Abstract
An efficient and reproducible protocol for in vitro propagation of Dolichandraunguis-cati has been established for the first time from nodal segments. In order to enhance survival rate under ex vitro conditions, photosynthetic potential of in vitro grown plantlets was also studied through JIP test based analysis of polyphasic OJIP chlorophyll a fluorescence OJIP transients, density of active reaction centers, light harvesting efficiency, electron transfer rate, dissipation energy, maximum quantum yield of primary PSII photochemistry and photosynthetic performance index. The best morphogenetic in term of explants response (92.2 %), shoot number (3.43 ± 0.07) and shoot length (4.7 ± 0.31 cm) was obtained on Murashige and Skoog medium supplemented with 0.5 mg l−1 BAP and 1.0 mg l−1 TDZ. The shoots exhibited high frequency rhizogenesis on half strength medium augmented with 2.0 mg l−1 IAA. In vitro plantlets developed highest rate of photosynthesis on day 18 after the initiation of rhizogenesis. High survival rate (96.16%) under ex vitro conditions was observed when in vitro plantlets having high photosynthetic efficiency (Fv/Fm > 0.75) were subjected to hardening and acclimatization process. Plantlets with reduced photosynthetic performance exhibited low survival rate under natural conditions. The developed in vitro protocol will be useful for genetic improvement and multiplication of D. unguis-cati. The results of this study also show that photosynthetic screening of in vitro developed plantlets is highly essential after the rhizogenesis process to achieve higher survival rate under field conditions.
Keywords: In vitro propagation, Chlorophyll a fluorescence, JIP test, Fv/Fm, Performance index
In vitro propagation; Chlorophyll a fluorescence; IP test; Fv/Fm; Performance index
1. Introduction
Dolichandra unguis-cati (L.) Lohmann (Bignoniaceae) commonly known as ‘cat's claw’ is a perennial rampant liana which is found in diverse regions of the globe like Brazil, South America, Egypt, Mexico to northern Argentina and western India [1, 2]. The plant has been extensively utilized since time immemorial by the ancient Indian system of medicines for combating several ailments like dysentery and stomach bloating [3], flu, arthrosis, bronchitis, splenosis, headache [4], snake bite, diarrhea, fever, inflammatory reactions [5], rheumatism [6], uterus infection and cysts [7] due to the activities of diverse bioactive molecules like corimboside, vicenin-2, O-flavonol, chlorogenic acid, lupeol, vanillinic acid, quercetin, β-sitosterol, isochlorogenic acid, p-coumaric acid, β -sitosterilglycoside, lapachol, allantoin, decaffeoylacteoside, yoniresinol-3α-O-β-d-glucopyranside, cirsimarin, cirsimaritin, caffeic acid, ferulic acid, transcinnamic acid, luteolin, apigenin, rosmarinic acid, quercitrin and quercetin ursolic acid [2, 5, 8, 9]. Conventionally, D. unguis-cati is propagated by subterranean tubers and seeds [10, 11]. However, seasonal dependency of seed germination makes it an ineffective way for the conservation of this plant species [12]. Furthermore, development of in vitro protocol is one of the essential steps of genetic improvement and transformation of this high valued plant species. Therefore, immediate attention is highly required for the development of an efficient and reproducible in vitro protocol for mass propagation and genetic improvement of D. unguis-cati.
In vitro propagation of plants is an alternative for rapid and large-scale production of plants under control conditions [13]. The ultimate success of in vitro protocols relies on the ability to transfer the plantlets from in vitro to the ex vitro conditions with a high survival rate [14]. Low survival rate during hardening and acclimatization is the major constraint in the large-scale production of micropropagated plantlets [15]. The high concentration of exogenous sucrose in the nutrient medium induces photosynthetic down-regulation in plantlets raised under in vitro conditions [16, 17]. Therefore, prior to transfer under ex vitro conditions, photosynthetic performance of in vitro developed plantlets should be screened.
Chlorophyll fluorescence measurements provide valuable information of the physiological condition of photosystem II and components of photosynthetic electron transport chain [18] and have been widely used to screen photosynthetic performance of plantlets growing under in vitro conditions [19, 20, 21]. The JIP-test, based on the rise in polyphasic OJIP chlorophyll fluorescence, provides in depth information on the status and function of PSII reaction centers, antenna, as well as on donor and acceptor sides of PSII [22, 23, 24]. The O–J phase indicates the status of PSII, while the J–I step indicates the performance of the QB, plastoquinone, cytochrome b6f, and plastocyanin. The I–P part of the OJIP induction curve is correlated to the reduction of electron transporters of the PSI acceptor side [25].
