Abstract
This systematic review and meta-analysis aimed to evaluate thrombocytopenia as a prognostic biomarker in patients with coronavirus disease 2019 (COVID-19). We performed a systematic literature search using PubMed, Embase and EuropePMC. The main outcome was composite poor outcome, a composite of mortality, severity, need for intensive care unit care and invasive mechanical ventilation. There were 8963 patients from 23 studies. Thrombocytopenia occurred in 18% of the patients. Male gender (P = 0.037) significantly reduce the incidence. Thrombocytopenia was associated with composite poor outcome (RR 1.90 (1.43–2.52), P < 0.001; I2: 92.3%). Subgroup analysis showed that thrombocytopenia was associated with mortality (RR 2.34 (1.23–4.45), P < 0.001; I2: 96.8%) and severity (RR 1.61 (1.33–1.96), P < 0.001; I2: 62.4%). Subgroup analysis for cut-off <100 × 109/l showed RR of 1.93 (1.37–2.72), P < 0.001; I2: 83.2%). Thrombocytopenia had a sensitivity of 0.26 (0.18–0.36), specificity of 0.89 (0.84–0.92), positive likelihood ratio of 2.3 (1.6–3.2), negative likelihood ratio of 0.83 (0.75–0.93), diagnostic odds ratio of 3 (2, 4) and area under curve of 0.70 (0.66–0.74) for composite poor outcome. Meta-regression analysis showed that the association between thrombocytopenia and poor outcome did not vary significantly with age, male, lymphocyte, d-dimer, hypertension, diabetes and CKD. Fagan's nomogram showed that the posterior probability of poor outcome was 50% in patients with thrombocytopenia, and 26% in those without thrombocytopenia. The Deek's funnel plot was relatively symmetrical and the quantitative asymmetry test was non-significant (P = 0.14). This study indicates that thrombocytopenia was associated with poor outcome in patients with COVID-19.
PROSPERO ID: CRD42020213974
Keywords: Coronavirus, mortality, platelet, severe, thrombocyte
Introduction
The coronavirus disease 2019 (COVID-19) remains a significant concern in many countries throughout the world. The number of new cases continues to grow worldwide, and this situation expands the capacity of healthcare resources to its limit. A decisive mitigation approach and effective allocation of resources are essential in slowing the progress of the pandemic, therefore, early assessments to forecast the clinical course of the disease are critical. The levels of several inflammatory parameters are often elevated in the context of severe COVID-19, given the excessive immune response to viral infection resulting in a hyperinflammatory state [1]. Basic investigation such as complete blood count may prove useful in predicting the prognosis of COVID-19 patients. In addition to a reduced lymphocyte count [2], the platelet count can be found in low levels in individuals with severe COVID-19 infection. This systematic review and meta-analysis aimed to evaluate thrombocytopenia as a prognostic biomarker in patients with COVID-19. We performed a diagnostic test accuracy meta-analysis to evaluate whether thrombocytopenia can be a suitable prognostic biomarker.
Material and methods
This is a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) compliant systematic review and meta-analysis. The protocol was registered in International Prospective Register of Systematic Review (PROSPERO) with ID: CRD42020213974.
Eligibility criteria
In this study we include research articles (both prospective and retrospective observational studies) and letters containing research data that report COVID-19 confirmed adults with information on thrombocytopenia (categorical) and mortality/severity/intensive care unit (ICU) care/invasive mechanical ventilation (IMV) (categorical).
We excluded preprints, abstract-only articles, reviews, editorials/commentaries, non-research letters, studies with less than 20 patients, case reports and non-English language articles. Studies reporting only continuous variables of platelets were excluded. Preprint articles were excluded because of the varying credibility [3].
Search strategy and study selection
We performed a systematic literature search using PubMed, Embase and EuropePMC with keywords ‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘2019-nCoV’ AND ‘thrombocytopenia’ OR ‘low platelet’ AND ‘Mortality’ OR ‘Death’ OR ‘non-survivor’ OR ‘severity’ OR ‘disease progression’ or ‘intubation’ OR ‘mechanical ventilation’ OR ‘intensive care unit’ from inception up until 12 October 2020. The PubMed (MEDLINE) search strategy was ((COVID-19) OR (SARS-CoV-2) OR (2019-nCoV)) AND ((thrombocytopenia) OR (low platelet)) AND ((mortality) OR (death) OR (non-survivor) OR (severity) OR (disease progression) or (intubation) OR (mechanical ventilation) OR (intensive care unit)). After recording the initial search and removal of duplicates, two independent authors performed title/abstract screening on the basis of inclusion and exclusion criteria.
Data extraction
Two authors independently performed data extraction of the included studies with the help of standardised extraction forms containing the rows and columns related to the first author, year of publication, study design, age, gender, hypertension, diabetes, chronic kidney diseases (CKD), lymphocyte counts, d-dimer levels and outcome of interests.
The main outcome was composite poor outcome, which is a composite of mortality, severity, ICU care and IMV. Mortality was defined as clinically validated mortality/death/non-survivor.
Severity was defined according to the included studies' parameters, which might be WHO−China Joint Mission on coronavirus disease definition, disease progression or others. ICU care and mechanical ventilation were defined as the need for ICU admission and IMV/intubation, respectively. The pooled effect estimates were presented as risk ratios (RRs). Additionally, diagnostic meta-analysis will use sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and area under curve (AUC) for the effect estimates.
