Abstract
Heatwaves have increased in intensity, duration and frequency over the last decades due to climate change. Intertidal species, living in a highly variable environment, are likely to be exposed to such heatwaves since they can be emerged for more than 6 h during a tidal cycle. Little is known, however, on how temperature affects species traits (e.g. locomotion and behaviour) of slow-moving organisms such as benthic foraminifera (single-celled protists), which abound in marine sediments. Here, we examine how temperature influences motion-behaviour and metabolic traits of the dominant temperate foraminifera Haynesina germanica by exposing individuals to usual (6, 12, 18, 24, 30 °C) and extreme (high; i.e. 32, 34, 36 °C) temperature regimes. Our results show that individuals reduced their activity by up to 80% under high temperature regimes whereas they remained active under the temperatures they usually experience in the field. When exposed to a hyper-thermic stress (i.e. 36 °C), all individuals remained burrowed and the photosynthetic activity of their sequestered chloroplasts significantly decreased. Recovery experiments subsequently revealed that individuals initially exposed to a high thermal regime partially recovered when the hyper-thermic stress ceased. H. germanica contribution to surface sediment reworking substantially diminished from 10 mm3 indiv−1 day−1 (usual temperature) to 0 mm3 indiv−1 day−1 when individuals were exposed to high temperature regimes (i.e. above 32 °C). Given their role in sediment reworking and organic matter remineralisation, our results suggest that heatwaves may have profound long-lasting effects on the functioning of intertidal muddy ecosystems and some key biogeochemical cycles.
Subject terms: Behavioural ecology, Climate-change ecology, Ecosystem ecology
Introduction
Over the last decades, anthropogenic pressures such as industrial activity, intensive agriculture, pollution, deforestation and overfishing have altered the terrestrial and marine biosphere1–3. Greenhouse gas emissions have risen substantially, affecting the global climate and the frequency and magnitude of extreme weather or climatic events such as storms, floods, droughts and heatwaves2,4–9. Over the period 1982–2010, extremely hot days have been more frequent along 38% of the world’s coastlines10 and a recent study suggests that 50% of the ocean surface may suffer from a permanent marine heatwave state by the late twenty-first century3. Marine heatwaves, which result from the warming of both air and seawater temperature11,12, have caused unprecedented mass mortalities of a wide range of intertidal species such as mussels and limpets13–17. In the intertidal environment, sessile and slow-moving invertebrates are more likely to be exposed to extreme temperature events. Noticeably, in temperate ecosystems, surface soft-sediment temperature (i.e. within the first centimetre) can frequently reach up to 30 °C18 and sometimes even 40 °C at low tide19,20 during spring and summer. Typically, in European Atlantic mudflats, organisms can experience daily rise in sediment temperature up to 20 °C in 2 h at emersion19. Consequently, intertidal species are more eurytherm than their subtidal counterparts21–23. However, these organisms often live close to the upper limit of their thermal tolerance window, which make them also sensitive to thermal stress21,24. Outside their thermal range, temperature may have adverse effects on behaviour (e.g. locomotion), metabolism and reproductive strategy, which ultimately affect species survival1,21,25. To alleviate a thermal stress, organisms typically decrease their metabolic rate by reducing their activity such as locomotion and feeding, which decrease the space they explore and hamper their foraging strategy21,26–28. Thermal stress may have substantial implications for soft-bottom ecosystem functioning and services. Indeed, the movements of benthic species affect biogeochemical or ecosystem processes since they contribute to sediment reworking and dissolved material fluxes29–33. In this context, assessing how temperature might affect movements, activity and metabolic rate of intertidal organisms is a critical prerequisite to better understand how their contribution to ecosystem functioning may be affected by the increasing occurrence of marine heatwaves in the context of global warming.
In soft sediment, macrofaunal taxa such as molluscs, shrimps or crabs have been well-studied since they play a key role in habitat structuration28,34–36. Meiobenthic organisms such as benthic foraminifera also play a major role in biogeochemical or ecosystem processes37–41. Yet, little is known about their behavioural and metabolic response to changing temperatures. Many studies have shown that temperature can affect intertidal foraminifera survival, diversity, growth, morphology and feeding20,42–46 and that some foraminiferal species also increase their locomotion speed and oxygen consumption up to a point where temperature negatively impede movement, behaviour and metabolism42,47. Under moderate temperature, Haynesina germanica is the most active species (i.e. with an important time allocated to motion) amongst dominant European mudflat foraminifera and may be a key contributor to sediment reworking48,49. Furthermore, H. germanica can sequester chloroplasts from diatoms to use them for photosynthesis, which implies that this species contributes to both oxygen consumption and production in the sediment47. In contrast to tropical species45,46,50,51, the metabolic response of H. germanica to changing temperatures remains unknown. Given its high abundance in temperate intertidal mudflats52–55, high level of activity and subsequent putative contribution to sediment reworking, H. germanica is a good candidate to experimentally assess the effects of temperature on soft-bottom ecosystem functioning, especially in the context of global warming.
The objectives of this study are (i) to experimentally describe the responses of H. germanica to temperature in terms of motion behaviour and metabolic rate using a thermal gradient usually encountered in temperate intertidal environments (i.e. 6–30 °C), (ii) to characterize the effects of experimentally-induced heatwaves ranging from 32 to 36 °C and (iii) to experimentally assess the ability of the species to recover after being exposed to extreme temperatures i.e. 6 and 36 °C. We also discuss possible consequences of an acute hyperthermic stress on H. germanica and its putative effects on benthic ecosystem functioning and services.
Methods
Collection
Surface sediment (0–1 cm) were gently scrapped off with a spoon in April, May and June 2019 in two intertidal mudflats located on the French coasts of the eastern English Channel, i.e. Authie Bay (50° 22′ 20′′ N, 1° 35′ 45′′ E) and Boulogne-sur-Mer harbour (50° 43′ 6′′ N, 1° 34′ 25′′ E). Both sampling sites showed similar grain size (20% sand, 80 silt), TOC contents (between 1 and 2%)55, temperature and salinity values (18 °C, 33.8 PSU)56. Samples were stored in plastic containers (100 ml) and transported to the laboratory, then washed through a 125 µm mesh sieve. Living H. germanica of similar size were sorted individually with a brush and subsequently kept for 24 h in temperature-controlled incubators (MIR-154, Panasonic, Japan; temperature fluctuation ± 0.3 °C, light intensity 170 µmol m−2 s−1). Temperatures at which individuals were acclimated corresponded to those used for the experiments (i.e. 6, 12, 18, 24, 30, 32, 34 and 36 °C, see section below). Additionally, the temperature was monitored inside each incubator with a temperature logger (DSL1922L iButttons, resolution 0.1 °C, Supplementary Fig. S1). Only active individuals (i.e. producing a displacement track on a thin layer of sediment)57–59 were chosen and subsequently imaged to assess the shell size parameter measurements (Olympus SZX16, Japan, TC capture software with a calibrated tool for the estimation of the maximum length and width of each individual) prior to each experiment.
Motion behaviour and recovery experiments
Active individuals were transferred into a 400 ml aquarium containing 25–30 ml of de-frozen sediment (i.e. ~ 1 cm thick) corresponding to their sampling site, free of moving animals with oxygenated overlaying natural seawater (33PSU; Supplementary Fig. S2). Eight temperatures (6, 12, 18, 24, 30, 32, 34, and 36 °C; see Supplementary Fig. S1 for temperature records) were tested. The ranges 6–30 °C and 32–36 °C were respectively considered as usual (i.e. temperature regularly experienced in the field) and extreme (i.e. temperature rarely or never reached so far in the field) temperatures in the intertidal mudflats located along the French side of the eastern English Channel. Fifteen experiments containing between 20 and 30 individuals were performed in temperature-controlled incubators (MIR-154, Panasonic, Japan, temperature fluctuation ± 0.3 °C, light intensity 170 µmol m−2 s−1) in April, May and June 2019 (Supplementary Table S1). Living foraminifera were randomly placed on the sediment surface and the displacement of each individual in and on the sediment was recorded using time-lapse photography (i.e. one image every 10 min during 24 h; Nikon V1 with a Nikkor 10–30 mm lens). Then, the images were analysed by using the software Fiji60. Such a method allowed us to visually follow each individual and extract the coordinates from each of the ~ 144 images combined by the computer program. The coordinates thereby gave the individual’s trajectory during the time of the experiment.
Additional recovery experiments were performed on one of each experiment carried out at 6 and 36 °C to assess specifically the resilience of H. germanica at extreme temperatures i.e. near the limit of their thermal range. To do so, one of each 24-h experiments carried out at 6 °C and 36 °C were pursued for extra 24-h by increasing or decreasing the temperature until 18 °C, respectively. Displacements were subsequently recorded every 10 min for 24 h. The mean distance travelled within 10 min was calculated with a 3-order simple moving average to reduce the influence of short-term fluctuations.
Motion-traits
A total of 713 active (i.e. moving) individuals was initially selected for the experiment. During the experiment, it was not possible to track all individuals (i) because some burrowed into the sediment up to a depth where their paths were not visible and/or (ii) because some paths crossed and consequently individual trajectories were lost. We therefore only kept individuals that exhibited visible tracks throughout the whole 24 h experiment. In total we followed the trajectories of 246 individuals.
