Abstract
Natural killer (NK) cells, as a potential source for off-the-shelf cell therapy, attack tumor cells with low risk of severe cytokine release syndrome (CRS) or graft-versus-host disease (GvHD). Fcγ receptor IIIA, also known as CD16, further confers NK cells with antibody-dependent cell-mediated cytotoxicity (ADCC), one mechanism of action of antibody-based immunotherapy. Here, we establish a novel human NK cell line, oNK-1, endogenously expressing CD16 along with high levels of NK activation markers and low levels of NK inhibitory markers. The long-term expansion and CD16 expression of oNK-1 cells were demonstrated. Furthermore, oNK-1 cells elicit superior cytotoxicity against cancer cells than primary NK cells. In conclusion, this study suggests that endogenous CD16-expressing oNK-1 has the potential to develop an effective NK-based therapy.
Keywords: Natural killer cells, oNK-1, CD16, FCGRIIIA, ADCC, Cell therapy
Highlights
-
●
oNK-1 is a stable endogenous CD16-expressing human natural killer cell line.
-
●
oNK-1 cells express high levels of activation markers and low levels of inhibitory markers.
-
●
oNK-1 cells elicit elevated cytotoxicity against different types of cancers.
-
●
oNK-1 cells harbor antibody-dependent cellular cytotoxicity.
1. Introduction
Over the last few decades, the clinical relevance of immune cells observed in several types of malignancies has been shown by the blooming of cancer immunotherapy [1]. Among them, T cells have been widely investigated and succeeded as cell therapeutic agents. For example, the clinical significance of Kymriah and Yescarta based on chimeric antigen receptor T cells (CAR-T) platforms for treating acute lymphoblastic leukemia and non-Hodgkin's lymphoma has been realized [2,3]. Nevertheless, accumulating cases of severe adverse events, including severe to fatal cytokine release syndrome (CRS) and graft versus host disease (GvHD), have been reported [4]. As for cell therapies based on natural killer (NK) cells, severe adverse events are less likely to occur because of the limited in vivo persistence and lack of clonal expansion [5]. The clinical use of autologous NK cells is confronted by the limitation of expansion efficiency and cytotoxic function, whereas ex vivo expanded allogeneic NK cells provide a promising alternative for clinical application [6]. In addition to the well-established NK-92 cell line [7], cord blood with proper HLA-type selection can be an alternative source of allogeneic NK cells [8,9]. Haploidentical and unrelated donor CD34+ progenitor cells or patient HLA-matched NK cells isolated from leukapheresis elicit cytotoxic effects [10,11]. More recently, controlled differentiation of induced pluripotent stem cells (iPSC) provide another source of allogeneic NK cells [12]. These allogeneic human NK cells possess efficient cytotoxicity against liquid and solid tumors [[13], [14], [15]].
It has been noted that the balance between NK activation and inhibitory signals determines the function of NK cells [16,17]. Among NK activators, Fcγ receptor IIIA, also known as CD16, is a well-characterized activation receptor and mediates the antibody-dependent cell-mediated cytotoxicity (ADCC) to eradicate “stressed” cells in antibody-based immunotherapy [18,19]. There are polymorphisms of the CD16 gene distributed in the normal population of NK cells, which encode CD16 proteins with differential binding affinity to the Fc region of antibodies [20]. Interestingly, some sources of allogeneic NK cells express no CD16, whereas others express CD16 with low antibody binding affinity [21]. Exogenous expression of CD16 variants, especially those with high affinity to antibodies, restores the ADCC activity in NK cells [[22], [23], [24]].
In this study, we establish a novel NK cell line oNK-1 with endogenous expression of CD16 as well as high expression of NK activation receptors and low expression of NK inhibitory receptors. CD16 expression of oNK-1 cells is stable during a long-term culture in the presence of IL-2. Further characterization demonstrates strong cytotoxicity of oNK-1 against different types of cancer cells and preferred ADCC activity, suggesting the potential of oNK-1 cells to be an effective NK-based therapy against cancer.
2. Materials and methods
2.1. Antibodies and cell lines
Fluorescent dye-conjugated antibodies against CD56, CD3, CD16, CD25, NKp30, NKG2D, NKp44, NKp46, CD107a, NKG2A, PD-1, TIGIT, and pan-KIR were purchased from BioLegend, USA. Human ovarian (SK-OV-3 and OVCAR-3), breast (SK-BR-3 and MCF-7), lung (A549), bladder (T24), and blood (Raji) cancer cell lines were obtained from American Type Cell Collection (ATCC, USA). These cell lines were cultured according to ATCC guidelines. Male donor-derived primary NK cells were purchased from Cellero, USA. The cryovial of primary NK cells was thawed under manufacturer's instructions and applied for subsequent cytotoxicity assay.
