Abstract
Kamchatka geothermal fields should be considered not only as heat and energy resources but also as possible sources of chemical compounds. Boron is one of such chemical elements. The Pauzhetskaya, Nalychevskoe, Pushchinskoe, Kireunskoe steam-hydrothermal fields are the most promising for boron recovery. Data obtained from experimental studies on boric acid sorption using the industrial ion exchangers KU-2-8, AV-17-8, EDE-10P, and AN-31 are presented in this article. The experiments are conducted in a dynamic mode, and the results showed that the method of sequential/consecutive two-stage sorption is most effective for: 1 – removal of the main part of cations using KU-2-8 cation exchanger; 2 – boric acid recovery using weakly basic AN-31 anion exchanger. The sorption capacity of the studied anion exchangers increases in the order as follows: AV-17-8 <EDE-10P < AN-31.
Keywords: Complex use, Geothermal heat carries, Boron, Boric acid, Sorption, Anion exchange resin
Complex use, Geothermal heat carries, Boron, Boric acid, Sorption, Anion exchange resin.
1. Introduction
Boron and its compounds are widely used industrially. Boron is a non-conventional material, although further studies of this material would be required to harness its use. The American US Borax and the Turkish EtiMaden companies control the majority of the world market of boron, that is, 90% of boron products. Dalnegorsk field (Primorsky Region) is the largest boron deposit in Russia, and it is estimated to contain about3% of the world's boron reserve. MCC is a mining and chemical enterprise of boron compounds, it produces boron-bearing products (boric acid, calcium borate, boric anhydride) of which 75% of the products are exported to Korea, Japan, and China.
Recently, large reserves of boron have been discovered in China where they are successfully processed. The surfaces of steel parts overstretched are saturated with boron. Boron saturation of surface layers of metal increases the resistance to wear-, heat- and corrosion. The strength and wear resistance of cast iron has been enhanced by adding 0.4% of boron. Borax, a compound of sodium and boron is a major imported material in Russia. It is used for melting and soldering of precious metals and their corresponding products. Boric acid is obtained from borax. Boric acid is used as an antiseptic in medicine.
Boron recovery from hydrominerals as the raw materials is a promising task. Hydromineral raw materials are practically inexhaustible sources of boron compounds, and they include separate geothermal station of various boron productions, freezing mixture, wastewater, sea, oceanic, and underground water. Other sources also include salt lakes, petroleum waters, hot springs, volcanic and mud volcanic breccia, and many soil types.
Most researchers focus on boron recovery from solutions. The most common methods for processing boron-containing solutions are reverse osmosis and ion exchange. However, a few other researches are conducted with electrodialysis, electro- and chemical coagulation, and various combinations of these methods. Researches by Z. Guan et al. [1] mainly focused on the recovery of boron by adsorption compared to the other methods of boron recovery. The earliest adsorbent materials used are made of chelating substances. Most of the commercial resins are modified by N-methyl-D-glucamine (NMDG) functional group such as Diaion WA30, Diaion CRB 02, Purolite S108, Dowex 2 × 8, Dowex XUS 43594.00, Amberlite IRA 743, and XSC-700.
Chelating resins that have functional groups, in which the hydroxyl groups in cis position show high selectivity to boron, were also used to remove boron from wastewaters of geothermal units/systems. The Diaion CRB 01, Diaion CRB 02, Purolite S 108 (1), and Purolite S 108 (2) take on the role of the chelating N-glucamine resins. Wastewaters from the geothermal power station of Kizildere field (Turkey) were investigated for the boron content. The analyses indicate the boron concentration ranges from 18–20 mg/l. The authors of [2] consider that a resin with concentration of 3 g resin per litre of wastewater is required to remove more than 90% of boron for all chelating resins. Before this breakthrough, the Diaion CRB 02 has the highest capacity among the tested resins [2].
M. Badruk and N. Kabay [3] recovered boron from Kizildere geothermal brine which contains 30.2 mg/dm3 of boron 1300 mg/dm3 of sodium and 0.39 mg/dm3 of calcium. The effect of Ca (II) and Na (I) ions on the sorption of boron was also studied using Diaion CRB02.
The authors I. Ismailet al. [4] used Diaion CRB02 chelating resin to recover boron from the bittern solution left after the extraction of sodium sulfate and sodium chloride from the salt water of Lake Qarun, Egypt. They also studied the effect of current flow on ion exchange as a variable parameter.