Present study was aimed (1) to develop a reproducible protocol for in vitro regeneration of D. unguis-cati and (2) to perform photosynthetic screening of in vitro grown plantlets to achieve high survival rate under ex vitro conditions.
2. Materials and methods
2.1. Plant material and surface sterilization
Young shoots of Dolichandra unguis-cati (L.) L.G.Lohmann (Bignoniaceae) were harvested from Botanical Garden of Department of Botany, Mohanlal Sukhadia University, Udaipur, India. The excised shoots were washed thoroughly under running tap water for 3 min to eliminate dust particles and then treated with 0.1% bavistin and rinsed twice with sterile distilled water. Thereafter, surface sterilization of explants was done under a laminar flow chamber with aqueous solution of 0.1% (w/v) HgCl2 for 3 min. After rinsing with double distilled water, nodal segments were cut into small pieces (2 cm) and used as the explants.
2.2. Culture media and growth conditions
The sterilized nodal explants were placed vertically on solid MS Medium [26] supplemented with 3 % sucrose, 0.8% (w/v) agar (Hi-Media, India) and various combinations/concentrations of plant growth regulators. The pH of the media was adjusted 5.8 before autoclaving at 121 °C for 15 min. All cultures were kept in a growth chamber at 25 ± 2 °C, 65–70 % relative humidity with photoperiod of 16-h using a photosynthetic photon flux density (PPFD) of 40 mmol m2s−1 provided by cool white fluorescent tube lights (Philips, India). After 4 weeks of culture response percentage of the explants, numbers of shoots per explant, length of the shoots were evaluated.
2.3. In vitro rhizogenesis
Well developed shoots were subcultured on MS enriched with various concentrations (0.1–5.0 mg l−1) of auxins viz. IBA, IAA and NAA to induce rhizogenesis in vitro. Prior to hardening process, the photosynthetic performance of well rooted plantlets was regularly measured by the analysis of polyphasic chlorophyll a fluorescence kinetics.
2.4. Measurement of photosynthetic performance
Plant Efficiency Analyser, PEA (Hansatech Instruments, U.K.) was used to analyze the photosynthetic potential of in vitro developed plantlets. Before the measurements, well developed plantlets were kept to darkness for 1 h. Fluorescence transients were induced over a leaf-lamina area of 4 mm diameter by a red light of 3000 μmolm−2s−1 provided by a high intensity LED array of three light emitting diodes. A total measuring time of one second was used thought out the experiments. Fluorescence values were used to calculate phenomenological fluxes (ABS/CSm, ETo/CSm and DIo/CSm), RC/CSm, Fv/Fm (ϕPo) and performance index on cross section basis (PIcs) using following equations (see Eqs. (1), (2), (3), (4), (5), and (6)) of JIP test [27, 28] –
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
where Ψ0 is calculated as 1- VJ (Vj is relative variable fluorescence at the J-step and calculated as (F2ms - F0)/(FM - F0), Fv is variable fluorescence between Fm and Fo) and M0 (approximated initial slope of the fluorescence transient) is calculated as 4 x (F300μs-F0)/(FM-F0).
2.5. Hardening and acclimatization
Only plantlets with functional photosynthetic apparatus and autotrophic potential were transplanted to plastic cups containing autoclaved soil, sand and coco peat (1:2:1) and then kept for 2 weeks in same growth chamber. On the other hand, plantlets with low photosynthetic potential were maintained on nutrient medium until the development of functional photosynthetic apparatus and autotrophic potential. The hardened plantlets were watered once a week. After hardening, the plantlets were subsequently transferred and maintained in the green net house (50% light transparency) with relative humidity 40–50% and 32 ± 2 °C.
2.6. Experimental design and statistical analysis
All tissue culture experiments were conducted with a minimum of 30 replicates per treatment and each experiment was repeated thrice. All data were analyzed statistically using GraphPad Prism 8. Differences were considered significant when the p value was <0.05. Photosynthetic data were analyzed using Biolyzer Software ver. 3.06 [29].
3. Results and discussion
3.1. In vitro establishment and multiplication
BAP at 0.5–5.0 mg l-1 concentrations could not evoke any significant morphogenetic response in nodal explants. After one week of inoculation on MS fortified with 0.5 mg l-1 BAP, explants showed swelling. At elevated concentration of BAP (5.0 mg l-1), formation of single shoot bud followed by the swelling was observed after 11 days of culture (Figure 1a). The shoots emerged on BAP containing medium failed to elongate. Low frequency of shoot bud proliferation was noted on MS augmented with KIN at high concentration (5.0 mg l-1) (Figure 1b). In the present studies, KIN at low 0.5 mg l-1 was found most effective in multiple shoot bud induction from nodal explants.