Four motion traits were investigated following Seuront and Bouchet49 and Deldicq et al.48.
First, the level of activity (i.e. time allocated to locomotion by each individual) was estimated with the activity index Ai which is based on the ratio tmove and tactive as follows:
where tmove includes the total time taken by an individual to move from its initial to its final position, which thereby includes the time periods when individual remains inactive. In contrast, tactive only considers the time periods when an individual actually moves between its initial and final position.
The distance travelled by each individual between two images (i.e. 10 min) was assessed as follows:
where (xt,yt) and (xt+1, yt+1) are the coordinates between two successive images taken at times t and t + 10 min and the total distance travelled within 24 h was then calculated (D24) and normalized by the experiment duration to obtain velocity.
The complexity of the trajectory of each individual was assessed using fractal dimension analysis. Because the principles behind fractal theory, fractal analysis techniques and their applications to behavioural data, including foraminifera behaviour48, have all been described in detail elsewhere61–63, we only briefly describe hereafter the basic principles of the box-counting method, which is likely among the most widely applied and intuitive methods available to date to characterize the geometric complexity of movement paths. This method superimposes a regular grid of squares of length l on a path and counts the number of occupied squares, N(l). This procedure is repeated using different values of l. The surface occupied by a trajectory is then estimated using a series of boxes spanning a range of surfaces down to some small fraction of the entire space, typically the size of the organism considered. The number of occupied squares fundamentally increases with decreasing square size, and the presence of a fractal structure manifests itself by a power–law relationship of the form N(l) = k × l−D, where k is an empirical constant and D the fractal dimension. The fractal dimension D, estimated from the slope of the linear trend of the log–log plot of N(l) versus l, fundamentally measures the degree to which the trajectory fills the available space and is bounded between D = 1 for a line (i.e. the simplest instance of a trajectory) and D = 2 for a movement so complex that it actually fills the whole available space.
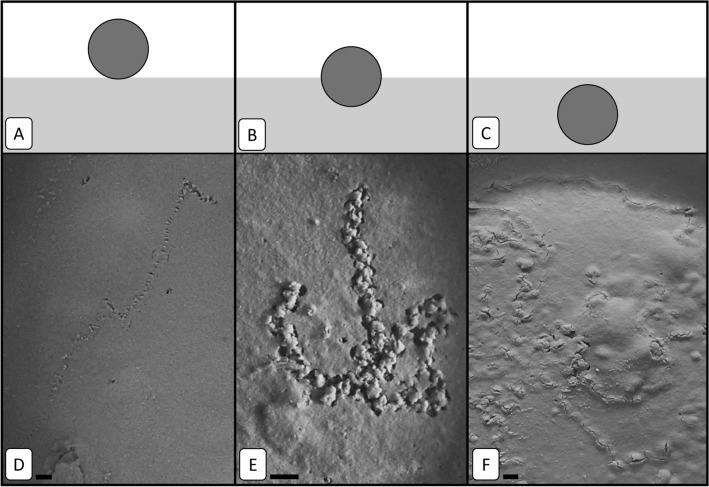
Following the method newly described in Deldicq et al.48, the vertical position of H. germanica in the sediment for every individual and picture was determined based on a classification with three depth categories. When part of the test remained visible at the surface and the width of the path was indistinguishable an individual was considered to be crawling on the sediment surface (Fig. 1A,D). When an individual was burrowing into the sediment, its position was divided into two categories: it was considered (i) as moving at the sediment–water interface when half of the test was visible (Fig. 1B,E) and (ii) as fully burrowed into the sediment when a swelling at the sediment surface was the only indication of the presence of a test in the sediment (Fig. 1C,F). The number of individuals was estimated for each position and each 10-min period during the time of the experiment.
Figure 1.
Schematic side-view representation of the vertical position (A–C) and top-view images of the sediment surface showing actual trajectories of foraminifera (D–F) related to the three vertical position categories, which can be taken by a foraminifera, i.e. surface (A,D) sediment–water interface, (B,E), and burrowed (C,F). Scale bars = 0.2 mm. From Deldicq et al.48.
Surface sediment reworking rate
To assess H. germanica contribution to surface sediment reworking, the test surface TSi (mm2) of each individual was estimated by measuring individual maximum length and width and assuming that the species has an ellipse-shape shell:
Since there was no significant difference in term of individual size between each set of experiment (Kruskal–Wallis test, p < 0.05), the mean test surface TS was calculated for each set of experiment and used for the calculation of the Individual Surface Sediment Reworking Rate, SSRRi (mm3 indiv−1 day−1):
where D24 is the total distance travelled (in mm) within 24h by each individual.
Oxygen consumption and production
Active individuals used for respiration measurements were acclimated overnight with artificial seawater (35 g of Red Sea salt per litter of MilliQ ultrapure water, and referred to as ASW hereafter) at the temperature corresponding to the experimental condition (i.e. 6, 12, 18, 24, 30 and 36 °C). Three sets of five active individuals (with homogenised shell length ranging from 340 to 420 µm, Kruskal–Wallis test, p < 0.05) were transferred to a 1-mm wide and 1-cm high glass microtube containing ASW for each chosen temperature (6, 12, 18, 24, 30 and 36 °C, Supplementary Table S2). Measurements within the microtube were carried out in a temperature-controlled water bath (Huber CC-K12, Germany) to estimate oxygen fluxes at 6, 12, 18, 24 and 30 °C. To this end, a 50-µm Clark-type oxygen microelectrode (Unisense, Denmark) was 2-point calibrated64 using oxygen-saturated seawater (considering O2 saturation at 35 PSU and at the chosen temperatures) and an anoxic solution (20 g of sodium ascorbate per litter of 0.1 mol l−1 NaOH solution). The electrode was then placed in the measurement microtube about 300 µm above the 5 individuals. Oxygen profiles were realized with a 50-µm vertical resolution to determine the oxygen consumption gradient (dC/dz, in pmol cm−4) in the first millimetre above the foraminifera38,59.
Oxygen consumption gradients were first measured in the dark to estimate foraminiferal respiration and then oxygen production gradients were estimated under homogeneous light conditions to determine net photosynthesis (photosynthetically active radiation 170 µmol photon m−2 s−1; SA-190 quantum sensor, LI-COR, USA, provided by two arrays of LEDs (YN-160 III, Yongnuo, China). Given that previous studies show that ASW alone does not produce nor consume oxygen59,65,66, no further blank controls were performed for this experiment and the measured oxygen production of consumption was assumed to originate from the foraminifera themselves.
Respiration and photosynthesis calculations
Oxygen fluxes J (pmolO2 cm−1 s−1) were calculated using Fick’s first law of free diffusion, as follows:
where D is the free diffusion coefficient for oxygen in seawater at a given temperature49 and dC/dz the oxygen gradient 1 mm above the foraminifera in the microtube. Oxygen solubility and free diffusion coefficients (D) were selected from tables compiled by Ramsing and Gundersen67 (Unisense, Denmark). All respiration measurements were performed in the dark in a temperature-controlled water bath (Huber CC-K12, Germany).
Individual respiration rate R (pmolO2 indiv−1 h−1) and net photosynthesis rate NP (pmolO2 indiv−1 h−1) were subsequently calculated as:
where S is the microtube inner section (S = 7.9 × 10–3 cm2), n the number of individuals (i.e. n = 5) and J the fluxes estimated under dark and light conditions, respectively.
Gross photosynthesis (GP) was estimated from respiration (R) and net photosynthesis (NP) rates as follow:
In addition, to estimate the influence of temperature on H. germanica physiological rate, Q10 was calculated within the ranges 6–24 °C and 24–36 °C. The Q10 values quantify changes in the metabolic rate for a 10 °C increase:
where R(T1) and R(T2) (nmolO2 indiv−1 h−1) are the metabolic rate (i.e. respiration or gross photosynthesis) respectively measured at extreme tested temperatures (i.e. 6 and 36 °C) and 24 °C.
To estimate the daily oxygen budget, i.e. the balance between oxygen consumption (respiration) and production (photosynthesis) within a day, we calculated the amount of oxygen produced in a day for a 12-h light exposure duration (to account for diurnal cycles) and 6-h light exposure duration (to account for both diurnal and tidal cycles, assuming that coastal seawater turbidity is so high that no light is reaching the sediment during immersion). Such calculations were done by pondering net photosynthesis with respiration rates with a 0.5 and 0.75 ratio for 12-h and 6-h light exposure, respectively.
Data analysis
Because behavioural parameters were non-normally distributed (Shapiro–Wilk test, p < 0.05). Kruskal–Wallis tests were conducted for activity and surface sediment reworking rate in order to discriminate temperatures. In case of significant differences a Dunn post-hoc test was applied for two-sample comparisons68. In turn, metabolic parameters rate were normally distributed (Shapiro–Wilk test, p > 0.05) and an analysis of variance (ANOVA) was conducted on respiration rates and photosynthesis followed by a two-sample comparison (Tukey test) to identify distinct groups of measurement68. The presence of significant differences between fractal dimensions was assessed using an analysis of covariance. All statistical analyses were performed using R.3.5.2. software69.