The authors P. Santander et al. [5] also recovered boron from thermal water of the geothermal heating center in Izmir City (Turkey) using novel boron selective resin obtained by modifying Diaion CRB02 chelating resin. The total capacity is 2.68 mg B/ml resin.
The effect of various parameters such as resin particle size, flow rate, and resin concentration on boron removal has been investigated [6]. For example, in the recovery of boron from Balcova geothermal water, the sorption characteristics of Diaion CRB02 and Dowex were compared (XUS 43594.00).
Boron and arsenic were recovered from the thermal water of Hachoubaru (Japan) geothermal water using Lewatit MK 51 and Diaion CRB03. The Diaion CRB03 resin showed greater efficiency in the static and column modes [7].
Researches on the removal of boron from aqueous solutions were carried out in this study [8]. The anion exchange resin Dowex 2 × 8 with a strong base was taken as the sorbent. The functional group in the resin is benzyl-dimethyl-ethanolamine.
Several studies have been conducted on the removal and recovery of boron from wastewater. In previous studies, the boron selective resin, Amberlite IRA 743, was used as the sorbent for recovery of boron from drinking water, boron-containing waters, and geothermal wastewaters [9, 10, 11]. Amberlite XE 243 resin was used to recover boric acid from wastewaters, and to protect plants from high concentrations of borax in the soil [12].
There are many geothermal resources in Romania. In this article, two of these resources are discussed. Our samples were collected from geothermal wells in northwest of Romania where high borate content was found. The content of the metaboric acid ranges from 59 to 100 mg/l. Boric acid was recovered from the geothermal waters by ion exchange methods, using Vionit AS-116 ion exchange resin. Geothermal waters contain organic compounds such as phenol, which affects the sorption process of boric acid. The copolymer CA-30 resin is used to remove the phenols. The sorption of boric acid from the water using Vionit AS-116 resin was carried out under conducive conditions after the phenol removal. A solution containing 6 g/l of boric acid was obtained. This solution can be concentrated to obtain high purity crystals of boric acid [13].
A large number of works are devoted to boron recovery from seawater. A detailed review is presented by N. Kabay et al. [14]. In particular, the use of single-stage reverse osmosis to desalinate seawater using a (SWRO) membrane can reduce the boron concentration from 6 mg/l to about 0.9–1.8 mg/l [15]. However, the required value of a boron content of 0.5 mg/l may not always be achieved. Therefore, the adsorption membrane filtration or ion exchange is proposed in addition to the SWRO membrane.
In a study, the efficiency of boron removal from seawater using a two-pass SWRO system with various membranes of reverse osmosis on water samples from Qingdao Jiaozhou Bay, Yellow Sea (China) was evaluated [16]. The system was affected by various negative factors as follows: low pH, low working pressure, and high boron concentration. Never the less, good index numbers for boron reduction have been achieved.
Boron content in the hydrothermal waters of Kamchatka varies: from unity to hundreds of mg/l (1.3–200). The researches to recover boron from natural hydromineral materials by industrial, natural and modified sorbents have been ongoing for years at the Research Geotechnological center, of the Far Eastern Branch of the Russian Academy of Sciences [17, 18, 19, 20]. Boric acid recovery from a separate Pauzhetskaya Geothermal Station, whose content reaches up to 200 mg/l, has been reported in previous studies [17, 18].
Anions are known to possess competing effect during boron acid sorption. Chief among these anions are the chloride and sulphate ions. The chloride ion contents separately reach up to1000–1100 mg/l. The sulfate ions have a rather insignificant effect on boric acid sorption and their content does not usually exceed 80 mg/l [19, 20].
The work aims to study the possibility of sequential use of industrial ion exchangers to recover boron compounds from geothermal heat carriers. This study aims to compare the characteristics of boron sorption from geothermal sources using anion exchangers of different basicity: strongly basic – AV-17-8, weakly basic – AN-31 and EDE-10P. In this process, the eluate of solutions is directly passed through a sorption column containing anion exchangers without preliminary preparation of the model solution and sequential elution of the solution, first through the cation resin, and then through the anion resin respectively.
2. Materials and methods
Investigating the ion exchangers’ properties: the first step involved plotting the titration/pH curves (Figure 1). With a trial experiment, the balance between the solid phase of the ion exchanger and solution is established within 10–12 h. All experiments were at room temperature 20 ± 2°С. The flasks were installed in a piston Wise shaker with a swing amplitude of 10 mm at a speed of 100 swings per minute. Consequently, sufficient phases contact time was selected, that is, 1 day. 1 g weight of ion exchangers was placed into different conical flasks to which was added various amounts of hydrochloric acid in increasing order from 0.3 to 3.0 mmol per 1 g of the sorbent. The solution volume was stepped up to 100 ml in order to maintain the solid to liquid (S:L) ratio = 1:100. The рН was measured after some time.