2.2. Generation of oNK-1 cell line
NK-92 cell line was established from a 50 years old Caucasian male with rapidly progressive non-Hodgkin's lymphoma. Parental NK-92 cells with early passages in original medium described previously [25] were cultured in the adaption medium X–VIVO 10 (Lonza, Switzerland) with human platelet lysates (Helios, USA) and recombinant human interleukin 2 (IL-2, Miltenyi Biotec, Germany) for 6 passages. CD16-expressing population of Adapted NK-92 cells were enriched by NKselect strategy through fluorescence-activated cell sorting using FACSAria IIIu (Becton Dickinson, USA), designated as oNK-1.
2.3. Flow cytometry analysis
All flow cytometry analysis was performed by the Attune NxT flow cytometer installed with Attune NxT software 3.1.0. Half a million cells were stained with 1 μg of fluorescent dye-conjugated antibody stock in 100 μL of reaction volume at room temperature for 10 min. The stained cell mixture was centrifuged at 400×g for 3 min. Cell pellets were washed with 1 mL of DPBS (Gibco, USA). After repeating centrifugation, cell pellets were resuspended with 0.5 mL of DPBS and loaded up for flow cytometry analysis.
2.4. Droplet digital polymerase chain reaction
The droplet digital polymerase chain reaction (ddPCR) was performed by QX200 Droplet Digital PCR System (Bio-Rad, USA) according to manufacturer's instruction. In brief, genomic DNA of oNK-1 cells was extracted by Blood and Cell Culture DNA Mini Kit (Qiagen, Germany). Fifty nanograms of extracted genomic DNA digested by BstXI restriction enzyme (New England BioLabs, USA) were mixed with the probe of TaqMan® SNP Genotyping Assays (assay ID: C__25815666_10, Applied Biosystem, USA) in ddPCR™ Supermix for Probe at a final volume of 20 μL. The nanoliter-sized droplet in the mixture generated by QX200 Droplet Generator was loaded in the DG8 Cartridge (Bio-Rad, USA) for the following polymerase chain reaction and analysis. No template control (NTC) was included to validate no contamination of master reaction mixture.
2.5. Cytotoxicity assay
The xCELLigence real-time cell analysis system and flow cytometry was applied under the manufacturer's instruction for analyzing cytotoxicity of adherent and floating cell cultures, respectively. In brief, adherent target cells were seeded into wells of E-plate and settled for cell attachment. At specific E:T ratio, effector cells were seeded into wells in the presence and absence of target cells. The cell index (CI) was recorded throughout the study. The cytotoxicity was translated by xCELLigence RTCA software automatically. For floating culture, target cells pre-stained with carboxyfluorescein succinimidyl ester (CFSE, ThermoFisher, USA) was co-cultured with effector cells at specific E:T ratios in 5% of CO2 at 37 °C for 2 h. The harvested cells were stained with propidium iodide (PI). The percentage of dead Raji cells designated as CFSE+PI+ was analyzed in gated CFSE+ cells by flow cytometry.
3. Results
3.1. Endogenous CD16-expressing oNK-1 cells exhibit enhanced cytotoxicity
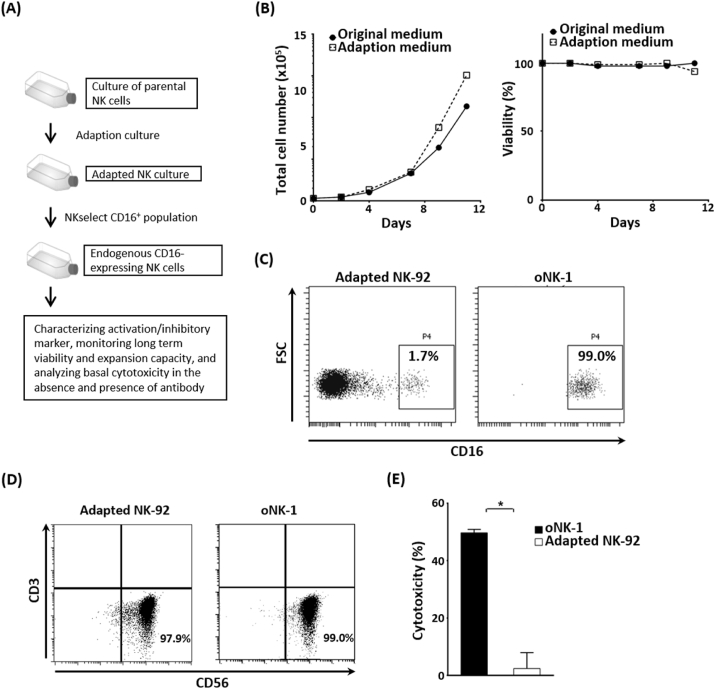
Exogenous CD16 expression of NK-92, CD16-negative cell line, has been shown to confers ADCC to NK-92 [23,24]. To obtain an endogenous CD16-expressing NK cell line, the adaption culture was applied to parental NK-92 and the data showed no significant change in viable cell density and viability of Adapted NK-92 cells (Fig. 1A and B). The endogenous CD16-expressing population, oNK-1, were isolated from Adapted NK-92 culture by NKselect strategy (Fig. 1C). NK characteristics of oNK-1 showed no significant change with Adaptive NK-92 (Fig. 1D), while the cytotoxicity of oNK-1 against human ovarian cancer cell line SK-OV-3 was significantly higher compared to that of Adapted NK-92 (Fig. 1E), indicating superior basal anti-cancer cytotoxicity of oNK-1.