Results
Motion traits
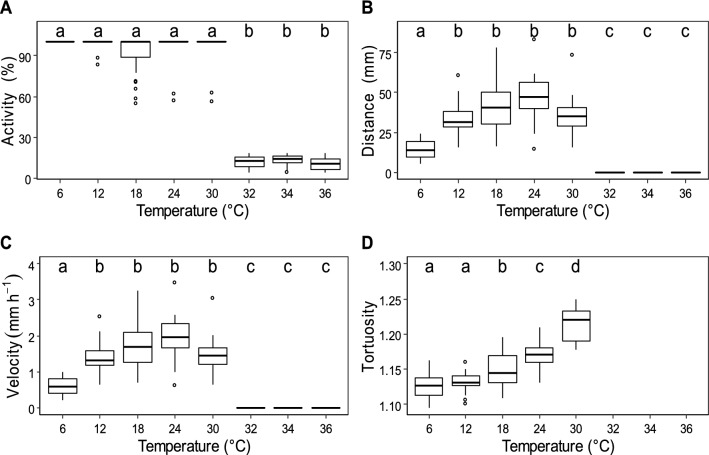
Individuals were most active between 6 and 30 °C, spending more than 90% of their time moving into the sediment (Fig. 2A). Individuals exposed to extremely high temperatures (i.e. 32–36 °C) significantly decreased their activity from circa 90% to ca. 15% (Dunn test, p < 0.01; Fig. 2A).
Figure 2.
The influence of temperature on (A) the activity (B) the distance travelled (over 24 h), (C) the velocity and (D) the fractal dimension of H. germanica. The box represents the first, second and third quartiles and the whiskers extend to 1.5 times the interquartile range; values outside this range are represented by open circles. Number of replicates are 30, 23, 30, 25, 15, 69, 28 and 26 for 6, 12, 18, 24, 30, 32, 34 and 36 °C respectively. Due to the absence of motion it was impossible to estimate fractal dimension at 32, 34 and 36 °C. Letters above the boxes (‘a’, ‘b’, ‘c’ and ‘d’) identify significant different groups (Dunn test, p < 0.05).
The highest velocities and the longest distances travelled during the 24-h experiment were observed in the range 12–30 °C (Fig. 2B,C). The longest trajectories were measured at 24 °C with a mean travelled distance of 46.9 mm (Fig. 2B,C). Beyond 32 °C, individuals started burrowing into the sediment at the beginning of the experiment but there was no subsequent displacement throughout the rest of the experiment (Fig. 2B,C). More specifically, the travelled distance of H. germanica trajectories were discriminated into several groups, i.e. Dt(36 °C) = Dt(34 °C) = Dt(32 °C) < Dt(6 °C) < Dt(12 °C) = Dt(18 °C) = Dt(24 °C) = Dt(30 °C) (Dunn test, p < 0.01).
Since there were no displacements between 32 and 36 °C, the complexity of movement (i.e. fractal analysis) was not assessed for these temperatures. However, all trajectories considered at cooler temperature (i.e. 6, 12, 18, 24, 30 °C) were characterized by a fractal property, i.e. a highly significantly linear behaviour of N(l) vs. l in log–log plots (r2 > 0.99, p < 0.01). The fractal dimension D ranged from 1.09 to 1.22 and significantly differed between treatments (Fig. 2D; Kruskal–Wallis test, p < 0.01). The trajectories of H. germanica was subsequently discriminated into several homogeneous groups, i.e. D6 °C = D12 °C < D18 °C < D24 °C < D30 °C, which overall indicated an increase in movement complexity with rising temperature.
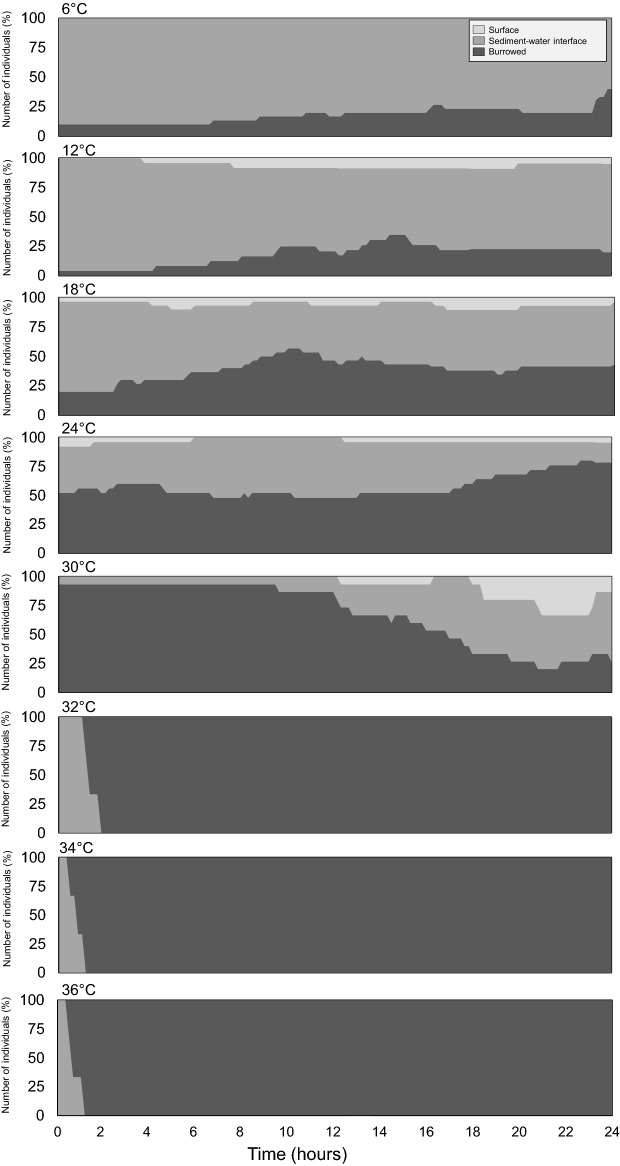
For intermediate temperatures (18, 24, 30 °C), individuals were alternatively observed at the sediment–water interface or burrowed in the sediment during the experiment (Fig. 3). At the hottest temperatures e.g. 32–36 °C, individuals moved rapidly from the surface down to the sub-surface and stayed buried during the remaining time of the experiment. In contrast, they were observed at the sediment–water interface between 6 and 12 °C (Fig. 3).
Figure 3.
Temporal changes in the vertical position of H. germanica for each tested temperature. Number of individuals are shown in Supplementary Table S1.
Recovery experiment
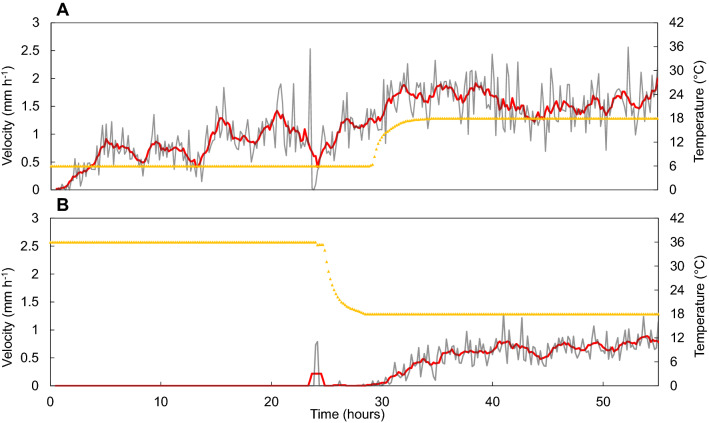
After a 24-h exposure to a temperature of 6 °C, individuals exposed to 18 °C increased their velocity from an average of 0.8 mm h−1 in the first 24 h of the experiment up to approximately 1.6 mm h−1 in average over the 30–55 h time interval (Fig. 4A). This increase started as soon as the temperature rose in the experiment container (Fig. 4A). Noticeably, the recovered velocity at 18 °C (1.6 mm h−1) was close to the value observed for individuals solely exposed to 18 °C (1.74 mm h−1, Fig. 2C).
Figure 4.
Temporal changes in the mean velocity of 9 H. germanica individuals previously exposed at (A) 6 °C then 18 °C and (B) 36 °C then 18 °C. The grey line is the instantaneous velocity and the red line is the 3-order simple moving average of the velocity. Yellow triangles correspond to water-temperature changes through time.
At 36 °C, the distance travelled was nearly nil during the first day of the experiment. Individuals exposed to 36 °C for a 24-h period started to move only 4 h after the decrease in temperature from 36 to 18 °C (Fig. 4B). The recovered mean velocity at 18 °C (0.57 mm h−1) never reached the mean velocity where individuals were solely exposed to a thermal regime of 18 °C (1.7 mm h−1; Fig. 2C).
Respiration and photosynthesis
Oxygen respiration rates did not significantly differ between 6 and 12 °C (Tukey test, p < 0.01). However, respiration rates were significantly higher for warmer temperatures (Tukey test, p < 0.01). Hence, oxygen consumption increased from 24.5 pmolO2 indiv−1 h−1 (12 °C) to 55.7 pmolO2 indiv−1 h−1 (24 °C), then decreased down to 48.5 pmolO2 indiv−1 h−1 at 36 °C (Fig. 5A). Gross photosynthesis also increased up to 77 pmolO2 indiv−1 h−1 when temperature warmed from 6 to 24 °C. A significant diminution was subsequently observed from 24 °C to 30 °C (Tukey test, p < 0.01; Fig. 5B).