Figure 1.
Nodal explants of D. unguis-cati showing the development of one shoot/node (a) on MS+5.0 mg l−1 BAP, low frequency shoot bud proliferation on MS+5.0 mg l−1 KIN (b), multiple shoot induction on MS+0.5 mg l−1 KIN and 1.0 mg l−1 1 TDZ (c) rhizogenesis on half-strength MS + 2.0 mg l−1 IAA (d), hardened (e) and acclimatized plant (f).
TDZ, a cytokinin-like compound, promotes a diverse array of morphogenic responses including shoot bud proliferation [30, 31]. Apart from its cytokinin-like activity, TDZ plays important role in modulation of endogenous hormone levels especially auxin/cytokinin ratio [32]. In the present study, our results revealed that the effect of KIN and TDZ combination on multiple shoot proliferation is more compared to KIN and TDZ when used separately. The best morphogenetic response in terms of explants response (92.2 %), shoot length (4.7 cm) and maximum number of shoots (4.33 shoots per node) was observed when 0.5 mg l-1 KIN was incorporated in MS along with 1.0 mg l-1 1 TDZ (Figure 1c), whereas high concentration of TDZ, either singly or in combination with KIN markedly decreased the frequency of shoot bud proliferation (Table 1). High cytokinin activity of TDZ inhibits in vitro shoot bud proliferation [33].
Table 1.
Influence of KIN and TDZ on explant response (%) and shoot length (cm) after 3 weeks of culture of nodal explants of D. unguis-cati. Values within the columns are highly significant at p < 0.05 and represented as mean of 30 replicates ± SD. Different characters indicate significant differences among the results (p ≤ 0.05).
| Plant growth regulators (mg l−1) |
Explant response (%) | Mean shoot length (cm) | Shoots per node | |
|---|---|---|---|---|
| KIN | TDZ | |||
| Control | 0.0d | 0.0d | 0.0c | |
| 0.0 | 0.5 | 0.0d | 0.0d | 0.0c |
| 0.0 | 1.0 | 0.0d | 0.0d | 0.0c |
| 0.0 | 2.5 | 0.0d | 0.0d | 0.0c |
| 0.5 | 0.0 | 52.0 ± 3.5b | 2.8 ± 0.02ab | 2.66 ± 0.52ab |
| 0.5 | 0.5 | 51.21 ± 1.7b | 2.9 ± 0.06bc | 2.63 ± 0.65ab |
| 0.5 | 1.0 | 92.2 ± 4.4a | 4.7 ± 0.31a | 4.33 ± 0.50a |
| 0.5 | 2.5 | 13.81 ± 2.3c | 0.9 ± 0.06cd | 1.41 ± 0.24bc |
3.2. In vitro rhizogenesis
Inadequate rooting is one of the major constraints to the survival rate of plantlets under ex vitro conditions [34]. In the present study, Full strength MS singly or in combination with various concentrations of IAA (1.0–3.0 mg l−1) could not evoke significant response in term of rhizogenesis in vitro. Half-strength MS supplemented with IAA proved best in term of initiating rhizogenesis in vitro as compared to full strength MS (Table 2). Half strength MS supplemented with low concentration of IAA (<1.0 mg l−1) could promote rhizogenesis in vitro at low frequency. Half strength MS along with IAA 2.0 mg l−1 exhibited highest mean number of roots per shoot (11.40) and increased root length to the maximum (4.03 cm) (Figure 1d), while other concentrations of IAA caused antagonist effect on rhizogenesis in vitro in D. unguis-cati. Our results are in accordance with the studies carried out on Cichorium intybus [35], Digitalis lanata [36], Prosopis laevigata [37] and Securidaca longipedunculata [38]. The superiority of IAA over other auxins for induction of rhizogenesis has also been reported in Vanda pumila [39], Dendrobium chryseum [40], Phyllanthus tenellus [34].
Table 2.
