mTBI may be primarily regional, implying that global CBF measures alone may obscure important effects. It is important to note that nine of 23 studies did not correct for multiple comparisons (Barlow et al. 2017; Coverdale et al. 2020; Doshi et al. 2015; Lin et al. 2016; Militana et al. 2016; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018; Peng et al. 2016; Sours et al. 2015). Caution should be exercised when interpreting results from these studies.
Although there was limited convergence in terms of anatomical location of CBF changes after injury and its relationship with clinical assessments, significantly reduced CBF was identified in acute, subacute, and chronic stages of mTBI (Churchill et al. 2017a; Churchill et al. 2017b; Ge et al. 2009; Y. Wang et al. 2016; Y. Wang et al. 2019; Y. Wang et al. 2015; Lin et al. 2016; Churchill et al. 2019b; Hamer et al. 2019). The findings of decreased CBF after mTBI from the majority of ASL studies included in this review are largely in accord with existing preclinical studies (Forbes et al. 1997; Hayward et al. 2010; Hendrich et al. 1999; Kochanek et al. 2002). Reduced CBF signifies one of the most lasting markers in animal models of TBI (Pasco et al. 2007; McGoron et al. 2008; Giza and Hovda 2014), as CBF was shown to decrease immediately after injury in an animal model (Ginsberg et al. 1997; Muir et al. 1992), and decreased regional CBF persisted after injury (Yamakami and McIntosh 1989). However, increased CBF after mTBI was also highlighted in two ASL studies on mTBI at acute or subacute stages (Doshi et al. 2015; Stephens et al. 2018), though one of them had limited interpretation due to a small sample size (i.e., findings that would not survive correction for multiple comparisons) (Doshi et al. 2015). One possible explanation with regard to contradicting results may be that patients with more symptoms might have higher CBF values (Stephens et al. 2018). Indeed, Churchill et al. found that specific symptom clusters may have distinct patterns of altered CBF (Churchill et al. 2017a), where concussed athletes with more cognitive symptoms showed significantly lower CBF and athletes with more somatic symptoms had significantly higher CBF relative to control subjects. Furthermore, Barlow and associates observed globally increased CBF in symptomatic mTBI and globally reduced CBF in asymptomatic mTBI relative to control subjects (Barlow et al. 2017), where concussed children with decreased CBF clinically recovered quickly, suggesting clinical recovery precedes cerebral recovery (Barlow et al. 2017). The observed variability in ASL results necessitates additional research, particularly on the role of post-injury CBF with regard to symptom status and recovery (Stephens et al. 2018). Nonetheless, CBF perfusion may be a marker of physiological status after mTBI. Recent research suggests SRC might be primarily a neurophysiologic injury mainly affecting CBF (Maugans et al. 2012). Existing data indicate ASL perfusion MRI has diagnostic potential as a sensitive method of detecting CBF abnormalities, even 24 hours up to one year after injury in concussed individuals.
Currently, only a few longitudinal studies have tested the prognostic ability of ASL in an mTBI or concussion sample. Meier et al. (Meier et al. 2015) reported both cross-sectional and longitudinal evidence of regional CBF recovery. Importantly, CBF in the dorsal mid-insular cortex was both decreased at one month post-concussion in slower-to-recover athletes and was inversely related to the magnitude of initial psychiatric symptoms, suggesting a potential prognostic indication for CBF as a biomarker (Meier et al. 2015). Peng and colleagues also described that CBF in various brain regions as measured using ASL may play a reference role in evaluating a patient’s condition and estimating prognosis after mTBI (Peng et al. 2016). Moreover, Sours and associates found that patients with chronic PCS demonstrated an imbalance in the ratio of CBF between the DMN nodes and task positive network nodes across multiple stages of recovery after mTBI, which suggests the altered network perfusion with the associated changes in resting-state FC may be a predictor of which mTBI patients will develop chronic PCS (Sours et al. 2015). Churchill et al. found that regional CBF reliably decreased as a function of days post-injury within the first week after SRC (Churchill et al. 2017b), and the most recent longitudinal report from the same group showed that the effects of concussion on CBF may persist over a longer time scale (perhaps one year after RTP) (Churchill et al. 2019b). Collectively, these examples highlight the prognostic potential of ASL perfusion MRI in mTBI. However, how CBF metrics change as the injury progresses or recovers remains unclear. Therefore, whether an observed change in CBF in the acute phase following injury or at any time over the course of recovery is predictive of poorer outcome merits far more longitudinal investigation.