Fig. 1.
Endogenous CD16-expressing oNK-1 cells elicit high cytotoxicity. (A) A flow chart of establishing oNK-1 cells was illustrated. (B) Growth of NK-92 in original and adaption media. Viable total cell number (left) and viability (right) in both culture media were monitored. (C) CD16+ population in Adapted NK-92 (left) and oNK-1 (right) cells were shown by flow cytometry. (D) NK characteristics (CD56+CD3−) of Adapted NK-92 and oNK-1 cells were analyzed by flow cytometry. (E) oNK-1 or Adapted NK-92 cells (Effector) were co-cultured with SK-OV-3 cells (Target). Cytotoxicity analyzed by xCELLigence real-time cell analysis was performed as described in Materials and Methods. A comparison of the cytotoxicity between oNK-1 and Adapted NK-92 cells was performed by t-test. *, p < 0.05.
3.2. oNK-1 cells harbor elevated expression of activation markers
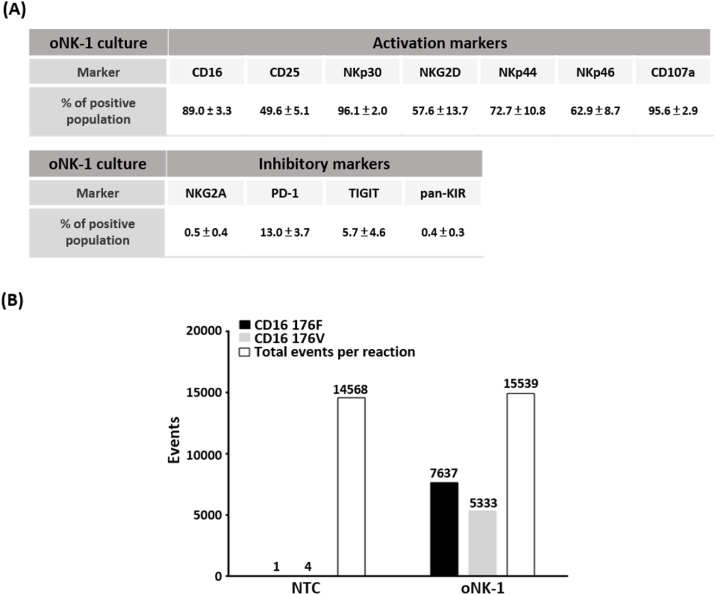
Accumulating studies have revealed that NK activity is tightly regulated by a suite of activation/co-stimulatory molecules and inhibitory signaling receptors [8]. To profile the expression of activation/co-stimulatory and inhibitory markers, flow cytometry was performed. As shown in Fig. 2A, oNK-1 cells highly expressed activation/co-stimulatory marker CD16, CD25, NKp30, NKG2D, NKp44, NKp46 and degranulation marker CD107a. It is worth noting that inhibitory receptors including NKG2A, PD-1, TIGIT, and pan-KIR were found only in a small portion of oNK-1 cells (Fig. 2A). These results indicate that oNK-1 cells retain high levels of activation/co-stimulatory receptors and degranulation along with the lower expression of inhibitory receptors.
Fig. 2.
oNK-1 cells harbor a high expression of activation markers. (A) NK activation markers were preferentially expressed in oNK-1 cells. A panel of NK activation and inhibitory markers in oNK-1 cells were profiled by flow cytometry. Three independent experiments of NK activation and inhibitory markers profiling were performed. The results were shown in mean ± standard deviation. The representative data were shown in Supplementary Fig. 2. (B) Both CD16 with low and high affinity at amino acid residue 176F and 176V were detected in oNK-1 cells. The characterization was analyzed by digital droplet PCR. NTC, no template control.