Figure 5.
Mean values of (A) respiration and (B) gross photosynthesis (pmolO2 indiv−1 h−1) of H. germanica under different thermal regime in 3 replicate measurements. The error bars are the standard errors of the mean. Letters ‘a’ and ‘b’ identify significant different groups (Tukey test, p < 0.05).
The increase in respiration and gross photosynthesis between 6 and 24 °C can be described with Q10 = 1.75 and Q10 = 1.22, respectively. However, the influence of the warmest temperatures on respiration decrease (Q10 = 0.89) was lower than for gross photosynthesis decrease (Q10 = 0.32) over the 24–36 °C range.
Surface sediment reworking rate and oxygen budget
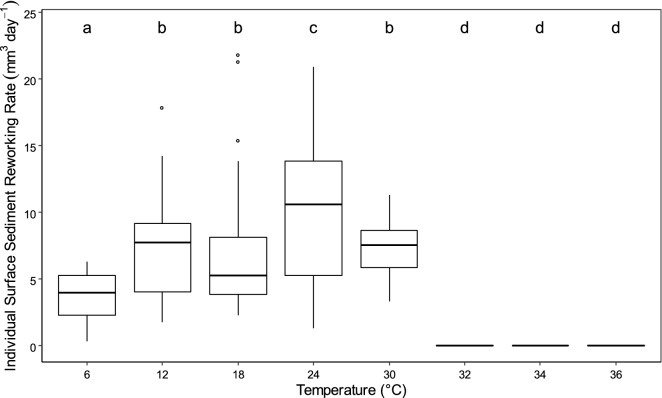
Due to low travelled distances, there was no surface sediment reworking beyond 32 °C. In contrast, for lower temperatures, individuals could rework between 3.7 and 10.1 mm3 indiv−1 day−1 (respectively 6 and 24 °C; Fig. 6). Statistical analyses showed significant differences in the SSRRi between temperatures (Kruskal–Wallis test, p < 0.05) and four groups were further identified as SSRRi(32 °C) = SSRRi(34 °C) = SSRRi(36 °C) < SSRRi(6 °C) < SSRRi(12 °C) = SSRRi(18 °C) = SSRRi(30 °C) < SSRRi(24 °C). Q10 of surface sediment reworking in the thermal range 6–24 °C was 1.75.
Figure 6.
Individual surface sediment reworking (mm3 indiv−1 day−1) of H. germanica under different thermal regime. Letters above plots (‘a’, ‘b’, ‘c’) indicate significant differences among measurements (Dunn test, p < 0.05). The box represents the first, second and third quartiles and the whiskers extend to 1.5 times the interquartile range; values outside this range are represented by open circles.
For a 6-h light exposure, daily oxygen budget was negative at all temperatures and significantly decreased above 12 °C (Fig. 7, Tukey test p < 0.05). When considering a 12 h light exposure cycle, average daily oxygen productive was positive at 6 and 12 °C and gradually decreased to reach negative values within the thermal range 18–36 °C.
Figure 7.
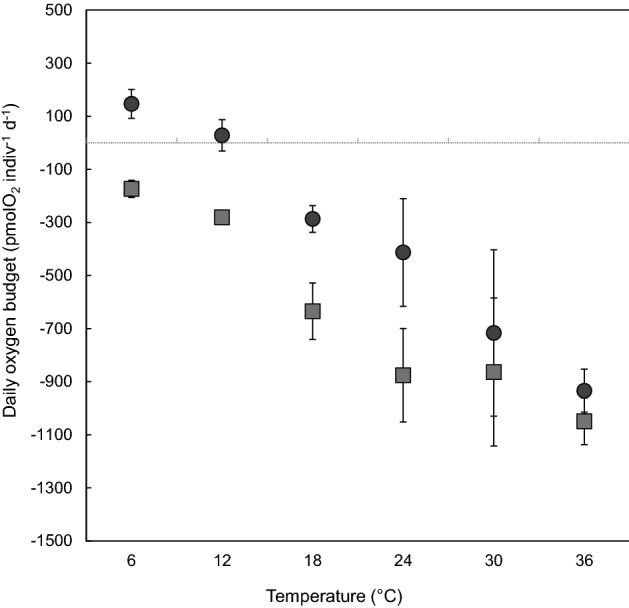
Daily oxygen budget of H. germanica (pmolO2 indiv−1 day−1) under 12 h (black dots) and 6 h (grey squares) light exposure and thermal regimes. The error bars are the standard errors calculated on the 3 replicates at each temperature.
Discussion
The resilience of H. germanica motion behaviour to temperature fluctuations reveals plasticity to seasonal thermal variations
Haynesina germanica was more active in the range 6–30 °C, with the highest velocities and distances travelled being in the range 12–24 °C. Specifically, individuals were 1.4 times faster at 24 °C than at 12 °C (Fig. 2C). This is consistent with previous measurements of locomotion speed on glass petri dish, velocity being nearly twice lower at 12 °C (~ 2 mm h−1)57 than at 22 °C (~ 4 mm h−1)70. This observation confirms that cold temperatures may reduce the activity of temperate foraminifera42. In our experiments, H. germanica explored actively its environment from 6 to 30 °C by consistently moving vertically and horizontally into the sediment between 22 and 24 h. However, specimens remained only active between 3 and 4 h in the sediment at temperatures above 30 °C with velocities and travelled distances being nil above 32 °C. Increasing fractal dimensions in the range 12–30 °C were also indicative of more intensive foraging behaviour consistent with the more complex trajectories and more intensive foraging behaviour exhibited by unstressed organisms49,71–73. Note that these results may also indicate that foraging behaviour may differ at the sediment–water interface and within the sediment. The observed adaptive responses to a range of temperatures typically encountered in temperate intertidal mudflats (i.e. 6–30 °C19,74,75) as well as more extreme and rare temperature (36 °C) suggest that H. germanica behavioural flexibility specifically evolved to optimize the timing of their response to thermal stress at temporal scales typical of the tidal alternance of immersion and emersion. In fact, many studies have shown that intertidal invertebrates often live close to the upper limit of their thermal tolerance windows24,76–78. Our findings therefore suggest that irrespective of species physiological and behavioural plasticity, unusual temperatures such as those caused by heatwaves may affect species performance and perhaps survival. After being exposed to extremely hot temperatures, H. germanica was nevertheless able to quickly recover. After bringing them back to 18 °C, all individual exposed to cold and hot temperatures (6 °C and 36 °C), started exploring all potential habitats i.e. both surface and deeper sediment, suggesting that the protist can exhibit a thermotactic behaviour.
Thermal control of the position of H. germanica in the sediment
At temperatures corresponding to autumn and winter (i.e. 6–12 °C), H. germanica preferably remained at the sediment–water interface. At intermediate temperatures (18 and 24 °C) corresponding to spring and summer conditions, individuals alternatively moved in and on the sediment during the whole experiment with a proportion of burrowed individuals increasing with temperatures. For instance, at 30 °C more than 90% of the individuals were observed below the sediment–water interface. Habitat selection as a function of environmental conditions has also been reported in a wide range of organisms such as crabs, worms and gastropods28,79,80. Organisms inhabiting intertidal mudflats move toward a more favourable habitat following the vertical thermal gradient they experience in soft sediments81,82. Under low temperatures (here 6, 12 °C), basking behaviour, i.e. a common thermoregulatory behaviour observed in many ectotherms, might allow species to live in the limited-oxygenated zone to draw benefit from solar heating83–87. In contrast, burrowing deep into the sediment may provide cooler environment and leads to a decrease in cell temperature79,88,89. Considering that the thin sediment layer used in our experiments is unlikely to generate a thermal gradient, our results strongly suggest that benthic foraminifera, in particular H. germanica, may have an intrinsically basking- and burrowing behaviour to regulate their inner body temperature.