Figure 1.
The titration curves of anion exchange resins with hydrochloric acid – 0,1 mol/l: АВ-17-8 – strongly basic; АN-31 – multipurpose anion exchange resin; EDE-10P – weakly basic.
Calculation [25] of the conditional constants of acid-base ionization рК was done using data of potentiometric titration, formula (1):
| (1) |
where the degree of neutralization of the ion exchanger resin α is calculated from the results of potentiometric titration with the formula: , N is the normality of the titrant (HCl), V is the value of titrant used for the titration; А is the full exchange capacity of the anion exchanger; m is the coefficient of proportionality that equals the tangent of slope angle of the straight line constructed in coordinates.
The chloride form of anion exchanger resin was used to determine the ratio of strongly basic and weakly basic exchange centers by washing an amount (g) of the anion exchange resin with a solution of ammonium hydroxide and then with a solution of sodium sulfate. The amount of desorbed chloride ions was determined by argentometric titration in the eluates. The error in determining the concentration of chloride ions was 7%.
The model solution similar to a natural/real separation of the Pauzhetskaya Geothermal Station according to a content of boric acid, sodium ions and chloride ions was prepared for experimental studies. The model solutions were prepared by dissolution of the sample weight of boric acid (chemically clean) and sodium chloride (chemically clean) in distilled water inside a measuring flask. The pH of the solutions was equal to 8.5 which corresponds to the pH value of a separate Pauzhetskaya Geothermal Station. Sodium hydroxide (chemically clean) was used for this purpose.
The experiments were carried out as follows: the first involved passing the model solution through the sorption column with anion exchange resin. In the second step, at first, the model solution was passed through the sorption column with KU-2-8 cation exchanger and thereafter through the sorption column with anion exchange resins. The composition of the model solution (1) and eluate after sorption on the KU-2-8 cation exchanger (2) is presented in Table 1.
Table 1.
The composition of model solution (1) and eluate after sorption on KU-2-8 (2).
| No | Solution name | рН | Components content, mg/l |
Ionic strength, mol/l | ||
|---|---|---|---|---|---|---|
| H3BO3 | Na+ | Cl– | ||||
| 1 | 1 – Model solution | 5.33 | 198 ± 49.5 | 795 ± 135 | 1240 ± 87 | 0.0348 |
| 2 | 2 – Eluate | 1.46 | 198 ± 49.5 | 3.33 ± 0.57 | 1240 ± 87 | 0.0349 |
The solutions ionic strength were calculated using the well-known Lewis formula (2). In the calculation, it was assumed that the dissociation of boric acid is insignificant [25]. Also, the concentration of hydrogen ions was taken into account while calculating the ionic strength of eluate since the pH = 1.46
| (2) |
where C – ion concentration, mol/l; Z – ion charge.
By the downflow method, the solutions were passed through sorption columns that have a layer height of 200 mm and diameter of 10 mm, at a flow rate of 1 ml/min. The content of sodium ions, pH, chloride ions and boric acid was controlled at the exit from the columns. Thereafter, the desorption was carried out using sodium hydroxide.
The chemical analysis for the sodium content was carried out using ion chromatography (IC) method on a liquid chromatograph LC-20 (Shimadzu) with a chromatography column Shodex IC YK-421 (Japan). The mixture containing 5 mmol/l of tartaric acid, 1 mmol/l of dipicolinic acid, and 24 mmol/l of boric acid was used as eluent; a conductometric detector was also used. The error in determining the concentration of sodium was 17%. The solution's acidity was controlled using an ionomer « Expert – 001» with universal electrode, EGC – 10601/7, which has a sensitivity of ±0.02 pH.
Boric acid standardization of the model solution and eluates was carried out by method of potentiometric titration. The method is based on the fact that when mannite is introduced as a complexing agent into the test boron-containing solution previously adjusted to pH = 7.0, the pH of the mixture decreases, corresponding to the content of boric acid in the test solution. Boric acid is potentiometrically titrated by alkali solution. The error in determining the concentration of boric acid was 25%.The titer of sodium hydroxide for boron is previously set under similar conditions for a standard solution of boric acid (7337-96, С = 1.03 g/dm3). The determination of chloride ions was performed by argentometric method of back titration.