Influence of IAA on rhizogenesis in vitro in D. unguis-cati after 3 weeks of culture. Values within the columns are highly significant at p < 0.05 and represented as mean of 30 replicates ± SD. Different characters indicate significant differences among the results (p ≤ 0.05).
| Media Combinations | % Rooting | Mean no. of root/shoot | Mean root length (cm) |
|---|---|---|---|
| MS full strength | 0g | 0d | 0d |
| MS full strength + 1.0 mg l−1 IAA | 13 ± 3.1ef | 1.16 ± 0.6cd | 1.02 ± 0.4cd |
| MS full strength + 2.0 mg l−1 IAA | 26 ± 2.6d | 4.16 ± 1.0bc | 2.81 ± 0.9ab |
| MS full strength + 3.0 mg l−1 IAA | 21 ± 3.2de | 4.14 ± 0.8bc | 2.87 ± 0.9ab |
| MS half strength | 8 ± 1.8fg | 2.82 ± 0.4bcd | 1.01 ± 0.4cd |
| MS half strength + 1.0 mg l−1 IAA | 57 ± 3.2b | 4.73 ± 1.6b | 1.42 ± 0.5bcd |
| MS half strength + 2.0 mg l−1 IAA | 94 ± 4.8a | 11.40 ± 2.4a | 4.03 ± 0.8a |
| MS half strength + 3.0 mg l−1 IAA | 39 ± 4.1c | 4.72 ± 1.8b | 1.72 ± 0.6abc |
3.3. Chlorophyll a fluorescence analysis
After the visible appearance of root primodia on Half strength MS + IAA 2.0 mg l−1, the polyphasic chlorophyll a fluorescence analysis was done prior to hardening process to evaluate the photosynthetic potential of in vitro developed plantlets. Fluorescence parameters (F0, Fm), phenomenological energy fluxes, RC/CSm, ϕPo and PIcs remarkably altered with increasing days after root primodia formation (DAR). During the initiation days of rhizogenesis (days 0–6), the plantlets were failed to form a complete OJIP curve. On 12 and 18 DAR, chl a fluorescence OJIP curve of in vitro grown plantlets showed three apparent intermediate phases namely OJ, JI and IP (Figure 2a). Photochemical phase OJ [27] and the thermal phase JI [41] are linked to the accumulation of QA and the status of PSII RCs. IP phase displays PQ pool reduction [25, 42]. The minimal (Fo) and maximal fluorescence (Fm) enhanced continuously with increasing the subculture duration on RIM. Sucrose acts as fuel source for growth and development of in vitro plantlets. The decreased Fm level and disappearance of O-J and J-I phases indicates the presence of dissociated light harvesting complexes [43] and undeveloped photosynthetic apparatus during the initial days of rhizogenesis. The values of Fm apparently increased with increasing the subculture duration or sucrose consumption rate on RIM. Highest Fm level was noted on 18 DAR (Figure 3a). The concentration of exogenous sucrose progressively reduces with increasing the subculture duration as in vitro grown plantlets utilize it as carbon source for their growth and development.
Figure 2.
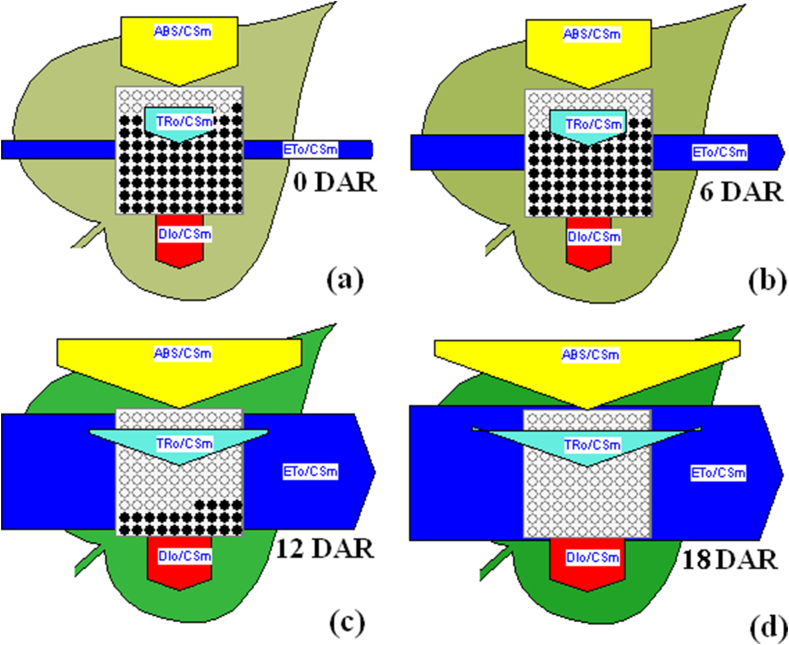
Leaf models showing changes in ABS/CSm, ETo/CSm and DIo/CSm and RC/CSm with increasing days after the emergence of root primodia (DAR) on rooting inducing medium (blacks dots represent inactive PSII RCs).
