Abstract
An efficient procedure for the synthesis of novel thiazolidinone triazoles through 32 cycloaddition reactions in the presence of copper(I) species was described, and the molecular mechanism of this 32CA was investigated computationally. Different possible pathways for CA process have been studied to achieve this goal, including one-step pathways for both regioisomers 1,4- and 1,5-triazoles (uncatalyzed, mono-copper, di-copper) and also mono- and di-copper stepwise pathways for 1,4-disubstituted triazole. It was exhibited that the most convenient route in terms of energy barriers includes two copper ions. Based on the calculation, the reaction follows a di-copper stepwise mechanism involving the formation of a six-membered ring and then undergoes a ring contraction to a five-membered ring. The regiochemistry of the reaction was investigated based on local and global reactivity indices of reactants, the transition state stabilities calculation. The electron reorganization along the uncatalyzed one-step mechanism has been investigated by the ELF topological analysis of the bonding changes along with the CA reaction.
Keywords: Novel triazoles, Thiazolidinone, Click reaction, Mechanism, DFT, ELF
Novel Triazoles; Thiazolidinone; Click Reaction; Mechanism; DFT; ELF.
1. Introduction
One of the most efficient synthetic tools for triazole derivatives' convenient preparation in a regioselective manner is the click reaction [1, 2]. Click reaction proceeds with regioselectivity and has an optimal atom economy, which is influential and crucial in synthetic chemistry [3, 4]. 1,2,3–Triazole scaffolds are utilized for treatment of HIV [5], tumors [6, 7] allergy [8], fungal infection [9, 10] and microbial diseases [11, 12, 13, 14, 15]. Huisgen, introduced uncatalyzed cycloaddition (CA) of alkynes and azides through thermal click reaction, with usually a mixture of 1,4- and 1,5- triazoles products in variable yields [16]. As shown in Scheme 1, Sharpless [17] and Meldal [18] reported the regioselective synthesis of 1,4-disubstituted triazoles through copper catalyzed azide-alkyne CA (CuAAC) reaction, in short time reactions and under mild conditions [19, 20, 21, 22]. The essential Cu(I) species for CuAAC reactions have been added as a cuprous salt with stabilizing ligands [18, 23, 24, 25], or were produced from copper (II) salts by using reducing agents [26]. Recently, several computational studies were presented on the mechanism of click reaction in the presence of different catalyst including Ru, Cu, Mg which results different regioselectivity [19, 27, 28, 29, 30, 31, 32, 33]. On the other hand, thiazolidinone derivatives have been the subject to considerable studies, because of important biological applications such as antibacterial [34, 35, 36, 37, 38], antiviral [39, 40, 41], antifungal [42, 43, 44] and antituberculosis properties [35, 45, 46].
Scheme 1.
CuAAC reaction between alkyne and benzyl azide.
To extend bioactive structures and overwhelm the inherent defects, merging scaffolds and introducing the various functional groups onto the backbone of heterocycles have been considered [47]. Based on the pharmacological properties associated with thiazolidinone and triazole derivatives [19, 48, 49], we were interested in the combine these heterocyclic moieties through click reaction [50, 51, 52]. Therefore, the alkyne fragment was generated through the Knoevenagel condensation of 4-thiazolidinones derivatives with propargylated salicylaldehyde. In the following, the triazole derivatives have been prepared through CuAAC reactions in aqueous/DMSO solution (Scheme 1). Besides, the regioselectivity and mechanism of this CA reaction were studied at B3LYP and wB97XD levels of theory [53]. Accordingly, a theoretical study on the five possible pathways involving the one-step mechanism with uncatalyzed, mono-copper and di-copper catalyzed processes. Also, the stepwise di-copper and mono-copper catalyzed mechanism were carried out.
1.1. Experimental
Initially, 5-arylidene-4-thiazolidinone 3 was prepared as the tautomeric mixtures under the literature procedure [54, 55]. Then, compound 5 was synthesized from the propargyl bromide and 2-hydroxy benzaldehyde 4 in the presence of K2CO3 [56]. The organic azides preparation procedure is shown in Scheme 2 [57].
Scheme 2.
Preparation of starting materials.
The dipolarophiles 8 were synthesized by the reaction of thiazolidinone 3 with propargylated salicylaldehyde 5 and malononitrile as a catalyst under reflux in good yield. Then, dipolarophile 8 was formed through a Michael addition reaction between the active CH2 group of the 4-thiazolidinones 3 with the C=C bond of malononitrile arylidene (Scheme 3) [58,59].
Scheme 3.
Synthesis of the alkyne derivatives 8.
1.2. Computational details
All calculation investigations were performed by DFT theory with wB97XD [61] and B3LYP [60]functionals as implemented in the Gaussian 09 program package [62]. The geometries of all transition states (TSs) and ground states (GSs) were optimized utilizing the LANL2DZ pseudopotential basis set for Cu element and 6-311+G(d,p) basis set for the C, N, H, O elements [60]. Solvent effects were studied using CPCM calculations in the mixed solvents (Water: DMSO) [63, 64, 65, 66, 67, 68, 69]. Based on optimized geometries, single-point (SP) calculations were performed with the full basis set (6-311+G(2d, 2p)) for all elements. Selected reaction pathway for the uncatalyzed scenario was subjected to IRC calculations with the intention of tracing their pathways and confirming that the optimized TS structure connects the accurate reactants and products [70, 71]. The 1H chemical shift was also investigated through the GIAO method using the TMS as 1H reference at the B3LYP/6-311+G(2d,2p) level [72]. The Gibbs free energies (G) reported, which include zero-point vibrational corrections (E), entropy (S), and thermal (H) corrections, and solvation energies at 353 K are considered in ΔG. All energies were amended with the SP dispersion effect through the DFT-D2 method [73]. It has revealed that the inclusion of these effects could improve the accuracy of the B3LYP method [74]. The ELF's topological analysis was accomplished with the TopMod program for the uncatalyzed cycloaddition step [75].
2. Results and discussion
2.1. Experiment
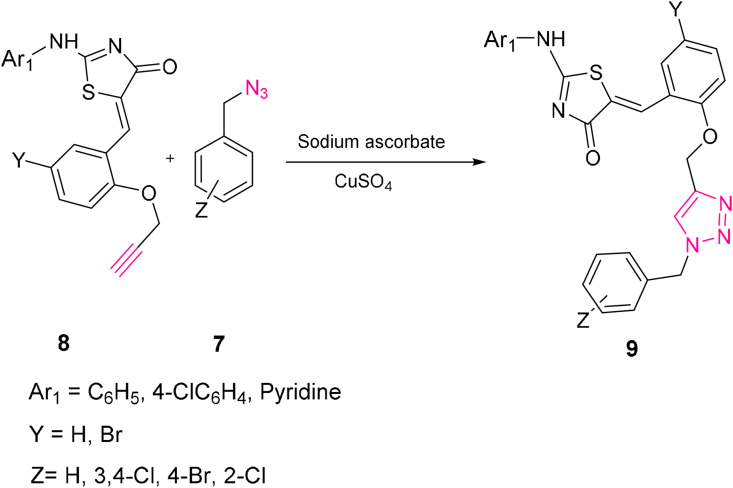
In the first endeavor, (Z)-5-(2-((1-benzyl-1H-1,2,3-triazole-4-yl)methoxy)benzylidene)-2-(phenylamino) thiazol-4(5H)-one 9a was prepared from CuAAC reaction of benzyl azide 7a and (Z)-2-(phenylamino)-5-(2-(prop-2-yn-1-yloxy)benzylidene)thiazol-4(5H)-one 8a. The synthetic route is outlined in Scheme 1.