Risk of bias assessment
Risk of bias assessment of individual studies was performed by two independent authors using the Newcastle−Ottawa Scale [4], and any discrepancies were resolved by discussion.
Statistical analysis
To perform statistical analysis, we used STATA 16 (Stata Corp, College Station, TX, USA). We performed the meta-analysis of proportion for patients with thrombocytopenia. To perform pooled analysis for RRs, we used the DerSimonian & Laird method and Mantel−Haenszel with random-effects model regardless of heterogeneity. P-values were considered as statistically significant if ≤0.05. Heterogeneity of the pooled estimate was assessed using I-squared (I2) and Cochrane Q test, a value of >50% or P-value <0.10 indicates a statistically significant heterogeneity. Restricted-maximum likelihood random-effects meta-regression was performed using one covariate at a time for age, gender, hypertension, diabetes mellitus, lymphocyte and d-dimer [5]. For the diagnostic meta-analysis, we displayed forest plot of sensitivity and specificity (a bivariate model) along with a summary receiver operating characteristic (SROC) curve [6]. Fagan's nomogram was plotted to evaluate relationship between the prior probability and posterior test probability [7]. Deek's funnel plot and asymmetry test were used to evaluate publication bias. We performed univariable meta-regression and subgroup analyses for the output of diagnostic meta-analysis using age, male, lymphocytes, d-dimer, hypertension, diabetes and CKD as covariates.
Results
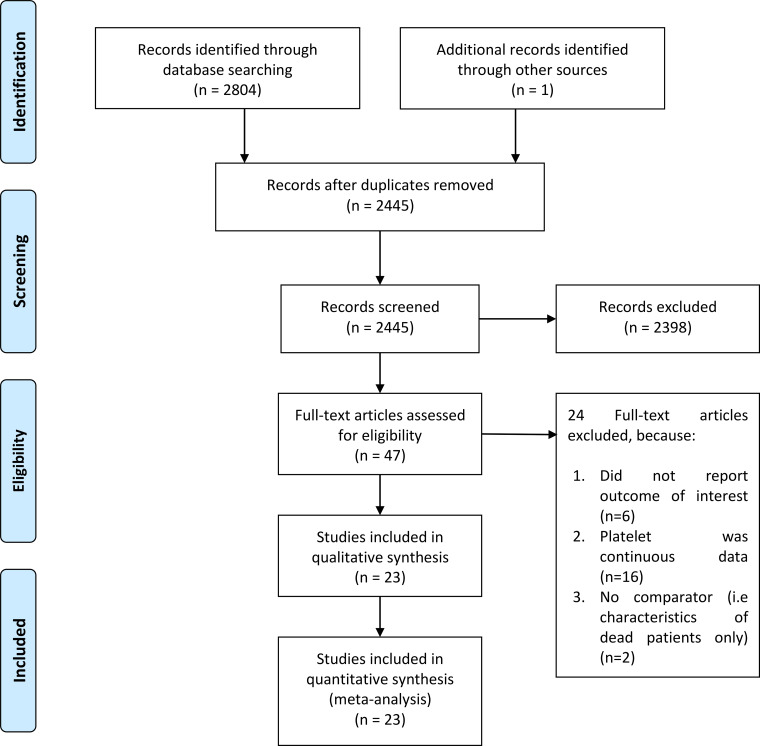
There were 8963 patients from 23 eligible studies included in the systematic review and meta-analysis (Fig. 1) [8–30]. The baseline characteristics of the included studies can be seen in Table 1. Thrombocytopenia occurred in 18% (13–23%) of the patients. Male gender (coefficient: −0.004, P = 0.034), but not age (P = 0.290), lymphocyte (P = 0.905), d-dimer (P = 0.195), hypertension (P = 0.355), diabetes (P = 0.450) and CKD (P = 0.658) significantly influence the incidence. The incidence of composite poor outcome was 30%.
Fig. 1.
PRISMA flowchart.
Table 1.