It is an established notion that numerous factors interact dynamically to influence an individual’s recovery trajectory after mTBI (Kenzie et al. 2017). When explaining CBF findings, two important biological variables—age and gender—should always be considered. For example, there are well-accepted effects of age and gender on CBF values in healthy subjects (Detre et al. 2009), and age may play a critical role in recovery after concussion as well (Maugans et al. 2012). Two of three pediatric cross-sectional studies have reported both regional increased and decreased CBF in subacute or chronic mTBI (Barlow et al. 2017; Brooks et al. 2019). In contrast, two of three studies with a mixed sample of adolescents and young adults (W. A. Mutch et al. 2016; Y. Wang et al. 2019) only showed decreased CBF at the acute or chronic stage of SRC. Unfortunately, no study directly compared different age groups. Furthermore, one study explicitly investigated gender effects on CBF and found clear gender differences at the chronic SRC recovery stage (Hamer et al. 2019). While most ASL studies attempted to match groups on both age and gender or control for them in data analysis, interpretation of the effects of age and gender on CBF changes is still limited in the mTBI sample. In addition, other factors including pre-existing conditions, such as genetics, biomechanics, and premorbid psychological functioning, all likely come into play, which might drive premorbid CBF difference and impact postinjury CBF (Kenzie et al. 2017). The effects of those factors have not been elucidated in existing ASL studies on mTBI. The inclusion of pre-participation data would be ideal and help better clarify the diverse findings on post-injury CBF.
Brooks et al. recently found that youth with a history of concussion had hypoperfusion in posterior and inferior regions and hyperperfusion in anterior, frontal, and temporal regions (Brooks et al. 2019), although the regional CBF alteration was not significantly associated with clinical measures. Though there is increasing recognition of the potential effects of previous concussion, only six studies in this review evaluated the concussion history and only one of them found a specific effect of previous concussion on decreased CBF in chronic mTBI (Y. Wang et al. 2015). The lack of data in the context of the effects of repetitive mTBI or subconcussive exposure of recurrent head impacts on an individual’s CBF results is notable. Due to the heterogeneity and complexity seen within mTBI, it is likely that a wide variety of destructive and restorative processes are at work in parallel (Kenzie et al. 2017; Giza and Hovda 2014). Although longitudinal follow-up data have been published, the question of how to identify proper time points to detect underlying pathophysiology as measured using ASL to help diagnosis or recovery trajectory prediction in mTBI remains a vital question for future clinical trials.
Observed heterogeneity in mTBI arguably stems from the complexity of the condition itself, as the mechanisms of injury, clinical features, and patient experiences are all extremely heterogenous (Kenzie et al. 2017). Generally, SRC falls at the mild end of the TBI severity continuum (Mayer et al. 2017). The majority of ASL studies in this review focused on SRC, and all but one of the non-SRC studies examined civilian (vs. military) mTBI. Due to the vast methodological diversity across studies, it is enormously challenging to compare results from these studies to reveal the effect of different mechanisms of injury (e.g., blast or impact) or contexts of injury (e.g., football game, car accident, or fall). Additional research should evaluate CBF dose response based on injury type and severity (Y. Wang et al. 2016). Moreover, biomechanics and other injury characteristics further interact with individual variation in physiology, along with other personal characteristics such as age, gender, preinjury conditions, and genetics (Kenzie et al. 2017). In light of this, exact regions showing significant CBF alterations in mTBI varied across reports, which might be due to different sample characteristics or the use of different methodology in assessments. Similarly, it was not totally surprising to see no positive CBF results from direct voxelwise or ROI-wise comparison between mTBI patients and controls in a few studies (Sours et al. 2015; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018; Militana et al. 2016). Instead, patient-specific abnormalities were identified by detecting voxels that responded less than or greater than the mean healthy control group from the atlas (i.e., abnormal voxel counts) (W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018). Of note, certain key FC networks (e.g., DMN) are reported more often than others despite very different approaches across studies (Militana et al. 2016; Sours et al. 2015; Liu et al. 2016), which may further suggest the vulnerability of these networks to mTBI. (See the review by Mayer and colleagues (Mayer et al. 2015) for discussion.)