Only six sorption columns with anion exchange resins were prepared. The mass of each anion exchange resin was 10 g. The ion exchangers were transformed in a hydroxyl form. For this purpose, the 10–15 column volumes of 2% sodium hydroxide were passed in a dynamic mode. After that, the ion exchanger was washed with distilled water to achieve a neutral reaction of the medium.
3. Results and discussions
There are various methods of boron ions recovery. The method of reverse osmosis is widely applicable for desalination of seawater. Boron selective membranes are used in this method. The membrane selectivity for boron varies depending on the water's pH and it increases with decreasing temperature. Electrodialysis is another method for boron recovery. In this method, boric acid as a weak electrolyte dominates the diluted solutions at pH less than or equal to 9.0 while the borate ions dominate at a higher pH.
Sorption method of boron recovery from solutions using industrial organic ion exchangers and sorbents, such as EDE-10P, AN-2F, AN-31, AV-17, SB-1, etc., is well known. The disadvantage of this method is that not all the above-mentioned ion exchangers can completely recover boron ions, because of their weak selectivity with respect to the ions.
In this work, AV-17-8, EDE-10P, AN-31 anion exchange resins were used. Their general properties are presented in Table 2. AV-17-8 anion exchange resin has a characteristic gel structure, and it is widely used in water treatment technologies [21]. It is characterized by high basicity, and reagent resistance. The matrix of the resin АV-17-8 is a copolymer of styrene and divinylbenzene. The functional group present is a trimethylammonium base. EDE-10P anion exchange resin is produced in the industry in form of irregular shaped red-brown granules [22]. It was prepared first in the USSR in the 50s from monomers of ethylenediamine and epichlorohydrin [23]. The functional groups are secondary and tertiary amino groups of the aliphatic series, as well as quaternary ammonium base. AN-31 anion exchange resin has a similar preparation method asEDE-10P anion exchange resin, but with ammonia present.
Table 2.
Characterization of ion exchange resin.
| No | Sorbent name | Matrix composition | Basicity | Functional group | Bulk density, g/ml | Specific volume, ml/g | Dynamic exchange capacity, mol/m3, no less | Maximum temperature (t°max), °C | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Сation exchange resin KU-2-8 | Styrene-divinylbenzene | Strong-acid cation-exchange resin | Sulphonic-acid groups (–SO3H) | 0.75–0.90 | 2.6–3.0 | 526 | 130 | – |
| 2 | Anion exchange resin АV-17-8 | Styrene-divinylbenzene | Strongly basic | Quaternary trimethylammonium groups (–N+(CH3)3 | 0.74 | 3.3 | 700 | 50 | [21] |
| 3 | Anion exchange resin АN-31 | 2.5-methyl-divinylpyridine, divinylbenzene | Weakly basic | Secondary and tertiary aliphatic amino groups =NH; ≡N |
0.60 | 3.2 | 1280 | 100 | [23] |
| 4 | Anion exchange resin EDE-10P | Polyethylene polyamines, epichlorhydrines | Weakly basic | Secondary and tertiaryamino groups of the aliphatic series and about 20% of the groups of quaternary ammonium bases =NH; ≡N; –N+(R)3 |
0.60–0.72 | 28.0–35.0 | 1000 | 60 | [22, 23], |
| 5 | DiaionWA 30 | Styrene-divinylbenzene | Weakly basic | Quaternary trimethylammonium groups (–N+(CH3)3 | 0.635 | – | 1500 | 100 | [24], |
| 6 | Diaion CRB 02 | Styrene-divinylbenzene | Free base | N-MethylGlucamine | 0.7 | – | – | 100 | [3, 5, 6] |
| 7 | Purolite S 108 | Styrene-divinylbenzene | Free base | N-MethylGlucamine | 0.67–0.73 | – | 600 | 60 | [3] |
| 8 | Dowex 2 × 8 | Styrene-divinylbenzene | Stronglybasic | N-MethylGlucamine | 0.7 | – | 1200 | 80 | [8] |
| 9 | Amberlite IRA 743 | Styrene-divinylbenzene | Weakly basic | N-MethylGlucamine | 0.7 | – | 700 | 75 | [1] |
Figure 1 presents the titration/pH curves of known weights of anion exchanger resins with hydrochloric acid. The titration/pH curve of strongly basic AV-17-8 with quaternary trimethylammonium present as functional group begins the curve at a pH = 6.76, since the hydroxyl groups are in the ion exchanger phase and their dissociation is insignificant. While adding hydrochloric acid, an ion exchange reaction occurs according to Eq. (1). Initially, the exchange occurs on the surface of the grains of anion exchange resin causinga decrease of pH. The hydroxide ions released from the ion exchange resin phase immediately binds to hydrogen ions forming water. As soon as all hydroxide ions are displaced from the ion exchanger phase, the solution pH decreases due to the addition of excess acid. However, the titration variation or alteration is not precise, because exchange reaction is limited by internal interdiffusion of exchanging ions.