To find optimum conditions to maximize the yields of the 1,2,3-triazoles 9a-h, various solvents were investigated for the CA reaction between 8a and benzyl azide 7a, using CuSO4 as copper source and sodium ascorbate as reductant (Scheme 1). According to Table 1, the most effective solvent system is the water-DMSO (3:1) system, which is consistent with the previous report by Candelon [25]. This procedure was applied to benzyl azide derivatives and a series of terminal alkynes 8b-h under the same conditions to form triazole products in acceptable yields (Scheme 1).
Table 1.
Optimizing the CA reaction in order to generate 9a.
| Entry | catalyst | Solvent | Yield (%) | Time (h) |
|---|---|---|---|---|
| 1 | CuSO4 | DMSOa | 70 | 24 |
| 2 | CuSO4 | DMSO-Watera | 94 | 15 |
| 3 | CuSO4 | Waterb | 65 | 20 |
1,4-triazole as a regioselective cycloadducts.
a mixture of 1,5- and 1,4- triazole cycloadducts.
The active Cu (I) is produced in situ through the reduction of the Cu(II) salt (0.5mol %) with sodium ascorbate. Furthermore, a small extra of sodium ascorbate prevents the coupling reaction that can be observed when a copper (I) source is used directly (Table 1 and 2) [18].
Table 2.
Cu-catalyzed 32CA reaction of azide and alkyne.
| Entry | X | Y | Z | Yield (%) |
|---|---|---|---|---|
| 9a | H | H | H | 95 |
| 9b | H | H | 3,4-Cl | 75 |
| 9c | H | H | 4-Br | 71 |
| 9d | Py | H | 4-Br | 82 |
| 9e | Py | H | 2-Cl | 81 |
| 9f | Py | 5-Br | H | 85 |
| 9g | Py | H | 3,4-Cl | 86 |
| 9h | 4-Cl | H | 2-Cl | 58 |
As shown in Figure 1, a mixture of tautomeric cycloadducts 9a and 9a′ was shown by the cycloaddition reaction in DMSO-Water. Several spectroscopic techniques assigned the structure of the cycloadduct 9a. For the solid products 9a and 9a′, the demonstrated absorptions at 3354 cm−1, 1686 cm−1, and 1176 cm−1 are ascribed to NH, CO, and ether groups. The NMR spectral data of isolated products were in good agreement with the 9a and 9a′s assigned structure. The 1H NMR spectrum of 9a and 9a′ exhibited a singlet at δ = 7.90 for Hd, and two singlet peaks of –CH2 groups at 5.26 and 5.30 ppm for Ha belong to 9a and 9a′, respectively. Two singlet peaks at 8.30 ppm and 8.34 ppm of CH are referred to as methine groups (Hc). Also, two signals at 11.54 and 12.34 ppm for NH protons are evidence for forming a mixture of tautomer's 9a and 9a'. The 13C NMR of 9a displayed two peaks at 53.32 and 62.13 ppm because of the CH2 group and the attached CH2 to the oxygen group. The preparation of the cycloadduct 9 was also confirmed by mass spectral data, which exhibited a molecular ion peak at 467.1 (M+). This procedure was applied to a series of organic azides and terminal alkynes under similar conditions to form the corresponding triazole products in good yields.
Figure 1.
The structures of 9a and 9a′.
In 2009, Khoshkholg and coworkers [76] described a one-pot reaction to form 6H-Indeno[2′,1':5,6]pyrano [3,4-c]chromen-13(13bH)-one under a CA reaction to give the corresponding pyran. In 2012, Pałasz also reported a multi-component, one-pot reaction for pyrano[2,3-d] pyrimidine derivative synthesis through domino Knoevenagel/Diels-Alder reactions [59]. Accordingly, we had planned the synthesis of N-phenyl-6H,11bH-chromeno [4′,3':4,5] pyrano[2,3-d]thiazol-2-amine 12 from an intramolecular hetero Diels-Alder (HAD) reaction of α,β-unsaturated carbonyl compound 8a (Scheme 4). This reaction was investigated under various conditions and solvents, and unfortunately, all attempts to isolate the desired product failed.
Scheme 4.
Possible hetero Diels-Alder alder and click reactions of 8a.
The regioselectivity and mechanism of this CuAAC from a computational point of view are investigated utilizing DFT.
2.2. Computational
2.2.1. Catalyzed vs. uncatalyzed one-step 32CA
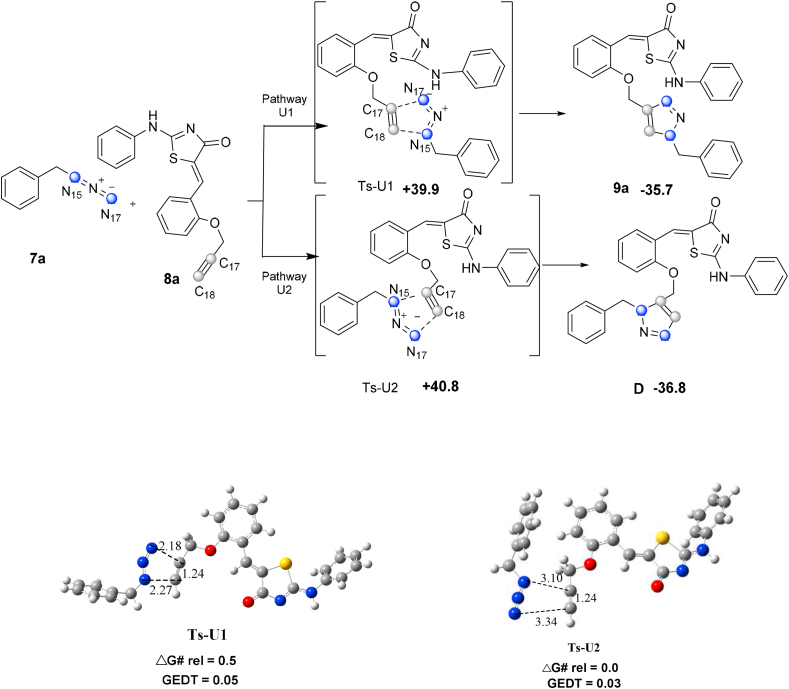
Two regioisomeric cycloadducts could be formed in the 32CA reactions of 7a and 8a owing to the asymmetry of reagents. Analysis of the stationary points found in this cycloaddition reaction indicates that it occurs through a one-step mechanism. Consequently, two transition states, Ts-U1 and Ts-U2, and corresponding triazoles 9a and F were characterized. As represented in Figure 2, both regioisomeric paths have high activation free-energy barriers (Ea) in the absence of the copper catalyst. The 32CA reaction path U1 in the cycloadduct 9a that is exergonic by 35.7 kcal/mol has a 39.9 kcal/mol barrier. However, the pathway U2, which leads to cycloadduct D with a more barrier (40.8 kcal/mol), is exergonic by 36.8 kcal/mol. The optimized geometries of the uncatalyzed TSs are shown in Figure 2.
Figure 2.
The uncatalyzed CA pathways. Distances in angstroms (Å). Energies are in (kcal/mol).