3.3. oNK-1 cells carry CD16 polymorphism
A wide spectrum of polymorphism has been found in CD16 gene FCGRIIIA [20] and associated with disease responses to antibody therapy [[26], [27], [28]]. To characterize polymorphism of FCGRIIIA in oNK-1 cells, corresponding T/G substitution in FCGRIIIA codon leading to phenylalanine (F)/valine (V) substitution at amino acid residue 176 of CD16 was analyzed by droplet digital PCR. It was shown that 7637 events of 176F and 5333 events of 176V in total 15,539 events were observed (Fig. 2B). The event ratio of 176F/176V was 1.43. These results suggest that oNK-1 cell line harbor both CD16 polymorphisms for low and high affinity to the Fc region of antibodies.
3.4. CD16 expression and proliferation of oNK-1 require IL-2
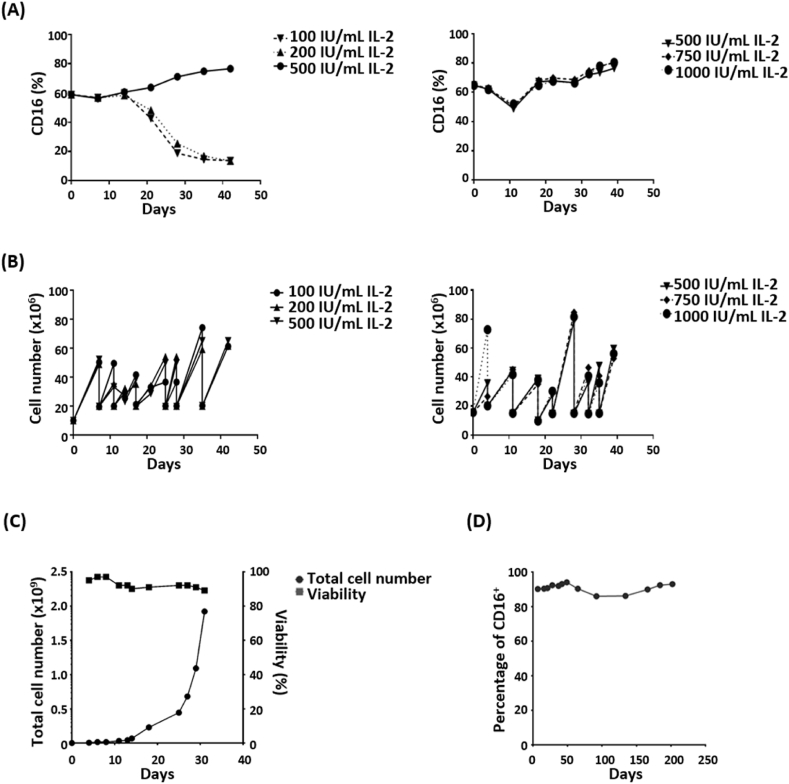
The essential role of IL-2 in the growth of T cells and NK cells has been broadly investigated [29]. To test the roles of IL-2 in oNK-1 cells, oNK-1 cells were cultured in the medium supplemented with different concentrations of IL-2. As shown in Fig. 3A, the percentage of CD16+ population in oNK-1 cultured in 100 or 200 IU/mL IL-2 was gradually decreased (left panel), while equal to or higher than 500 IU/mL IL-2 maintained CD16 expression of oNK-1 (right panel). Additionally, 100 IU/mL of IL-2 was enough to maintain the proliferation of oNK-1 cells (Fig. 3B, left panel). The proliferation rate of oNK-1 cells among groups showed no significant difference throughout the study, while an increased cell number was observed in oNK-1 culture with 1000 IU/mL of IL-2 during the first four days post seeding (Fig. 3B, right panel). The viability and proliferation rate of oNK-1 cells was stable during a 30-day expansion (Fig. 3C). The CD16 expression of oNK-1 cells was maintained during a long-term culture (Fig. 3D). These results support that the oNK-1 culture has the capacity to be continuous.
Fig. 3.
Maintenance of oNK-1 culture is IL-2 dependent. (A) CD16+ population of oNK-1 cells at different concentrations of IL-2 was monitored by flow cytometry. (B) The expansion of oNK-1 cells at different concentrations of IL-2 was recorded. (C) Cell viability (filled square) and total cell number (filled circle) were determined by the trypan blue exclusion method. The viability and cell number of oNK-1 cells were monitored for 30 days. (D) The percentage of CD16-expressing oNK-1 cells was monitored by flow cytometry for more than 200 days.