| Eq.(1) |
The curve follows a stepped wise configuration while titrating the anion exchange resin of AN-31. In the first stage, the anion exchange resin behaves as a strongly basic one. After the strongly basic centers are filled, the weak basic groups come into exchange. The expressed three steps on the titration curve confirm that the anion exchange resin AN-31 comprises three types of exchange centers. The anion exchange resin, EDE-10P, behaves as a weakly basic anion exchange resin. It has a curve that is gently sloping.
The model solution No 1 was passed through anion exchange resins while executing single-stage sorption. Data are presented in Table 3. The breakthrough chromatogram for АV-17-8 anion exchanger resin has a typical S-shape form. The boric acid slip was instantaneous, with 16.8 mg/l boric acid detected in the first portion of the eluate, which is 8.5% of the initial content. The chloride absorption was complete in the first seven column volumes. At this equilibrium level, the chloride ion concentration detected in the eluent is 100 mg/l, and it remained at this level until the end of the experiment. The experiment was stopped when boric acid concentration at the entrance and exit of the column reached equilibrium. The sorption capacity for boric acid was 0.098 mmol/g. Following, the model solution No 1 was passed through KU-2-8 cation exchange resin in a hydrogen form during two-stage sorption. Data are presented in Table 4. The cation free eluate was obtained at the exit of the column. The solution's pH decreased from 5.33 to 1.46.
Table 3.
Values of equilibrium concentrations of boric acid and pH during the direct elution of the model solution (single-stage sorption in a dynamic mode).
| Sample number | B/V | pH |
С (H3BO3), mg/l P = 0.95 |
pH |
С (H3BO3), mg/l P = 0.95 |
pH |
С (H3BO3), mg/l P = 0.95 |
|---|---|---|---|---|---|---|---|
| AV-17-8 | EDE-10P | AN-31 | |||||
| 1 – Model solution | 0 | 5.33 | 198 | 5.33 | 198 | 5,33 | 198 |
| 1 | 0.75 | 12.50 | 0.00 | 12.3 | 0.00 | 10.46 | 16.8 |
| 3 | 2.25 | 12.57 | 16.8 | 12.38 | 16.8 | 11.08 | 65.1 |
| 8 | 3.75 | 12.58 | 21.0 | 12.45 | 54.6 | 11.28 | 79.9 |
| 10 | 7.50 | 12.60 | 71.5 | 12.45 | 63.0 | 10.99 | 118 |
| 12 | 9.00 | 12.62 | 122 | 12.4 | 68.0 | 10.72 | 120 |
| 15 | 11.25 | 12.70 | 183 | 12.33 | 86.0 | 10.56 | 122 |
| 16 | 12.00 | 12.72 | 198 | 12.29 | 96.6 | 10.24 | 122 |
| 17 | 12.75 | 12.70 | 198 | 12.27 | 110 | 10.10 | 127 |
| 18 | 13.50 | 12.70 | 198 | 12.25 | 127 | 9.70 | 126 |
| 20 | 15.00 | – | – | 12.15 | 150 | 9.63 | 130 |
| 24 | 18.00 | – | – | 11.9 | 198 | 9.32 | 132 |
| 35 | 26.25 | – | – | – | – | 8.24 | 137 |
| 42 | 31.50 | – | – | – | – | 8.44 | 193 |
| 43 | 32.25 | – | – | – | – | 8.44 | 198 |
Table 4.