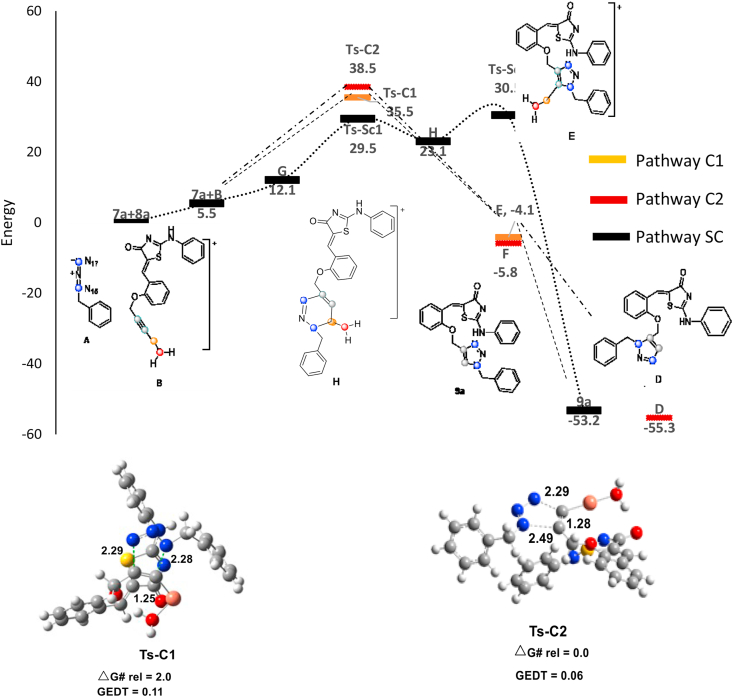
The CA reaction of azide 7a toward alkyne B in water as ligand for Cu(L), was investigated utilizing DFT calculations (Figure 3). Study on the stationary points found along the two 1,4- and 1,5-triazole preparation reveals that the proposed cycloaddition reaction through a one-step mechanism takes place through two transition states Ts-C1 and Ts-C2 and the corresponding metallated triazoles E and F, along with the 1,4- and 1,5-regioisomeric reaction pathways were placed and characterized. As forecast in the absence of the catalyst, the calculated Ea for both regioisomers is more than of that the presence of Cu catalyst (ΔΔGǂ = 11.3 and 7.8 kcal/mol for Pathways C1 and C2, respectively). Both paths are exergonic, respectively by 4.1 and 5.8 kcal/mol (Scheme 1). The calculations exhibited that the Ea for Pathway C1 is nearly similar to pathway C2 (35.5 and 38.5 kcal/mol, respectively). The intermediate F has a lower barrier, and it is more endergonic (1.7 kcal/mol), which is in agreement with the experimental observations. The optimized geometries of TSs TS-C1 and Ts-C2 are displayed in Figure 3.
Figure 3.
The mono-copper catalyzed CA pathways. Distances in angstroms (Å). Energies are in (kcal/mol).
In the namely reaction conditions, by coordinating of Cu(L)+alkyne 8a is considerably acidified. The calculations show that the coordination process is exergonic by 3.1 kcal/mol. The deprotonation process of complex A to acetylide C, using a base in the solvent, is also exergonic by 3.4 kcal/mol, shown in Scheme 5. On the other hand, the overall deprotonation process with two copper ions is exergonic by 6.5 kcal/mol. In intermediate C, one copper ion coordinates the π system, while the other copper is bound to the σ bound of the alkynyl ligand.
Scheme 5.
The proposed mechanism for alkyne deprotonation.
The reaction of azide 7a with alkyne C in the presence of two Cu(L) was also calculated through DFT calculations to explain the total regioselectivity experimentally observed (Figure 4). Analysis of the stationary structures found along the two 1,4- and 1,5-regioisomeric reaction pathways indicates that this di-copper one-step cycloaddition reaction takes place through two transition states, Ts-D1 and Ts-D2 and also the corresponding metallated-triazoles I and L, along with the 1,4- and 1,5-regioisomeric reaction pathways were characterized. Accordingly, in the presence of two copper ions, both of calculated Eas for two different regioisomeric pathways are lower than that of mono-copper and without Cu catalyst pathways, Ts-D1 (23.5 kcal/mol) and Ts-D2 (28.3 kcal/mol) <Ts-C1 (35.5 kcal/mol) <Ts-C2 (38.5 kcal/mol) <Ts-U1 (39.9 kcal/mol) <Ts-U2 (40.8 kcal/mol). In contrast, the pathway DC1, which afford triazole 9a, has a much lower energy barrier than the second pathway DC2 by 20.4 kcal/mol vs. 25.2 kcal/mol. As well, the intermediate L formation is exergonic by 9.8 kcal/mol. These results are along with the experimentally observed regioselectivity of reaction. The optimized geometries of TSs TS-D1 and Ts-D2 are shown in reaction of azide 7a with alkyne C in the presence of two Cu(L) was also calculated through DFT calculations (Figure 4). These results indicated that the di-copper catalyzed reaction's overall barriers are lower than the mono-copper catalyzed, and that of the mono-copper catalyzed reaction is lower than the uncatalyzed. In the presence of a two-copper, Ea for the formation of the 1,4-triazoles decreases more than the 1,5-triazoles.
Figure 4.
The di-copper catalyzed CA pathways. Distances in angstroms (Å). Energies are in (kcal/mol).
For the di-copper case, it is estimated that the favorable product is 1,4-triazole in line with experimental results. In the di-copper pathway, considering weak interactions such as the π/π interactions, Ts-D1 and Ts-D2 have the lower energy, and pathway DC1 is more preferred than DC2, which is in accordance with the experimental result (calculation in wB97XD functional). It is worth revealing that more theoretical studies about CA reactions will be efficient to understand the accuracy of wB97XD and B3LYP functionals in the prediction of regioselectivity (Supporting information Table 1).
2.2.2. Copper-catalyzed stepwise cycloaddition
In continuation of previous works [77, 78], the analysis of plausible reaction paths between both metallated alkynes (B and C) and azide 7a also displayed that the 32CA could occur through a stepwise mechanism (Figures 3 and 4, Scheme 6). Therefore, we investigated the stepwise mono- and di-copper catalyzed mechanisms of 32CA reaction of azide 7a and alkynes (B and C), and the reactants, TSs, and intermediates were optimized and characterized by frequency calculations (SI).
Scheme 6.
The plausible mechanism of di-copper catalyzed stepwise CA.
The reaction starts with coordinating the Nitrogen atom N15 of azide 7a with one Cu ion of C to result in intermediate J. However, the Cu–N coordination is weak because of low amount of the gained energy (2.2 kcal/mol). The following formal CA reaction happens in a stepwise manner, which the first step includes a C–N bond construction (Ts-DC1). This Ts is directed to the six-membered ring intermediate K, in which one of the Cu species is formally Cu(III) with a barrier of 8.2 kcal/mol. This intermediate is endergonic by 8.5 kcal/mol compared with J. Afterward, the five-membered ring intermediate L formation happens through TS Ts-DC2, which can be considered as a proper reductive elimination leding to a Cu(I) species again. The method is exergonic by 49.8 kcal/mol relative to C, and the overall barrier leading to cycloadduct 9a is 4.3 kcal/mol. As shown in Figure 4, our calculations demonstrate that the rate-determining step of this reaction is the formation of six-membered ring K with an uncommon Cu(III) species, which has a lower energy barrier than a one-step manner (14.7 vs. 20.4 kcal/mol). Then, the five-membered metallated triazole L is prepared by ring contraction of transition states Ts-DC2. The activation energy barrier accompanying the coordination of azide 7a to the di-copper acetylide C via J is 14.7 kcal/mol. The corresponding intermediate K formation is endergonic, 7.3 kcal/mol. The energy barrier for ring formation of intermediate L is 8.2 kcal/mol via Ts-DC2, and this step is exergonic by 9.8 kcal/mol. The Ea for the preparation of cycloadduct 9a through M is 4.3 kcal/mol; also, this step is exergonic (43.4 kcal/mol). This di-copper stepwise CA pathway's computed energies predict an exergonic overall reaction (ΔG = −53.2 kcal/mol).