Baseline characteristics of the included studies
| References | Study design | Sample size (n) | Mean age (years) | Male (%) | HT (%) | DM (%) | CKD (%) | Lymphocytes (109) | D-dimer (mcg/ml) | Outcome | Cut-off for Thrombocytopenia (*109/l) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] | PO | 373 | 52.78 | 67 | – | – | – | – | – | Mortality | <150 | 7 |
| [9] | PO | 178 | 64 | 59.6 | 32.6 | 17.4 | 1.7 | 1.2 | 0.56 | Severity | <100 | 7 |
| [20] | RO | 21 | 56 | 81 | 23.8 | 14.3 | – | 0.9 | 0.5 | Severity | <100 | 7 |
| [24] | RO | 44 | >65 (56.8) | 36.3 | 34.1 | 15.9 | – | – | – | Severity | <150 | 7 |
| [25] | RO | 393 | 62.2 | 60.6 | 50.1 | 25.2 | – | – | Dimer >0.5 (36.4) | IMV | <150 | 6 |
| [26] | RO | 1099 | 47 | 59.1 | 15 | 7.4 | 0.7 | 1 | Dimer >0.5 (46.4) | Severity | <150 | 7 |
| [27] | RO | 98 | 55.4 | 38.8 | 30.6 | 9.2 | – | 1.4 | – | ICU Care | ? | 7 |
| [28] | RO | 41 | 49 | 73 | 15 | 20 | – | 0.8 | 0.5 | ICU Care | <100 | 6 |
| [29] | RO | 110 | 56.9 | 43.6 | 33.6 | 26.4 | – | 1.4 | 4.4 Dimer >0.5 (76.3) | Severity | <140 | 9 |
| [30] | RO | 59 | 64 | 49 | 42 | 15 | – | 1.1 | 6.1 | ICU Care | <100 | 7 |
| [10] | RO | 63 | 48 | 85 | 32 | 32 | 6.4 | 1.1 | 0.6 Dimer >0.55 (59) | ICU Care | <125 | 6 |
| [23] | RO | 611 | 64 | 54 | 30 | 16 | 1 | 1.24 | Severity | <100 | 7 | |
| [11] | RO | 548 | 60 | 50.9 | 30.3 | 15.1 | 1.8 | - | – | Severity | <150 | 9 |
| [12] | RO | 1190 | 57 | 53.4 | 26.1 | 12.2 | 2.6 | 1.2 | 0.9 | Mortality | <100 | 9 |
| [13] | RO | 756 | 69 | 62.2 | 37.8 | 28.6 | 13.5 | Dimer >0.50 (87.8) | Mortality | <130 | 9 | |
| [14] | RO | 113 | 53.8 | 62.8 | 19.5 | 14.2 | 5.3 | 1.2 | – | Mortality | <150 | 7 |
| [15] | RO | 135 | 47 | 53.3 | 9.6 | 8.9 | – | 1.1 | 0.4 | Severity | <125 | 7 |
| [16] | RO | 276 | 51 | 56.2 | 17 | 5.1 | – | 1.1 | Dimer >0.50 (53.7) | Severity | <150 | 7 |
| [17] | RO | 239 | 62.5 | 59.8 | 43.9 | 18.4 | – | 0.6 | – | Mortality | <125 | 9 |
| [18] | RO | 1476 | 58.7 | 52.3 | – | – | – | – | – | Mortality | <125 | 8 |
| [19] | RO | 788 | 45.8 | 51.6 | 16 | 7.2 | 0.9 | 1.2 | – | Severity | <100 | 9 |
| [21] | RO | 161 | 45 | 49.7 | 13.7 | 4.3 | 1.1 | – | Severity | <100 | 7 | |
| [22] | RO | 191 | 56 | 62 | 30 | 19 | 1 | 1 | 0.8 Dimer >0.50 (68) | Mortality | <100 | 7 |
CKD, chronic kidney disease; DM, diabetes mellitus; HT, hypertension; ICU, intensive care unit; IMV, invasive mechanical ventilation; PO, prospective observational; RO, retrospective observational; NOS, Newcastle−Ottawa scale.
Thrombocytopenia and composite outcome
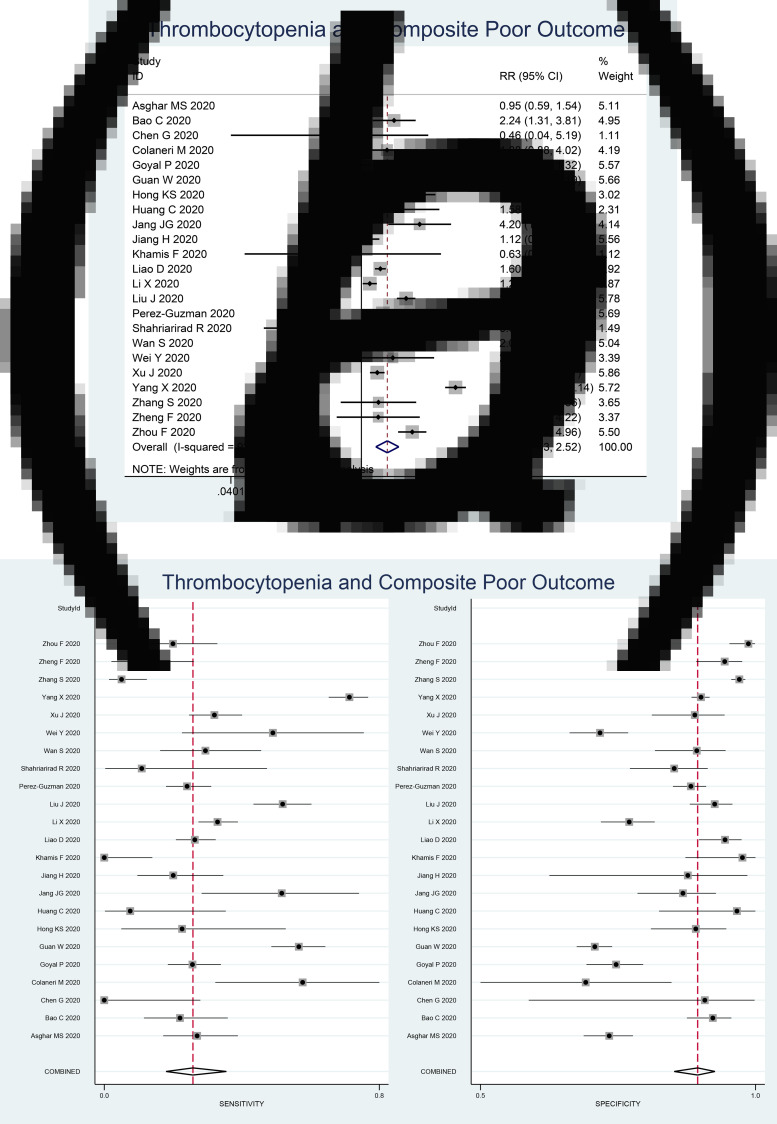
Thrombocytopenia was associated with composite poor outcome (RR 1.90 (1.43–2.52), P < 0.001; I2: 92.3%, P < 0.001) (Fig. 2a). Subgroup analysis showed that thrombocytopenia was associated with mortality (RR 2.34 (1.23–4.45), P < 0.001; I2: 96.8%, P < 0.001) and severity (RR 1.61 (1.33–1.96), P < 0.001; I2: 62.4%, P < 0.001). Subgroup analysis for cut-off <100 × 109/l (9 studies) showed RR of 1.93 (1.37–2.72), P < 0.001; I2: 83.2%, P < 0.001). Subgroup analysis for cut-off <150 × 109/l (seven studies) was borderline significant (RR 1.39 (0.99–1.95), P = 0.058; I2: 76.2%, P < 0.001).