As an alternative to resting CBF, five studies reported positive changes in CVR (Militana et al. 2016; W. A. Mutch et al. 2016; W. A. C. Mutch et al. 2018; Churchill et al. 2019a; Coverdale et al. 2020), where CVR was assessed as the change in CBF that occurs in response to a vasoactive stimulus, such as during hypercapnia using controlled CO2. CO2 is a potent cerebral vasomotor agent, as indicated by the typical rapid increase or decrease in CBF in response to changes in the partial pressure of arterial carbon dioxide (PaCO2), and thus represents a significant mediator of CVR (Len et al. 2013; Len et al. 2011). Although animal studies have demonstrated mTBI impairs CBF response to CO2 (Pop and Badaut 2011), previous works suggest patient-specific alterations in cerebrovascular physiology can be safely and reliably assessed using validated MRI-based CVR assessment techniques such as BOLD fMRI or ASL (Ellis et al. 2016). These demonstrated the sensitivity in localizing subtle brain injuries (W. A. Mutch et al. 2014). Moreover, other than the resting CBF, insight into individuals’ responses to physiological stress (e.g., hypercapnia) may prove very useful toward the development of markers for recovery from mTBI (A. J. Gardner et al. 2015; Len and Neary 2011; Len et al. 2013; Len et al. 2011; Tan et al. 2014; W. A. Mutch et al. 2014). However, the ability of CVR assessment to accurately confirm recovery following concussion has not been fully determined using a longitudinal design (Ellis et al. 2016; W. A. Mutch et al. 2014). Because the aim of this review was to investigate ASL measures of CBF, we did not comprehensively target studies of CVR. More thorough review of CVR–mTBI literature, as well as further study of CVR as a candidate biomarker of mTBI, may be fruitful.
As the quality of ASL-derived perfusion maps has reached a level that makes the method useful for many clinical and research applications, ASL is still a rapidly developing field in view of technical innovation and applications (Alsop et al. 2015). Although the 3D pCASL technique with a higher signal-to-noise ratio and efficiency has been recommended as the workhorse labeling approach, with ASL images collected at a single post-labeling delay (Chen et al. 2011; Vidorreta et al. 2013; Alsop et al. 2015), the 2D PASL is still widely available and has demonstrated good test–retest reliability (Y. Wang et al. 2011). One advantage of 2D PASL is that single-shot acquisition is immune to inconsistency between excitations due to motion that can affect multishot methods such as 3D pCASL (Alsop et al. 2015). As summarized in this review, various ASL methods and protocols have been applied in mTBI research. The disparity of ASL imaging protocols across sites could contribute to present inconsistent findings between research sites. Nevertheless, Wang et al. directly compared results from two sites using very different 3D pCASL and 2D PASL methods and found very similar decreased CBF in acute concussion across sites with robust correlations between reduced rCBF and cognitive deficits (Y. Wang et al. 2019). It is also important to note that as a result of the low perfusion and long arterial transit times (ATT) in WM, the signal-to-noise ratio of the ASL signal is typically very low in WM; the common ASL protocol with single post-labeling delay is not specifically optimized to reliably detect both GM and WM perfusion (Haller et al. 2016). In addition, the WM ASL signal can easily be overwhelmed by GM signal owing to blurring in either in-plane or through-plane directions (van Gelderen et al. 2008; Alsop et al. 2015). Moreover, the ATT itself is a critical physiological parameter that varies between individuals, regionally, and between healthy and pathological tissue (Alsop et al. 2015). How brain injury affects regional ATT, CBF, and associated brain function remains to be fully explored. To date, there is no well accepted ASL protocol for TBI. More advanced ASL techniques, such as the recently developed 3D pCASL sequence with multiple post-labeling delay, can be used to estimate regional ATT and correct CBF based on ATT (Cohen et al. 2019). Though more complex post-processing is required, such techniques can more accurately determine regional CBF and should be considered in future TBI applications (Alsop et al. 2015; Cohen et al. 2019). (See the recent reviews (Alsop et al. 2015; Haller et al. 2016) for more detailed discussion of the ASL perfusion MRI technique.)
Conclusion
This systematic review of 23 studies of ASL perfusion MRI found reasonably strong evidence that mTBI is associated with changes in ASL-derived CBF, which correlate to some degree with clinical recovery. The need for further investigation is apparent, however, in light of heterogeneity among study findings and complexity of mTBI. Methodological uniformity is recommended to enable more consistent interpretation of data across studies, particularly given the anticipated proliferation of research employing this imaging technique within the field. Comparing characteristics of the various ASL techniques and developing a consensus in the field regarding the most reliable and valid acquisition and processing techniques for mTBI are certainly recommended. Future work should continue to assess how CBF perfusion relates to symptomology and recovery after injury, especially at the individual level. Further systematic investigation will provide more information on ASL perfusion MRI techniques for the diagnosis of mTBI, monitoring the course of recovery (i.e., prognosis), and informing the management of patients with single and recurrent mTBI.
Funding:
This study was partially supported by NIH grant (R01NS110600-01 to Y.W)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest.