The proposed mechanism, including two Cu ions, is shown in Scheme 6. The Cu-catalyst is contained in each step of the reaction, which efficiently lowers all of them' energy barriers. Primary, Cu coordination raises the alkyne's acidity, facilitating a relatively weak base's deprotonation process. As discussed above, a second Cu ion's involvement in this step makes the deprotonation more comfortable. Second, Cu species allows the CA to occur in a stepwise manner, with much lower energy barriers than those found for the uncatalyzed CA reaction.
Also, a probable mechanism involving one Cu ion was studied in Figure 3. The energy profile for this scenario is considered in a stepwise manner, which is analogous to the one found for the reaction involving two Cu species. This manner was started by C–N bond formation affording a six-membered ring intermediate H, tailed by a ring contraction leading to metallated triazole E. In the mono-copper stepwise pathway SC, intermediate E is formed through Ts-Sc1, an asynchronous transition state with a shorter distance for the C–N bond forming. We also could locate a transition state Ts-Sc2 for the ring contraction of metallated triazole, which would lead to the formation of 1,4-disubstituted triazole 9a. However, all these possibilities have high barriers to be viable under the experimental reaction conditions. The stepwise mechanism has a 17.4 kcal/mol barrier for Ts-Sc1, while the one-step mechanism C1 has barriers of 30.0, or 36.0 kcal/mol, depending on the regiochemistry of the cycloaddition reaction.
2.2.3. Local and global properties analysis
The regioselectivity of CA could be studied through the global and local indices defined in the DFT context. Global electrophilicity index, which measured the total electron attraction abilities from the environment, is defined as ω = μ2/(2η). Chemical hardness (η) is the differences between LUMO (εL) and HOMO (εH) energies, and the global softness computed as S = 1/2η. Electronic chemical potential μ is a relative measure of the molecular capacity to donate electron density, defined the mean value of εL and εH as and the relative global nucleophilicity index N, based on εH well-defined as where TCE is tetracyanoethylene [79, 80, 81, 82]. As presented in Table 3, the static global properties, namely global nucleophilicity N, global electrophilicity ω, chemical hardness η, and electronic chemical potential μ indices of di-copper acetylide C, copper acetylide B, alkyne 8a, and azide 7a are reported. The electrophilicity of alkyne 8a is more significant than azide 7a. The electronic chemical potential of azide 7a is more significant than alkyne 8a, representing charge transfer (CT) flux from azide 7a toward alkyne 8a. The mono-copper acetylide B and dicopper acetylide C have higher electronic chemical potential and nucleophilicity than azide 7a in Table 4, suggesting that along with a polar 32CA reaction, the GEDT can occur from alkynes B and C to azide 7a. More to say, in the nucleophilicity scale, the di-copper acetylide C being classified as a strong nucleophile because of the high value of nucleophilicity, N = 4.21 eV [83].
Table 3.
Electronics Chemical Potential, μ, Global Electrophilicity, ω, Chemical Hardness, η, and Nucleophilicity index, N in eV, Values of azide 7a, alkyne 8a, B and C.
| Structure | Atoms | μ | η | ω | N | S | ||
|---|---|---|---|---|---|---|---|---|
| 7a | 15 | −3.31 | 5.01 | 0.96 | 3.15 | 0.09 | 0.47 | 0.21 |
| 17 | 0.25 | 0.57 | ||||||
| 8a | 17 | −3.73 | 7.20 | 0.98 | 1.85 | 0.06 | 0.20 | 0.45 |
| 18 | 0.28 | 0.23 | ||||||
| B | 17 | −3.42 | 5.63 | 1.05 | 2.88 | 0.08 | 0.19 | 0.10 |
| 18 | 0.14 | 0.27 | ||||||
| C | 17 | −2.89 | 4.01 | 1.1 | 4.21 | 0.12 | 0.30 | 0.22 |
| 18 | 0.19 | 0.06 |
Table 4.
Comparison of the theoretical and experimental 1H-NMR chemical shifts data (δ/ppm) of Ha, Hb, Hc and He of each tautomeric cycloadducts.
 | |||||
|---|---|---|---|---|---|
| Atom number | 3a | 3a′ | 9a | 9a′ | Experimental |
| He | 11.40 | 11.57 | 11.83 | ||
| 11.28 | |||||
| Ha | 4.07 | 4.26 | 4.01 | ||
| 3.97 | |||||
| He | 11.80 | 12.25 | 12.34 | ||
| 11.54 | |||||
| Hb | 5.06 | 5.23 | 5.26 | ||
| 5.30 | |||||
| Hc | 8.70 | 8.49 | 8.34 | ||
| 8.30 | |||||
Overall, the polar character of 32CA was assessed by calculating the GEDT at TSs. In this study, the GEDT [84] was calculated for all the optimized TSs using Natural Population Analysis (NPA) [85, 86], which are given in Figures 2, 3, and 4. Accordingly, the GEDT for Pathways U1 and U2 of the azide 7a with alkyne B reaction is 0.05e and 0.03e, respectively. On the other hand, the GEDT that fluxes from alkynes B and C towards azide 7a for Ts-C1, Ts-C2, Ts-DC1, and Ts-DC2 are 0.11e, 0.06e, 0.43e, and 0.21e, respectively. These GEDT values show no polarity character for pathways U1, U2, and C2, whereas pathways C1, DC1, and DC2 are involved in polar 32CAs [85, 86].
By approaching asymmetric nucleophilic/electrophilic pair and a polar process, the most favorable pathway is related to the initial interaction between the most nucleophilic centers of the nucleophile and the most electrophilic centers of the electrophile. Lately, the nucleophilic Parr function Pk− and electrophilic function Pk+ are proposed by Domingo et al. as powerful tools to study the local interaction in ionic and polar processes. Pk+ and Pk− functions are gained from the atomic spin density maps (ASD) at the radical cations and the radical anions of the reagents [87, 88], which are powerful tools to study the local interaction in ionic and polar processes [89, 90, 91, 92, 93, 94].
The ASD maps of the radical anions of azide 7a, alkynes B, and C and that of the radical cations of azide 7a and alkyne 8a are shown in Figure 5. As shown in Table 3, analysis of the nucleophilic and electrophilic functions at the reactive sites of azide 7a indicates that the N15 (Pk− = 0.47 vs. 0.25) and N17 (Pk+ = 0.57 vs. 0.21) are the most nucleophilic and electrophilic centers, respectively. Alkyne 8a, without any copper catalyst, has the highest electrophilic activation at the C17 atom. Therefore, it is predictable that the C17 of alkyne 8a will be the most favored position for a nucleophilic attack of the N15 atom of azide 7a. The analysis of the ASD maps of mono- and di-copper acetylide B and C indicate that the C17 has the more extensive nucleophilic activation than C18, so it is predictable that the C17 of alkynes B and C will be the favored position for a nucleophilic attack to the N17 of azide 7a which these interactions are in good agreement with the experimental regioselectivity.