Values of equilibrium concentrations of boric acid and pH during sorption of boric acid with anion exchangers after previous elution through cation exchanger (two-stage sorption in a dynamic mode).
| Sample number | B/V | pH |
С (H3BO3), mg/l P = 0.95 |
pH |
С (H3BO3), mg/l P = 0.95 |
pH |
С (H3BO3), mg/l P = 0.95 |
|---|---|---|---|---|---|---|---|
| AV-17-8 | EDE-10P | AN-31 | |||||
| 2 – Eluate | 0 | 1.46 | 198 | 1.46 | 198 | 1.46 | 198 |
| 18 | 13.50 | 6.82 | 0.00 | – | 0 | – | 0 |
| 19 | 14.25 | 6.52 | 14.20 | – | 0 | – | 0 |
| 20 | 15.00 | 6.30 | 147 | – | 0.0 | – | 0 |
| 21 | 15.75 | 6.30 | 198 | 4.99 | 0.0 | – | 0 |
| 43 | 32.25 | – | – | 7.35 | 0.00 | – | 0 |
| 45 | 33.75 | – | – | 6.75 | 12.6 | – | 0 |
| 47 | 35.25 | – | – | 6.47 | 42.0 | – | 0 |
| 50 | 37.50 | – | – | – | 198 | – | 0 |
| 58 | 43.50 | – | – | – | – | 7.15 | 0 |
| 60 | 45.00 | – | – | – | – | 7.30 | 16.8 |
| 62 | 46.50 | – | – | – | – | 6.70 | 25.2 |
| 66 | 49.50 | – | – | – | – | 6.30 | 126 |
| 70 | 52.50 | – | – | – | – | 5.38 | 198 |
The resulting eluate thereafter was directed into a column with anion exchange resin. The result showed that the capacity ofAV-17-8 anion exchange resin for boric acid doubled and reached a value of 0.185 mmol/g. Thus, the breakthrough of boric acid occurred in 18 column volumes.
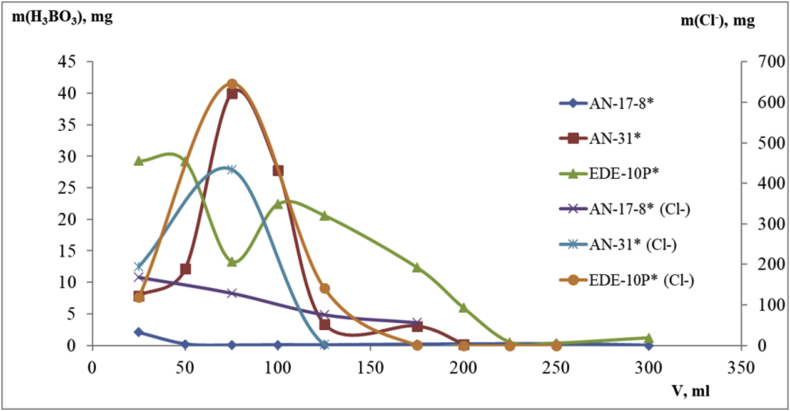
As a result of the single-stage sorption using EDE-10P anion exchange resin, the breakthrough chromatogram also has a characteristic S-shape form, however, at the midpoint of the curve, there is a gentle section (curve 2, Figure 1), which is assumed to be due to the formation of polyborate anionic complexes [B3O5(OH)4]– or [B4O5(OH)4]2–. The capacity ofEDE-10P anion exchange resin for boric acid is 0.172 mmol/g, which is almost two times higher than AV-17-8 anion exchange resin under the same conditions. The capacity of EDE-10P anion exchange resin for boric acid is 0.587 mmol/g under two-stage sorption. This is 3.2 times higher than the sorption capacity of AV-1,7–8 anion exchange resin under two-stage sorption.
The best values/features were obtained by sorption using AN-31 anion exchange resin. Curve 3 (curve 3, Figure 2) is a double S-shape curve under single-stage sorption. The concentration of boron acid becomes enhanced as the solution passes through the column at the initial stage. It is assumed that at this stage the ion exchange takes place only at the surface of the ion exchanger grains. At the exit of the column, the boron acid reaches a stable concentration, at which the curve forms a plateau. This stage corresponds to the formation of polyborate complexes in the resin. Its capacity for boric acid under single-stage and two-stage sorption is 0.209 mmol/g and 0.918 mmol/g, respectively. No significant change in pH is observed.
Figure 2.
Dynamics of boric acid sorption by anions exchange from model solutions. Single-stage sorption: 1 –АV-17-8, 2 – EDE-10P; 3 – АN-31. Subsequent two-stage sorption: 4 –АV-17-8; 5 – EDE-10P; 6 – АN-31.
Desorption of boric acid was carried out in columns of two-stage sorption (Figure 3). The desorption was carried out with a 2% sodium hydroxide solution at a flow rate of 1 ml/min. The eluates were collected in 25 ml volumetric flasks at the column exit, and the contents of boric acid and chloride ions were determined.