Per personal communication with Dr. Churchill, the 26 concussed participants and 26 control subjects in Churchill et al. (2017b) were also in the Churchill et al. (2017a) study. In Churchill et al. (2019a), 6/21 concussed athletes overlapped with the sample in Churchill (2017a). In Churchill et al. (2019b), 21/24 concussed subjects were also in Churchill et al. (2017a) and 15/24 were also in Churchill et al. (2017b). The control subjects included in both 2017 studies by Churchill et al. were included in the large, normative dataset reported in Churchill et al. (2019b).
References
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, et al. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med, 73(1), 102–116, doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Borogovac A, & Brown TR (2008). Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med, 60(6), 1362–1371, doi: 10.1002/mrm.21670. [DOI] [PubMed] [Google Scholar]
- Audenaert K, Jansen HM, Otte A, Peremans K, Vervaet M, Crombez R, et al. (2003). Imaging of mild traumatic brain injury using 57Co and 99mTc HMPAO SPECT as compared to other diagnostic procedures. Med Sci Monit, 9(10), MT112–117. [PubMed] [Google Scholar]
- Barlow K, Marcil L, Dewey D, Carlson H, MacMaster F, Brooks B, et al. (2017). Cerebral Perfusion Changes in Post-Concussion Syndrome: a Prospective Controlled Cohort Study. J Neurotrauma, 34(5), 996–1004. doi: 10.1089/neu.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2013). Neuroinflammation and the dynamic lesion in traumatic brain injury. Brain, 136(Pt 1), 9–11, doi: 10.1093/brain/aws342. [DOI] [PubMed] [Google Scholar]
- Brooks BL, Low TA, Plourde V, Virani S, Jadavji Z, MacMaster FP, et al. (2019). Cerebral blood flow in children and adolescents several years after concussion. [Research Support, Non-U.S. Gov’t]. Brain Inj, 33(2), 233–241, doi: 10.1080/02699052.2018.1540798. [DOI] [PubMed] [Google Scholar]
- Buxton RB (2005). Quantifying CBF with arterial spin labeling. J Magn Reson Imaging, 22(6), 723–726, doi: 10.1002/jmri.20462. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang DJ, & Detre JA (2011). Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging, 33(4), 940–949, doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill NW, Hutchison MG, Graham SJ, & Schweizer TA (2017a). Symptom correlates of cerebral blood flow following acute concussion. Neuroimage Clin, 16, 234–239, doi: 10.1016/j.nicl.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill NW, Hutchison MG, Graham SJ, & Schweizer TA (2019a). Evaluating Cerebrovascular Reactivity during the Early Symptomatic Phase of Sport Concussion. J Neurotrauma, 36(10), 1518–1525, doi: 10.1089/neu.2018.6024. [DOI] [PubMed] [Google Scholar]
- Churchill NW, Hutchison MG, Graham SJ, & Schweizer TA (2019b). Mapping brain recovery after concussion: From acute injury to 1 year after medical clearance. Neurology, 93(21), e1980–e1992, doi: 10.1212/WNL.0000000000008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill NW, Hutchison MG, Richards D, Leung G, Graham SJ, & Schweizer TA (2017b). The first week after concussion: Blood flow, brain function and white matter microstructure. Neuroimage Clin, 14, 480–489, doi: 10.1016/j.nicl.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Agarwal M, Jagra AS, Nencka AS, Meier TB, Lebel RM, et al. (2019). Longitudinal Reproducibility of MR Perfusion Using 3D Pseudocontinuous Arterial Spin Labeling With Hadamard-Encoded Multiple Postlabeling Delays. J Magn Reson Imaging, doi: 10.1002/jmri.27007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, et al. (2012). Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J Safety Res, 43(4), 299–307, doi: 10.1016/j.jsr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Coverdale NS, Fernandez-Ruiz J, Champagne AA, Mark CI, & Cook DJ (2020). Co-localized impaired regional cerebrovascular reactivity in chronic concussion is associated with BOLD activation differences during a working memory task. Brain Imaging Behav, doi: 10.1007/s11682-019-00194-5. [DOI] [PubMed] [Google Scholar]