Fig. 2.
Forest-plot for thrombocytopenia and composite poor outcome (a) and pooled sensitivity and specificity (b).
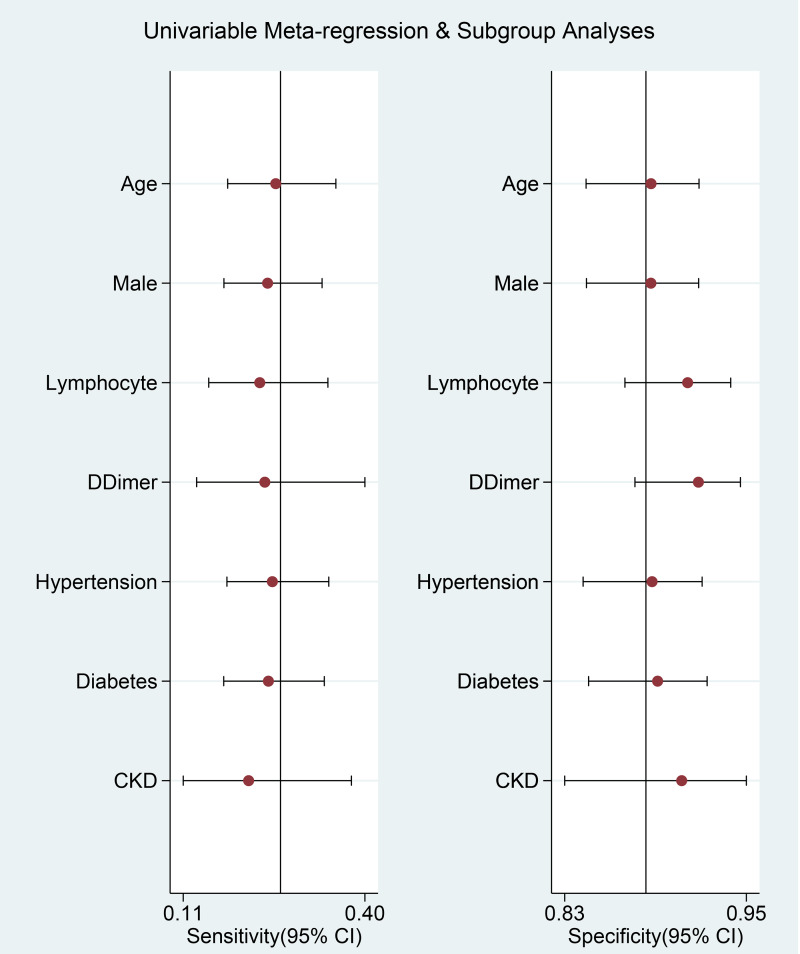
Meta-regression analysis showed that the association did not vary significantly with age (P = 0.534), male (P = 0.117), lymphocyte (P = 0.910), d-dimer (P = 0.751), hypertension (P = 0.0647), diabetes (P = 0.812) and CKD (P = 0.509).