Figure 3.
Chl fluorescence OJIP induction curves measured at regular intervals after the emergence of root primodia on rooting inducing medium (a), and radar plot showing the alternations in various photosynthetic parameters with increasing the subculture duration on rooting inducing medium (b).
The effects of subculture duration on fluorescence values Fo and Fm, phenomenological fluxes i.e ABS/CS, ET/CS, DI/CS, RC/CSm, ϕPo and performance index (PIcs) is represented in radar plot (Figure 3b). The flux of absorption and electron transfer per cross section (CS) of PSII, defined as ABS/CSm and ETo/CSm, respectively, were significantly increased in plantlets with increasing subculture duration on RIM as ES get exhausts over time. ABS/CSm which represents light harvesting efficiency of active PSII RCs, enhanced progressively and reached to highest level on 18 DAR. Low values of ABS/CSm during the emergence of root promidia indicate reduced antenna size and low chl concentration. Similarly, ETo/CSm progressively increased with progression of subculture duration on RIM. Reduced values of ABS/CSm and ETo/CSm during the appearance of root promidia (0 DAR) shows the antagonist effect of higher ES concentration on light harvesting and electron transfer potential in Dolichandra unguis-cati. Decline in DIo/CSm with progression of subculture duration on RIM was associated with reduced light harvesting potential (ABS/CSm). The effects of subculture duration on RIM on phenomenological energy fluxes (ABS/CSm, ETo/CSm and DIo/CSm) are diagrammatically represented through the leaf models.
Density of active PSII RCs (RC/CSm) increased as subculture duration increased (inactive PSII RCs are denoted as black dots in leaf models. The quantum yield potential of photosystem II (at t = 0) (ϕPo = Fv/Fm = TR0/ABS) was found lowest (0.603) on 0 DAR, which further increased gradually with increasing subculture duration on RIM (Table 3). Highest Fv/Fm (0.755) was observed on 18 DAR. A reduction in Fv/Fm ratio is associated to photoinhibition or damage of PSII complex [25]. PIcsm, which displays the functionality of active PSII RCs markedly enhanced with increasing the duration on RIM. During the initial days of rhizogenesis, the presence of sugar in the medium lowers the photosynthesis through feedback inhibition. Increment of ABS/CS, ET/CS, DI/CS, RC/CSm, ϕPo and PIcs with increasing the subculture duration is linked to the gradual depletion of exogenous sucrose into the medium. Presents study indicates that in vitro plantlets are unable to develop their photosynthetic apparatus until the presence of exogenous sucrose into the medium. In vitro plantlets develop their photosynthetic apparatus after the complete consumption of exogenous sucrose from the medium. Exogenous sugars mediated inhibition of photosynthesis has been proven by many researchers [44, 45, 46, 47], and is consistent with Koch's theory on the inhibitory influence of sugars on photosynthesis [48]. Exogenous sugar inhibits the expression of photosynthetic genes and reduces the activities of enzymes involved in CBB cycle [16]. Therefore, photosynthetic screening of in vitro grown plantlets is highly required before transferring plants from growth chamber to ex vitro conditions.
Table 3.
Changes in various photosynthetic parameters with increasing the subculture duration after the initiation of root-primodia (days after rhizogenesis-DAR) on RIM. Values within the columns are highly significant at p < 0.05 and represented as mean of 30 replicates ± SD. Different characters indicate significant differences among the results (p ≤ 0.05).