Figure 5.
(a) ASD map of the azide radical anion 7a·-, (b) the azide radical cation 7a·+ (c) the alkyne radical cation 8a·+, (d) the mono-copper acetylide radical anion B·- and (e) the di-copper acetylide radical anion C·-.
2.2.4. 1H NMR spectral analysis
The use of 1H-NMR spectroscopy enhanced the characterization of the arylaminothiazol-4-one 3a and triazole 9a. The arylaminothiazol-4-one compounds exist as a mixture of tautomeric equilibrium 3a and 3a′ in DMSO solution; thus, the CA reaction products also include both of the tautomeric cycloadducts 9a and 9a'. The evaluation between the calculated chemical shifts and possible products' experimental data could be useful due to a similar splitting pattern for the two tautomeric cycloadducts. Thus, the 1H-NMR chemical shifts of compounds 3a, 3a′, 9a, and 9a′ were calculated using the GIAO method, and the results were summarized in Table 4. The theoretical values of Ha, Hb, Hc, and He of compounds 3a and 3a′, as well as 9a and 9a′ for 32CA reaction, are close to the experimental values.
2.2.5. ELF topological analysis of the mechanism of the uncatalyzed cycloaddition pathway U1
A well-known powerful tool for studying the bonding changes along the organic reactions is ELF [95]. Recent ELF studies revealed that non-polar [96] and polar [97] 32CA reactions occur through the pseudo radical center. Here, the uncatalyzed one-step pathway has been studied by the ELF topological analysis of the bonding changes along with the reaction. The ELF attractors and calculated valence basin populations of selected points of the IRC pathway of U1 are shown in Figure 6. The ELF topological analysis of the attractors at transition state Ts-U1 demonstrates a monosynaptic basin at C17 of azide fragment, at a C17–N17 distance of 2.23 Å, with a population of 0.01e. Then, at C18–N15 distance of 1.98 Å, two monosynaptic basins emerge with the populations of 0.20 e for V(C18) and 1.13 e for V(N15), while the population of V(C17) increased to 0.58e. Consequently, after passing the TS two pseudoradical centers, which are necessary for the formation of the C18–N15 single bond, have been emerged. Then, the first new C–N single bond is formed through the combination of two monosynaptic basins V(C18) and V(N15) at a C18–N15 distance of 1.70 Å and a disynaptic basin with the integration of 1.81e is created. Along with this point, two monosynaptic basins V(C17) and V(N17) are also observed with populations of 0.72e and 0.66 e, respectively. Finally, at a C17–N17 distance 1.63 Å, a disynaptic V(C17,N17) basin is observed and the triazole ring is formed. A two-stage one-step mechanism characterized this reaction because of the two new single bonds' formations at two differentiated phases of IRC (Figure 6) [84].
Figure 6.
The calculated ELF valence basins populations of the IRC path of the U1 pathway.
3. Discussion
In summary, novel thiazolidinone 1,4-triazole analogs were synthesized through azide-alkyne cycloaddition reaction using click chemistry with a 63–95% yield. The alkynes were synthesized via the Knoevenagel condensation of propargylated salicylaldehyde with 4-thiazolidinones derivatives. The regiochemistry and mechanism of the reaction have been studied in terms of local and global reactivity indices at the B3LYP level of theory. FMO analysis are along with the experimental results. The stepwise mono-copper and di-copper catalyzed cycloaddition mechanisms are also studied. As expected, the di-copper catalyzed stepwise cycloaddition step exhibits a lower Ea than the mono-copper catalyzed stepwise cycloaddition, catalyzed and uncatalyzed one step mechanisms. The DFT-D results have the lowest energy as a result of the π/π interaction in accordance with the experiment. The calculations show that the inclusion of these effects can significantly upgrade the accuracy of the results. The ELF analysis, performed on the uncatalyzed 32CA reaction, showed the two-stage one-step mechanism in which the formation of the second single bond, C17–N17, takes place when the first single bond, C18–N15, was almost entirely formed, along with the local and global analysis.
Declarations
Author contribution statement
Mahdieh Darroudi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, Materials, Analysis tools or data; Wrote the paper.
Yaghoub Sarrafi: Contributed reagents, Materials, Analysis tools or data.
Mahshid Hamzehloueian: Conceived and designed the experiments; Analyzed and interpreted the data; Analysis tools or data, Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The theoretical calculations reported in this paper were carried out at SANCAR, Turkish-German University System, and Nodes of Computational Applications and Research.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Padwa Albert. Wiley-Blackwell; 1986. 1,3-Dipolar Cycloaddition Chemistry. [Google Scholar]
- 2.Naga Siva Rao J., Raghunathan R. An expedient diastereoselective synthesis of pyrrolidinyl spirooxindoles fused to sugar lactone via [3+2] cycloaddition of azomethine ylides. Tetrahedron Lett. 2012;53:854–858. [Google Scholar]
- 3.Rouh H., Liu Y., Katakam N., Pham L., Zhu Y.L., Li G. Synthesis of functionalized chromene and chroman derivatives via cesium carbonate promoted formal [4 + 2] annulation of 2′-hydroxychalcones with allenoates. J. Org. Chem. 2018;83:15372–15379. doi: 10.1021/acs.joc.8b02627. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Ahmed S., Qin X.Y., Rouh H., Wu G., Li G., Jiang B. Synthesis of diastereoenriched α-aminomethyl enaminones via a brønsted acid-catalyzed asymmetric Aza-Baylis-Hillman reaction of chiral N-phosphonyl imines. Chem. - An Asian J. 2020;15:1125–1131. doi: 10.1002/asia.201901734. [DOI] [PubMed] [Google Scholar]
- 5.Buckle D.R., Rockell C.J., Smith H., Spicer B.A. Studies on 1,2,3-triazoles. 13. (Piperazinylalkoxy) [1]benzopyrano[2,3-d]-1,2,3-triazol-9(1H)-ones with combined H1-antihistamine and mast cell stabilizing properties. J. Med. Chem. 1986;29:2262–2267. doi: 10.1021/jm00161a022. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez R., Velazquez S., San-Felix A., Aquaro S., De Clercq E., Perno C.-F., Karlsson A., Balzarini J., Camarasa M.J. 1,2,3-Triazole-[2,5-Bis-O-(tert-butyldimethylsilyl)-.beta.-D-ribofuranosyl]-3’-spiro-5’’-(4’’-amino-1’’,2’’-oxathiole 2’’,2’’-dioxide) (TSAO) analogs: synthesis and anti-HIV-1 activity. J. Med. Chem. 1994;37:4185–4194. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- 7.da Silva F. de C., V de Souza M.C.B.V., Frugulhetti I.I.P.P., Castro H.C., de O. Souza S.L., de Souza T.M.L., Rodrigues D.Q., Souza A.M.T., Abreu P.A., Passamani F. Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009;44:373–383. doi: 10.1016/j.ejmech.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Fung-Tomc J.C., Huczko E., Minassian B., Bonner D.P. In vitro activity of a new oral triazole, BMS-207147 (ER-30346) Antimicrob. Agents Chemother. 1998;42:313–318. doi: 10.1128/aac.42.2.313. http://www.ncbi.nlm.nih.gov/pubmed/9527778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Périon R., Ferrières V., Isabel García-Moreno M., Ortiz Mellet C., Duval R., García Fernández J.M., Plusquellec D. 1,2,3-Triazoles and related glycoconjugates as new glycosidase inhibitors. Tetrahedron. 2005;61:9118–9128. [Google Scholar]