Effect of temperature on H. germanica metabolism: an adaptation to variable thermal forcing
In our experiments, highest respiration and photosynthesis rates were recorded between 18 and 24 °C. Outside this range, H. germanica respiration rates strongly decreased at cooler temperatures (6, 12 °C) while there was a decrease in gross photosynthesis at 30 °C. Metabolic change is a common response to temperature in ectothermic species90, including benthic and planktonic foraminifera42,91. Instability in metabolism affects macro-invertebrate species performance such as feeding, mating and locomotion92–95, which is consistent with our observations on H. germanica motion-behaviour, where travelled distances, and hence velocities, consistently decreased at cooler and warmer temperatures. Our results open a new perspective on our understanding of the physiology of H. germanica. In our experiments, the Q10 values reported in the range 6–24 °C for respiration (Q10 = 1.75) and photosynthesis (Q10 = 1.22) suggest (i) a maximum performance level and a relatively low thermal dependence of respiration and (ii) that photosynthesis is not affected by temperature inside this thermal range. Low Q10 values have been interpreted as characteristic of the optimal temperature range of a species in its natural habitat96. Noticeably, our Q10 calculated on respiration is substantially lower than previous direct Q10 estimates for planktonic foraminifera91 (Q10 = 3.18) and for the intertidal foraminifera Ammonia beccarii tepida42 (Q10 = 3.2 in the north-eastern regions of the Pacific) but in the same order of magnitude as Arcachon Basin mudflats for Ammonia tepida and Haynesina germanica58 (Q10 = 1.4 and Q10 = 1.8 respectively). Compared to other meiobenthic species from the English Channel mudflats, H. germanica respiration Q10 in the 6–24 °C range is lower than those reported in the 0–20 °C range in the sabellid polychaete Manayunkia aestuarina (Q10 = 2.19) and in the copepod Tachidius discipes (Q10 = 2.17)97. Our findings suggest that the protist is particularly well adapted to the frequently-occurring thermal range 6–24 °C in intertidal soft-sediments in temperate environments. Similarly, a vast majority of intertidal macro-invertebrates can easily tolerate thermal variation with no adverse effects on their physiological rates76,98, like on metabolic rates of fiddler crabs99.
Fast behavioural and metabolic responses of H. germanica to extreme temperatures: a key for survival in an era of climate change?
At high temperatures (32, 34 and 36 °C), H. germanica individuals immediately burrowed in the sediment and then remained inactive throughout the rest of the experiment. These two successive behaviours (i.e. burrowing then inactivity) are typically observed in macro-invertebrate intertidal species exposed to temperatures outside their tolerance thermal range79,89,100. Note that this strategy may also be detrimental given the low oxygen penetration depth and the intense hydrogen sulphide production in coastal marine sediments101,102, which are known to hamper benthic foraminifera103–105. Noticeably, the lethal limit of H. germanica was never reached since after being inactive for 24-h at 36 °C, all individuals started to move (though they never recovered their baseline behaviour and activity during the time of the experiment) when temperature decreased at 18 °C. The distance travelled at 18 °C by individuals previously exposed at 36 °C was twice lower than the distance travelled by individuals previously exposed to 6 °C, suggesting that although not lethal, the 24 h spent by H. germanica individuals at 36 °C had long-lasting harmful consequences. In the literature, temperature LT50 (i.e. the temperature for which 50% of individuals die) for intertidal foraminifera typically ranged from 37.5 to 45 °C42. Exposure to high temperatures have important adverse effects such as production of reactive oxygen species and DNA degradation26,76. These is confirmed by the metabolic Q10 value, which dropped below 1 in the range 24–36 °C (respectively Q10 = 0.89 and Q10 = 0.32 for respiration and gross photosynthesis), suggesting that biological functions are altered in H. germanica above 24 °C. Our respiration Q10 is similar to the one of the intertidal nematode Pellioditis marina from the south-western regions of the Netherlands106 (Q10 = 0.76 in the range 25–35 °C), although thermal dependence is much higher in Ammonia beccarii tepida from the eastern Pacific (Q10 = 0.17 in the 34–45 °C range)42 suggesting that H. germanica respiration might also be inhibited beyond 36 °C. Photosynthetic activity of H. germanica is more affected than respiration, a result that has been found in other symbiont-bearing benthic foraminifera50,51,107. Our results therefore suggest that H. germanica may not benefit from autotrophic nutrition since sequestered chloroplast photosynthetic activity was strongly inhibited beyond 24 °C. Further analyses are needed to identify whether the plastids could recover after being exposed to high temperatures and whether individuals maintain them in their cell or use them as a source of food.
Consequences of marine heatwaves on H. germanica contribution to benthic ecosystem functioning and services
The shifts in metabolism and motion behaviour observed in this study provide evidence that heatwaves may alter the contribution of H. germanica to benthic ecosystem functioning. Specifically, sediment reworking directly depends on motion-behaviour (e.g. crawling, burrowing), which leads to sediment particle displacements36,108. The Q10 value reported in the range 6–24 °C for surface sediment reworking rate (Q10 = 1.75) indicated a thermal dependence in the range 6–24 °C. Hence, H. germanica can rework a larger amount of sediment within the range 18–30 °C. In addition, individuals intensively explored the environment by moving vertically and horizontally into the sediment. This diversity of movements would most likely lead to more intense sediment mixing since particles are carried out in both directions. In contrast, at lower temperatures, H. germanica remained in the upper millimetres of sediment inducing a space-scale limited contribution to surface sediment reworking. The intertidal polychaete species Neanthes virens also showed a lower bioturbation rate at 6 °C, which limits sediment transport and dissolved fluxes109. At temperatures > 32 °C, H. germanica surface sediment reworking activity fully ceased. Such temperatures can be reached during summer in temperate intertidal mudflats18,19,110. Heatwaves may therefore limit H. germanica contribution to surface sediment reworking. Although heatwaves have limited duration, they actually continue to increase in frequency and intensity3. The repetition of such extreme events over successive periods has dramatic consequences on species’ survival and associated ecosystem functions1,6,14,17. As previously evidenced for macro-invertebrates inhabiting the Eastern English Channel coastlines17, we suggest that the thermal tolerance of H. germanica and therefore its contribution to ecosystem functions could be altered by the successive exposition to extreme temperatures. It would be interesting to perform successive thermal exposures to high temperatures (i.e. chronic stress) to further investigate the ability of H. germanica to acclimate and assess its resistance and resilience to several expositions to extreme temperatures.
Benthic foraminifera may also affect benthic fluxes directly by consuming or producing oxygen. Our results suggest that foraminiferal oxygen uptake increases in the 6–24 °C range and that high temperatures may most likely limit the contribution of H. germanica to oxygen fluxes. Noticeably, oxygen production by photosynthesis, and to a lesser extent oxygen consumption, decreased at 30 °C and above. It further co-occurred with individuals reduced-surface sediment reworking activity during heatwaves. Our daily oxygen budget calculations under realistic light exposure revealed that H. germanica oxygen production was closed to 0 or negative at all measured temperatures. Specimens from Atlantic mudflats showed similar negative oxygen production under 12 h light exposure (i.e. − 283 at 13 °C and − 327 pmolO2 indiv−1 day−1 at 18 °C; recalculated respectively from Jauffrais et al.111 and Cesbron et al.58). Within European waters kleptoplastic intertidal species, only Cribroelphidium williamsoni showed positive oxygen production budget under a 12 h dark–light cycle (5165 pmolO2 indiv−1 day−1; recalculated from Jauffrais et al.112). This result confirms that H. germanica has a minimal impact on benthic oxygen production (up to 0.2%).
Conclusion
Global climate change has now unambiguous effects on many marine biological and ecological systems of the world. Among observed consequences of global climate change, marine heatwaves have become more frequent and prominent. In this context, we have examined some biological responses of the temperate foraminifera H. germanica to thermal changes in soft-sediment habitats over a short period. Although some thermal plasticity is observed for temperatures commonly observed in the field, we show that a hyper-thermic stresses typical of a marine heatwave strongly affects the behaviour and the metabolism of this protist, triggering responses that were not entirely reversed during the time of the experiments. Our results also suggest that these biological alterations have consequences on the species contribution to surface sediment reworking.
Supplementary Information
Acknowledgements
The authors thank the Région Hauts-de-France, the Ministère de l’Enseignement Supérieur et de la Recherche and the European fund for regional economic development for their financial support through the attribution of the CPER research project CLIMIBIO. Noémie Deldicq PhD fellowship is funded by the Ministère de l’Enseignement Supérieur et de la Recherche. Dewi Langlet Post-doctoral fellowship is supported by the STARS research project COFFEE of the Région Hauts-de-France and the CPER research project CLIMIBIO. The authors are grateful to E. Metzger, F. Mermillod-Blondin and E. Armynot du Châtelet members of N. Deldicq’s PhD thesis survey committee for suggesting to test the effect of temperature on benthic foraminifera. The authors would like to thank the three anonymous reviewers and the editor for their comments that helped to improve the manuscript.
Author contributions
N.D. and V.M.P.B. conceived the idea of this study and V.M.P.B. obtained financial support. N.D., D.L. and V.M.P.B. provided significant input on experimental design. N.D. and C.D. performed the behavioural experiments and analysed the images. D.L. and C.D. performed the respiration experiments. N.D., D.L. and L.S. analysed the data and performed the statistical analyses. N.D., D.L., L.S. and V.M.P.B. contributed to the interpretation of the data and the discussion of the results presented in the manuscript. N.D. wrote the draft manuscript. N.D., D.L., C.D., G.B., L.S. and V.M.P.B. revised the article critically for intellectual content and gave final approval of the submitted version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83311-z.