| DAR | Fo | Fm | RC/CSm | ABS/CSm | ETo/CSm | Fv/Fm | PIcsm | Survival rate under ex vitro condition (%) |
|---|---|---|---|---|---|---|---|---|
| 0 | 164 ± 8.33c | 492 ± 14.63d | 119.04 ± 5.72d | 492 ± 11.76d | 86.99 ± 7.14d | 0.603 ± 0.05d | 750.9 ± 7.14d | 0d |
| 6 | 176 ± 6.56c | 521 ± 10.87c | 146.43 ± 11.92c | 521 ± 14.82c | 153.02 ± 8.61c | 0.629 ± 0.06c | 2176.0 ± 17.34c | 4.81 ± 1.03c |
| 12 | 256 ± 9.24b | 1028 ± 11.51b | 702.78 ± 12.52b | 1028 ± 23.39b | 495.98 ± 15.17b | 0.738 ± 0.05b | 37400.4 ± 92.87b | 56.82 ± 3.62b |
| 16 | 293 ± 6.33a | 1278 ± 19.47a | 996.17 ± 20.53a | 1278 ± 17.90a | 570.06 ± 14.89a | 0.755 ± 0.07a | 44402.4 ± 289.90a | 96.16 ± 6.68a |
3.4. Hardening and acclimatization
Rooted plantlets were having high photosynthesis in term of ABS/CSm, ET0/CSm, DI0/CSm, RC/CSm, ϕPo and PIcsm showed high survival rate (96.16%) during hardening and acclimatization process (Figure 2e, f). In vitro grown plantlets with reduced photosynthetic potential showed declined rate of survival under natural conditions. Plants produced through in vitro method exhibited similar morphologically to mother plants. The results of this study show that photosynthetic screening of in vitro developed plantlets is highly essential prior to hardening process and the fast Chl a fluorescence transient measurement with high time resolution provide a non-invasive and rapid method to screen the photosynthetic potential of in vitro propagated plantlets to achieve higher survival rate under ex-vitro condition. In future, the developed in vitro protocol can be used for large scale propagation and genetic improvement of D. unguis-cati.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Fonseca L.H., Cabral S.M., Agra M.D., Lohmann L.G. Taxonomic revision of Dolichandra (Bignonieae, bignoniaceae) Phytotaxa. 2017;301(1):1–70. [Google Scholar]
- 2.Brondani J.C., Reginato F.Z., da Silva Brum E., de Souza, Vencato M., Lhamas C.L., Viana C., Manfron M.P. Evaluation of acute and subacute toxicity of hydroethanolic extract of Dolichandra unguis-cati L. leaves in rats. J. Ethnopharmacol. 2017;202:147–153. doi: 10.1016/j.jep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Hilgert N.I. Plants used in home medicine in the Zenta River basin, Northwest Argentina. J. Ethnopharmacol. 2001;76(1):11–34. doi: 10.1016/s0378-8741(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 4.Duke J.A. CRC Press; Boca Raton (FL): 2008. Duke's Handbook of Medicinal Plants of Latin America. [Google Scholar]
- 5.Aboutabl E.A., Hashem F.A., Sleem A.A., Maamoon A.A. Flavonoids, antinflammatory activity and cytotoxicity of Macfadyena uniguis-cati L. Afr. J. Tradit. Complement. Altern. Med. 2008;5(1):18–26. doi: 10.4314/ajtcam.v5i1.31251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanz-Biset J., Campos-de-la-Cruz J., Epiquién-Rivera M.A., Cãnigueral S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon) J. Ethnopharmacol. 2009;122(2):333–362. doi: 10.1016/j.jep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Flor A.S.S.O., Barbosa W.L.R. Sabedoria popular no uso de plantas medicinais pelos moradores do bairro do sossego no distrito de Marudá - PA. Rev. Bras. Plantas Med. 2015;17(4):757–768. [Google Scholar]
- 8.Duarte D.S., Dolabela M.F., Salas C.E., Raslan D.S., Oliveiras A.B., Nenninger A., Wiedemann B., Wagner H., Lombardi J., Lopes M.T.P. Chemical characterization and biological activity of Macfadyena unguis-cati (Bignoniaceae) J. Pharm. Pharmacol. 2000;52(3):347–352. doi: 10.1211/0022357001773904. [DOI] [PubMed] [Google Scholar]
- 9.Chen L., Chen D., Zheng Z., Liu S., Tong Q., Xiao J., Lin H., Ming Y. Cytotoxic and antioxidant activities of Macfadyena unguis-cati L. aerial parts and bioguided isolation of the antitumor active components. Ind. Crop. Prod. 2017;107:531–538. [Google Scholar]
- 10.Downey P.O., Turnbull I. The biology of Australian weeds 48. Macfadyena unguis-cati (L.) A.H. Gentry. Plant Protect. Q. 2007;22(3):82–91. [Google Scholar]
- 11.Osunkoya O.O., Pyle K., Scharaschkin T., Dhileepan K. What lies beneath? The pattern and abundance of the subterranean tuber bank of the invasive liana cat’s claw creeper, Macfadyena unguis-cati(Bignoniaceae) Aust. J. Bot. 2009;57:132–138. [Google Scholar]