- 10.Anderson D.J., Barbachyn M.R., Emmert D.E., a Garmon S., Graber D.R. Substituent effects on the antibacterial activity of Nitrogen−Carbon-linked (Azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms haemophilusinfluenzaeand moraxellacatarrhalis. J. Med. Chem. 2000;43:953–970. doi: 10.1021/jm990373e. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino M. Effect of 3-Amino-1,2,4-triazole on the experimental production of liver cancer. Nature. 1960;186:174–175. doi: 10.1038/186174a0. [DOI] [PubMed] [Google Scholar]
- 12.Thompson A.M., Blaser A., Anderson R.F., Shinde S.S., Franzblau S.G., Ma Z., Denny W.A., Palmer B.D. Synthesis, reduction potentials, and antitubercular activity of ring A/B analogues of the bioreductive synthesis, reduction potentials, and antitubercular activity of ring A/B. J. Med. Chem. 2009;52:637–645. doi: 10.1021/jm801087e. [DOI] [PubMed] [Google Scholar]
- 13.Jordão A.K., Ferreira V.F., Lima E.S., V de Souza M.C.B.V.B., Carlos E.C.L.L., Castro H.C., Geraldo R.B., Rodrigues C.R., Almeida M.C.B., Cunha A.C. Synthesis, antiplatelet and in silico evaluations of novel N-substituted-phenylamino-5-methyl-1H-1,2,3-triazole-4-carbohydrazides. Bioorg. Med. Chem. 2009;17:3713–3719. doi: 10.1016/j.bmc.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Hou D.-R., Alam S., Kuan T.-C., Ramanathan M., Lin T.-P., Hung M.-S. 1,2,3-Triazole derivatives as new cannabinoid CB1 receptor antagonists. Bioorg. Med. Chem. Lett. 2009;19:1022–1025. doi: 10.1016/j.bmcl.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Shen J., Woodward R., Kedenburg J.P., Liu X., Chen M., Fang L., Sun D., Wang P.G. Histone deacetylase inhibitors through click chemistry. J. Med. Chem. 2008;51:7417–7427. doi: 10.1021/jm8005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padwa A. Wiley-Blackwell; New York: 1984. 1, 3–Dipolar Cycloaddition Chemistry.https://www.abebooks.com/9780471083641/3-Dipolar-Cycloaddition-Chemistry-General-Heterocyclic-047108364X/plp [Google Scholar]
- 17.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise huisgen cycloaddition process: copper(I)-Catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chemie Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Tornøe C.W., Christensen C., Meldal M. Peptidotriazoles on solid phase:-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 19.Himo F., Lovell T., Hilgraf R., Rostovtsev V.V., Noodleman L., Sharpless K.B., Fokin V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 20.Díez-González S., Kolb H.C., Finn M.G., Sharpless K.B. Well-defined copper(i) complexes for Click azide–alkyne cycloaddition reactions: one Click beyond. Catal. Sci. Technol. 2011;1:166. [Google Scholar]
- 21.Hein J.E., Fokin V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LIu Z.X., Bin Chen B., Liu M.L., Zou H.Y., Huang C.Z. Cu(I)-doped carbon quantum dots with zigzag edge structure for high efficient catalysis of azide-alkyne cycloadditions. Green Chem. 2017;46:6473–6475. [Google Scholar]
- 23.Chan T.R., Hilgraf R., Sharpless K.B., V Fokin V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 24.Díez-González S., Correa A., Cavallo L., Nolan S.P. (NHC)Copper(I)-Catalyzed [3+2] cycloaddition of azides and mono- or disubstituted alkynes. Chem. Eur. J. 2006;12:7558–7564. doi: 10.1002/chem.200600961. [DOI] [PubMed] [Google Scholar]
- 25.Candelon N., Lastécouères D., Diallo A.K., Ruiz Aranzaes J., Astruc D. A highly active and reusable copper( i )-tren catalyst for the “click” 1,3-dipolar cycloaddition of azides and alkynes, Chem. Commun. 2008;41:741–743. doi: 10.1039/b716306a. [DOI] [PubMed] [Google Scholar]
- 26.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise huisgen cycloaddition process: copper(I)-Catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Ikhlef D., Wang C., Kahlal S., Maouche B., Astruc D., Saillard J.Y. Reaction mechanisms of transition-metal-catalyzed azide-alkyne cycloaddition “click” reactions: a DFT investigation, Comput. Theor. Chem. 2015;1073:131–138. [Google Scholar]
- 28.Hosseinnejad T., Fattahi B., Heravi M.M. Computational studies on the regioselectivity of metal-catalyzed synthesis of 1,2,3 triazoles via click reaction: a review. J. Mol. Model. 2015;21:1–37. doi: 10.1007/s00894-015-2810-2. [DOI] [PubMed] [Google Scholar]
- 29.Özklllç Y., Tüzün N.S. A DFT study on the binuclear CuAAC reaction: mechanism in light of new experiments, Organometallics. 2016;35:2589–2599. [Google Scholar]
- 30.Boz E., Tüzün N.Ş. Reaction mechanism of ruthenium-catalyzed azide-alkyne cycloaddition reaction: a DFT study, J. Organomet. Chem. 2013;724:167–176. [Google Scholar]
- 31.Hosseinnejad T., Dinyari M. Computational study on stereoselective synthesis of substituted 1H-tetrazoles via a click reaction: DFT and QTAIM approaches. Comput. Theor. Chem. 2015;1071:53–60. [Google Scholar]
- 32.Domingo L.R., Acharjee N. A molecular electron density theory study of the Grignard reagent-mediated regioselective direct synthesis of 1,5-disubstituted-1,2,3-triazoles, J. Phys. Org. Chem. 2020;33 [Google Scholar]
- 33.Meldal M., Diness F. Recent fascinating aspects of the CuAAC click reaction. Trends Chem. 2020;2:569–584. [Google Scholar]
- 34.Bonde C.G., Gaikwad N.J. Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Bioorg. Med. Chem. 2004;12:2151–2161. doi: 10.1016/j.bmc.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Kucukguzel S.G., Oruc E.E., Rollas S., Sahin F., Ozbek A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1, 3, 4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002;37:197–206. doi: 10.1016/s0223-5234(01)01326-5. http://www.sciencedirect.com/science/article/pii/S0223523401013265 [DOI] [PubMed] [Google Scholar]
- 36.Croatica A., Hrvatske G. Acta medica Vol 67 Supl 1 WEB. Pdf. Acta Medica Cordoba. 2013;67 http://paperzz.com/doc/5154181/acta-medica-vol-67-supl-1-web.pdf [Google Scholar]