3.5. oNK-1 cells elicit anti-cancer and ADCC activity
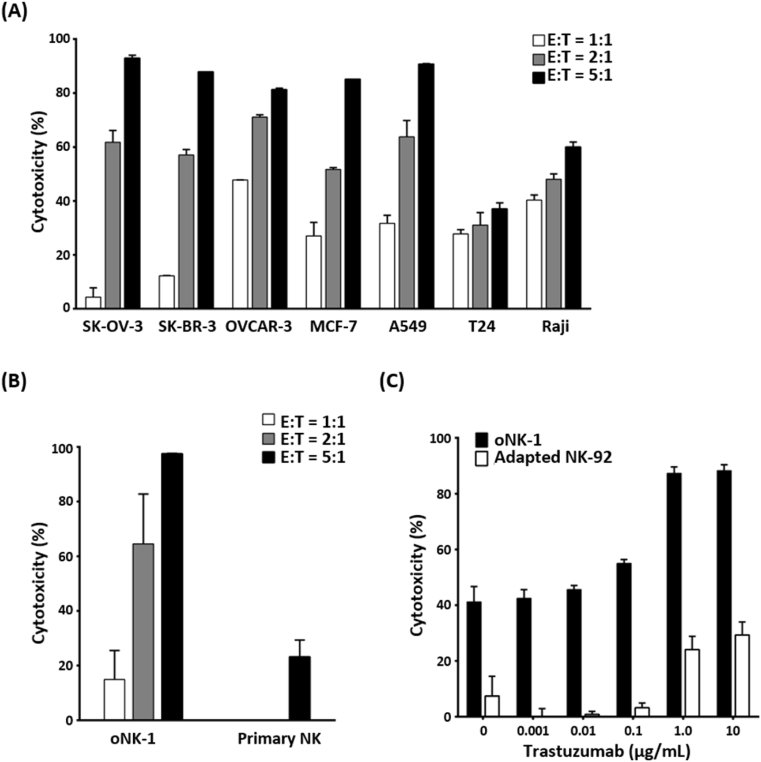
To examine if oNK-1 cells exert cytotoxicity against different cancer types, cancer cell lines derived from ovarian (SK-OV-3 and OVCAR-3), breast (SK-BR-3 and MCF-7), lung (A549), bladder (T24) and, blood (Raji) were co-cultured with oNK-1 cells at different E:T ratios of 1:1, 2:1 and 5:1. As shown in Fig. 4A, oNK-1 cells exhibited strong dose-dependent cytotoxicity against SK-OV-3, OVCAR-3, SK-BR-3, MCF-7, and A549 and moderate against T24 and Raji. Importantly, oNK-1 cells exerted stronger cytotoxicity than donor-derived primary NK cells against OVCAR-3 cells (Fig. 4B). Moreover, the ADCC of oNK-1 cells was examined in the presence of anti-human epidermal growth factor receptor 2 antibodies (trastuzumab). The results showed a higher ADCC activity of oNK-1 against SK-OV-3 cells than that of Adapted NK-92 cells (Fig. 4C). These findings demonstrate that oNK-1 cells elicit efficient cytotoxicity against diverse cancers and exhibit ADCC activity against ovarian cancer cells in the presence of trastuzumab.
Fig. 4.
oNK-1 exerts cytotoxic activity against cancer cells in antibody-dependent cellular cytotoxicity. (A) Cytotoxicity of oNK-1 cells against SK-OV-3, OVCAR-3, SK-BR-3, MCF-7, A549, T24 and, Raji cancer cell lines at E:T ratio of 1:1, 2:1, and 5:1 was analyzed. (B) Cytotoxicity of oNK-1 than donor-derived primary NK cells against OVCAR-3 cells was analyzed. Cryopreserved oNK-1 and primary NK cells were cocultured with OVCAR-3 cells at E:T ratio of 1:1, 2:1 and 5:1. (C) ADCC of oNK-1 and Adapted NK-92 cells against SK-OV-3 cells in the presence of trastuzumab (0.001, 0.01, 0.1, 1.0, and 10 μg/mL) at E:T ratio of 2 was analyzed. The cytotoxicity assay was performed by xCELLigence real-time cell analysis. All experiments were performed in triplicate.
4. Discussion
We report here a novel human NK cell line oNK-1 with high levels of endogenous CD16 that is a key NK receptor of ADCC. oNK-1 cells not only harbor elevated activation markers but also exhibit the stability of long-term culture. In addition to ADCC activity, oNK-1 cells further show cytotoxicity against different types of cancer cells at a relatively low E:T ratio. This study reveals the exceptional potency of oNK-1 against cancer cells.