- 12.Buru J.C., Dhileepan K.,J., Osunkoya O.O., Scharaschkin T. Germination biology and occurrence of polyembryony in two forms of cats claw creeper vine, Dolichandra unguis-cati (bignoniaceae): implications for its invasiveness and management. Am. J. Plant Sci. 2016;7:657–670. [Google Scholar]
- 13.Espinosa-Leal C.A., Puente-Garza C.A., García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248(1):1–18. doi: 10.1007/s00425-018-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shekhawat M.S., Manokari M. In vitro multiplication, micromorphological studies and ex vitro rooting of Hybanthus enneaspermus (L.) F. Muell.–a rare medicinal plant. Acta Bot. Croat. 2018;77(1):80–87. [Google Scholar]
- 15.da Silva J.A.T., Hossain M.M., Sharma M., Dobránszki J., Cardoso J.C., Songjun Z.E.N.G. Acclimatization of in vitro-derived Dendrobium. Hortic. Plant J. 2017;3(3):110–124. [Google Scholar]
- 16.Matysiak B., Gabryszewska E. The effect of in vitro culture conditions on the pattern of maximum photochemical efficiency of photosystem II during acclimatisation of Helleborus niger plantlets to ex vitro conditions. Plant Cell Tissue Organ Cult. 2016;125(3):585–593. [Google Scholar]
- 17.Soni V., Kumari R., Swarnkar P.L. High frequency in vitro regeneration system for conservation of Barleria prionitis L., a threatened medicinal shrub. 2017;6(1):45–49. [Google Scholar]
- 18.Kalaji H.M., Schansker G., Brestic M., Bussotti F., Calatayud A., Ferroni L., Losciale P. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017;132(1):13–66. doi: 10.1007/s11120-016-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genoud C., Coudret A., Amalric C., Sallanon H. Effects of micropropagation conditions of rose shootlets on chlorophyll fluorescence. Photosynthetica. 1999;36(1-2):243–251. [Google Scholar]
- 20.Mazurek M., Siekierzyńska A., Jacek B., Litwińczuk W. Differences in response to drought stress among highbush blueberry plants propagated conventionally and by tissue culture. Plant Biosyst. 2020:1–7. [Google Scholar]
- 21.Xu Y., Yang M., Cheng F., Liu S., Liang Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020;20(1):1–12. doi: 10.1186/s12870-020-02480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heber U., Soni V., Strasser R.J. Photoprotection of reaction centers: thermal dissipation of absorbed light energy vs charge separation in lichens. Physiol. Plantarum. 2011;142(1):65–78. doi: 10.1111/j.1399-3054.2010.01417.x. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt U., Singh H., Kumar D., Soni V. Rehydration quickly assembles photosynthetic complexes in desiccation tolerant Riccia gangetica. Biomed. J. Sci. Tech. Res. 2020;30(1):23034–23037. [Google Scholar]
- 24.Bhatt U., Singh H., Kumar D., Soni V. Rehydration induces quick recovery of photosynthesis in desiccation tolerant moss Semibarbula orientalis. J. Plant Sci. Res. 2019;35(2):183–187. [Google Scholar]
- 25.Banks J.M. Continuous excitation chlorophyll fluorescence parameters: a review for practitioners. Tree Physiol. 2017;37(8):1128–1136. doi: 10.1093/treephys/tpx059. [DOI] [PubMed] [Google Scholar]
- 26.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 1962;15:473–497. [Google Scholar]
- 27.Strasser R.J., Srivastava A., Tsimilli-Michael M. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G.C., Govindjee, editors. Chlorophyll Fluorescence a Signature of Photosynthesis, Advances in Photosynthesis and Respiration Series. Kluwer Academic Publishers; Dordrecht: 2004. pp. 321–362. [Google Scholar]
- 28.Heber U., Soni V., Strasser R.J. Photoprotection of reaction centers: thermal dissipation of absorbed light energy vs charge separation in lichens. Physiol. Plantarum. 2011;142:65–78. doi: 10.1111/j.1399-3054.2010.01417.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez R.M. Laboratory of Bioenergetics, University of Geneva; Switzerland: 2002. Biolyzer Program to Calculate Fluorescent Transient. [Google Scholar]
- 30.Talla S.K., Madam E., Manga S., Aileni M., Mamidala P. Efficient TDZ-induced regeneration from capitulum explants of Gerbera jamesonii Bolus ex Hooker F.-an ornamental plant with high aesthetic value. Plant Biosyst. 2019;153(5):679–685. [Google Scholar]