Figure 3.
The desorption of boric acid by sodium hydroxide.
Figure 3 indicates a graphical representation of the desorption of boric acid from these anion exchange resins AV-17-8, AN-31, EDE-10P.The boric acid slip occurred instantaneously, as far back as in the first portion of the eluate. The process was stopped when the concentration of the sorbed boric acid and desorbed boric acid was equilibrated. The desorption of boric acid in the column with AN-31 cation exchange resin completely occurred and reached 100%, while the boric acid desorption in the column with EDE-10P cation exchange resin reached 98.2% completion. However, the desorption of boric acid in the column with AV-17-8 cation exchange resin was carried out sequentially using two solutions: 2% solution of sodium hydroxide and 2% solution of sodium sulfate at a flow rate of 1 ml/min. Here the boric acid desorption was 5.29%. It is worthy to note that the desorption of chloride ions occurred completely in all three sorption columns with the anion exchange resins (Figure 3).
4. Conclusion
Thermal waters and separate of geothermal stations should be considered as possible sources of boron compounds.
The application of industrial anion exchangers for boric acid recovery is justified based on experimental studies.
It seems like a good idea to implement sequential two-stage sorption:
-
1
– removal of the main part of cations using KU-2-8 cation exchanger;
-
2
– boric acid recovery using low basic AN-31 anion exchanger.
-
3
– desorption – regeneration.
The sorption capacity of the studied anion exchangers increases in the series: AV-17-8 <EDE-10P < AN-31.
The constant dissociation of active center of hydroxide form of anion exchangers were determined: KD (AV-17-8) – 1.20 × 10−11; KD1 (AN-31) – 2.63 × 10−11; KD2 (AN-31) – 7.24 × 10−12; KD1 (EDE-10P) – 2.75 × 10−11; KD2 (EDE-10P) – 2.14 × 10−12. We proved that the anion exchangers EDE-10P and AN-31 possess two types of active centers.
As a result of the desorption, solutions were obtained and enriched in borate ions compared to the initial solutions. The concentration coefficient was 8. Solutions, in which the boric acid content reaches 1200–1400 mg/l were obtained in two-stage sorption and desorption. Further concentrations are obtained by repeating the cycles of the sorption-desorption with anion exchangers. The methods of concentration by evaporation are also known.
Declarations
Author contribution statement
Belova T.P: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ershova L.S: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to appreciate the director of the Research Geotechnological Center, Far Eastern Branch of Russian Academy of Sciences Pashkevich R.I. for contributed reagents, materials and to appreciate science officers for carrying out chemical analyses.
References
- 1.Guan Z., Lv J., Bai P., Guo X. Boron removal from aqueous solutions by adsorption. Desalination. 2016;383:29–37. [Google Scholar]
- 2.Kabay N., Yılmaz I., Yamac¸ S., Samatya S., Y¨uksel M., Y¨uksel U., Arda M., Sa˘glam M., Iwanaga T., Hirowatari K. Removal and recovery of boron from geothermal wastewater by selective ion exchange resin. I. Laboratory tests. React. Funct. Polym. 2004;60:163–170. [Google Scholar]
- 3.Badruk M., Kabay N., editors. International Geothermal Conference, Reykjavík. Sept. 2003. Removal of boron from Kizildere-Denizli geothermal brines using ion-exchange method; pp. 8–13. [Google Scholar]
- 4.Ismail I., Abdel-Salam O., Barakat F., Fateen S., Soliman A., Nogami M. Removal of boron from the bittern solution of Lake Qarun water by electrically assisted ion exchange. Port. Electrochim. Acta. 2014;32(2):125–136. [Google Scholar]
- 5.Santander P., Rivas B.L., Urbano B.F., İpek İ.Y., Özkula G., Arda M., Yüksel M., Bryjak M., Kozlecki T., Kabay N. Removal of boron from geothermal water by a novel boron selective resin. Desalination. 2013;310:102–108. [Google Scholar]
- 6.Kabay N., Yilmaz-Ipeka I., Sorokob I., Makowskib M., Kirmizisakala O., Yaga S., Bryjakb M., Yuksela M. Removal of boron from Balcova geothermal water by ion exchange microfiltration hybrid process. Desalination. 2009;241:167–173. [Google Scholar]