- Detre JA, Rao H, Wang DJ, Chen YF, & Wang Z (2012). Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging, 35(5), 1026–1037, doi: 10.1002/jmri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, & Rao H (2009). Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol, 22(4), 348–355, doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM (2011). Advances in MRI-Based Detection of Cerebrovascular Changes after Experimental Traumatic Brain Injury. Transl Stroke Res, 2(4), 524–532, doi: 10.1007/s12975-011-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi H, Wiseman N, Liu J, Wang W, Welch RD, O’Neil BJ, et al. (2015). Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS One, 10(2), e0118061, doi: 10.1371/journal.pone.0118061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MJ, Ryner LN, Sobczyk O, Fierstra J, Mikulis DJ, Fisher JA, et al. (2016). Neuroimaging Assessment of Cerebrovascular Reactivity in Concussion: Current Concepts, Methodological Considerations, and Review of the Literature. [Hypothesis & Theory]. Front Neurol, 7, 61, doi: 10.3389/fneur.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing JR, Cao Y, Knight RA, & Fenstermacher JD (2005). Arterial spin labeling: validity testing and comparison studies. J Magn Reson Imaging, 22(6), 737–740, doi: 10.1002/jmri.20451. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald M, & Coronado V (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Google Scholar]
- Forbes ML, Hendrich KS, Kochanek PM, Williams DS, Schiding JK, Wisniewski SR, et al. (1997). Assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact by perfusion magnetic resonance imaging using arterial spin-labeling in rats. J Cereb Blood Flow Metab, 17(8), 865–874, doi: 10.1097/00004647-199708000-00005. [DOI] [PubMed] [Google Scholar]
- Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, et al. (2012). A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma, 29(16), 2521–2538, doi: 10.1089/neu.2012.2628. [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Tan CO, Ainslie PN, van Donkelaar P, Stanwell P, Levi CR, et al. (2015). Cerebrovascular reactivity assessed by transcranial Doppler ultrasound in sport-related concussion: a systematic review. Br J Sports Med, 49(16), 1050–1055, doi: 10.1136/bjsports-2014-093901. [DOI] [PubMed] [Google Scholar]
- Ge Y, Patel MB, Chen Q, Grossman EJ, Zhang K, Miles L, et al. (2009). Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj, 23(7), 666–674, doi: 10.1080/02699050903014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers S, Majoie CB, van den Tweel XW, Lavini C, & Nederveen AJ (2009). Acquisition time and reproducibility of continuous arterial spin-labeling perfusion imaging at 3T. AJNR Am J Neuroradiol, 30(5), 968–971, doi: 10.3174/ajnr.A1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, & Busto R (1997). Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats (Vol. 272, Vol. 6). [DOI] [PubMed] [Google Scholar]
- Giza CC, & Hovda DA (2014). The new neurometabolic cascade of concussion. Neurosurgery, 75 Suppl 4, S24–33, doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Toweill D, Lai S, Sonnenthal K, & Kimberly B (1998). Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol, 275(4), R1287–1292, doi: 10.1152/ajpregu.1998.275.4.R1287. [DOI] [PubMed] [Google Scholar]
- Gowda NK, Agrawal D, Bal C, Chandrashekar N, Tripati M, Bandopadhyaya GP, et al. (2006). Technetium Tc-99m ethyl cysteinate dimer brain single-photon emission CT in mild traumatic brain injury: a prospective study. AJNR Am J Neuroradiol, 27(2), 447–451. [PMC free article] [PubMed] [Google Scholar]
- Gronseth GS, Cox J, & Getchius TS (2015). Amendments to the 2011 American Academy of Neurology Clinical Practice Guideline Process Manual. Minneapolis, MN: American Academy of Neurology. [Google Scholar]
- Gronseth GS, Cox J, Gloss D, Merillat S, Dittman J, Amstrong MJ, et al. (2017). 2017 Edition Clinical Practice Guideline Process Manual. Minneapolis, MN: American Academy of Neurology. [Google Scholar]
- Gronseth GS, Woodroffe LM, & Getchius TS (2011). Clinical practice guideline process manual. St. Paul, MN: American Academy of Neurology. [Google Scholar]
- Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, & Golay X (2016). Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology, 281(2), 337–356, doi: 10.1148/radiol.2016150789. [DOI] [PubMed] [Google Scholar]
- Hamer J, Churchill NW, Hutchison MG, Graham SJ, & Schweizer TA (2019). Sex Differences in Cerebral Blood Flow Associated with a History of Concussion. J Neurotrauma, 0(0), null, doi: 10.1089/neu.2019.6800. [DOI] [PubMed] [Google Scholar]
- Hayward NM, Immonen R, Tuunanen PI, Ndode-Ekane XE, Grohn O, & Pitkanen A (2010). Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J Neurotrauma, 27(12), 2203–2219, doi: 10.1089/neu.2010.1448. [DOI] [PubMed] [Google Scholar]
- Hendrich KS, Kochanek PM, Williams DS, Schiding JK, Marion DW, & Ho C (1999). Early perfusion after controlled cortical impact in rats: Quantification by arterial spin-labeled MRI and the influence of spin-lattice relaxation time heterogeneity. Magnetic Resonance in Medicine, 42(4), 673–681, doi:. [DOI] [PubMed] [Google Scholar]