Upon the formation of immune synapses, NK activation receptors bind to their ligands on target cells and trigger downstream polarization and degranulation signaling [19,30,31]. Nevertheless, self-antigens on target cells usually become the hurdle of the clinical application of autologous NK cells due to the interaction of NK inhibitory receptors with self-antigens [32]. To circumvent this problem, allogeneic source of NK cells, such as human NK cell lines and donor-derived hematopoietic cells, are currently under development for therapeutic interventions [8,13,25]. Human NK cell lines derived from different sources show a prevalence of lacking or minimal CD16 expression, which leads to compromised ADCC activity [15,25]. Ectopically expression of CD16 in those cells restores ADCC activity [23,33]. Besides, CD16 expression confers NK cell lines with higher cytotoxicity against target cancer cells in the absence of antibodies [34]. This study shows the up-regulation of endogenous CD16 and preferential expression of NK activation receptors in oNK-1 cells (Figs. 1C and 2A), contributing to increased cytotoxicity of oNK-1 cells against various cancer cell lines (Figs. 1E and 4A). Furthermore, oNK-1 cells exhibited ADCC activity against human epidermal growth factor receptor 2 (HER2)-expressing SK-OV-3 cells in the presence of trastuzumab, whereas low CD16-expressing Adapted NK-92 cells harbored reduced ADCC activity (Fig. 4C). These results indicate that oNK-1 cells are strong effector cells which elicit cytotoxicity through activation receptor-ligand interaction and ADCC-dependent manner.
CD16, a receptor for the Fc region of IgG, plays a pivotal role in ADCC of NK cells, and polymorphisms of CD16 lead to different levels of the binding capability to antibodies [26,27]. It has been well-documented that the polymorphism of phenylalanine to valine at amino acid residue 158 or 176 homozygotes leads to high binding affinities to antibodies and enhanced ADCC activity [35,36]. CD16 polymorphisms with high affinities to antibodies (CD16 158V and 176V) have been deliberately expressed in human NK cell lines to enhance ADCC activity and subsequent clinical applications [[21], [22], [23]]. In this study, oNK-1 cells harbor both CD16 polymorphisms (Fig. 2B). The presence of high-affinity CD16 with 176V polymorphism may contribute to increased ADCC activity against trastuzumab-bound SK-OV-3 cells (Fig. 4C). The intrinsic CD16 expression may provide the potential for clinical application of oNK-1 cells in combination with cancer-targeting antibodies.
The binding of activation and inhibitory receptors with their ligands plays a crucial role in shaping the functions of NK cells [16,17]. In addition to CD16, studies demonstrated that interaction between activation receptors, such as NKG2D, NKp44, NKp46, and NKp30, and their ligands enable NK cells to eliminate stressed cells [16]. Furthermore, the high expression of degranulation marker CD107a also implicates expansion and enhanced cytotoxicity of NK cells [37,38]. When the ligands bind to inhibitory receptors, including NKG2A/HLA-E, PD-1/PD-L1, TIGIT/PVR, or KIR/HLA, at the immune synapse, functions of NK cells are substantially suppressed [[39], [40], [41], [42]]. Absent or low expression of the inhibitory receptors in human NK cell lines are more likely to overcome the immunosuppressive tumor microenvironment [32,43]. This study demonstrates a high expression of activation receptors along with a low expression of the inhibitory receptors of oNK-1 cells (Fig. 2A), which contributes to the high cytotoxicity of oNK-1 cells against different types of cancer cells.
A stable supply of therapeutic immune cells promises off-the-shelf potential. Human NK cell lines as well as cord blood-, peripheral blood- and iPSC-derived NK cells have been reported as successful sources of allogeneic applications [5,12,44]. Among those NK cells, NK cell lines are effective in the manufacturing process and well-tolerated in cancer patients [21,25]. No significant infusion-related toxicity and promising efficacy of cord blood-derived NK cells are also reported in patients with multiple myeloma [45]. In this study, stable expansion of oNK-1 cells was demonstrated in a long-term timeframe (Fig. 3C, D), ensuring a stable oNK-1-based therapies.
In summary, we established an endogenous CD16-expressing human NK cell line, oNK-1, that has continuous expansion capability as well as higher cytotoxicity and robust ADCC activity against cancer cell.
Author statement
Zih-Fei Cheng: Methodology, Investigation, Formal analysis, Resources. Hao-Kang Li: Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Hsiu-Ping Yang: Investigation, Resources, Formal analysis, Data curation, Visualization. Chia-Yun Lee: Investigation, Resources, Formal analysis, Data curation, Visualization. Sai-Wen Tang: Validation, Investigation, Writing – review & editing. Yan-Liang Lin: Resources. Shih-Chia Hsiao: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Zih-Fei Cheng, Hao-Kang Li, Hsiu-Ping Yang, Chia-Yun Lee, Sai-Wen Tang, and Yan-Liang Lin are employees of Acepodia Biotech Inc. Shih-Chia Hsiao is the co-founder of Acepodia Biotech Inc. Zih-Fei Cheng, Hao-Kang Li, Hsiu-Ping Yang, Chia-Yun Lee, Yan-Liang Lin and Shih-Chia Hsiao are inventors of the patent of international application number PCT/US20/13,883 related to this study.
Acknowledgement
We would like to thank Dr. Yi-Hung Ou, Mr. Yan-Da Lai and Ms. Stephanie Hsiao for providing helpful scientific review and discussion. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100935.