Diagnostic meta-analysis
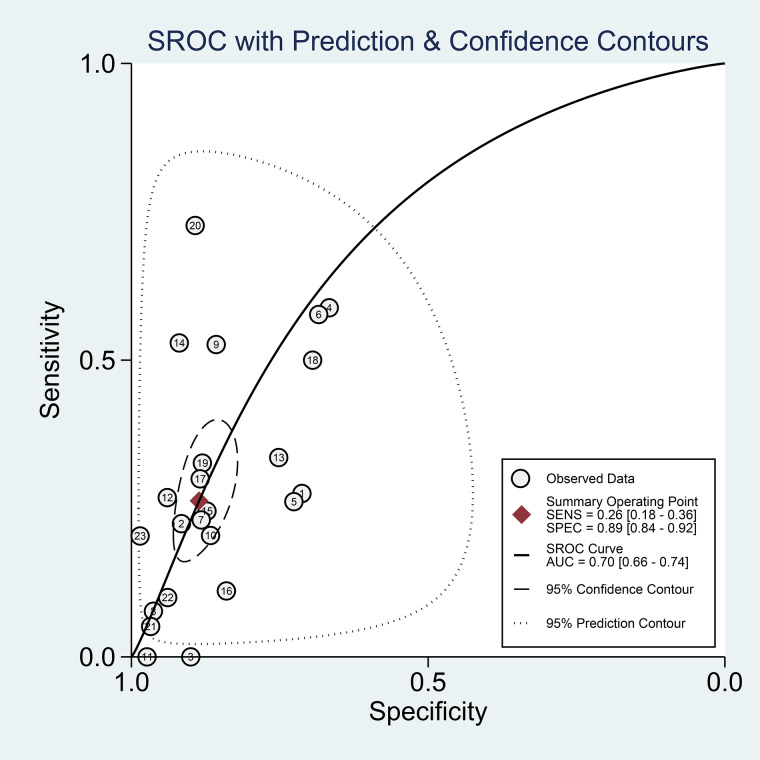
Thrombocytopenia had a sensitivity of 0.26 (0.18–0.36), specificity of 0.89 (0.84–0.92), PLR of 2.3 (1.6–3.2), NLR of 0.83 (0.75–0.93), DOR of 3 (2, 4) and AUC of 0.70 (0.66–0.74). Pooled sensitivity and specificity is displayed in Figure 2b.
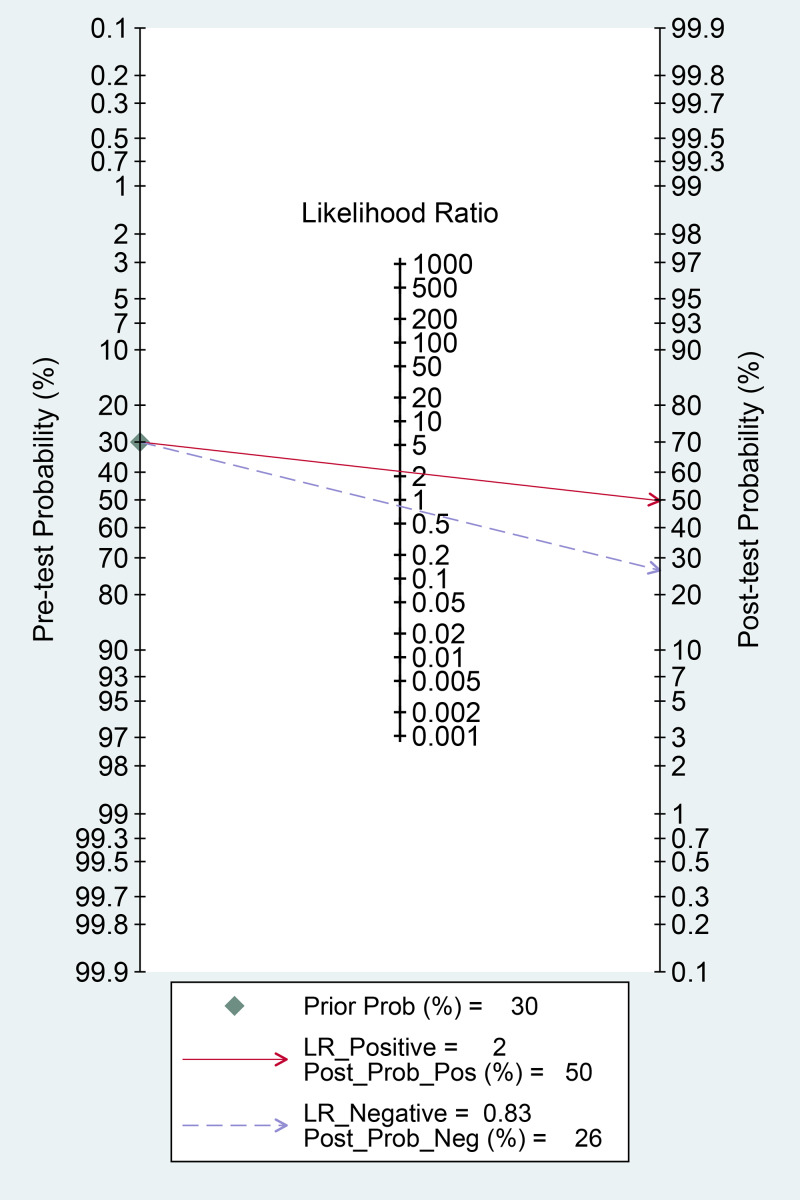
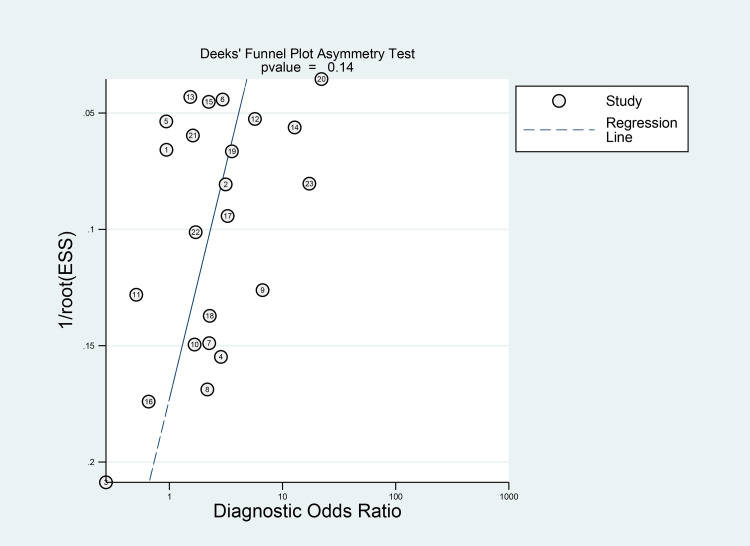
Fagan's nomogram showed that the posterior probability of poor outcome was 50% in patients with thrombocytopenia, and 26% in those without thrombocytopenia (Fig. 3). The Deek's funnel plot was relatively symmetrical with respect to the regression line and the asymmetry test was non-significant (P = 0.14) (Fig. 4).