- Iadecola C, & Nedergaard M (2007). Glial regulation of the cerebral microvasculature. Nat Neurosci, 10(11), 1369–1376, doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, & El Khoury J (2017). Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron, 95(6), 1246–1265, doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger EC, Newell DW, Grant GA, Avellino AM, Ghatan S, Douville CM, et al. (1997). Cerebral autoregulation following minor head injury. J Neurosurg, 86(3), 425–432, doi: 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, et al. (1993). Definition of Mild Traumatic Brain Injury. Journal of Head Trauma Rehabilitation, 8(3), 86–87. [Google Scholar]
- Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, et al. (2016). Cerebral Vascular Injury in Traumatic Brain Injury. Exp Neurol, 275 Pt 3, 353–366, doi: 10.1016/j.expneurol.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Kenzie ES, Parks EL, Bigler ED, Lim MM, Chesnutt JC, & Wakeland W (2017). Concussion As a Multi-Scale Complex System: An Interdisciplinary Synthesis of Current Knowledge. [Review]. Front Neurol, 8(513), 513, doi: 10.3389/fneur.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Hendrich KS, Dixon CE, Schiding JK, Williams DS, & Ho C (2002). Cerebral Blood Flow at One Year after Controlled Cortical Impact in Rats: Assessment by Magnetic Resonance Imaging. J Neurotrauma, 19(9), 1029–1037, doi: 10.1089/089771502760341947. [DOI] [PubMed] [Google Scholar]
- Koretsky AP (2012). Early development of arterial spin labeling to measure regional brain blood flow by MRI. Neuroimage, 62(2), 602–607, doi: 10.1016/j.neuroimage.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, & Loane DJ (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun, 26(8), 1191–1201, doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Len TK, & Neary JP (2011). Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging, 31(2), 85–93, doi: 10.1111/j.1475-097X.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- Len TK, Neary JP, Asmundson GJ, Candow DG, Goodman DG, Bjornson B, et al. (2013). Serial monitoring of CO2 reactivity following sport concussion using hypocapnia and hypercapnia. Brain Inj, 27(3), 346–353, doi: 10.3109/02699052.2012.743185. [DOI] [PubMed] [Google Scholar]
- Len TK, Neary JP, Asmundson GJ, Goodman DG, Bjornson B, & Bhambhani YN (2011). Cerebrovascular reactivity impairment after sport-induced concussion. Med Sci Sports Exerc, 43(12), 2241–2248, doi: 10.1249/MSS.0b013e3182249539. [DOI] [PubMed] [Google Scholar]
- Lin CM, Tseng YC, Hsu HL, Chen CJ, Chen DY, Yan FX, et al. (2016). Arterial Spin Labeling Perfusion Study in the Patients with Subacute Mild Traumatic Brain Injury. [Article]. PLoS One, 11(2), e0149109, doi: 10.1371/journal.pone.0149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Li B, Qian S, Jiang Q, Li L, Wang W, et al. (2016). Mental fatigue after mild traumatic brain injury: a 3D-ASL perfusion study. [Article]. Brain Imaging Behav, 10(3), 857–868, doi: 10.1007/s11682-015-9492-3. [DOI] [PubMed] [Google Scholar]
- Loane DJ, & Faden AI (2010). Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci, 31(12), 596–604, doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, & Maas AI (2013). Traumatic brain injury: an international knowledge-based approach. JAMA, 310(5), 473–474, doi: 10.1001/jama.2013.169158. [DOI] [PubMed] [Google Scholar]
- Maugans TA, Farley C, Altaye M, Leach J, & Cecil KM (2012). Pediatric Sports-Related Concussion Produces Cerebral Blood Flow Alterations. Pediatrics, 129(1), 28–37, doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Bellgowan PS, & Hanlon FM (2015). Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci Biobehav Rev, 49, 8–18, doi: 10.1016/j.neubiorev.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Quinn DK, & Master CL (2017). The spectrum of mild traumatic brain injury: A review. Neurology, 89(6), 623–632, doi: 10.1212/WNL.0000000000004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW (2011). Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci, 13(3), 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, et al. (2017). Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med, 51(11), 838–847, doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- McGoron AJ, Capille M, Georgiou MF, Sanchez P, Solano J, Gonzalez-Brito M, et al. (2008). Post traumatic brain perfusion SPECT analysis using reconstructed ROI maps of radioactive microsphere derived cerebral blood flow and statistical parametric mapping. BMC Med Imaging, 8, 4, doi: 10.1186/1471-2342-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes K, Friesen CL, MacKenzie DE, Westwood DA, & Boe SG (2017). Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One, 12(4), e0174847, doi: 10.1371/journal.pone.0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Bellgowan PS, Singh R, Kuplicki R, Polanski DW, & Mayer AR (2015). Recovery of cerebral blood flow following sports-related concussion. [Article]. JAMA Neurol, 72(5), 530–538, doi: 10.1001/jamaneurol.2014.4778. [DOI] [PubMed] [Google Scholar]