References
- 1.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353:169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 2.Oliver ECJ, et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018;9:1324. doi: 10.1038/s41467-018-03732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver ECJ, et al. Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Mar. Sci. 2019;6:734. doi: 10.3389/fmars.2019.00734. [DOI] [Google Scholar]
- 4.Bond NA, Cronin MF, Freeland H, Mantua N. Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys. Res. Lett. 2015;42:3414–3420. doi: 10.1002/2015GL063306. [DOI] [Google Scholar]
- 5.Smale DA, Wernberg T, Vanderklift MA. Regional-scale variability in the response of benthic macroinvertebrate assemblages to a marine heatwave. Mar. Ecol. Prog. Ser. 2017;568:17–30. doi: 10.3354/meps12080. [DOI] [Google Scholar]
- 6.Benthuysen JA, Oliver ECJ, Feng M, Marshall AG. Extreme marine warming across tropical Australia during austral summer 2015–2016. J. Geophys. Res. Oceans. 2018;123:1301–1326. doi: 10.1002/2017JC013326. [DOI] [Google Scholar]
- 7.Della-Marta P, Haylock M, Luterbacher J, Wanner H. Doubled length of western European summer heat waves since 1880. J. Geophys. Res. 2007;112:D15103. doi: 10.1029/2007JD008510. [DOI] [Google Scholar]
- 8.Oswald E, Rood R. A trend analysis of the 1930–2010 extreme heat events in the Continental United States. J. Appl. Meteorol. Climatol. 2014;53:565–582. doi: 10.1175/JAMC-D-13-071.1. [DOI] [Google Scholar]
- 9.Perkins S, Alexander LV. On the measurement of heat waves. J. Clim. 2013;26:4500–4517. doi: 10.1175/JCLI-D-12-00383.1. [DOI] [Google Scholar]
- 10.Lima FP, Wethey DS. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 2012;3:704. doi: 10.1038/ncomms1713. [DOI] [PubMed] [Google Scholar]
- 11.Hobday AJ, et al. Categorizing and naming marine heatwaves. Oceanography. 2018;31:162. doi: 10.5670/oceanog.2018.205. [DOI] [Google Scholar]
- 12.Hobday AJ, et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016;141:227–238. doi: 10.1016/j.pocean.2015.12.014. [DOI] [Google Scholar]
- 13.Harley CDG, et al. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 14.Garrabou J, et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Change Biol. 2009;15:1090–1103. doi: 10.1111/j.1365-2486.2008.01823.x. [DOI] [Google Scholar]
- 15.Caputi N, et al. Management adaptation of invertebrate fisheries to an extreme marine heat wave event at a global warming hot spot. Ecol. Evol. 2016;6:3583–3593. doi: 10.1002/ece3.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caputi N, et al. Factors affecting the recovery of invertebrates stocks from the 2011 Western Australian extreme marine heatwave. Front. Mar. Sci. 2019;6:484. doi: 10.3389/fmars.2019.00484. [DOI] [Google Scholar]
- 17.Seuront L, Nicastro KR, Zardi GI, Goberville E. Decreased thermal tolerance under recurrent heat stress conditions explains summer mass mortality of the blue mussel Mytilus edulis. Sci. Rep. 2019;9:17498. doi: 10.1038/s41598-019-53580-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy EAK, Reidenbach MA. Oxygen transport in periodically ventilated polychaete burrows. Mar. Biol. 2016;163:208. doi: 10.1007/s00227-016-2983-y. [DOI] [Google Scholar]
- 19.Goulletquer, P. et al. Summer mortality of the Pacific cupped oyster Crassostrea gigas in the Bay of Marennes-Oléron (France). In Mariculture Committee CM 1998/CC: 14 (1998).
- 20.Li M, Lei Y, Li T, Jian Z. Impact of temperature on intertidal foraminifera: Results from laboratory culture experiment. J. Exp. Mar. Biol. Ecol. 2019;520:151224. doi: 10.1016/j.jembe.2019.151224. [DOI] [Google Scholar]
- 21.Pörtner HO. Climate change and temperature-dependent biogeography: Oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- 22.Pörtner HO. Integrating climate-related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 2012;470:273–290. doi: 10.3354/meps10123. [DOI] [Google Scholar]
- 23.Straub SC, et al. Resistance, extinction, and everything in between—The diverse responses of seaweeds to marine heatwaves. Front. Mar. Sci. 2019;6:763. doi: 10.3389/fmars.2019.00763. [DOI] [Google Scholar]
- 24.Stillman JH, Somero GN. Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): Correlation of physiology, biochemistry and morphology with vertical distribution. J. Exp. Biol. 1996;199:1845–1855. doi: 10.1242/jeb.199.8.1845. [DOI] [PubMed] [Google Scholar]
- 25.Joint I, Smale DA. Marine heatwaves and optimal temperatures for microbial assemblage activity. FEMS Microbiol. Ecol. 2017;93:243. doi: 10.1093/femsec/fiw243. [DOI] [PubMed] [Google Scholar]
- 26.Pörtner HO, Farrell AP. Physiology and climate change. Nature. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, et al. Effects of seawater pH and temperature on foraging behavior of the Japanese stone crab Charybdis japonica. Mar. Pollut. Bull. 2017;120:99–108. doi: 10.1016/j.marpolbul.2017.04.053. [DOI] [PubMed] [Google Scholar]
- 28.da Vianna BS, Miyai CA, Augusto A, Costa TM. Effects of temperature increase on the physiology and behavior of fiddler crabs. Physiol. Behav. 2020;215:112765. doi: 10.1016/j.physbeh.2019.112765. [DOI] [PubMed] [Google Scholar]
- 29.François F, Poggiale J-C, Durbec J-P, Stora G. A new approach for the modelling of sediment reworking induced by a macrobenthic community. Acta. Biotheor. 1997;45:295–319. doi: 10.1023/A:1000636109604. [DOI] [Google Scholar]
- 30.Kristensen E, et al. What is bioturbation? the need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012;446:285–302. doi: 10.3354/meps09506. [DOI] [Google Scholar]
- 31.Piot A, Nozais C, Archambault P. Meiofauna affect the macrobenthic biodiversity—Ecosystem functioning relationship. Oikos. 2013;123:1–11. [Google Scholar]
- 32.Bonaglia S, et al. Meiofauna improve oxygenation and accelerate sulfide removal in the seasonally hypoxic seabed. Mar. Environ. Res. 2020;159:104968. doi: 10.1016/j.marenvres.2020.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonaglia S, Nascimento FJA, Bartoli M, Klawonn I, Brüchert V. Meiofauna increases bacterial denitrification in marine sediments. Nat. Commun. 2014;5:5133. doi: 10.1038/ncomms6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mermillod-Blondin F, Rosenberg R. Ecosystem engineering: The impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat. Sci. 2006;68:434–442. doi: 10.1007/s00027-006-0858-x. [DOI] [Google Scholar]
- 35.Kristensen E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008;59:30–43. doi: 10.1016/j.seares.2007.05.004. [DOI] [Google Scholar]
- 36.Pascal L, Maire O, Deflandre B, Romero-Ramirez A, Grémare A. Linking behaviours, sediment reworking, bioirrigation and oxygen dynamics in a soft-bottom ecosystem engineer: The mud shrimp Upogebia pusilla (Petagna 1792) J. Exp. Mar. Biol. Ecol. 2019;516:67–78. doi: 10.1016/j.jembe.2019.05.007. [DOI] [Google Scholar]
- 37.Risgaard-Petersen N, et al. Evidence for complete denitrification in a benthic foraminifer. Nature. 2006;443:93–96. doi: 10.1038/nature05070. [DOI] [PubMed] [Google Scholar]