Fig. 3.
Fagan's nomogram.
Fig. 4.
Deek's funnel plot and asymmetry test. The vertical axis displays the inverse of the square root of the effective sample size (1/root(ESS)).
A curve for prediction of composite poor outcome was generated (Fig. 5). The 95% prediction region was wide, however, meta-regression analysis showed that no reasons for the heterogeneity in the selected covariates, age, male, lymphocytes, d-dimer, hypertension, diabetes and CKD. Univariable meta-regression and subgroup analyses are displayed in Figure 6.
Fig. 5.
Summary receiver operating characteristic (SROC) with prediction and confidence contours.
Fig. 6.
Univariable meta-regression and subgroup analyses. Vertical lines represent pooled sensitivity and specificity, respectively.
Discussion
This study indicates that thrombocytopenia was associated with poor outcome in patients with COVID-19. Diagnostic test accuracy indicates sensitivity of 26% and specificity of 89% with AUC of 0.70. Meta-regression analysis showed that the association or diagnostic accuracy was not significantly influenced by age, male, lymphocytes, d-dimer level, hypertension, diabetes and CKD. There are varying cut-off points for thrombocytopenia, a cut-off point of <100 × 109/l showed a stronger association compared to <150 × 109/l. The varying cut-off point might also cause heterogeneity. Meta-regression analysis also showed that male gender was associated with reduced incidence of thrombocytopenia.
Comorbidities, such as diabetes mellitus, hypertension, chronic lung disease, cardiac disease and CKD [31–36] as well as individuals with advanced age and obesity are at higher risk for severe COVID-19 and mortality [37, 38]. Several parameters such as C-reactive protein (CRP), procalcitonin (PCT), ferritin and D-dimer are frequently elevated in patients with poor prognosis [1]. Based on the aforementioned possible factors, meta-regression analyses were performed for several variables that may affect the prediction derived from thrombocytopenia, these include age, male, lymphocytes, d-dimer, hypertension, diabetes and CKD. On admission, a complete blood count is almost always ordered and often repeated at the discretion of the treating doctor, thus, did not add to the treatment costs. Since platelet count is simple, easy and inexpensive to perform, it is an ideal clinical prognostication tools. Low platelet count is a common laboratory finding in patients with severe COVID-19, in our meta-analysis, we found that thrombocytopenia was present in 18% of the patients [9, 18].
Coagulopathy is one of the serious complications that can develop in patients with COVID-19, as well as acute respiratory distress syndrome (ARDS), cardiopulmonary collapse and multi-organ failure (MOF) [39]. Alteration in bleeding and clotting parameters can contribute to the development of coagulopathy-related events, such as pulmonary emboli (PE) and disseminated intravascular coagulation (DIC). In some patients, the prothrombin time (PT) and activated partial thromboplastin time (aPTT) is prolonged, while others may have a PT [40, 41]. Haematological changes, including lymphopenia and thrombocytopenia but normal white blood cell count, are also commonly observed in individuals contracted to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. However, it is still unclear how the novel coronavirus influence the haematopoietic system.
The pathophysiology of thrombocytopenia in COVID-19 is hypothetically caused by the alteration of platelets' production and consumption (and/or destruction) [42]. It affects platelets' production by either directly or indirectly affecting the haematopoietic stem cells (HSCs), reduction of thrombopoietin production, and megakaryocyte maturation due to increase of specific inflammatory cytokines [43], and decrease of supplementary haematopoietic progenitor in the pulmonary vessels due to COVID-19-induced lung damage [44]. Moreover, extensive lung damage and disseminated intravascular coagulation due to hyperinflammation cause further thrombocytopenia in COVID-19 due to the increase of platelet consumption [42].
Coronaviruses may directly infect haematopoietic and bone marrow cells and cause aberrant haematopoiesis. Human aminopeptidase N (CD13) is a metalloprotease found on lung epithelial cells' surface, smooth muscle cells, fibroblasts and epithelial cells in the kidneys and small intestine, may serve as a receptor for the entry of novel coronavirus. It is speculated that the virus may invade bone marrow cells and platelets through CD13 receptors and cause growth inhibition and apoptosis, resulting in haematopoiesis dysfunction, reduced primary platelet production and eventual thrombocytopenia [18, 40, 45].