- 7.Koseoglu P., Yoshizuka K., Nishihama S., Yuksel U., Kabay N. Removal of boron and arsenic from geothermal water in kyushu island, Japan, by using selective ion exchange resins. Solvent Extr. Ion Exch. 2011;29:440–457. [Google Scholar]
- 8.Köse Te., Oztürk N. Boron removal from aqueous solutions by ion-exchange resin: column sorption–elution studies. J. Hazard Mater. 2008;152:744–749. doi: 10.1016/j.jhazmat.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Simonnot M., Castel C., Nicolai M., Rasin C., Sardin M., Jauffret H. Boron removal from drinking water with a boron selective resin: is the treatment really selective. Water Res. 2000;34:109–116. [Google Scholar]
- 10.Yılmaz A.E., Boncukcuoglu R., Yılmaz M.T., Kocakerim M.M. Adsorption of boron from boron-containing wastewaters by ion exchange in a continuous reactor. J. Hazard Mater. 2005;B117:221–226. doi: 10.1016/j.jhazmat.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Recepog˘lu O., Beker Ü. A preliminary study on boron removal from Kızıldere, Turkey geothermal wastewater. Geothermics. 1991;20:83–89. [Google Scholar]
- 12.Sahin S. A mathematical relationship for the explanation of ion exchange for boron adsorption. Desalination. 2002;143:35–43. [Google Scholar]
- 13.Gilǎu Ludovic, Stǎnǎşel Oana. Proceedings World Geothermal Congress. 2010. Experimental study of boron recovery from geothermal water on ion exchange. Bali, Indonesia, 25-29 April 2010. 4 P. [Google Scholar]
- 14.Kabay N., Güler E., Bryjak M. Boron in seawater and methods for its separation. Desalination. 2010;261:212–217. [Google Scholar]
- 15.Felder A., Epstein R., Villensky N., Gitis V. Optimization of coagulation step in membrane treatment of municipal secondary effluents. Desal. Water Treatment. 2011;35:62–67. [Google Scholar]
- 16.Jia Xu, Gao Xueli, Chen Guohua, Zou Linda, Gao Congjie. High performance boron removal from seawater by two-pass SWRO system with different membranes. Water Supply. 2010;10(3):327–336. [Google Scholar]
- 17.Belova T.P. The use of sorption methods for the purification of waste thermal waters// Natural Tech. Sci. 2013;6(68):212–216. [Google Scholar]
- 18.Belova T.P. Experimental studies in the sorptive extraction of boron and lithium from thermal waters. J. Volcanol. Seismol. 2017:136–142. [Google Scholar]
- 19.Belova T.P., Ershova L.S., Ratchina T.I. In the Book of Reports: 2nd International Geothermal Conference, GEOHEAT 2018 Electronic Publishing. IOP Conference Series: Earth and Environmental Science; 2019. Boron recovery from separate of Pauzhetskaya geothermal station based on modified zeolites of Yagodninsky deposit of Kamchatka. 012031. [Google Scholar]
- 20.Belova T.P., Ershova L.S. The effect of sulfate and chloride ions on the sorption of boric acid from the separate of Pauzhetskaya GeoPP with modified zeolite. Mt. Info. Analyt. Bullet. (scientific and technical journal) 2018;S57:354–360. [Google Scholar]
- 21.Vinnitsky V.A., Chugunov A.S., Nechaev A.F. The boric acid sorption with hydroxyl form of highly basic anion exchange resin AV-17-8 and direction modernization of special water purification system for Atomic Power Station. Adv. Chem. Chem. Technol. 2013;XXVII(6):84–87. [Google Scholar]
- 22.Kovaleva O.V., Azarnyh L.N., Slavinskaya G.V. The study of the physicochemical properties of anion exchange resin EDE-10P//scientific herald of the voronezh state university of civil engineering. Series. Physicochemical problems of construction engineering. 2009;2:87–90. [Google Scholar]
- 23.Ivanov V.A., Gorshkov V.N. 70 years of the history of the ion-exchange resins production//Sorption and chromatographic processes. 2006;6(1):5–31. [Google Scholar]
- 24.Product line brochure diaion mitsubishi chemical corporation. Rev. 2013;12:10. https://www.lenntech.com/Data-sheets/Mitsubishi-DIAION-Cataloque.pdf [Google Scholar]
- 25.Polyansky N.G., Gorbunov G.V., Polyanskaya N.L. 1976. Research Methods of Ionexchangers M.: Chemistry; p. 208. [Google Scholar]