- 38.Høgslund S, Revsbech NP, Cedhagen T, Nielsen LP, Gallardo VA. Denitrification, nitrate turnover, and aerobic respiration by benthic foraminiferans in the oxygen minimum zone off Chile. J. Exp. Mar. Biol. Ecol. 2008;359:85–91. doi: 10.1016/j.jembe.2008.02.015. [DOI] [Google Scholar]
- 39.Pike J, Bernhard JM, Moreton S, Butler I. Microbiorrigation of marine sediments in dysoxic environments: Implication for early sediment fabric formation and diagenetic processes. Geology. 2001;29:923–926. doi: 10.1130/0091-7613(2001)029<0923:MOMSID>2.0.CO;2. [DOI] [Google Scholar]
- 40.Woulds C, et al. Oxygen as a control on seafloor biological communities and their roles in sedimentary carbon cycling. Limnol. Oceanogr. 2007;52:1698–1709. doi: 10.4319/lo.2007.52.4.1698. [DOI] [Google Scholar]
- 41.Bernhard JM, Mollo-Christensen E, Eisenkolb N, Starczak VR. Tolerance of allogromid Foraminifera to severaly elevated carbon dioxide concentrations: Implications to future ecosystem functioning and paleoceanographic interpretations. Glob. Planet. Change. 2009;65:107–114. doi: 10.1016/j.gloplacha.2008.10.013. [DOI] [Google Scholar]
- 42.Bradshaw J. Laboratory experiments on the ecology of foraminifera. Contrib. Cushman Found. Foramin. Res. 1961;12:87–106. [Google Scholar]
- 43.Pascal P-Y, Dupuy C, Richard P, Niquil N. Bacterivory in the common foraminifer Ammonia tepida: Isotope tracer experiment and the controlling factors. J. Exp. Mar. Biol. Ecol. 2008;359:55–61. doi: 10.1016/j.jembe.2008.02.018. [DOI] [Google Scholar]
- 44.Wukovits J, Enge AJ, Wanek W, Watzka M, Heinz P. Increased temperature causes different carbon and nitrogen processing patterns in two common intertidal foraminifera (Ammonia tepida and Haynesina germanica) Biogeosciences. 2017;14:2815–2829. doi: 10.5194/bg-14-2815-2017. [DOI] [Google Scholar]
- 45.Schmidt C, Heinz P, Kucera M, Uthicke S. Temperature-induced stress leads to bleaching in larger benthic foraminifera hosting endosymbiotic diatoms. Limnol. Oceanogr. 2011;56:1587–1602. doi: 10.4319/lo.2011.56.5.1587. [DOI] [Google Scholar]
- 46.Stuhr M, et al. Variable thermal stress tolerance of the reef-associated symbiont-bearing foraminifera Amphistegina linked to differences in symbiont type. Coral Reefs. 2018;37:811–824. doi: 10.1007/s00338-018-1707-9. [DOI] [Google Scholar]
- 47.Gross O. Influence of temperature, oxygen and food availability on the migrational activity of bathyal benthic foraminifera: Evidence by microcosm experiments. Hydrobiologia. 2000;426:123–137. doi: 10.1023/A:1003930831220. [DOI] [Google Scholar]
- 48.Deldicq N, Seuront L, Langlet D, Bouchet V. Assessing behavioural traits of benthic foraminifera: Implications for sediment mixing. Mar. Ecol. Prog. Ser. 2020;643:21–31. doi: 10.3354/meps13334. [DOI] [Google Scholar]
- 49.Seuront L, Bouchet VMP. The devil lies in details: New insights into the behavioural ecology of intertidal foraminifera. J. Foramin. Res. 2015;45:390–401. doi: 10.2113/gsjfr.45.4.390. [DOI] [Google Scholar]
- 50.van Dam JW, Negri AP, Mueller JF, Altenburger R, Uthicke S. Additive pressures of elevated sea surface temperatures and herbicides on symbiont-bearing foraminifera. PLoS ONE. 2012;7:e33900. doi: 10.1371/journal.pone.0033900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinutok S, Hill R, Kühl M, Doblin MA, Ralph PJ. Ocean acidification and warming alter photosynthesis and calcification of the symbiont-bearing foraminifera Marginopora vertebralis. Mar. Biol. 2014;161:2143–2154. doi: 10.1007/s00227-014-2494-7. [DOI] [Google Scholar]
- 52.Alve E, Murray JW. Temporal variability in vertical distributions of live (stained) intertidal foraminifera, Southern England. J. Foramin. Res. 2001;31:12–24. doi: 10.2113/0310012. [DOI] [Google Scholar]
- 53.Debenay J-P, Bicchi E, Goubert E, Armynot-du-Châtelet E. Spatio-temporal distribution of benthic foraminifera in relation to estuarine dynamics (Vie estuary, Vendée, W France) Estuar. Coast. Shelf Sci. 2006;67:181–197. doi: 10.1016/j.ecss.2005.11.014. [DOI] [Google Scholar]
- 54.Morvan J, et al. Patchiness and life cycle of intertidal foraminifera: Implication for environmental and paleoenvironmental interpretation. Mar. Micropaleontol. 2006;61:131–154. doi: 10.1016/j.marmicro.2006.05.009. [DOI] [Google Scholar]
- 55.Francescangeli F, et al. Multidisciplinary study to monitor consequences of pollution on intertidal benthic ecosystems (Hauts de France, English Channel, France): Comparison with natural areas. Mar. Environ. Res. 2020;160:105034. doi: 10.1016/j.marenvres.2020.105034. [DOI] [PubMed] [Google Scholar]
- 56.Amara R, Meziane T, Gilliers C, Hermel G, Laffargues P. Growth and condition indices in juveniles sole Solea solea measured to assess the quality of essential fish habitat. Mar. Ecol. Prog. Ser. 2007;351:201–208. doi: 10.3354/meps07154. [DOI] [Google Scholar]
- 57.Langlet D, Bouchet VMP, Delaeter C, Seuront L. Motion behavior and metabolic response to microplastic leachates in the benthic foraminifera Haynesina germanica. J. Exp. Mar. Biol. Ecol. 2020;529:151395. doi: 10.1016/j.jembe.2020.151395. [DOI] [Google Scholar]
- 58.Cesbron F, et al. Sequestered chloroplasts in the benthic foraminifer Haynesina germanica: Cellular organization, oxygen fluxes and potential ecological implications. J. Foramin. Res. 2017;47:268–278. doi: 10.2113/gsjfr.47.3.268. [DOI] [Google Scholar]
- 59.Geslin E, et al. Oxygen respiration rates of benthic foraminifera as measured with oxygen microsensors. J. Exp. Mar. Biol. Ecol. 2011;396:108–114. doi: 10.1016/j.jembe.2010.10.011. [DOI] [Google Scholar]
- 60.Schindelin J, et al. Fiji : An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seuront L. Fractals and Multifractals in Ecology and Aquatic Science. Boca Raton: CRC Press; 2010. [Google Scholar]
- 62.Seuront L. On uses, misuses and potential abuses of fractal analysis in zooplankton behavioral studies: A review, a critique and a few recommendations. Phys. A. 2015;432:410–434. doi: 10.1016/j.physa.2015.03.007. [DOI] [Google Scholar]
- 63.Seuront L, Cribb N. Fractal analysis provides new insights into the complexity of marine mammal behavior: A review, two methods, their application to diving and surfacing patterns, and their relevance to marine mammal welfare assessment. Mar. Mamm. Sci. 2017;33:847–879. doi: 10.1111/mms.12399. [DOI] [Google Scholar]
- 64.Revsbech NP. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 1989;34:474–478. doi: 10.4319/lo.1989.34.2.0474. [DOI] [Google Scholar]
- 65.Glock N, et al. Metabolic preference of nitrate over oxygen as an electron acceptor in foraminifera from the Peruvian oxygen minimum zone. PNAS. 2019;116:2860–2865. doi: 10.1073/pnas.1813887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choquel C, et al. Total nitrate uptake by an invasive benthic foraminifer in marine sediments. Biogeosci. Discuss. 2020;18:327–341. doi: 10.5194/bg-18-327-2021. [DOI] [Google Scholar]
- 67.Ramsing, N. & Gundersen, J. Seawater and Gases-Tabulated Physical Parameters of Interest to People Working with Microsensors in Marine Systems. (Unisense Internal Report, 1994).
- 68.Zar J. Biostatistical Analysis. 5. London: Pearson Education; 2009. [Google Scholar]
- 69.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2019).