Contributor Information
Hao-Kang Li, Email: howard@acepodiabio.com.
Shih-Chia Hsiao, Email: sonny@acepodiabio.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weber E.W., Maus M.V., Mackall C.L. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. doi: 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan C. First approval in sight for Novartis' CAR-T therapy after panel vote. Nat. Biotechnol. 2017;35:691–693. doi: 10.1038/nbt0817-691. [DOI] [PubMed] [Google Scholar]
- 3.Dushenkov A., Jungsuwadee P. Chimeric antigen receptor T-cell therapy: foundational science and clinical knowledge for pharmacy practice. J. Oncol. Pharm. Pract. 2019;25:1217–1225. doi: 10.1177/1078155219836480. [DOI] [PubMed] [Google Scholar]
- 4.Acharya U.H., Dhawale T., Yun S., Jacobson C.A., Chavez J.C., Ramos J.D., Appelbaum J., Maloney D.G. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expet Rev. Hematol. 2019;12:195–205. doi: 10.1080/17474086.2019.1585238. [DOI] [PubMed] [Google Scholar]
- 5.Veluchamy J.P., Kok N., van der Vliet H.J., Verheul H.M.W., de Gruijl T.D., Spanholtz J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front. Immunol. 2017;8:631. doi: 10.3389/fimmu.2017.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koehl U., Kalberer C., Spanholtz J., Lee D.A., Miller J.S., Cooley S., Lowdell M., Uharek L., Klingemann H., Curti A., Leung W., Alici E. Advances in clinical NK cell studies: donor selection, manufacturing and quality control. OncoImmunology. 2016;5:e1115178. doi: 10.1080/2162402X.2015.1115178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonn T., Becker S., Esser R., Schwabe D., Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematother. Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 8.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020 doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 9.Sarvaria A., Jawdat D., Madrigal J.A., Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front. Immunol. 2017;8:329. doi: 10.3389/fimmu.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koepsell S.A., Miller J.S., McKenna D.H., Jr. Natural killer cells: a review of manufacturing and clinical utility. Transfusion. 2013;53:404–410. doi: 10.1111/j.1537-2995.2012.03724.x. [DOI] [PubMed] [Google Scholar]
- 11.Rizzieri D.A., Storms R., Chen D.F., Long G., Yang Y., Nikcevich D.A., Gasparetto C., Horwitz M., Chute J., Sullivan K., Hennig T., Misra D., Apple C., Baker M., Morris A., Green P.G., Hasselblad V., Chao N.J. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2010;16:1107–1114. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar K., Capitini C.M., Saha K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res. Ther. 2020;11:234. doi: 10.1186/s13287-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Hermanson D.L., Moriarity B.S., Kaufman D.S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–192 e185. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezvani K., Rouce R., Liu E., Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunesch J.T., Angelo L.S., Mahapatra S., Deering R.P., Kowalko J.E., Sleiman P., Tobias J.W., Monaco-Shawver L., Orange J.S., Mace E.M. Genome-wide analyses and functional profiling of human NK cell lines. Mol. Immunol. 2019;115:64–75. doi: 10.1016/j.molimm.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morvan M.G., Lanier L.L. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Canc. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 17.Paul S., Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., Erbe A.K., Hank J.A., Morris Z.S., Sondel P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiossone L., Dumas P.Y., Vienne M., Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018;18:671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 20.Mahaweni N.M., Olieslagers T.I., Rivas I.O., Molenbroeck S.J.J., Groeneweg M., Bos G.M.J., Tilanus M.G.J., Voorter C.E.M., Wieten L. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci. Rep. 2018;8:15983. doi: 10.1038/s41598-018-34258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klingemann H., Boissel L., Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front. Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S., Chauhan S.K.S., Daly J., Natoni A., Fairfield H., Henderson R., Nolan E., Swan D., Hu J., Reagan M.R., O'Dwyer M. The CD38(low) natural killer cell line KHYG1 transiently expressing CD16(F158V) in combination with daratumumab targets multiple myeloma cells with minimal effector NK cell fratricide. Cancer Immunol. Immunother. 2020;69:421–434. doi: 10.1007/s00262-019-02477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jochems C., Hodge J.W., Fantini M., Fujii R., Morillon Y.M., 2nd, Greiner J.W., Padget M.R., Tritsch S.R., Tsang K.Y., Campbell K.S., Klingemann H., Boissel L., Rabizadeh S., Soon-Shiong P., Schlom J. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–86373. doi: 10.18632/oncotarget.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams B.A., Wang X.H., Leyton J.V., Maghera S., Deif B., Reilly R.M., Minden M.D., Keating A. CD16(+)NK-92 and anti-CD123 monoclonal antibody prolongs survival in primary human acute myeloid leukemia xenografted mice. Haematologica. 