- 37.Ateş Ö., Altintaş H., Ötük G. Synthesis and antimicrobial activity of 4-carbethoxymethyl-2- thiazoles and 5-Nonsubstituted/substituted 2-[(4-Carbethoxymethylthiazol-2-yl) Arzneimittelforschung. 2000;50:569–575. doi: 10.1055/s-0031-1300251. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0031-1300251 [DOI] [PubMed] [Google Scholar]
- 38.Ateş O., Kocabalkanli A., Saniş G., Ekinci A. Synthesis and antibacterial activity of 5-aryl-2-[(alpha-chloro-alpha-phenylacetyl/alpha-bromopropionyl) amino]-1, 3, 4-oxadiazoles and 2-[(5-aryl-1, 3, 4-oxadiazol-2-yl) imino]-5-phenyl/methyl-4-thiazolidinone s. Arzneimittel-forschung. 1997;47:1134–1138. http://europepmc.org/abstract/med/9368708 [PubMed] [Google Scholar]
- 39.Rao A., Carbone A., Chimirri A., De Clercq E., Monforte A.M., Monforte P., Pannecouque C., Zappalà M. Synthesis and anti-HIV activity of 2,3-diaryl-1,3-thiazolidin-4-(thi)one derivatives. Farm. 2002;57:747–751. doi: 10.1016/s0014-827x(02)01268-5. [DOI] [PubMed] [Google Scholar]
- 40.Barreca M.L., Chimirri A., De Luca L., Monforte A.M., Monforte P., Rao A., Zappal M., Balzarini J., De Clercq E., Pannecouque C., Witvrouw M. Discovery of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV-1 agents. Bioorg. Med. Chem. Lett. 2001;11:1793–1796. doi: 10.1016/s0960-894x(01)00304-3. [DOI] [PubMed] [Google Scholar]
- 41.Rao A., Balzarini J., Carbone A., Chimirri A., De Clercq E., Monforte A.M., Monforte P., Pannecouque C., Zappalà M. Synthesis of new 2,3-diaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Farmaco. 2004;59:33–39. doi: 10.1016/j.farmac.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Fahmy H.T. Synthesis of some new triazoles as potential antifungal agents. Boll. Chim. Farm. 2000;140:422–427. http://www.ncbi.nlm.nih.gov/pubmed/11822232 [PubMed] [Google Scholar]
- 43.Karali N., İllhan E., Gürsoy A., Kiraz M. New cyclohexylidenehydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of 3-phenyl-4(3H)-quinazolinones. Farm. 1998;53:346–349. [Google Scholar]
- 44.Cesur N., Cesur Z., Ergenc N., Uzun M. Synthesis and Antifungal Activity of Some 2-Aryl-3-substituted 4-Thiazolidinones. Synthese und antimykotische Aktivität einiger 2-Aryl-3-substituierter 4- Arch. Plus. 1994;327:271–272. doi: 10.1002/ardp.19943270414. [DOI] [PubMed] [Google Scholar]
- 45.Babaoglu K., Page M.A., Jones V.C., McNeil M.R., Dong C., Naismith J.H., Lee R.E. Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorganic & medicinal chemistry letters. 2003;13(19):3227–3230. doi: 10.1016/s0960-894x(03)00673-5. [DOI] [PubMed] [Google Scholar]
- 46.Ulusoy N. Synthesis and antituberculosis activity of cycloalkylidenehydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of imidazo[2,1-b]thiazole. Arzneimittelforschung. 2002;52:565–571. doi: 10.1055/s-0031-1299931. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0031-1299931 [DOI] [PubMed] [Google Scholar]
- 47.Chen Q., Yu H., Wang L., ul Abdin Z., Chen Y., Wang J., Zhou W., Yang X., Khan R.U., Zhang H., Chen X. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015;5:67459–67474. [Google Scholar]
- 48.Boren B.C., Narayan S., Rasmussen L.K., Zhang L., Zhao H., Lin Z., Jia G., Fokin V.V. Ruthenium-catalyzed azide-alkyne cycloaddition: scope and mechanism. J. Am. Chem. Soc. 2008;130:8923–8930. doi: 10.1021/ja0749993. [DOI] [PubMed] [Google Scholar]
- 49.Cantillo D., Ávalos M., Babiano R., Cintas P., Jiménez J.L., Palacios J.C. Assessing the whole range of CuAAC mechanisms by DFT calculations—on the intermediacy of copper acetylides. Org. Biomol. Chem. 2011;9:2952. doi: 10.1039/c0ob01001d. [DOI] [PubMed] [Google Scholar]
- 50.Darroudi M., Ranjbar S., Esfandiar M., Khoshneviszadeh M., Hamzehloueian M., Khoshneviszadeh M., Sarrafi Y. Synthesis of novel triazole incorporated thiazolone motifs having promising antityrosinase activity through green nanocatalyst CuI-Fe 3 O 4 @SiO 2 (TMS-EDTA) Appl. Organomet. Chem. 2020 [Google Scholar]
- 51.Ranjbar S., Shahvaran P., Edraki N., Khoshneviszadeh M., Darroudi M., Sarrafi Y., Hamzehloueian M., Khoshneviszadeh M. 1,2,3-Triazole-linked 5-benzylidene (thio)barbiturates as novel tyrosinase inhibitors and free-radical scavengers. Arch. Pharm. (Weinheim) 2020 doi: 10.1002/ardp.202000058. [DOI] [PubMed] [Google Scholar]
- 52.Darroudi M., Sarrafi Y., Hamzehloueian M. An efficient synthesis of novel triazoles incorporating barbituric motifs via [3+2] cycloaddition reaction: experimental and theoretical study. J. Serb. Chem. Soc. 2018;83:821–835. [Google Scholar]
- 53.Darroudi M., Sarrafi Y., Hamzehloueian M. Theoretical exploration of mechanism of carbapenam formation in catalytic Kinugasa reaction. Tetrahedron. 2017;73:1673–1681. [Google Scholar]
- 54.Behbehani H., Ibrahim H.M. 4-Thiazolidinones in heterocyclic synthesis: synthesis of novel enaminones, azolopyrimidines and 2-arylimino-5-arylidene-4-thiazolidinones. Molecules. 2012;17:6362–6385. doi: 10.3390/molecules17066362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Behbehani H., Ibrahim H., Makhseed S. Studies with 3-oxoalkanonitriles: synthesis and reactivity of 3-Oxo-3-(1-methylindoloyl) propanenitrile. Heterocycles. 2009;78:3081–3090. http://ci.nii.ac.jp/naid/40016842016/ [Google Scholar]
- 56.Bashiardes G., Safir I., Barbot F., Laduranty J. An expedient synthesis of diversified pyrrolizines and indolizines. Tetrahedron Lett. 2004;45:1567–1570. [Google Scholar]
- 57.Kitamura Y., Taniguchi K., Maegawa T., Monguchi Y., Kitade Y., Sajiki H. Copper/HP20: novel and polymer-supported copper catalyst for huisgen cycloaddition. Heterocycles. 2009;77:521–532. [Google Scholar]
- 58.Behbehani H., Ibrahim H.M., Makhseed S., Elnagdi M.H., Mahmoud H. 2-Aminothiophenes as building blocks in heterocyclic synthesis: synthesis and antimicrobial evaluation of a new class of pyrido[1,2-a]thieno[3,2-e]pyrimidine, quinoline and pyridin-2-one derivatives. Eur. J. Med. Chem. 2012;52:51–65. doi: 10.1016/j.ejmech.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Pałasz A. Synthesis of fused uracils: pyrano[2,3-d]pyrimidines and 1,4-bis(pyrano[2,3-d]pyrimidinyl)benzenes by domino Knoevenagel/Diels-Alder reactions. Monatshefte Für Chemie - Chem. Mon. 2012;143:1175–1185. doi: 10.1007/s00706-012-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hay P.J., Wadt W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985;82:270. [Google Scholar]
- 61.Da Chai J., Head-Gordon M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008;128:84106. doi: 10.1063/1.2834918. [DOI] [PubMed] [Google Scholar]
- 62.Robert M. 40 sleep hacks. Fhjgfd. 1990;11:79–170. [Google Scholar]