- 70.Bouchet VMP, Seuront L. Strength may lie in numbers: Intertidal foraminifera non-negligible contribution to surface sediment reworking. OJMS. 2020;10:131–140. doi: 10.4236/ojms.2020.103010. [DOI] [Google Scholar]
- 71.Seuront L. Behavioral fractality in marine copepods: Endogenous rhythms versus exogenous stressors. Phys. A. 2011;390:250–256. doi: 10.1016/j.physa.2010.09.025. [DOI] [Google Scholar]
- 72.Seuront L. Hydrocarbon contamination decreases mating success in a marine planktonic copepod. PLoS ONE. 2011;6:e26283. doi: 10.1371/journal.pone.0026283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seuront L. When complexity rimes with sanity: Loss of fractal and multifractal behavioural complexity as an indicator of sub-lethal contaminations in zooplankton. In: Ceccaldi H-J, Hénocque Y, Koike Y, Komatsu T, Stora G, Tusseau-Vuillemin M-H, editors. Marine Productivity: Perturbation and Resilience of Socio-ecosystems. Berlin: Springer; 2015. pp. 129–137. [Google Scholar]
- 74.Harrison S, Phizacklea A. Vertical temperature gradient in muddy intertidal sediments in the Forth estuary, Scotland. Limnol. Oceanogr. 1987;32:954–963. doi: 10.4319/lo.1987.32.4.0954. [DOI] [Google Scholar]
- 75.Bouchet VMP, Debenay J-P, Sauriau P-G, Radford-Knoery J, Soletchnik P. Effects of short-term environmental disturbances on living benthic foraminifera during the Pacific oyster summer mortality in the Marennes-Oléron Bay (France) Mar. Environ. Res. 2007;64:358–383. doi: 10.1016/j.marenvres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Somero GN. Thermal physiology and vertical zonation of intertidal animals: Optima, limits, and costs of living. Integr. Comp. Biol. 2002;42:780–789. doi: 10.1093/icb/42.4.780. [DOI] [PubMed] [Google Scholar]
- 77.Stillman JH. Causes and consequences of thermal tolerance limits in rocky intertidal porcelain crabs, genus Petrolisthes. Integr. Comp. Biol. 2002;42:790–796. doi: 10.1093/icb/42.4.790. [DOI] [PubMed] [Google Scholar]
- 78.Pörtner HO, Peck L, Somero G. Thermal limits and adaptation in marine Antarctic ectotherms: An integrative view. Phil. Trans. R. Soc. B. 2007;362:2233–2258. doi: 10.1098/rstb.2006.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Przeslawski R, Zhu Q, Aller R. Effects of abiotic stressors on infaunal burrowing and associated sediment characteristics. Mar. Ecol. Prog. Ser. 2009;392:33–42. doi: 10.3354/meps08221. [DOI] [Google Scholar]
- 80.Chapperon C, Seuront L. Behavioral thermoregulation in a tropical gastropod: Links to climate change scenarios. Glob. Change Biol. 2011;17:1740–1749. doi: 10.1111/j.1365-2486.2010.02356.x. [DOI] [Google Scholar]
- 81.Tsubokura T, Goshima S, Nakao S. Seasonal horizontal and vertical distribution patterns of the supralittoral amphipod Trinorchestia trinitatis in relation to environmental variables. J. Crust. Biol. 1997;17:674–686. doi: 10.2307/1549370. [DOI] [Google Scholar]
- 82.Lardies MA, Clasing E, Navarro JM, Stead RA. Effects of environmental variables on burial depth of two infaunal bivalves inhabiting a tidal flat in southern Chile. J. Mar. Biol. Assoc. U.K. 2001;81:809–816. doi: 10.1017/S0025315401004635. [DOI] [Google Scholar]
- 83.Diaz JA, Cabezas-Diaz S. Seasonal variation in the contribution of different behavioural mechanisms to lizard thermoregulation. Funct. Ecol. 2004;18:867–875. doi: 10.1111/j.0269-8463.2004.00916.x. [DOI] [Google Scholar]
- 84.Lencioni V. Survival strategies of freshwater insects in cold environments. J. Limnol. 2004;63:45–55. doi: 10.4081/jlimnol.2004.s1.45. [DOI] [Google Scholar]
- 85.Dubois Y, Blouin-Demers G, Shipley B, Thomas D. Thermoregulation and habitat selection in wood turtles Glyptemys insculpta: Chasing the sun slowly. J. Anim. Ecol. 2009;78:1023–1032. doi: 10.1111/j.1365-2656.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- 86.Chapperon C, Seuront L. Keeping warm in the cold: On the thermal benefits of aggregation behaviour in an intertidal ectotherm. J. Therm. Biol. 2012;37:640–647. doi: 10.1016/j.jtherbio.2012.08.001. [DOI] [Google Scholar]
- 87.Koo BJ, Kim S-H, Hyun J-H. Feeding behavior of the ocypodid crab Macrophthalmus japonicus and its effects on oxygen-penetration depth and organic-matter removal in intertidal sediments. Estuar. Coast. Shelf Sci. 2019;228:106366. doi: 10.1016/j.ecss.2019.106366. [DOI] [Google Scholar]
- 88.Gosling E. Bivalve Molluscs Biology, Ecology and Culture. Oxford: Blackwell Publishing Ltd; 2004. [Google Scholar]
- 89.Verdelhos T, Marques JC, Anastácio P. Behavioral and mortality responses of the bivalves Scrobicularia plana and Cerastoderma edule to temperature, as indicator of climate change’s potential impacts. Ecol. Ind. 2015;58:95–103. doi: 10.1016/j.ecolind.2015.05.042. [DOI] [Google Scholar]
- 90.Angilletta MJ. Looking for answers to questions about heat stress: Researchers are getting warmer. Funct. Ecol. 2009;23:231–232. doi: 10.1111/j.1365-2435.2009.01548.x. [DOI] [Google Scholar]
- 91.Lombard F, Labeyrie L, Michel E, Spero HJ, Lea DW. Modelling the temperature dependent growth rates of planktic foraminifera. Mar. Micropaleontol. 2009;70:1–7. doi: 10.1016/j.marmicro.2008.09.004. [DOI] [Google Scholar]
- 92.Fraser KPP, Clarke A, Peck LS. Low-temperature protein metabolism: Seasonal changes in protein synthesis and RNA dynamics in the Antarctic limpet Nacella concinna Strebel 1908. J. Exp. Biol. 2002;205:3077–3086. doi: 10.1242/jeb.205.19.3077. [DOI] [PubMed] [Google Scholar]
- 93.Gilbert C, et al. One for all and all for one: The energetic benefits of huddling in endotherms. Biol. Rev. 2010;85:545–569. doi: 10.1111/j.1469-185X.2009.00115.x. [DOI] [PubMed] [Google Scholar]
- 94.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change. 2012;2:686–690. doi: 10.1038/nclimate1539. [DOI] [Google Scholar]
- 95.Lou F, Gao T, Han Z. Transcriptome analyses reveal alterations in muscle metabolism, immune responses and reproductive behavior of Japanese mantis shrimp (Oratosquilla oratoria) at different cold temperature. Comp. Biochem. Physiol. D Genomics Proteomics. 2019;32:100615. doi: 10.1016/j.cbd.2019.100615. [DOI] [PubMed] [Google Scholar]
- 96.Wieser W. Temperature relations of ectotherms: A speculative review. In: Wieser W, editor. Effects of Temperature on Ectothermic Organisms: Ecological Implications and Mechanisms of Compensation. Berlin: Springer; 1973. pp. 1–23. [Google Scholar]
- 97.Price R, Warwick RM. The effect of temperature on the respiration rate of meiofauna. Oecologia. 1980;44:145–148. doi: 10.1007/BF00572671. [DOI] [PubMed] [Google Scholar]
- 98.Stillman JH, Somero GN. A comparative analysis of the upper thermal tolerance limits of Eastern Pacific porcelain crabs, genus Petrolisthes : Influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 2000;73:200–208. doi: 10.1086/316738. [DOI] [PubMed] [Google Scholar]
- 99.Vernberg W, Vernberg F. Environmental Physiology of Marine Organisms. Berlin: Springer; 1972. [Google Scholar]
- 100.Mestre NC, Brown A, Thatje S. Temperature and pressure tolerance of larvae of Crepidula fornicata suggest thermal limitation of bathymetric range. Mar. Biol. 2013;160:743–750. doi: 10.1007/s00227-012-2128-x. [DOI] [Google Scholar]
- 101.Meysman FJR, Galaktionov OS, Glud RN, Middelburg JJ. Oxygen penetration around burrows and roots in aquatic sediments. J. Mar. Res. 2010;68:309–336. doi: 10.1357/002224010793721406. [DOI] [Google Scholar]
- 102.Mouret A, et al. Oxygen and organic carbon fluxes in sediments of the Bay of Biscay. Deep-Sea Res. 2010;I(57):528–540. doi: 10.1016/j.dsr.2009.12.009. [DOI] [Google Scholar]
- 103.Bernhard JM. Experimental and field evidence of Antarctic foraminiferal tolerance to anoxia and hydrogen sulfide. Mar. Micropaleontol. 1993;20:203–213. doi: 10.1016/0377-8398(93)90033-T. [DOI] [Google Scholar]
- 104.Maire O, et al. How does macrofaunal bioturbation influence the vertical distribution of living benthic foraminifera? Mar. Ecol. Prog. Ser. 2016;561:83–97. doi: 10.3354/meps11929. [DOI] [Google Scholar]
- 105.Richirt J, et al. Foraminiferal community response to seasonal anoxia in Lake Grevelingen (the Netherlands) Biogeosciences. 2020;17:1415–1435. doi: 10.5194/bg-17-1415-2020. [DOI] [Google Scholar]
- 106.Moens T, Vincx M. Temperature, salinity and food thresholds in two brackish-water bacterivorous nematode species: Assessing niches from food absorption and respiration experiments. J. Exp. Mar. Biol. Ecol. 2000;243:137–154. doi: 10.1016/S0022-0981(99)00114-8. [DOI] [Google Scholar]
- 107.Pinko D, Abramovich S, Titelboim D. Foraminiferal holobiont thermal tolerance under climate change—Roommates problems or successful collaboration? Biogeosciences. 2020;17:2341–2348. doi: 10.5194/bg-17-2341-2020. [DOI] [Google Scholar]
- 108.Maire O, Duchêne J, Bigot L, Grémare A. Linking feeding activity and sediment reworking in the deposit-feeding bivalve Abra ovata with image analysis, laser telemetry, and luminophore tracers. Mar. Ecol. Prog. Ser. 2007;351:139–150. doi: 10.3354/meps07147. [DOI] [Google Scholar]
- 109.Ouellette D, et al. Effects of temperature on in vitro sediment reworking processes by a gallery biodiffusor, the polychaete Neanthes virens. Mar. Ecol. Prog. Ser. 2004;266:185–193. doi: 10.3354/meps266185. [DOI] [Google Scholar]
- 110.Guarini J, Blanchard G, Gros P, Gouleau D, Bacher C. Dynamic model of the short-term variability of microphytobenthic biomass on temperate intertidal mudflats. Mar. Ecol. Prog. Ser. 2000;195:291–303. doi: 10.3354/meps195291. [DOI] [Google Scholar]
- 111.Jauffrais T, et al. Effect of light on photosynthetic efficiency of sequestered chloroplasts in intertidal benthic foraminifera (Haynesina germanica and Ammonia tepida) Biogeosciences. 2016;13:2715–2726. doi: 10.5194/bg-13-2715-2016. [DOI] [Google Scholar]
- 112.Jauffrais T, et al. Response of a kleptoplastidic foraminifer to heterotrophic starvation: Photosynthesis and lipid droplet biogenesis. FEMS Microbiol. Ecol. 2019 doi: 10.1093/femsec/fiz046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.