- Metting Z, Spikman JM, Rodiger LA, & van der Naalt J (2014). Cerebral perfusion and neuropsychological follow up in mild traumatic brain injury: acute versus chronic disturbances? Brain Cogn, 86(0), 24–31, doi: 10.1016/j.bandc.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Militana AR, Donahue MJ, Sills AK, Solomon GS, Gregory AJ, Strother MK, et al. (2016). Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: a pilot study. Brain Imaging Behav, 10(2), 559–568, doi: 10.1007/s11682-015-9407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & Group P (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6(7), e1000097, doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller MC, Nordin LE, Bartfai A, Julin P, & Li TQ (2017). Fatigue and Cognitive Fatigability in Mild Traumatic Brain Injury are Correlated with Altered Neural Activity during Vigilance Test Performance. [Article]. Front Neurol, 8, 496, doi: 10.3389/fneur.2017.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JK, Boerschel M, & Ellis EF (1992). Continuous monitoring of posttraumatic cerebral blood flow using laser-Doppler flowmetry. J Neurotrauma, 9(4), 355–362, doi: 10.1089/neu.1992.9.355. [DOI] [PubMed] [Google Scholar]
- Mutch WA, Ellis MJ, Graham MR, Wourms V, Raban R, Fisher JA, et al. (2014). Brain MRI CO2 stress testing: a pilot study in patients with concussion. PLoS One, 9(7), e102181, doi: 10.1371/journal.pone.0102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch WA, Ellis MJ, Ryner LN, Graham MR, Dufault B, Gregson B, et al. (2016). Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J Neurosurg, 125(3), 648–660, doi: 10.3171/2015.6.JNS15972. [DOI] [PubMed] [Google Scholar]
- Mutch WAC, Ellis MJ, Ryner LN, McDonald PJ, Morissette MP, Pries P, et al. (2018). Patient-Specific Alterations in CO2 Cerebrovascular Responsiveness in Acute and Sub-Acute Sports-Related Concussion. [Article]. Front Neurol, 9(JAN), 23, doi: 10.3389/fneur.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, & Elmagarmid A (2016). Rayyan-a web and mobile app for systematic reviews. [journal article]. Syst Rev, 5(1), 210, doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, & Tofts PS (2004). Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med, 51(4), 736–743, doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Pasco A, Lemaire L, Franconi F, Lefur Y, Noury F, Saint-Andre JP, et al. (2007). Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion-weighted magnetic resonance imaging. J Neurotrauma, 24(8), 1321–1330, doi: 10.1089/neu.2006.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, et al. (2017). Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. [journal article]. Cell Mol Neurobiol, 37(4), 571–585, doi: 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SP, Li YN, Liu J, Wang ZY, Zhang ZS, Zhou SK, et al. (2016). Pulsed arterial spin labeling effectively and dynamically observes changes in cerebral blood flow after mild traumatic brain injury. Neural Regen Res, 11(2), 257–261, doi: 10.4103/1673-5374.177733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertab JL, Merkley TL, Cramond AJ, Cramond K, Paxton H, & Wu T (2018). Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation, 42(4), 397–427, doi: 10.3233/NRE-172298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen ET, Mouridsen K, Golay X, & all named co-authors of the, Q. t.-r. s. (2010). The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage, 49(1), 104–113, doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, & Badaut J (2011). A neurovascular perspective for long-term changes after brain trauma. Transl Stroke Res, 2(4), 533–545, doi: 10.1007/s12975-011-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae-Grant A, Messé S, Potrebic S, Cox J, O’Brien MD, Merillat S, et al. (2019). Amendments to the 2017 American Academy of Neurology Clinical Practice Guideline Process Manual. Minneapolis, MN: American Academy of Neurology. [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. [Research Support, Non-U.S. Gov’t]. Ann Neurol, 70(3), 374–383, doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rangel-Castilla L, Gasco J, Nauta HJ, Okonkwo DO, & Robertson CS (2008). Cerebral pressure autoregulation in traumatic brain injury. Neurosurg Focus, 25(4), E7, doi: 10.3171/FOC.2008.25.10.E7. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Gay M, Johnson B, & Zhang K (2012). Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav, 6(2), 224–243, doi: 10.1007/s11682-012-9167-2. [DOI] [PubMed] [Google Scholar]