2018;103:1720–1729. doi: 10.3324/haematol.2017.187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong J.H., Maki G., Klingemann H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 26.Treon S.P., Hansen M., Branagan A.R., Verselis S., Emmanouilides C., Kimby E., Frankel S.R., Touroutoglou N., Turnbull B., Anderson K.C., Maloney D.G., Fox E.A. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. J. Clin. Oncol. 2005;23:474–481. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 27.Musolino A., Naldi N., Bortesi B., Pezzuolo D., Capelletti M., Missale G., Laccabue D., Zerbini A., Camisa R., Bisagni G., Neri T.M., Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 28.Talathi S.P., Shaikh N.N., Pandey S.S., Saxena V.A., Mamulwar M.S., Thakar M.R. FcgammaRIIIa receptor polymorphism influences NK cell mediated ADCC activity against HIV. BMC Infect. Dis. 2019;19:1053. doi: 10.1186/s12879-019-4674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y., Tian Z., Wei H. Developmental and functional control of natural killer cells by cytokines. Front. Immunol. 2017;8:930. doi: 10.3389/fimmu.2017.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long E.O., Kim H.S., Liu D., Peterson M.E., Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orange J.S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C., Oberoi P., Oelsner S., Waldmann A., Lindner A., Tonn T., Wels W.S. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and Induction of protective antitumor immunity. Front. Immunol. 2017;8:533. doi: 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemenceau B., Vivien R., Pellat C., Foss M., Thibault G., Vie H. The human natural killer cytotoxic cell line NK-92, once armed with a murine CD16 receptor, represents a convenient cellular tool for the screening of mouse mAbs according to their ADCC potential. mAbs. 2013;5:587–594. doi: 10.4161/mabs.25077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., You F., Jiang L., Li J., Zhu X., Bao Y., Sun X., Tang X., Meng H., An G., Zhang B., Yang L. Gene-modified NK-92MI cells expressing a chimeric CD16-BB-zeta or CD64-BB-zeta receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Oncotarget. 2017;8:37128–37139. doi: 10.18632/oncotarget.16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J., Edberg J.C., Redecha P.B., Bansal V., Guyre P.M., Coleman K., Salmon J.E., Kimberly R.P. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J. Clin. Invest. 1997;100:1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatjiharissi E., Xu L., Santos D.D., Hunter Z.R., Ciccarelli B.T., Verselis S., Modica M., Cao Y., Manning R.J., Leleu X., Dimmock E.A., Kortsaris A., Mitsiades C., Anderson K.C., Fox E.A., Treon S.P. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood. 2007;110:2561–2564. doi: 10.1182/blood-2007-01-070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clausen J., Vergeiner B., Enk M., Petzer A.L., Gastl G., Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207:85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Lomeli P., Bravo-Cuellar A., Hernandez-Flores G., Jave-Suarez L.F., Aguilar-Lemarroy A., Lerma-Diaz J.M., Dominguez-Rodriguez J.R., Sanchez-Reyes K., Ortiz-Lazareno P.C. Increase of IFN-gamma and TNF-alpha production in CD107a + NK-92 cells co-cultured with cervical cancer cell lines pre-treated with the HO-1 inhibitor. Canc. Cell Int. 2014;14:100. doi: 10.1186/s12935-014-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath P.R., Pal-Nath D., Mandal A., Cam M.C., Schwartz A.L., Roberts D.D. Natural killer cell recruitment and activation are regulated by CD47 expression in the tumor microenvironment. Cancer Immunol Res. 2019;7:1547–1561. doi: 10.1158/2326-6066.CIR-18-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q., Bi J., Zheng X., Chen Y., Wang H., Wu W., Wang Z., Wu Q., Peng H., Wei H., Sun R., Tian Z. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 41.Hsu J., Hodgins J.J., Marathe M., Nicolai C.J., Bourgeois-Daigneault M.C., Trevino T.N., Azimi C.S., Scheer A.K., Randolph H.E., Thompson T.W., Zhang L., Iannello A., Mathur N., Jardine K.E., Kirn G.A., Bell J.C., McBurney M.W., Raulet D.H., Ardolino M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamiya T., Seow S.V., Wong D., Robinson M., Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Invest. 2019;129:2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 44.Mehta R.S., Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 2018;9:283. doi: 10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah N., Li L., McCarty J., Kaur I., Yvon E., Shaim H., Muftuoglu M., Liu E., Orlowski R.Z., Cooper L., Lee D., Parmar S., Cao K., Sobieiski C., Saliba R., Hosing C., Ahmed S., Nieto Y., Bashir Q., Patel K., Bollard C., Qazilbash M., Champlin R., Rezvani K., Shpall E.J. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br. J. Haematol. 2017;177:457–466. doi: 10.1111/bjh.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.