Cytokine storm, a condition in which human body releases large amounts of inflammatory cytokines and cause hyperinflammation, is thought to play a central role in the pathophysiology of COVID-19 [1, 39]. Excessive activation and proliferation of mononuclear macrophage system leads to secondary haemophagocytic lymphohistiocytosis (sHLH), in which cytokines are released excessively and an enormous number of blood cells are engulfed, contributing to reduced peripheral blood platelets. This proinflammatory state also causes immune damage to various organs, including the lungs, and is thought to affect the haematopoietic progenitor cells in the bone marrow, leading to reduced platelet synthesis [40, 41].
The lung is a reservoir for haematopoietic progenitors and a site of platelet biogenesis. Lung injury in COVID-19 pneumonia may be exacerbated by persistent hypertension and oxygen toxicity, leading to consolidation changes and pulmonary fibrosis. It is found that a considerable number of megakaryocytes releases platelet dynamically into pulmonary circulation [44]. Injured capillary beds and diffuse alveolar damage in the lungs cause trapping of megakaryocyte and inhibit platelets' release from megakaryocytes, which causes reduced platelet formation in the systemic circulation [18, 45]. Also, injured pulmonary endothelial cells and lung tissues will activate the coagulation system, resulting in platelet aggregation and micro-thrombosis in the lungs, which increases platelet consumption [40].
Elevated levels of autoantibodies and immune complexes in patients with SARS-CoV-2 infection may result in platelet destruction and clearance by the immune system. The exact pathogenesis of immune-mediated thrombocytopenia in COVID-19 is still unclear, however, reticuloendothelial cells may recognise platelets as target tissue given the presence of antibodies and immune complexes deposited on the platelet surface. Furthermore, antibodies generated when the virus invades human cells may specifically bind to platelet antigens via molecular mimicry, causing substantial damage to the circulating platelets [40, 45, 46]. The reason why thrombocytopenia is independently associated with poor outcome is biologically plausible because it might reflect a greater viral load of SARS-CoV-2, higher inflammatory response and extensive lung damage, as we previously described the hypothetical mechanism behind this phenomenon.
Sociodemographic may influence the findings in this meta-analysis, for example, certain infections such as dengue virus may cause thrombocytopenia [47]. Thrombocytopenia caused by dengue virus may differ from those caused by sepsis and disseminated intravascular coagulation, or COVID-19 [40, 48, 49]. Concurrent infections with such diseases were not reported among the studies. The prevalence of these infections may be higher in tropical regions [50, 51]. Clinical applications should not be generalised without considering these factors.
Clinical implications
This meta-analysis indicate that thrombocytopenia was associated with increased composite poor outcome with RR of 1.90. It has a low sensitivity but high specificity, thus is best used to rule in rather to rule out the possibility of composite poor outcome. The sensitivity and specificity are highly heterogeneous as demonstrated by Figure 2. In a 30% pre-test probability for composite poor outcome, thrombocytopenia may increase the probability to 50%. Meanwhile, Deek's funnel plot asymmetry test indicate low risk of publication bias. SROC curve showed a AUC of 0.70, indicating an acceptable diagnostic performance. Meta-regression analysis indicate that several assigned comorbidities did not affect the sensitivity and specificity for thrombocytopenia to predict poor outcome. Thus, thrombocytopenia might be used to rule in composite poor outcome, but not to rule it out. Finding from subgroup analysis based on cut-off points may indicate that a lower cut-off point was associated with a higher risk. To enhance the sensitivity and specificity, it is better to construct a prediction model using several variables.
Limitations
Most of the studies did not report the number of possible co-infections that can potentially cause thrombocytopenia or the number of bacterial infections. The definition for thrombocytopenia still varies among the included studies. Drug therapy, such as anticoagulants use were rarely reported by the studies.
Conclusion
This study indicates that thrombocytopenia was associated with poor prognosis in patients with COVID-19. Diagnostic test accuracy indicates sensitivity of 26% and specificity of 89% with AUC of 0.70.
Acknowledgement
None
Authors contribution
Raymond Pranata: This author helped in concept development, manuscript drafting, data acquisition, data analysis and statistical analysis. Michael Anthonius Lim: This author helped in data acquisition, data analysis and manuscript drafting. Emir Yonas: Investigation, extensive review and editing of the manuscript. Ian Huang: Data acquistition, investigation, extensive review and editing of the manuscript. Sally Aman Nasution: Investigation, extensive review and editing. Siti Setiati: Investigation, extensive review and editing. Idrus Alwi: Investigation, extensive eeview and editing. Tuty Kuswardhani: Investigation, extensive eeview and editing.
Financial support
None.
Conflict of interest
None.