- Smith C, Gentleman SM, Leclercq PD, Murray LS, Griffin WS, Graham DI, et al. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol, 39(6), 654–666, doi: 10.1111/nan.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C, Zhuo J, Roys S, Shanmuganathan K, & Gullapalli RP (2015). Disruptions in Resting State Functional Connectivity and Cerebral Blood Flow in Mild Traumatic Brain Injury Patients. PLoS One, 10(8), e0134019, doi: 10.1371/journal.pone.0134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Liu P, Lu H, & Suskauer SJ (2018). Cerebral Blood Flow after Mild Traumatic Brain Injury: Associations between Symptoms and Post-Injury Perfusion. J Neurotrauma, 35(2), 241–248, doi: 10.1089/neu.2017.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel S, Lam AM, Matta BF, & Newell DW (1997). Impaired cerebral autoregulation after mild brain injury. Surg Neurol, 47(2), 128–131, doi: 10.1016/s0090-3019(96)00459-4. [DOI] [PubMed] [Google Scholar]
- Talagala SL, & Noll DC (1998). Functional MRI using steady-state arterial water labeling. Magn Reson Med, 39(2), 179–183, doi: 10.1002/mrm.1910390203. [DOI] [PubMed] [Google Scholar]
- Tan CO, Meehan WP, Iverson GL, & Taylor JA (2014). Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology, 83(18), 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Szarka N, Farkas E, Ezer E, Czeiter E, Amrein K, et al. (2016). Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanisms, perspectives, and therapeutic implications. Am J Physiol Heart Circ Physiol, 311(5), H1118–H1131, doi: 10.1152/ajpheart.00267.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, & Duyn JH (2008). Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med, 59(4), 788–795, doi: 10.1002/mrm.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidorreta M, Wang Z, Rodriguez I, Pastor MA, Detre JA, & Fernandez-Seara MA (2013). Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage, 66, 662–671, doi: 10.1016/j.neuroimage.2012.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, & Detre JA (2003). Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med, 49(5), 796–802, doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang H, Lee DH, Yu J, Cheng T, Hong M, et al. (2017a). Using functional and molecular MRI techniques to detect neuroinflammation and neuroprotection after traumatic brain injury. Brain Behav Immun, 64(Supplement C), 344–353, doi: 10.1016/j.bbi.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhang H, Lee DH, Yu J, Cheng T, Hong M, et al. (2017b). Using functional and molecular MRI techniques to detect neuroinflammation and neuroprotection after traumatic brain injury. Brain Behav Immun, 64, 344–353, doi: 10.1016/j.bbi.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nelson LD, LaRoche AA, Pfaller AY, Nencka AS, Koch KM, et al. (2016). Cerebral Blood Flow Alterations in Acute Sport-Related Concussion. J Neurotrauma, 33(13), 1227–1236, doi: 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nencka AS, Meier TB, Guskiewicz K, Mihalik JP, Alison Brooks M, et al. (2019). Cerebral blood flow in acute concussion: preliminary ASL findings from the NCAA-DoD CARE consortium. Brain Imaging Behav, 13(5), 1375–1385, doi: 10.1007/s11682-018-9946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, et al. (2011). Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage, 54(2), 1188–1195, doi: 10.1016/j.neuroimage.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, West JD, Bailey JN, Westfall DR, Xiao H, Arnold TW, et al. (2015). Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. Dev Neuropsychol, 40(1), 40–44, doi: 10.1080/87565641.2014.979927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C, & Engelhard K (2007a). Pathophysiology of traumatic brain injury. [Review]. Br J Anaesth, 99(1), 4–9, doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Werner C, & Engelhard K (2007b). Pathophysiology of traumatic brain injury. Br J Anaesth, 99(1), 4–9, doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Wong EC (2014). An introduction to ASL labeling techniques. J Magn Reson Imaging, 40(1), 1–10, doi: 10.1002/jmri.24565. [DOI] [PubMed] [Google Scholar]
- Yamakami I, & McIntosh TK (1989). Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J Cereb Blood Flow Metab, 9(1), 117–124, doi: 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, & Levine BD (2002). Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation, 106(14), 1814–1820, doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]