- 31.Novikova T.I., Asbaganov S.V., Ambros E.V., Zaytseva Y.G. Vitro Cellular & Developmental Biology-Plant. 2020. TDZ-induced axillary shoot proliferation of Rhododendron mucronulatum Turcz and assessment of clonal fidelity using DNA-based markers and flow cytometry; pp. 1–11. [Google Scholar]
- 32.Novikova T.I., Zaytseva Y.G. Thidiazuron: from Urea Derivative to Plant Growth Regulator. Springer; Singapore: 2018. TDZ-induced morphogenesis pathways in woody plant culture; pp. 61–94. [Google Scholar]
- 33.Huetteman C.A., Preece J.E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult. 1993;33:105–119. [Google Scholar]
- 34.Nikule H.A., Nitnaware K.M., Chambhare M.R., Kadam N.S., Borde M.Y., Nikam T.D. In-vitro propagation, callus culture and bioactive lignan production in Phyllanthus tenellus Roxb: a new source of phyllanthin, hypophyllanthin and phyltetralin. Sci. Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-67637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yucesan B., Turker A.U., Gurel E. TDZ-induced high frequency plant regeneration through multiple shoot formation in witloof chicory (Cichorium intybus L.) Plant Cell Tissue Organ Cult. 2007;91(3):243–250. [Google Scholar]
- 36.Bhusare B.P., John C.K., Bhatt V.P., Nikam T.D. In vitro propagation of Digitalis lanata Ehrh. through direct shoot regeneration–A source of cardiotonic glycosides. Ind. Crop. Prod. 2018;121:313–319. [Google Scholar]
- 37.Morales-Domínguez J.F., de León D.S., Garcidueñas-Piña C., Pérez-Molphe-Balch E. Germination, in vitro propagation and soil acclimatization of Acacia farnesiana and Prosopis laevigata. South Afr. J. Bot. 2019;124:345–349. [Google Scholar]
- 38.Lijalem T., Feyissa T. In vitro propagation of Securidaca longipedunculata (Fresen) from shoot tip: an endangered medicinal plant. J. Genetic Eng. Biotechnol. 2020;18(1):3. doi: 10.1186/s43141-019-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maharjan S., Pradhan S., Thapa B.B., Pant B. In vitro propagation of endangered orchid, Vanda pumila Hook. f. through protocorms culture. Am. J. Plant Sci. 2019;10(7):1220. [Google Scholar]
- 40.Maharjan S., Thakuri L.S., Thapa B.B., Pradhan S., Pant K.K., Joshi G.P., Pant B. In vitro propagation of the endangered orchid Dendrobium chryseum Rolfe from protocorms culture. Nepal J. Sci. Technol. 2020;19(1):39–47. [Google Scholar]
- 41.Schansker G., Tóth S.Z., Holzwarth A.R., Garab G. Chlorophyll a fluorescence: beyond the limits of the QA model. Photosynth. Res. 2014;120(1-2):43–58. doi: 10.1007/s11120-013-9806-5. [DOI] [PubMed] [Google Scholar]
- 42.Boisvert S., Joly D., Carpentier R. Quantitative analysis of the experimental O–J–I–P chlorophyll fluorescence induction kinetics: apparent activation energy and origin of each kinetic step. FEBS J. 2006;273(20):4770–4777. doi: 10.1111/j.1742-4658.2006.05475.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamane Y., Kashino Y., Koike H., Satoh K. Increases in the fluorescence Fo level and reversible inhibition of photosystem II reaction center by high-temperature treatments in higher plants. Photosynth. Res. 1997;52(1):57–64. [Google Scholar]
- 44.Fuentes G., Talavera C., Oropeza C., Desjardins Y., Santamaria J.M. Exogenous sucrose can decrease in vitro photosynthesis but improve field survival and growth of coconut (Cocos nucifera L.) in vitro plantlets. In Vitro Cell. Dev. Biol. Plant. 2005;41(1):69–76. [Google Scholar]
- 45.Lobo A.K., de Oliveira M.M., Neto M.C., Machado E.C., Ribeiro R.V., Silveira J.A. Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J. Plant Physiol. 2015;179:113–121. doi: 10.1016/j.jplph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Rybczyński J.J., Borkowska B., Fiuk A., Gawrońska H., Śliwińska E., Mikuła A. Effect of sucrose concentration on photosynthetic activity of in vitro cultures Gentiana kurroo (Royle) germlings. Acta Physiol. Plant. 2007;29(5):445–453. [Google Scholar]
- 47.Meng C., Liu X., Chai Y., Xu J., Yue M. Another choice for measuring tree photosynthesis in vitro. Peer J. 2019;7 doi: 10.7717/peerj.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.