Data availability
All data generated or analysed during this study are included in this published article
References
- 1.Huang I et al. (2020) C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Therapeutic Advances in Respiratory Disease 14, 175346662093717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang I and Pranata R (2020) Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Journal of Intensive Care 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henrina J, Lim MA and Pranata R (2020) COVID-19 and misinformation: how an infodemic fueled the prominence of vitamin D. British Journal of Nutrition 125, 359–360. doi: 10.1017/S0007114520002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells G et al. (2009) The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
- 5.Higgins JP and Green S (2011) 9.6.4 Meta-regression. Cochrane Handbook for Systematic Reviews of Interventions. UK: Cochrane. [Google Scholar]
- 6.Macaskill P et al.l (2010) Chapter 10 analysing and presenting results. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. UK: Cochrane. [Google Scholar]
- 7.Caraguel CGB and Vanderstichel R (2013) The two-step Fagan's nomogram: ad hoc interpretation of a diagnostic test result without calculation. Evidence Based Medicine 18, 125–128. [DOI] [PubMed] [Google Scholar]
- 8.Asghar MS et al. (2020) Role of biochemical markers in invasive ventilation of coronavirus disease 2019 patients: multinomial regression and survival analysis. Cureus 12, Published online: 26 August 2020. doi: 10.7759/cureus.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao C et al. (2020) SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Experimental Hematology & Oncology 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khamis F et al. (2020) Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. Journal of Infection and Public Health 13, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X et al. (2020) Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology 146, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J et al. (2020) Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Annals of Intensive Care 10, Published online: 2020. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Guzman PN et al. (2020) Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 29, 2341–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahriarirad R et al. (2020) Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infectious Diseases 20, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan S et al. (2020) Clinical features and treatment of COVID-19 patients in northeast Chongqing. Journal of Medical Virology 92, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Y et al. (2020) Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infectious Diseases 20, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J et al. (2020) Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Critical Care 24, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X et al. (2020) Thrombocytopenia and its association with mortality in patients with COVID-19. Journal of Thrombosis and Haemostasis 18, 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang SY et al. (2020) Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China. Infectious Diseases of Poverty 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G et al. (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation 130, 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng F et al. (2020) Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. European Review for Medical and Pharmacological Sciences 24, 3404–3410. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao D et al. (2020) Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. The Lancet Haematology 7, e671–e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colaneri M et al. (2020) Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveillance: Bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 25, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal P et al. (2020) Clinical characteristics of Covid-19 in New York City. The New England Journal of Medicine 382, 2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan W et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong KS et al. (2020) Clinical features and outcomes of 98 patients hospitalized with SARS-COV-2 infection in daegu, South Korea: a brief descriptive study. Yonsei Medical Journal 61, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The Lancet 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang JG et al. (2020) Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. Journal of Korean Medical Science 35, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H et al. (2020) Clinical imaging characteristics of inpatients with coronavirus disease-2019 in Heilongjiang Province, China: a retrospective study. Aging 12, 13860–13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pranata R et al. (2020) Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. Journal of the Renin-Angiotensin-Aldosterone System 21, 147032032092689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang I, Lim MA and Pranata R (2020) Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pranata R et al. (2020) Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. International Journal of Tuberculosis and Lung Disease 24, 838–843. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 34.Pranata R et al. (2020) Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19 – systematic review, meta-analysis, and meta-regression. Journal of Stroke and Cerebrovascular Diseases 29, 104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonas E et al. (2020) Effect of heart failure on the outcome of COVID-19 – a meta analysis and systematic review. The American Journal of Emergency Medicine, Published online: July 2020. doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pranata R et al. (2020) The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine 14, 117954842095916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pranata R et al. (2020) Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes & Metabolism Published online: July 2020. doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim MA and Pranata R (2020) Coronavirus disease 2019 (COVID-19) markedly increased mortality in patients with hip fracture – a systematic review and meta-analysis. Journal of Clinical Orthopaedics and Trauma Published online: 2020. doi: 10.1016/j.jcot.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim MA et al. (2020) Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Canadian Journal of Kidney Health and Disease 7 Published online: 2020. doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu P, Zhou Q and Xu J (2020) Mechanism of thrombocytopenia in COVID-19 patients. Annals of Hematology 99, 1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iba T et al. (2020) Coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis : JTH 18, 2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y et al. (2020) Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thrombosis Research, 193, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prompetchara E, Ketloy C and Palaga T (2020) Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pacific Journal of Allergy and Immunology 38, 1–9. [DOI] [PubMed] [Google Scholar]
- 44.Lefrançais E et al. (2017) The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amgalan A and Othman M (2020) Exploring possible mechanisms for COVID-19 induced thrombocytopenia: unanswered questions. Journal of Thrombosis and Haemostasis : JTH 18, 1514–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed MZ et al. (2020) Thrombocytopenia as an initial manifestation of COVID-19; case series and literature review. British Journal of Haematology 189, 1057–1058. [DOI] [PubMed] [Google Scholar]
- 47.Martina BEE, Koraka P and Osterhaus ADME (2009) Dengue virus pathogenesis: an integrated view. Clinical Microbiology Reviews 22, 564–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venugopal A (2014) Disseminated intravascular coagulation. Indian Journal of Anaesthesia 58, 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedet A et al. (2018) Mechanisms of thrombocytopenia during septic shock: a Multiplex cluster analysis of endogenous sepsis mediators. Shock 49, 641–648. [DOI] [PubMed] [Google Scholar]
- 50.Murray NEA, Quam MB and Wilder-Smith A (2013) Epidemiology of dengue: past, present and future prospects. Clinical Epidemiology 5, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatt S et al. (2013) The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article