- 63.MacGregor W.S. The chemical and physical properties of DMSO. Ann. N. Y. Acad. Sci. 1967;141:3–12. doi: 10.1111/j.1749-6632.1967.tb34860.x. [DOI] [PubMed] [Google Scholar]
- 64.Luzar A., Stefan J. Dielectric behaviour of DMSO-water mixtures. A hydrogen-bonding model, J. Mol. Liq. 1990;46:221–238. [Google Scholar]
- 65.Jie Q., Guo-Zhu J. Dielectric constant of polyhydric alcohol-DMSO mixture solution at the microwave frequency. J. Phys. Chem. 2013;117:12983–12989. doi: 10.1021/jp4082245. [DOI] [PubMed] [Google Scholar]
- 66.Klamt A., Schüürmann G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc., Perkin Trans. 1993;2:799–805. [Google Scholar]
- 67.Andzelm J., Kölmel C., Klamt A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995;103:9312. [Google Scholar]
- 68.Barone V., Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. 1998;102:1995–2001. [Google Scholar]
- 69.Cossi M., Rega N., Scalmani G., Barone V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003;24:669–681. doi: 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez C., Schlegel H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989;90:2154. [Google Scholar]
- 71.Gonzalez C., Schlegel H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990;94:5523–5527. [Google Scholar]
- 72.Wolinski K., Hinton J., Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. 1990;112:8251–8260. [Google Scholar]
- 73.Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006;27:1787–1799. doi: 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
- 74.Xu X., Liu P., Lesser A., Sirois L.E., Wender P.A., Houk K.N. Ligand effects on rates and regioselectivities of Rh(I)-catalyzed (5 + 2) cycloadditions: a computational study of cyclooctadiene and dinaphthocyclooctatetraene as ligands. J. Am. Chem. Soc. 2012;134:11012–11025. doi: 10.1021/ja3041724. [DOI] [PubMed] [Google Scholar]
- 75.Becke A.D., Edgecombe K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990;92:5397. [Google Scholar]
- 76.Khoshkholgh M., Balalaie S., Bijanzadeh H. Intramolecular domino-Knoevenagel-hetero-Diels-Alder reaction with terminal acetylenes. Arkivoc. 2009;9:114–121. https://www.researchgate.net/profile/Hamid_Bijanzadeh/publication/267723170_Intramolecular_domino-Knoevenagel-hetero-Diels-Alder_reaction_with_terminal_acetylenes/links/54b4be570cf28ebe92e48263.pdf [Google Scholar]
- 77.Kolb H.C., Finn M.G., Sharpless K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 78.Rodionov V.O., Fokin V.V., Finn M.G. Mechanism of the ligand-free CuI-catalyzed azide-alkyne cycloaddition reaction, angew. Chemie. 2005;117:2250–2255. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
- 79.Rahm M., Brinck T. Novel 1,3-dipolar cycloadditions of dinitraminic acid: implications for the chemical stability of ammonium dinitramide. J. Phys. Chem. 2008;112:2456–2463. doi: 10.1021/jp710559g. [DOI] [PubMed] [Google Scholar]
- 80.Gothelf K.V., Jørgensen K.A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 1998;98:863–910. doi: 10.1021/cr970324e. [DOI] [PubMed] [Google Scholar]
- 81.Andrada D.M., Granados A.M., Solà M., Fernández I. DFT study of thermal 1,3-dipolar cycloaddition reactions between alkynyl metal(0) fischer carbene complexes and 3 H-1,2-dithiole-3-thione derivatives. Organometallics. 2011;30:466–476. [Google Scholar]
- 82.Chen Z., Lin L., Wang M., Liu X., Feng X. Asymmetric synthesis of trans-??-lactams by a Kinugasa reaction on water, Chem. Eur. J. 2013;19:7561–7567. doi: 10.1002/chem.201204373. [DOI] [PubMed] [Google Scholar]
- 83.Jaramillo P., Domingo L.R., Chamorro E., Pérez P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials, J. Mol. Struct. THEOCHEM. 2008;865:68–72. [Google Scholar]
- 84.Domingo L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014;4:32415. [Google Scholar]
- 85.Domingo L.R., Sáez J.A. Understanding the mechanism of polar Diels–Alder reactions. Org. Biomol. Chem. 2009;7:3576. doi: 10.1039/b909611f. [DOI] [PubMed] [Google Scholar]
- 86.Reed A.E., Weinstock R.B., Weinhold F. Natural population analysisa. J. Chem. Phys. 1985;83:735. [Google Scholar]
- 87.Parr R.G., Pearson R.G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983;105:7512–7516. [Google Scholar]
- 88.Parr W., Yang R.G. Density-functional theory of atoms and molecules. Int. J. Quant. Chem. 1993;47:101. [Google Scholar]
- 89.Domingo L.R., Pérez P., Viehe H.G., Merenyi R., Stella L., Janousek Z., Sustmann R. Global and local reactivity indices for electrophilic/nucleophilic free radicals. Org. Biomol. Chem. 2013;11:4350. doi: 10.1039/c3ob40337h. [DOI] [PubMed] [Google Scholar]
- 90.Domingo L.R., Arnó M., Contreras R., Pérez P. Density functional theory study for the cycloaddition of 1,3-butadienes with dimethyl acetylenedicarboxylate. Polar stepwise vs concerted mechanisms. J. Phys. Chem. 2002;106:952–961. [Google Scholar]
- 91.Domingo L.R. A density functional theory study for the Diels–Alder reaction between N-acyl-1-aza-1,3-butadienes and vinylamines. Lewis acid catalyst and solvent effects, Tetrahedron. 2002;58:3765–3774. [Google Scholar]
- 92.Domingo L.R., José Aurell M. Density functional theory study of the cycloaddition reaction of furan derivatives with masked o-benzoquinones. Does the furan act as a dienophile in the cycloaddition reaction? J. Org. Chem. 2002;67:959–965. doi: 10.1021/jo011003c. [DOI] [PubMed] [Google Scholar]
- 93.Domingo L.R., José Aurell M., Pérez P., Contreras R. Origin of the synchronicity on the transition structures of polar Diels−Alder reactions. Are these reactions [4 + 2] processes? J. Org. Chem. 2003;68:3884–3890. doi: 10.1021/jo020714n. [DOI] [PubMed] [Google Scholar]
- 94.Domingo L.R., Andrés J. Enhancing reactivity of carbonyl compounds via hydrogen-bond formation. A DFT study of the hetero-Diels−Alder reaction between butadiene derivative and acetone in chloroform, J. Org. Chem. 2003;68:8662–8668. doi: 10.1021/jo030156s. [DOI] [PubMed] [Google Scholar]
- 95.Savin A. The electron localization function (ELF) and its relatives: interpretations and difficulties. J. Mol. Struct. THEOCHEM. 2005;727:127–131. [Google Scholar]
- 96.Domingo L.R., Sáez J.A. Understanding the electronic reorganization along the nonpolar [3 + 2] cycloaddition reactions of carbonyl ylides. J. Org. Chem. 2011;76:373–379. doi: 10.1021/jo101367v. [DOI] [PubMed] [Google Scholar]
- 97.Domingo L.R., Chamorro E., Perez P. Understanding the high reactivity of the azomethine ylides in [3 + 2] cycloaddition reactions. Lett. Org. Chem. 2010;7:432–439. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.